Thermodynamics Evaluation and Verification of High-Sulfur Copper Slag Composite Agglomerate in Oxidation-Roasting-Separation-Leaching Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Thermodynamic Calculation
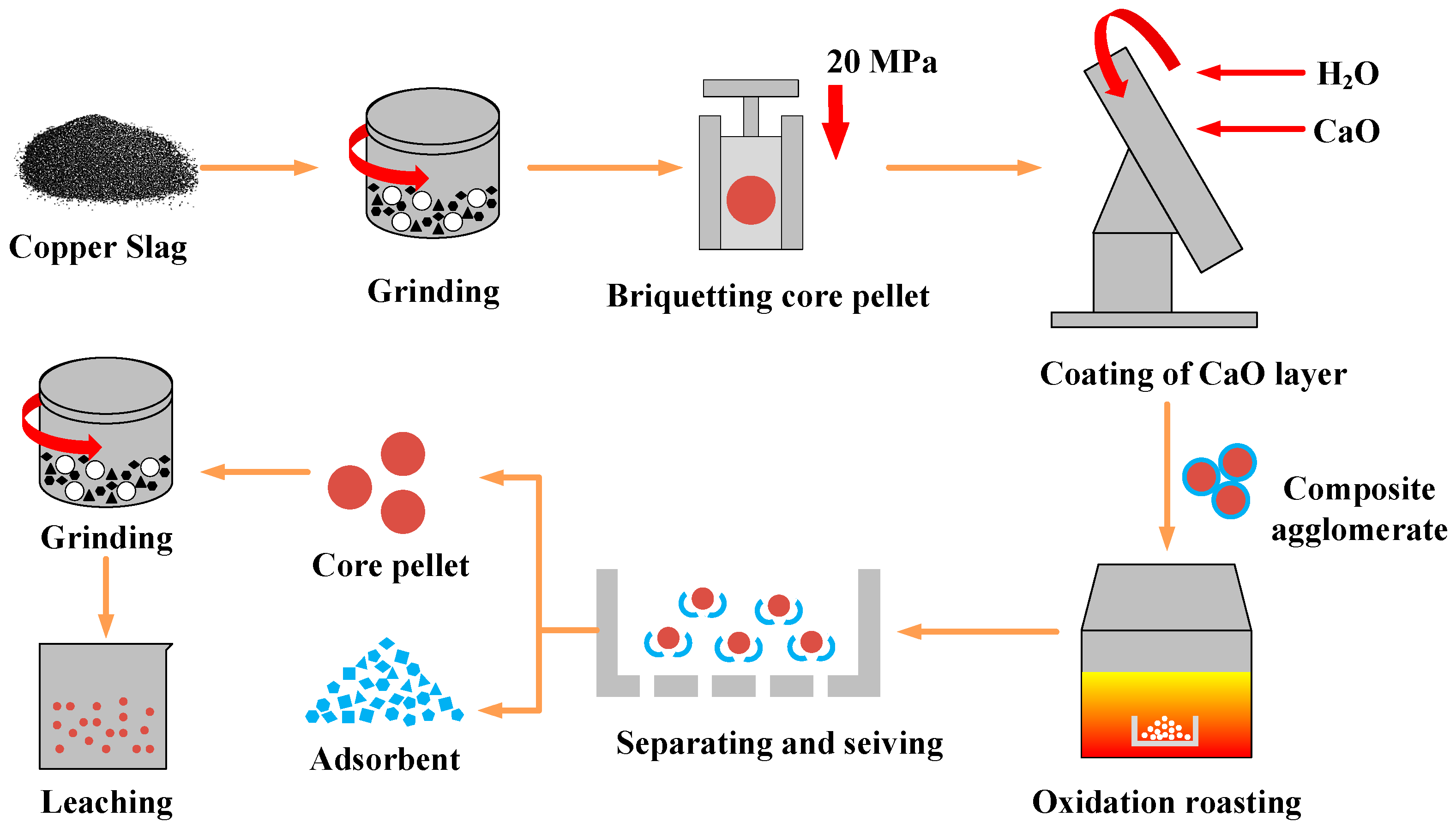
2.3. Experimental Design
3. Results
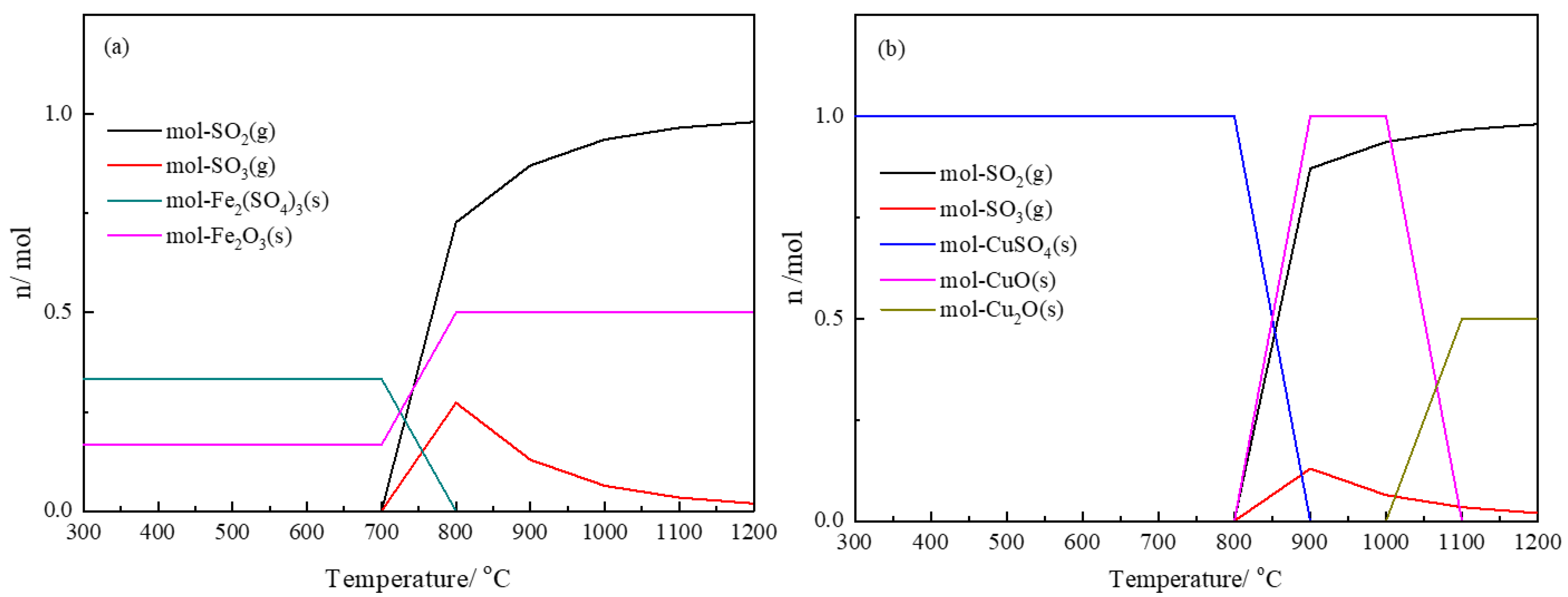
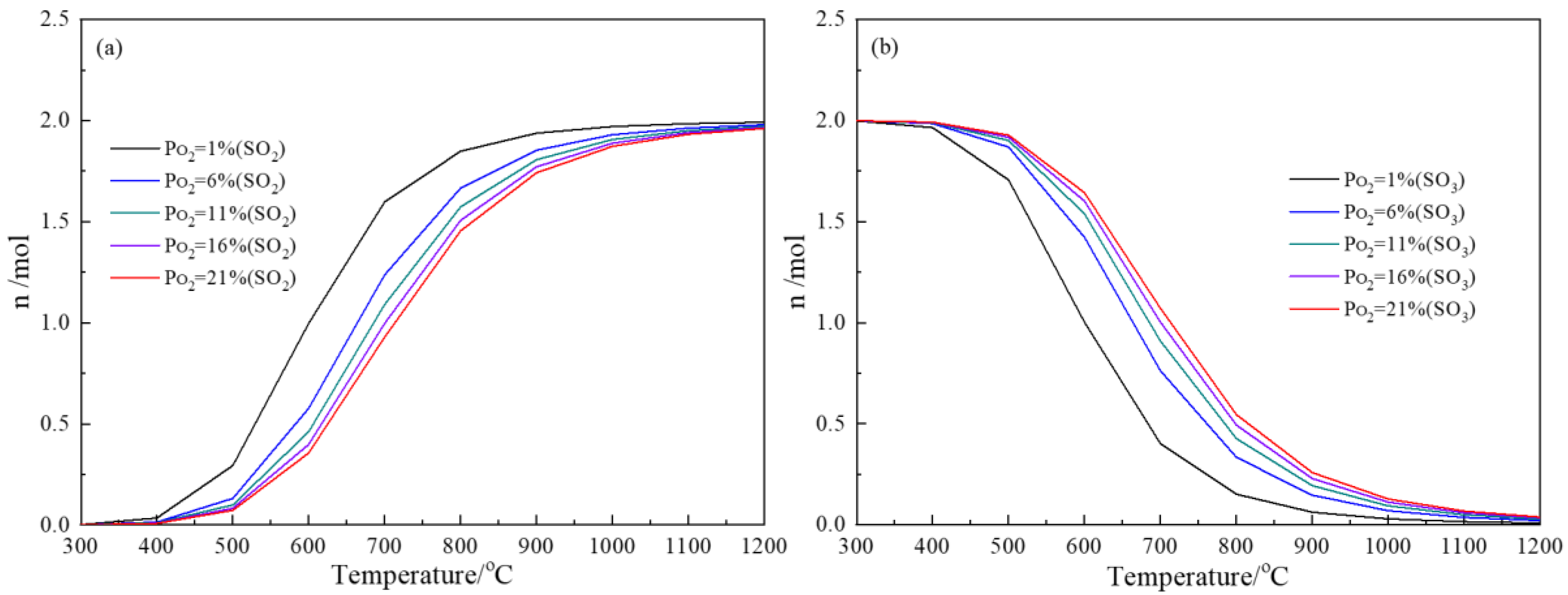
3.1. Thermodynamics of Sulfur Oxidation
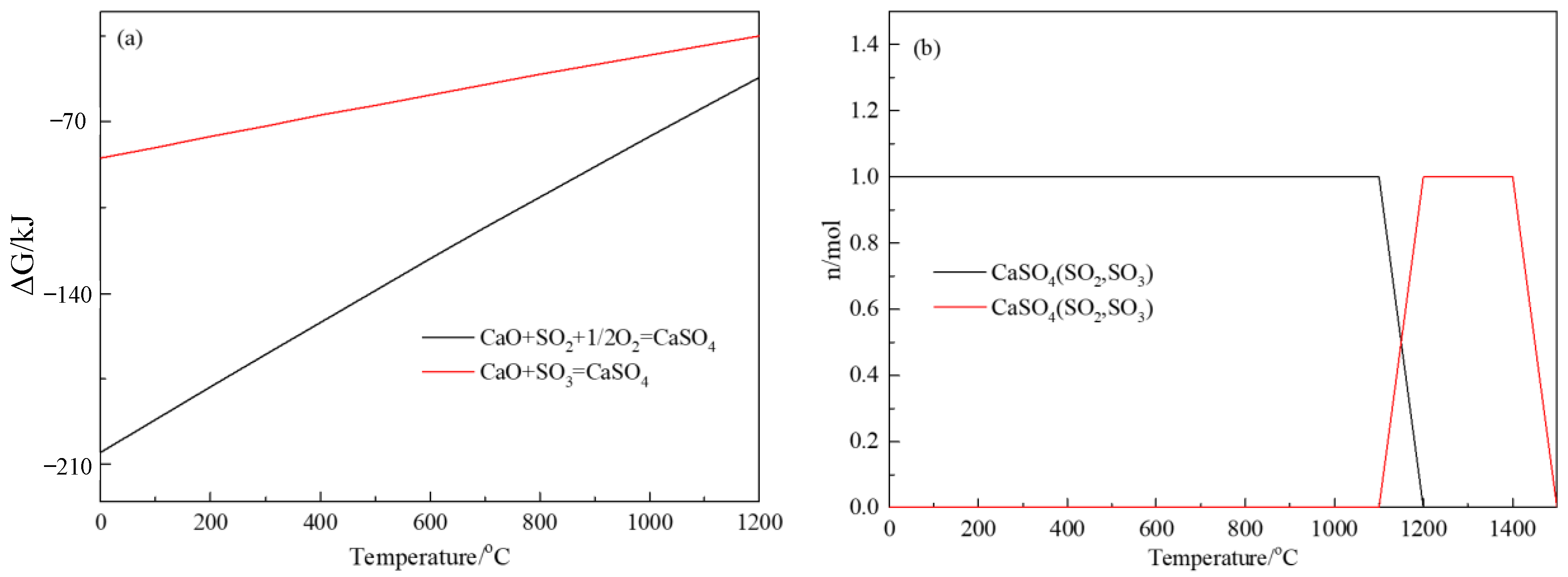
3.2. Thermodynamics of Fe2SiO4 Decomposition and SiO2 Leaching
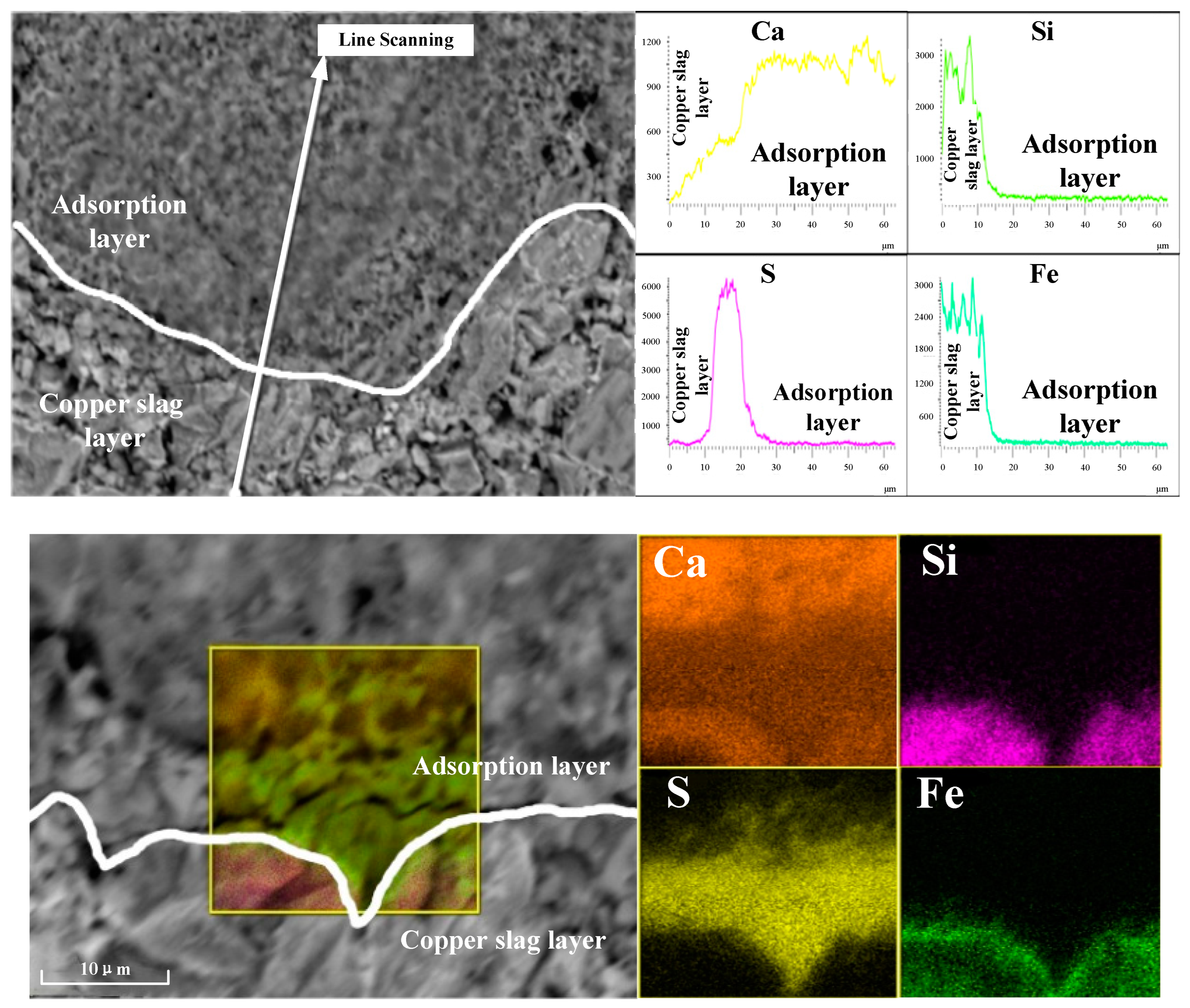
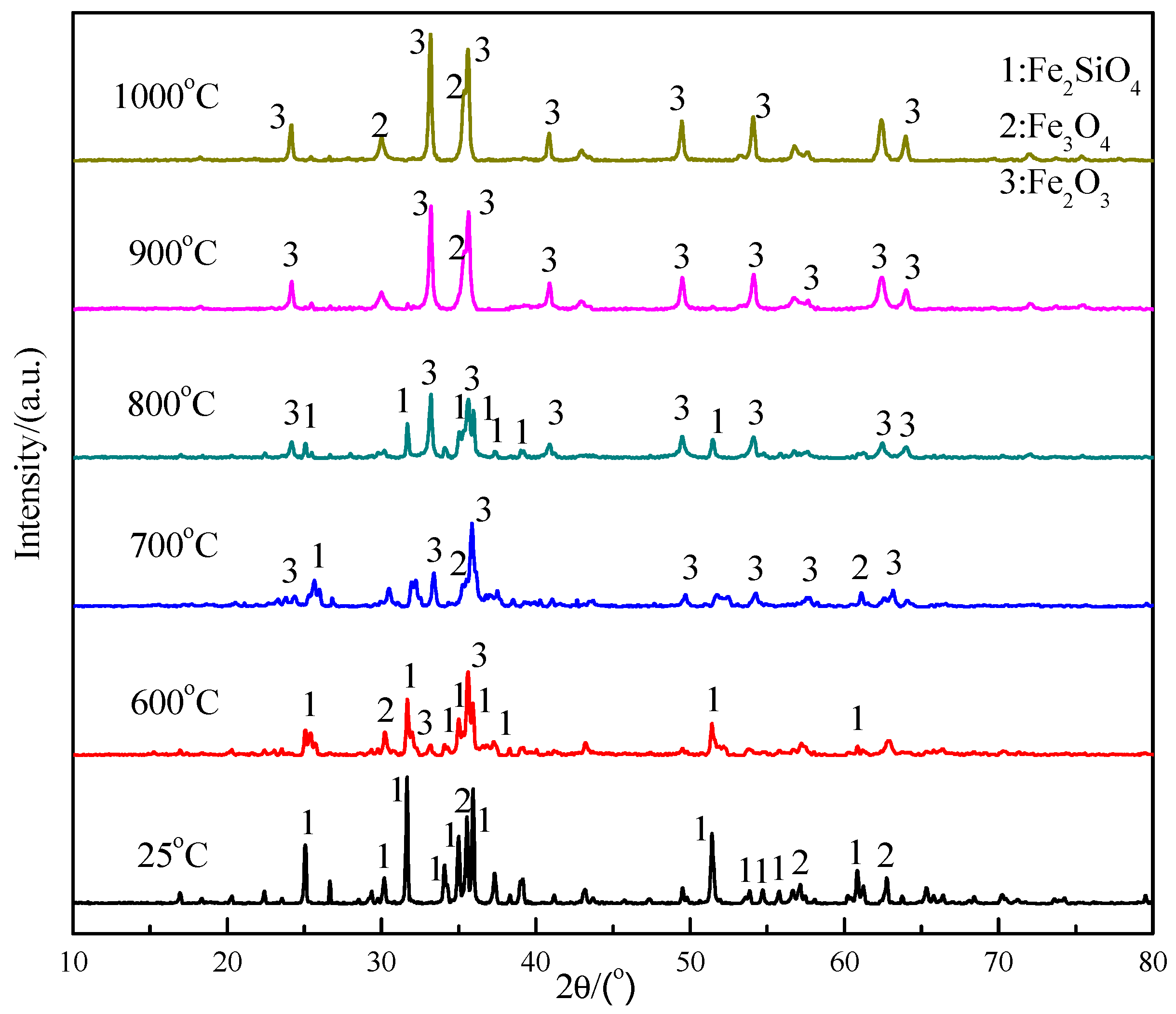
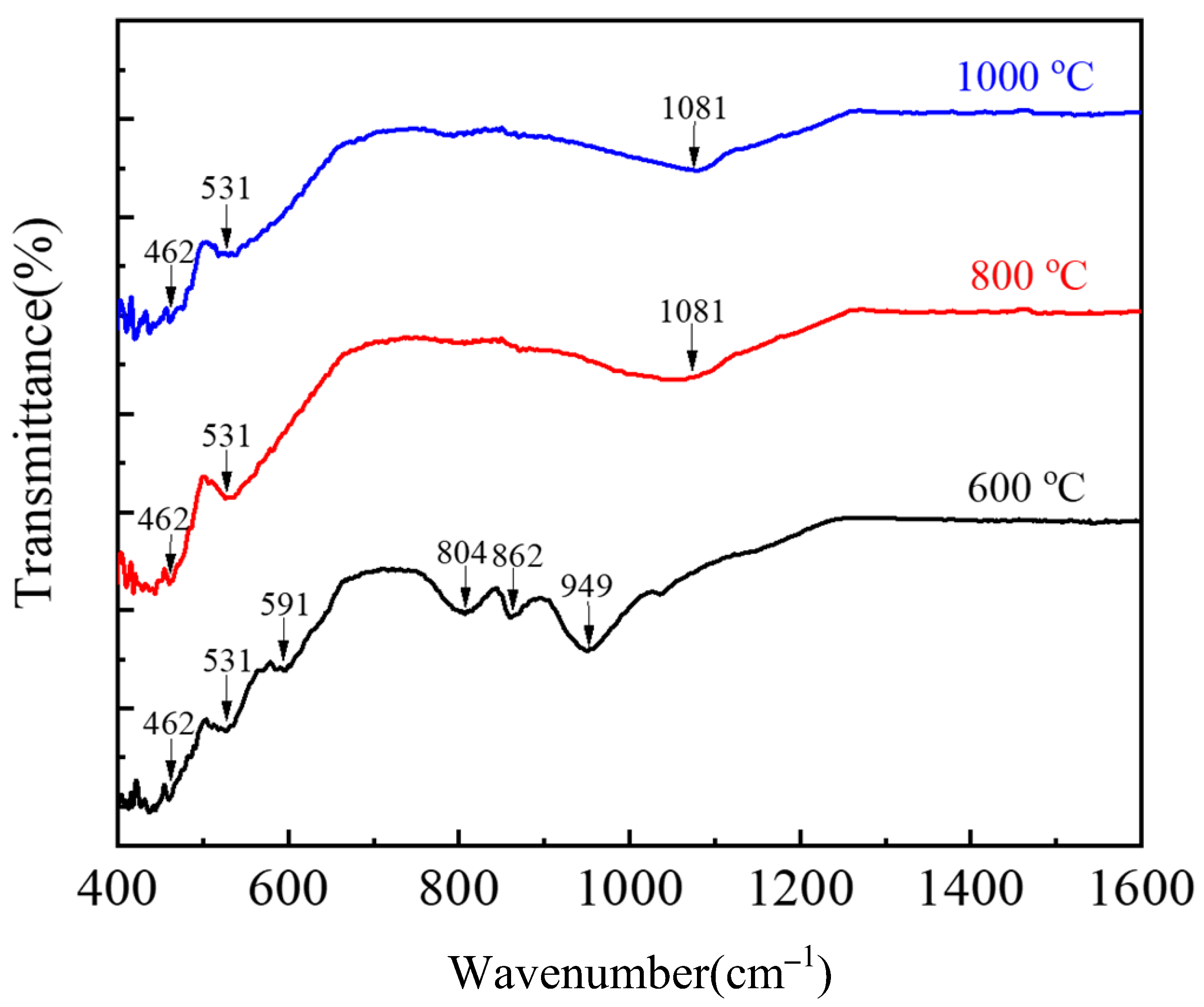
3.3. Desulfurization and Adsorption Behavior in Oxidation-Roasting Process
3.4. Leaching Behavior of Copper Slag in Alkaline Aqueous
