One-Step Synthesis of Sulfur-Doped Nanoporous Carbons from Lignin with Ultra-High Surface Area, Sulfur Content and CO2 Adsorption Capacity
Abstract
:1. Introduction
2. Experimental
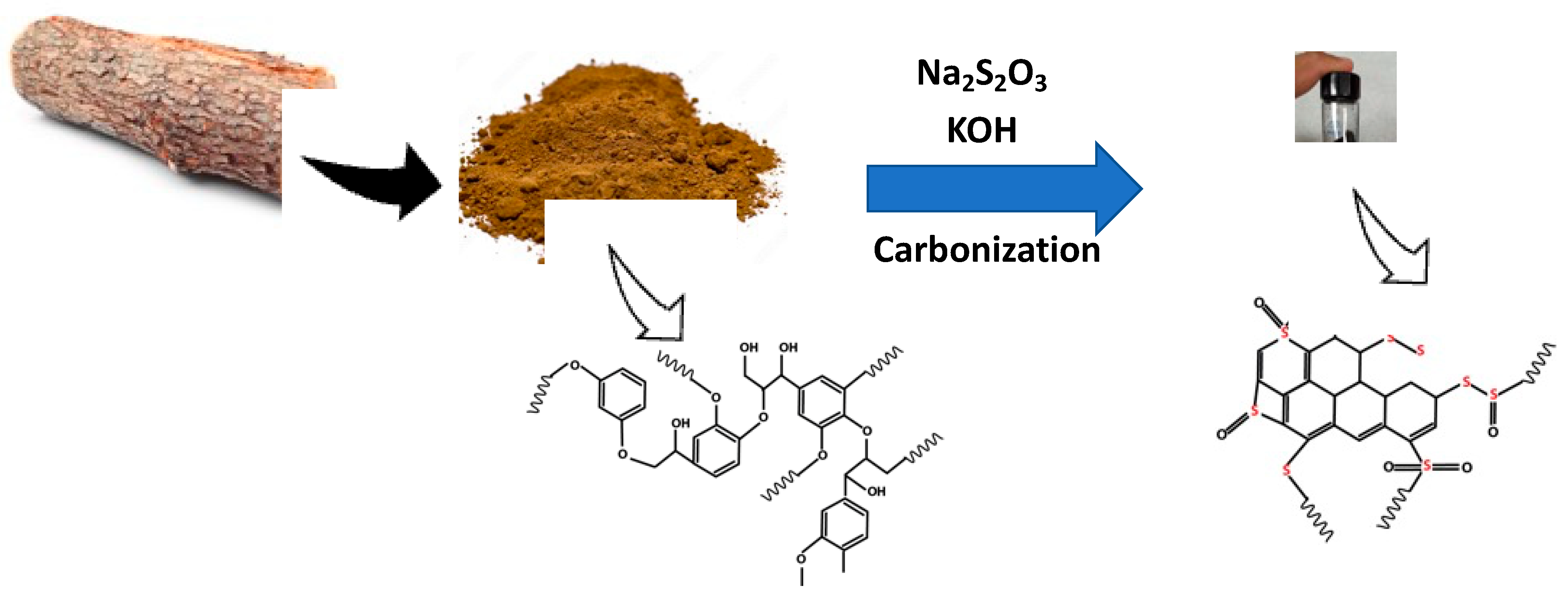
2.1. Synthesis of Sulfur-Doped Carbons
2.2. Characterization of Sulfur-Doped Carbons
2.3. Gas Adsorption Studies
3. Results and Discussion
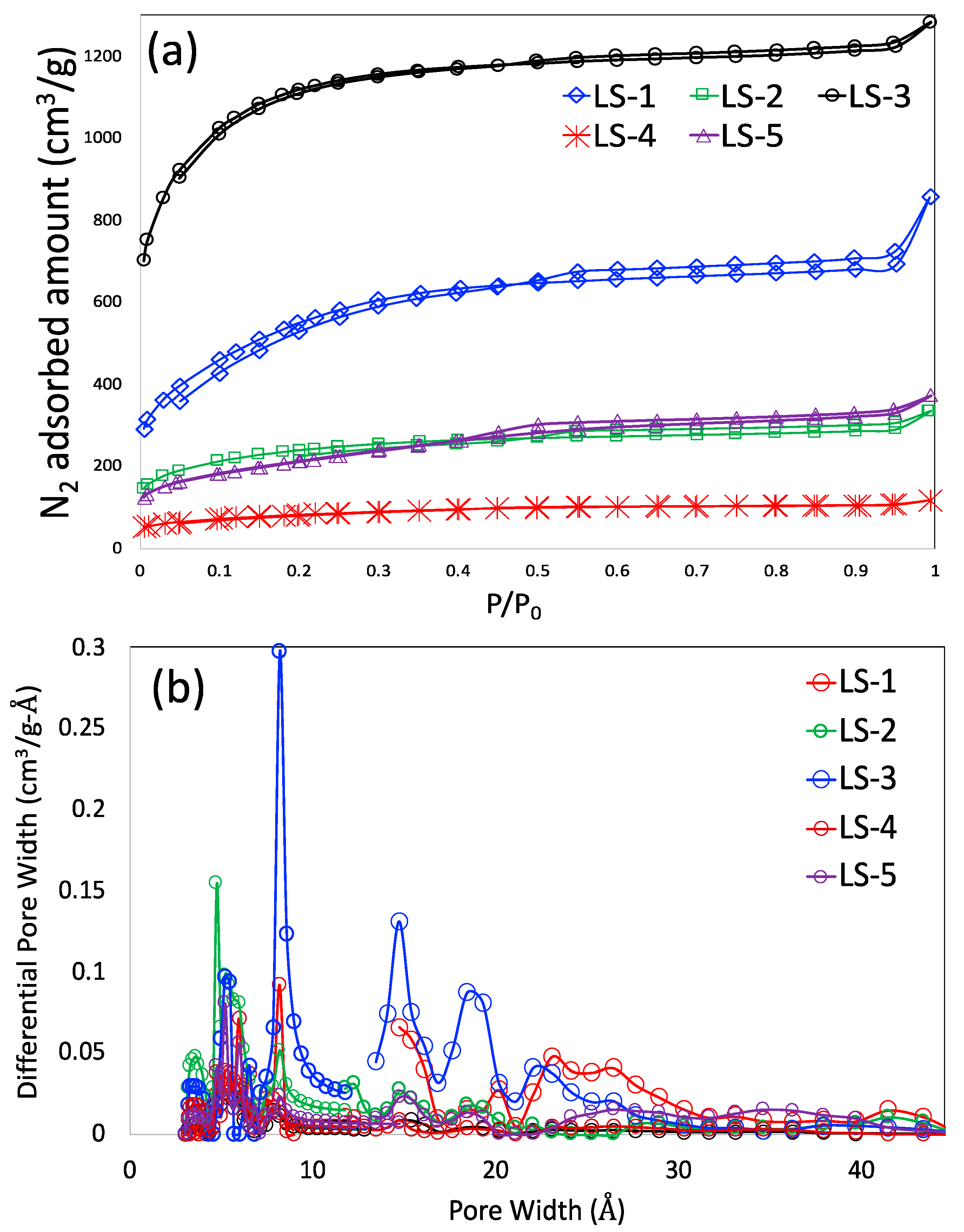
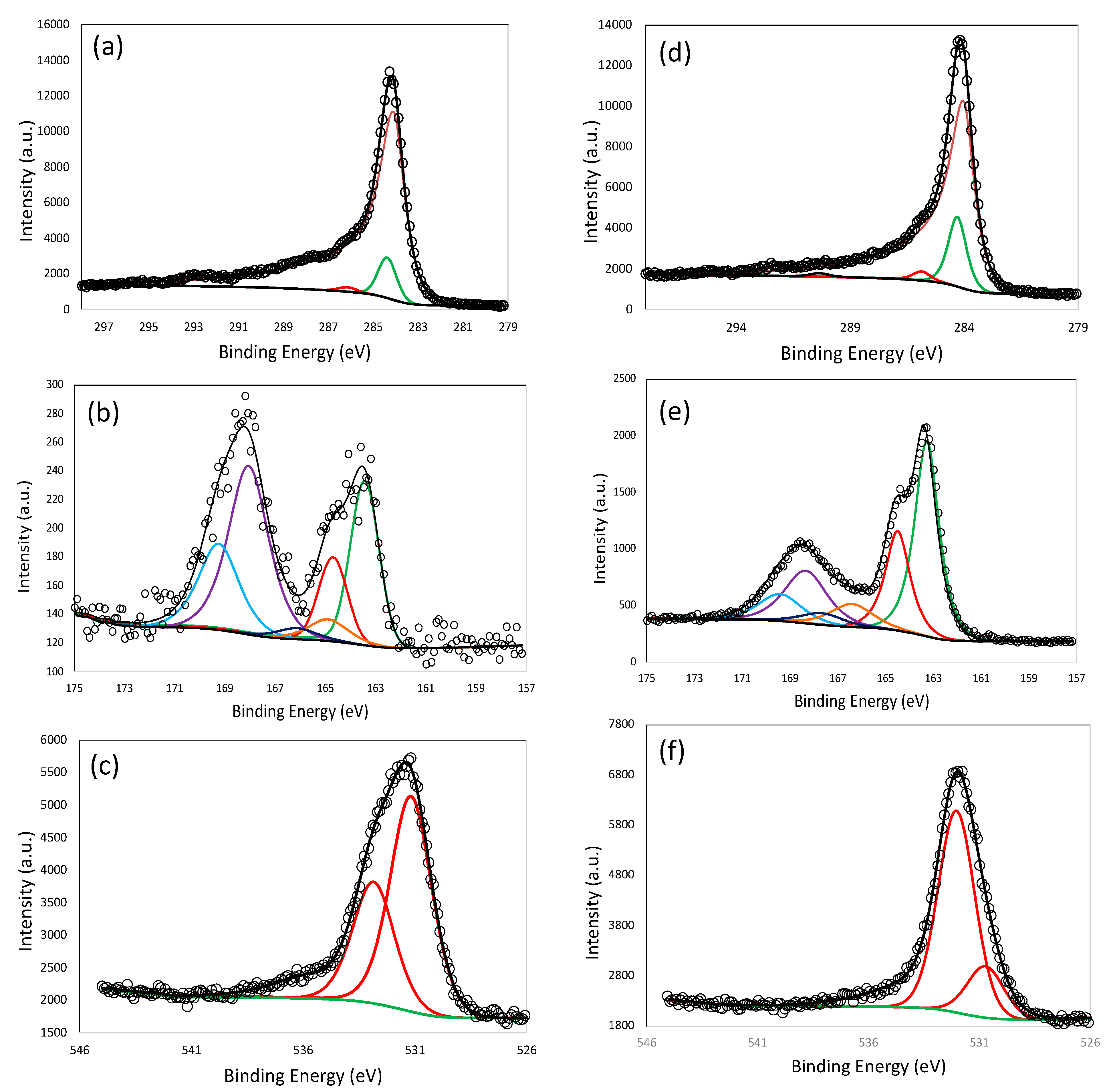
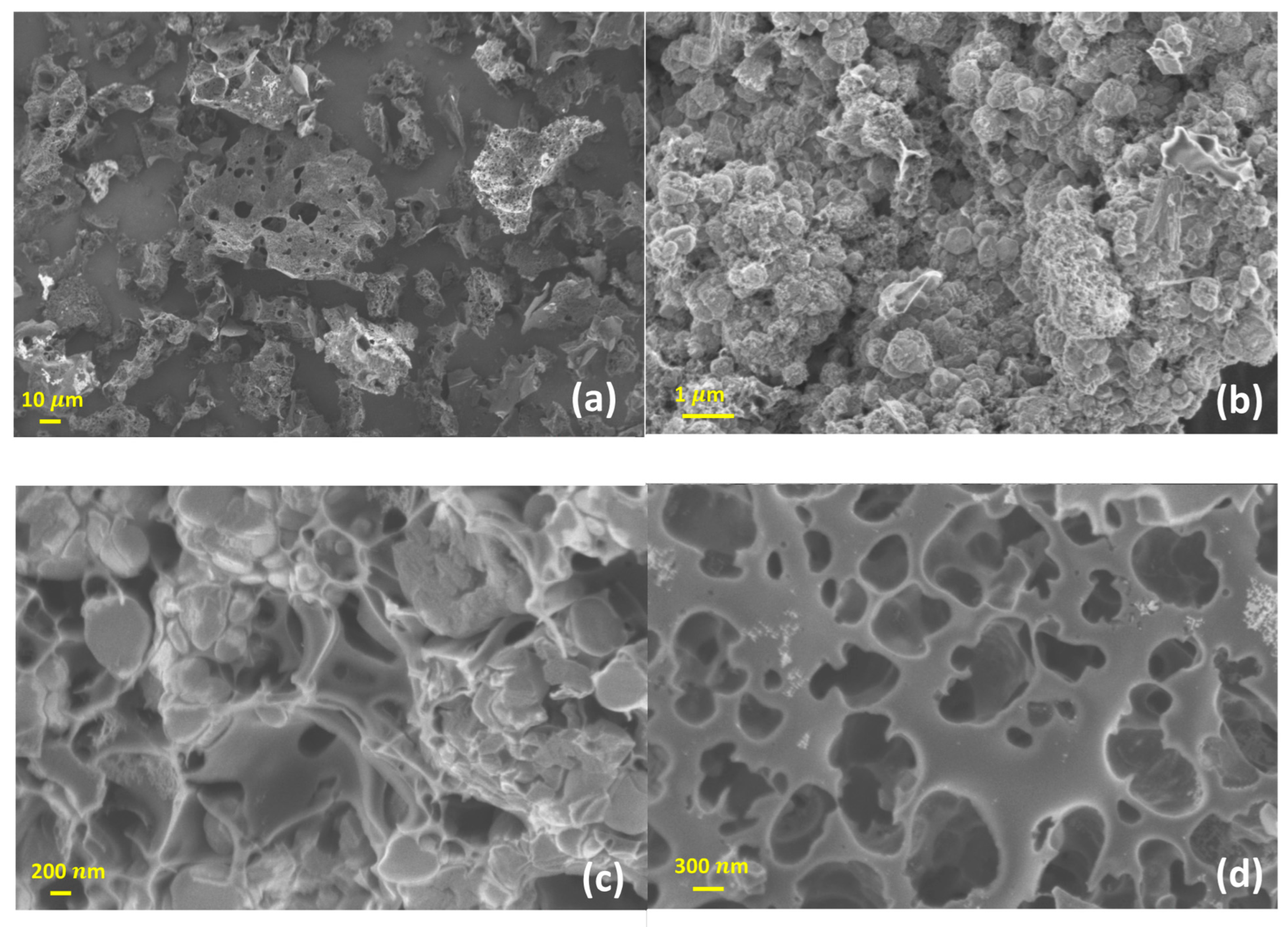
3.1. Material Characteristics
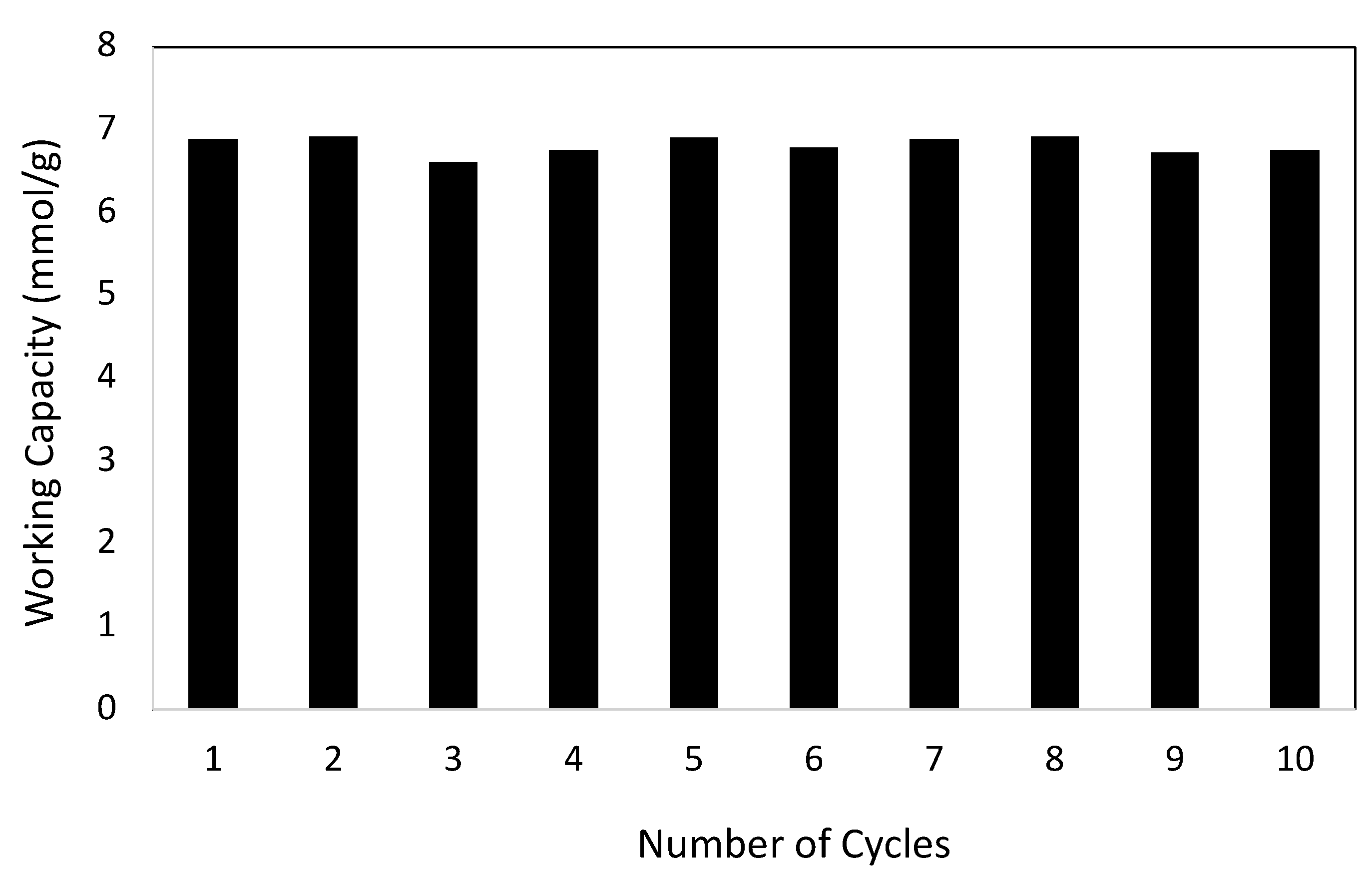
3.2. Gas Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silva, R.R.; Torres, J.H.S.; Kopelevich, Y. Indication of superconductivity at 35 K in graphite-sulfur composites. Phys. Rev. Lett. 2001, 87, 147001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurmaev, E.; Galakhov, A.; Moewes, A.; Moehlecke, S.; Kopelevich, Y. Inter- layer conduction band states in graphite-sulfur composites. Phys. Rev. B 2002, 66, 193402. [Google Scholar] [CrossRef] [Green Version]
- Paraknowitsch, J.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Nie, H.; Chen, X.; Chen, X.; Huang, S. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J. Power Sources 2013, 236, 238–249. [Google Scholar] [CrossRef]
