Corrosion Resistance and Titanium Ion Release of Hybrid Dental Implants
Abstract
:1. Introduction
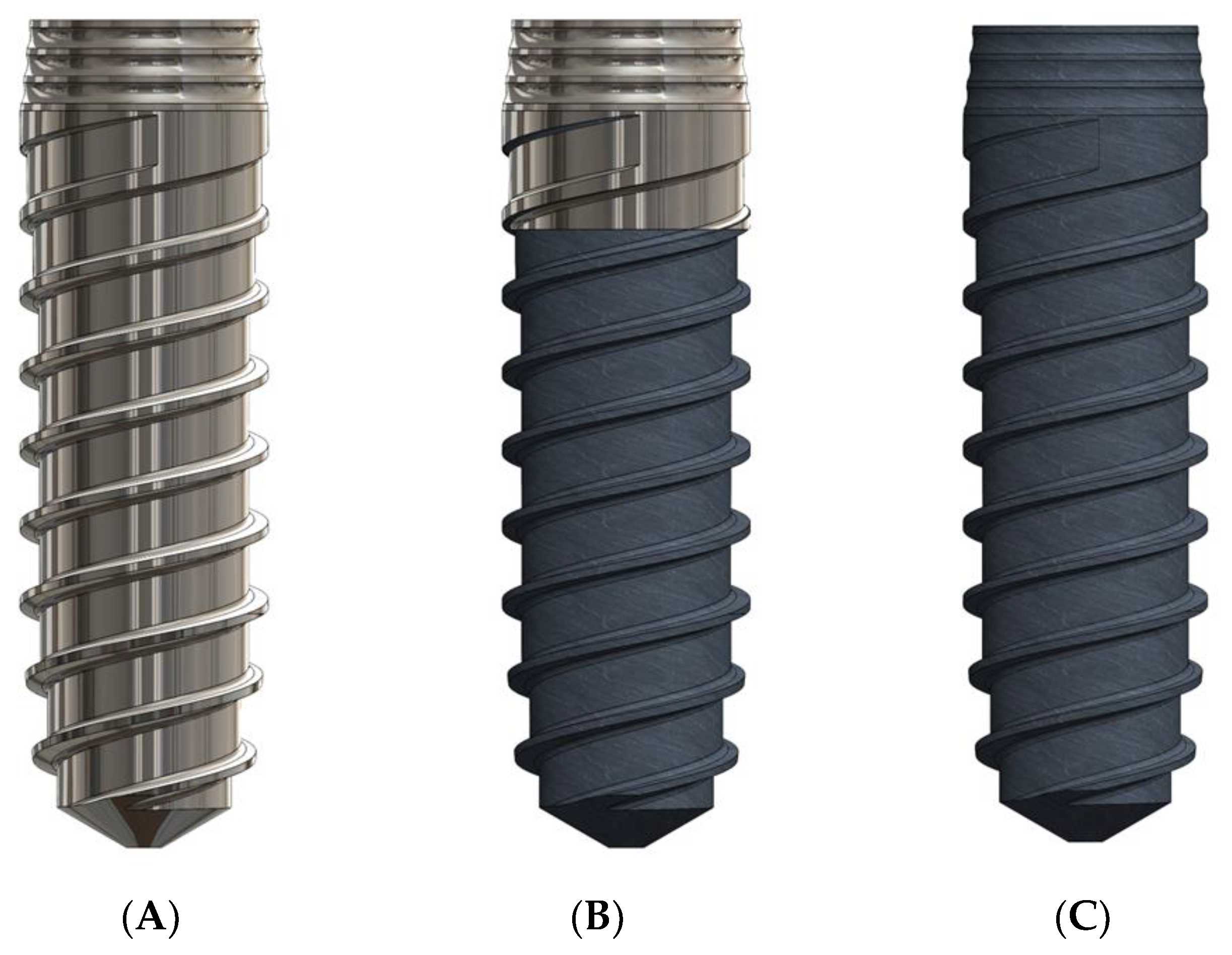
2. Materials and Methods
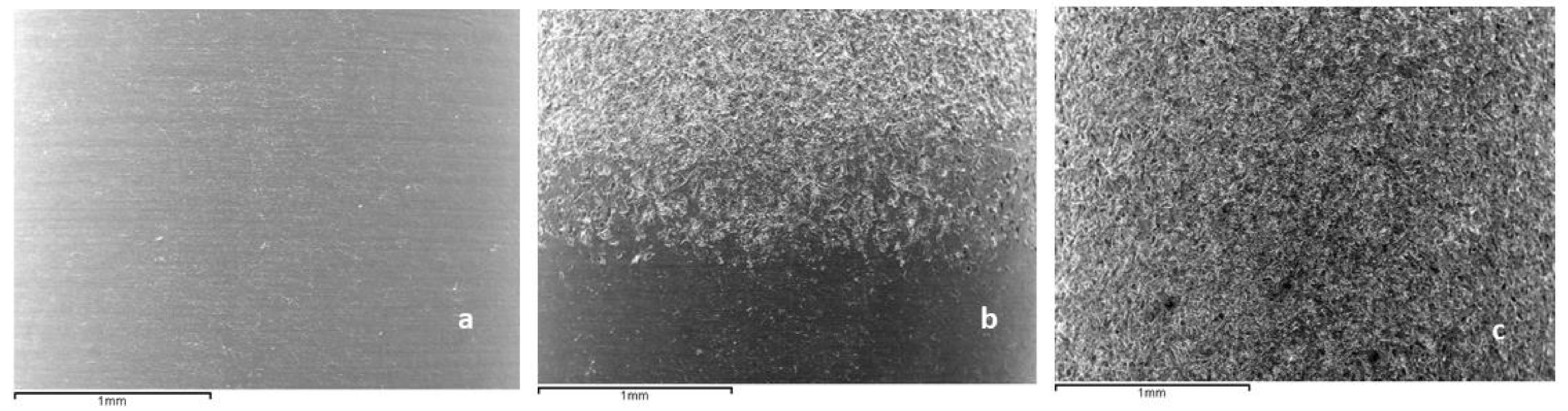
2.1. Roughness
2.2. Residual Stresses
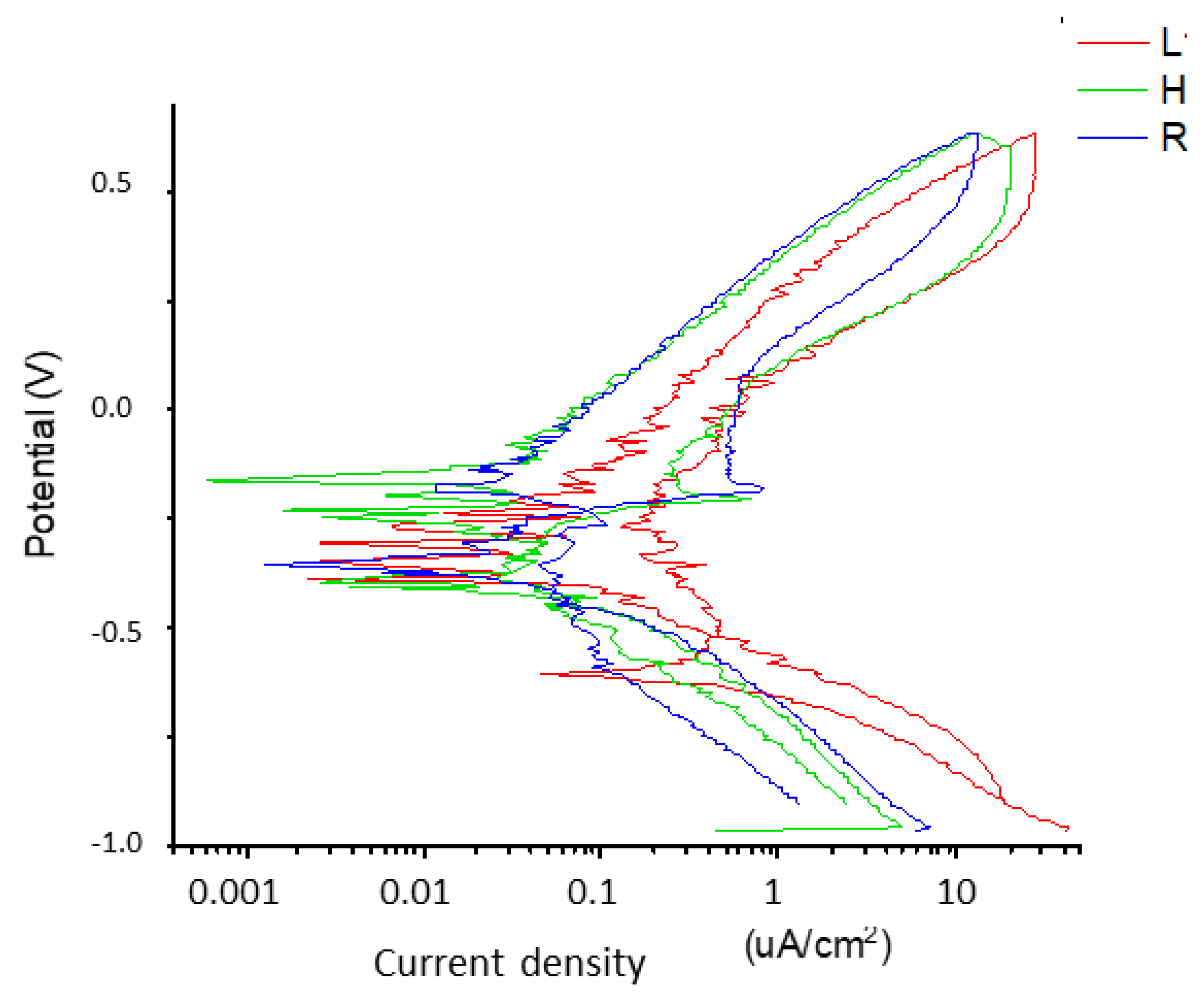
2.3. Corrosion Resistance
2.4. Ion Release
2.5. Scanning Electron Microscopy
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Dmd, S.F.M.J.; Wittneben, J.; Brägger, U.; Dmd, C.A.R.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Gotfredsen, K. A 10-year prospective study of single tooth implants placed in the anterior maxilla. Clin. Implant Dent. Relat. Res. 2012, 14, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Berglundh, T.; Genco, R. Primary prevention of periimplantitis: Managing peri-implant mucositis. J. Clin. Periodontol. 2015, 42, S152–S157. [Google Scholar] [CrossRef]
- Schwartz, F.; Dersk, J.; Mone, A.; Wang, H. Peri-implantitis, 2017 World Workshop. J. Periodontol. 2018, 89, S267–S290. [Google Scholar]
- Derks, J.; Hakansson, J.; Wennstrom, J.L.; Tomasi, C.; Larsson, M.; Berglundh, T. Effectiveness of implant therapy analyzed in a swedish population: Early and late implantloss. J. Dent. Res. 2015, 94, 44S–51S. [Google Scholar] [CrossRef]
- Derks, J.; Schaller, D.; Hakansson, J.; Wennstrom, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of implant therapy analyzed in a swedish population: Prevalence of peri-implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Klinge, B.; Flemming, T.; Cosyn, J.; De