Modification of Some Structural and Functional Parameters of Living Culture of Arthrospira platensis as the Result of Selenium Nanoparticle Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Determination of Selenium Content in Biomass
2.3. Determination of the Amount of Spirulina Biomass
2.4. Determination of the Biochemical Composition of Spirulina Biomass
2.5. RNA Extraction and Quantitative RT-PCR (RT-qPCR)
2.6. Transmission Electron Microscopy (TEM) Analysis and Energy Dispersive X-ray Analysis
3. Results and Discussion
3.1. Uptake of Se and Its Accumulation in the Arthrospira platensis Biomass
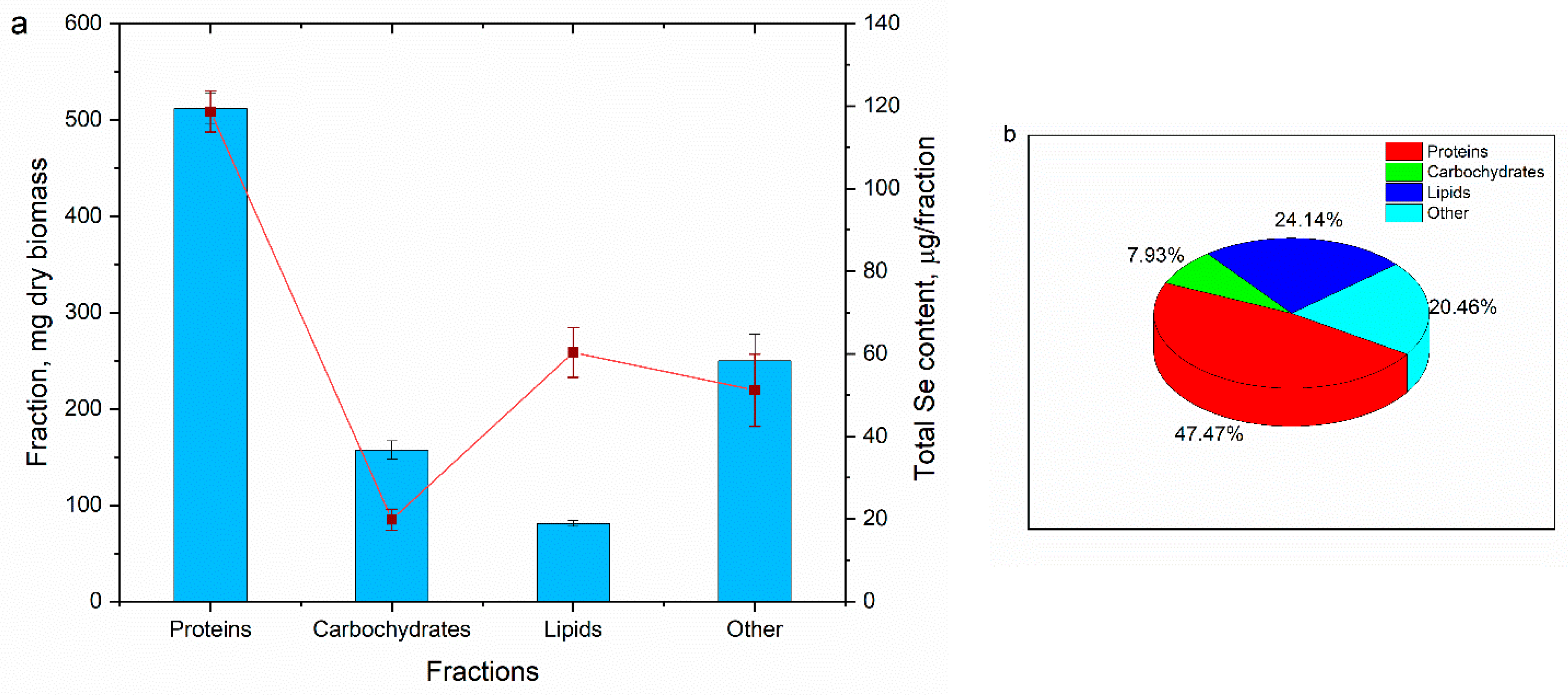
3.2. The Content of Selenium in Arthrospira platensis Biomass Fractions
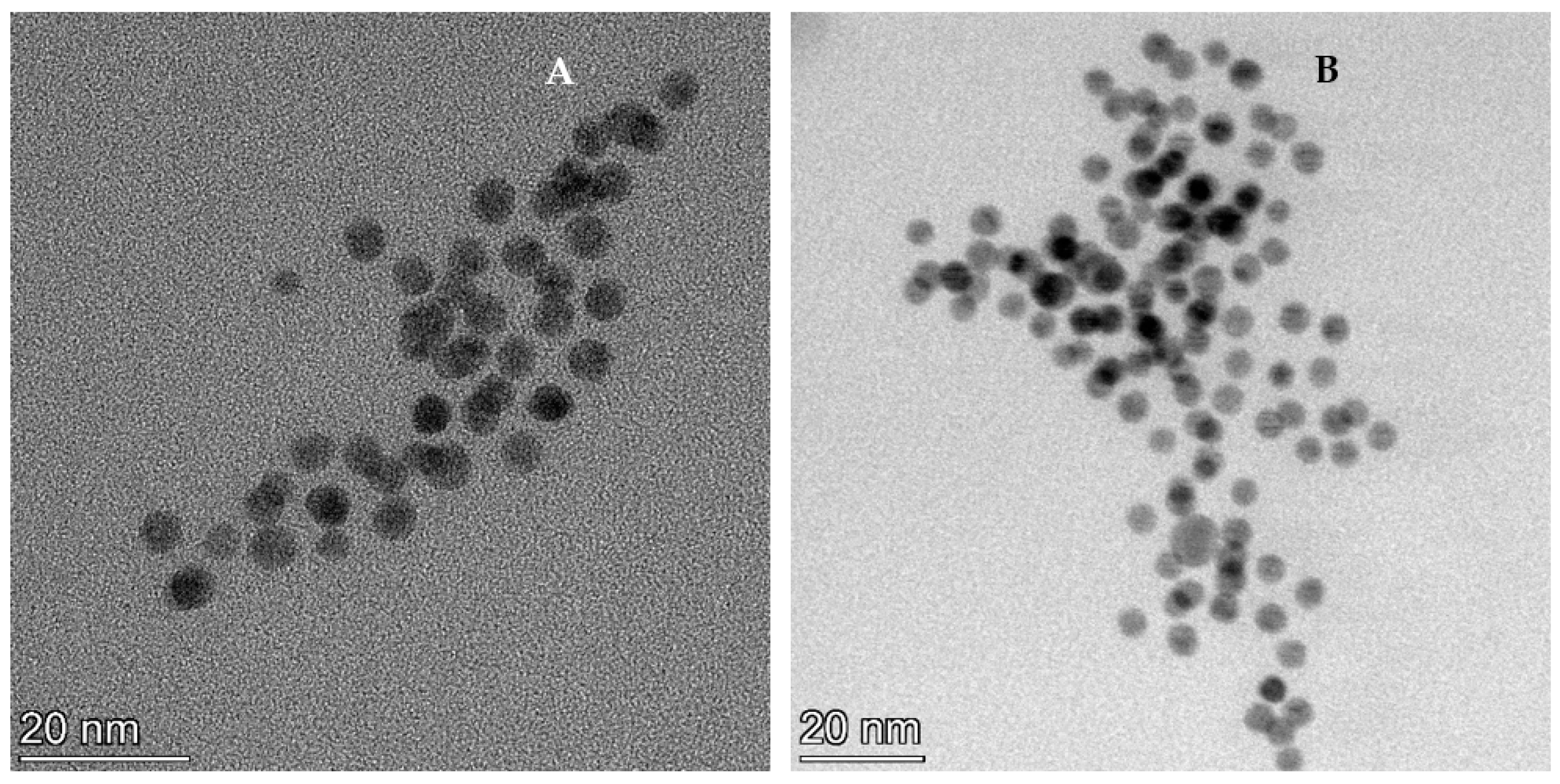
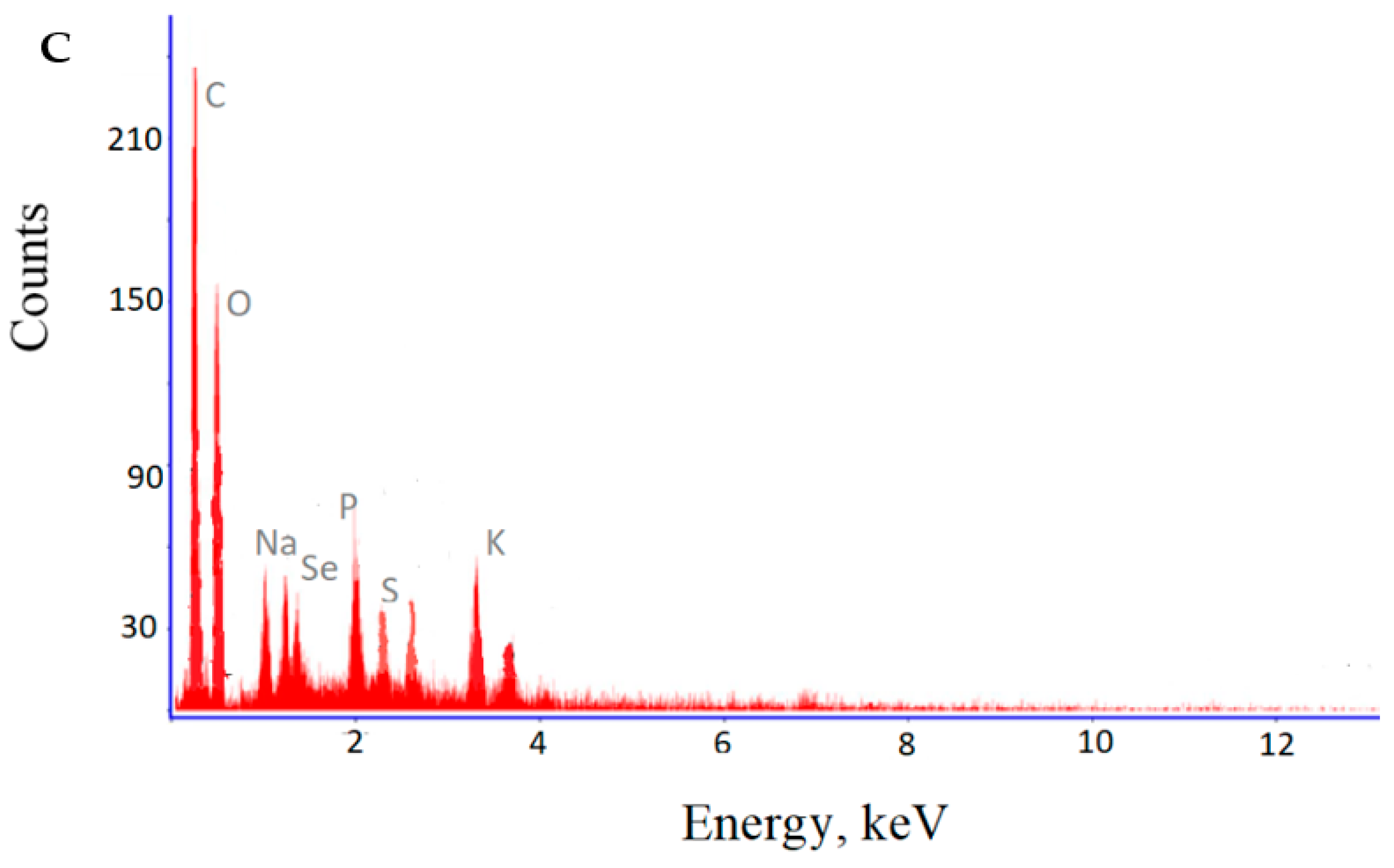
3.3. Formation of Selenium Nanoparticles
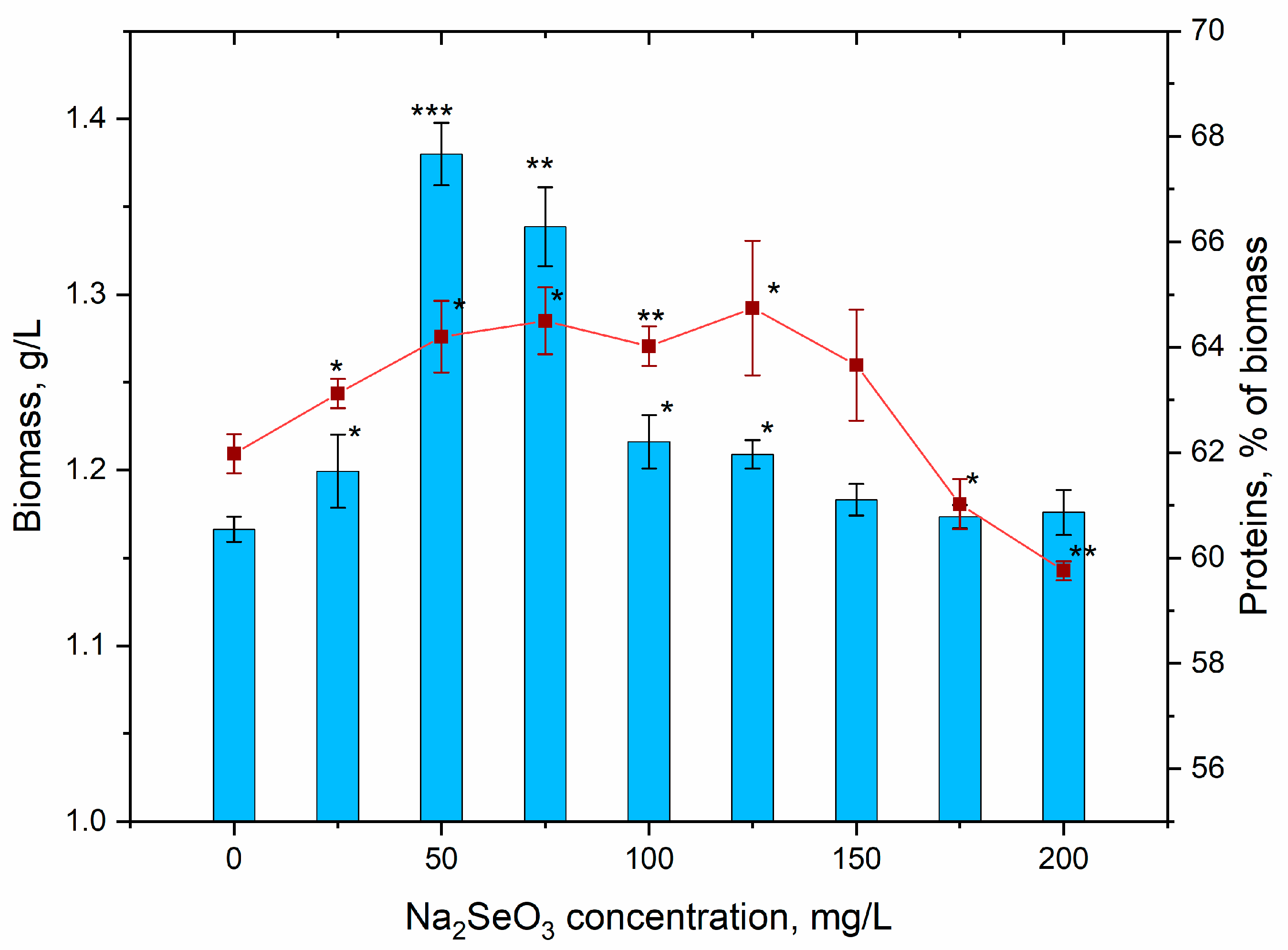
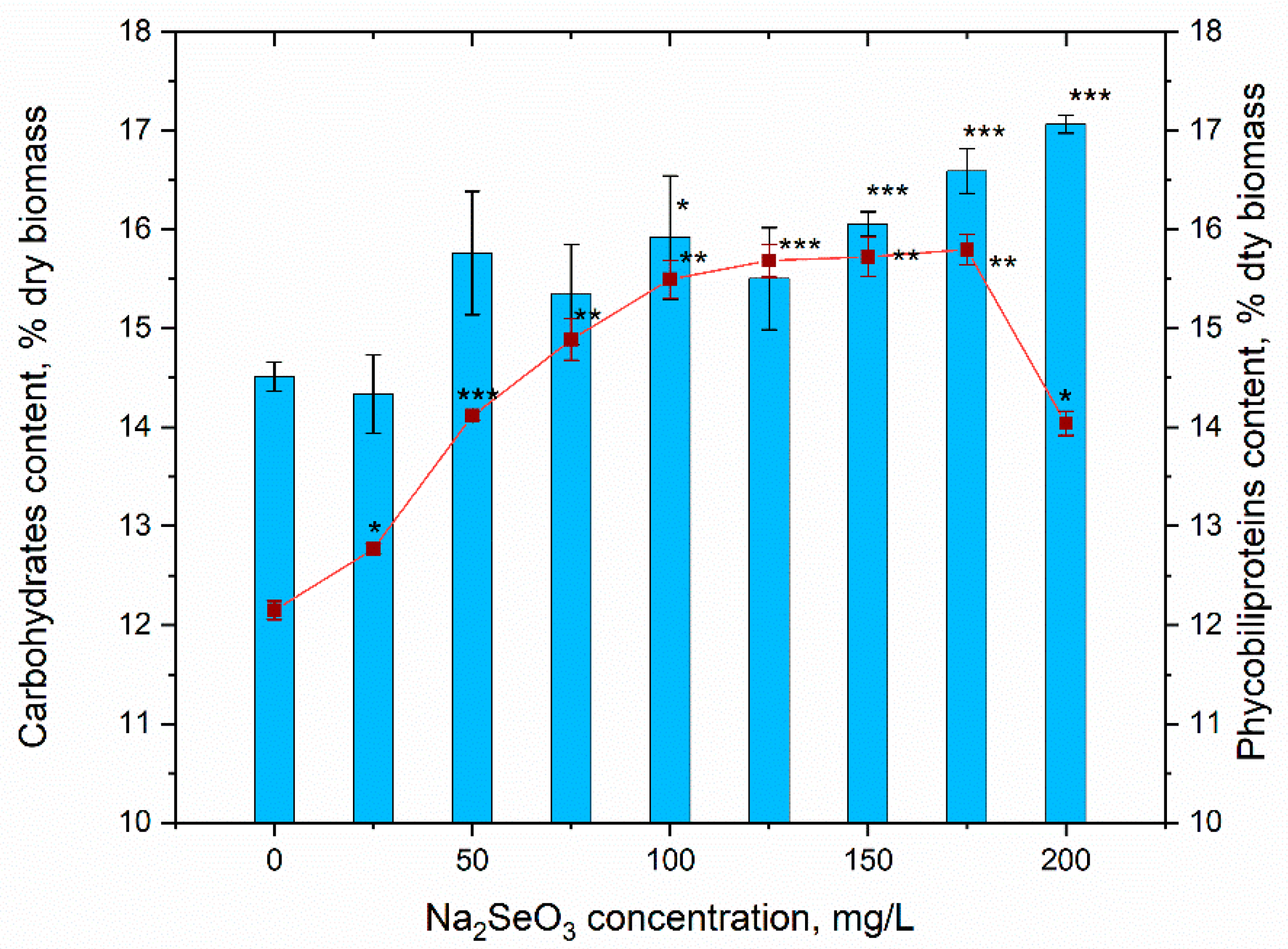
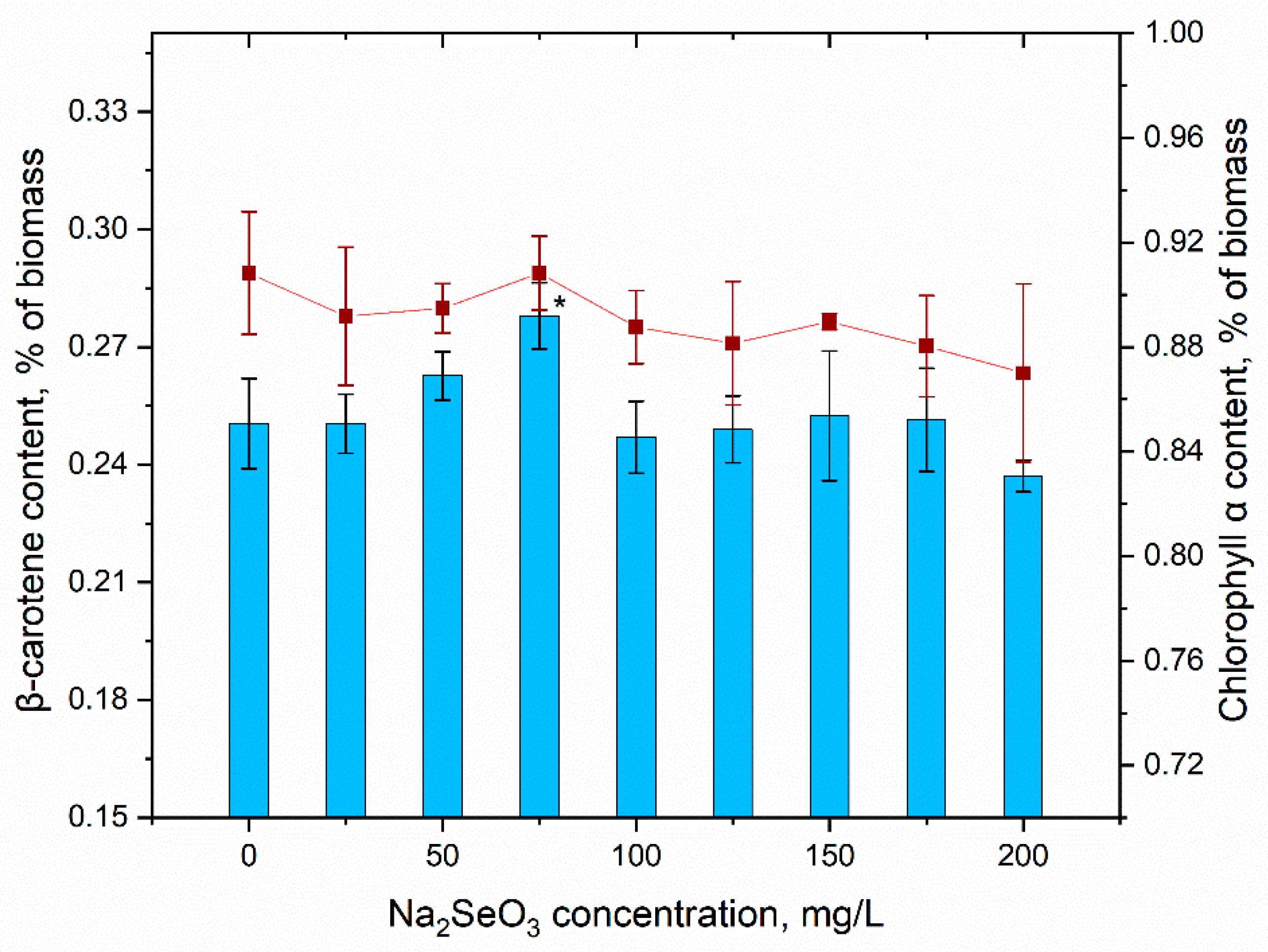
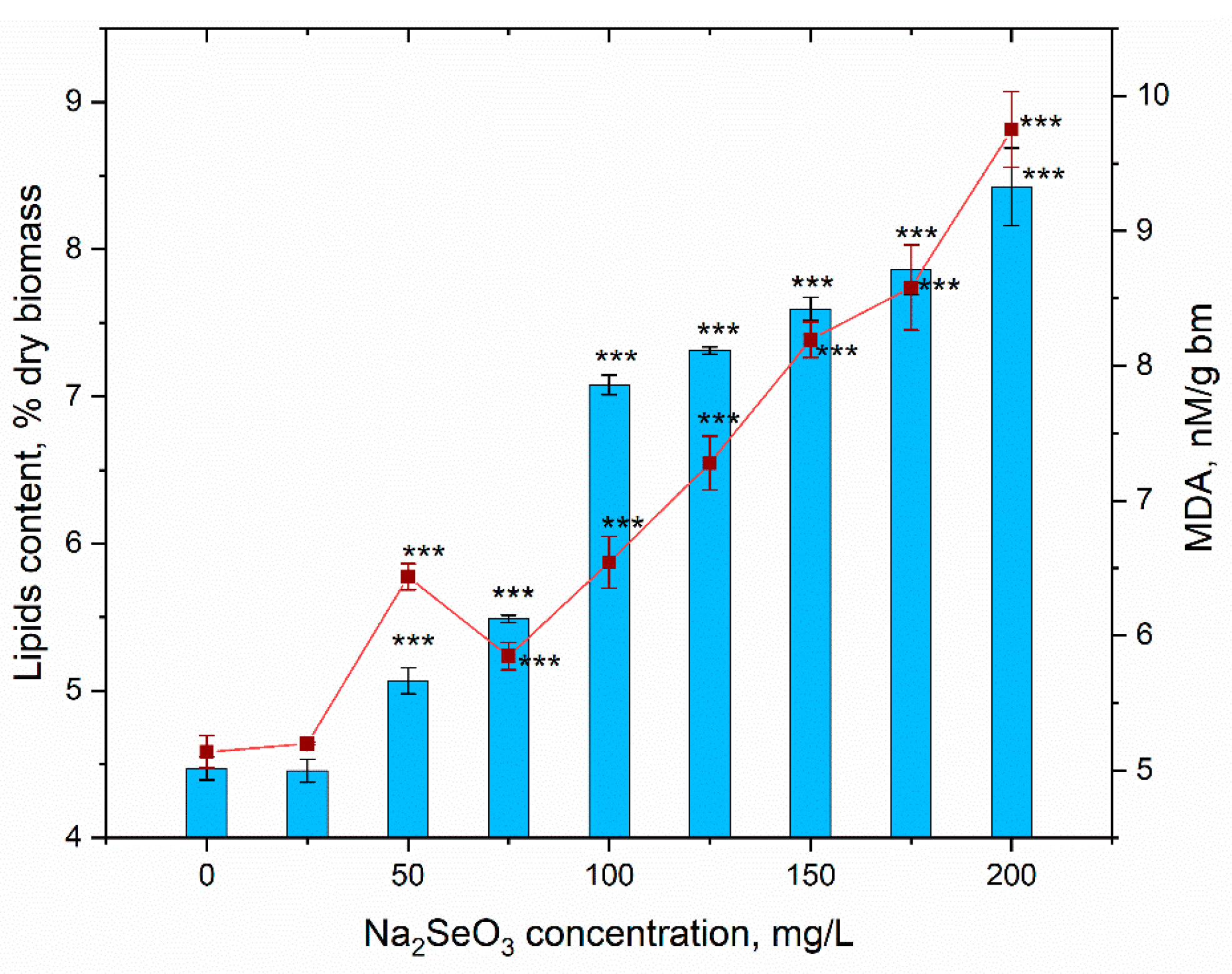
3.4. Content of Biomass and Its Biochemical Composition
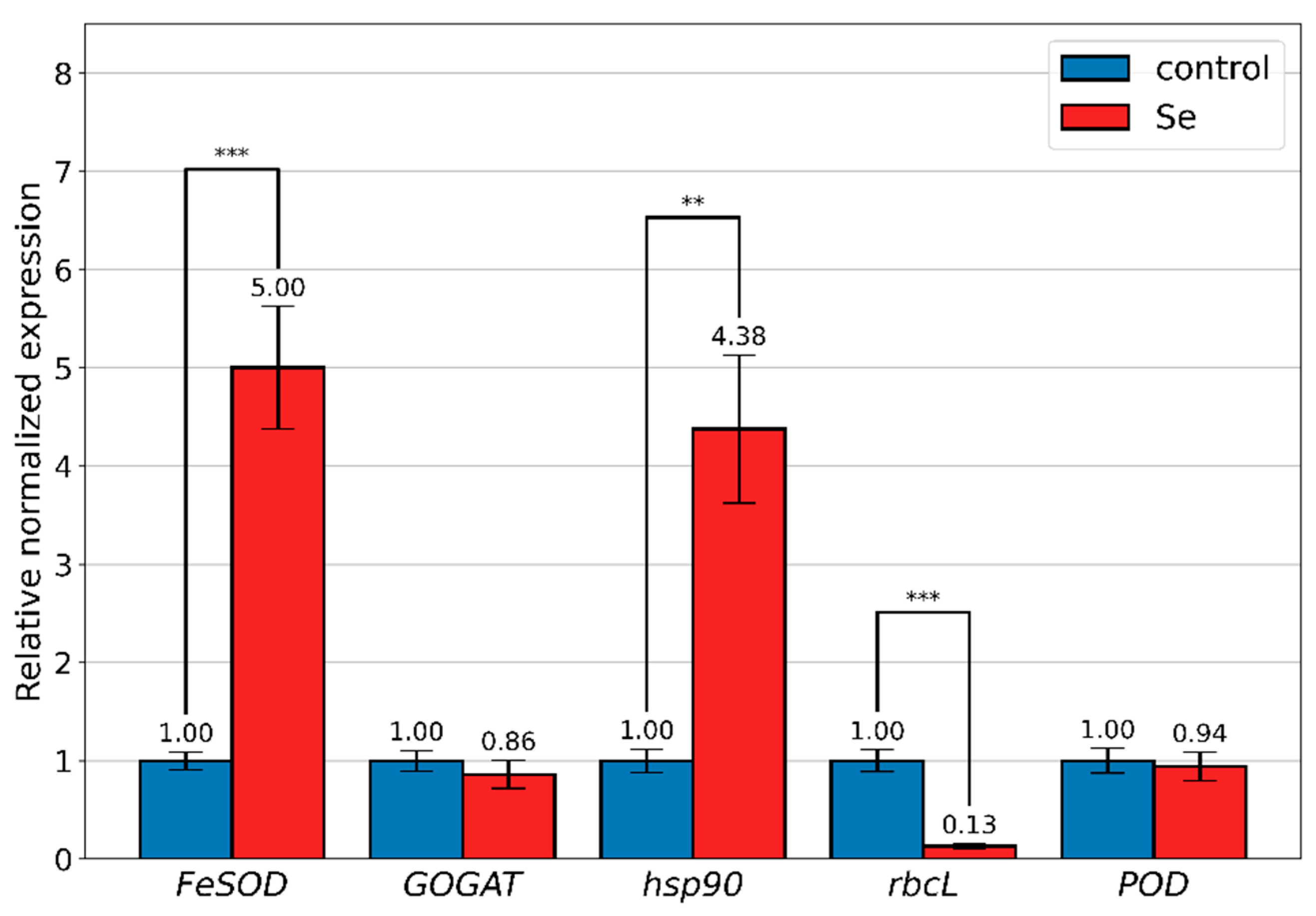
3.5. Gene Expression Analysis
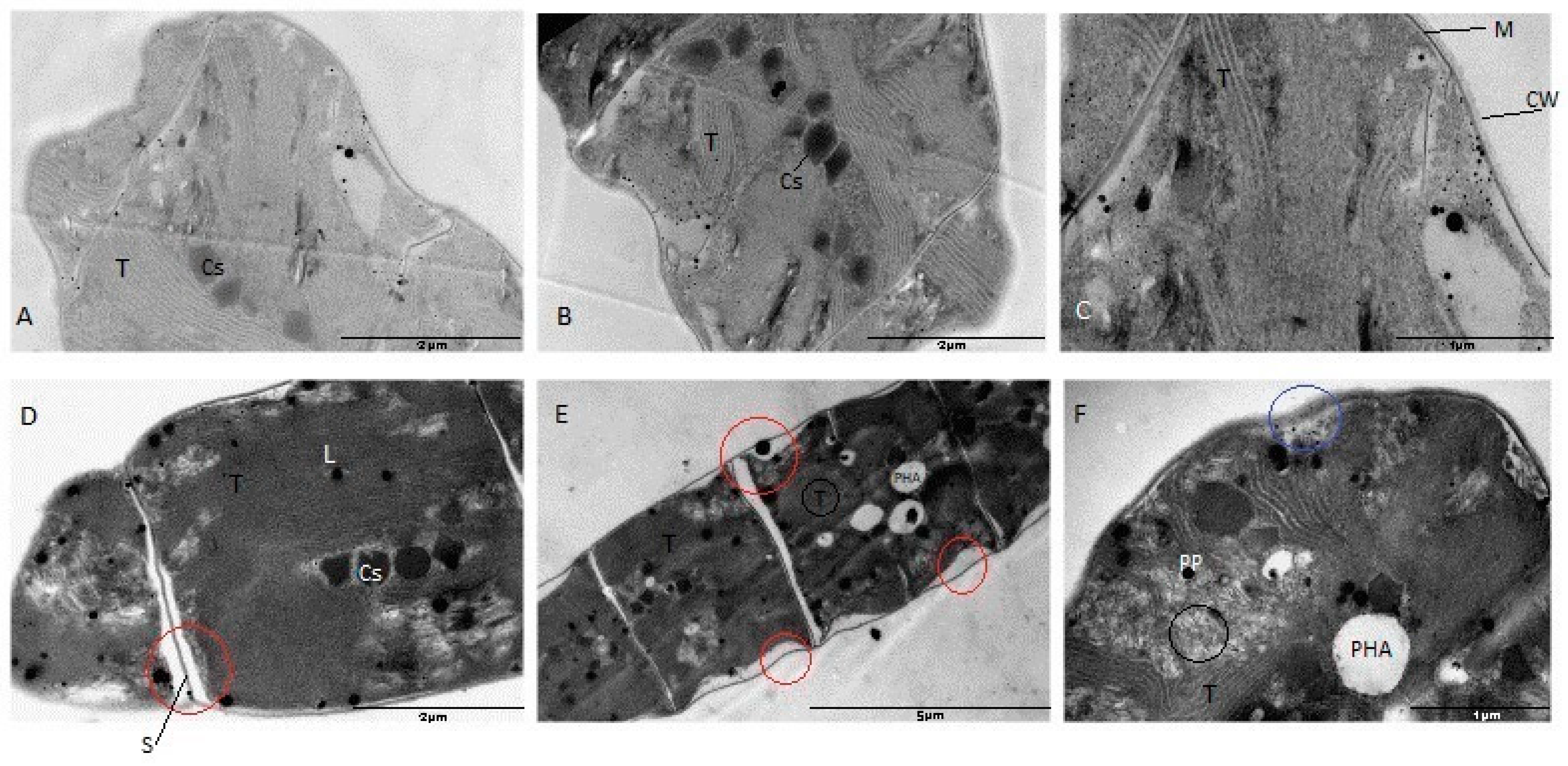
3.6. Ultrastructural Changes in Arthrospira platensis under the Influence of Selenium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic Applications of Selenium Nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium Nanoparticles for Biomedical Applications: From Development and Characterization to Therapeutics. Adv. Healthc. Mater. 2021, 10, 2100598. [Google Scholar] [CrossRef] [PubMed]