- Hasegawa, G.; Aoki, M.; Kanamori, K.; Nakanishi, K.; Hanada, T.; Tadanaga, K. Monolithic electrode for electric double-layer capacitors based on macro/meso/microporous S-containing activated carbon with high surface area. J. Mater. Chem. 2011, 21, 2060–2063. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Murata, T.; Hasegawa, M.; Bito, Y.; Toyoguchi, Y. Study on CXN and CXS with disordered carbon structure as the anode materials for secondary lithium batteries. J. Power Sources 1997, 68, 245–248. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Q.; Tao, Z.; Chen, J. Composite of sulfur impregnated in porous hollow carbon spheres as the cathode of Li–S batteries with high performance. Nano Res. 2013, 6, 38–46. [Google Scholar] [CrossRef]
- Saha, D.; Barakat, S.; Van Bramer, S.E.; Nelson, K.A.; Hensley, D.K.; Chen, J. Noncompetitive and competitive adsorption of heavy metals in sulfur-functionalized ordered mesoporous carbon. ACS Appl. Mater. Interfaces 2016, 8, 34132–34142. [Google Scholar] [CrossRef]
- Petit, C.; Kante, K.; Bandosz, T.J. The role of sulfur-containing groups in ammonia retention on activated carbons. Carbon 2010, 48, 654–667. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B.; Mokaya, R. Preparation and hydrogen storage capacity of highly porous activated carbon materials derived from polythiophene. Int. J. Hydrog. Energy 2011, 36, 15658–15663. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, Y.; Tang, Y. Preparation of sulfur-doped microporous carbons for the storage of hydrogen and carbon dioxide. Carbon 2012, 50, 5543–5553. [Google Scholar] [CrossRef]
