In Situ Reactive Formation of Mixed Oxides in Additively Manufactured Cobalt Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
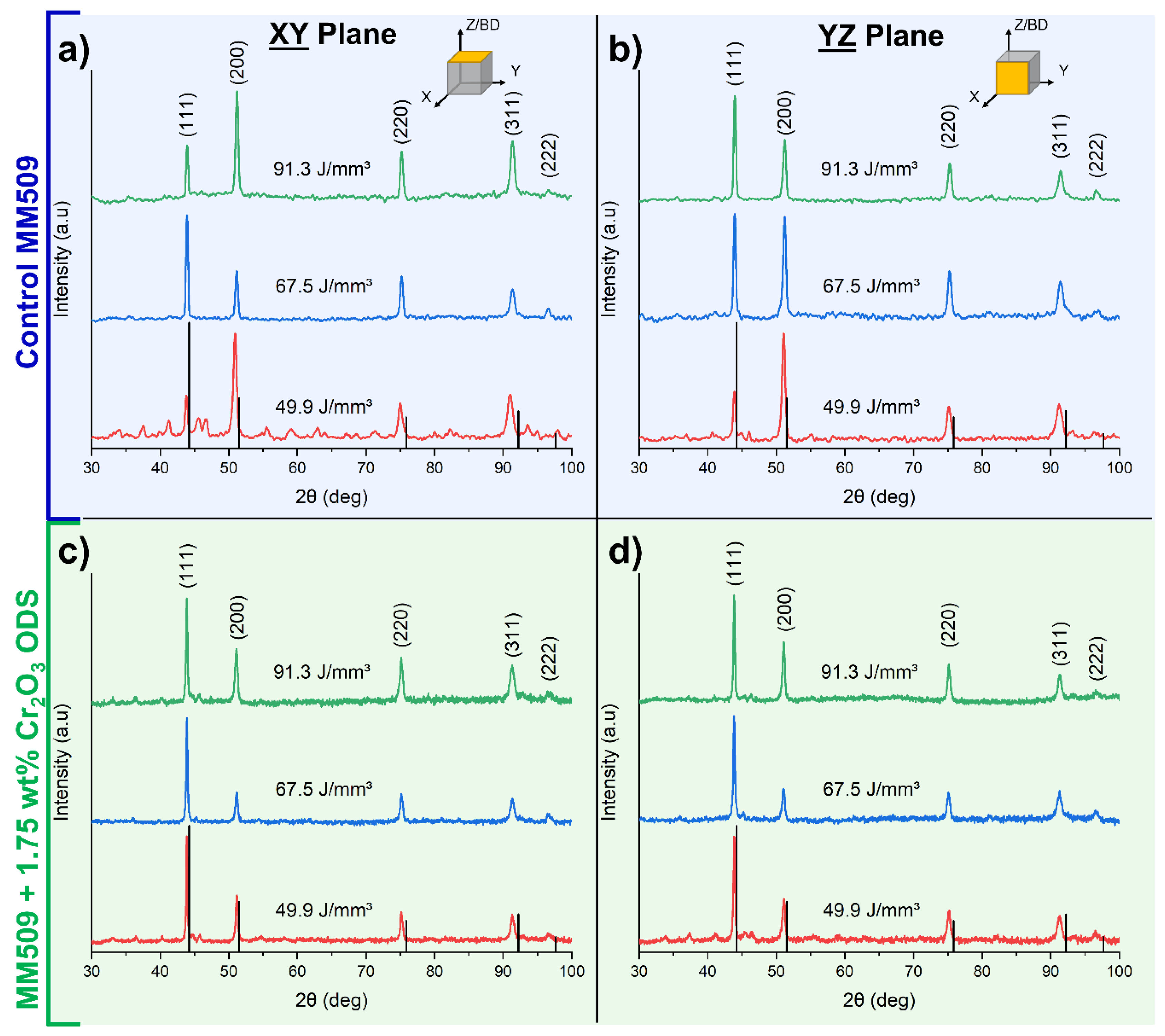
3.1. X-ray Diffraction and Texture Analysis
3.2. Microstructure Analysis
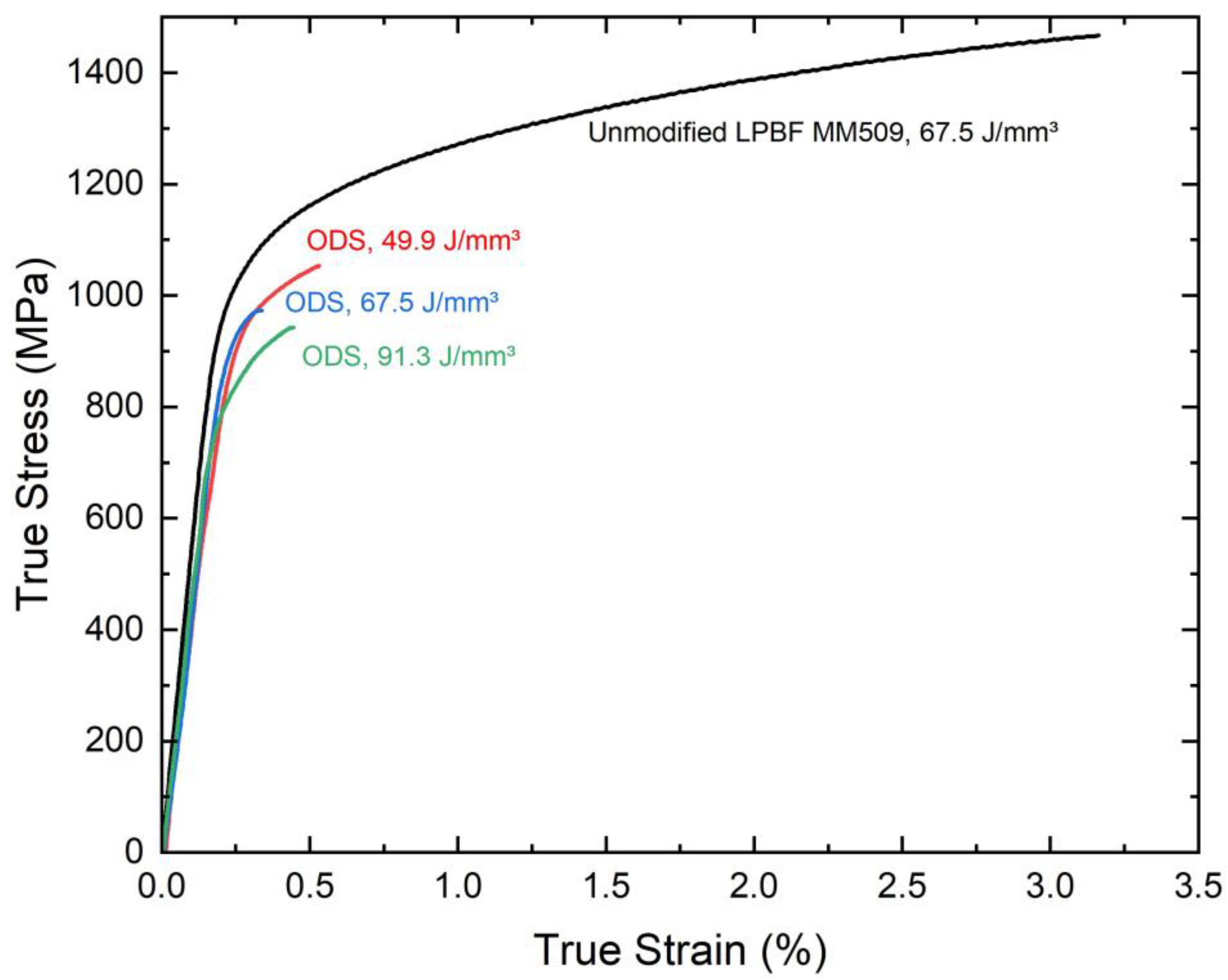
3.3. Mechanical Properties
4. Discussion
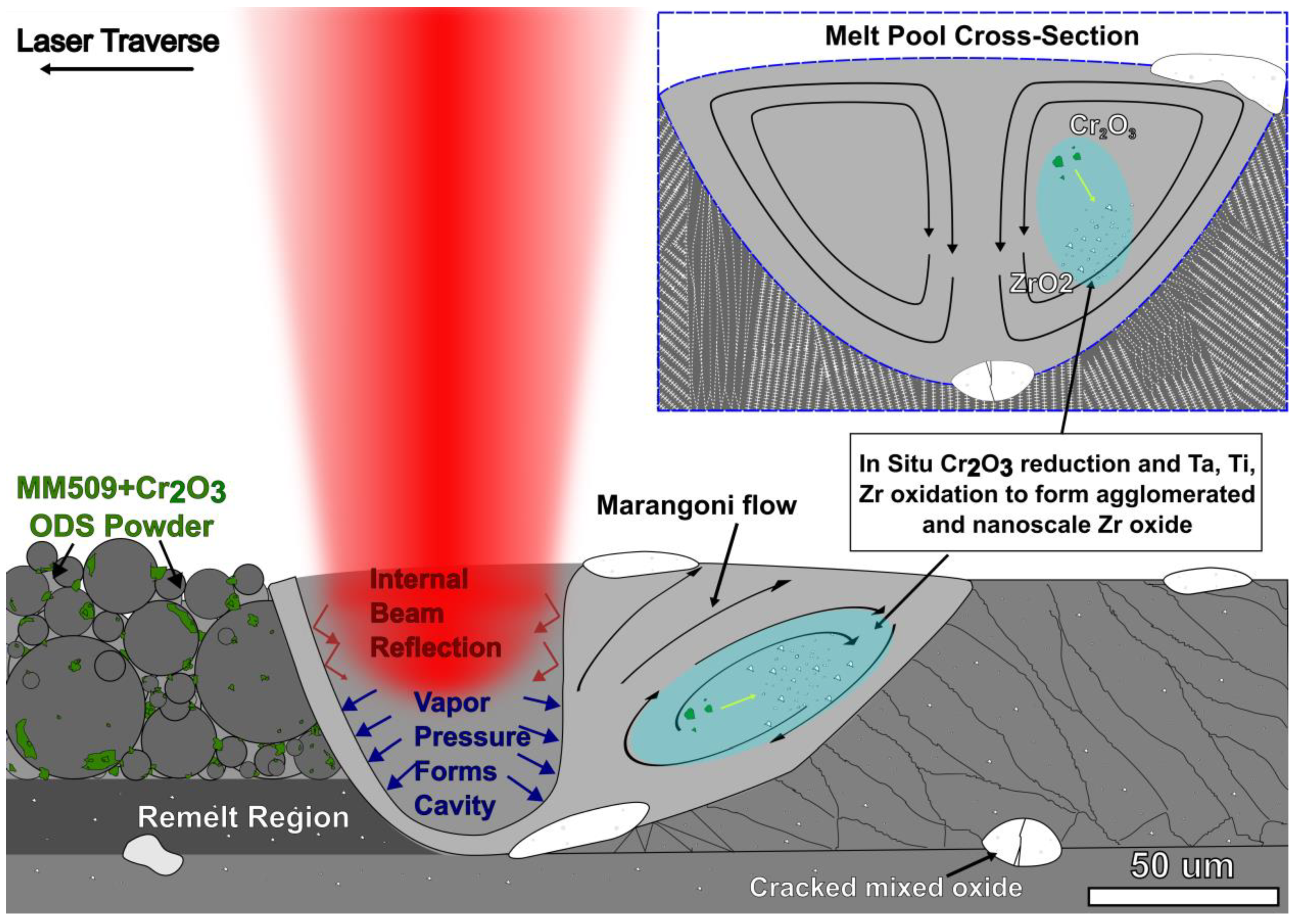
4.1. In Situ Reduction–Oxidation Reactions in LPBF ODS Mar-M 509
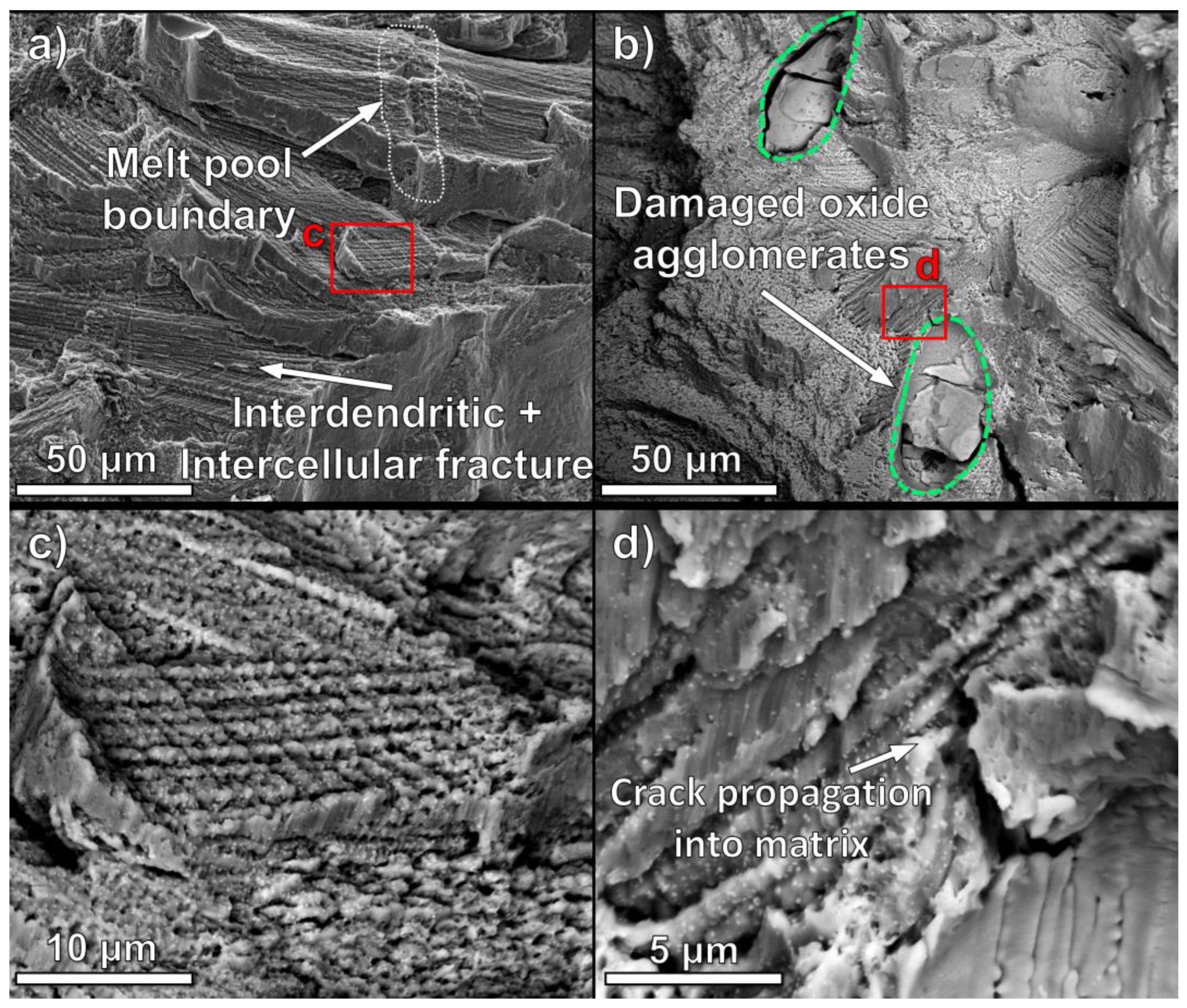
4.2. Oxide Particle Impact on Microstructure
4.3. Oxide Particle Impact on Mechanical Properties
5. Conclusions
- A reduction–oxidation transformation from Cr2O3 to a more stable mixed tantalum, titanium, and zirconium oxide is observed. These reactions could be used to achieve higher entropy mixed oxide compositions in situ.
- The macroscale brittle behavior of oxide dispersion of Mar-M 509 is caused by cracking near the 50–100 µm agglomerated oxide particles. Residual stress-induced cracks in the oxide particles propagate into the matrix and rapidly grow along the aligned carbide precipitates in the as-printed Mar-M 509.
- Zirconium-rich nanoscale oxides in the matrix provide potential for an idealized oxide-dispersion-strengthened system for high temperature applications due to the high thermal stability of the oxide particles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer International Publishing: Cham, Switzerland, 2021; Volume 89, ISBN 978-3-030-56126-0. [Google Scholar]
- Ghiaasiaan, R.; Muhammad, M.; Gradl, P.R.; Shao, S.; Shamsaei, N. Superior Tensile Properties of Hastelloy X Enabled by Additive Manufacturing. Mater. Res. Lett. 2021, 9, 308–314. [Google Scholar] [CrossRef]
- Singh, K. Advanced Materials for Land Based Gas Turbines. Trans. Indian Inst. Met. 2014, 67, 601–615. [Google Scholar] [CrossRef]
