The Usability of Sorbents in Restoring Enzymatic Activity in Soils Polluted with Petroleum-Derived Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Methodology of Physicochemical and Chemical Determinations
2.3. Methodology of Determination of the Activity of Soil Enzymes
2.4. Calculations and Statistical Analysis
- y—respectively: DO, P or sorbent (Ad);
- Po—weight of Zea mays biomass or activity of the analyzed enzyme in the soil polluted with DO or P;
- Co—weight of Zea mays biomass or activity of the analyzed enzyme in the control soil (uncontaminated).
3. Results
3.1. Effect of Diesel Oil, Petrol and Sorbents on Zea mays Biomass
3.2. Effect of Diesel Oil, Petrol and Sorbents on the Activity of Soil Enzymes
4. Discussion
4.1. Response of Zea mays to Soil Contamination with Petroleum-Derived Products
4.2. Response of Soil Enzymes to Soil Pollution with Petroleum-Derived Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Liu, Q.; Wu, Y.; Zhao, W.; Ma, J.; Qu, Y.; Chen, H.; Tian, Y.; Wu, F. Soil Environmental Criteria in Six Representative Developed Countries: Soil Management Targets, and Human Health and Ecological Risk Assessment. Crit. Rev. Environ. Sci. Technol. 2023, 53, 577–600. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.S.; Pereira, M.G. Is Environmental Contamination a Concern in Global Technosols? A Bibliometric Analysis. Water Air Soil Pollut. 2023, 234, 142. [Google Scholar] [CrossRef]
- Li, G.; Sun, G.-X.; Ren, Y.; Luo, X.-S.; Zhu, Y.-G. Urban Soil and Human Health: A Review. Eur. J. Soil Sci. 2018, 69, 196–215. [Google Scholar] [CrossRef] [Green Version]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and Health: A Progress Update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, E.; Karaca, A. Bioremediation of Crude Oil Polluted Soils. Asian J. Biotechnol. 2011, 3, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, B.; Surya, S.; Sivakumar, K.; AlSalhi, M.S.; Rao, T.N.; Devanesan, S.; Arunkumar, P.; Rajasekar, A. Influence of Bioaugmentation in Crude Oil Contaminated Soil by Pseudomonas Species on the Removal of Total Petroleum Hydrocarbon. Chemosphere 2023, 310, 136826. [Google Scholar] [CrossRef]
- Chunyan, X.; Qaria, M.A.; Qi, X.; Daochen, Z. The Role of Microorganisms in Petroleum Degradation: Current Development and Prospects. Sci. Total Environ. 2023, 865, 161112. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Prasad, S.; Gomez, F.H.; Franzetti, A.; Vaccari, M. Remediation of soil polluted with petroleum hydrocarbons and its reuse for agriculture: Recent progress, challenges, and perspectives. Chemosphere 2022, 293, 133572. [Google Scholar] [CrossRef]
- OMR 2023. IEA Oil Market Report. Available online: https://www.iea.org/reports/oil-market-report-april-2023 (accessed on 5 March 2023).
- OPEC 2022. Organization of the Petroleum Exporting Countries. Available online: https://www.opec.org/opec_web/en/publications/6809.htm (accessed on 5 March 2023).
- Song, X.; Wu, X.; Song, X.; Shi, C.; Zhang, Z. Sorption and desorption of petroleum hydrocarbons on biodegradable and non-degradable microplastics. Chemosphere 2021, 273, 128553. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Saeed, T.; Jupp, B.; Faizuddin, M. Petroleum hydrocarbon pollution in sediments from the Gulf and Omani waters: Status and review. Mar. Pollut. Bull. 2021, 173, 112913. [Google Scholar] [CrossRef]
- Pérez, A.P.; Eugenio, N.R. Status of Local Soil Contamination in Europe: Revision of the Indicator ‘Progress in the Management Contaminated Sites in EUROPE’. JRC. 2018. Available online: https://Data.Europa.Eu/Doi/10.2760/093804 (accessed on 5 March 2023).
- EC—European Commission the Implementation of the Soil Thematic Strategy and Ongoing Activities. COM/2012/046 Final 2012. Available online: https://Eur-Lex.Europa.Eu/Legal-Content/EN/TXT/PDF/?Uri=CELEX:52012DC0046&from=EN (accessed on 5 March 2023).
- Abu-Khasan, M.S.; Makarov, Y.I. Analysis of Soil Contamination with Oil and Petroleum Products. IOP Conf. Ser. Earth Environ. Sci. 2021, 937, 022046. [Google Scholar] [CrossRef]
- Abousnina, R.; Allister, R.L.; Abousnina, R.; Allister, R.L. Oil Contaminated Sand: Sources, Properties, Remediation, and Engineering Applications. IntechOpen: Sydney, Australia, 2022; ISBN 978-1-80355-586-7. [Google Scholar]
- Mirjani, M.; Soleimani, M.; Salari, V. Toxicity assessment of total petroleum hydrocarbons in aquatic environments using the bioluminescent bacterium Aliivibrio fischeri. Ecotoxicol. Environ. Saf. 2021, 207, 111554. [Google Scholar] [CrossRef]
- Truskewycz, A.; Gundry, T.D.; Khudur, L.S.; Kolobaric, A.; Taha, M.; Aburto-Medina, A.; Ball, A.S.; Shahsavari, E. Petroleum Hydrocarbon Contamination in Terrestrial Ecosystems—Fate and Microbial Responses. Molecules 2019, 24, 3400. [Google Scholar] [CrossRef] [Green Version]
- Borowik, A.; Wyszkowska, J.; Kucharski, J. Microbiological Study in Petrol-Spiked Soil. Molecules 2021, 26, 2664. [Google Scholar] [CrossRef]
- Ssenku, J.E.; Walusansa, A.; Oryem-Origa, H.; Ssemanda, P.; Ntambi, S.; Omujal, F.; Mustafa, A.S. Bacterial Community and Chemical Profiles of Oil-Polluted Sites in Selected Cities of Uganda: Potential for Developing a Bacterial-Based Product for Remediation of Oil-Polluted Sites. BMC Microbiol. 2022, 22, 120. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, J. The Resistance of Lolium Perenne L. × Hybridum, Poa Pratensis, Festuca Rubra, F. Arundinacea, Phleum Pratense and Dactylis Glomerata to Soil Pollution by Diesel Oil and Petroleum. Plant Soil Environ. 2019, 65, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.; Krupnik, T.J.; Timsina, J.; Mahboob, M.G.; Chaki, A.K.; Farooq, M.; Bhatt, R.; Fahad, S.; Hasanuzzaman, M. Agricultural Land Degradation: Processes and Problems Undermining Future Food Security. In Environment, Climate, Plant and Vegetation Growth; Fahad, S., Hasanuzzaman, M., Alam, M., Ullah, H., Saeed, M., Ali Khan, I., Adnan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–61. ISBN 978-3-030-49732-3. [Google Scholar]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. Implications of Soil Pollution with Diesel Oil and BP Petroleum with ACTIVE Technology for Soil Health. Int. J. Environ. Res. Public Health 2019, 16, 2474. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Chen, H.; Su, Y.; Sun, S.; Zhao, C.; Zhang, X.; Gu, Y.; Li, L. Enhanced Crude Oil Degradation by Remodeling of Crude Oil-Contaminated Soil Microbial Community Structure Using Sodium Alginate/Graphene Oxide/Bacillus C5 Immobilized Pellets. Environ. Res. 2023, 223, 115465. [Google Scholar] [CrossRef]
- Borah, G.; Deka, H. Crude Oil Associated Heavy Metals (HMs) Contamination in Agricultural Land: Understanding Risk Factors and Changes in Soil Biological Properties. Chemosphere 2023, 310, 136890. [Google Scholar] [CrossRef]
- Karlen, D.L.; Rice, C.W. Soil Degradation: Will Humankind Ever Learn? Sustainability 2015, 7, 12490–12501. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Wang, Q.; Liu, W.; Zhong, D.; Ge, Y.; Christie, P.; Luo, Y. Soil microbial community and association network shift induced by several tall fescue cultivars during the phytoremediation of a petroleum hydrocarbon-contaminated soil. Sci. Total Environ. 2021, 792, 148411. [Google Scholar] [CrossRef]
- Steliga, T.; Kluk, D. Application of Festuca arundinacea in phytoremediation of soils contaminated with Pb, Ni, Cd and petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 2020, 194, 110409. [Google Scholar] [CrossRef]
- Hunt, L.J.; Duca, D.; Dan, T.; Knopper, L.D. Petroleum Hydrocarbon (PHC) Uptake in Plants: A Literature Review. Environ. Pollut. 2019, 245, 472–484. [Google Scholar] [CrossRef]
- Gawryluk, A.; Stępniowska, A.; Lipińska, H. Effect of Soil Contamination with Polycyclic Aromatic Hydrocarbons from Drilling Waste on Germination and Growth of Lawn Grasses. Ecotoxicol. Environ. Saf. 2022, 236, 113492. [Google Scholar] [CrossRef]
- Novakovskiy, A.B.; Kanev, V.A.; Markarova, M.Y. Long-Term Dynamics of Plant Communities after Biological Remediation of Oil-Contaminated Soils in Far North. Sci. Rep. 2021, 11, 4888. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The Concept and Future Prospects of Soil Health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and Human Security in the 21st Century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef] [Green Version]
- Wyszkowski, M.; Wyszkowska, J.; Kordala, N.; Borowik, A. Applicability of Ash Wastes for Reducing Trace Element Content in Zea Mays L. Grown in Eco-Diesel Contaminated Soil. Molecules 2022, 27, 897. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the Intensification of Agriculture for Global Food Security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on Pollution and Health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [Green Version]
- IEA 2022. International Energy Agency, Word Energy Outlook 2022. Available online: https://iea.blob.core.windows.net/assets/b67ce2ff-5fae-4d98-a6fb-001645f3a911/WEO2022_ES_Polish.pdf (accessed on 5 March 2023).