- Geoffrion, L.D.; Hesabizadeh, T.; Medina-Cruz, D.; Kusper, M.; Taylor, P.; Vernet-Crua, A.; Chen, J.; Ajo, A.; Webster, T.J.; Guisbiers, G. Naked Selenium Nanoparticles for Antibacterial and Anticancer Treatments. ACS Omega 2020, 5, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Shoeibi, S.; Mashreghi, M. Biosynthesis of Selenium Nanoparticles Using Enterococcus Faecalis and Evaluation of Their Antibacterial Activities. J. Trace Elem. Med. Biol. 2017, 39, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Vinković Vrček, I. Selenium Nanoparticles: Biomedical Applications. In Selenium Molecular and Integrative Toxicology; Michalke, B., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 393–412. ISBN 978-3-319-95389-2. [Google Scholar]
- Nayak, V.; Singh, K.R.; Singh, A.K.; Singh, R.P. Potentialities of Selenium Nanoparticles in Biomedical Science. New J. Chem. 2021, 45, 2849–2878. [Google Scholar] [CrossRef]
- Cristallini, C.; Vitale, E.; Giachino, C.; Rastaldo, R. Nanoengineering in Cardiac Regeneration: Looking Back and Going Forward. Nanomaterials 2020, 10, 1587. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Selvamani, S.; Prasanna, M.; Muthukumaran, A. A Novel Biocompatible Chitosan–Selenium Nanoparticles (SeNPs) Film with Electrical Conductivity for Cardiac Tissue Engineering Application. Mater. Sci. Eng. C 2018, 92, 151–160. [Google Scholar] [CrossRef]
- Yang, C.; Tian, A.; Li, Z. Reversible Cardiac Hypertrophy Induced by PEG-Coated Gold Nanoparticles in Mice. Sci. Rep. 2016, 6, 20203. [Google Scholar] [CrossRef]
- Xiao, S.; Mao, L.; Xiao, J.; Wu, Y.; Liu, H. Selenium Nanoparticles Inhibit the Formation of Atherosclerosis in Apolipoprotein E Deficient Mice by Alleviating Hyperlipidemia and Oxidative Stress. Eur. J. Pharmacol. 2021, 902, 174120. [Google Scholar] [CrossRef]
- Weekley, C.M.; Harris, H.H. Which Form Is That? The Importance of Selenium Speciation and Metabolism in the Prevention and Treatment of Disease. Chem. Soc. Rev. 2013, 42, 8870. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of Selenium Nanoparticles Using Plant Extracts. J. Nanostruct. Chem. 2022, 12, 467–480. [Google Scholar] [CrossRef]
