Recent Progress in Electrochemical Upgrading of Bio-Oil Model Compounds and Bio-Oils to Renewable Fuels and Platform Chemicals
Abstract
:1. Introduction
2. Electrochemical Upgrading Reactions
2.1. Electrochemical Hydrogenation/Hydrogenolysis
2.1.1. Fundamentals of the Hydrogen Evolution Reaction (HER) and ECH
2.1.2. Impact of Functional Groups on ECH Performance
2.2. Electrochemical Oxidation (ECO)
2.3. Electrochemical Dimerization
3. Factors That Influence Bio-Oil ECH Reactions
3.1. Catalysts for ECH Reactions
3.1.1. Platinum Group Metal (PGM) Catalysts
3.1.2. Non-PGM Catalysts
3.1.3. Electrode and Catalyst Support Materials
3.2. Electrochemical Cell Designs
3.2.1. Experimental Electrochemical Cell
3.2.2. Flow Type Cells
3.3. Effect of Temperature
3.4. Effect of Voltage and Current
3.5. Effect of Electrolyte
4. Electrochemical Upgrading of Real Oils
5. Environmental and Economic Analysis
6. Effect of Hydrogen Source on Hydrogenation Reactions
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma’Arof, N.A.N.B.; Hindryawati, N.; Khazaai, S.N.M.; Bhuyar, P.; Rahim, M.H.A.; Maniam, G.P. Exploitation of cost-effective renewable heterogeneous base catalyst from banana (Musa paradisiaca) peel for effective methyl ester production from soybean oil. Appl. Nanosci. 2021, 1–12. [Google Scholar] [CrossRef]
- Fankhauser, S.; Smith, S.M.; Allen, M.; Axelsson, K.; Hale, T.; Hepburn, C.; Kendall, J.M.; Khosla, R.; Lezaun, J.; Mitchell-Larson, E.; et al. The meaning of net zero and how to get it right. Nat. Clim. Chang. 2022, 12, 15–21. [Google Scholar] [CrossRef]
- United States Energy Information Agency. August 2022 Mon. Energy Rev. 2022. Available online: https://www.eia.gov/totalenergy/data/monthly/previous.php (accessed on 30 December 2022).
- Yana, S.; Nizar, M.; Irhamni; Mulyati, D. Biomass waste as a renewable energy in developing bio-based economies in Indonesia: A review. Renew. Sustain. Energy Rev. 2022, 160, 112268. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Xia, X.; Lin, C.-X.; Tong, D.-S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Lopez, G.; Santamaria, L.; Lemonidou, A.; Zhang, S.; Wu, C.; Sipra, A.T.; Gao, N. Hydrogen generation from biomass by pyrolysis. Nat. Rev. Methods Prim. 2022, 2, 1–13. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Abbasi, S.A.; Hashmi, Z.; Shah, A.K.; Alam, M.S.; Bhatti, Z.A.; Maitlo, G.; Hussain, S.; Khandro, G.A.; Usto, M.A.; et al. Recent trends and future perspectives of lignocellulose biomass for biofuel production: A comprehensive review. Biomass-Convers. Biorefinery 2021, 1–13. [Google Scholar] [CrossRef]
- Rasapoor, M.; Young, B.; Brar, R.; Sarmah, A.; Zhuang, W.-Q.; Baroutian, S. Recognizing the challenges of anaerobic digestion: Critical steps toward improving biogas generation. Fuel 2020, 261, 116497. [Google Scholar] [CrossRef]
- Kumar, G.; Dharmaraja, J.; Arvindnarayan, S.; Shoban, S.; Bakonyi, P.; Saratale, G.D.; Nemestothy, N.; Bélafi–Bakó, K.; Yoon, J.-J.; Kim, S.-H. A comprehensive review on thermochemical, biological, biochemical and hybrid conversion methods of bio-derived lignocellulosic molecules into renewable fuels. Fuel 2019, 251, 352–367. [Google Scholar] [CrossRef]
- LeClerc, H.O.; Atwi, R.; Niles, S.F.; McKenna, A.M.; Timko, M.T.; West, R.H.; Teixeira, A.R. Elucidating the role of reactive nitrogen intermediates in hetero-cyclization during hydrothermal liquefaction of food waste. Green Chem. 2022, 24, 5125–5141. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Batalha, N.; Mahmudul, H.M.; Perkins, G.; Konarova, M. A review on advanced catalytic co-pyrolysis of biomass and hydrogen-rich feedstock: Insights into synergistic effect, catalyst development and reaction mechanism. Bioresour. Technol. 2020, 310, 123457. [Google Scholar] [CrossRef]
- Ahamed, T.S.; Anto, S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Upgrading of bio-oil from thermochemical conversion of various biomass—Mechanism, challenges and opportunities. Fuel 2020, 287, 119329. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Patel, M.; Kumar, M.; Kumar, A. The Upgrading of Bio-Oil via Hydrodeoxygenation. In Chemical Catalysts for Biomass Upgrading; Wiley-VCH: Weinheim, Germany, 2020; pp. 35–60. ISBN 9783527814794. [Google Scholar]
- Raheem, A.; Wan Azlina, W.; Yap, Y.T.; Danquah, M.; Harun, R. Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Bollas, G.M.; Valla, J.A. Bifunctional Ni–ZSM–5 Catalysts for the Pyrolysis and Hydropyrolysis of Biomass. Energy Technol. 2017, 5, 172–182. [Google Scholar] [CrossRef]
- Yu, L.; Farinmade, A.; Ajumobi, O.; Su, Y.; John, V.T.; Valla, J.A. MCM-41/ZSM-5 composite particles for the catalytic fast pyrolysis of biomass. Appl. Catal. A Gen. 2020, 602, 117727. [Google Scholar] [CrossRef]