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durinck, D.; Engström, F.; Arnout, S.; Heulens, J.; Jones, P.T.; Björkman, B.; Blanpain, B.; Wollants, P. Hot stage processing of metallurgical slags. Resour. Conserv. Recycl. 2008, 52, 1121. [Google Scholar] [CrossRef]
- Zhao, K.; Gong, X.-R.; Li, J.; Liu, W.-X.; Xing, H.-W. Mineralogical characteristics and comprehensive utilization of rapid cooling copper slag. China Min. Mag. 2015, 24, 102–106. [Google Scholar]
- Yu, W.; Sun, Y.; Lei, M.; Chen, S.; Qiu, T.; Tang, Q. Preparation of micro-electrolysis material from flotation waste of copper slag and its application for degradation of organic contaminants in water. J. Hazard. Mater. 2019, 361, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-L.; Xiong, J.-C.; Hu, Z.-B.; Tong, C.-Q.; Wang, R.-X. Research progress in resource utilization of iron and copper in copper smelting slag. Des. Res. Non-Ferr. Metall. 2016, 37, 15. [Google Scholar]
- Zhao, K.; Gong, X.-R.; Li, J.; Liu, W.; Xing, H. Thermodynamics of recovering iron, copper, zinc in copper slag by direct reduction method. J. Environ. Eng. 2016, 10, 2638. [Google Scholar]
- Wang, P.; Gao, L.; Dong, F.; Chen, L.; Ma, F. Recovery status of copper recovery by flotation from copper smelting slag. Compr. Util. Miner. Resour. 2017, 1, 16–20. [Google Scholar]
- Wei, Z.-F.; Zhao, K.; Zhang, Q.-R.; Shi, X.-F.; Ji, H.-J.; Gong, X.-R.; Xing, H.-W. The effect of carbon thermal reduction modification on the dissolution behavior of Cu and S in iron. Compr. Util. Miner. Resour. 2021, 2, 44–48. [Google Scholar]
- Jing, W.-B.; Li, S.-L. Experimental study on direct reduction iron-making with copper slag. China Nonferrous Metall. Gold 2018, 47, 78. [Google Scholar]