- Oono, N.; Ukai, S.; Kondo, S.; Hashitomi, O.; Kimura, A. Irradiation Effects in Oxide Dispersion Strengthened (ODS) Ni-Base Alloys for Gen. IV Nuclear Reactors. J. Nucl. Mater. 2015, 465, 835–839. [Google Scholar] [CrossRef]
- Raman, L.; Gothandapani, K.; Murty, B.S. Austenitic Oxide Dispersion Strengthened Steels: A Review. Def. Sci. J. 2016, 66, 316–322. [Google Scholar] [CrossRef]
- Courtney, T.H. Mechanical Behavior of Materials, 2nd ed.; Waveland Press: Long Grove, IL, USA, 2005; ISBN 978-1-57766-425-3. [Google Scholar]
- Amirjan, M. Development of Ni-Base Oxide Dispersion Strengthened Alloys Using Yttria-Stabilised Zirconia Nanoparticles: Powder Preparation and Spark Plasma Sintering Processing. Powder Metall. 2022, 65, 222–234. [Google Scholar] [CrossRef]
- Pasebani, S.; Dutt, A.K.; Burns, J.; Charit, I.; Mishra, R.S. Oxide Dispersion Strengthened Nickel Based Alloys via Spark Plasma Sintering. Mater. Sci. Eng. A 2015, 630, 155–169. [Google Scholar] [CrossRef]
- Ghasali, E.; Fazili, A.; Alizadeh, M.; Shirvanimoghaddam, K.; Ebadzadeh, T. Evaluation of Microstructure and Mechanical Properties of Al-TiC Metal Matrix Composite Prepared by Conventional, Microwave and Spark Plasma Sintering Methods. Materials 2017, 10, 1255. [Google Scholar] [CrossRef]
- Rieken, J.R. Gas Atomized Precursor Alloy Powder for Oxide Dispersion Strengthened Ferritic Stainless Steel. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2011. [Google Scholar]
- Horn, T.; Rock, C.; Kaoumi, D.; Anderson, I.; White, E.; Prost, T.; Rieken, J.; Saptarshi, S.; Schoell, R.; DeJong, M.; et al. Laser Powder Bed Fusion Additive Manufacturing of Oxide Dispersion Strengthened Steel Using Gas Atomized Reaction Synthesis Powder. Mater. Des. 2022, 216, 110574. [Google Scholar] [CrossRef]
- Zhang, D.; Darsell, J.T.; Wang, J.; Ma, X.; Grant, G.J.; Anderson, I.E.; Rieken, J.R.; Edwards, D.J.; Setyawan, W.; Horn, T.J.; et al. No Ball Milling Needed: Alternative ODS Steel Manufacturing with Gas Atomization Reaction Synthesis (GARS) and Friction-Based Processing. J. Nucl. Mater. 2022, 566, 153768. [Google Scholar] [CrossRef]