- Du, S.; Gamliel, D.P.; Valla, J.A.; Bollas, G.M. The effect of ZSM-5 catalyst support in catalytic pyrolysis of biomass and compounds abundant in pyrolysis bio-oils. J. Anal. Appl. Pyrolysis 2016, 122, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Sun, Y.; Gamliel, D.P.; Valla, J.A.; Bollas, G.M. Catalytic pyrolysis of miscanthus × giganteus in a spouted bed reactor. Bioresour. Technol. 2014, 169, 188–197. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Du, S.; Bollas, G.M.; Valla, J.A. Investigation of in situ and ex situ catalytic pyrolysis of miscanthus × giganteus using a PyGC–MS microsystem and comparison with a bench-scale spouted-bed reactor. Bioresour. Technol. 2015, 191, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Valla, J.A.; Bollas, G.M. Characteristics and origin of char and coke from fast and slow, catalytic and thermal pyrolysis of biomass and relevant model compounds. Green Chem. 2013, 15, 3214–3229. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Cho, H.J.; Fan, W.; Valla, J.A. On the effectiveness of tailored mesoporous MFI zeolites for biomass catalytic fast pyrolysis. Appl. Catal. A Gen. 2016, 522, 109–119. [Google Scholar] [CrossRef] [Green Version]
- LeClerc, H.O.; Tompsett, G.A.; Paulsen, A.D.; McKenna, A.M.; Niles, S.F.; Reddy, C.M.; Nelson, R.K.; Cheng, F.; Teixeira, A.R.; Timko, M.T. Hydroxyapatite catalyzed hydrothermal liquefaction transforms food waste from an environmental liability to renewable fuel. Iscience 2022, 25, 104916. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Hisham, M.W.; Yarmo, M.A.; Hin, T.-Y.Y. A review on bio-oil production from biomass by using pyrolysis method. Renew. Sustain. Energy Rev. 2012, 16, 5910–5923. [Google Scholar] [CrossRef]
- Valle, B.; Remiro, A.; García-Gómez, N.; Gayubo, A.G.; Bilbao, J. Recent research progress on bio-oil conversion into bio-fuels and raw chemicals: A review. J. Chem. Technol. Biotechnol. 2018, 94, 670–689. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Karakalos, S.; Valla, J.A. Liquid phase hydrodeoxygenation of anisole, 4-ethylphenol and benzofuran using Ni, Ru and Pd supported on USY zeolite. Appl. Catal. A Gen. 2018, 559, 20–29. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Bollas, G.M.; Valla, J.A. Two-stage catalytic fast hydropyrolysis of biomass for the production of drop-in biofuel. Fuel 2018, 216, 160–170. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Baillie, B.P.; Augustine, E.; Hall, J.; Bollas, G.M.; Valla, J.A. Nickel impregnated mesoporous USY zeolites for hydrodeoxygenation of anisole. Microporous Mesoporous Mater. 2018, 261, 18–28. [Google Scholar] [CrossRef]
- Yu, L.; Farinmade, A.; Ajumobi, O.; John, V.T.; Valla, J.A. One-Step Hydropyrolysis and Hydrotreating Tandem Reactions of Miscanthus × giganteus Using Ni Impregnated ZSM-5/MCM-41 Composites. Energy Fuels 2021, 35, 20117–20130. [Google Scholar] [CrossRef]
- Page, J.R.; LeClerc, H.O.; Smolitsky, P.; Esposito, J.P.; Theberge, D.P.; Zaker, A.; Maag, A.R.; Sabnis, S.; Ledford, E.B.; Coleman, J.; et al. Improving Yields and Catalyst Reuse for Palmitic Acid Aromatization in the Presence of Pressurized Water. ACS Sustain. Chem. Eng. 2022, 10, 5659–5673. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Cheng, C.K.; Bhuyar, P.; Atabani, A.; Pugazhendhi, A.; Chi, N.T.L.; Witoon, T.; Lim, J.W.; Juan, J.C. Effect of reaction conditions on the lifetime of SAPO-34 catalysts in methanol to olefins process—A review. Fuel 2020, 283, 118851. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Wang, H.; Li, H.; Han, X.; Zeng, Y.; Xu, C.C. A review of bio-oil upgrading by catalytic hydrotreatment: Advances, challenges, and prospects. Mol. Catal. 2021, 504, 111438. [Google Scholar] [CrossRef]
- Iribarren, D.; Peters, J.F.; Dufour, J. Life cycle assessment of transportation fuels from biomass pyrolysis. Fuel 2012, 97, 812–821. [Google Scholar] [CrossRef]
- Zhu, C.; Gamliel, D.P.; Valla, J.A.; Bollas, G.M. Fischer-tropsch synthesis in monolith catalysts coated with hierarchical ZSM-5. Appl. Catal. B Environ. 2020, 284, 119719. [Google Scholar] [CrossRef]
- Akorede, M.F.; Hizam, H.; Pouresmaeil, E. Distributed energy resources and benefits to the environment. Renew. Sustain. Energy Rev. 2010, 14, 724–734. [Google Scholar] [CrossRef]
- Resasco, D.E.; Wang, B.; Sabatini, D. Distributed processes for biomass conversion could aid UN Sustainable Development Goals. Nat. Catal. 2018, 1, 731–735. [Google Scholar] [CrossRef]
- Chen, G.; Liang, L.; Li, N.; Lu, X.; Yan, B.; Cheng, Z. Upgrading of Bio-Oil Model Compounds and Bio-Crude into Biofuel by Electrocatalysis: A Review. ChemSusChem 2021, 14, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Akhade, S.A.; Singh, N.; Gutiérrez, O.Y.; Lopez-Ruiz, J.; Wang, H.; Holladay, J.D.; Liu, Y.; Karkamkar, A.; Weber, R.S.; Padmaperuma, A.B.; et al. Electrocatalytic Hydrogenation of Biomass-Derived Organics: A Review. Chem. Rev. 2020, 120, 11370–11419. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Colmenares, J.C.; Tsiplakides, D.; Triantafyllidis, K.S. Nanoengineered Electrodes for Biomass-Derived 5-Hydroxymethylfurfural Electrocatalytic Oxidation to 2,5-Furandicarboxylic Acid. ACS Sustain. Chem. Eng. 2021, 9, 1970–1993. [Google Scholar] [CrossRef]
- Das, S.; Anderson, J.E.; De Kleine, R.; Wallington, T.J.; Jackson, J.E.; Saffron, C.M. Technoeconomic analysis of corn stover conversion by decentralized pyrolysis and electrocatalysis. Sustain. Energy Fuels 2022, 6, 2823–2834. [Google Scholar] [CrossRef]
- Yang, Z.; Chou, X.; Kan, H.; Xiao, Z.; Ding, Y. Nanoporous Copper Catalysts for the Fluidized Electrocatalytic Hydrogenation of Furfural to Furfuryl Alcohol. ACS Sustain. Chem. Eng. 2022, 10, 7418–7425. [Google Scholar] [CrossRef]