- Kicin, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Shin, Y.; Fryxell, G.E.; Um, W.; Parker, K.; Mattigod, S.V.; Skaggs, R. Sulfur-functionalized mesoporous carbon. Adv. Funct. Mater. 2007, 17, 2897–2901. [Google Scholar] [CrossRef]
- Choi, C.H.; Park, S.H.; Woo, S.I. Heteroatom doped carbons prepared by the pyrolysis of bio-derived amino acids as highly active catalysts for oxygen electro-reduction reactions. Green Chem. 2011, 13, 406–412. [Google Scholar] [CrossRef]
- Saha, D.; Thorpe, R.; Van Bramer, S.; Alexander, N.; Hensley, D.; Orkoulas, G.; Chen, J. Synthesis of Nitrogen and Sulfur Co-Doped Nanoporous Carbons from Algae: Role in CO2 Separation. ACS Omega 2018, 3, 18592–18602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paraknowitsch, J.P.; Wienert, B.; Zhang, Y.; Thomas, A. Intrinsically sulfur- and nitrogen-co-doped carbons from thiazolium salts. Chem. Eur. J. 2012, 18, 15416–15423. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Highly porous S-doped carbons. Microporous Mesoporous Mater. 2012, 158, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Toof, B.; Krishna, R.; Orkoulas, G.; Gismondi, P.; Thorpe, R.; Comroe, M. Separation of ethane-ethylene and propane-propylene in Ag(I)-doped and sulfurized microporous carbon. Microporous Mesoporous Mater. 2020, 299, 110099. [Google Scholar] [CrossRef]
