Combined DFT-D3 Computational and Experimental Studies on g-C3N4: New Insight into Structure, Optical, and Vibrational Properties
Abstract
1. Introduction
2. Computational Set Up
3. Materials and Methods
4. Results and Discussion
4.1. Crystal Structure by XRD Analysis and Modelling
4.1.1. Experimental XRD Analysis
4.1.2. Modelling the Crystal Structure
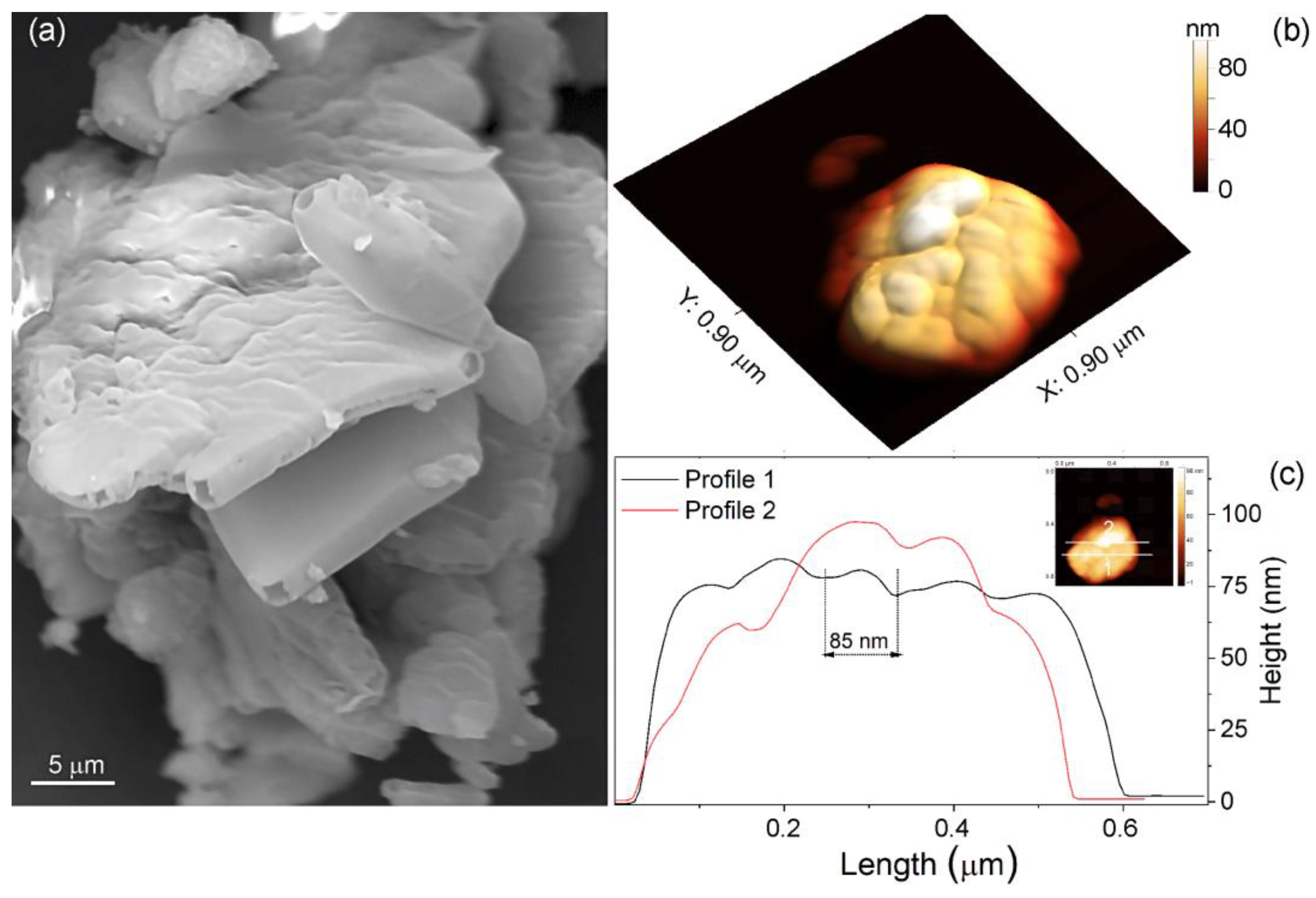
4.2. Morphology by SEM and AFM Microscopies
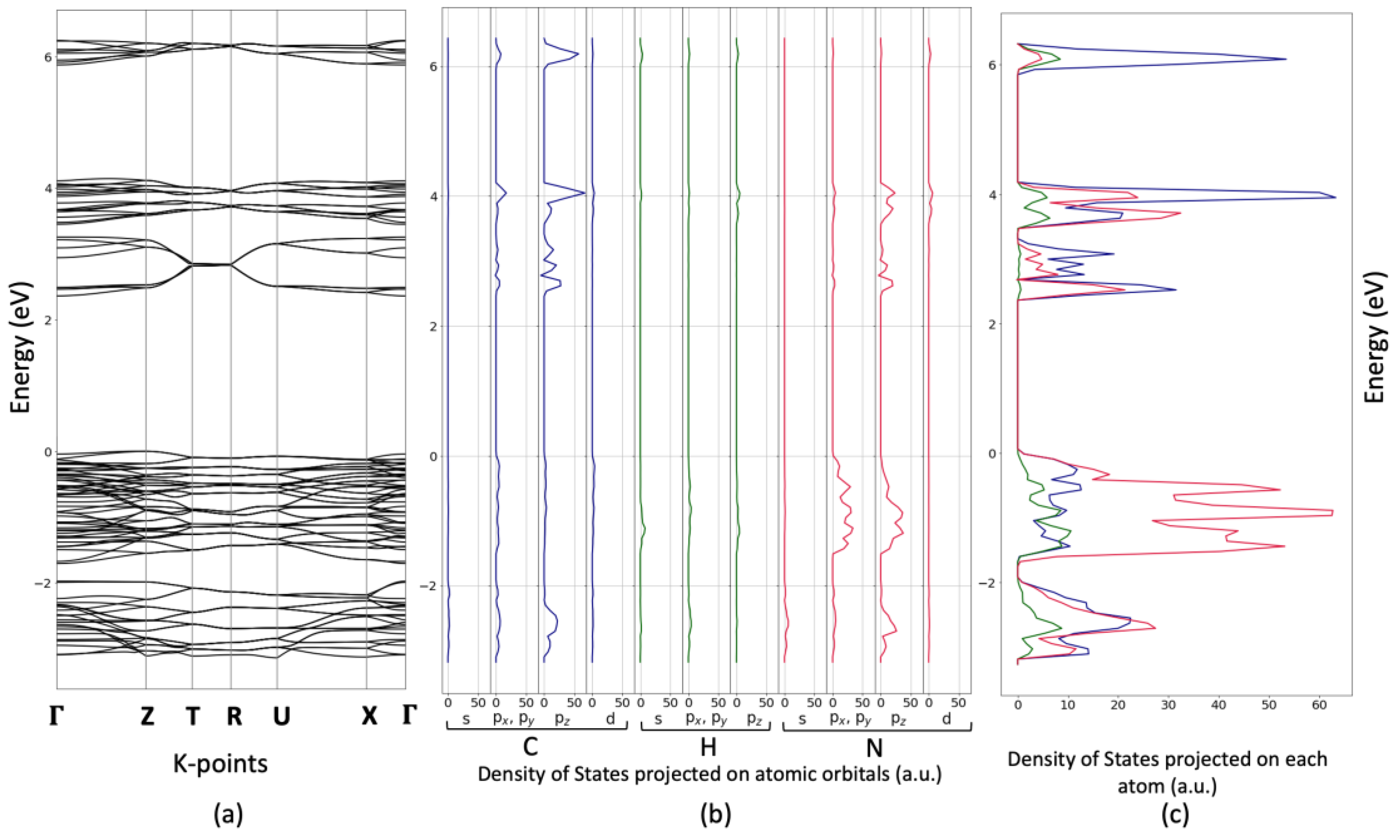
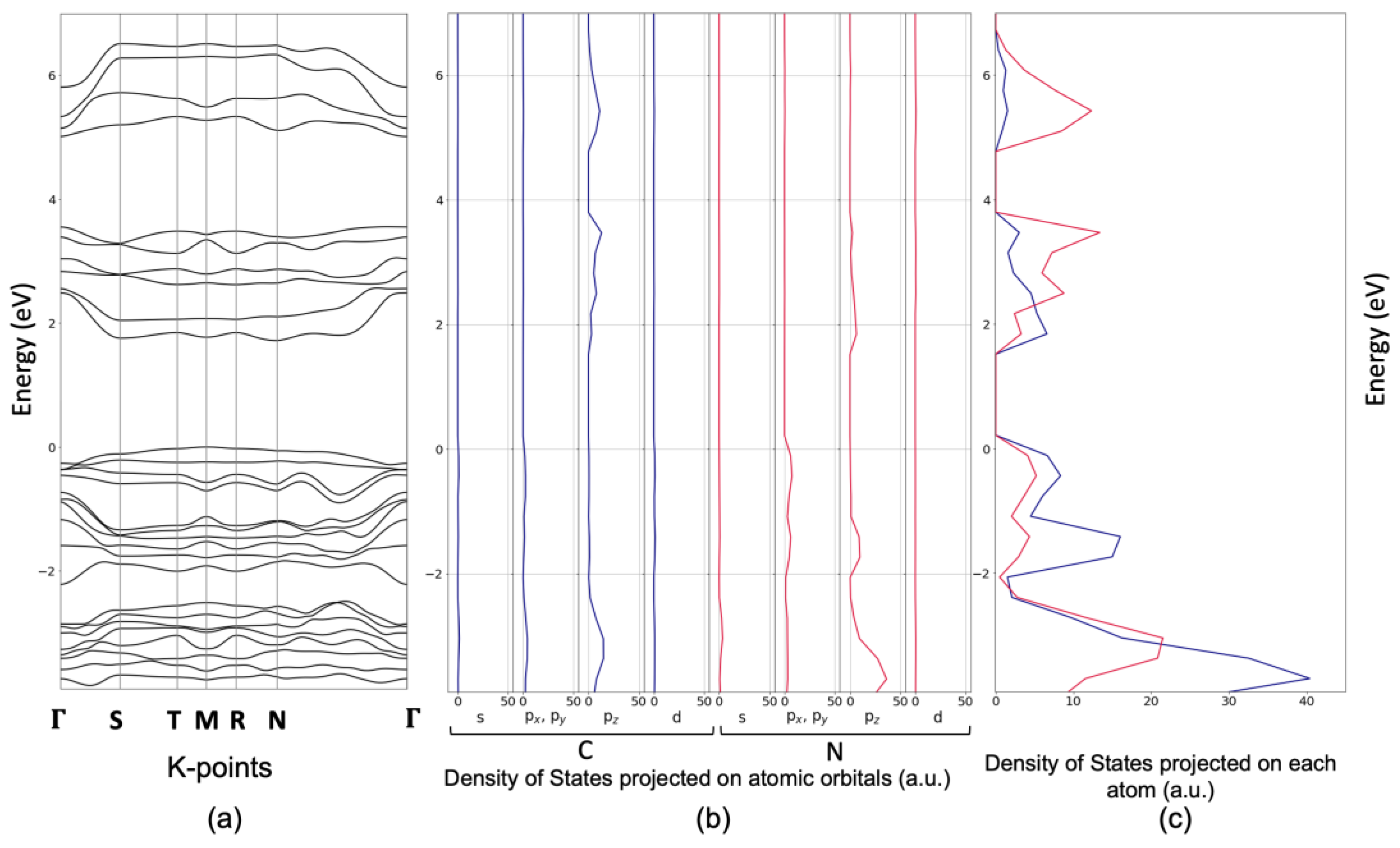
4.3. Optical and Electronic Properties Determined via UV-Vis Spectroscopy and DFT Calculations
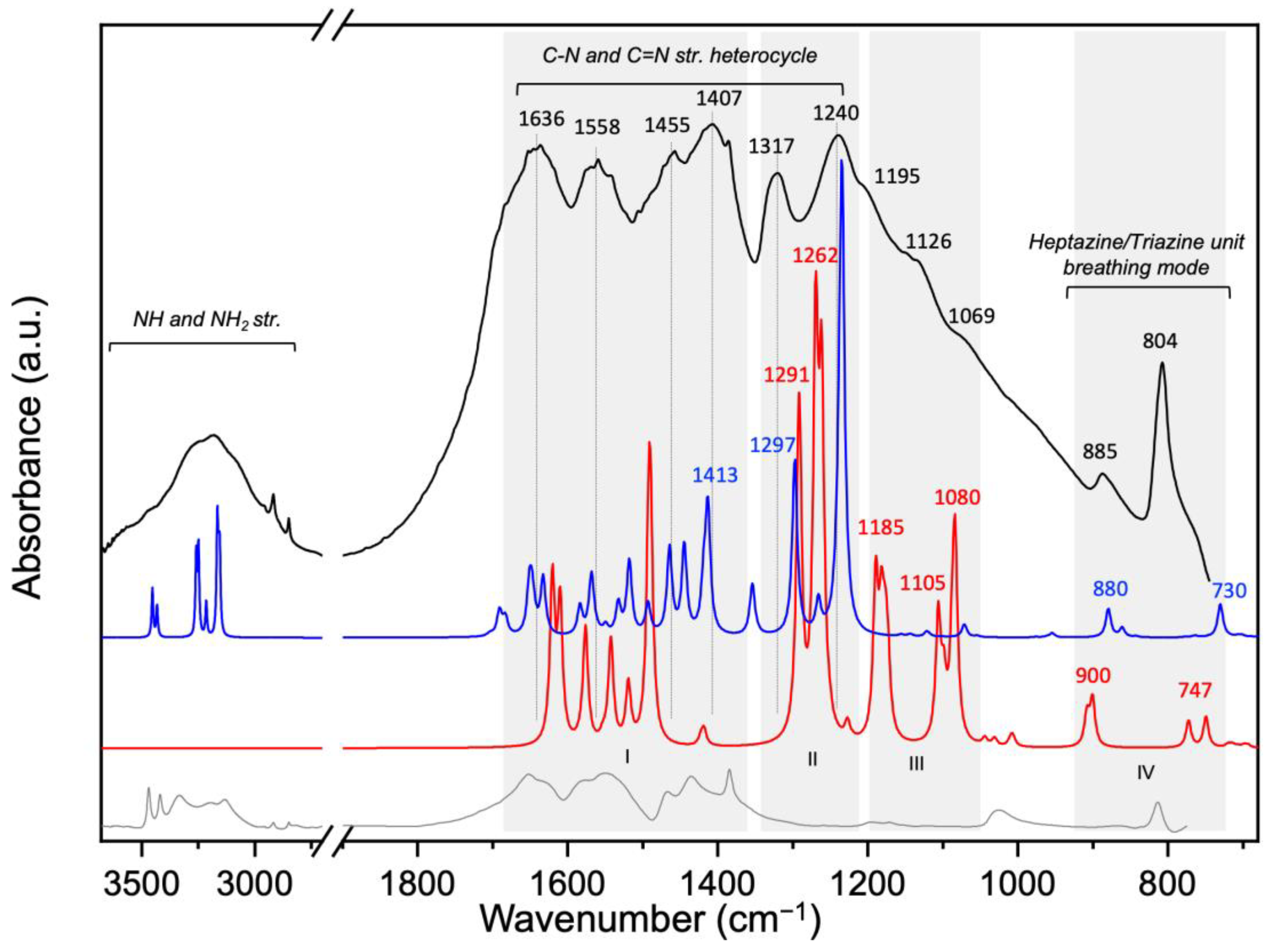
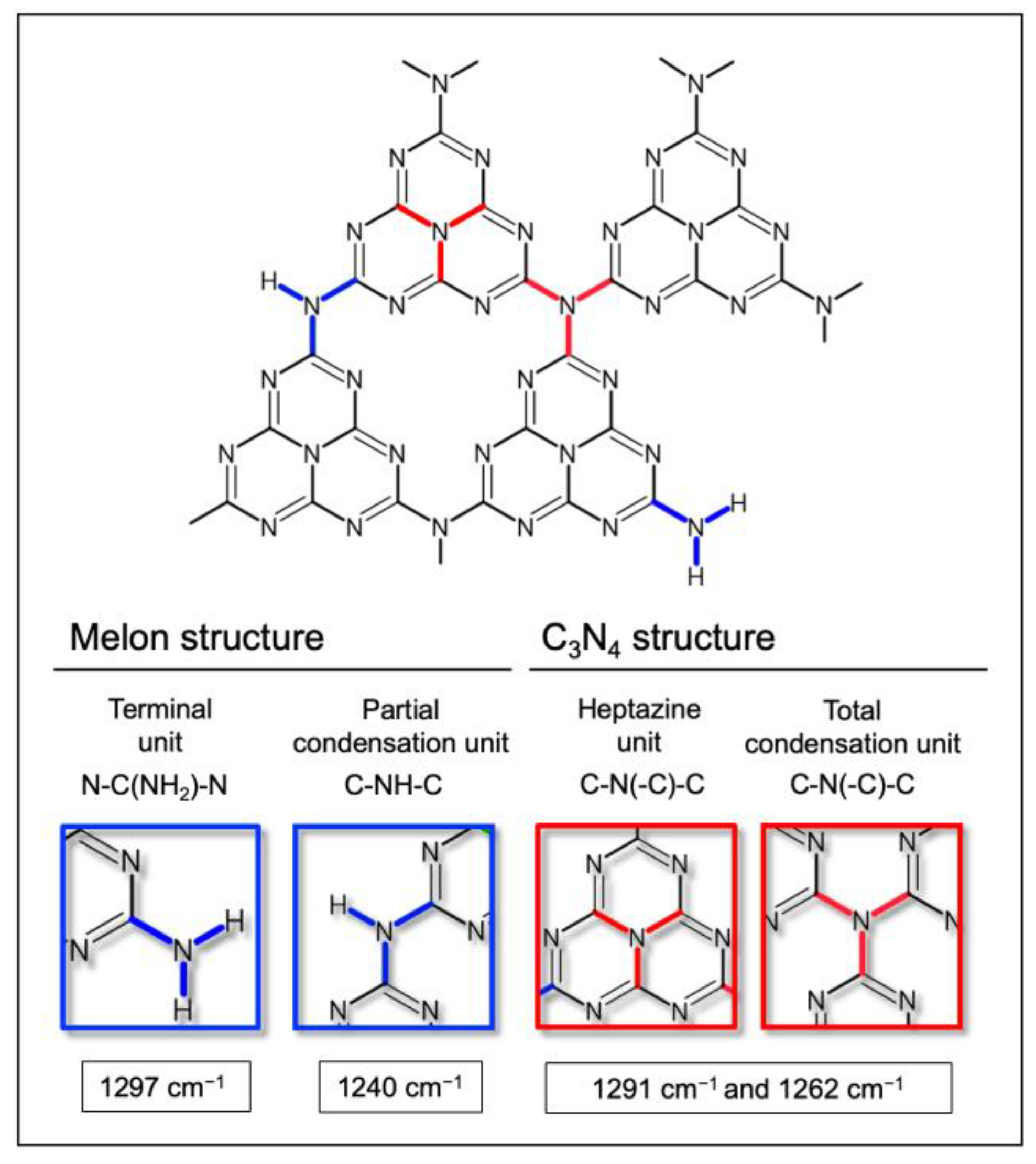
4.4. Surface Vibrational Properties Determined via FTIR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Wang, J.; Hao, D.; Ye, J.; Umezawa, N. Determination of Crystal Structure of Graphitic Carbon Nitride: Ab Initio Evolutionary Search and Experimental Validation. Chem. Mater. 2017, 29, 2694–2707. [Google Scholar] [CrossRef]
- Liu, N.; Li, T.; Zhao, Z.; Liu, J.; Luo, X.; Yuan, X.; Luo, K.; Luo, K.; He, J.; Yu, D.; et al. From Triazine to Heptazine: Origin of Graphitic Carbon Nitride as a Photocatalyst. ACS Omega 2020, 5, 12557–12567. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, B.; Luo, K.; Shi, K.; Zhang, L.; Li, Y.; Yu, T.; Hu, W.; Xie, C.; et al. Restacked melon as highly-efficient photocatalyst. Nano Energy 2020, 77, 105124. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Döblinger, M.; Sehnert, J.; Seyfarth, L.; Senker, J.; Oeckler, O.; Schnick, W. Unmasking melon by a complementary approach employing electron diffraction, solid-state NMR spectroscopy, and theoretical calculations—Structural characterization of a carbon nitride polymer. Chem.-A Eur. J. 2007, 13, 4969–4980. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural investigation of graphitic carbon nitride via XRD and neutron diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloy. Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Safaei, J.; Mohamed, N.A.; Mohamad Noh, M.F.; Soh, M.F.; Ludin, N.A.; Ibrahim, M.A.; Roslam Wan Isahak, W.N.; Mat Teridi, M.A. Graphitic carbon nitride (g-C3N4) electrodes for energy conversion and storage: A review on photoelectrochemical water splitting, solar cells and supercapacitors. J. Mater. Chem. A 2018, 6, 22346–22380. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent advances of graphitic carbon nitride-based structures and applications in catalyst, sensing, imaging, and leds. Nano-Micro Lett. 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Rhimi, B.; Wang, C.; Bahnemann, D.W. Latest progress in g-C3N4 based heterojunctions for hydrogen production via photocatalytic water splitting: A mini review. J. Phys. Energy 2020, 2, 042003. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Ali Shah Syed, J.; Al-Sehemi, A.G.; Mohammed, M.H.; Al-Ghamdi, A.A.; Taha, T.A.; Salem AlSalem, H.; Alenad, A.M.; Amin, M.A.; et al. Recent Advancement of the Current Aspects of g-C3N4 for its Photocatalytic Applications in Sustainable Energy System. Chem. Rec. 2022, 22, 202100310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Liu, G.; Wang, T.; Zhang, H.; Meng, X.; Hao, D.; Chang, K.; Li, P.; Kako, T.; Ye, J. Nature-Inspired Environmental “Phosphorylation” Boosts Photocatalytic H2 Production over Carbon Nitride Nanosheets under Visible-Light Irradiation. Angew. Chem. (Int. Ed. Engl.) 2015, 54, 13561–13565. [Google Scholar] [CrossRef]
- Bhunia, M.K.; Yamauchi, K.; Takanabe, K. Harvesting solar light with crystalline carbon nitrides for efficient photocatalytic hydrogen evolution. Angew. Chem. (Int. Ed. Engl.) 2014, 53, 11001–11005. [Google Scholar] [CrossRef]