- Bafghi, M.H.; Darroudi, M.; Zargar, M.; Zarrinfar, H.; Nazari, R. Biosynthesis of Selenium Nanoparticles by Aspergillus Flavus and Candida Albicans for Antifungal Applications. Micro Nano Lett. 2021, 16, 656–669. [Google Scholar] [CrossRef]
- Diko, C.S.; Zhang, H.; Lian, S.; Fan, S.; Li, Z.; Qu, Y. Optimal Synthesis Conditions and Characterization of Selenium Nanoparticles in Trichoderma Sp. WL-Go Culture Broth. Mater. Chem. Phys. 2020, 246, 122583. [Google Scholar] [CrossRef]
- Srivastava, N.; Mukhopadhyay, M. Biosynthesis and Structural Characterization of Selenium Nanoparticles Using Gliocladium Roseum. J. Clust. Sci. 2015, 26, 1473–1482. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, H.; Bai, J.; Li, Y.; Yang, J.; Ma, Q.; Qu, Y. Biosynthesis of Selenium Nanoparticles Mediated by Fungus Mariannaea Sp. HJ and Their Characterization. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 571, 9–16. [Google Scholar] [CrossRef]
- Presentato, A.; Piacenza, E.; Anikovskiy, M.; Cappelletti, M.; Zannoni, D.; Turner, R.J. Biosynthesis of Selenium-Nanoparticles and -Nanorods as a Product of Selenite Bioconversion by the Aerobic Bacterium Rhodococcus Aetherivorans BCP1. New Biotechnol. 2018, 41, 1–8. [Google Scholar] [CrossRef]
- Shakibaie, M.; Khorramizadeh, M.R.; Faramarzi, M.A.; Sabzevari, O.; Shahverdi, A.R. Biosynthesis and Recovery of Selenium Nanoparticles and the Effects on Matrix Metalloproteinase-2 Expression. Biotechnol. Appl. Biochem. 2010, 56, 7–15. [Google Scholar] [CrossRef]
- Ullah, A.; Yin, X.; Wang, F.; Xu, B.; Mirani, Z.A.; Xu, B.; Chan, M.W.H.; Ali, A.; Usman, M.; Ali, N.; et al. Biosynthesis of Selenium Nanoparticles (via Bacillus Subtilis BSN313), and Their Isolation, Characterization, and Bioactivities. Molecules 2021, 26, 5559. [Google Scholar] [CrossRef]