Bruyn, H.; Eisner, B.M.; Hultin, M.; Schliephake, H. The patient undergoing implant therapy. Summary and consensus statements. The 4th EAO consensus conference 2015. Clin. Oral Implant. Res. 2015, 26, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35, 292–304. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Muller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implant. Res. 2012, 23, 67–76. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2012, 23, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Gallego, L.; Sicilia, A.; Sicilia, P.; Mallo, C.; Cuesta, S.; Sanz, M. A retrospective study on the crestal bone loss associated with different implant surfaces in chronic periodontitis patients. Clin. Oral Impl. Res. 2018, 29, 557–567. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Dennison, D.K.; Huerzeler, M.B.; Quinones, C.; Caffesse, R.G. Contaminated implant surfaces: An in vitro comparison of implant surface coating and treatment modalities for decontamination. J. Periodontol. 1994, 65, 942–948. [Google Scholar] [CrossRef]
- Berglundh, T.; Gotfredsen, K.; Zitzmann, N.U.; Lang, N.P.; Lindhe, J. Spontaneous progression of ligature induced peri-implantitis at implants with different surface roughness: An experimental study in dogs. Clin. Oral Implant. Res 2007, 18, 655–661. [Google Scholar] [CrossRef]
- Esposito, M.; Murray-Curtis, L.; Grusovin, M.G.; Coulthard, P.; Worthington, H.V. Interventions for replacing missing teeth: Different types of dental implants. Cochrane Database Syst. Rev. 2007, 2019, CD003815. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implants. 2010, 25, 63–74. [Google Scholar]