- Zhang, Y.; Schnepp, Z.; Cao, J.; Ouyang, S.; Li, Y.; Ye, J.; Liu, S. Biopolymer-Activated Graphitic Carbon Nitride towards a Sustainable Photocathode Material. Sci. Rep. 2013, 3, 2163. [Google Scholar] [CrossRef]
- Merschjann, C.; Tyborski, T.; Orthmann, S.; Yang, F.; Schwarzburg, K.; Lublow, M.; Lux-Steiner, M.C.; Schedel-Niedrig, T. Photophysics of polymeric carbon nitride: An optical quasimonomer. Phys. Rev. B 2013, 87, 205204. [Google Scholar] [CrossRef]
- Zhang, Y.; Mori, T.; Niu, L.; Ye, J. Non-covalent doping of graphitic carbon nitride polymer with graphene: Controlled electronic structure and enhanced optoelectronic conversion. Energy Environ. Sci. 2011, 4, 4517–4521. [Google Scholar] [CrossRef]
- Akhundi, A.; Zaker Moshfegh, A.; Habibi-Yangjeh, A.; Sillanpää, M. Simultaneous Dual-Functional Photocatalysis by g-C3N4-Based Nanostructures. ACS EST Eng. 2022, 2, 564–585. [Google Scholar] [CrossRef]
- Im, C.; Kirchhoff, B.; Krivtsov, I.; Mitoraj, D.; Beranek, R.; Jacob, T. Structure and Optical Properties of Polymeric Carbon Nitrides from Atomistic Simulations. Chem. Mater. 2023, 35, 1547–1559. [Google Scholar] [CrossRef]
- Suyana, P.; Ganguly, P.; Nair, B.N.; Pillai, S.C.; Hareesh, U.S. Structural and compositional tuning in g-C3N4 based systems for photocatalytic antibiotic degradation. Chem. Eng. J. Adv. 2021, 8, 100148. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C 3 N 4 -based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Wang, B.; Wang, X. Graphitic Carbon Nitride Polymers toward Sustainable Photoredox Catalysis. Angew. Chem.-Int. Ed. 2015, 54, 12868–12884. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, X.; Yu, Z.; Yao, L.; Guo, S.; Zhao, W. Environmentally Friendly Non-Metal Solar Photocatalyst C3N4 for Efficient Nitrogen Fixation as Foliar Fertilizer. ChemistrySelect 2020, 5, 7720–7727. [Google Scholar] [CrossRef]