- Bergner, F.; Hilger, I.; Virta, J.; Lagerbom, J.; Gerbeth, G.; Connolly, S.; Hong, Z.; Grant, P.S.; Weissgärber, T. Alternative Fabrication Routes toward Oxide-Dispersion-Strengthened Steels and Model Alloys. Metall. Mater. Trans. A 2016, 47, 5313–5324. [Google Scholar] [CrossRef]
- Mirzababaei, S.; Ghayoor, M.; Doyle, R.P.; Pasebani, S. In-Situ Manufacturing of ODS FeCrAlY Alloy via Laser Powder Bed Fusion. Mater. Lett. 2021, 284, 129046. [Google Scholar] [CrossRef]
- Chen, P.; Yang, C.; Li, S.; Attallah, M.M.; Yan, M. In-Situ Alloyed, Oxide-Dispersion-Strengthened CoCrFeMnNi High Entropy Alloy Fabricated via Laser Powder Bed Fusion. Mater. Des. 2020, 194, 108966. [Google Scholar] [CrossRef]
- Xu, R.; Geng, Z.; Wu, Y.; Chen, C.; Ni, M.; Li, D.; Zhang, T.; Huang, H.; Liu, F.; Li, R.; et al. Microstructure and Mechanical Properties of In-Situ Oxide-Dispersion-Strengthened NiCrFeY Alloy Produced by Laser Powder Bed Fusion. Adv. Powder Mater. 2022, 1, 100056. [Google Scholar] [CrossRef]
- Smith, T.M.; Thompson, A.C.; Gabb, T.P.; Bowman, C.L.; Kantzos, C.A. Efficient Production of a High-Performance Dispersion Strengthened, Multi-Principal Element Alloy. Sci. Rep. 2020, 10, 9663. [Google Scholar] [CrossRef]
- Ashby, M.F. Materials Selection in Mechanical Design, 4th ed.; Butterworth-Heinemann: Burlington, MA, USA, 2011; ISBN 978-1-85617-663-7. [Google Scholar]
- Haines, M.P.; Peter, N.J.; Babu, S.S.; Jägle, E.A. In-Situ Synthesis of Oxides by Reactive Process Atmospheres during L-PBF of Stainless Steel. Addit. Manuf. 2020, 33, 101178. [Google Scholar] [CrossRef]
- Chia, E.; Kang, B.; Zheng, M.; Li, Y.; Chyu, M. Development of Ods Coating for High Temperature Turbine Components Using Ded Additive Manufacturing. In Proceedings of the ASME Turbo Expo 2018: Turbomachinery Technical Conference and Exposition, Oslo, Norway, 11 June 2018. [Google Scholar]