- Atieh, M.A.; Alsabeeha, N.H.; Faggion, C.M., Jr.; Duncan, W.J. The frequency of periimplant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [PubMed]
- Zetterqvist, L.; Feldman, S.; Rotter, B.; Vincenzi, G.; Wennström, J.L.; Chierico, A.; Stach, R.M.; Kenealy, J.N. A prospective, multicenter, randomizedcontrolled 5-year study of hybrid and fully etched implants for the incidence of periimplantitis. J. Periodontol. 2010, 81, 493–501. [Google Scholar] [CrossRef]
- Lee, C.-T.; Tran, D.; Jeng, M.-D.; Shen, Y.-T. Survival rates of hybrid rough surface implants and their alveolar bone level alterations. J. Periodontol. 2018, 89, 1390–1399. [Google Scholar]
- Schwarz, F.; Mihatovic, I.; Golubovic, V.; Eick, S.; Iglhaut, T.; Becker, J. Experimental periimplant mucositis at different implant surfaces. J. Clin. Periodontol. 2014, 41, 513–520. [Google Scholar] [CrossRef]
- Hermann, J.S.; Jones, A.A.; Bakaeen, L.G.; Buser, D.; Schoolfield, J.D.; Cochran, D.L. Influence of a machined collar on crestal bone changes around titanium implants: A histometric study in the canine mandible. J. Periodontol. 2011, 82, 1329–1338. [Google Scholar] [CrossRef]
- Mombelli, A.; Hashim, D.; Cionca, N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin. Oral Implant. Res. 2018, 29, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Kelk, P.; Belibasakis, G.N.; Bylund, D.; Molin Thorén, M.; Johansson, A. Titanium ions form particles that activate and execute interleukin-1β