- Dandia, A.; Gupta, S.L.; Saini, P.; Sharma, R.; Meena, S.; Parewa, V. Structure couture and appraisal of catalytic activity of carbon nitride (g-C3N4) based materials towards sustainability. Curr. Res. Green Sustain. Chem. 2020, 3, 100039. [Google Scholar] [CrossRef]
- Hartley, G.O.; Martsinovich, N. Computational design of graphitic carbon nitride photocatalysts for water splitting. Faraday Discuss. 2021, 227, 341–358. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, J.; Lin, B.; Ding, D.; Xu, H.; Wang, P.; Chen, Y. Understanding the Surface of g-C3N4, an Experimental Investigation of the Catalytic Active Site on the Interface. Catal. Lett. 2019, 149, 3296–3303. [Google Scholar] [CrossRef]
- Seyfarth, L.; Seyfarth, J.; Lotsch, B.V.; Schnick, W.; Senker, J. Tackling the stacking disorder of melon—Structure elucidation in a semicrystalline material. Phys. Chem. Chem. Phys. 2010, 12, 2227–2237. [Google Scholar] [CrossRef]
- Schwinghammer, K.; Tuffy, B.; Mesch, M.B.; Wirnhier, E.; Martineau, C.; Taulelle, F.; Schnick, W.; Senker, J.; Lotsch, B.V. Triazine-based carbon nitrides for visible-light-driven hydrogen evolution. Angew. Chem. (Int. Ed. Engl.) 2013, 52, 2435–2439. [Google Scholar] [CrossRef] [PubMed]
- Algara-Siller, G.; Severin, N.; Chong, S.Y.; Björkman, T.; Palgrave, R.G.; Laybourn, A.; Antonietti, M.; Khimyak, Y.Z.; Krasheninnikov, A.V.; Rabe, J.P.; et al. Triazine-based graphitic carbon nitride: A two-dimensional semiconductor. Angew. Chem. (Int. Ed. Engl.) 2014, 53, 7450–7455. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, L.F.; Vahdat, M.T.; Dakhchoune, M.; Nadizadeh, Z.; Mensi, M.; Oveisi, E.; Campi, D.; Marzari, N.; Agrawal, K.V. Large-scale synthesis of crystalline g-C3N4 nanosheets and high-temperature H2 sieving from assembled films. Sci. Adv. 2022, 6, eaay9851. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y.; Cohen, M.L. Prediction of New Low Compressibility Solids. Science 1989, 245, 841–842. [Google Scholar] [CrossRef]
