Comparing the Corrosion Resistance of 5083 Al and Al2O33D/5083 Al Composite in a Chloride Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Characterization
3. Results
3.1. Sample of Polished Surface of the Two Materials
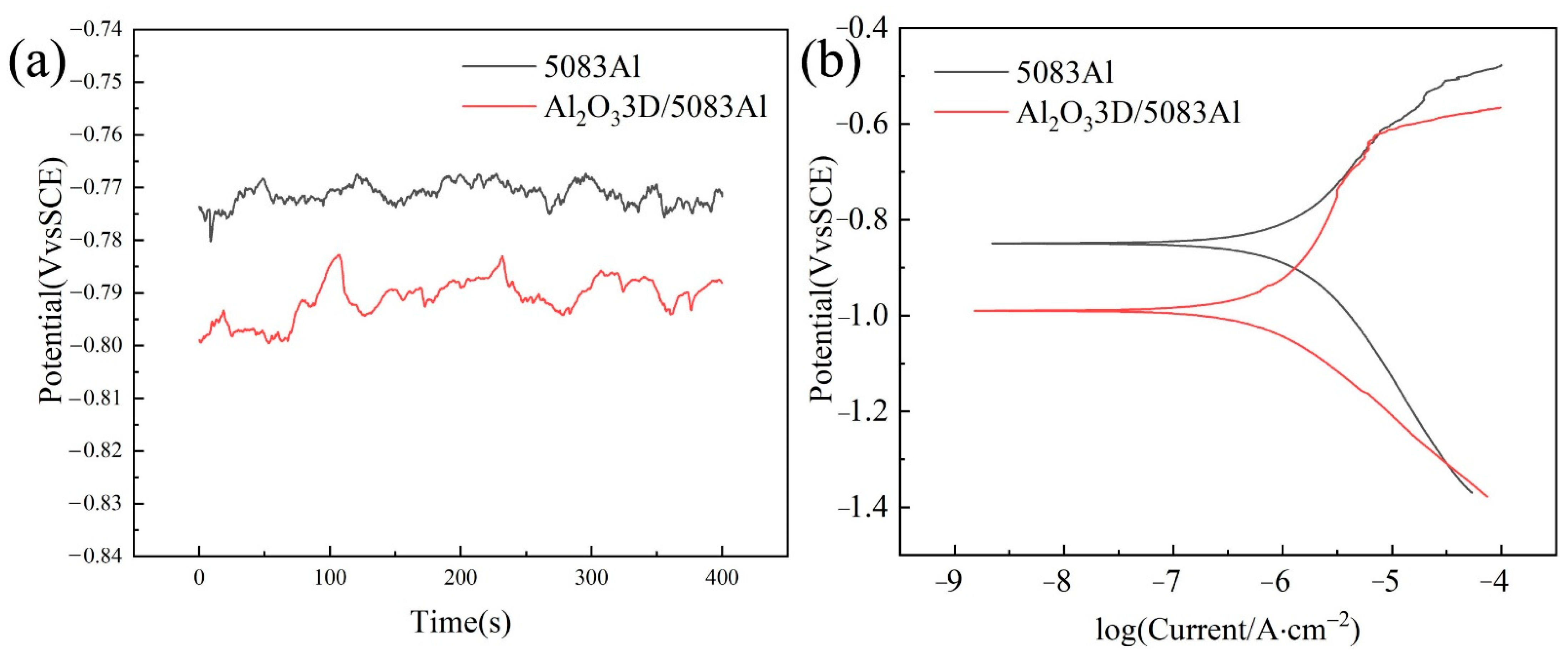
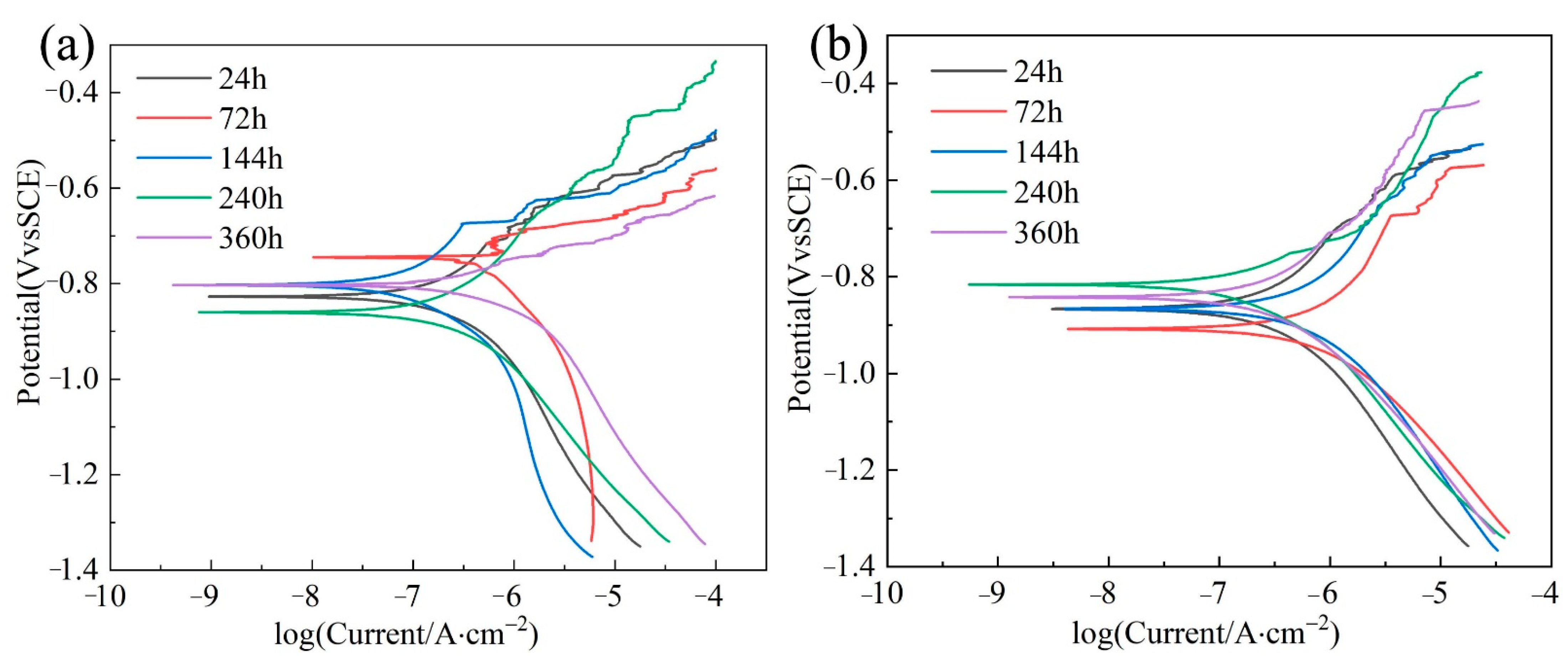
3.2. Polarization Curve
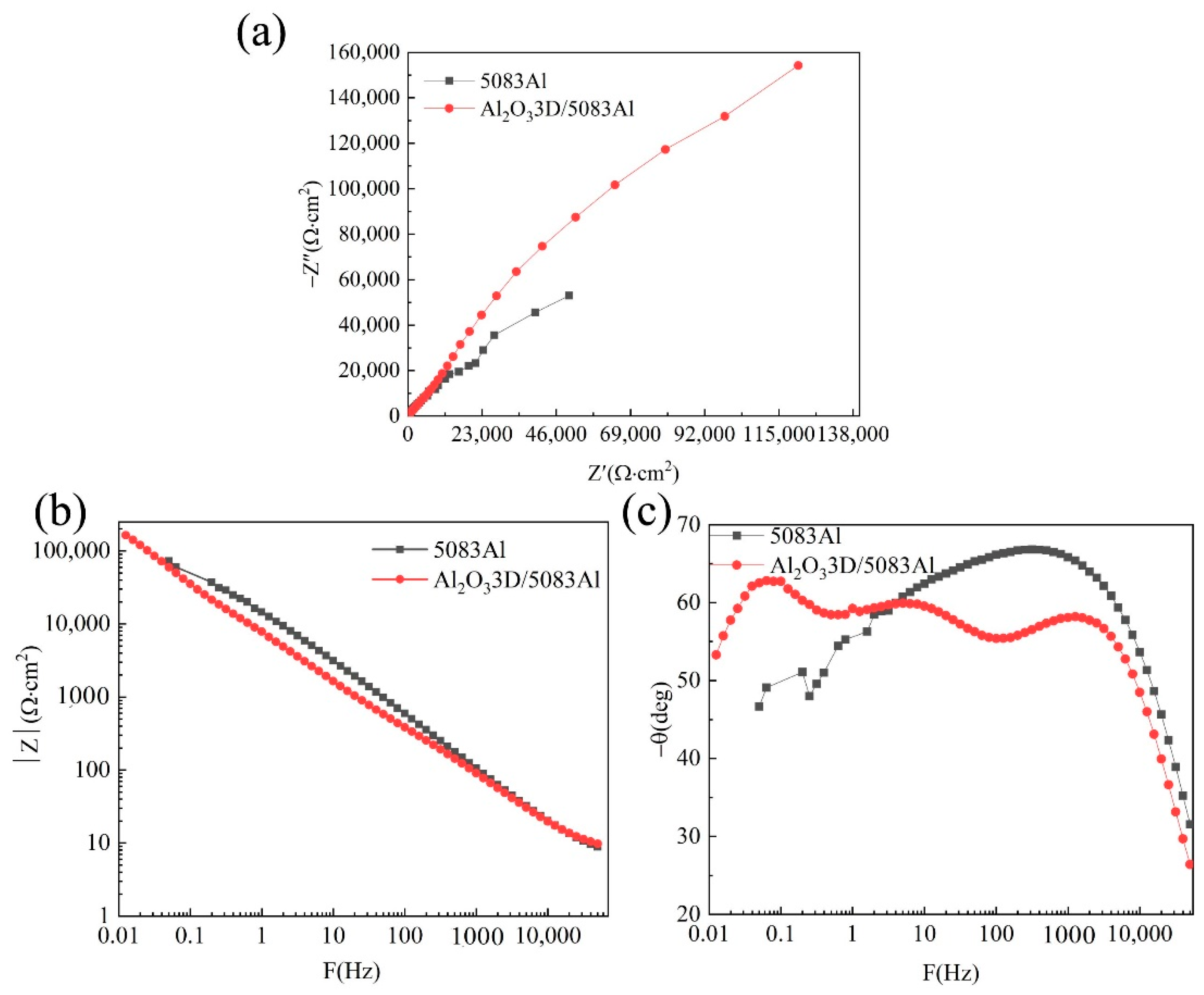