- Lin, F.; Tse, H.-Y.; Erythropel, H.C.; Petrovic, P.; Garedew, M.; Chen, J.; Lam, J.C.-H.; Anastas, P. Development of a Ni-Promoted, Selective Electrochemical Reductive Cleavage of the C–O bond in Lignin Model Compound Benzyl Phenyl Ether. Green Chem. 2022, 24, 6295–6305. [Google Scholar] [CrossRef]
- Xu, W.; Yu, C.; Chen, J.; Liu, Z. Electrochemical hydrogenation of biomass-based furfural in aqueous media by Cu catalyst supported on N-doped hierarchically porous carbon. Appl. Catal. B Environ. 2022, 305, 121062. [Google Scholar] [CrossRef]
- Wu, R.; Meng, Q.; Yan, J.; Liu, H.; Zhu, Q.; Zheng, L.; Zhang, J.; Han, B. Electrochemical Strategy for the Simultaneous Production of Cyclohexanone and Benzoquinone by the Reaction of Phenol and Water. J. Am. Chem. Soc. 2022, 144, 1556–1571. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Syed-Hassan, S.S.A.; Lam, C.H.; Hu, X.; Wang, X.; Xiong, Z.; Han, H.; Xu, J.; Jiang, L.; Su, S.; et al. Polymerization during low-temperature electrochemical upgrading of bio-oil: Multi-technique characterization of bio-oil evolution. Energy Convers. Manag. 2022, 253, 115165. [Google Scholar] [CrossRef]
- Deng, W.; Wang, X.; Lam, C.H.; Xiong, Z.; Han, H.; Xu, J.; Jiang, L.; Su, S.; Hu, S.; Wang, Y.; et al. Evolution of coke structures during electrochemical upgrading of bio-oil. Fuel Process. Technol. 2022, 225, 107036. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Z.; Sun, C.; Ren, X.; Li, Q. High-efficiency electrochemical hydrogenation of biomass-derived benzaldehyde compounds via a durable and versatile dendritic-like Pd/Cu-CF electrocatalyst. Fuel Process. Technol. 2022, 237, 107436. [Google Scholar] [CrossRef]
- Liu, W.; You, W.; Gong, Y.; Deng, Y. High-efficiency electrochemical hydrodeoxygenation of bio-phenols to hydrocarbon fuels by a superacid-noble metal particle dual-catalyst system. Energy Environ. Sci. 2020, 13, 917–927. [Google Scholar] [CrossRef]
- Brosnahan, J.T.; Zhang, Z.; Yin, Z.; Zhang, S. Electrocatalytic reduction of furfural with high selectivity to furfuryl alcohol using AgPd alloy nanoparticles. Nanoscale 2021, 13, 2312–2316. [Google Scholar] [CrossRef]
- May, A.S.; Watt, S.M.; Biddinger, E.J. Kinetics of furfural electrochemical hydrogenation and hydrogenolysis in acidic media on copper. React. Chem. Eng. 2021, 6, 2075–2086. [Google Scholar] [CrossRef]
- Song, Y.; Gutiérrez, O.Y.; Herranz, J.; Lercher, J.A. Aqueous phase electrocatalysis and thermal catalysis for the hydrogenation of phenol at mild conditions. Appl. Catal. B Environ. 2015, 182, 236–246. [Google Scholar] [CrossRef]
- Singh, N.; Song, Y.; Gutiérrez, O.Y.; Camaioni, D.M.; Campbell, C.T.; Lercher, J.A. Electrocatalytic Hydrogenation of Phenol over Platinum and Rhodium: Unexpected Temperature Effects Resolved. ACS Catal. 2016, 6, 7466–7470. [Google Scholar] [CrossRef]
- Li, Z.; Garedew, M.; Lam, C.H.; Jackson, J.E.; Miller, D.J.; Saffron, C.M. Mild electrocatalytic hydrogenation and hydrodeoxygenation of bio-oil derived phenolic compounds using ruthenium supported on activated carbon cloth. Green Chem. 2012, 14, 2540–2549. [Google Scholar] [CrossRef]
- Cyr, A.; Chiltz, F.; Jeanson, P.; Martel, A.; Brossard, L.; Lessard, J.; Ménard, H. Electrocatalytic hydrogenation of lignin models at Raney nickel and palladium-based electrodes. Can. J. Chem. 2000, 78, 307–315. [Google Scholar] [CrossRef]
- Song, Y.; Chia, S.H.; Sanyal, U.; Gutiérrez, O.Y.; Lercher, J.A. Integrated catalytic and electrocatalytic conversion of substituted phenols and diaryl ethers. J. Catal. 2016, 344, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Garedew, M.; Lam, C.H.; Petitjean, L.; Huang, S.; Song, B.; Lin, F.; Jackson, J.E.; Saffron, C.M.; Anastas, P.T. Electrochemical upgrading of depolymerized lignin: A review of model compound studies. Green Chem. 2021, 23, 2868–2899. [Google Scholar] [CrossRef]
- Lam, C.H.; Deng, W.; Lang, L.; Jin, X.; Hu, X.; Wang, Y. Minireview on Bio-Oil Upgrading via Electrocatalytic Hydrogenation: Connecting Biofuel Production with Renewable Power. Energy Fuels 2020, 34, 7915–7928. [Google Scholar] [CrossRef]
- Miller, L.L.; Christensen, L. Electrocatalytic hydrogenation of aromatic compounds. J. Org. Chem. 2002, 43, 2059–2061. [Google Scholar] [CrossRef]
- Sanyal, U.; Lopez-Ruiz, J.A.; Padmaperuma, A.B.; Holladay, J.; Gutiérrez, O.Y. Electrocatalytic Hydrogenation of Oxygenated Compounds in Aqueous Phase. Org. Process Res. Dev. 2018, 22, 1590–1598. [Google Scholar] [CrossRef]
- Deng, W.; Xu, K.; Xiong, Z.; Chaiwat, W.; Wang, X.; Su, S.; Hu, S.; Qiu, J.; Wang, Y.; Xiang, J. Evolution of Aromatic Structures during the Low-Temperature Electrochemical Upgrading of Bio-oil. Energy Fuels 2019, 33, 11292–11301. [Google Scholar] [CrossRef]
- Garedew, M.; Young-Farhat, D.; Jackson, J.E.; Saffron, C.M. Electrocatalytic Upgrading of Phenolic Compounds Observed after Lignin Pyrolysis. ACS Sustain. Chem. Eng. 2019, 7, 8375–8386. [Google Scholar] [CrossRef]
- Huang, S.; Wu, X.; Chen, W.; Wang, T.; Wu, Y.; He, G. A bilateral electrochemical hydrogen pump reactor for 2-propanol dehydrogenation and phenol hydrogenation. Green Chem. 2016, 18, 2353–2362. [Google Scholar] [CrossRef]