- Basu, N.; Lanphear, B.P. The challenge of pollution and health in Canada. Can. J. Pub. Health 2019, 110, 159–164. [Google Scholar] [CrossRef]
- COM 2021. Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and the Committee of the Regions EU Soil Strategy for 2030 Reaping the Benefits of Healthy Soils for People, Food, Nature and Climate 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52021DC0699 (accessed on 2 May 2023).
- Chinedu, E.; Chukwuemeka, C.K. Oil Spillage and Heavy Metals Toxicity Risk in the Niger Delta, Nigeria. J. Health Pollut. 2018, 8, 180905. [Google Scholar] [CrossRef] [Green Version]
- Overton, E.B.; Wade, T.L.; Radović, J.R.; Meyer, B.M.; Miles, M.S.; Larter, S.R. Chemical Composition of Macondo and Other Crude Oils and Compositional Alterations During Oil Spills. Oceanography 2016, 29, 50–63. [Google Scholar] [CrossRef] [Green Version]
- De la Cueva, S.C.; Rodríguez, C.H.; Cruz, N.O.S.; Contreras, J.A.R.; Miranda, J.L. Changes in Bacterial Populations during Bioremediation of Soil Contaminated with Petroleum Hydrocarbons. Water Air Soil Pollut. 2016, 227, 91. [Google Scholar] [CrossRef]
- Bidja Abena, M.T.; Li, T.; Shah, M.N.; Zhong, W. Biodegradation of Total Petroleum Hydrocarbons (TPH) in Highly Contaminated Soils by Natural Attenuation and Bioaugmentation. Chemosphere 2019, 234, 864–874. [Google Scholar] [CrossRef]
- Zhong, L.; Qing, J.W.; Chen, H.Y.; Li, G.; Guanyi Chen, G.; Sun, Y.; Jinlei Li, J.; Song, Y.; Yan, B. Advances in microbial remediation of petroleum hydrocarbon soil contamination. J. Biol. Eng. 2021, 37, 3636–3652. [Google Scholar] [CrossRef]
- Tetteh, K.E.; Rathilal, S. Evaluating Pre- and Post-Coagulation Configuration of Dissolved Air Flotation Using Response Surface Methodology. Processes 2020, 8, 383. [Google Scholar] [CrossRef] [Green Version]
- Adedeji, J.A.; Tetteh, E.K.; Opoku Amankwa, M.; Asante-Sackey, D.; Ofori-Frimpong, S.; Armah, E.K.; Rathilal, S.; Mohammadi, A.H.; Chetty, M. Microbial Bioremediation and Biodegradation of Petroleum Products—A Mini Review. Appl. Sci. 2022, 12, 12212. [Google Scholar] [CrossRef]
- Shahsavari, E.; Poi, G.; Aburto-Medina, A.; Haleyur, N.; Ball, A.S. Bioremediation Approaches for Petroleum Hydrocarbon-Contaminated Environments. In Enhancing Cleanup of Environmental Pollutants; Anjum, N., Gill, S., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 21–41. [Google Scholar] [CrossRef]
- Tomei, M.C.; Daugulis, A.J. Ex Situ Bioremediation of Contaminated Soils: An Overview of Conventional and Innovative Technologies. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2107–2139. [Google Scholar] [CrossRef] [Green Version]
- Tekere, M. Microbial Bioremediation and Different Bioreactors Designs Applied. In Biotechnology and Bioengineering; Jacob, E.-L., Zepka, L.Q., Eds.; Intech Publishers: London, UK, 2019; p. 188. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Naidoo, D.B. Photocatalytic degradation of oily waste and phenol from a local South Africa oil refinery wastewater using response methodology. Sci. Rep. 2020, 10, 8850. [Google Scholar] [CrossRef]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Inoculation with bacteria in floating treatment wetlands positively modulates the phytoremediation of oil field wastewater. J. Hazard. Mater. 2018, 349, 242–251. [Google Scholar] [CrossRef]
- Sui, X.; Wang, X.; Li, Y.; Ji, H. Remediation of petroleum-contaminated soils with microbial and microbial combined methods: Advances, mechanisms, and challenges. Sustainability 2021, 13, 9267. [Google Scholar] [CrossRef]
- Rahayu, Y.S. Bioremediation model of oil-contaminated soil in Lapindo mud using multisymbiotic organism. Manag. Environ. Qual. Int. J. 2020, 31, 586–601. [Google Scholar] [CrossRef]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of Soil and Water Contaminated with Petroleum Hydrocarbon: A Review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Mitigation of the Adverse Impact of Copper, Nickel, and Zinc on Soil Microorganisms and Enzymes by Mineral Sorbents. Materials 2022, 15, 5198. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt. Materials 2022, 15, 5738. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation Technologies and Their Mechanism for Removal of Heavy Metal from Contaminated Soil: An Approach for a Sustainable Environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Yurak, V.; Apakashev, R.; Dushin, A.; Usmanov, A.; Lebzin, M.; Malyshev, A. Testing of Natural Sorbents for the Assessment of Heavy Metal Ions’ Adsorption. Appl. Sci. 2021, 11, 3723. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Preparation, Characterization and Application of Biosurfactant in Various Industries: A Critical Review on Progress, Challenges and Perspectives. Environ. Technol. Innov. 2021, 24, 102090. [Google Scholar] [CrossRef]
- Usman, M.; Jellali, S.; Anastopoulos, I.; Charabi, Y.; Hameed, B.H.; Hanna, K. Fenton Oxidation for Soil Remediation: A Critical Review of Observations in Historically Contaminated Soils. J. Hazard. Mater. 2022, 424, 127670. [Google Scholar] [CrossRef]
- Ma, C.; Ci, K.; Zhu, J.; Sun, Z.; Liu, Z.; Li, X.; Zhu, Y.; Tang, C.; Wang, P.; Liu, Z. Impacts of exogenous mineral silicon on cadmium migration and transformation in the soil-rice system and on soil health. Sci. Total Environ. 2021, 759, 143501. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rizvi, H.; Rinklebe, J.; Tsang, D.C.W.; Meers, E.; Ok, Y.S.; Ishaque, W. Phytomanagement of heavy metals in contaminate soils using sunflower–A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1498–1528. [Google Scholar] [CrossRef]
- Liu, H.; Ding, W.; Lei, S.; Tian, X.; Zhou, F. Selective Adsorption of CH4/N2 on Ni-based MOF/SBA-15 Composite Materials. Nanomaterials 2019, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Oueslati, W.; Ammar, M.; Chorfi, N. Quantitative XRD Analysis of the Structural Changes of Ba-Exchanged Montmorillonite: Effect of an in Situ Hydrous Perturbation. Minerals 2015, 5, 507–526. [Google Scholar] [CrossRef] [Green Version]
- Roces, E.; Muñiz-Menéndez, M.; González-Galindo, J.; Estaire, J. Lightweight expanded clay aggregate properties based on laboratory testing. Constr. Build. Mater. 2021, 313, 125486. [Google Scholar] [CrossRef]