- Bhowmik, A.; Zhai, W.; Zhou, W.; Nai, S.M.L. Characterization of Carbide Particle-Reinforced 316L Stainless Steel Fabricated by Selective Laser Melting. Mater. Charact. 2021, 179, 111360. [Google Scholar] [CrossRef]
- Li, J.; Qu, H.; Bai, J. Grain Boundary Engineering during the Laser Powder Bed Fusion of TiC/316L Stainless Steel Composites: New Mechanism for Forming TiC-Induced Special Grain Boundaries. Acta Mater. 2022, 226, 117605. [Google Scholar] [CrossRef]
- Lin, T.C.; Cao, C.; Sokoluk, M.; Jiang, L.; Wang, X.; Schoenung, J.M.; Lavernia, E.J.; Li, X. Aluminum with Dispersed Nanoparticles by Laser Additive Manufacturing. Nat. Commun. 2019, 10, 4124. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xi, X.; Tan, C.; Song, X. Recent Progress in Laser Additive Manufacturing of Aluminum Matrix Composites. Curr. Opin. Chem. Eng. 2020, 28, 28–35. [Google Scholar] [CrossRef]
- Arora, A.; Mula, S. Effect of Ti Addition on Thermal Stability and Phase Evolution of Super-Invar Based Yttria Added ODS Alloys Developed by Mechanical Alloying and Spark Plasma Sintering. J. Alloys Compd. 2022, 899, 163336. [Google Scholar] [CrossRef]
- Ghayoor, M.; Lee, K.; He, Y.; Chang, C.; Paul, B.K.; Pasebani, S. Selective Laser Melting of Austenitic Oxide Dispersion Strengthened Steel: Processing, Microstructural Evolution and Strengthening Mechanisms. Mater. Sci. Eng. A 2020, 788, 139532. [Google Scholar] [CrossRef]
- Zhang, L.; Ukai, S.; Hoshino, T.; Hayashi, S.; Qu, X. Y2O3 Evolution and Dispersion Refinement in Co-Base ODS Alloys. Acta Mater. 2009, 57, 3671–3682. [Google Scholar] [CrossRef]
- Wheaton, H.L. MAR-M 509, A New Cast Cobalt-Base Alloy for High-Temperature Service. Cobalt 1965, 29, 163–170. [Google Scholar]
- Woulds, M.J. Recent Developments in Mar M 509. Cobalt 1969, 42, 3–13. [Google Scholar]
- Sims, C. Contemporary View of Cobalt-Base Alloys. J. Met. 1969, 21, 27–42. [Google Scholar] [CrossRef]