- Saha, D.; Richards, C.P.; Haines, R.G.; D’Alessandro, N.D.; Kienbaum, M.J.; Griffaton, C.A. Competitive Adsorption of Lead in Sulfur and Iron Dual-Doped Mesoporous Carbons. Molecules 2020, 25, 403. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, G.; Saha, D.; Chakraborty, S. Why Ag(I) grafted porous carbon matrix prefers alkene over alkane? An inside view from ab-initio study. Microporous Mesoporous Mater. 2021, 316, 110940. [Google Scholar] [CrossRef]
- Saha, D.; Comroe, M.L.; Krishna, R. Synthesis of Cu(I)-doped Mesoporous Carbon for Selective Capture of Ethylene from Reaction Products of Oxidative Coupling of Methane. Microporous Mesoporous Mater. 2021, 328, 111488. [Google Scholar] [CrossRef]
- Saha, D.; Orkoulas, G.; Chen, J.; Hensley, D. Adsorptive separation of CO2 in sulfur-doped nanoporous carbons: Selectivity and breakthrough simulation. Microporous Mesoporous Mater. 2017, 241, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Van Bramer, S.E.; Orkoulas, G.; Ho, H.-C.; Chen, J.; Henley, D.K. CO2 capture in lignin-derived and nitrogen-doped hierarchical porous carbons. Carbon 2017, 121, 257–266. [Google Scholar] [CrossRef]