- Bandura, L.; Woszuk, A.; Kołodyńska, D.; Franus, W. Application of Mineral Sorbents for Removal of Petroleum Substances: A Review. Minerals 2017, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Lipińska, A.; Wyszkowska, J.; Kucharski, J. Diversity of Organotrophic Bacteria, Activity of Dehydrogenases and Urease as Well as Seed Germination and Root Growth Lepidium Sativum, Sorghum Saccharatum and Sinapis Alba under the Influence of Polycyclic Aromatic Hydrocarbons. Environ. Sci. Pollut. Res. Int. 2015, 22, 18519–18530. [Google Scholar] [CrossRef] [Green Version]
- Gospodarek, J.; Rusin, M.; Barczyk, G.; Nadgórska-Socha, A. The Effect of Petroleum-Derived Substances and Their Bioremediation on Soil Enzymatic Activity and Soil Invertebrates. Agronomy 2021, 11, 80. [Google Scholar] [CrossRef]
- Achuba, F.I.; Okoh, P.N. Effect of Petroleum Products on Soil Catalase and Dehydrogenase Activities. Open J. Soil Sci. 2014, 4, 399. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yang, F.; Yang, Y.; Xing, G.; Deng, C.; Shen, Y.; Luo, L.; Li, B.; Yuan, H. A Proposal of “Core Enzyme” Bioindicator in Long-Term Pb-Zn Ore Pollution Areas Based on Topsoil Property Analysis. Environ. Pollut. 2016, 213, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Martinez, V.; Cano, A.; Johnson, J. Simultaneous Determination of Multiple Soil Enzyme Activities for Soil Health-Biogeochemical Indices. Appl. Soil Ecol. 2018, 126, 121–128. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, B.; Lyu, H.; Duan, Y.; Yang, Y.; Wang, S.; Li, Z. Rhizosphere Enzyme Activities and Microorganisms Drive the Transformation of Organic and Inorganic Carbon in Saline–Alkali Soil Region. Sci. Rep. 2022, 12, 1314. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, M.; El Chami, A.; Iovieno, P.; Rouphael, Y.; Bonini, P.; Colla, G. Organic Fertilizer Sources Distinctively Modulate Productivity, Quality, Mineral Composition, and Soil Enzyme Activity of Greenhouse Lettuce Grown in Degraded Soil. Agronomy 2023, 13, 194. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, M.; Kucharski, J. Effect of Cadmium, Copper and Zinc on Plants, Soil Microorganisms and Soil Enzymes. J. Elem. 2013, 18, 769–796. [Google Scholar] [CrossRef]
- Dindar, E.; Topaç Şağban, F.O.; Başkaya, H.S. Variations of Soil Enzyme Activities in Petroleum-Hydrocarbon Contaminated Soil. Int. Biodeterior. Biodegrad. 2015, 105, 268–275. [Google Scholar] [CrossRef]
- Kaczyńska, G.; Borowik, A.; Wyszkowska, J. Soil Dehydrogenases as an Indicator of Contamination of the Environment with Petroleum Products. Water Air Soil Pollut. 2015, 226, 372. [Google Scholar] [CrossRef] [Green Version]
- Keller, N.; Bol, R.; Herre, M.; Marschner, B.; Heinze, S. Catchment Scale Spatial Distribution of Soil Enzyme Activities in a Mountainous German Coniferous Forest. Soil Biol. Biochem. 2023, 177, 108885. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Li, Z.; Zhou, Z.; Wang, J.; Wang, S.; Cao, Y. Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China. Sustainability 2020, 12, 10095. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Effect of Separate and Combined Toxicity of Bisphenol A and Zinc on the Soil Microbiome. Int. J. Mol. Sci. 2022, 23, 5937. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bisphenol A—A Dangerous Pollutant Distorting the Biological Properties of Soil. Int. J. Mol. Sci. 2021, 22, 12753. [Google Scholar] [CrossRef]
- Yadav, R.; Tripathi, P.; Singh, R.P.; Khare, P. Assessment of Soil Enzymatic Resilience in Chlorpyrifos Contaminated Soils by Biochar Aided Pelargonium Graveolens L. Plantation. Environ. Sci. Pollut. Res. 2023, 30, 7040–7055. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. The Impact of Permethrin and Cypermethrin on Plants, Soil Enzyme Activity, and Microbial Communities. Int. J. Mol. Sci. 2023, 24, 2892. [Google Scholar] [CrossRef]
- Dindar, E.; Topac, F.O.; Baskaya, H.S.; Kaya, T. Effect of Wastewater Sludge Application on Enzyme Activities in Soil Contaminated with Crude Oil. J. Soil Sci. Plant Nutr. 2017, 17, 180–193. [Google Scholar] [CrossRef] [Green Version]
- Wyszkowska, J.; Kucharski, M.; Kucharski, J. Application of the Activity of Soil Enzymes in the Evaluation of Soil Contamination by Diesel Oil. Pol. J. Environ. Stud. 2006, 15, 501–506. [Google Scholar]
- IUSS Working Group WRB World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. In World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015.
- Borowik, A.; Wyszkowska, J. Bioaugmentation of Soil Contaminated with Diesel Oil. J. Elem. 2018, 23, 1161–1178. [Google Scholar] [CrossRef]
- OECD-FAO. OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021; ISBN 978-92-64-43607-7. [Google Scholar]
- Yi, L.; Shenjiao, Y.; Shiqing, L.; Xinping, C.; Fang, C. Growth and Development of Maize (Zea Mays L.) in Response to Different Field Water Management Practices: Resource Capture and Use Efficiency. Agric. For Meteorol. 2010, 150, 606–613. [Google Scholar] [CrossRef]
- Morales-Máximo, C.N.; López-Sosa, L.B.; Rutiaga-Quiñones, J.G.; Corral-Huacuz, J.C.; Aguilera-Mandujano, A.; Pintor-Ibarra, L.F.; López-Miranda, A.; Delgado-Domínguez, S.N.; Rodríguez-Magallón, M.d.C.; Morales-Máximo, M. Characterization of Agricultural Residues of Zea Mays for Their Application as Solid Biofuel: Case Study in San Francisco Pichátaro, Michoacán, Mexico. Energies 2022, 15, 6870. [Google Scholar] [CrossRef]
- Liao, C.; Xu, W.; Lu, G.; Liang, X.; Guo, C.; Yang, C.; Dang, Z. Accumulation of Hydrocarbons by Maize (Zea Mays L.) in Remediation of Soils Contaminated with Crude Oil. Int. J. Phytoremediation 2015, 17, 693–700. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of Aerobic Microorganisms and Soil Enzyme Response to Soil Contamination with Ekodiesel Ultra Fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef] [Green Version]
- RStudio Team RStudio: Integrated Development for R. RStudio, Inc.: Boston, MA, USA. 2019. Available online: http://www.Rstudio.Com/ (accessed on 8 December 2022).
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-Project.Org/ (accessed on 23 February 2020).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, M.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data. R Package Version 2.17.0. Available online: https://CRAN.R-Project.Org/Package=gplots (accessed on 8 December 2022).
- Tibco Software Inc. Statistica, Version 13; Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA, 2021; Available online: http://statistica.io (accessed on 18 October 2022).