- Anibal, J.; Xu, B. Electroreductive C–C Coupling of Furfural and Benzaldehyde on Cu and Pb Surfaces. ACS Catal. 2020, 10, 11643–11653. [Google Scholar] [CrossRef]
- Li, Z.; Kelkar, S.; Lam, C.H.; Luczek, K.; Jackson, J.; Miller, D.J.; Saffron, C.M. Aqueous electrocatalytic hydrogenation of furfural using a sacrificial anode. Electrochim. Acta 2012, 64, 87–93. [Google Scholar] [CrossRef]
- Doherty, A.P.; A Brooks, C. Electrosynthesis in room-temperature ionic liquids: Benzaldehyde reduction. Electrochim. Acta 2004, 49, 3821–3826. [Google Scholar] [CrossRef]
- Song, Y.; Sanyal, U.; Pangotra, D.; Holladay, J.D.; Camaioni, D.M.; Gutiérrez, O.Y.; Lercher, J.A. Hydrogenation of benzaldehyde via electrocatalysis and thermal catalysis on carbon-supported metals. J. Catal. 2018, 359, 68–75. [Google Scholar] [CrossRef]
- Mortensen, J.; Heinze, J. The Electrochemical Reduction of Benzene—First Direct Determination of the Reduction Potential. Angew. Chem. Int. Ed. 1984, 23, 84–85. [Google Scholar] [CrossRef]
- Fichter, F.; Stocker, R. Die elektrochemische Oxydation aromatischer Kohlenwasser-stoffe und Phenole. Eur. J. Inorg. Chem. 1914, 47, 2003–2019. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, Q.; Fu, Y. Electrocatalytic Hydrogenation of Lignin-Derived Phenol into Alkanes by Using Platinum Supported on Graphite. Electrochemistry 2014, 82, 954–959. [Google Scholar] [CrossRef] [Green Version]
- Wijaya, Y.P.; Grossmann-Neuhaeusler, T.; Putra, R.D.D.; Smith, K.J.; Kim, C.S.; Gyenge, E.L. Electrocatalytic Hydrogenation of Guaiacol in Diverse Electrolytes Using a Stirred Slurry Reactor. Chemsuschem 2020, 13, 629–639. [Google Scholar] [CrossRef]
- Lam, C.H.; Lowe, C.B.; Li, Z.; Longe, K.N.; Rayburn, J.T.; Caldwell, M.A.; Houdek, C.E.; Maguire, J.B.; Saffron, C.M.; Miller, D.J.; et al. Electrocatalytic upgrading of model lignin monomers with earth abundant metal electrodes. Green Chem. 2014, 17, 601–609. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Putra, R.D.D.; Smith, K.J.; Kim, C.S.; Gyenge, E.L. Guaiacol Hydrogenation in Methanesulfonic Acid Using a Stirred Slurry Electrocatalytic Reactor: Mass Transport and Reaction Kinetics Aspects. ACS Sustain. Chem. Eng. 2021, 9, 13164–13175. [Google Scholar] [CrossRef]
- Peng, T.; Zhuang, T.; Yan, Y.; Qian, J.; Dick, G.R.; de Bueren, J.B.; Hung, S.-F.; Zhang, Y.; Wang, Z.; Wicks, J.; et al. Ternary Alloys Enable Efficient Production of Methoxylated Chemicals via Selective Electrocatalytic Hydrogenation of Lignin Monomers. J. Am. Chem. Soc. 2021, 143, 17226–17235. [Google Scholar] [CrossRef]
- Wang, M.; Peng, T.; Yang, C.; Liang, B.; Chen, H.; Kumar, M.; Zhang, Y.; Zhao, W. Electrocatalytic hydrogenation of lignin monomer to methoxy-cyclohexanes with high faradaic efficiency. Green Chem. 2021, 24, 142–146. [Google Scholar] [CrossRef]
- Akhade, S.A.; Lee, M.-S.; Meyer, L.C.; Yuk, S.F.; Nguyen, M.-T.; Sanyal, U.; Egbert, J.D.; Gutiérrez, O.Y.; Glezakou, V.-A.; Rousseau, R. Impact of functional groups on the electrocatalytic hydrogenation of aromatic carbonyls to alcohols. Catal. Today 2022, 397–399, 63–68. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Akhade, S.A.; Cantu, D.C.; Lee, M.-S.; Glezakou, V.-A.; Rousseau, R. Electro-reduction of organics on metal cathodes: A multiscale-modeling study of benzaldehyde on Au (111). Catal. Today 2020, 350, 39–46. [Google Scholar] [CrossRef]
- Singh, N.; Sanyal, U.; Ruehl, G.; Stoerzinger, K.A.; Gutiérrez, O.Y.; Camaioni, D.M.; Fulton, J.L.; Lercher, J.A.; Campbell, C.T. Aqueous phase catalytic and electrocatalytic hydrogenation of phenol and benzaldehyde over platinum group metals. J. Catal. 2020, 382, 372–384. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chou, T.-C. Electrochemical reduction of benzaldehyde using Pt-Pb/Nafion® as electrode. J. Appl. Electrochem. 1994, 24, 434–438. [Google Scholar] [CrossRef]
- Sanyal, U.; Yuk, S.F.; Koh, K.; Lee, M.; Stoerzinger, K.; Zhang, D.; Meyer, L.C.; Lopez-Ruiz, J.A.; Karkamkar, A.; Holladay, J.D.; et al. Hydrogen Bonding Enhances the Electrochemical Hydrogenation of Benzaldehyde in the Aqueous Phase. Angew. Chem. Int. Ed. 2020, 60, 290–296. [Google Scholar] [CrossRef]
- Birkett, M.; Kuhn, A. The electrochemical reduction of Benzaldehyde. Electrochim. Acta 1980, 25, 273–278. [Google Scholar] [CrossRef]
- Lopez-Ruiz, J.A.; Sanyal, U.; Egbert, J.D.; Gutiérrez, O.Y.; Holladay, J. Kinetic Investigation of the Sustainable Electrocatalytic Hydrogenation of Benzaldehyde on Pd/C: Effect of Electrolyte Composition and Half-Cell Potentials. ACS Sustain. Chem. Eng. 2018, 6, 16073–16085. [Google Scholar] [CrossRef]