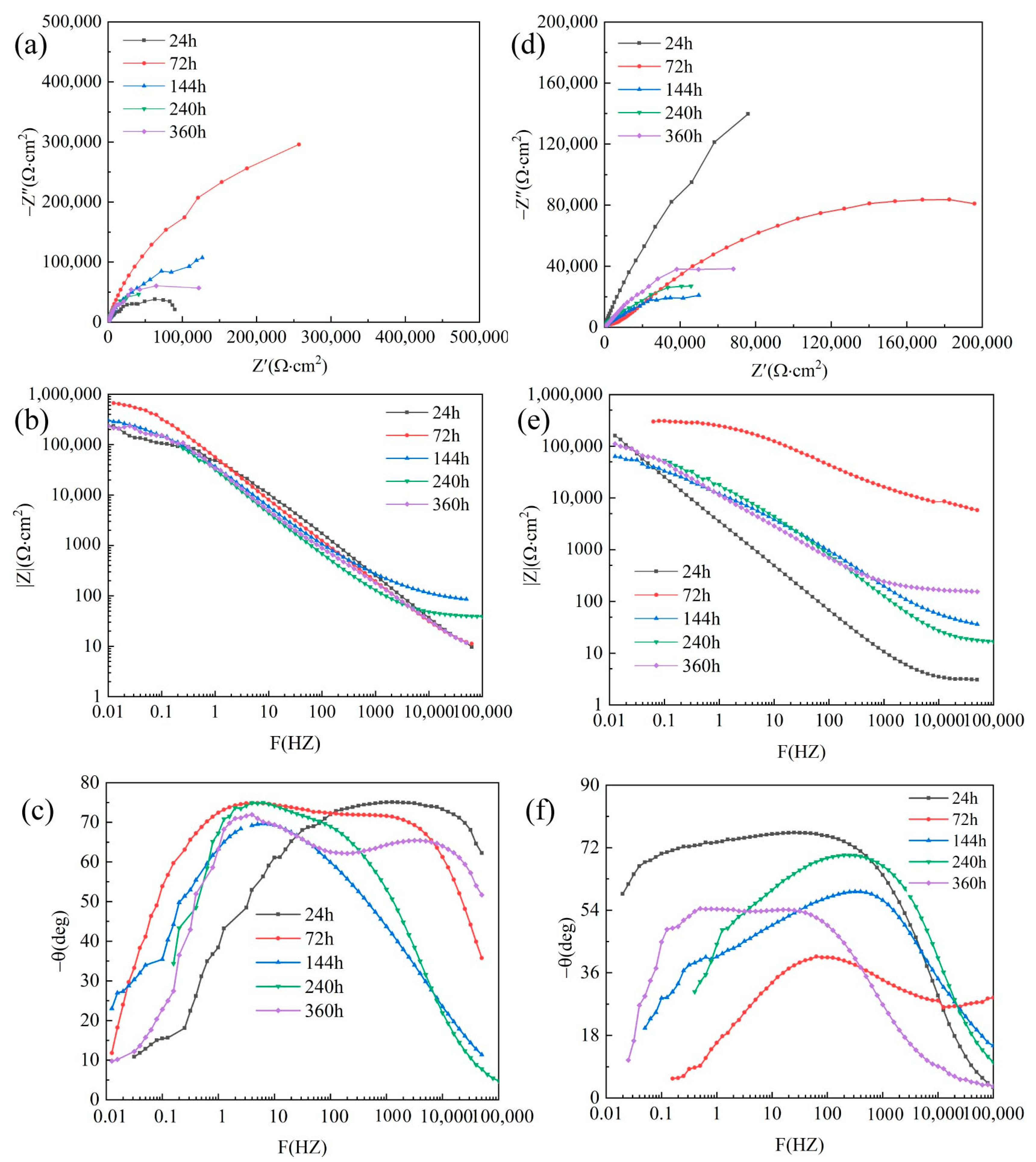
3.3. EIS of Polished Surface Materials
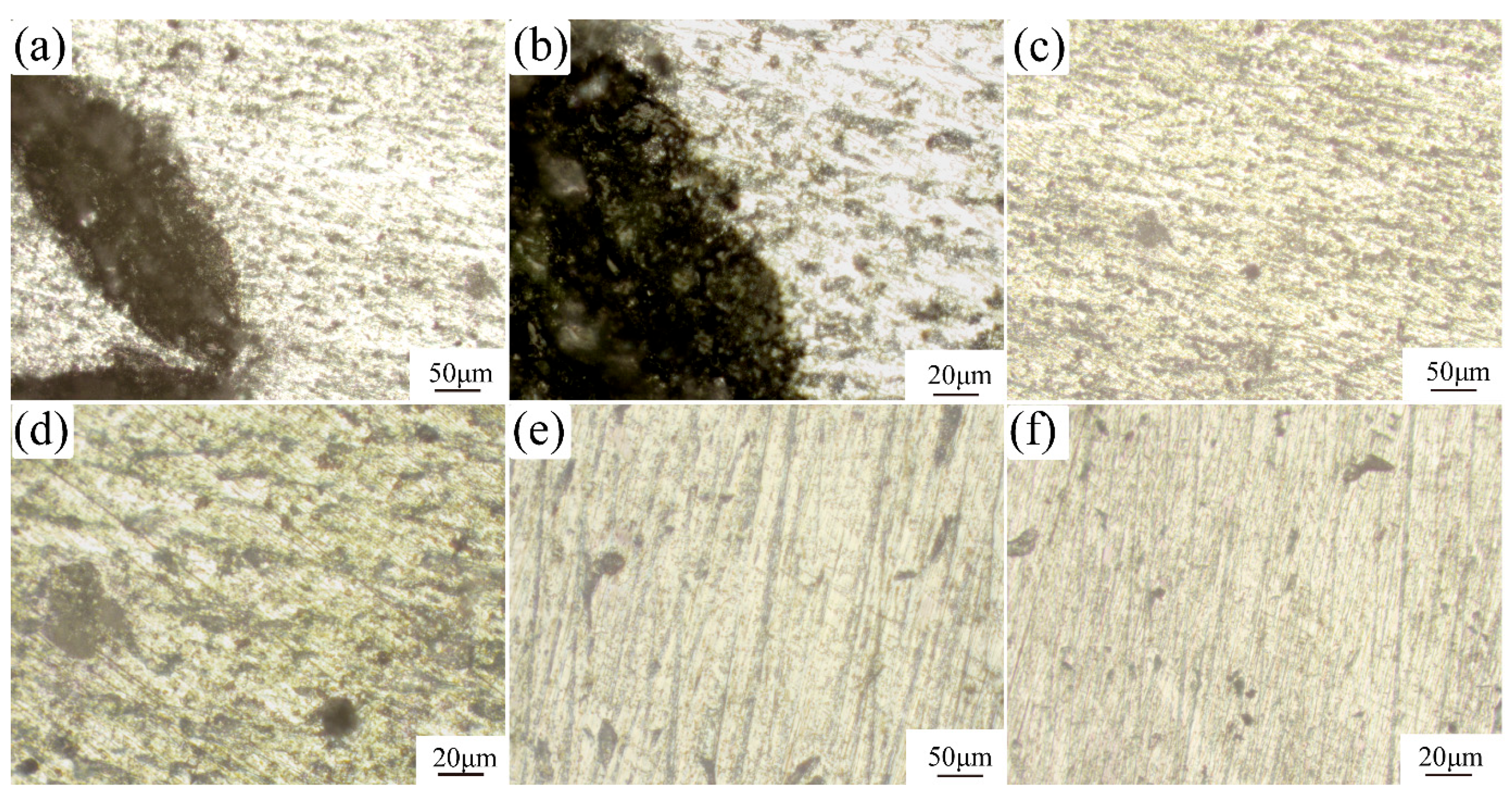
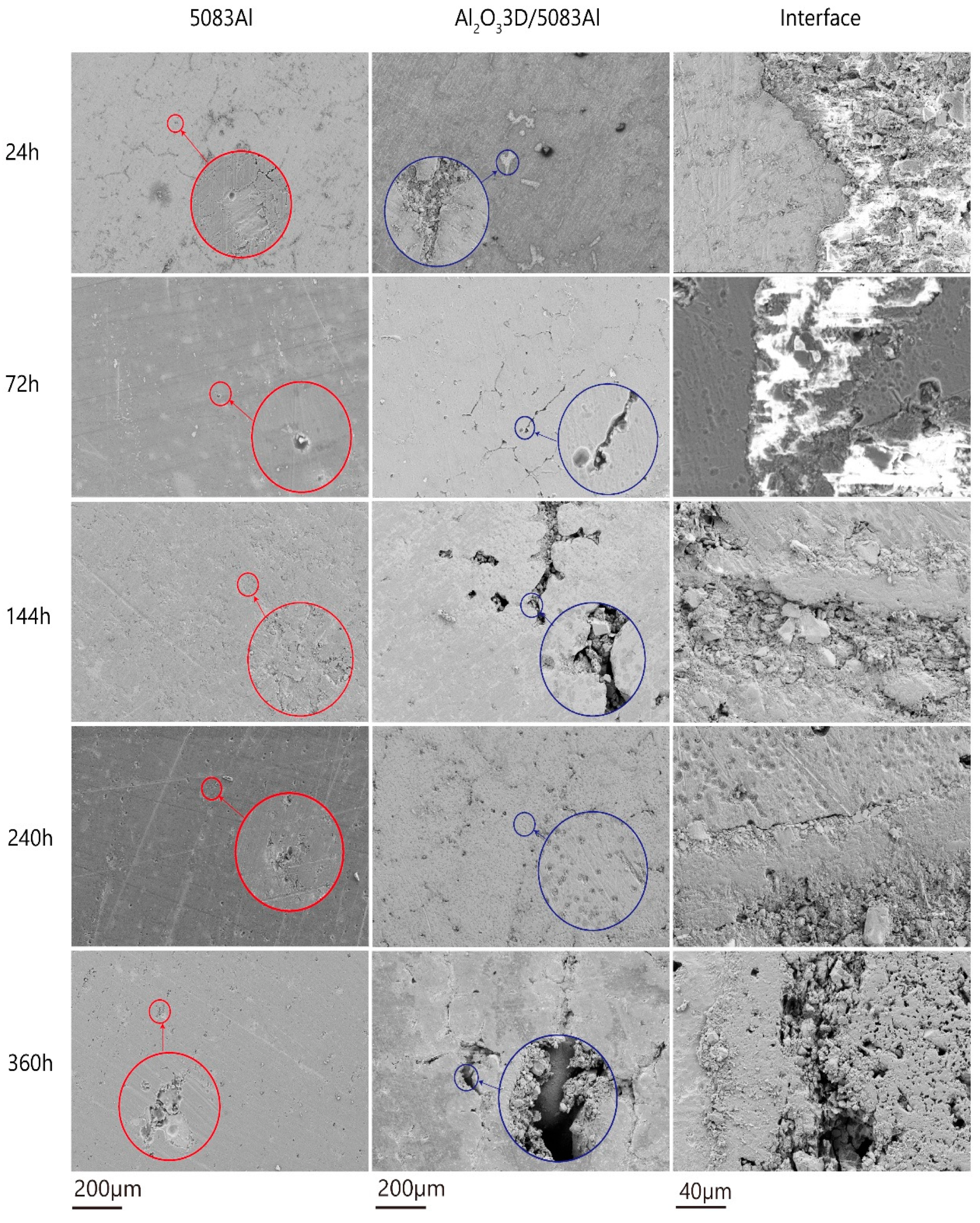
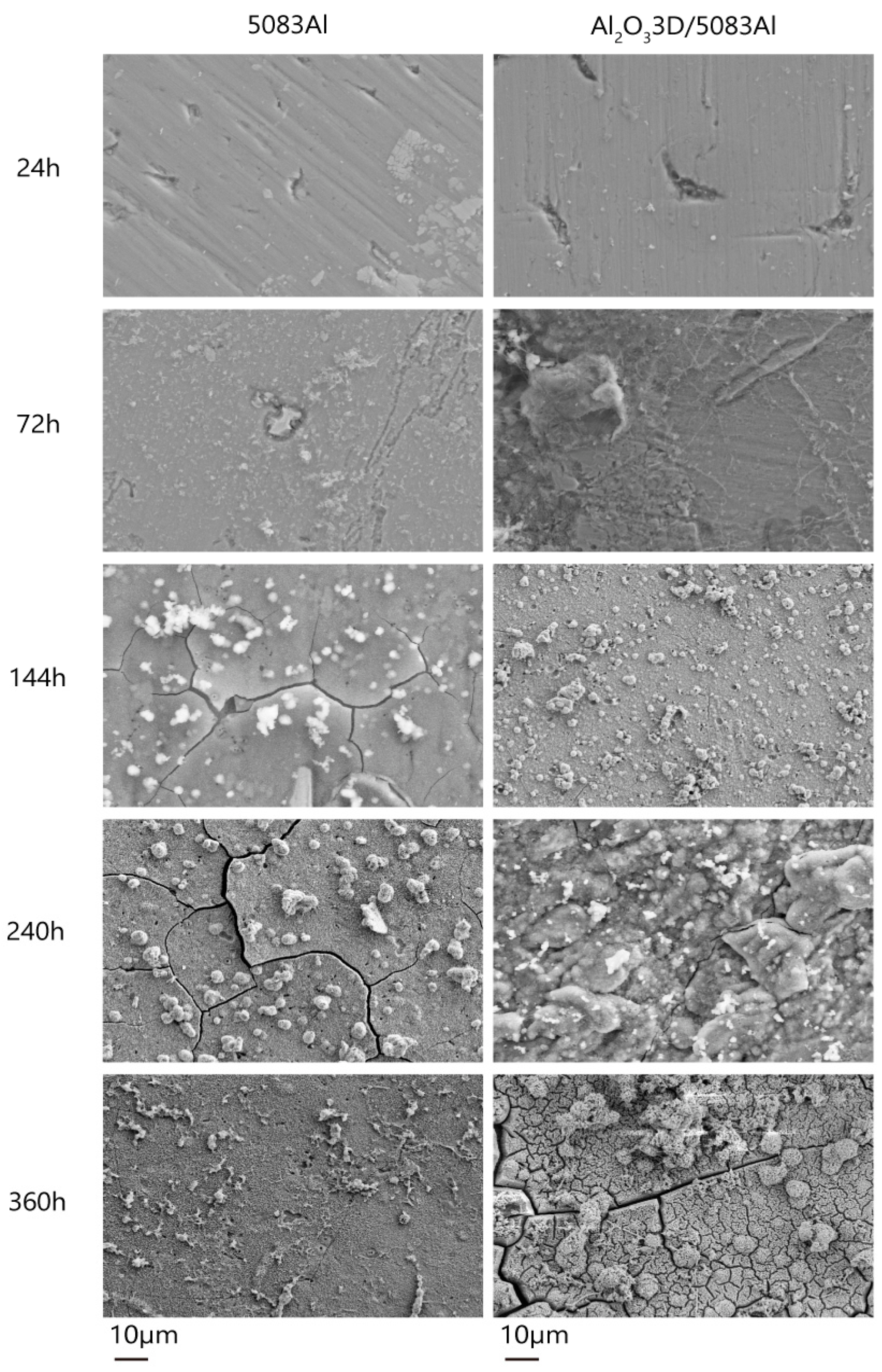