- Davis, J.R. Cobalt and Cobalt Alloys. In Metals Handbook Desk Edition; ASM International: Almere, The Netherlands, 1998; pp. 616–620. [Google Scholar]
- Morral, F.R. Cobalt-Base Alloys in Aerospace. Space Congr. Proc. 1970, 1970, 53–64. [Google Scholar]
- Krewinkel, R.; Such, A.; de la Torre, A.O.; Wiedermann, A.; Castillo, D.; Rodriguez, S.A.; Schleifenbaum, J.H.; Blaswich, M. Design and Characterization of Additively Manufactured NGVs Operated in a Small Industrial Gas Turbine. Int. J. Gas Turbine Propuls. Power Syst. 2020, 11, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.C. The Superalloys; Cambridge University Press: Cambridge, UK, 2006; ISBN 978-0-521-85904-2. [Google Scholar]
- Klarstrom, D.L. Heat Treatment of Cobalt-Base Alloys. In Heat Treating of Nonferrous Alloys; ASM International: Almere, The Netherlands, 2018; Volume 4, pp. 625–633. [Google Scholar] [CrossRef]
- Sato, J.; Omori, T.; Oikawa, K.; Ohnuma, I.; Kainuma, R.; Ishida, K. Cobalt-Base High-Temperature Alloys. Science 2006, 312, 90–91. [Google Scholar] [CrossRef]
- Pollock, T.M.; Dibbern, J.; Tsunekane, M.; Zhu, J.; Suzuki, A. New Co-Based γ-Γ′ High-Temperature Alloys. JOM 2010, 62, 58–63. [Google Scholar] [CrossRef]
- Pollock, T.M.; Clarke, A.J.; Babu, S.S. Design and Tailoring of Alloys for Additive Manufacturing. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 6000–6019. [Google Scholar] [CrossRef]
- Suzuki, A.; Inui, H.; Pollock, T.M. L12-Strengthened Cobalt-Base Superalloys. Annu. Rev. Mater. Res. 2015, 45, 345–368. [Google Scholar] [CrossRef]
- Cacciamani, G.; Roncallo, G.; Wang, Y.; Vacchieri, E.; Costa, A. Thermodynamic Modelling of a Six Component (C-Co-Cr-Ni-Ta-W) System for the Simulation of Cobalt Based Alloys. J. Alloys Compd. 2018, 730, 291–310. [Google Scholar] [CrossRef]
- Opiekun, Z.; Chrusciel, K. Structural Studies of Partial Meltings of Casting Surfaces Made of Cobalt Alloy. Arch. Foundry Eng. 2009, 9, 197–200. [Google Scholar]