- Chavan, R.B.; Bhattacharjee, M.B. Role of Alginate and Oxalic Acid in Ameliorating Se Toxicity in Hapalosiphon Cyanobacterium. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 132–139. [Google Scholar] [CrossRef]
- Gouget, B.; Avoscan, L.; Sarret, G.; Collins, R.; Carrière, M. Resistance, Accumulation and Transformation of Selenium by the Cyanobacterium Synechocystis Sp. PCC 6803 after Exposure to Inorganic Se VI or Se IV. Radiochim. Acta 2005, 93, 683–689. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, J.-C.; Gan, X.; He, S.; Zhou, W. Selenium Uptake, Volatilization, and Transformation by the Cyanobacterium Microcystis Aeruginosa and Post-Treatment of Se-Laden Biomass. Chemosphere 2021, 280, 130593. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Kalwani, P.; Chakravarty, D.; Singh, B.; Ballal, A. Functional and Mechanistic Insights into the Differential Effect of the Toxicant ‘Se(IV)’ in the Cyanobacterium Anabaena PCC 7120. Aquat. Toxicol. 2021, 236, 105839. [Google Scholar] [CrossRef] [PubMed]
- Afzal, B.; Yasin, D.; Husain, S.; Zaki, A.; Srivastava, P.; Kumar, R.; Fatma, T. Screening of Cyanobacterial Strains for the Selenium Nanoparticles Synthesis and Their Anti-Oxidant Activity. Biocatal. Agric. Biotechnol. 2019, 21, 101307. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Chiriac, T.; Cepoi, L.; Rudi, L.; Culicov, O.; Frontasyeva, M.; Rudic, V. Selenium Uptake and Assessment of the Biochemical Changes in Arthrospira ( Spirulina ) Platensis Biomass during the Synthesis of Selenium Nanoparticles. Can. J. Microbiol. 2017, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Kalari, S.; Morowvat, M.H.; Sabahi, Z.; Dehshahri, A. Green Synthesis of Selenium Nanoparticles by Cyanobacterium Spirulina Platensis (Abdf2224): Cultivation Condition Quality Controls. BioMed Res. Int. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Afzal, B.; Fatma, T. Selenium Nanoparticles: Green Synthesis and Exploitation. In Emerging Technologies for Nanoparticle Manufacturing; Patel, J.K., Pathak, Y.V., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 473–484. ISBN 978-3-030-50702-2. [Google Scholar]
- Cepoi, L.; Zinicovscaia, I.; Zosim, L.; Chiriac, T.; Rudic, V.; Rudi, L.; Djur, S.; Elenciuc, D.; Miscu, V.; Ludmila, B.; et al. Metals Removal by Cyanobacteria and Accumulation in Biomass. In Cyanobacteria for Bioremediation of Wastewaters; Zinicovscaia, I., Cepoi, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 61–111. ISBN 978-3-319-26749-4. [Google Scholar]
- Cepoi, L.; Chiriac, T.; Rudi, L.; Djur, S.; Zosim, L.; Bulimaga, V.; Batir, L.; Elenciuc, D.; Rudic, V. Spirulina as a Raw Material for Products Containing Trace Elements. In Recent Advances in Trace Elements; Chojnacka, K., Saeid, A., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2018; pp. 403–420. ISBN 978-1-119-13378-0. [Google Scholar]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Rotari, I.; Turchenko, V.; Djur, S. Effects of PEG-Coated Silver and Gold Nanoparticles on Spirulina Platensis Biomass during Its Growth in a Closed System. Coatings 2020, 10, 717. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, W.; Wong, Y.-S.; Yang, F.; Bai, Y. Accumulation of Selenium in Mixotrophic Culture of Spirulina Platensis on Glucose. Bioresour. Technol. 2006, 97, 2260–2265. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Guo, S.-Y.; Li, L. Bioeffects of Selenite on the Growth of Spirulina Platensis and Its Biotransformation. Bioresour. Technol. 