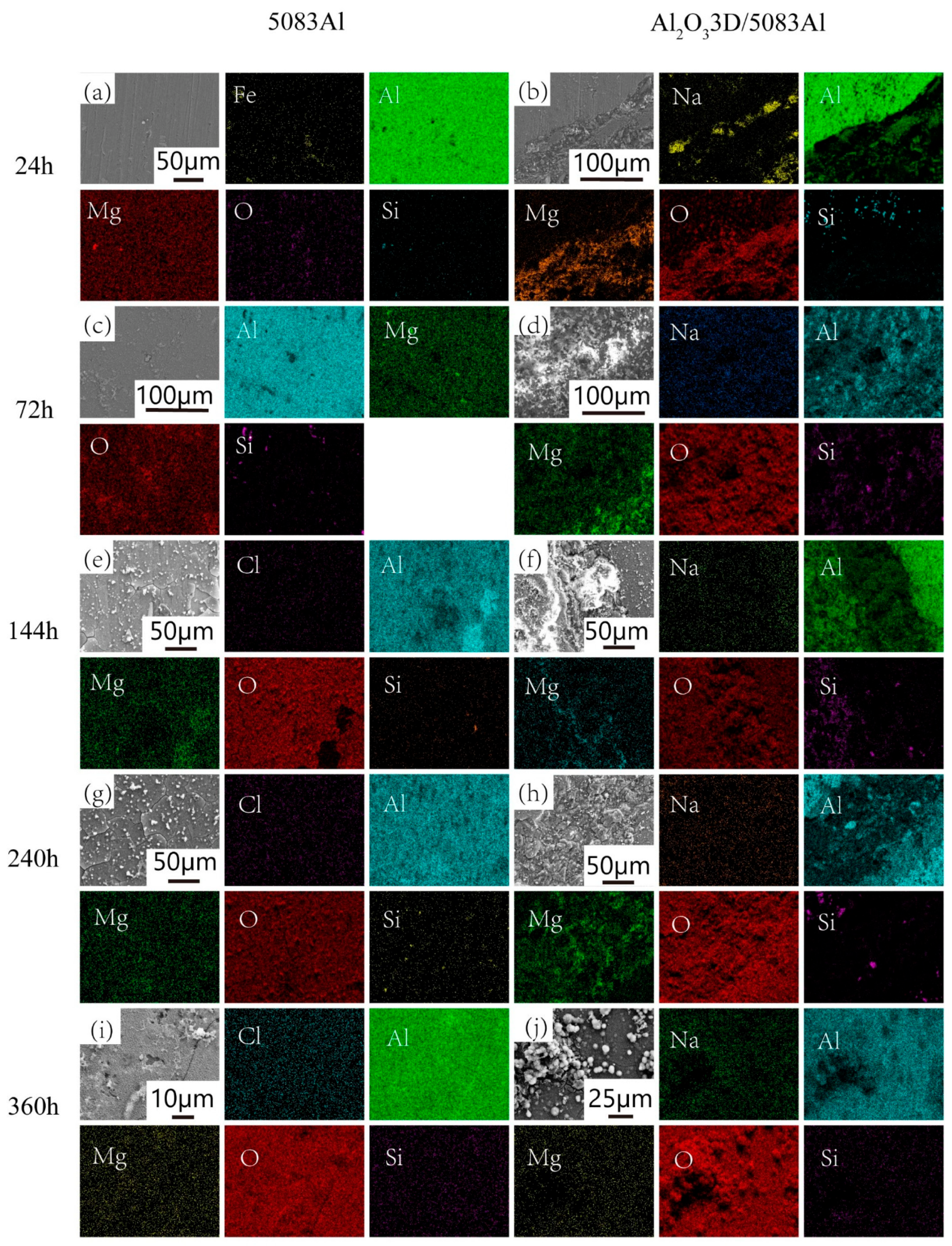
3.4. Corrosion Morphology Analysis
3.5. EIS of Corrosion Product
3.6. Tafel of Corrosion Product
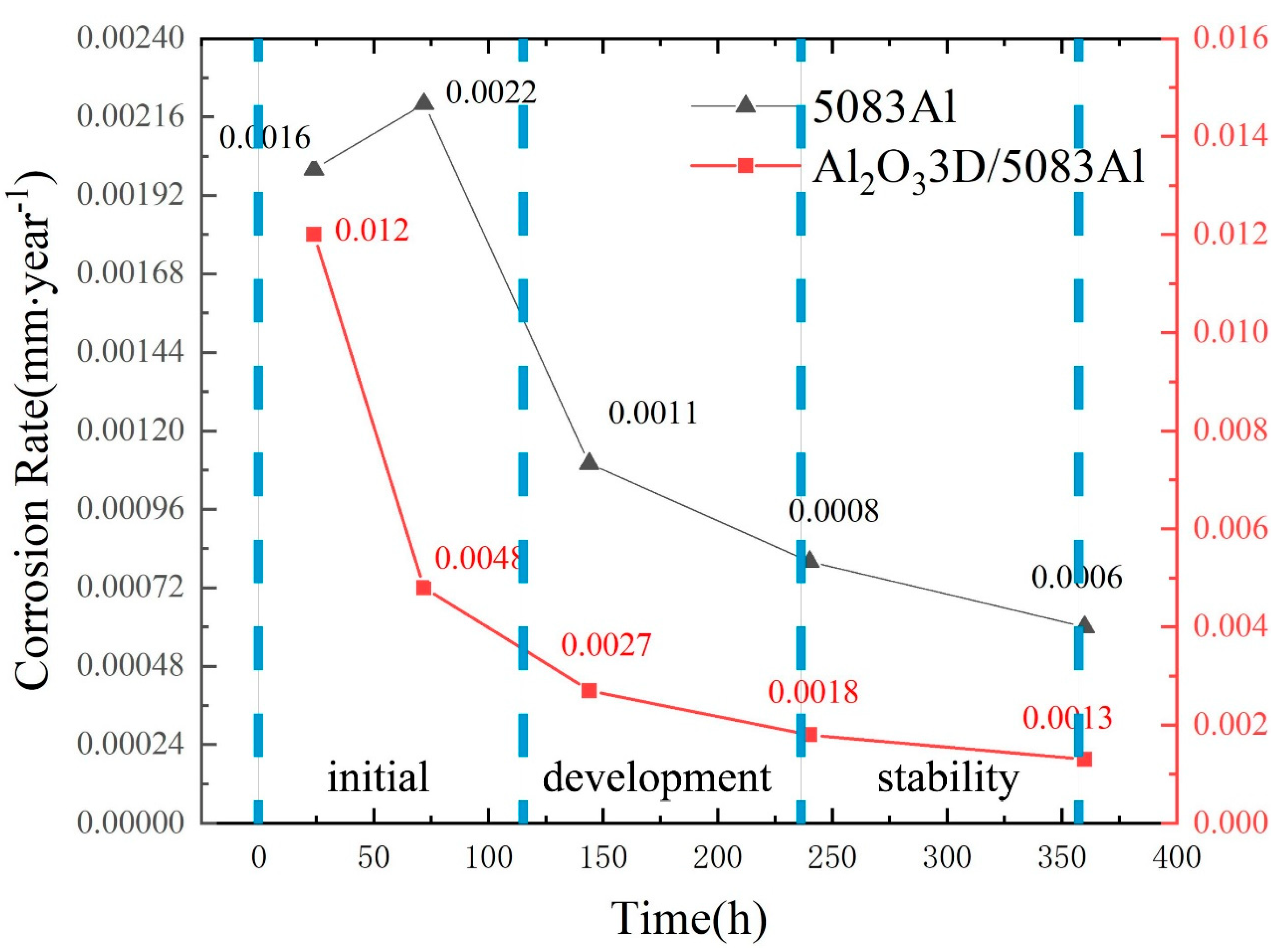
3.7. Analysis of Corrosion Rate (CR)
4. Discussion
5. Conclusions
- The corrosion development process can be divided into the initial period, the development period, and the stability period.