- Teter, D.M.; Hemley, R.J. Low-Compressibility Carbon Nitrides. Science 1996, 271, 53–55. [Google Scholar] [CrossRef]
- Erba, A.; Desmarais, J.K.; Casassa, S.; Civalleri, B.; Donà, L.; Bush, I.J.; Searle, V.; Maschio, L.; Daga, L.-E.; Cossard, A.; et al. CRYSTAL23: A Program for Computational Solid State Physics and Chemistry. J. Chem. Theory Comput. 2022. [CrossRef]
- CRYSTAL—Basis Sets Library. Available online: https://www.crystal.unito.it/basis-sets.php (accessed on 24 March 2023).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Dovesi, R.; Saunders, V.; Roetti, C.; Orlando, R.; Zicovich-Wilson, C.M.; Pascale, F.; Civalleri, B.; Doll, V.; Harrison, N.M.; Bush, V.; et al. CRYSTAL23 User’s Manual. 2023. Available online: https://www.crystal.unito.it/include/manuals/crystal23.pdf (accessed on 24 March 2023).
- Shanno, D.F. Conditioning of Quasi-Newton Methods for Function Minimization. Math. Comput. 1970, 24, 647–656. [Google Scholar] [CrossRef]
- Zicovich-Wilson, C.M.; Dovesi, R. On the use of symmetry-adapted crystalline orbitals in SCF-LCAO periodic calculations. II. Implementation of the self-consistent-field scheme and examples. Int. J. Quantum Chem. 1998, 67, 311–320. [Google Scholar] [CrossRef]
- Pascale, F.; Zicovich-Wilson, C.M.; López Gejo, F.; Civalleri, B.; Orlando, R.; Dovesi, R. The calculation of the vibrational frequencies of crystalline compounds and its implementation in the CRYSTAL code. J. Comput. Chem. 2004, 25, 888–897. [Google Scholar] [CrossRef]
- Zicovich-Wilson, C.M.; Pascale, F.; Roetti, C.; Saunders, V.R.; Orlando, R.; Dovesi, R. Calculation of the vibration frequencies of alpha-quartz: The effect of Hamiltonian and basis set. J. Comput. Chem. 2004, 25, 1873–1881. [Google Scholar] [CrossRef]
- Gatti, C.; Casassa, S. TOPOND 14 User ’ s Manual. Available online: https://www.crystal.unito.it/topond/topond.pdf (accessed on 24 March 2023).