- Saha, D.; Comroe, M.; Krishna, R.; Rascavage, M.; Larwa, J.; You, V.; Standhart, G.; Bingnear, B. Separation of propylene from propane and nitrogen by Ag(I)-doped nanoporous carbons obtained from hydrothermally treated lignin. Diam. Relat. Mater. 2022, 121, 108750. [Google Scholar] [CrossRef]
- Saha, D.; Payzant, E.A.; Kumbhar, A.S.; Naskar, A.K. Sustainable Mesoporous Carbons as Storage and Controlled-Delivery Media for Functional Molecules. ACS Appl. Mater. Interfaces 2013, 5, 5868–5874. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Warren, K.E.; Naskar, A.K. Controlled release of antipyrine from mesoporous carbons. Microporous Mesoporous Mater. 2014, 196, 327. [Google Scholar] [CrossRef]
- Kadia, J.F.; Kubo, S.; Gilbert, R.A.V.R.D.; Compere, A.L.; Giriffith, W. Lignin-based carbon fibers for composite fiber applications. Carbon 2002, 40, 2913–2920. [Google Scholar]
- Rong, K.; Wei, J.; Wang, Y.; Liu, J.; Qiao, Z.-A.; Fang, Y.; Dong, S. Deep eutectic solvent assisted zero-waste electrospinning of lignin fiber aerogels. Green Chem. 2021, 23, 6065–6075. [Google Scholar] [CrossRef]
- Srinivas, G.; Krungleviciute, V.; Guo, Z.-X.; Yildirim, T. Exceptional CO2 capture in a hierarchically porous carbon with simultaneous high surface area and pore volume. Energy Environ. Sci. 2014, 7, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Yang, S.; Yu, K.; Zhao, J.; Wang, Z.; Xu, H. The preparation and mechanism studies of rice husk based porous carbon. Mater. Chem. Phys. 2002, 74, 320–323. [Google Scholar] [CrossRef]