- Xu, D.; Chun, T.-J.; Chen, J.-A. Iron recovery from copper slag by a combined technique of high-temperature reduction roasting and magnetic separation. Min. Metall. Eng. 2017, 37, 89. [Google Scholar]
- Yang, H.-F.; Jing, L.-L.; Dang, C.-G. Iron recovery from copper-slag with lignite-based direct reduction followed by magnetic separation. China J. Nonferrous Met. 2011, 21, 1165–1170. [Google Scholar]
- Cao, Z.-C.; Sun, T.-C.; Wu, D.-H.; Xue, X.; Liu, Z.-H. Technology of recovery of iron and zinc from copper slag by RHF direct reduction. J. Mater. Metall. 2017, 16, 38. [Google Scholar]
- Cao, Z.-C.; Sun, T.-C.; Xue, X.; Liu, Z.-H. Recovery of Iron and zinc from copper slag by rotary hearth furnacewith anthracite as reductant. Min. Metall. Eng. 2017, 37, 74–78. [Google Scholar]
- Zhang, X.-H.; Wang, B.; Zhao, K.; Zhang, Q.-R.; Gong, X.-R.; Xing, H.-W. Formation and growth of iron and grains in direct reduction process of high silicon and iron waste slag. Iron Steel 2021, 56, 21–27. [Google Scholar]
- Li, S.; Pan, J.; Zhu, D.; Guo, Z.; Xu, J.; Chou, J. A novel process to upgrade the copper slag by direct reduction-magnetic separation with the addition of Na2CO3 and CaO. Powder Technol. 2019, 347, 159–169. [Google Scholar] [CrossRef]
- Chen, Q.-Z.; Zhu, R.; Wu, J.-D. Experimental study on desulfurization of copper slag by gasification. Ind. Heat. 2017, 46, 15–16+20. [Google Scholar]
- Tian, H.; Guo, Z.; Pan, J.; Zhu, D.; Yang, C.; Xue, Y.; Li, S.; Wang, D. Comprehensive review on metallurgical recycling and cleaning of copper slag. Resour. Conserv. Recycl. 2021, 168, 105366. [Google Scholar] [CrossRef]