- Krzywiński, M.; Schein, J.; Birol, İ.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Pant, G. Phytoremediation: Low Input-Based Ecological Approach for Sustainable Environment. Appl. Water Sci. 2023, 13, 85. [Google Scholar] [CrossRef]
- Meištininkas, R.; Vaškevičienė, I.; Dikšaitytė, A.; Pedišius, N.; Žaltauskaitė, J. Potential of Eight Species of Legumes for Heavy Fuel Oil-Contaminated Soil Phytoremediation. Sustainability 2023, 15, 4281. [Google Scholar] [CrossRef]
- Latif, A.; Abbas, A.; Iqbal, J.; Azeem, M.; Asghar, W.; Ullah, R.; Bilal, M.; Arsalan, M.; Khan, M.; Latif, R.; et al. Remediation of Environmental Contaminants Through Phytotechnology. Water Air Soil Pollut. 2023, 234, 139. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, H.; Kim, J.-G. Current Status of and Challenges for Phytoremediation as a Sustainable Environmental Management Plan for Abandoned Mine Areas in Korea. Sustainability 2023, 15, 2761. [Google Scholar] [CrossRef]
- Bedair, H.; Ghosh, S.; Abdelsalam, I.M.; Keerio, A.A.; AlKafaas, S.S. Potential Implementation of Trees to Remediate Contaminated Soil in Egypt. Environ. Sci. Pollut. Res. 2022, 29, 78132–78151. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Response of Avena Sativa L. and the Soil Microbiota to the Contamination of Soil with Shell Diesel Oil. Plant Soil Environ. 2018, 64, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Wyszkowski, M.; Ziółkowska, A. Content of Polycyclic Aromatic Hydrocarbons in Soils Polluted with Petrol and Diesel Oil after Remediation with Plants and Various Substances. Plant Soil Environ. 2013, 59, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Panchenko, L.; Muratova, A.; Dubrovskaya, E.; Golubev, S.; Turkovskaya, O. Natural and Technical Phytoremediation of Oil-Contaminated Soil. Life 2023, 13, 177. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Angela, J. Phytoremediation of Crude Oil-Contaminated Soil with Local Plant Species. IOP Conf. Ser. Mater. Sci. Eng. 2019, 495, 012054. [Google Scholar] [CrossRef]
- He, M.; Li, Z.; Chen, C.; Mei, P. Impact of Soil Types and Root Exudates on Cadmium and Petroleum Hydrocarbon Phytoremediation by Sorghum Sudanense, Festuca Arundinace, and Lolium Perenne. Front. Ecol. Evol. 2022, 10, 1036765. [Google Scholar] [CrossRef]
- Lin, M.-S.; Huang, C.-Y.; Lin, Y.-C.; Lin, S.-L.; Hsiao, Y.-H.; Tu, P.-C.; Cheng, P.-C.; Cheng, S.-F. Green Remediation Technology for Total Petroleum Hydrocarbon-Contaminated Soil. Agronomy 2022, 12, 2759. [Google Scholar] [CrossRef]
- Abdel-Moghny, T.; Mohamed, R.S.A.; El-Sayed, E.; Mohammed Aly, S.; Snousy, M.G. Effect of Soil Texture on Remediation of Hydrocarbons-Contaminated Soil at El-Minia District, Upper Egypt. Int. Sch. Res. Notices 2012, 2012, e406598. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Xu, J.; Zhou, J.; Ren, L.; Li, J.; Zhang, Z.; Xia, J.; Xie, H.; Wu, T. Using Sweet Sorghum Varieties for the Phytoremediation of Petroleum-Contaminated Salinized Soil: A Preliminary Study Based on Pot Experiments. Toxics 2023, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Zhou, J.; Wang, Y.; Ai, Y.; Li, X.; Zhang, P.; Zhou, S. Responses of the Root Morphology and Photosynthetic Pigments of Ryegrass to Fertilizer Application under Combined Petroleum-Heavy Metal Stress. Environ. Sci. Pollut. Res. Int. 2022, 29, 87874–87883. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, X.; Fan, X.; Wang, Q.; Liu, Y.; Wei, H.; He, J. Phytoremediation of Crude Oil-Contaminated Sediment Using Suaeda Heteroptera Enhanced by Nereis Succinea and Oil-Degrading Bacteria. Int. J. Phytoremediation 2023, 25, 322–328. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Su, Y.; He, W.; He, F.; Song, H. Phytoremediation of Petroleum Polluted Soil. Pet. Sci. 2008, 5, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Sharonova, N.; Breus, I. Tolerance of Cultivated and Wild Plants of Different Taxonomy to Soil Contamination by Kerosene. Sci. Tot. Environ. 2012, 424, 121–129. [Google Scholar] [CrossRef]