- Meyer, L.C.; Sanyal, U.; Stoerzinger, K.A.; Koh, K.; Fulton, J.L.; Camaioni, D.M.; Gutiérrez, O.Y.; Lercher, J.A. Influence of the Molecular Structure on the Electrocatalytic Hydrogenation of Carbonyl Groups and H2 Evolution on Pd. ACS Catal. 2022, 11910–11917. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Michel, F.C.; Co, A.C. Efficient Electrochemical Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Bis(hydroxymethyl)furan on Ag-Displaced Nanotextured Cu Catalysts. ChemElectroChem 2019, 6, 4739–4749. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.; Yang, Y.; Chang, J.; Borgna, A. Hydrogenation of Furfural as Model Reaction of Bio-Oil Stabilization under Mild Conditions Using Multiwalled Carbon Nanotube (MWNT)-Supported Pt Catalysts. Ind. Eng. Chem. Res. 2014, 53, 11284–11291. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Parpot, P.; Bettencourt, A.; Chamoulaud, G.; Kokoh, K.; Belgsir, E. Electrochemical investigations of the oxidation–reduction of furfural in aqueous medium: Application to electrosynthesis. Electrochim. Acta 2004, 49, 397–403. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Wang, B.-X.; Qin, L.; Li, Q.; Fan, Y.-M. A non-noble bimetallic alloy in the highly selective electrochemical synthesis of the biofuel 2,5-dimethylfuran from 5-hydroxymethylfurfural. Green Chem. 2019, 21, 1108–1113. [Google Scholar] [CrossRef]
- Ji, K.; Xu, M.; Xu, S.; Wang, Y.; Ge, R.; Hu, X.; Sun, X.; Duan, H. Electrocatalytic Hydrogenation of 5-Hydroxymethylfurfural Promoted by a Ru 1 Cu Single-Atom Alloy Catalyst. Angew. Chem. Int. Ed. 2022, 61. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Birdja, Y.; Raoufmoghaddam, S.; Koper, M.T.M. Electrocatalytic Hydrogenation of 5-Hydroxymethylfurfural in Acidic Solution. ChemSusChem 2015, 8, 1745–1751. [Google Scholar] [CrossRef]
- Das, L.; Kolar, P.; Sharmashivappa, R.R. Heterogeneous catalytic oxidation of lignin into value-added chemicals. Biofuels 2012, 3, 155–166. [Google Scholar] [CrossRef]
- Kuznetsov, B.; Sudakova, I.; Garyntseva, N.; Tarabanko, V.; Yatsenkova, O.; Djakovitch, L.; Rataboul, F. Processes of catalytic oxidation for the production of chemicals from softwood biomass. Catal. Today 2021, 375, 132–144. [Google Scholar] [CrossRef]
- Yang, Y.; Mu, T. Electrochemical oxidation of biomass derived 5-hydroxymethylfurfural (HMF): Pathway, mechanism, catalysts and coupling reactions. Green Chem. 2021, 23, 4228–4254. [Google Scholar] [CrossRef]
- Luo, H.; Barrio, J.; Sunny, N.; Li, A.; Steier, L.; Shah, N.; Stephens, I.E.L.; Titirici, M. Progress and Perspectives in Photo- and Electrochemical-Oxidation of Biomass for Sustainable Chemicals and Hydrogen Production. Adv. Energy Mater. 2021, 11. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Zhu, B.; Zhou, Z.; El-Hout, S.I.; Yang, J.; Zhang, J. 2,5-Furandicarboxylic acid production via catalytic oxidation of 5-hydroxymethylfurfural: Catalysts, processes and reaction mechanism. J. Energy Chem. 2020, 54, 528–554. [Google Scholar] [CrossRef]
- Andrews, E.M.; Egbert, J.D.; Sanyal, U.; Holladay, J.D.; Weber, R.S. Anode-Boosted Electrolysis in Electrochemical Upgrading of Bio-oils and in the Production of H2. Energy Fuels 2019, 34, 1162–1165. [Google Scholar] [CrossRef]
- You, B.; Liu, X.; Jiang, N.; Sun, Y. A General Strategy for Decoupled Hydrogen Production from Water Splitting by Integrating Oxidative Biomass Valorization. J. Am. Chem. Soc. 2016, 138, 13639–13646. [Google Scholar] [CrossRef] [PubMed]
- Bambagioni, V.; Bevilacqua, M.; Bianchini, C.; Filippi, J.; Lavacchi, A.; Marchionni, A.; Vizza, F.; Shen, P.K. Self-Sustainable Production of Hydrogen, Chemicals, and Energy from Renewable Alcohols by Electrocatalysis. ChemSusChem 2010, 3, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, D.; Zhao, Y.; Song, C.; Wang, D. In-suit growth of NiS quantum dots embedded in ultra-thin N,O,S-tri-doped carbon porous nanosheets on carbon cloth for high-efficient HMF oxidation coupling hydrogen evolution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129597. [Google Scholar] [CrossRef]
- Huang, X.; Zou, Y.; Jiang, J. Electrochemical Oxidation of Glycerol to Dihydroxyacetone in Borate Buffer: Enhancing Activity and Selectivity by Borate–Polyol Coordination Chemistry. ACS Sustain. Chem. Eng. 2021, 9, 14470–14479. [Google Scholar] [CrossRef]
- Zhang, X.; Han, M.; Liu, G.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Simultaneously high-rate furfural hydrogenation and oxidation upgrading on nanostructured transition metal phosphides through electrocatalytic conversion at ambient conditions. Appl. Catal. B Environ. 2019, 244, 899–908. [Google Scholar] [CrossRef]
- Kiss, L.; Kunsági-Máté, S. Electrochemical oxidation of benzaldehyde and hydroxybenzaldehydes in acetonitrile on platinum and glassy carbon electrodes. C. R. Chim. 2019, 22, 557–561. [Google Scholar] [CrossRef]
- Hui, W.; Xiujuan, Y.; Lan, W.; Qiang, W.; Dezhi, S. A Comparative Study on Electrochemical Oxidation of Phenol in Two Types of Cells. Russ. J. Electrochem. 2005, 41, 719–724. [Google Scholar] [CrossRef]
- Qin, M.; Fan, S.; Li, X.; Tade, M.O.; Liu, S. Electroreductive C O coupling of benzaldehyde over SACs Au–NiMn2O4 spinel synergetic composites. J. Colloid Interface Sci. 2022, 625, 305–316. [Google Scholar] [CrossRef]
- Anibal, J.; Malkani, A.; Xu, B. Stability of the ketyl radical as a descriptor in the electrochemical coupling of benzaldehyde. Catal. Sci. Technol. 2020, 10, 3181–3194. [Google Scholar] [CrossRef]
- Kloth, R.; Vasilyev, D.V.; Mayrhofer, K.J.J.; Katsounaros, I. Electroreductive 5-Hydroxymethylfurfural Dimerization on Carbon Electrodes. Chemsuschem 2021, 14, 5245–5253. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; del Pozo, M.; Calvo, E.J. Electrocatalytic hydrogenation of acetophenone and benzophenone using palladium electrodes. Electrochim. Acta 2015, 164, 125–131. [Google Scholar] [CrossRef]