- Gatti, C. Chemical bonding in crystals: New directions. Z. Krist.-Cryst. Mater. 2005, 220, 399–457. [Google Scholar] [CrossRef]
- Casassa, S.; Erba, A.; Baima, J.; Orlando, R. Electron density analysis of large (molecular and periodic) systems: A parallel implementation. J. Comput. Chem. 2015, 36, 1940–1946. [Google Scholar] [CrossRef]
- Eugen Schwarz, W.H.; Richard, F. Bader: Atoms in Molecules (A Quantum Theory) Clarendon Press 1990, Oxford. ISBN 019-855–1681, 438 pages. Ber. Bunsenges. Phys. Chem. 1991, 95, 1308. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, L.; Xing, J.; Utama, M.I.B.; Lu, X.; Du, K.; Li, Y.; Hu, X.; Wang, S.; Genç, A.; et al. High-yield synthesis and optical properties of g-C3N4. Nanoscale 2015, 7, 12343–12350. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, W.; Wang, S.; Peng, H.; Hu, X.; Yu, Y. Monolayer g-C3N4 Fluorescent Sensor for Sensitive and Selective Colorimetric Detection of Silver ion from Aqueous Samples. J. Fluoresc. 2016, 26, 739–744. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Shen, L.; Ma, Y.; Lei, W.; Cui, Q.; Zou, G. Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine. Appl. Phys. A Mater. Sci. Process. 2009, 94, 387–392. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, E.; Hu, X.; Tang, C.; Wan, J.; Li, J.; Fan, J. A simple process to prepare few-layer g-C 3 N 4 nanosheets with enhanced photocatalytic activities. Appl. Surf. Sci. 2015, 358, 246–251. [Google Scholar] [CrossRef]
- Alizadeh, T.; Nayeri, S.; Hamidi, N. Graphitic carbon nitride (g-C3N4)/graphite nanocomposite as an extraordinarily sensitive sensor for sub-micromolar detection of oxalic acid in biological samples. RSC Adv. 2019, 9, 13096–13103. [Google Scholar] [CrossRef]
- Jmol: An Open-Source Java Viewer for Chemical Structures in 3D. Available online: https://jmol.sourceforge.net (accessed on 24 March 2023).