- Malik, B.; Pirzadah, T.B.; Hakeem, K.R. Chapter 20–Phytoremediation of Persistent Organic Pollutants (POPs). In Phytoremediation; Bhat, R.A., Tonelli, F.M.P., Dar, G.H., Hakeem, K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 415–436. ISBN 978-0-323-89874-4. [Google Scholar]
- Koshlaf, E.; Shahsavari, E.; Haleyur, N.; Osborn, A.M.; Ball, A.S. Impact of Necro phytoremediation on Petroleum Hydrocarbon Degradation, Ecotoxicity and Soil Bacterial Community Composition in Diesel-Contaminated Soil. Environ. Sci. Pollut. Res. 2020, 27, 31171–31183. [Google Scholar] [CrossRef]
- Huang, L.; Ye, J.; Jiang, K.; Wang, Y.; Li, Y. Oil Contamination Drives the Transformation of Soil Microbial Communities: Co-Occurrence Pattern, Metabolic Enzymes and Culturable Hydrocarbon-Degrading Bacteria. Ecotoxicol. Environ. Saf. 2021, 225, 112740. [Google Scholar] [CrossRef]
- Udume, O.A.; Abu, G.O.; Stanley, H.O.; Vincent-Akpu, I.F.; Momoh, Y.; Eze, M.O. Biostimulation of Petroleum-Contaminated Soil Using Organic and Inorganic Amendments. Plants 2023, 12, 431. [Google Scholar] [CrossRef]
- Varjani, S.; Upasani, V.N. Comparing Bioremediation Approaches for Agricultural Soil Affected with Petroleum Crude: A Case Study. Indian J. Microbiol. 2019, 59, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Klamerus-Iwan, A.; Błońska, E.; Lasota, J.; Kalandyk, A.; Waligórski, P. Influence of Oil Contamination on Physical and Biological Properties of Forest Soil After Chainsaw Use. Water Air Soil Pollut. 2015, 226, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. The Role of Dactylis Glomerata and Diesel Oil in the Formation of Microbiome and Soil Enzyme Activity. Sensors 2020, 20, 3362. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shen, J.; Liu, Q.; Fang, F.; Cai, H.; Guo, C. Risk Assessment of Petroleum-Contaminated Soil Using Soil Enzyme Activities and Genotoxicity to Vicia Faba. Ecotoxicology 2014, 23, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, S.I.; Tatosyan, M.L.; Aznaur Fyan, D.K. Change in Enzymatic Activity of Common Chernozem Polluted with Crude Oil and Its Products in Model Experiments. Russ. Agricult. Sci. 2007, 33, 318–320. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Oszust, K. Functional Diversity of Fungal Communities in Soil Contaminated with Diesel Oil. Front. Microbiol. 2017, 8, 01862. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lan, T.; Lu, D.; Liu, Z. Ecological and Enzymatic Responses to Petroleum Contamination. Environ. Sci. Process. Impacts 2014, 16, 1501–1509. [Google Scholar] [CrossRef]
- Galiulin, R.V.; Galiulina, R.A. Remediation of Polar Ecosystems Polluted by Gas Condensate and Oil Hydrocarbons by Biological Preparations. Open J. Ecol. 2015, 8, 40–43. [Google Scholar] [CrossRef]
- Serrano, A.; Tejada, M.; Gallego, M.; Gonzalez, J.L. Evaluation of Soil Biological Activity after a Diesel Fuel Spill. Sci. Total. Environ. 2009, 407, 4056–4061. [Google Scholar] [CrossRef]
- Shen, Y.; Ji, Y.; Li, C.; Luo, P.; Wang, W.; Zhang, Y.; Nover, D. Effects of Phytoremediation Treatment on Bacterial Community Structure and Diversity in Different Petroleum-Contaminated Soils. Inter. J. Environ. Res. Public Health 2018, 15, 2168. [Google Scholar] [CrossRef] [Green Version]
- Song, N.; Hursthouse, A.; McLellan, I.; Wang, Z. Treatment of Environmental Contamination Using Sepiolite: Current Approaches and Future Potential. Environ. Geochem. Health 2021, 43, 2679–2697. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.M.; Poolpak, T.; Pokethitiyook, P.; Kruatrachue, M.; Saengwilai, P. Responses of Oil Degrader Enzyme Activities, Metabolism and Degradation Kinetics to Bean Root Exudates during Rhizoremediation of Crude Oil Contaminated Soil. Int. J. Phytoremediation 2022, 24, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Ilyas, N.; Jayachandran, K.; Gaffar, S.; Arshad, M.; Sheeraz Ahmad, M.; Bibi, F.; Jeddi, K.; Hessini, K. Biostimulation Potential of Biochar for Remediating the Crude Oil Contaminated Soil and Plant Growth. Saudi J. Biol. Sci. 2021, 28, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Bandura, L.; Franus, M.; Józefaciuk, G.; Franus, W. Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- Kenes, K.; Yerdos, O.; Zulkhair, M.; Yerlan, D. Study on the effectiveness of thermally treated rice husks for petroleum adsorption. J. Non-Cryst. Solids 2012, 358, 2964–2969. [Google Scholar] [CrossRef]