2003, 89, 171–176. [Google Scholar] [CrossRef]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal Clearance of Quantum Dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Liu, F.; Nasr, K.; Misra, P.; Bawendi, M.G.; Frangioni, J.V. Design Considerations for Tumour-Targeted Nanoparticles. Nat. Nanotech. 2010, 5, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, A.M.; Usseglio, S.; Mondini, S.; Drago, C.; La Mattina, R.; Chini, B.; Verderio, C.; Leonzino, M.; Cagnoli, C.; Joshi, P.; et al. Towards Bio-Compatible Magnetic Nanoparticles: Immune-Related Effects, in-Vitro Internalization, and in-Vivo Bio-Distribution of Zwitterionic Ferrite Nanoparticles with Unexpected Renal Clearance. J. Colloid Interface Sci. 2021, 582, 678–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, J.; Huang, B.; Ying, H.; He, J.; Jiang, L. Selenium Metabolism and Selenoproteins in Prokaryotes: A Bioinformatics Perspective. Biomolecules 2022, 12, 917. [Google Scholar] [CrossRef]
- Chen, T.-F.; Zheng, W.-J.; Wong, Y.-S.; Yang, F. Selenium-Induced Changes in Activities of Antioxidant Enzymes and Content of Photosynthetic Pigments in Spirulina Platensis. J. Integr. Plant Biol. 2008, 50, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Jeamton, W.; Mungpakdee, S.; Sirijuntarut, M.; Prommeenate, P.; Cheevadhanarak, S.; Tanticharoen, M.; Hongsthong, A. A Combined Stress Response Analysis of Spirulina Platensis in Terms of Global Differentially Expressed Proteins, and MRNA Levels and Stability of Fatty Acid Biosynthesis Genes: Combined Stress Response Analysis of Spirulina Platensis. FEMS Microbiol. Lett. 2008, 281, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Bryant, D.A. Synechococcus Sp. Strain PCC 7002 Transcriptome: Acclimation to Temperature, Salinity, Oxidative Stress, and Mixotrophic Growth Conditions. Front. Microbio. 2012, 3, 354. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, H.; Suzuki, N.; Roy, S.K. Constitutive Expression of a Small Heat-Shock Protein Confers Cellular Thermotolerance and Thermal Protection to the Photosynthetic Apparatus in Cyanobacteria. FEBS Lett. 2000, 483, 169–174. [Google Scholar] [CrossRef]