release from lipopolysaccharide-primed macrophages. J. Periodontal. Res. 2017, 52, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shah, K.; Dong, S.; Peterson, L.; La Plante, E.C.; Sant, G. Elucidating the corrosion-related degradation mechanism of a Ti-6Al-4V dental implant. Dent. Mater. 2020, 36, 431–441. [Google Scholar] [CrossRef]
- Suito, H.; Iwawaki, Y.; Goto, T.; Tomotake, Y.; Ichikawa, T. Oral factors affecting titanium elution and corrosion: An in vitro study using simulated body fluid. PLoS ONE 2013, 8, e66052. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.T.; Kerwell, S.; Lundberg, H.J.; Sukotjo, C.; Mercuri, L.G. Tribocorrosion and oral and maxillofacial surgical devices. Br. J. Oral Maxillofac. Surg. 2014, 52, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef]
- Leonhardt, Å.; Dahlén, G. Effect of titanium on selected oral bacterial species in vitro. Eur. J. Oral Sci. 1995, 103, 382–387. [Google Scholar] [CrossRef]
- Schwarz, F.; Alcoforado, G.; Guerrero, A.; Jönsson, D.; Klinge, B.; Lang, N.; Mattheos, N.; Mertens, B.; Pitta, J.; Ramanauskaite, A.; et al. Peri-implantitis: Summary and consensus statements of group 3. The 6th EAO Consensus Conference 2021. Clin. Oral Implant. Res. 2021, 32, 245–253. [Google Scholar] [CrossRef]
- ASTM-E3-11; Standard Guide for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM G5-14e1; Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2014.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- ASTM G-102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2010.