- The OCP, PDP curves, and EIS tests on the sample of polished surface show that the corrosion resistance of the Al2O33D/5083 Al is better than that of 5083 Al.
- The NSS test shows that the corrosion resistance of Al2O33D/5083 Al was lower than that of the 5083 Al alloy during the initial period of corrosion and higher than that of the 5083 Al alloy during the corrosion development period.
- Al2O33D used as a reinforcement in the Al2O33D/5083 Al composite improves the corrosion resistance of the Al2O33D/5083 Al composite. The interpenetrating structures of Al2O33D and the 5083 Al matrix, combined with the strong interface, are not easy to corrode. Al2O33D and the 5083 Al matrix are combined tightly to promote excellent corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kota, N.; Charan, M.S.; Laha, T.; Roy, S. Review on development of metal/ceramic interpenetrating phase composites and critical analysis of their properties. Ceram. Int. 2022, 48, 1451–1483. [Google Scholar] [CrossRef]
- San Marchi, C.; Kouzeli, M.; Rao, R.; Lewis, J.A.; Dunand, D.C. Alumina–aluminum interpenetrating-phase composites with three-dimensional periodic architecture. Scr. Mater. 2003, 49, 861–866. [Google Scholar] [CrossRef]
- Xie, F.; Lu, Z.; Yuan, Z. Numerical analysis of elastic and elastoplastic behavior of interpenetrating phase composites. Comput. Mater. Sci. 2015, 97, 94–101. [Google Scholar] [CrossRef]
- Roy, S.; Gibmeier, J.; Kostov, V.; Weidenmann, K.A.; Nagel, A.; Wanner, A. Internal load transfer in a metal matrix composite with a three-dimensional interpenetrating structure. Acta Mater. 2011, 59, 1424–1435. [Google Scholar] [CrossRef]
- Dolata, A.J. Tribological Properties of AlSi12-Al2O3 Interpenetrating Composite Layers in Comparison with Unreinforced Matrix Alloy. Materials 2017, 10, 1045. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.H.D. Synthesis of SiC_Al Co-Continuous Composite by Spontaneous Melt Infiitration. J. Mater. Sci. Technol. 2000, 16, 466–470. [Google Scholar] [CrossRef]
- Sahin, I.; Akdogan Eker, A. Analysis of Microstructures and Mechanical Properties of Particle Reinforced AlSi7Mg2 Matrix Composite Materials. J. Mater. Eng. Perform. 2010, 20, 1090–1096. [Google Scholar] [CrossRef]
- Jhaver, R.; Tippur, H. Processing, compression response and finite element modeling of syntactic foam based interpenetrating phase composite (IPC). Mater. Sci. Eng. A 2009, 499, 507–517. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, Y.-l.; Yu, L.; Su, N.; Ding, Y.-d. Thermal analysis for brake disks of SiC/6061 Al alloy co-continuous composite for CRH3 during emergency braking considering airflow cooling. Trans. Nonferrous Met. Soc. China 2012, 22, 2783–2791. [Google Scholar] [CrossRef]
- Potoczek, M.Ś.R. Microstructure and Physical Properties of AlMg/Al2O3 Interpenetrating Composites Fabricated by Metal Infiltration into Ceramic Foams. Arch. Metall. Mater. 2011, 56, 1265–1269. [Google Scholar] [CrossRef]