- Ismaiel, M.M.S.; Piercey-Normore, M.D. Gene Transcription and Antioxidants Production in Arthrospira (Spirulina) Platensis Grown under Temperature Variation. J. Appl. Microbiol. 2021, 130, 891–900. [Google Scholar] [CrossRef]
- Deng, Y.; Zhan, Z.; Tang, X.; Ding, L.; Duan, D. Molecular Cloning and Expression Analysis of RbcL CDNA from the Bloom-Forming Green Alga Chaetomorpha Valida (Cladophorales, Chlorophyta). J. Appl. Phycol. 2014, 26, 1853–1861. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Han, Z.; Sun, X.; Li, H.; Zhang, J.; Lu, Y. Molecular Analyses of Tomato GS, GOGAT and GDH Gene Families and Their Response to Abiotic Stresses. Acta Physiol. Plant 2016, 38, 229. [Google Scholar] [CrossRef]
- Panyakampol, J.; Cheevadhanarak, S.; Sutheeworapong, S.; Chaijaruwanich, J.; Senachak, J.; Siangdung, W.; Jeamton, W.; Tanticharoen, M.; Paithoonrangsarid, K. Physiological and Transcriptional Responses to High Temperature in Arthrospira ( Spirulina ) Platensis C1. Plant Cell Physiol. 2015, 56, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Nakamoto, H. Role for the Cyanobacterial HtpG in Protection from Oxidative Stress. Curr. Microbiol. 2003, 46, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Castoreno, A.; Lammers, P.J. Transcript Levels of RbcR1, NtcA, and RbcL/S Genes in Cyanobacterium Anabaena Sp. PCC 7120 Are Downregulated in Response to Cold and Osmotic Stress. FEMS Microbiol. Lett. 2002, 213, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Rangsayatorn, N.; Upatham, E.S.; Kruatrachue, M.; Pokethitiyook, P.; Lanza, G.R. Phytoremediation Potential of Spirulina (Arthrospira) Platensis: Biosorption and Toxicity Studies of Cadmium. Environ. Pollut. 