- Dixit, R.J.; Bhattacharyya, K.; Ramani, V.K.; Basu, S. Electrocatalytic hydrogenation of furfural using non-noble-metal electrocatalysts in alkaline medium. Green Chem. 2021, 23, 4201–4212. [Google Scholar] [CrossRef]
- de Luna, G.S.; Ho, P.H.; Sacco, A.; Hernández, S.; Velasco-Vélez, J.-J.; Ospitali, F.; Paglianti, A.; Albonetti, S.; Fornasari, G.; Benito, P. AgCu Bimetallic Electrocatalysts for the Reduction of Biomass-Derived Compounds. ACS Appl. Mater. Interfaces 2021, 13, 23675–23688. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Zhong, X.; Jiang, W.; Liang, Y.; Niu, P.; Li, M.; Zhuang, G.; Li, X.; Wang, J. Electrocatalytic Upgrading of Lignin-Derived Bio-Oil Based on Surface-Engineered PtNiB Nanostructure. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Sanyal, U.; Koh, K.; Meyer, L.C.; Karkamkar, A.; Gutiérrez, O.Y. Simultaneous electrocatalytic hydrogenation of aldehydes and phenol over carbon-supported metals. J. Appl. Electrochem. 2020, 51, 27–36. [Google Scholar] [CrossRef]
- Barth, I.; Akinola, J.; Lee, J.; Gutiérrez, O.Y.; Sanyal, U.; Singh, N.; Goldsmith, B.R. Explaining the structure sensitivity of Pt and Rh for aqueous-phase hydrogenation of phenol. J. Chem. Phys. 2022, 156, 104703. [Google Scholar] [CrossRef]
- Lopez-Ruiz, J.A.; Andrews, E.M.; Akhade, S.A.; Lee, M.-S.; Koh, K.; Sanyal, U.; Yuk, S.F.; Karkamkar, A.J.; Derewinski, M.A.; Holladay, J.; et al. Understanding the Role of Metal and Molecular Structure on the Electrocatalytic Hydrogenation of Oxygenated Organic Compounds. ACS Catal. 2019, 9, 9964–9972. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, M.; Guo, Q.; Fu, Y. Electrocatalytic hydrogenation of furfural to furfuryl alcohol using platinum supported on activated carbon fibers. Electrochim. Acta 2014, 135, 139–146. [Google Scholar] [CrossRef]
- Amouzegar, K.; Savadogo, O. Electrocatalytic hydrogenation of phenol on dispersed Pt: Effect of metal electrochemically active surface area and electrode material. J. Appl. Electrochem. 1997, 27, 539–542. [Google Scholar] [CrossRef]
- Sasaki, K.; Kunai, A.; Harada, J.; Nakabori, S. Electrolytic hydrogenation of phenols in aqueous acid solutions. Electrochim. Acta 1983, 28, 671–674. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Smith, K.J.; Kim, C.S.; Gyenge, E.L. Synergistic effects between electrocatalyst and electrolyte in the electrocatalytic reduction of lignin model compounds in a stirred slurry reactor. J. Appl. Electrochem. 2020, 51, 51–63. [Google Scholar] [CrossRef]
- Andrews, E.M.; Lopez-Ruiz, J.A.; Egbert, J.D.; Koh, K.; Sanyal, U.; Song, M.; Li, D.; Karkamkar, A.J.; Derewinski, M.A.; E Holladay, J.; et al. Performance of Base and Noble Metals for Electrocatalytic Hydrogenation of Bio-Oil-Derived Oxygenated Compounds. ACS Sustain. Chem. Eng. 2020, 8, 4407–4418. [Google Scholar] [CrossRef]
- Koh, K.; Sanyal, U.; Lee, M.; Cheng, G.; Song, M.; Glezakou, V.; Liu, Y.; Li, D.; Rousseau, R.; Gutiérrez, O.Y.; et al. Electrochemically Tunable Proton-Coupled Electron Transfer in Pd-Catalyzed Benzaldehyde Hydrogenation. Angew. Chem. Int. Ed. 2020, 59, 1501–1505. [Google Scholar] [CrossRef] [Green Version]
- Laplante, F.; Brossard, L.; Ménard, H. Considerations about phenol electrohydrogenation on electrodes made with reticulated vitreous carbon cathode. Can. J. Chem. 2003, 81, 258–264. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Zhong, Z. Low-Energy Mild Electrocatalytic Hydrogenation of Bio-oil Using Ruthenium Anchored in Ordered Mesoporous Carbon. ACS Appl. Energy Mater. 2018, 1, 6758–6763. [Google Scholar] [CrossRef]
- Garedew, M.; Young-Farhat, D.; Bhatia, S.; Hao, P.; Jackson, J.E.; Saffron, C.M. Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon cloth. Sustain. Energy Fuels 2020, 4, 1340–1350. [Google Scholar] [CrossRef]
- Chu, Y.; Sanyal, U.; Li, X.S.; Qiu, Y.; Song, M.; Engelhard, M.H.; Davidson, S.D.; Koh, K.; Meyer, L.C.; Zheng, J.; et al. Tuning proton transfer and catalytic properties in triple junction nanostructured catalyts. Nano Energy 2021, 86, 106046. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Peng, M.; Zhang, Q.; Liu, J.; Zhang, J.; Fu, Y.; Li, W. Highly selective electrocatalytic hydrogenation of 5-hydroxymethylfurfural to 2,5-dihydroxymethylfuran over AgCu nanoalloys. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Amouzegar, K.; Savadogo, O. Electrocatalytic hydrogenation of phenol on highly dispersed Pt electrodes. Electrochim. Acta 1994, 39, 557–559. [Google Scholar] [CrossRef]
- Le Bras, J.; Chatterjee, D.; Muzart, J. A simple one-pot synthesis of β-alkoxy alcohols from alkenes. Tetrahedron Lett. 2005, 46, 4741–4743. [Google Scholar] [CrossRef]
- Menegazzo, F.; Canton, P.; Pinna, F.; Pernicone, N. Bimetallic Pd–Au catalysts for benzaldehyde hydrogenation: Effects of preparation and of sulfur poisoning. Catal. Commun. 2008, 9, 2353–2356. [Google Scholar] [CrossRef]