- Erdenebold, U.; Sung, C.M.; Wang, J.P. Manufacture of low sulphur pig iron from copper slag. Arch. Metall. Mater. 2020, 65, 349–355. [Google Scholar]
- Liao, Z.-L.; Tang, P.; Zhang, B.; Zhang, H.-W.; Shi, X.-Y.; Li, Q.-J.; Hong, X. Experimental study on oxidation modification of copper slag at medium and low temperature. China Nonferrous Metall. 2012, 41, 74–78. [Google Scholar]
- Li, L.; Hu, J.H.; Wang, H. Application of the chloridizing roasting method for the removal of copper and sulphur from copper slags. Miner. Process. Extr. Metall. 2018, 127, 49–55. [Google Scholar] [CrossRef]
- Cao, Z.-M.; Song, X.-Y.; Qiao, Z.-Y. Thermodynamic modeling software FactSage and its application. Chin. J. Rare Met. 2008, 32, 216–219. [Google Scholar]
- Wan, X.-Y.; Qi, Y.-H.; Gao, J.-J.; Wu, B.-Q. Behavior of arsenic in arsenic- bearing copper slag by pyrolysis roasting process. Nonferrous Met. Eng. 2017, 7, 40–45. [Google Scholar]
- Wan, X.-Y.; Zhao, K.; Qi, Y.-H.; Gao, J.-J.; Shangguan, F.-Q. Thermodynamics of arsenic removal from arsenic-bearing copper slag. Min. Metall. Eng. 2017, 37, 73–76+80. [Google Scholar]
- Wang, H.; Zhang, X.; Shen, L.; Yang, S.; Luo, L.; Song, S. Liberation and enrichment of metallic iron from reductively roasted copper slag. JOM 2021, 73, 1013–1022. [Google Scholar] [CrossRef]






| Items | TFe | MFe | FeO | SiO2 | Al2O3 | MgO | CaO |
|---|---|---|---|---|---|---|---|
| % | 36.10 | 0.23 | 42.74 | 38.01 | 3.92 | 1.80 | 3.41 |
| Items | K2O | Na2O | Pb | As | Cu | S | P |
| % | 0.77 | 0.44 | 0.31 | 0.14 | 0.68 | 1.19 | 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Zhang, X.; Zhao, W.; Guo, H.; Zhang, Q.; Zhen, C. Thermodynamics Evaluation and Verification of High-Sulfur Copper Slag Composite Agglomerate in Oxidation-Roasting-Separation-Leaching Process. Materials 2023, 16, 42. https://doi.org/10.3390/ma16010042
Zhao K, Zhang X, Zhao W, Guo H, Zhang Q, Zhen C. Thermodynamics Evaluation and Verification of High-Sulfur Copper Slag Composite Agglomerate in Oxidation-Roasting-Separation-Leaching Process. Materials. 2023; 16(1):42. https://doi.org/10.3390/ma16010042
Chicago/Turabian StyleZhao, Kai, Xinghua Zhang, Wei Zhao, Hongwei Guo, Qiaorong Zhang, and Changliang Zhen. 2023. "Thermodynamics Evaluation and Verification of High-Sulfur Copper Slag Composite Agglomerate in Oxidation-Roasting-Separation-Leaching Process" Materials 16, no. 1: 42. https://doi.org/10.3390/ma16010042