- Xue, M.; Chen, C.; Tan, Y.; Ren, Z.; Li, B.; Zhang, C. Mangosteen peel-derived porous carbon: Synthesis and its application in the sulfur cathode for lithium sulfur battery. J. Mater. Sci. 2018, 53, 11062–11077. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, S.; Peng, J.; Hong, Y.; Wang, Z.; Jin, X. Preparation of highly porous carbon through activation of NH4Cl induced hydrothermal microsphere derivation of glucose. RSC Adv. 2017, 7, 6486–6491. [Google Scholar] [CrossRef] [Green Version]
- Baumann, T.F.; Worslet, M.A.; Han, T.Y.-J.; Satcher, J.H. High surface area carbon aerogel monoliths with hierarchical porosity. J. Non-Cryst. Solids 2008, 354, 3513–3515. [Google Scholar] [CrossRef]
- Kim, H.R.; Yoon, T.-U.; Kim, S.-K.; An, J.; Bae, Y.-S.; Lee, C.Y. Beyond pristine MOFs: Carbon dioxide capture by metal–organic frameworks (MOFs)-derived porous carbon materials. RSC Adv. 2017, 7, 1266–1270. [Google Scholar] [CrossRef] [Green Version]
- Comroe, M.; Kolasinski, K.; Saha, D. Direct Ink 3D Printing of Porous Carbon Monoliths for Gas. Molecules 2022, 27, 5653. [Google Scholar] [CrossRef] [PubMed]
- Seema, H.; Kemp, K.; Le, N.; Park, S.-W.; Chandra, V.; Lee, J.; Kim, K. Highly selective CO2 capture by S-doped microporous carbon materials. Carbon 2014, 66, 320–326. [Google Scholar] [CrossRef]