- Ferreri, N.C.; Ghorbanpour, S.; Bhowmik, S.; Lussier, R.; Bicknell, J.; Patterson, B.M.; Knezevic, M. Effects of Build Orientation and Heat Treatment on the Evolution of Microstructure and Mechanical Properties of Alloy Mar-M-509 Fabricated via Laser Powder Bed Fusion. Int. J. Plast. 2019, 121, 116–133. [Google Scholar] [CrossRef]
- Wang, W.; Lee, P.D.; McLean, M. A Model of Solidification Microstructures in Nickel-Based Superalloys: Predicting Primary Dendrite Spacing Selection. Acta Mater. 2003, 51, 2971–2987. [Google Scholar] [CrossRef]
- Yuan, L.; Sabau, A.S.; StJohn, D.; Prasad, A.; Lee, P.D. Columnar-to-Equiaxed Transition in a Laser Scan for Metal Additive Manufacturing. IOP Conf. Ser. Mater. Sci. Eng. 2020, 861, 012007. [Google Scholar] [CrossRef]
- Paul, S.; Liu, J.; Strayer, S.T.; Zhao, Y.; Sridar, S.; Klecka, M.A.; Xiong, W.; To, A.C. A Discrete Dendrite Dynamics Model for Epitaxial Columnar Grain Growth in Metal Additive Manufacturing with Application to Inconel. Addit. Manuf. 2020, 36, 101611. [Google Scholar] [CrossRef]
- Quested, P.N.; McLean, M. Solidification Morphologies in Directionally Solidified Superalloys. Mater. Sci. Eng. 1984, 65, 171–180. [Google Scholar] [CrossRef]
- Yang, Y.; Doñate-Buendía, C.; Oyedeji, T.D.; Gökce, B.; Xu, B.X. Nanoparticle Tracing during Laser Powder Bed Fusion of Oxide Dispersion Strengthened Steels. Materials 2021, 14, 3463. [Google Scholar] [CrossRef] [PubMed]
- Ellingham, H.J.T. Reducibility of Oxides and Sulphides in Metallurgical Processes. J. Soc. Chem. Ind. 1944, 63, 125–160. [Google Scholar] [CrossRef]
- Stegman, B.; Yang, B.; Shang, Z.; Ding, J.; Sun, T.; Lopez, J.; Jarosinski, W.; Wang, H.; Zhang, X. Reactive Introduction of Oxide Nanoparticles in Additively Manufactured 718 Ni Alloys with Improved High Temperature Performance. J. Alloys Compd. 2022, 920, 165846. [Google Scholar] [CrossRef]