- Ji, Y.-y.; Xu, Y.-z.; Zhang, B.-b.; Behnamian, Y.; Xia, D.-h.; Hu, W.-b. Review of micro-scale and atomic-scale corrosion mechanisms of second phases in aluminum alloys. Trans. Nonferrous Met. Soc. China 2021, 31, 3205–3227. [Google Scholar] [CrossRef]
- Tao, J.; Xiang, L.; Zhang, Y.; Zhao, Z.; Su, Y.; Chen, Q.; Sun, J.; Huang, B.; Peng, F. Corrosion Behavior and Mechanical Performance of 7085 Aluminum Alloy in a Humid and Hot Marine Atmosphere. Materials 2022, 15, 7503. [Google Scholar] [CrossRef]
- Liew, Y.; Örnek, C.; Pan, J.; Thierry, D.; Wijesinghe, S.; Blackwood, D.J. In-Situ Time-Lapse SKPFM Investigation of Sensitized AA5083 Aluminum Alloy to Understand Localized Corrosion. J. Electrochem. Soc. 2020, 167, 141502. [Google Scholar] [CrossRef]
- Huang, Q.; He, R.; Wang, C.; Tang, X. Microstructure, Corrosion and Mechanical Properties of TiC Particles/Al-5Mg Composite Fillers for Tungsten Arc Welding of 5083 Aluminum Alloy. Materials 2019, 12, 3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatimah, S.; Nashrah, N.; Tekin, K.; Ko, Y.G. Improving Corrosion and Photocatalytic Properties of Composite Oxide Layer Fabricated by Plasma Electrolytic Oxidation with NaAlO2. Materials 2022, 15, 7055. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Tao, Y.; Han, X.; Cui, C. Enhanced corrosion resistance of 5083 aluminum alloy by refining with nano-CeB6/Al inoculant. Appl. Surf. Sci. 2019, 484, 403–408. [Google Scholar] [CrossRef]
- Roseline, S.; Paramasivam, V. Corrosion behaviour of heat treated Aluminium Metal Matrix composites reinforced with Fused Zirconia Alumina 40. J. Alloys Compd. 2019, 799, 205–215. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, Y.-l.; Yu, L.; Yang, H.-l.; Li, Z.-s.; Ding, Y.-d.; Fu, G.-f. Fabrication, microstructure, friction and wear properties of SiC3D/Al brake disc−graphite/SiC pad tribo-couple for high-speed train. Trans. Nonferrous Met. Soc. China 2019, 29, 1889–1902. [Google Scholar] [CrossRef]
- Yu, L.; Hao, S.; Nong, X.; Cao, X.; Zhang, C.; Liu, Y.; Yan, Y.; Jiang, Y. Comparative Study on the Corrosion Resistance of 6061Al and SiC3D/6061Al Composite in a Chloride Environment. Materials 2021, 14, 7730. [Google Scholar] [CrossRef]
- Liu, X.; Kong, D. Salt spray corrosion and electrochemical corrosion performances of Dacromet fabricated Zn–Al coating. Anti-Corros. Methods Mater. 2019, 66, 565–572. [Google Scholar] [CrossRef]
- Mosleh-Shirazi, S.; Hua, G.; Akhlaghi, F.; Yan, X.; Li, D. Interfacial valence electron localization and the corrosion resistance of Al-SiC nanocomposite. Sci. Rep. 2015, 5, 18154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Dang, J. A Summary of Corrosion Properties of Al-Rich Solid Solution and Secondary Phase Particles in Al Alloys. Metals 2017, 7, 84. [Google Scholar] [CrossRef]
- Sun, Y.; Li, C.; Yu, L.; Gao, Z.; Xia, X.; Liu, Y. Corrosion behavior of Al-15%Mg2Si alloy with 1% Ni addition. Results Phys. 2020, 17, 103129. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Gao, Z.; Liu, Y.; Liu, X.; Guo, Q.; Yu, L.; Li, H. Corrosion behavior of Al–Mg2Si alloys with/without addition of Al–P master alloy. Mater. Charact. 2015, 110, 170–174. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, X.-S. Influence of α-Al2O3 (0001) surface reconstruction on wettability of Al/Al2O3 interface: A first-principle study. Comput. Mater. Sci. 2014, 85, 193–199. [Google Scholar] [CrossRef]