- Carvalho, M.N.; da Motta, M.; Benachour, M.; Sales, D.C.S.; Abreu, C.A.M. Evaluation of BTEX and phenol removal from aqueous solution by multi-solute adsorption onto smectite organoclay. J. Hazard. Mater. 2012, 239, 95–101. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Remediation of Soil Contaminated with Diesel Oil. J. Elem. 2018, 23, 767–788. [Google Scholar] [CrossRef]
- De Lima, J.A.; Camilo, F.F.; Faez, R.; Cruz, S.A. A New Approach to Sepiolite Dispersion by Treatment with Ionic Liquids. Appl. Clay Sci. 2017, 143, 234–240. [Google Scholar] [CrossRef]

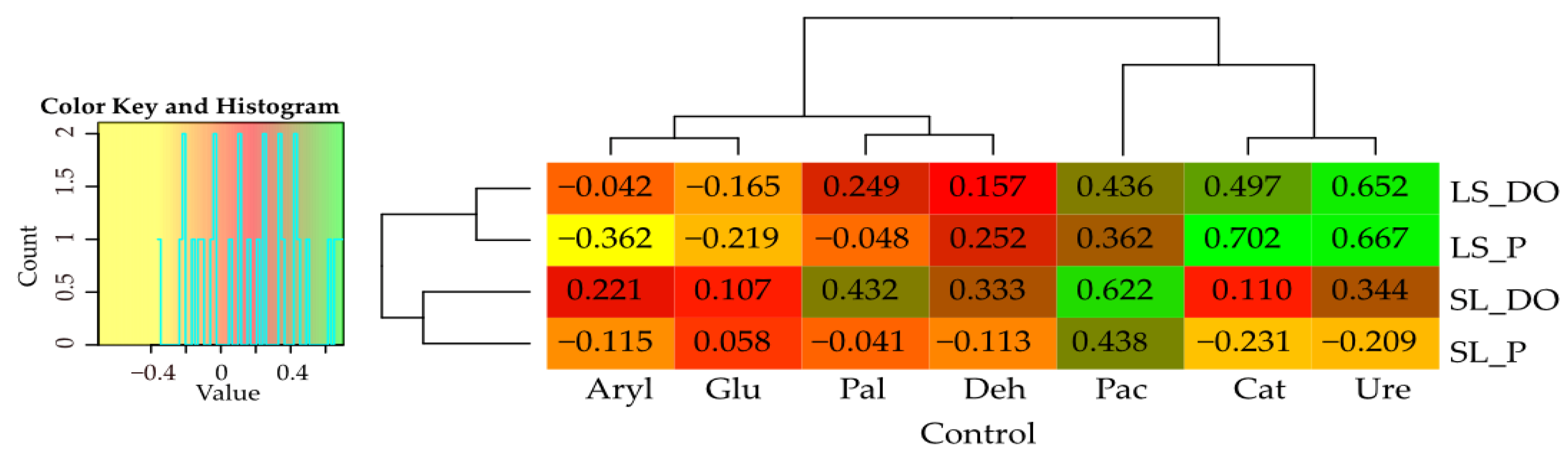
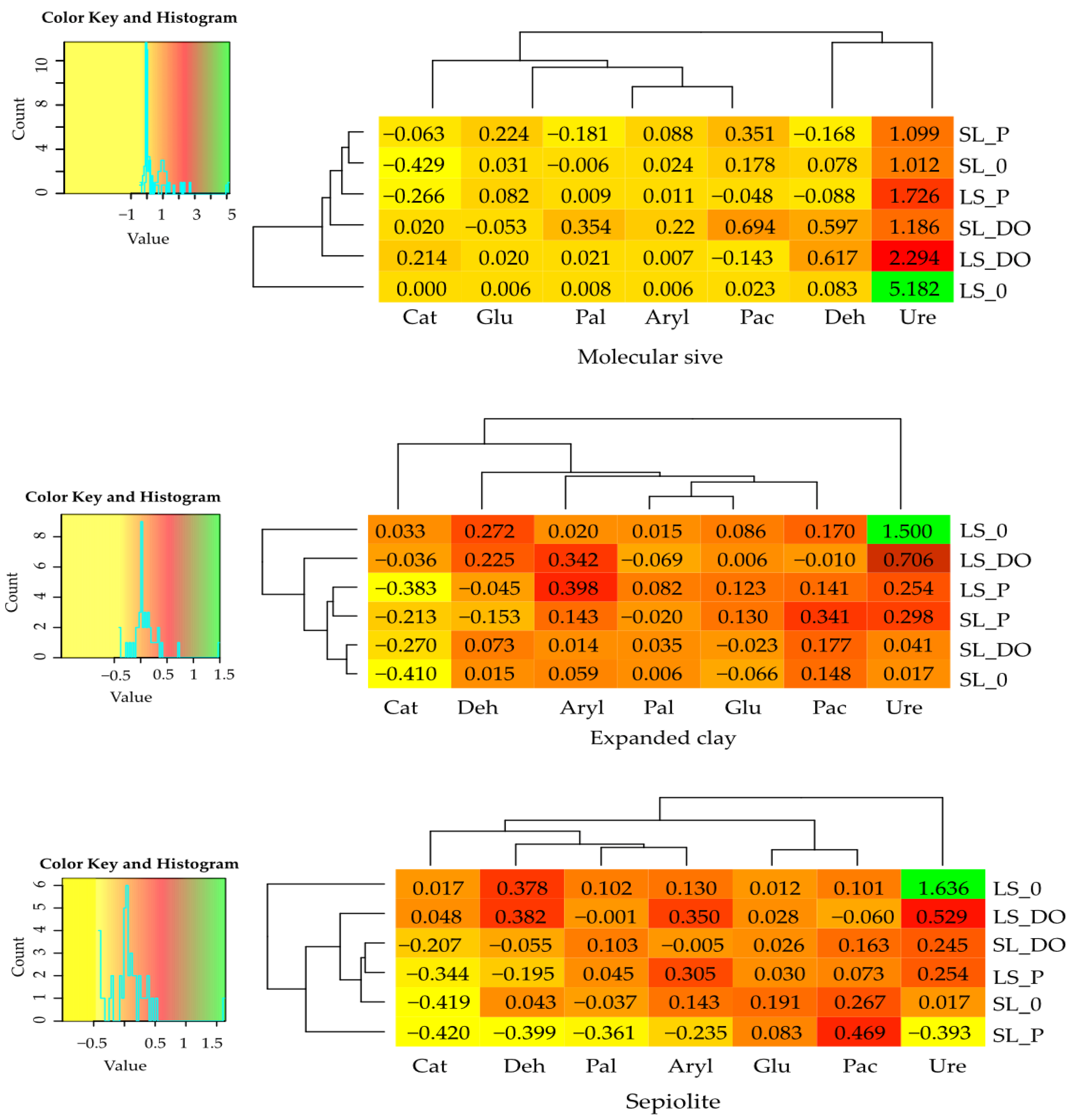

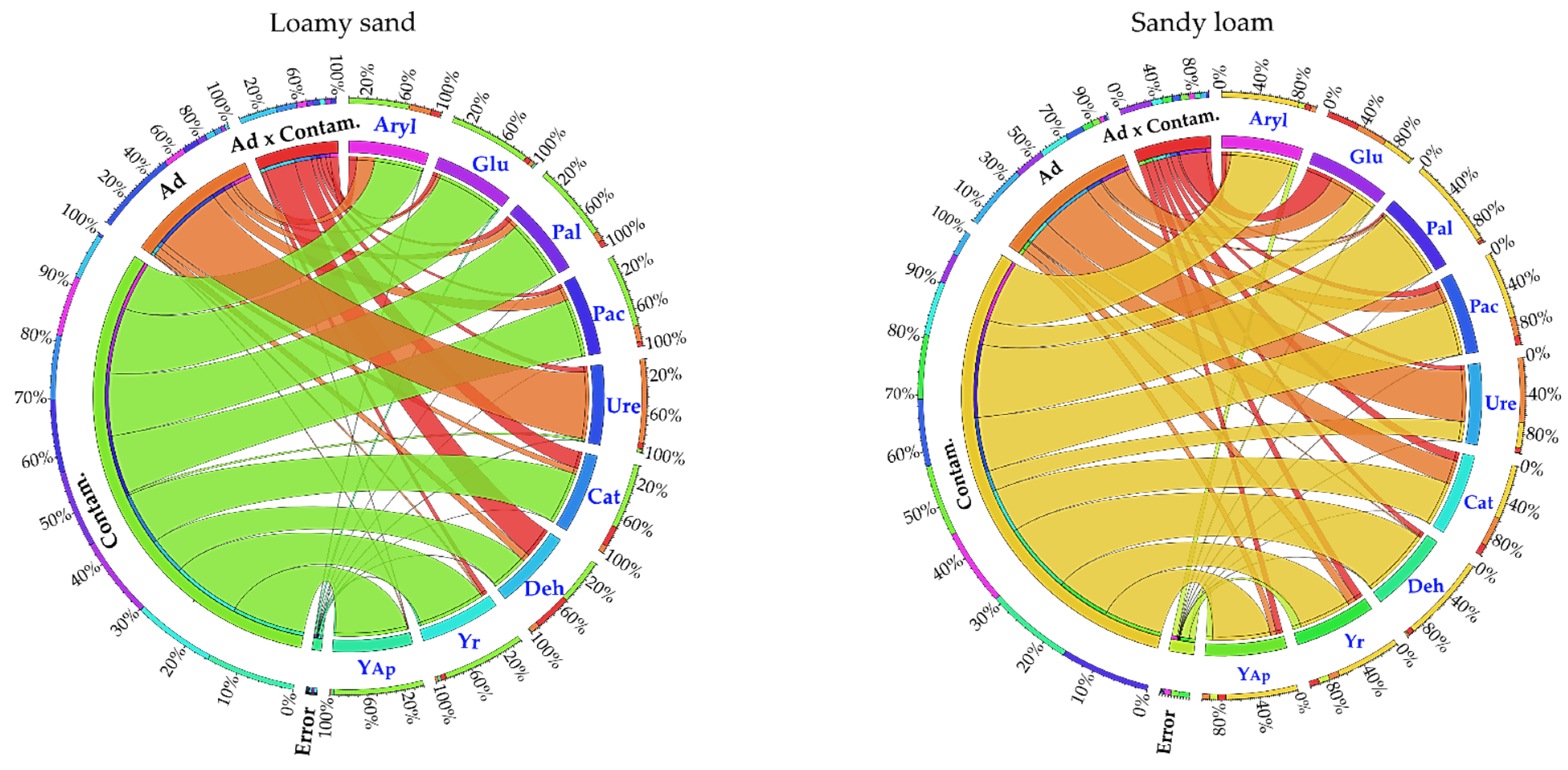
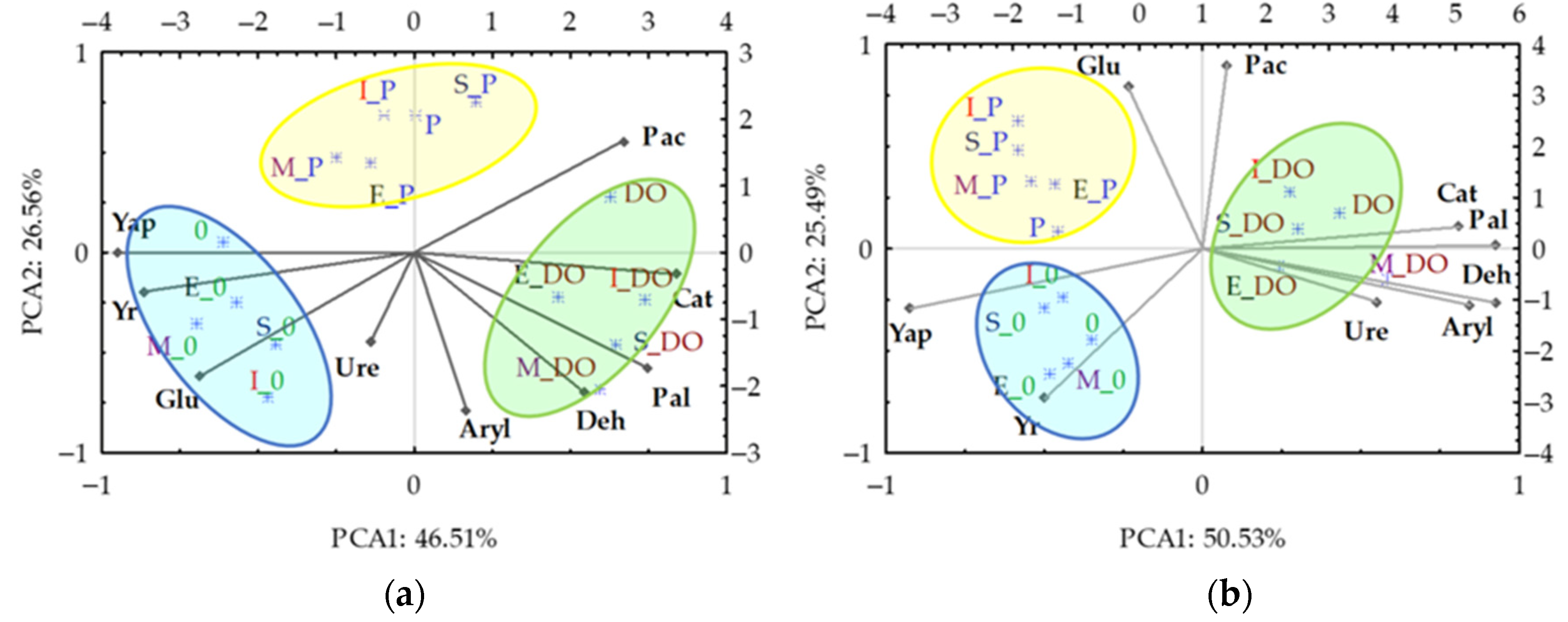
| Parameter | Description |
|---|---|
| Loamy sand (LS) | Sandy loam: sand 0.05–2.0 mm—74.30%; silt 0.02–0.05 mm—23.69%; and clay < 0.002 mm—2.01%. 0.89 g Ntot kg−1 d.m.; 11.20 g Corg kg−1 d.m.; 164.05 mg P kg−1 d.m.; 53.95 mg K kg−1 d.m.; 46.00 mg Mg kg−1 d.m.; pHKCl—6.98; EBC—84.20 mmol (+) kg−1 d.m.; HAC—8.00 mmol (+) kg−1 d.m.; CEC—92.20 mmol (+) kg−1 d.m.; ACS—91.32%. |
| Sandy loam (SL) | Sandy loam: sand 0.05–2.0 mm—70.38%; silt 0.02–0.05 mm—27.19%; and clay < 0.002 mm—2.43%. 1.01 g Ntot kg−1 d.m.; 11.50 g Corg kg−1 d.m.; 172.73 mg P kg−1 d.m.; 78.85 mg K kg−1 d.m.; 38.00 mg Mg kg−1 d.m.; pHKCl—7.13; EBC—181.80 mmol (+) kg−1 d.m.; HAC—5.70 mmol (+) kg−1 d.m.; CEC—187.50 mmol (+) kg−1 d.m.; ACS—96.96%. |
| Molecular sieve (M) | Hydrated aluminosilicate with a micropore size of 0.3 nm. Silosiv A3 manufactured by Grace, Columbia, SC, USA, was used in the study. It is a zeolite with a three-dimensional pore system with pHKCl = 8.5. |
| Expanded clay (E) | Lightweight ceramic aggregate produced by firing loamy clay at a temperature of about 1200 °C. It is characterized by high porosity. The aggregate used in the study was obtained from Garden Guru (Piła, Poland). It consists of 85% of particles with a size of 75 to 710 μm, with a pHKCl of 7.1. |
| Sepiolite (S) | Naturally occurring loamy material (Mg4[Si6O15(OH)2]·6H2O) with a pore diameter of 1.4 nm. The study was conducted using a Sepiolite 60/100, made by the Sepiolsa Minersa Group (Guadalajara, Spain). |
| Ikasorb (I) | Granulate with granule diameters of 0.3–1 mm. Porous material characterized by high sorption capacity were derived from roasted diatomaceous earth. The study was conducted with Ikasorb 1850 sorbent purchased from Ikaros (Espoo, Finland) |
| Diesel oil VERVA (DO) | Fuel for the latest generation compression ignition engines. This product meets the stringent European requirements for the so-called “sulfur-free” fuels. Diesel oil density is 820–845 kg m−3. It contains a mixture of C9–C25 petroleum-derived hydrocarbons. Diesel oil was purchased at a PKN Orlen (Olsztyn, Poland) gas station. The detailed characteristics of VERVA diesel oil is provided on the following website: http://www.orlen.pl/ (accessed on 5 March 2023) |
| petrol VERVA 98 (P) | Unleaded motor petrol with octane number 98. Its density is 720–775 kg m−3. It was purchased at a PKN Orlen (Poland) gas station. Its detailed characteristics are available on the following website: http://www.orlen.pl/ (accessed on 5 March 2023) |
| The Design of the Greenhouse Experiment with Zea mays | |
| Experiment Steps/Factors | Treatments |
| 1 factor soil type | The experiment was carried out in 3.0 dm3 polyethylene pots with 2.5 kg portions of loamy sand (LS) or sandy loam (SL). Before the physicochemical analyses, the soil was air-dried and sieved through a screen with a 5 mm mesh diameter. |
| 2 factor Soil contamination with of diesel oil (DO) or petrol (P) | Selected soil samples were thoroughly mixed with DO or P in doses of 0 and 7 cm3 of petroleum substances kg−1 d.m. of soil and with mineral fertilizers meeting nutritional demands of Zea mays and were placed in each pot. |