- Li, X.; Xue, Q.; Chang, X.; Zhu, L.; Ling, C.; Zheng, H. Effects of Sulfur Doping and Humidity on CO2 Capture by Graphite Split Pore: A Theoretical Study. ACS Appl. Mater. Interfaces 2017, 9, 8336–8343. [Google Scholar] [CrossRef]
- Yang, R.T. Gas Separation by Adsorption Processes; Imperial College Press: London, UK, 1997. [Google Scholar]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Saha, D.; Grappe, H.; Chakraborty, A.; Orkoulas, G. Post extraction separation, on-board storage and catalytic conversion of methane in natural gas: A review. Chem. Rev. 2016, 116, 11436–11499. [Google Scholar] [CrossRef]



| Sample Identity | Lignin: Na2S2O3: KOH |
|---|---|
| LS-1 | 3:2:2 |
| LS-2 | 3:3:1 |
| LS-3 | 3:1:3 |
| LS-4 | 3:6:0 |
| LS-5 | 3:6:3 |
| Sample Identity | BET SSA (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) |
|---|---|---|---|---|
| LS-1 | 1915 | 1.079 | 0.532 | 0.547 |
| LS-2 | 787 | 0.443 | 0.292 | 0.151 |
| LS-3 | 3626 | 1.741 | 1.44 | 0.301 |
| LS-4 | 280 | 0.157 | 0.089 | 0.068 |
| LS-5 | 741 | 0.501 | 0.214 | 0.287 |
| Elements (%) | LS-1 | LS-2 | LS-3 | LS-4 | LS-5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C (%) | C-C sp2 | 77.3 | 87.2 | 69.5 | 79.6 | 73.0 | 80.1 | 39.0 | 51.3 | 58.4 | 69.6 |
| C-C sp3 | 9.0 | 8.8 | 6.4 | 9.5 | 9.5 | ||||||
| C-O, C-S | 0.9 | 1.2 | 0.7 | 2.2 | 1.3 | ||||||