- Costa, L.; Camino, G. Thermal behaviour of melamine. J. Therm. Anal. 1988, 34, 423–429. [Google Scholar] [CrossRef]
- Inoki, H.; Seo, G.; Kanai, K. Synthesis of graphitic carbon nitride under low ammonia partial pressure. Appl. Surf. Sci. 2020, 534, 147569. [Google Scholar] [CrossRef]
- Martha, S.; Nashim, A.; Parida, K.M. Facile synthesis of highly active g-C3N4 for efficient hydrogen production under visible light. J. Mater. Chem. A 2013, 1, 7816–7824. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Wei, W.; Jacob, T. Strong excitonic effects in the optical properties of graphitic carbon nitride g-C3N4 from first principles. Phys. Rev. B-Condens. Matter Mater. Phys. 2013, 87, 1–7. [Google Scholar] [CrossRef]
- Wu, P.; Wang, J.; Zhao, J.; Guo, L.; Osterloh, F.E. Structure defects in g-C3N4 limit visible light driven hydrogen evolution and photovoltage. J. Mater. Chem. A 2014, 2, 20338–20344. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Wang, T.; Zhang, P.; Li, A.; Gong, J. Controllable synthesis of nanotube-type graphitic C3N 4 and their visible-light photocatalytic and fluorescent properties. J. Mater. Chem. A 2014, 2, 2885–2890. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Yu, D.L.; Zhou, H.W.; Tian, Y.J.; Yanagisawa, O. Turbostratic carbon nitride prepared by pyrolysis of melamine. J. Mater. Sci. 2005, 40, 2645–2647. [Google Scholar] [CrossRef]
- Qiu, P.; Chen, H.; Xu, C.; Zhou, N.; Jiang, F.; Wang, X.; Fu, Y. Fabrication of an exfoliated graphitic carbon nitride as a highly active visible light photocatalyst. J. Mater. Chem. A 2015, 3, 24237–24244. [Google Scholar] [CrossRef]
- Mitoraj, D.; Kisch, H. On the Mechanism of Urea-Induced Titania Modification. Chem.–A Eur. J. 2010, 16, 261–269. [Google Scholar] [CrossRef]



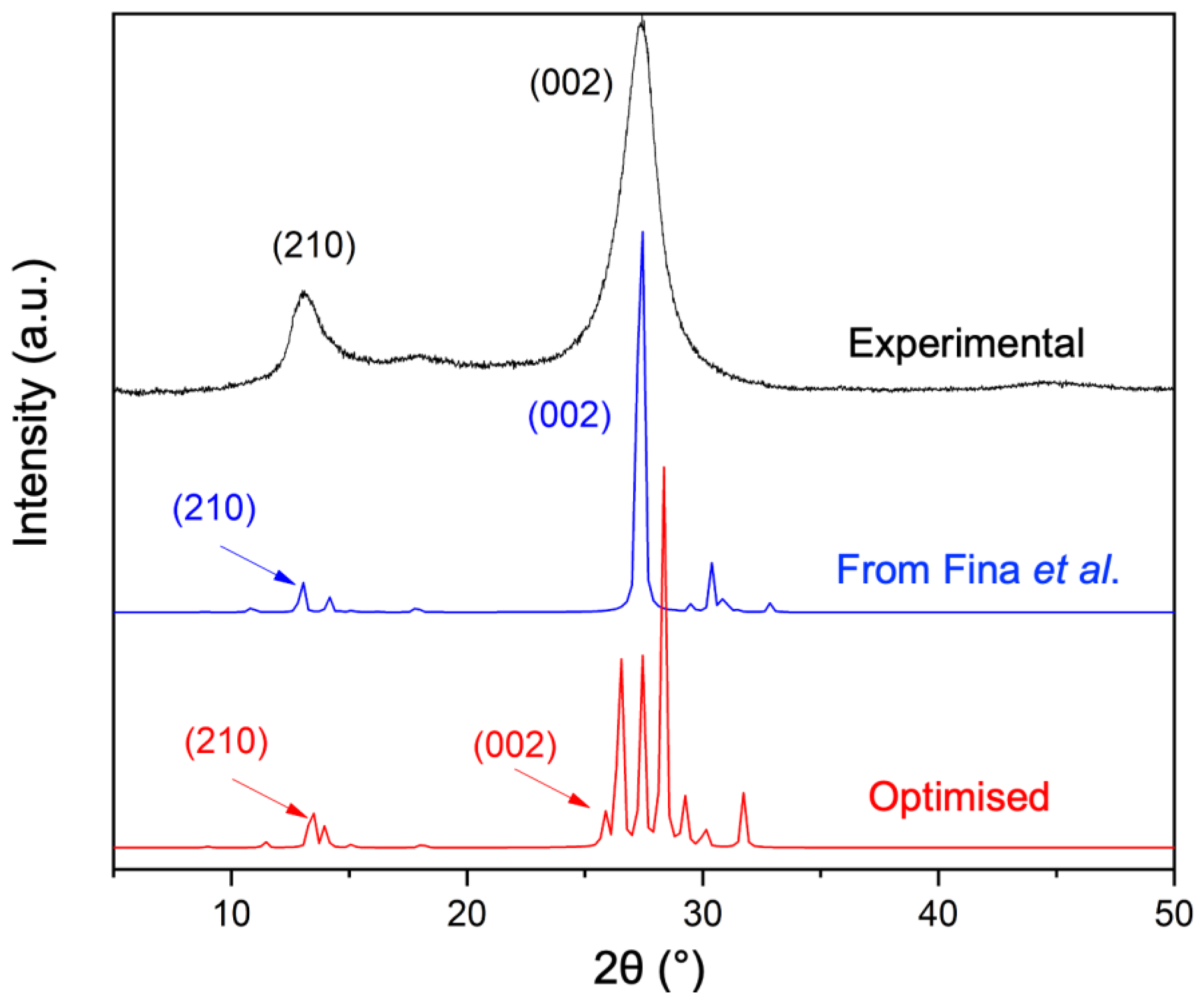
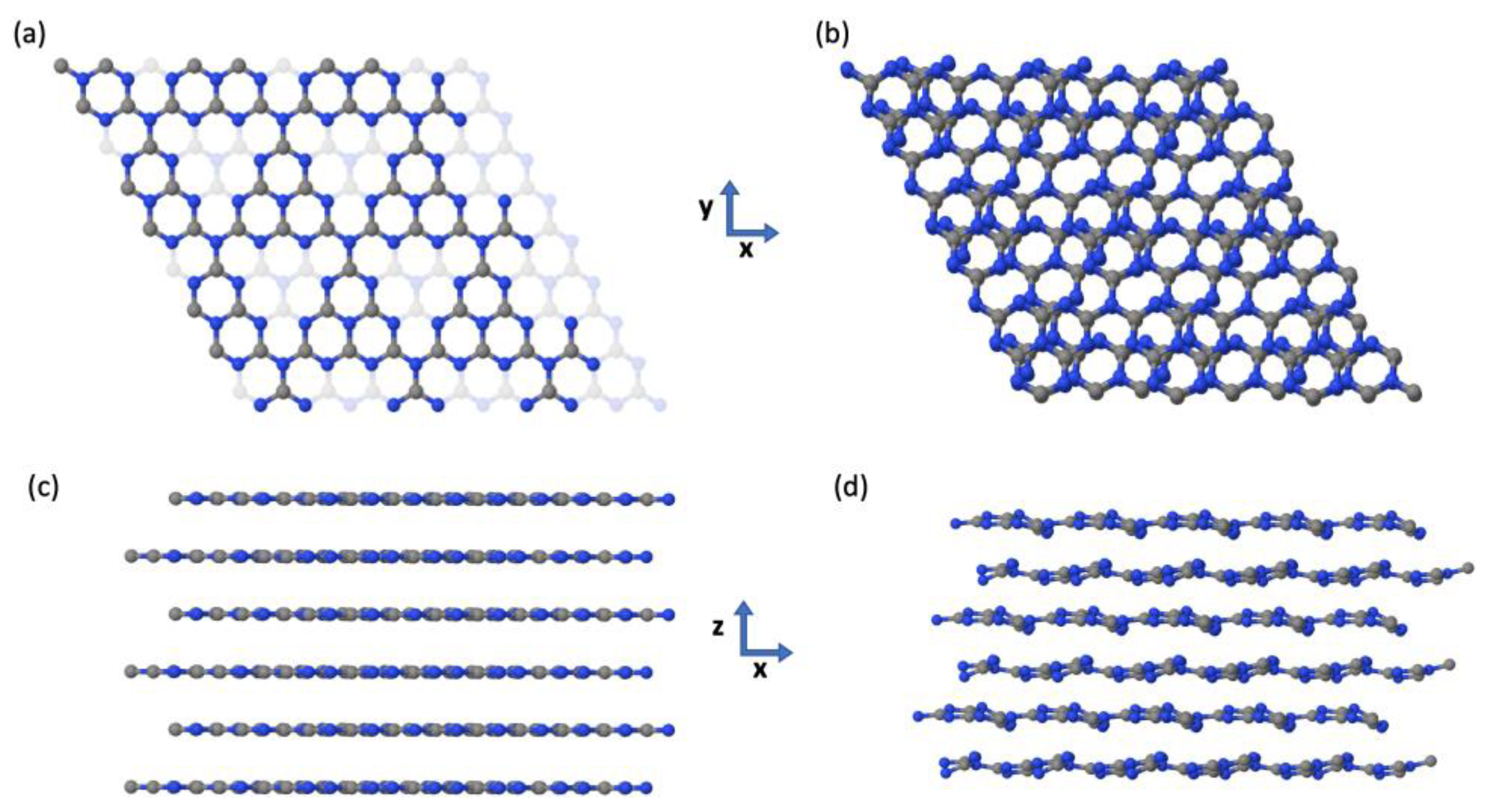