| Mineral fertilization | Nitrogen (112 mg N kg−1 d.m. of soil) was applied in the form of N2H4CO, phosphorus (39 mg P kg−1 d.m. of soil) in the form of KH2PO4 and potassium (112 mg K kg−1 d.m. of soil); likewise, the dose of phosphorus, in the form of KH2PO4 up to 112 mg kg−1 d.m. of soil was completed with KCl. Zea mays was also fertilized with a magnesium dose of 15 mg kg−1 d.m. of soil, in the form of MgSO4 × 7H2O. |
| 3 factor sorbent type | All sorbents, namely control, molecular sieve, expanded clay, sepiolite and Ikasorb, were used in amounts of 10 g·kg−1 d.m. of soil. |
| Maximum water capacity | Subsequently, 7 cm3 of a suitable petroleum substance, measured with a cylinder, was added to the individual pots and mixed with the soil material in order to homogenize the samples. Once the soil had been packed in pots, its moisture content was brought to 60% of the maximum water capacity. |
| An experimental plant | After 7 days, the soil was sown with eight seeds of Zea mays PR39H32 (variety registered in the European Union). Five plants were left in each pot after emergence. Vegetation growth was continued for 60 days, while maintaining the stable moisture content of the soil using distilled water. The pots were located in random complete blocks on tables. |
| Objects | Loamy Sand (LS) | Sandy Loam (SL) | ||||
|---|---|---|---|---|---|---|
| Aerial Parts (Ap) | Roots (R) | Ap/R | Aerial Parts (Ap) | Roots (R) | Ap/R | |
| g d.m. of pot−1 | g d.m. of pot−1 | |||||
| C | 40.712 a | 8.818 a | 4.617 | 46.708 a | 8.533 a | 5.474 |
| DO | 4.903 c | 1.656 c | 2.961 | 28.122 c | 4.513 c | 6.231 |
| P | 33.378 b | 6.826 b | 4.890 | 42.422 b | 5.723 b | 7.413 |
| Average | 26.331 | 5.767 | 4.566 | 39.084 | 6.256 | 6.247 |
| Object | Loamy Sand (LS) | Sandy Loam (SL) | ||||
|---|---|---|---|---|---|---|
| Aerial Parts (Ap) | Roots (R) | Ad/R | Aerial Parts (Ap) | Roots (R) | Ad/R | |
| g d.m. of pot−1 | g d.m. of pot−1 | |||||
| C | 40.712 c | 8.818 b | 4.617 | 46.708 c | 8.533 ab | 5.474 |
| M | 48.363 a | 9.074 b | 5.330 | 52.658 a | 8.646 ab | 6.090 |
| E | 44.856 b | 9.211 ab | 4.870 | 47.138 c | 9.489 a | 4.968 |
| S | 41.859 c | 9.699 ab | 4.316 | 49.990 b | 8.854 ab | 5.646 |
| I | 41.255 c | 10.369 a | 3.979 | 47.779 c | 7.986 b | 5.983 |
| Average | 43.409 | 9.434 | 4.622 | 48.855 | 8.702 | 5.614 |
| Object | Loamy Sand (LS) | Sandy Loam (SL) | ||||
|---|---|---|---|---|---|---|
| Aerial Parts (Ap) | Roots (R) | Ad/R | Aerial Parts (Ap) | Roots (R) | Ad/R | |
| g d.m. of pot−1 | g d.m. of pot−1 | |||||
| C | 4.903 c | 1.656 c | 2.961 | 28.122 bc | 4.513 c | 6.231 |
| M | 10.249 a | 5.629 a | 1.821 | 26.434 c | 4.982 c | 5.306 |
| E | 5.417 bc | 5.720 a | 0.947 | 32.500 a | 7.603 a | 4.275 |
| S | 6.012 b | 3.712 b | 1.620 | 32.494 a | 6.223 b | 5.222 |
| I | 6.205 b | 4.501 b | 1.379 | 31.449 ab | 6.192 b | 5.079 |
| Average | 6.557 | 4.244 | 1.745 | 30.200 | 5.903 | 5.222 |
| Objects | Loamy Sand (LS) | Sandy Loam (SL) | ||||
|---|---|---|---|---|---|---|
| Aerial Parts (Ap) | Roots (R) | Ad/R | Aerial Parts (Ap) | Roots (R) | Ad/R | |
| g d.m. of pot−1 | g d.m. of pot−1 | |||||
| C | 33.378 b | 6.826 b | 4.890 | 42.422 bc | 5.723 c | 7.413 |
| M | 35.863 ab | 7.424 b | 4.831 | 47.003 a | 6.572 a | 7.152 |
| E | 33.542 b | 8.555 a | 3.921 | 39.426 c | 6.226 abc | 6.332 |
| S | 34.018 b | 7.634 ab | 4.456 | 46.042 a | 6.415 ab | 7.177 |
| I | 38.242 a | 6.767 b | 5.651 | 44.933 ab | 5.934 bc | 7.572 |
| Average | 35.009 | 7.441 | 4.750 | 43.965 | 6.174 | 7.129 |
| Kind of Soil | Object | Deh μM TFF | Cat M O2 | Ure mM N-NH4 | Pac | Pal | Glu | Aryl mM PNS |
|---|---|---|---|---|---|---|---|---|
| mM PNP | ||||||||
| Loamy sand (LS) | 0 | 5.901 c | 0.227 c | 0.160 b | 2.473 c | 2.086 b | 0.571 a | 0.401 a |
| DO | 6.824 b | 0.339 b | 0.264 b | 3.551 a | 2.606 a | 0.476 b | 0.385 b | |
| P | 7.388 a | 0.386 a | 0.266 a | 3.369 b | 1.986 c | 0.446 c | 0.256 c | |
| Average | 6.705 | 0.317 | 0.230 | 3.131 | 2.226 | 0.498 | 0.347 | |
| Sandy loam (SL) | 0 | 8.550 b | 0.393 b | 1.238 b | 1.011 c | 1.619 b | 0.986 b | 0.765 b |
| DO | 11.395 a | 0.437 a | 1.664 a | 1.640 a | 2.318 a | 1.092 a | 0.934 a | |
| P | 7.588 c | 0.303 c | 0.979 c | 1.454 b | 1.553 b | 1.044 ab | 0.677 c | |