2002, 119, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Deschoenmaeker, F.; Facchini, R.; Cabrera Pino, J.C.; Bayon-Vicente, G.; Sachdeva, N.; Flammang, P.; Wattiez, R. Nitrogen Depletion in Arthrospira Sp. PCC 8005, an Ultrastructural Point of View. J. Struct. Biol. 2016, 196, 385–393. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cepoi, L.; Zinicovscaia, I.; Chiriac, T.; Rudi, L.; Yushin, N.; Grozdov, D.; Tasca, I.; Kravchenko, E.; Tarasov, K. Modification of Some Structural and Functional Parameters of Living Culture of Arthrospira platensis as the Result of Selenium Nanoparticle Biosynthesis. Materials 2023, 16, 852. https://doi.org/10.3390/ma16020852
Cepoi L, Zinicovscaia I, Chiriac T, Rudi L, Yushin N, Grozdov D, Tasca I, Kravchenko E, Tarasov K. Modification of Some Structural and Functional Parameters of Living Culture of Arthrospira platensis as the Result of Selenium Nanoparticle Biosynthesis. Materials. 2023; 16(2):852. https://doi.org/10.3390/ma16020852
Chicago/Turabian StyleCepoi, Liliana, Inga Zinicovscaia, Tatiana Chiriac, Ludmila Rudi, Nikita Yushin, Dmitrii Grozdov, Ion Tasca, Elena Kravchenko, and Kirill Tarasov. 2023. "Modification of Some Structural and Functional Parameters of Living Culture of Arthrospira platensis as the Result of Selenium Nanoparticle Biosynthesis" Materials 16, no. 2: 852. https://doi.org/10.3390/ma16020852
APA StyleCepoi, L., Zinicovscaia, I., Chiriac, T., Rudi, L., Yushin, N., Grozdov, D., Tasca, I., Kravchenko, E., & Tarasov, K. (2023). Modification of Some Structural and Functional Parameters of Living Culture of Arthrospira platensis as the Result of Selenium Nanoparticle Biosynthesis. Materials, 16(2), 852. https://doi.org/10.3390/ma16020852