- Zhou, P.; Li, L.; Mosali, V.S.S.; Chen, Y.; Luan, P.; Gu, Q.; Turner, D.R.; Huang, L.; Zhang, J. Electrochemical Hydrogenation of Furfural in Aqueous Acetic Acid Media with Enhanced 2-Methylfuran Selectivity Using CuPd Bimetallic Catalysts. Angew. Chem. Int. Ed. 2022, 61. [Google Scholar] [CrossRef]
- Bancroft, W.D.; George, A.B. The Reduction of Phenol. J. Electrochem. Soc. 1930, 57, 399–406. [Google Scholar] [CrossRef]
- Pacut, R.I.; Kariv-Miller, E. Birch-type reductions in aqueous media. Benzo[b]thiophene and diphenyl ether. J. Org. Chem. 2002, 51, 3468–3470. [Google Scholar] [CrossRef]
- Sabri, M.A.; Bharath, G.; Hai, A.; Abu Haija, M.; Nogueira, R.P.; Banat, F. Synthesis of molybdenum-cobalt nanoparticles decorated on date seed-derived activated carbon for the simultaneous electrochemical hydrogenation and oxidation of furfural into fuels. Fuel Process. Technol. 2022, 238, 107525. [Google Scholar] [CrossRef]
- Chiba, T.; Okimoto, M.; Nagai, H.; Takata, Y. Electrocatalytic Reduction Using Raney Nickel. Bull. Chem. Soc. Jpn. 1983, 56, 719–723. [Google Scholar] [CrossRef]
- Fujihira, M.; Yokozawa, A.; Kinoshita, H.; Osa, T. Asymmetric Synthesis by modified raney nickel powder electrodes. Chem Lett 1982, 1089–1092. [Google Scholar] [CrossRef] [Green Version]
- Behrouzi, L.; Zand, Z.; Fotuhi, M.; Kaboudin, B.; Najafpour, M.M. Water oxidation couples to electrocatalytic hydrogenation of carbonyl compounds and unsaturated carbon–carbon bonds by nickel. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Mahdavi, B.; Lafrance, A.; Martel, A.; Lessard, J.; Me’nard, H.; Brossard, L. Electrocatalytic hydrogenolysis of lignin model dimers at Raney nickel electrodes. J. Appl. Electrochem. 1997, 27, 605–611. [Google Scholar] [CrossRef]
- Nilges, P.; Schröder, U. Electrochemistry for biofuel generation: Production of furans by electrocatalytic hydrogenation of furfurals. Energy Environ. Sci. 2013, 6, 2925–2931. [Google Scholar] [CrossRef]
- Jung, S.; Karaiskakis, A.N.; Biddinger, E.J. Enhanced activity for electrochemical hydrogenation and hydrogenolysis of furfural to biofuel using electrodeposited Cu catalysts. Catal. Today 2019, 323, 26–34. [Google Scholar] [CrossRef]
- Jung, S.; Biddinger, E.J. Controlling Competitive Side Reactions in the Electrochemical Upgrading of Furfural to Biofuel. Energy Technol. 2018, 6, 1370–1379. [Google Scholar] [CrossRef]
- Jung, S.; Biddinger, E.J. Electrocatalytic Hydrogenation and Hydrogenolysis of Furfural and the Impact of Homogeneous Side Reactions of Furanic Compounds in Acidic Electrolytes. ACS Sustain. Chem. Eng. 2016, 4, 6500–6508. [Google Scholar] [CrossRef]
- May, A.S.; Biddinger, E.J. Modeling Competing Kinetics between Electrochemical Reduction of Furfural on Copper and Homogeneous Side Reactions in Acid. Energy Fuels 2022. [Google Scholar] [CrossRef]
- Kwon, Y.; De Jong, E.; Raoufmoghaddam, S.; Koper, M.T.M. Electrocatalytic Hydrogenation of 5-Hydroxymethylfurfural in the Absence and Presence of Glucose. ChemSusChem 2013, 6, 1659–1667. [Google Scholar] [CrossRef]
- Yang, G.; Jiao, Y.; Yan, H.; Xie, Y.; Tian, C.; Wu, A.; Wang, Y.; Fu, H. Unraveling the mechanism for paired electrocatalysis of organics with water as a feedstock. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Polcaro, A.M.; Palmas, S.; Dernini, S. Role of catalyst characteristics in electrocatalytic hydrogenation: Reduction of benzaldehyde and acetophenone on carbon felt/palladium electrodes. Ind. Eng. Chem. Res. 1993, 32, 1315–1322. [Google Scholar] [CrossRef]
- Wang, X.; Deng, W.; Lam, C.H.; Xiong, Y.; Xiong, Z.; Xu, J.; Jiang, L.; Su, S.; Hu, S.; Wang, Y.; et al. Coke formation and its impacts during electrochemical upgrading of bio-oil. Fuel 2021, 306, 121664. [Google Scholar] [CrossRef]
- May, A.S.; Biddinger, E.J. Strategies to Control Electrochemical Hydrogenation and Hydrogenolysis of Furfural and Minimize Undesired Side Reactions. ACS Catal. 2020, 10, 3212–3221. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Q.; Han, S.; Hse, C.-Y.; Jiang, J.; Xu, J. Electrocatalytic hydrogenation of mono- and dimeric lignin to hydrocarbons in fluidized electrocatalytic system. Fuel Process. Technol. 2022, 227, 107109. [Google Scholar] [CrossRef]
- Chen, W.; He, G.; Ge, F.; Xiao, W.; Benziger, J.; Wu, X. Effects of Hydrophobicity of Diffusion Layer on the Electroreduction of Biomass Derivatives in Polymer Electrolyte Membrane Reactors. ChemSusChem 2015, 8, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Guena, T.; Pletcher, D.; Qiao, X.; Pascal, J.R.A.; Bard, A.J.; Francis, G.W.; Szúnyog, J.; Långström, B. Electrosyntheses from Aromatic Aldehydes in a Flow Cell. Part II. The Cross-Coupling of Benzaldehydes to Unsymmetrical Diols. Acta Chem. Scand. 1998, 52, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Zhang, Z.; Qi, J.; Chadderdon, D.J.; Qiu, Y.; Warsko, K.M.; Li, W. Electricity Storage in Biofuels: Selective Electrocatalytic Reduction of Levulinic Acid to Valeric Acid or γ-Valerolactone. ChemSusChem 2013, 6, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Benziger, J.; Nehlsen, J. A Polymer Electrolyte Hydrogen Pump Hydrogenation Reactor. Ind. Eng. Chem. Res. 2010, 49, 11052–11060. [Google Scholar] [CrossRef]