| Average | 9.178 | 0.378 | 1.294 | 1.368 | 1.830 | 1.040 | 0.792 | |
| Type of Soil | Object | Deh μM TFF | Cat M O2 | Ure mM N-NH4 | Pac | Pal | Glu | Aryl mM PNS |
|---|---|---|---|---|---|---|---|---|
| mM PNP | ||||||||
| Loamy sand (LS) | C | 5.901 b | 0.227 b | 0.160 d | 2.473 e | 2.086 c | 0.571 b | 0.401 b |
| M | 6.392 b | 0.227 b | 0.988 a | 2.530 d | 2.104 c | 0.574 b | 0.404 b | |
| E | 7.509 a | 0.234 ab | 0.399 c | 2.893 a | 2.117 c | 0.620 a | 0.409 b | |
| S | 8.130 a | 0.231 b | 0.421 c | 2.724 c | 2.299 b | 0.577 b | 0.453 a | |
| I | 8.096 b | 0.246 a | 0.595 b | 2.842 b | 2.392 a | 0.632 a | 0.477 a | |
| Average | 7.206 | 0.233 | 0.513 | 2.692 | 2.200 | 0.595 | 0.429 | |
| Sandy loam (SL) | C | 8.550 b | 0.393 a | 1.238 b | 1.011 d | 1.619 a | 0.986 c | 0.765 b |
| M | 9.222 a | 0.225 c | 2.491 a | 1.191 c | 1.609 a | 1.016 c | 0.783 b | |
| E | 8.678 b | 0.232 bc | 1.260 b | 1.160 c | 1.628 a | 0.921 d | 0.810 b | |
| S | 8.919 ab | 0.229 c | 1.260 b | 1.281 b | 1.559 b | 1.174 a | 0.874 a | |
| I | 8.720 b | 0.244 b | 1.325 b | 1.393 a | 1.654 a | 1.087 b | 0.818 b | |
| Average | 8.818 | 0.265 | 1.515 | 1.207 | 1.614 | 1.037 | 0.810 | |
| Type of Soil | Object | Deh μM TFF | Cat M O2 | Ure mM N-NH4 | Pac | Pal | Glu | Aryl mM PNS |
|---|---|---|---|---|---|---|---|---|
| mM PNP | ||||||||
| Loamy sand (LS) | C | 6.824 d | 0.339 c | 0.264 c | 3.551 b | 2.606 c | 0.476 a | 0.385 c |
| M | 11.033 a | 0.412 a | 0.869 a | 3.044 d | 2.662 b | 0.486 a | 0.387 c | |
| E | 8.357 c | 0.327 d | 0.450 b | 3.517 b | 2.427d | 0.479 a | 0.516 a | |
| S | 9.429 b | 0.356 b | 0.404 b | 3.338 c | 2.604 c | 0.490 a | 0.519 a | |
| I | 9.144 b | 0.408 a | 0.404 b | 3.608 a | 2.713 a | 0.495 a | 0.422 b | |
| Average | 8.957 | 0.368 | 0.478 | 3.412 | 2.602 | 0.485 | 0.446 | |
| Sandy loam (SL) | C | 11.395 bc | 0.437 a | 1.664 c | 1.640 c | 2.318 c | 1.092 bc | 0.934 a |
| M | 13.653 a | 0.401 b | 2.707 a | 1.712 bc | 2.193 d | 0.934 d | 0.933 a | |
| E | 12.223 b | 0.319 d | 1.732 c | 1.930 a | 2.399 b | 1.067 c | 0.947 a | |
| S | 10.765 c | 0.346 c | 2.072 b | 1.906 a | 2.558 a | 1.120 b | 0.929 a | |
| I | 12.558 ab | 0.398 b | 1.437 d | 1.822 ab | 2.432 b | 1.257 a | 0.882 a | |
| Average | 12.119 | 0.380 | 1.922 | 1.802 | 2.380 | 1.094 | 0.925 | |
| Type of Soil | Object | Deh μM TFF | Cat M O2 | Ure mM N-NH4 | Pac | Pal | Glu | Aryl mM PNS |
|---|---|---|---|---|---|---|---|---|
| mM PNP | ||||||||
| Loamy sand (LS) | C | 7.388 a | 0.386 a | 0.266 c | 3.369 c | 1.986 d | 0.446 c | 0.256 c |
| M | 6.736 b | 0.283 b | 0.726 a | 3.208 d | 2.003 d | 0.483 ab | 0.259 c | |
| E | 7.058 ab | 0.238 d | 0.334 c | 3.842 a | 2.148 b | 0.501 a | 0.358 a | |
| S | 5.945 c | 0.253 cd | 0.334 c | 3.616 b | 2.076 c | 0.459 bc | 0.334 ab | |
| I | 5.616 c | 0.261 c | 0.421 b | 3.640 b | 2.198 a | 0.456 bc | 0.319 b | |
| Average | 6.549 | 0.284 | 0.416 | 3.535 | 2.082 | 0.469 | 0.305 | |
| Sandy loam (SL) | C | 7.588 a | 0.303 a | 0.979 c | 1.454 d | 1.553 a | 1.044 c | 0.677 b |
| M | 6.312 c | 0.283 b | 2.055 a | 1.964 c | 1.271 b | 1.277 a | 0.737 ab | |
| E | 6.426 c | 0.238 d | 1.271 b | 1.949 c | 1.522 a | 1.180 b | 0.774 a | |
| S | 6.854 b | 0.253 c | 1.009 c | 2.409 a | 1.482 b | 1.183 b | 0.714 ab | |
| I | 6.732 b | 0.261 c | 1.075 c | 2.328 b | 1.560 a | 1.278 a | 0.675 b | |
| Average | 6.782 | 0.268 | 1.278 | 2.021 | 1.478 | 1.192 | 0.715 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The Usability of Sorbents in Restoring Enzymatic Activity in Soils Polluted with Petroleum-Derived Products. Materials 2023, 16, 3738. https://doi.org/10.3390/ma16103738
Wyszkowska J, Borowik A, Zaborowska M, Kucharski J. The Usability of Sorbents in Restoring Enzymatic Activity in Soils Polluted with Petroleum-Derived Products. Materials. 2023; 16(10):3738. https://doi.org/10.3390/ma16103738
Chicago/Turabian StyleWyszkowska, Jadwiga, Agata Borowik, Magdalena Zaborowska, and Jan Kucharski. 2023. "The Usability of Sorbents in Restoring Enzymatic Activity in Soils Polluted with Petroleum-Derived Products" Materials 16, no. 10: 3738. https://doi.org/10.3390/ma16103738