- Gil, F.J.; Rodríguez, D.; Planell, J.A.; Cortada, M.; Giner, L.; Costa, S. Galvanic corrosion behaviour of Titanium implants coupled to dental alloys. J. Mater. Sci. Mater. Med. 2000, 11, 287–293. [Google Scholar]
- Gil, F.J.; Sánchez, L.A.; Espias, A.; Planell, J.A. In vitro corrosion behaviour and metallic ion release of different prosthodontic alloys. Int. Dent. J. 1999, 49, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Al-Hity, R.R.; Kappert, H.F.; Viennot, S.; Dalard, F.; Grosgogeat, B. Corrosion resistance measurements of dental alloys, are they correlated? Dent. Mater 2007, 23, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. Corrosion behaviour of commercially pure tianium shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials 2003, 24, 263–273. [Google Scholar] [CrossRef]
- Branemark, P.I. Tissue-Integrated Prostheses. In Osseointegration in Clinical Dentistry; Quintessence Publishing Co., Inc.: Loma Linda, CA, USA, 1985. [Google Scholar]
- Aparicio, C.; Rodríguez, D.; Gil, F.J. Variation of roughness and adhesion strength of deposited apatite layers on titanium dental implants. Mater. Sci. Eng. C 2011, 31, 320–324. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Ortiz-Garcia, I.; Jiménez-Guerra, A.; Núñez-Márquez, E.; Moreno-Muñoz, J.; Rondón-Romero, J.L.; Cabanillas-Balsera, D.; Gil, J.; Muñoz-Guzón, F.; Monsalve-Guil, L. Osseointegration of sandblasted and acid-etched implant surfaces. A histological and histomorphometric study in the rabbit. Int. J. Mol. Sci. 2021, 22, 8507. [Google Scholar] [CrossRef]
- Nicolas-Silvente, A.; Velasco-Ortega, E.; Ortiz-García, I.; Monsalve-Guil, L.; Gil, F.J.; Jimenez-Guerra, A. Influence of the Titanium implants surface treatment on the surface roughness and chemical composition. Materials 2020, 13, 314. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45, 246–266. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Galindo-Moreno, P.; Monje, A.; Radovanovic, S.; Wang, H.-L.; Cochran, D.; Sculean, A.; Canullo, L. How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 1805–1816. [Google Scholar] [CrossRef]
- Chan, H.L.; Lin, G.H.; Suarez, F.; MacEachern, M.; Wang, H.L. Surgical management of peri-implantitis: A systematic review and meta-analysis of treatment outcomes. J. Periodontol. 2014, 85, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pons, R.; Amerio, E.; Wang, H.L.; Nart, J. Resolution of peri-implantitis by means of implantoplasty as adjunct to surgical therapy: A retrospective study. J. Periodontol. 2021, 93, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Buxadera-Palomero, J.; Godoy-Gallardo, M.; Molmeneu, M.; Punset, M.; Gil, F.J. Antibacterial Properties of Triethoxysilylpropyl Succinic Anhydride Silane (TESPSA) on Titanium Dental Implants. Polymers 2020, 12, 773. [Google Scholar] [CrossRef]
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. The effect of shot blasting and heat treatment on the fatigue behavior of titanium for dental implant applications. Dent. Mater. 2007, 23, 486–491. [Google Scholar]
- Gil, F.J.; Espinar, E.; Llamas, J.M.; Sevilla, P. Fatigue life of bioactive titanium dental implants treated by means of Grit Blasting and Thermo-Chemical treatment. Clin. Impl. Dent. Rel. Res. 2014, 16, 273–281. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Monsalve-Guil, L.; Jiménez-Guerra, A.; Ortiz, I.; Moreno-Muñoz, J.; Nuñez-Marquez, E.; Pequeroles, M.; Perez, R.A.; Gil, F.J. Importance of the roughness and residual stresses of dental implants on fatigue and osseointegration behavior. In vivo study in rabbits. J. Oral Impl. 2016, 42, 469–476. [Google Scholar] [CrossRef]