- Backman, L.; Opila, E.J. Thermodynamic Assessment of the Group IV, V and VI Oxides for the Design of Oxidation Resistant Multi-Principal Component Materials. J. Eur. Ceram. Soc. 2019, 39, 1796–1802. [Google Scholar] [CrossRef]
- Zhao, C.; Fezzaa, K.; Cunningham, R.W.; Wen, H.; De Carlo, F.; Chen, L.; Rollett, A.D.; Sun, T. Real-Time Monitoring of Laser Powder Bed Fusion Process Using High-Speed X-ray Imaging and Diffraction. Sci. Rep. 2017, 7, 3602. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.J.; Guss, G.; Khairallah, S.A.; Rubenchik, A.M.; Depond, P.J.; King, W.E. Denudation of Metal Powder Layers in Laser Powder Bed Fusion Processes. Acta Mater. 2016, 114, 33–42. [Google Scholar] [CrossRef]
- Irrinki, H.; Nath, S.D.; Akilan, A.A.; Atre, S.V. Laser Powder-Bed Fusion Additive Manufacturing: Physics of Complex Melt Flow and Formation Mechanisms of Pores, Spatter, and Denudation Zones. In Additive Manufacturing Processes; ASM International: Almere, The Netherlands, 2020; pp. 209–219. [Google Scholar] [CrossRef]
- Kenel, C.; De Luca, A.; Joglekar, S.S.; Leinenbach, C.; Dunand, D.C. Evolution of Y2O3 Dispersoids during Laser Powder Bed Fusion of Oxide Dispersion Strengthened Ni-Cr-Al-Ti γ/γ’ Superalloy. Addit. Manuf. 2021, 47, 102224. [Google Scholar] [CrossRef]
- Doñate-Buendía, C.; Frömel, F.; Wilms, M.B.; Streubel, R.; Tenkamp, J.; Hupfeld, T.; Nachev, M.; Gökce, E.; Weisheit, A.; Barcikowski, S.; et al. Oxide Dispersion-Strengthened Alloys Generated by Laser Metal Deposition of Laser-Generated Nanoparticle-Metal Powder Composites. Mater. Des. 2018, 154, 360–369. [Google Scholar] [CrossRef]
- Mao, X.; Oh, K.H.; Kang, S.H.; Kim, T.K.; Jang, J. On the Coherency of Y2Ti2O7 Particles with Austenitic Matrix of Oxide Dispersion Strengthened Steel. Acta Mater. 2015, 89, 141–152. [Google Scholar] [CrossRef]