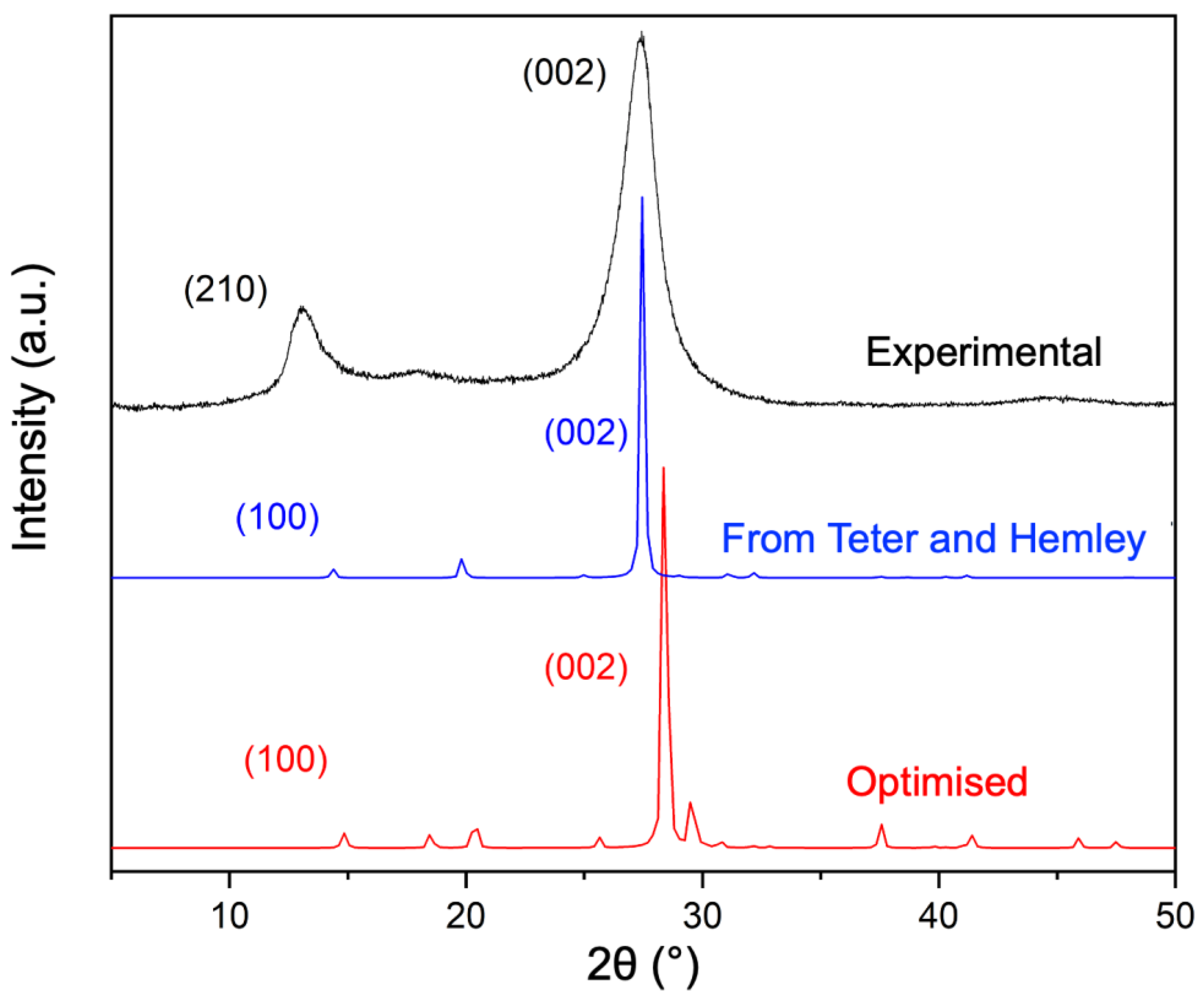

| Fina et al. | PBE-D3 | Difference [%] | |
|---|---|---|---|
| a [Å] | 16.24 | 15.18 | −6.53 |
| b [Å] | 12.52 | 12.60 | +0.64 |
| c [Å] | 6.52 | 6.55 | +0.46 |
| 𝛼 = 𝛽 = 𝛾 [°] | 90 | 90 | 0 |
| V [Å3] | 1324.90 | 1253.61 | −5.38 |
| Inter-layer distance [Å] | 3.28 | 3.26 | −0.61 |
| Bending angle N77–N86–N130 [°] | 175.0 | 150.5 | −14.0 |
| Teter and Hamley | PBE-D3 | Difference [%] | |
|---|---|---|---|
| a [Å] | 7.11 | 6.99 | −1.75 |
| b [Å] | 7.11 | 6.99 | −1.72 |
| c [Å] | 6.49 | 6.43 | −0.99 |
| 𝛼 [°] | 90.00 | 77.58 | −13.80 |
| 𝛽 [°] | 90.00 | 96.17 | +6.86 |
| 𝛾 [°] | 120.00 | 120.01 | 0.00 |
| V [Å3] | 284.37 | 265.47 | −6.64 |
| Inter-layer distance [Å] | 3.25 | 3.12 | −4.00 |
| Bending angle N6 –C21 –N3 [°] | 180.00 | 163.90 | −8.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negro, P.; Cesano, F.; Casassa, S.; Scarano, D. Combined DFT-D3 Computational and Experimental Studies on g-C3N4: New Insight into Structure, Optical, and Vibrational Properties. Materials 2023, 16, 3644. https://doi.org/10.3390/ma16103644
Negro P, Cesano F, Casassa S, Scarano D. Combined DFT-D3 Computational and Experimental Studies on g-C3N4: New Insight into Structure, Optical, and Vibrational Properties. Materials. 2023; 16(10):3644. https://doi.org/10.3390/ma16103644
Chicago/Turabian StyleNegro, Paolo, Federico Cesano, Silvia Casassa, and Domenica Scarano. 2023. "Combined DFT-D3 Computational and Experimental Studies on g-C3N4: New Insight into Structure, Optical, and Vibrational Properties" Materials 16, no. 10: 3644. https://doi.org/10.3390/ma16103644
APA StyleNegro, P., Cesano, F., Casassa, S., & Scarano, D. (2023). Combined DFT-D3 Computational and Experimental Studies on g-C3N4: New Insight into Structure, Optical, and Vibrational Properties. Materials, 16(10), 3644. https://doi.org/10.3390/ma16103644