- Nespoli, A.; Passaretti, F.; Szentmiklósi, L.; Maróti, B.; Placidi, E.; Cassetta, M.; Yada, R.Y.; Farrar, D.H.; Tian, K.V. Biomedical NiTi and β-Ti Alloys: From Composition, Microstructure and Thermo-Mechanics to Application. Metals 2022, 12, 406. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.A.; Gay-Escoda, C.; Valmaseda-Castellon, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical properties and corrosión behavior of Ti6Al4V particles obtained by Implatoplasty. An in vivo study. Part II Mater. 2021, 14, 6519. [Google Scholar] [CrossRef]
- Briceño, J.; Romeu, A.; Espinar, E.; Llamas, J.M.; Gil, F.J. Influence of the microstructure on electrochemical corrosion and nickel release in NiTi orthodontic archwires. Mater. Sci. Eng. 2013, 33, 4989–4993. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-J.; Shin, J.-S.; Cha, J.Y. Metal release from simulated fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2001, 120, 383–391. [Google Scholar] [CrossRef]
- Shruthi, D.; Patil, G.; Prithviraj, D.R. Comparative evaluation of ion release in bonded and nonbonded stainless steel brackets with use of different mouthwashes: An In vitro study. Contemp. Clin. Dent. 2020, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Contuzzi, N.; Casalino, G.; Boccaccio, A.; Ballini, A.; Charitos, I.A.; Bottalico, L.; Santacroce, L. Metals Biotribology and Oral Microbiota Biocorrosion Mechanisms. J. Funct. Biomater. 2023, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Alovisi, M.; Carossa, M.; Mandras, N.; Roana, J.; Costalonga, M.; Cavallo, L.; Pira, E.; Putzu, M.G.; Bosio, D.; Roato, I.; et al. Disinfection and Biocompatibility of Titanium Surfaces Treated with Glycine Powder Airflow and Triple Antibiotic Mixture: An In Vitro Study. Materials 2022, 15, 4850. [Google Scholar] [CrossRef] [PubMed]



| Component | Composition (mM) |
|---|---|
| K2HPO4 | 0.44 |
| KCl | 5.4 |
| CaCl2 | 1.3 |
| Na2HPO4 | 0.25 |
| NaCl | 137 |
| NaHCO3 | 4.2 |
| MgSO4 | 1.0 |
| C6H12O6 | 5.5 |
| Surface | Sa (µm) ± SD | Sm (µm) ± SD | Index Area ± SD |
|---|---|---|---|
| Smooth | 0.23 ± 0.02 | 0.33 ± 0.01 | 1.10 ± 0.02 |
| Rough | 1.98 ± 0.12 * | 5.40 ± 0.20 * | 1.16 ± 0.05 * |
| Dental Implant | Residual Stress (MPa) |
|---|---|
| Smooth | −20.2 (5.3) |
| Rough | −201.2 (11.2) * |
| Dental Implants | EOCP (mV) |
|---|---|
| L | −200.9 ± 13.3 |
| R | −192.2 ± 0.10 * |
| H | −186.4 ± 4.9 ** |
| Dental Implants | jCORR (µA/cm2) | ECORR (mV) |
|---|---|---|
| L | 0.014 ± 0.055 | −280 ± 53 |
| R | 0.019 ± 0.019 | −273 ± 34 |
| H | 0.069 ± 0.015 * | −223 ± 50 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles, D.; Brizuela, A.; Fernández-Domínguez, M.; Gil, J. Corrosion Resistance and Titanium Ion Release of Hybrid Dental Implants. Materials 2023, 16, 3650. https://doi.org/10.3390/ma16103650
Robles D, Brizuela A, Fernández-Domínguez M, Gil J. Corrosion Resistance and Titanium Ion Release of Hybrid Dental Implants. Materials. 2023; 16(10):3650. https://doi.org/10.3390/ma16103650
Chicago/Turabian StyleRobles, Daniel, Aritza Brizuela, Manuel Fernández-Domínguez, and Javier Gil. 2023. "Corrosion Resistance and Titanium Ion Release of Hybrid Dental Implants" Materials 16, no. 10: 3650. https://doi.org/10.3390/ma16103650