| C=O | 0 | 0.0 | 0.8 | 0.6 | 0.5 | ||||||
| S (%) | S-C | 1.9 | 3.0 | 3.5 | 6.5 | 0.4 | 1.0 | 6.5 | 12.6 | 5.5 | 8.9 |
| S=C-O | 0.2 | 0.5 | 0.1 | 1.1 | 1.0 | ||||||
| SOx | 0.9 | 2.5 | 0.6 | 5.0 | 2.4 | ||||||
| O (%) | S=O, C-O, OH | 6.0 | 8.8 | 9.8 | 11.5 | 8.6 | 13.5 | 26.5 | 30.9 | 8.9 | 11.2 |
| C-O-H | 2.8 | 1.8 | 4.8 | 4.5 | 2.3 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, D.; Orkoulas, G.; Bates, D. One-Step Synthesis of Sulfur-Doped Nanoporous Carbons from Lignin with Ultra-High Surface Area, Sulfur Content and CO2 Adsorption Capacity. Materials 2023, 16, 455. https://doi.org/10.3390/ma16010455
Saha D, Orkoulas G, Bates D. One-Step Synthesis of Sulfur-Doped Nanoporous Carbons from Lignin with Ultra-High Surface Area, Sulfur Content and CO2 Adsorption Capacity. Materials. 2023; 16(1):455. https://doi.org/10.3390/ma16010455
Chicago/Turabian StyleSaha, Dipendu, Gerassimos Orkoulas, and Dean Bates. 2023. "One-Step Synthesis of Sulfur-Doped Nanoporous Carbons from Lignin with Ultra-High Surface Area, Sulfur Content and CO2 Adsorption Capacity" Materials 16, no. 1: 455. https://doi.org/10.3390/ma16010455
APA StyleSaha, D., Orkoulas, G., & Bates, D. (2023). One-Step Synthesis of Sulfur-Doped Nanoporous Carbons from Lignin with Ultra-High Surface Area, Sulfur Content and CO2 Adsorption Capacity. Materials, 16(1), 455. https://doi.org/10.3390/ma16010455