- Cabrini, M.; Calignano, F.; Fino, P.; Lorenzi, S.; Lorusso, M.; Manfredi, D.; Testa, C.; Pastore, T. Corrosion Behavior of Heat-Treated AlSi10Mg Manufactured by Laser Powder Bed Fusion. Materials 2018, 11, 1051. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Kim, S.-K.; Park, J.-C. The corrosion and mechanical properties of Al alloy 5083-H116 in metal inert gas welding based on slow strain rate test. Surf. Coat. Technol. 2010, 205, S73–S78. [Google Scholar] [CrossRef]
- Gopinath K., B.R.; Murthy, V.S.R. Corrosion behavior of cast Al-Al2O3 particulate composites. J. Mater. Sci. Lett. 2001, 20, 793–794. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, Y.; Yu, L.; Yang, H.; Li, Z.; Ding, Y. Thermo-Mechanical Coupling Analyses for Al Alloy Brake Discs with Al(2)O(3)-SiC((3D))/Al Alloy Composite Wear-Resisting Surface Layer for High-Speed Trains. Materials 2019, 12, 3155. [Google Scholar] [CrossRef]

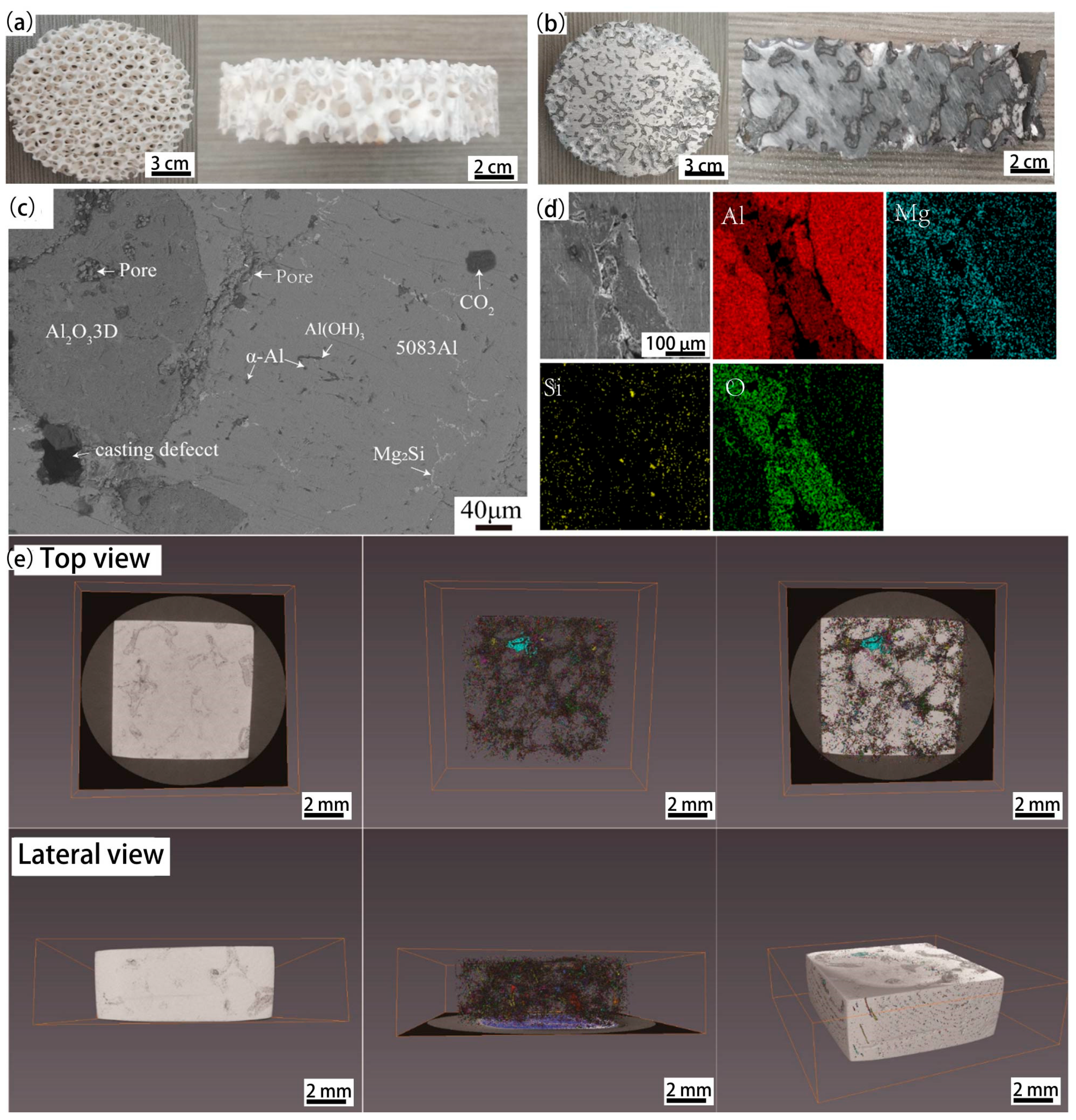
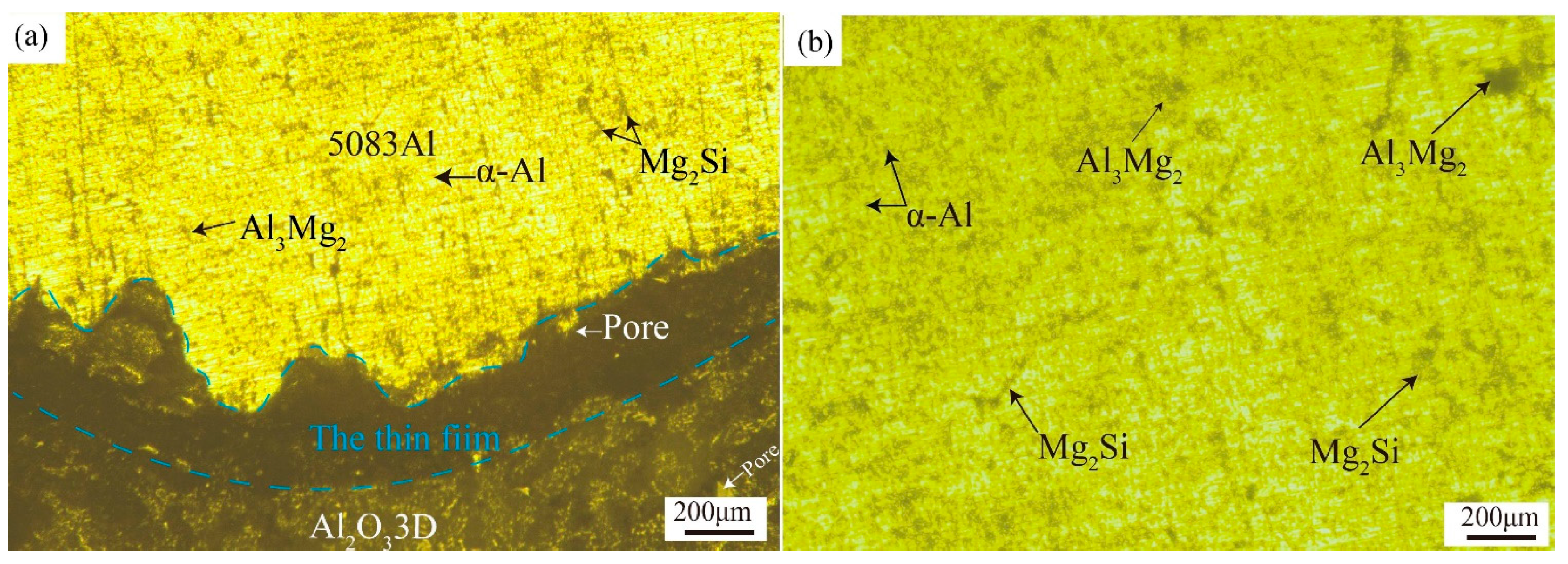
| Elements | Si | Cu | Mg | Zn | Mn | Ti | Cr | Fe | Al |
|---|---|---|---|---|---|---|---|---|---|
| Wt.% | 0.14 | 0.03 | 3.90 | 0.02 | 0.60 | 0.15 | 0.07 | 0.42 | Balance |
| Sample | Open Circuit Potential OPC (mV) | Ecorr (mV) | Icorr (µA·cm2) |
|---|---|---|---|
| Al2O33D/5083Al | −791.6 | −1069 | 6.410 |
| 5083Al | −773.9 | −849 | 9.879 |
| Tafel of 5083 Al after NSS at Different Times | |||||
|---|---|---|---|---|---|
| Time/h | 24 | 72 | 144 | 240 | 360 |
| Ecorr/mV | −827 | −720 | −803 | −860 | −803 |
| Icorr/(µA·cm2) | 2.115 | 1.024 | 1.086 | 2.114 | 2.580 |
| Tafel of Al2O33D/5083 Al after NSS at Different Times | |||||
|---|---|---|---|---|---|
| Time/h | 24 | 72 | 144 | 240 | 360 |
| Ecorr/mV | −867 | −908 | −866 | −816 | −842 |
| Icorr/(µA·cm2) | 2.808 | 7.048 | 5.343 | 1.094 | 2.823 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Zhang, C.; Liu, Y.; Yan, Y.; Xu, P.; Jiang, Y.; Cao, X. Comparing the Corrosion Resistance of 5083 Al and Al2O33D/5083 Al Composite in a Chloride Environment. Materials 2023, 16, 86. https://doi.org/10.3390/ma16010086
Yu L, Zhang C, Liu Y, Yan Y, Xu P, Jiang Y, Cao X. Comparing the Corrosion Resistance of 5083 Al and Al2O33D/5083 Al Composite in a Chloride Environment. Materials. 2023; 16(1):86. https://doi.org/10.3390/ma16010086
Chicago/Turabian StyleYu, Liang, Chen Zhang, Yuan Liu, Yulong Yan, Pianpian Xu, Yanli Jiang, and Xiuling Cao. 2023. "Comparing the Corrosion Resistance of 5083 Al and Al2O33D/5083 Al Composite in a Chloride Environment" Materials 16, no. 1: 86. https://doi.org/10.3390/ma16010086
APA StyleYu, L., Zhang, C., Liu, Y., Yan, Y., Xu, P., Jiang, Y., & Cao, X. (2023). Comparing the Corrosion Resistance of 5083 Al and Al2O33D/5083 Al Composite in a Chloride Environment. Materials, 16(1), 86. https://doi.org/10.3390/ma16010086