- Lemkey, F.D.; Thompson, E.R. Nickel and Cobalt Eutectic Alloys Reinforced by Refractory Metal Carbides. Metall. Trans. 1971, 2, 1537–1544. [Google Scholar] [CrossRef]




| Co | Cr | Ni | C | W | Ta | Ti | Zr | Fe | Si |
|---|---|---|---|---|---|---|---|---|---|
| Bal. | 23.82 | 10.19 | 0.60 | 6.98 | 3.37 | 0.23 | 0.45 | 0.02 | 0.07 |
| Parameters | Energy Density (J/mm3) | Laser Power (W) | Scan Speed (mm/s) | Hatch Spacing (µm) | Layer Thickness (µm) |
|---|---|---|---|---|---|
| Set 1 | 49.9 | 242 | 1104 | 110 | 40 |
| Set 2 | 67.5 | 285 | 960 | 110 | 40 |
| Set 3 | 91.3 | 328 | 816 | 110 | 40 |
| Sample | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Elongation at Fracture (%) |
|---|---|---|---|
| Control 67 J/mm3 | 954 | 1420 | 3.2 |
| ODS 49 J/mm3 | 797 | 1054 | 0.55 |
| ODS 67 J/mm3 | 756 | 973 | 0.34 |
| ODS 91 J/mm3 | 695 | 942 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, J.; Cerne, R.; Ho, D.; Madigan, D.; Shen, Q.; Yang, B.; Corpus, J.; Jarosinski, W.; Wang, H.; Zhang, X. In Situ Reactive Formation of Mixed Oxides in Additively Manufactured Cobalt Alloy. Materials 2023, 16, 3707. https://doi.org/10.3390/ma16103707
Lopez J, Cerne R, Ho D, Madigan D, Shen Q, Yang B, Corpus J, Jarosinski W, Wang H, Zhang X. In Situ Reactive Formation of Mixed Oxides in Additively Manufactured Cobalt Alloy. Materials. 2023; 16(10):3707. https://doi.org/10.3390/ma16103707
Chicago/Turabian StyleLopez, Jack, Rok Cerne, David Ho, Devin Madigan, Qing Shen, Bo Yang, Joseph Corpus, William Jarosinski, Haiyan Wang, and Xinghang Zhang. 2023. "In Situ Reactive Formation of Mixed Oxides in Additively Manufactured Cobalt Alloy" Materials 16, no. 10: 3707. https://doi.org/10.3390/ma16103707
APA StyleLopez, J., Cerne, R., Ho, D., Madigan, D., Shen, Q., Yang, B., Corpus, J., Jarosinski, W., Wang, H., & Zhang, X. (2023). In Situ Reactive Formation of Mixed Oxides in Additively Manufactured Cobalt Alloy. Materials, 16(10), 3707. https://doi.org/10.3390/ma16103707