- Sáez, A.; García-García, V.; Solla-Gullón, J.; Aldaz, A.; Montiel, V. Electrocatalytic hydrogenation of acetophenone using a Polymer Electrolyte Membrane Electrochemical Reactor. Electrochim. Acta 2013, 91, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Green, S.K.; Tompsett, G.A.; Kim, H.J.; Kim, W.B.; Huber, G.W. Electrocatalytic Reduction of Acetone in a Proton-Exchange-Membrane Reactor: A Model Reaction for the Electrocatalytic Reduction of Biomass. ChemSusChem 2012, 5, 2410–2420. [Google Scholar] [CrossRef]
- Green, S.K.; Lee, J.; Kim, H.J.; Tompsett, G.A.; Kim, W.B.; Huber, G.W. The electrocatalytic hydrogenation of furanic compounds in a continuous electrocatalytic membrane reactor. Green Chem. 2013, 15, 1869–1879. [Google Scholar] [CrossRef]
- Diaz, L.A.; Lister, T.E.; Rae, C.; Wood, N.D. Anion Exchange Membrane Electrolyzers as Alternative for Upgrading of Biomass-Derived Molecules. ACS Sustain. Chem. Eng. 2018, 6, 8458–8467. [Google Scholar] [CrossRef]
- Lister, T.E.; Diaz, L.A.; Lilga, M.A.; Padmaperuma, A.; Lin, Y.J.; Palakkal, V.M.; Arges, C.G. Low-Temperature Electrochemical Upgrading of Bio-oils Using Polymer Electrolyte Membranes. Energy Fuels 2018, 32, 5944–5950. [Google Scholar] [CrossRef]
- Shang, X.; Yang, Y.; Sun, Y. Electrohydrodimerization of biomass-derived furfural generates a jet fuel precursor. Green Chem. 2020, 22, 5395–5401. [Google Scholar] [CrossRef]
- Li, Z.; Kelkar, S.; Raycraft, L.; Garedew, M.; Jackson, J.E.; Miller, D.J.; Saffron, C.M. A mild approach for bio-oil stabilization and upgrading: Electrocatalytic hydrogenation using ruthenium supported on activated carbon cloth. Green Chem. 2014, 16, 844–852. [Google Scholar] [CrossRef]
- Tu, Q.; Parvatker, A.; Garedew, M.; Harris, C.; Eckelman, M.; Zimmerman, J.B.; Anastas, P.T.; Lam, C.H. Electrocatalysis for Chemical and Fuel Production: Investigating Climate Change Mitigation Potential and Economic Feasibility. Environ. Sci. Technol. 2021, 55, 3240–3249. [Google Scholar] [CrossRef]
- Lam, C.H.; Das, S.; Erickson, N.C.; Hyzer, C.D.; Garedew, M.; Anderson, J.E.; Wallington, T.J.; Tamor, M.A.; Jackson, J.E.; Saffron, C.M. Towards sustainable hydrocarbon fuels with biomass fast pyrolysis oil and electrocatalytic upgrading. Sustain. Energy Fuels 2017, 1, 258–266. [Google Scholar] [CrossRef]
- Orella, M.J.; Brown, S.M.; Leonard, M.E.; Román-Leshkov, Y.; Brushett, F.R. A General Technoeconomic Model for Evaluating Emerging Electrolytic Processes. Energy Technol. 2020, 8, 1900994. [Google Scholar] [CrossRef]

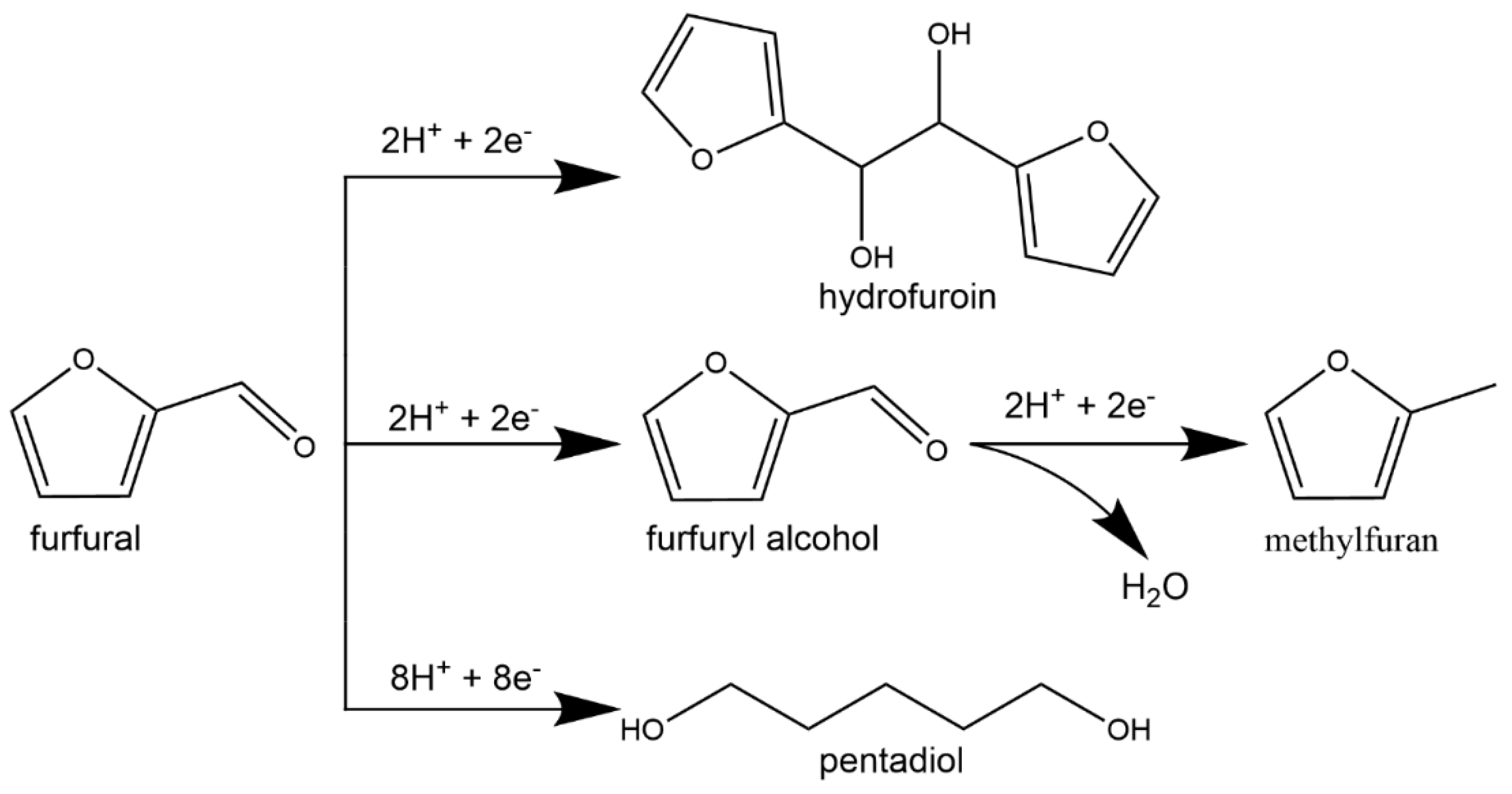
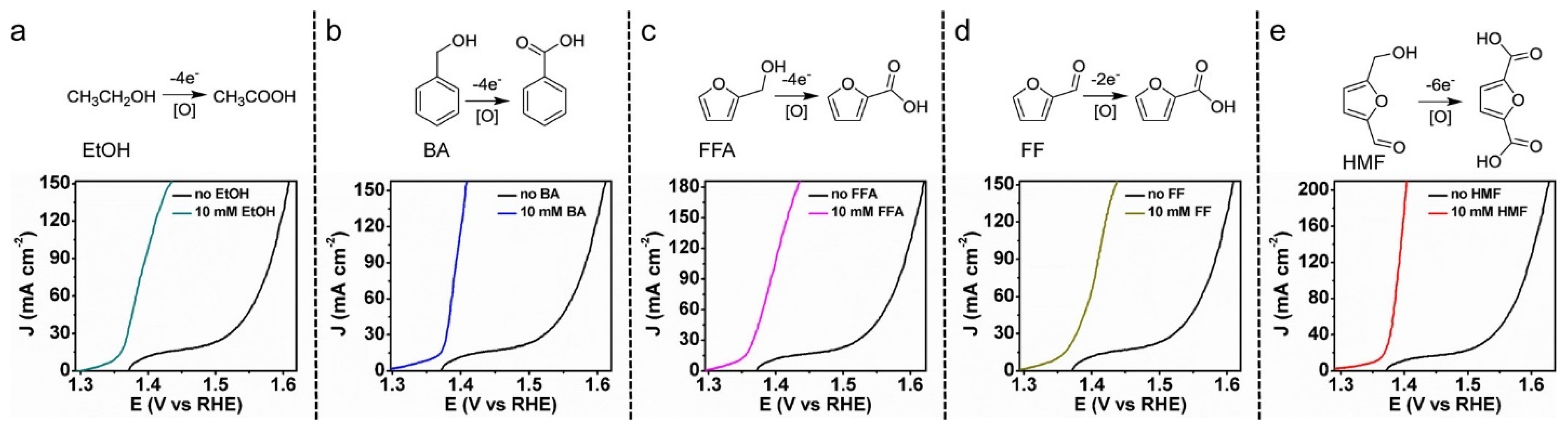
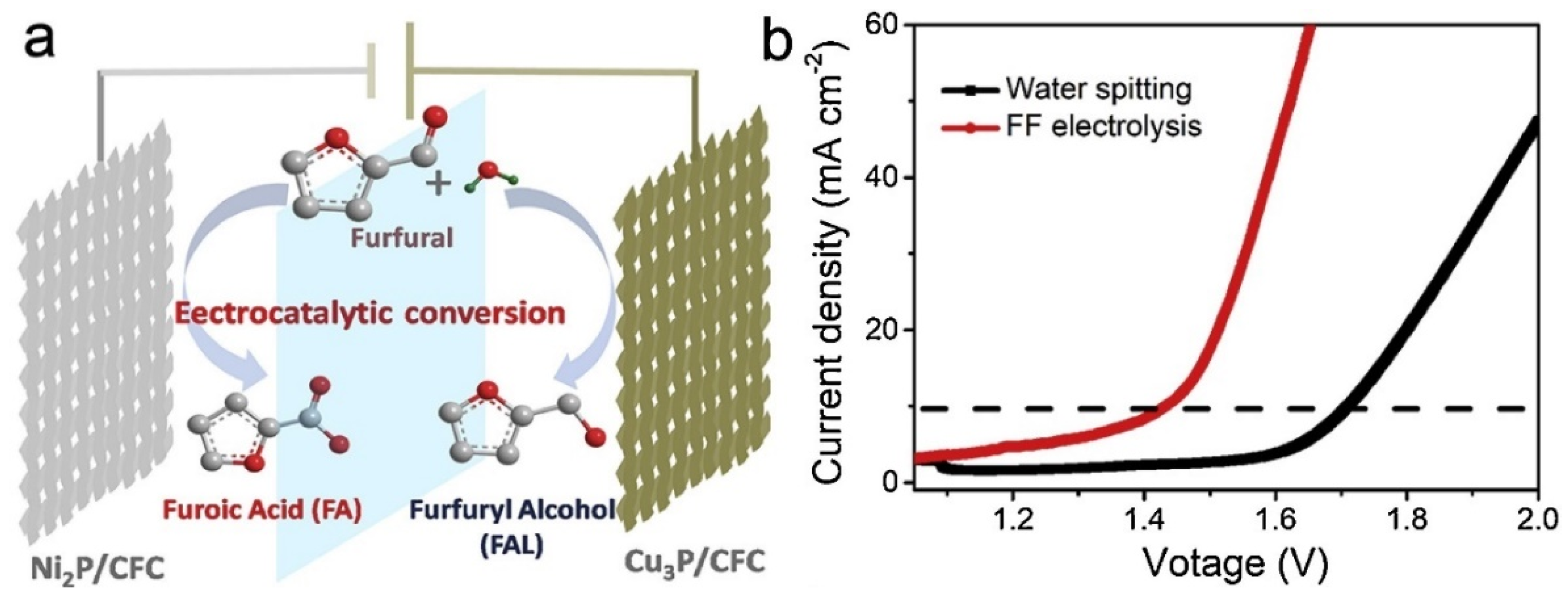
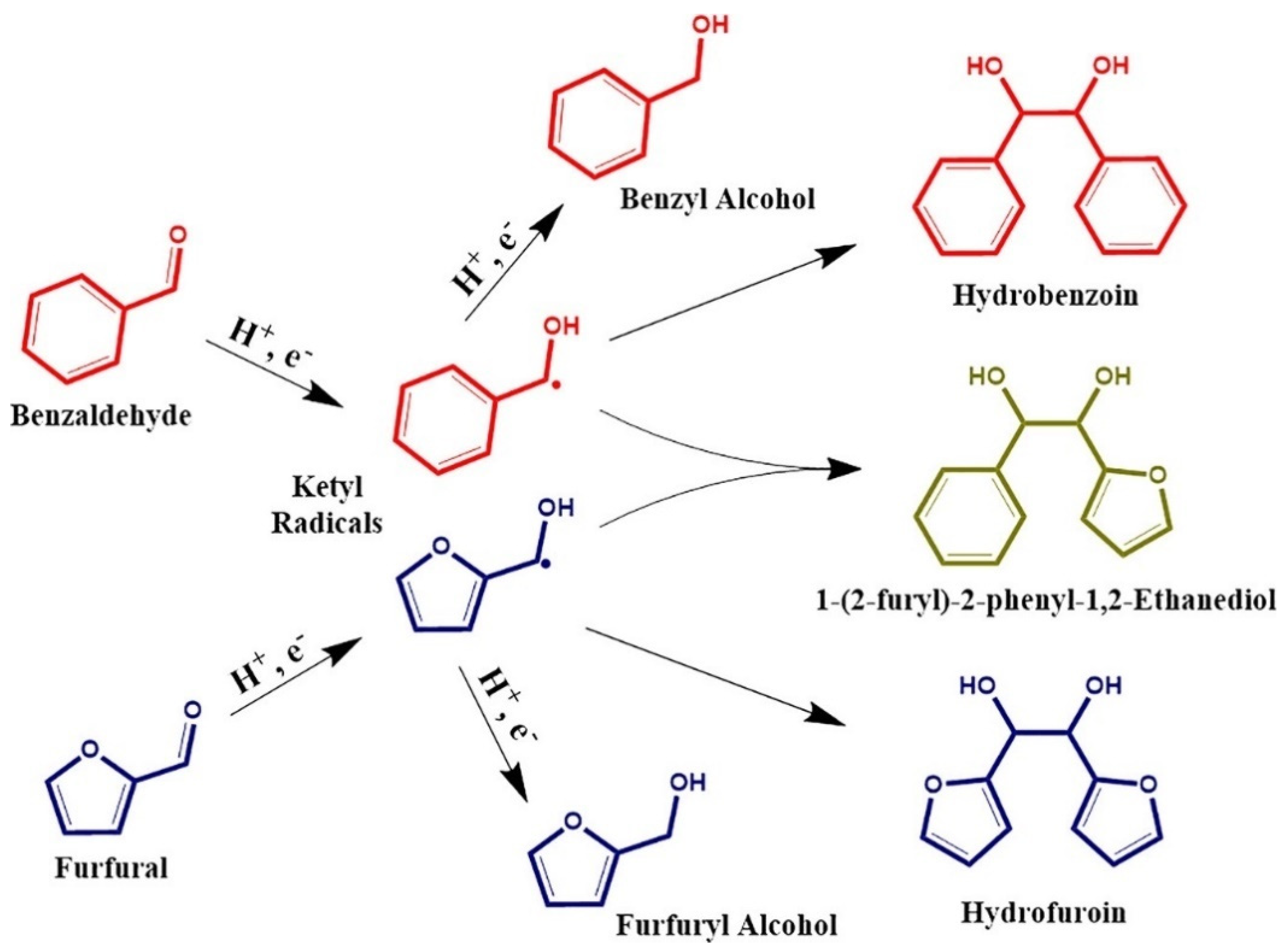
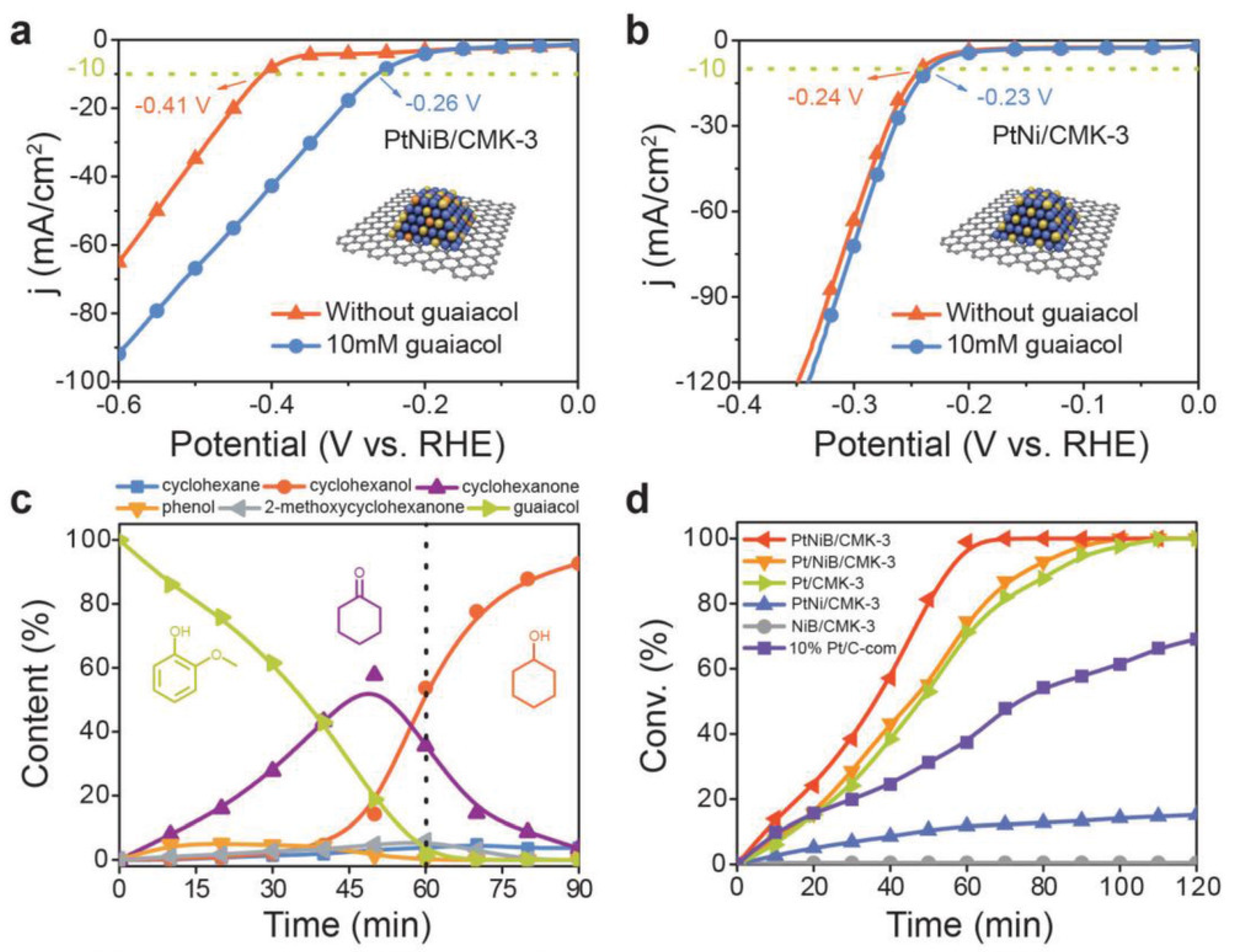
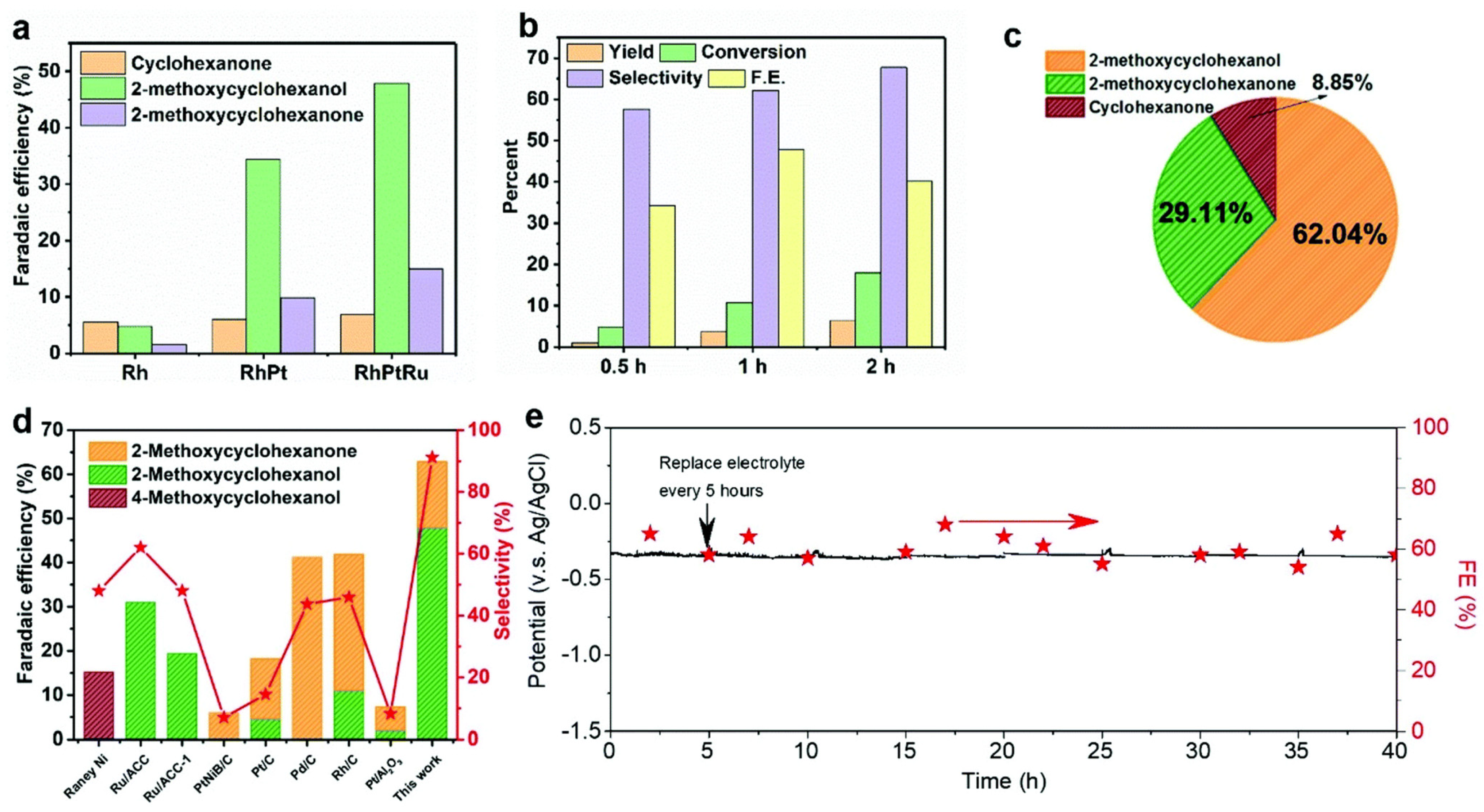
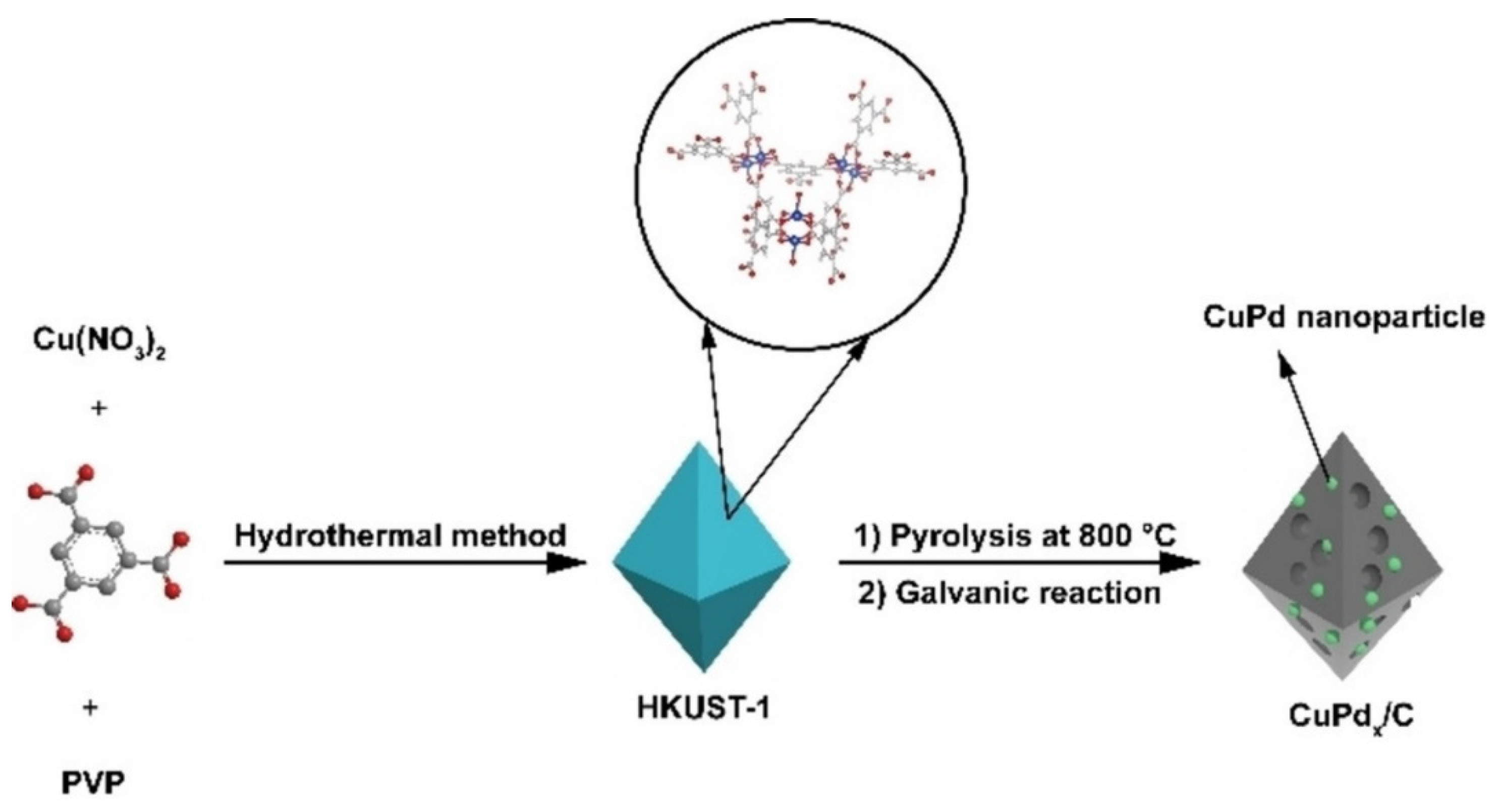
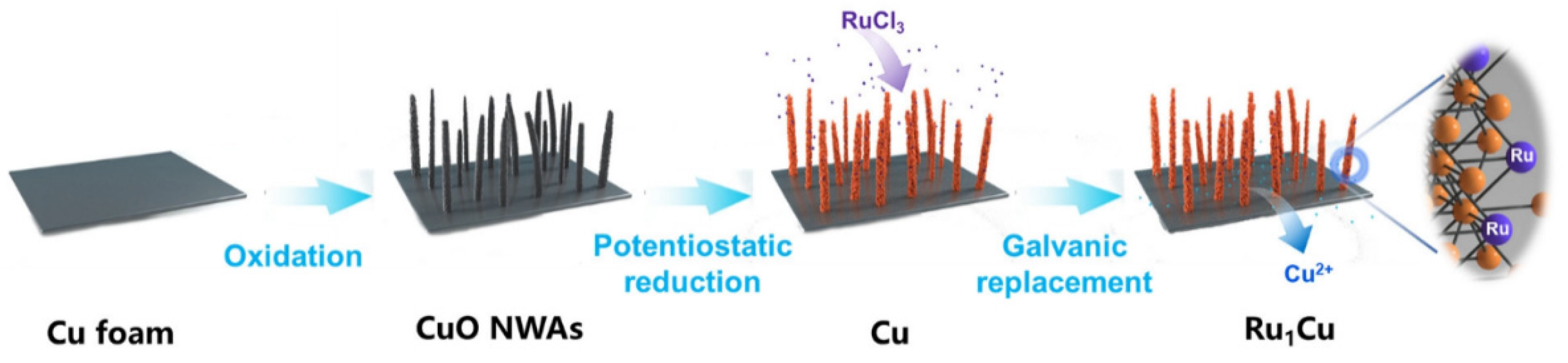
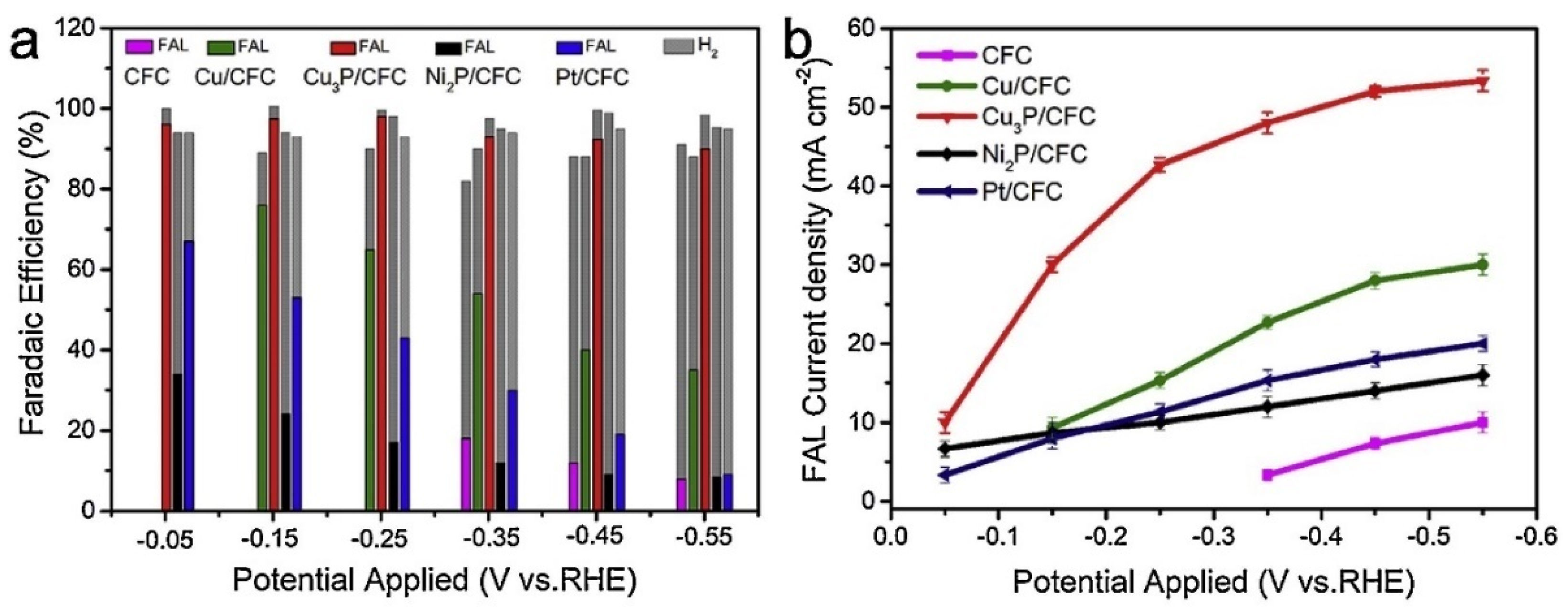
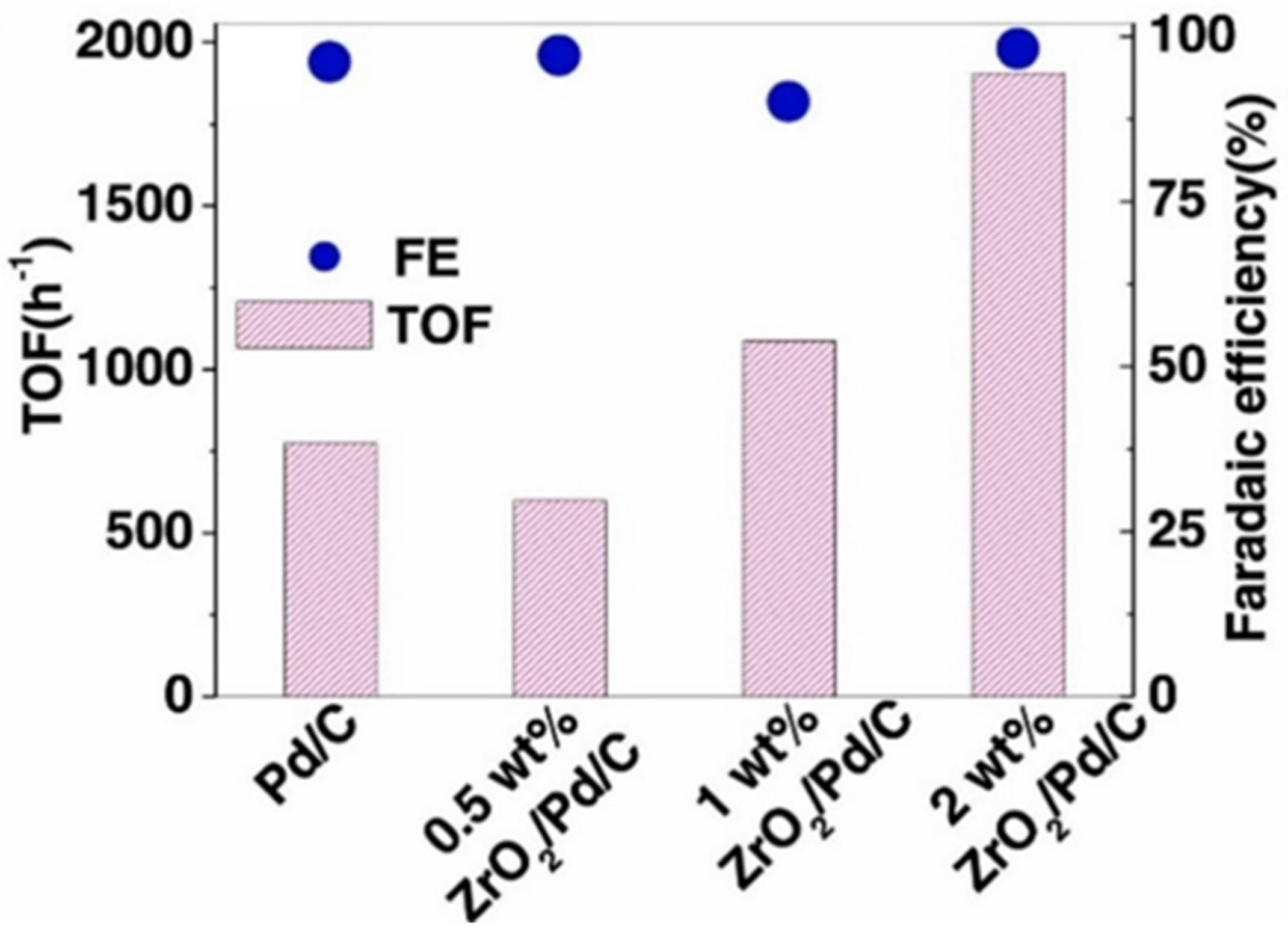
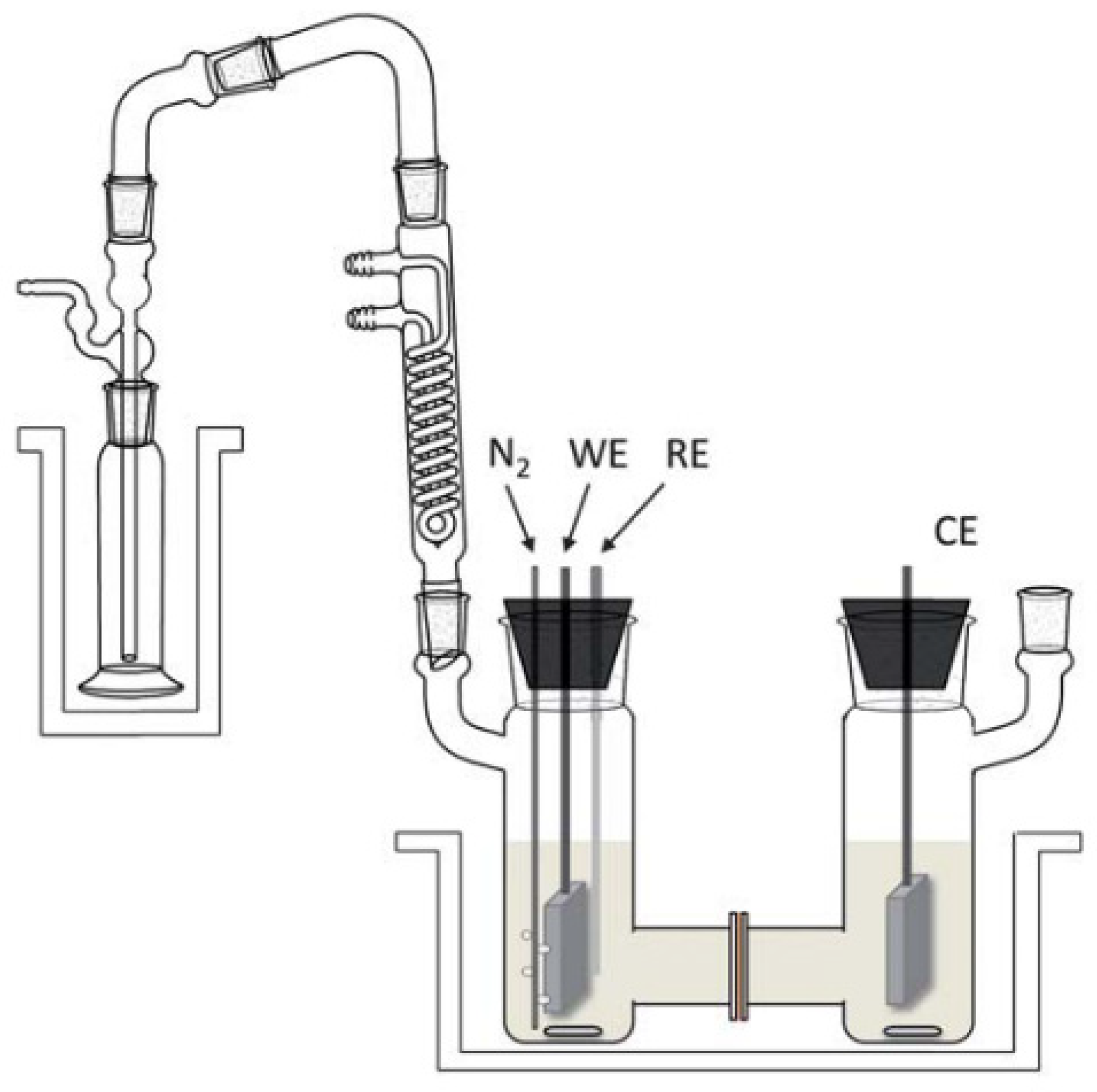
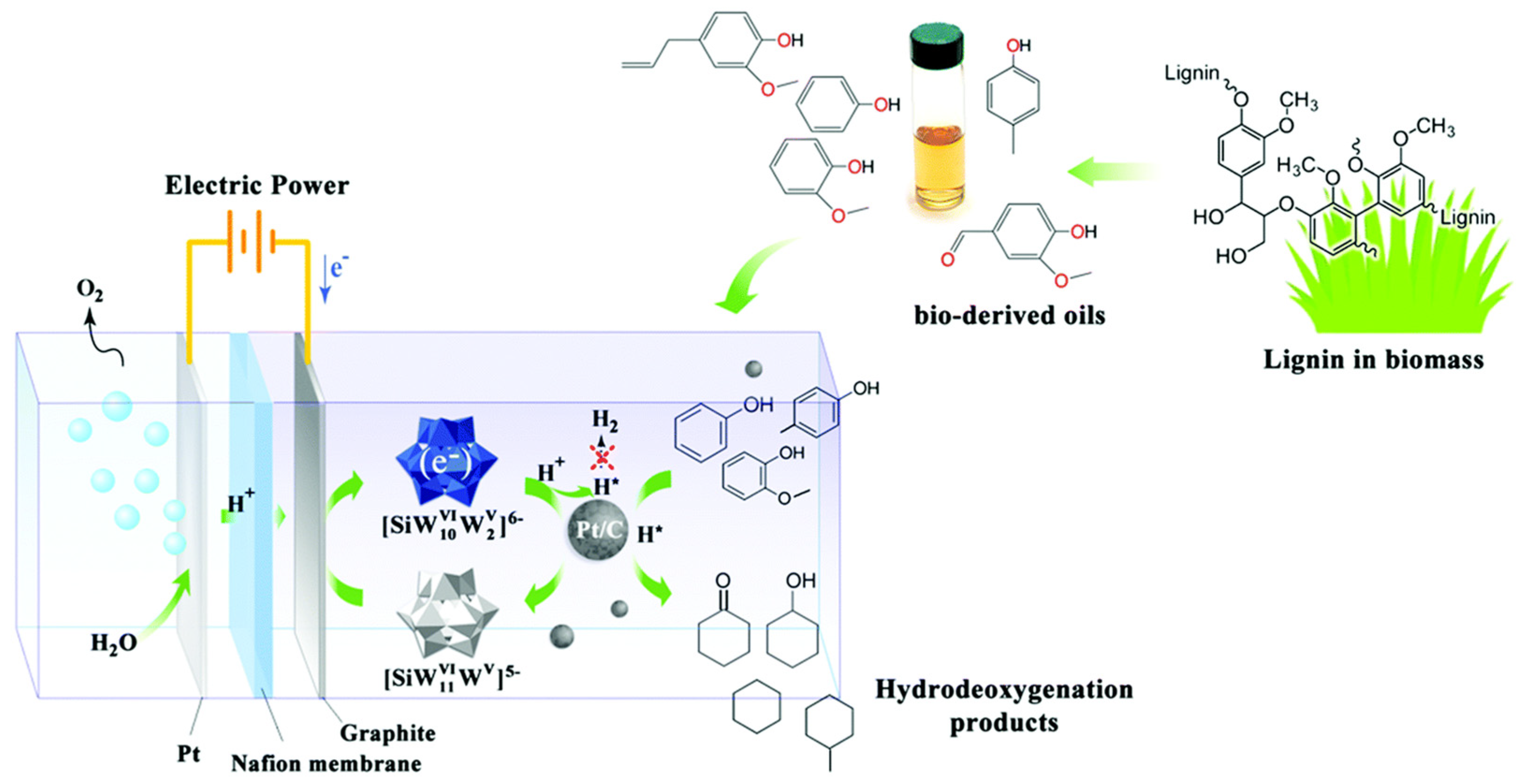
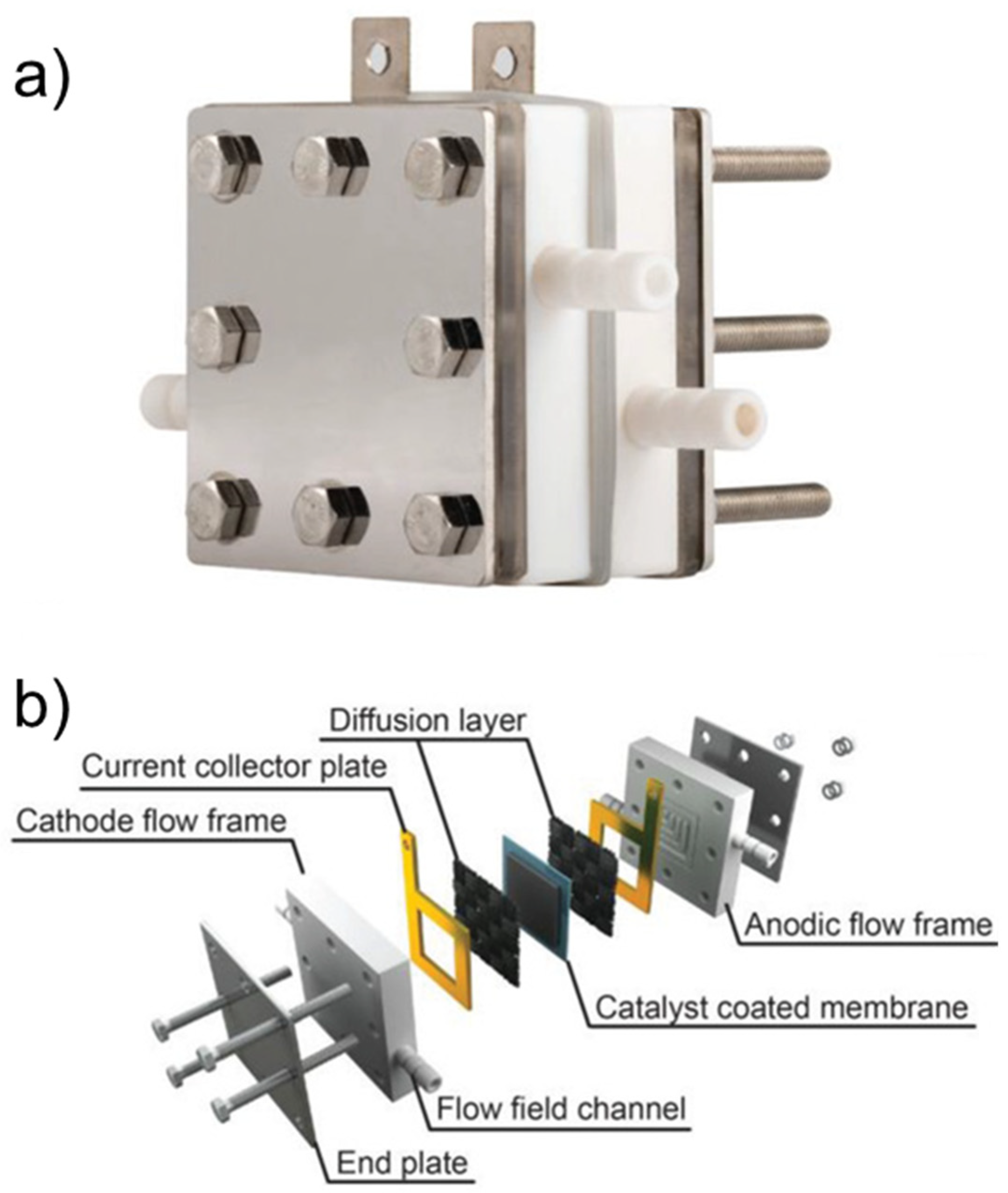
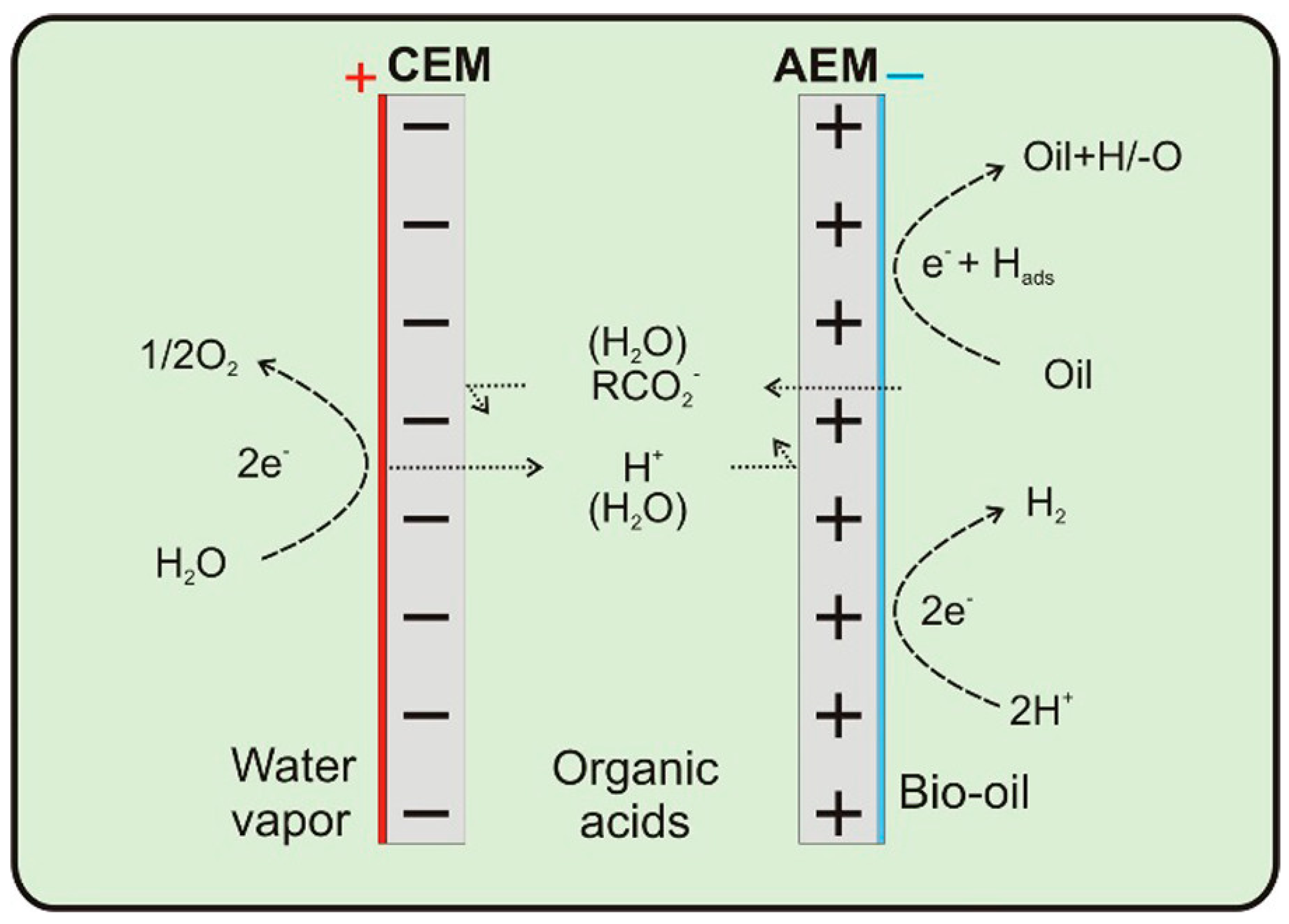
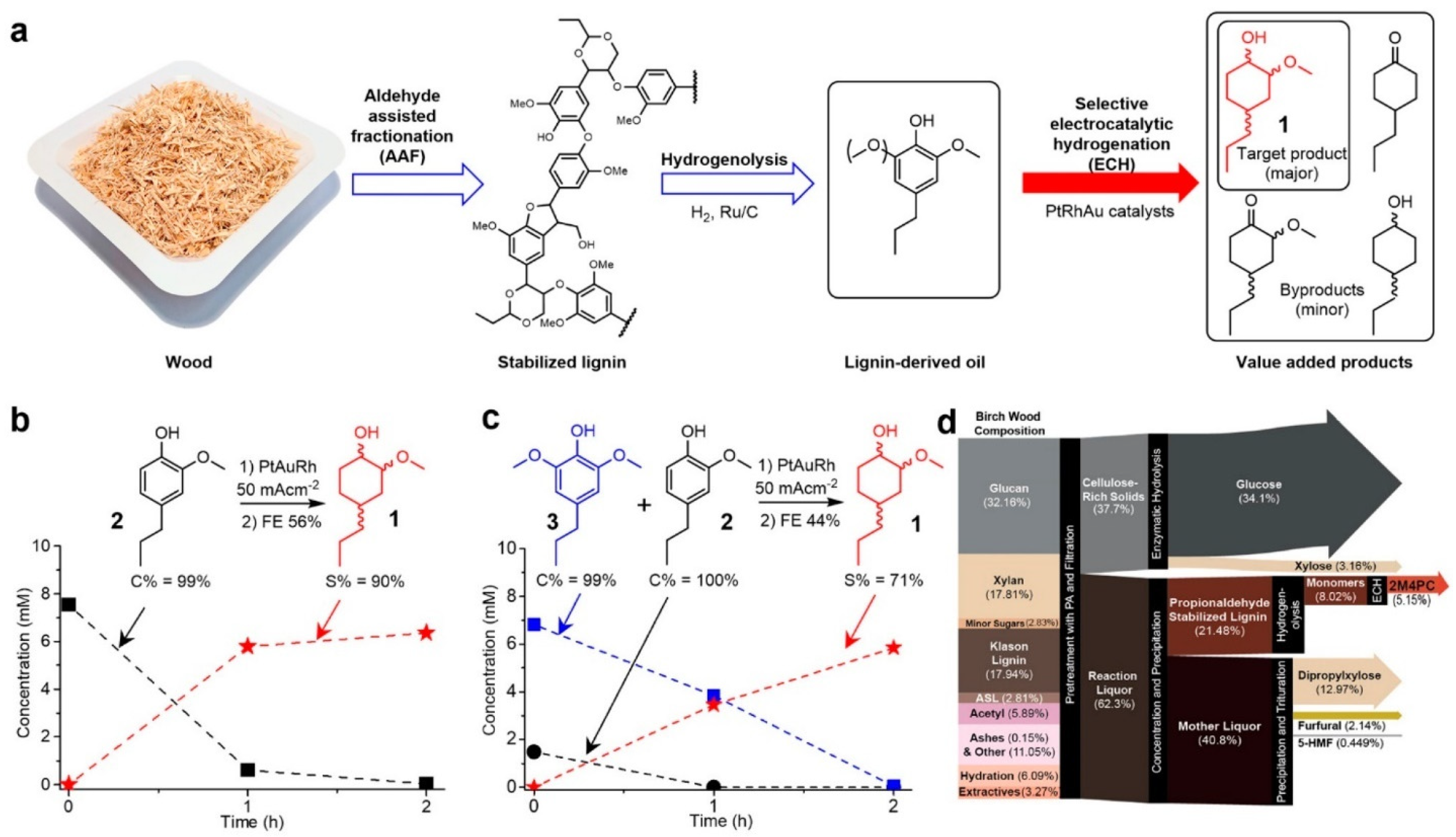
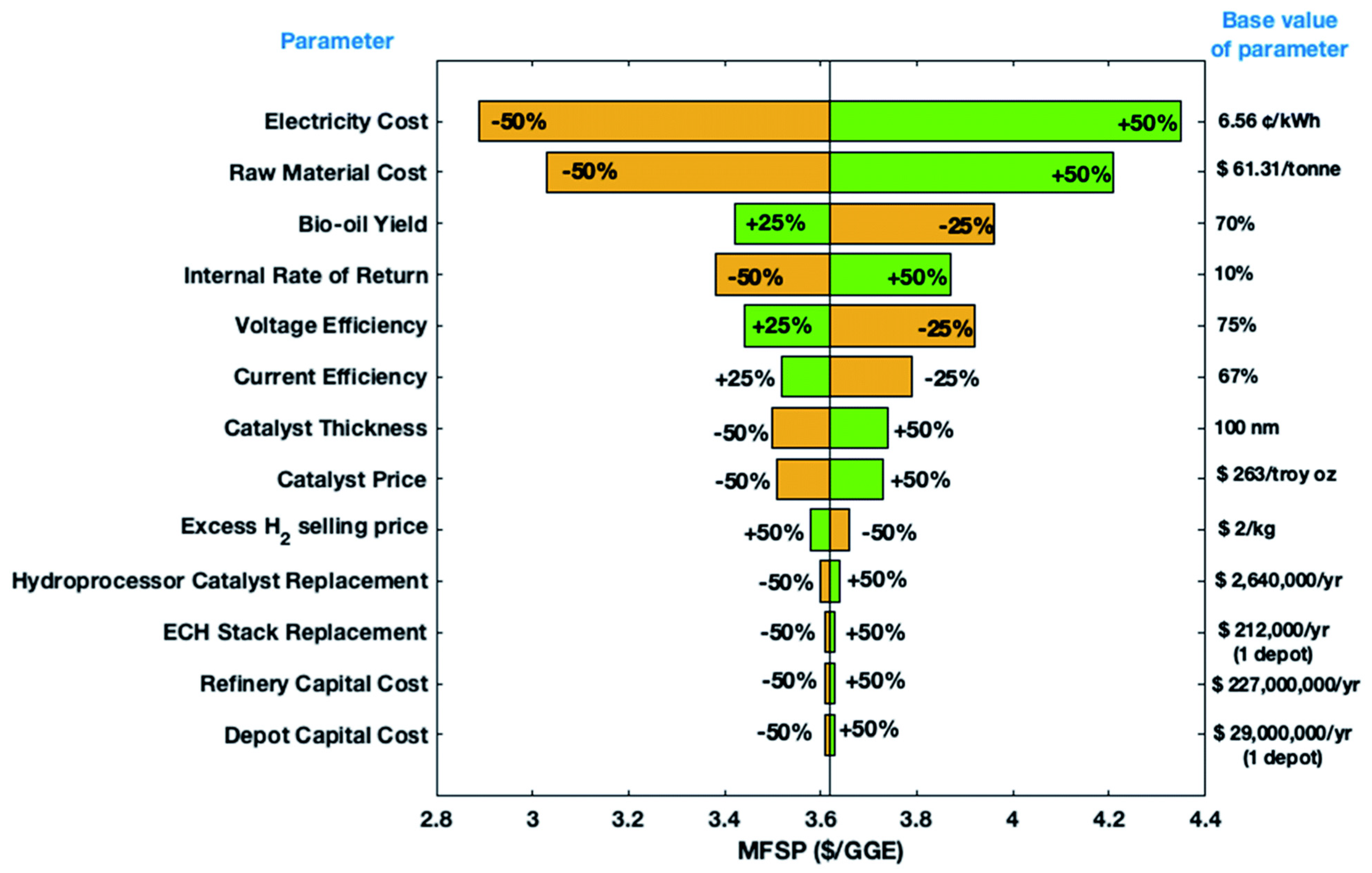
| Catalyst | Model Compound | Target Product (Selectivity %) | Electrolyte | Temp (°C) | TOF (h−1) | j (mA/cm2) | FE (%) | REF |
|---|---|---|---|---|---|---|---|---|
| PtNi/NHPC | Phenol | Cyclohexanone (99.9) | H2SO4 | 25 | 115.1 | 7.5 | 87.7 | [43] |
| PtNiB/CMK-3 | Guaiacol | Cyclohexanol (90.3) | HClO4 | 60 | 291.28 | 10 | 90.8 | [108] |
| Ru/ACC | Guaiacol | Cyclohexanol (53) | HCl | 80 | NR | 100 * | 30 | [52] |
| Pt/C | Phenol | Cyclohexanol (56) | H2SO4 | 25 | 77.7 | 7.5 | NR | [43] |
| Pt/C | Phenol | Cyclohexanol (NR) | Acetate Buffer | 25 | 270 | 100 * | 19 | [58] |
| Rh/C | Phenol | Cyclohexanol (NR) | Acetate Buffer | 25 | 296 | 100 * | 68 | |
| Pd/C | Phenol | Cyclohexanol (NR) | Acetate Buffer | 25 | <0.002 | 40 * | <1 | [50] |
| Pt-Graphite | Phenol | Cyclohexane (63%) | H2SO4, HClO4, HCl | 80 | NR | 30 | 23 ^ | [68] |
| Pt/C | Benzealdehyde | Benzyl alchol (NR) | Acetate Buffer | RT | 2189 | 200 * | 39 | [65] |
| Rh/C | Benzealdehyde | Benzyl alchol (NR) | Acetate Buffer | RT | 2267 | 185 * | 64 | |
| Pd/C | Benzealdehyde | Benzyl alchol (NR) | Acetate Buffer | RT | 3899 | 300 * | 99.6 | |
| Pt/C | Furfural | Furfuryl alchol (NR) | Acetate Buffer | RT | 250 | 0.7 V | NR | [58] |
| PdAg/C | Furfural | Furfuryl alchol (95) | Na3PO4 | 60 | NR | 0.5 V | >95% | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Page, J.R.; Manfredi, Z.; Bliznakov, S.; Valla, J.A. Recent Progress in Electrochemical Upgrading of Bio-Oil Model Compounds and Bio-Oils to Renewable Fuels and Platform Chemicals. Materials 2023, 16, 394. https://doi.org/10.3390/ma16010394
Page JR, Manfredi Z, Bliznakov S, Valla JA. Recent Progress in Electrochemical Upgrading of Bio-Oil Model Compounds and Bio-Oils to Renewable Fuels and Platform Chemicals. Materials. 2023; 16(1):394. https://doi.org/10.3390/ma16010394
Chicago/Turabian StylePage, Jeffrey R., Zachary Manfredi, Stoyan Bliznakov, and Julia A. Valla. 2023. "Recent Progress in Electrochemical Upgrading of Bio-Oil Model Compounds and Bio-Oils to Renewable Fuels and Platform Chemicals" Materials 16, no. 1: 394. https://doi.org/10.3390/ma16010394








