Recent Advances in Carbon Nitride-Based S-scheme Photocatalysts for Solar Energy Conversion
Abstract
:1. Introduction
2. Preparation of Carbon Nitride-Based S-scheme Photocatalyst
2.1. Design Principles

2.2. Preparation of Carbon Nitride-Based S-scheme Photocatalysts
2.3. Techniques for the Identification of Carbon Nitride-Based S-scheme Photocatalysts
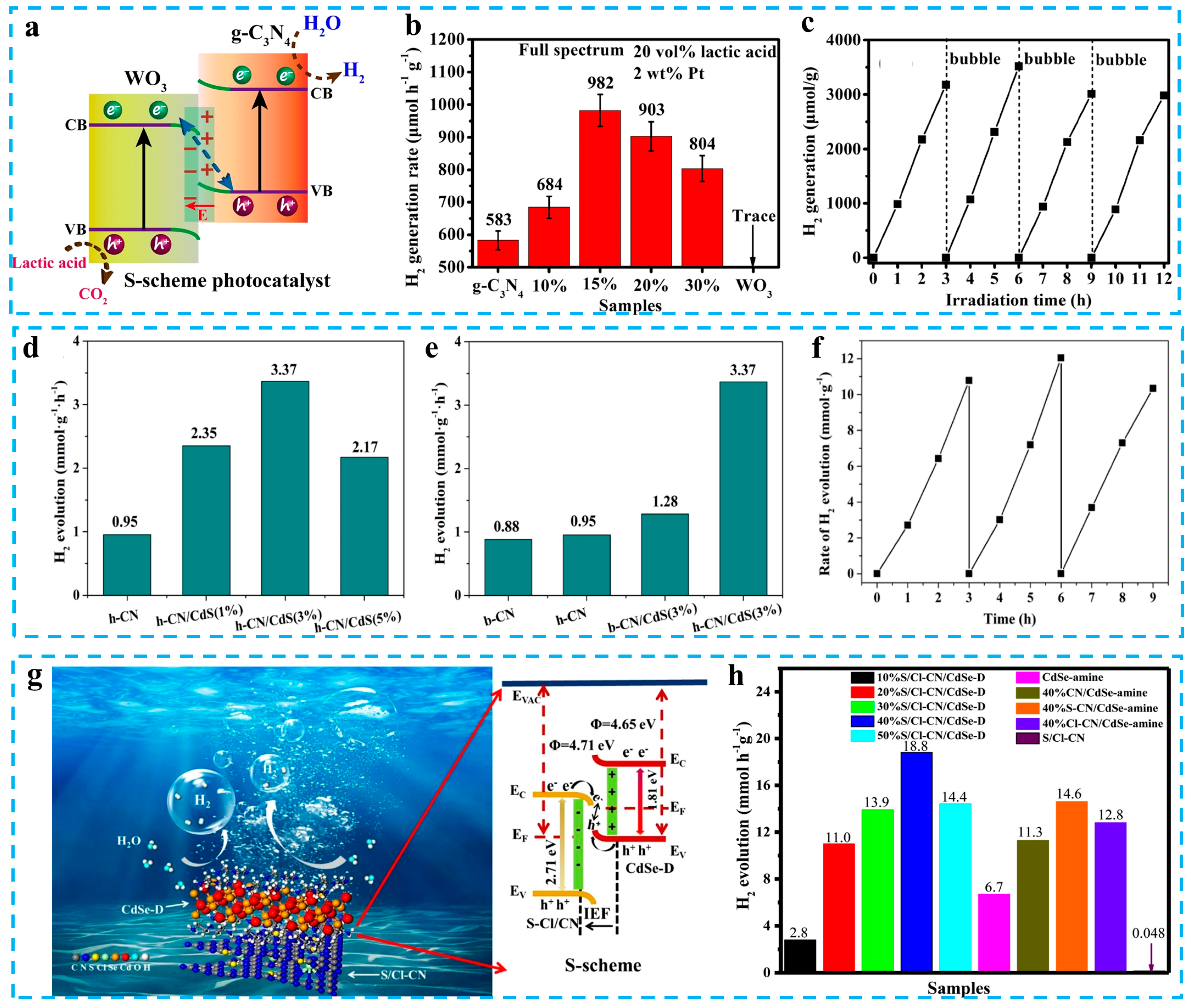
3. Application of Carbon Nitride-Based S-scheme Photocatalysts in Solar Energy Conversion
3.1. Photocatalytic H2 Evolution
3.2. Photocatalytic CO2 Reduction
3.3. Other Applications of Carbon Nitride-Based S-scheme Photocatalysts
4. Conclusions and Outlook
- The carbon nitride has a rather negative CB potential, so carbon nitride generally acts as a reduction photocatalyst matched to oxidation photocatalysts with a wide band gap to construct S-scheme heterojunctions. However, this design scheme is not conducive to improving the spectral response of heterojunction photocatalysts. The band structure of carbon nitride can be continuously and controllably adjusted via doping engineering [129,130]. The carbon nitride is expected to be used as oxidation semiconductors to construct broad-spectrum response S-scheme heterojunctions with narrow bandgap semiconductors such as Bi2S3 and CuInS2;
- The preparation method of a carbon nitride-based S-scheme photocatalyst often requires complex steps, so it is difficult to achieve large-scale production. In addition, conventional assembly methods, such as electrostatic self-assembly/physical adsorption, may also suffer from inadequate contact between the two components. Therefore, the simple one-step method or in situ growth process needs to be further studied. It is also necessary to expand the contact interface of the heterojunction, simplify the processes, and reduce the preparation cost of the photocatalyst;
- The related characterization techniques of S-scheme heterojunctions still need to be further developed. The built-in electric field is the key driver for rapid charge separation in S-scheme photocatalysts, but conventional characterization methods make it difficult to accurately characterize the strength of the internal electric field and its position relative to the active center. Li et al. used time-resolved photoelectron microscopy and surface photovoltage microscopy to directly observe the charge transfer characteristics in nanoseconds. [131,132] These advanced in situ characterization techniques are expected to further reveal the action mechanism of an internal electric field in a carbon nitride-based S-scheme photocatalyst;
- Photocatalytic solar energy conversion is a complex process. The carbon nitride-based S-scheme photocatalyst system usually includes defect engineering, surface plasmon resonance, photothermal effect, and so on. The synergistic mechanism of different factors needs to be further explored in depth;
- The carbon nitride-based S-scheme photocatalyst has many advantages in solar energy conversion. However, there is still a big gap between its catalytic efficiency and the requirements of commercial application. Therefore, it is necessary to explore more efficient improvements of carbon nitride-based S-scheme photocatalysts, such as photo-electric synergy, photo-thermal synergy, and photo-magnetic synergy, in order to substantially enhance the catalytic efficiency of carbon nitride-based S-scheme photocatalysts.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; López-Duarte, I.; Charalambidis, G.; Ladomenou, K.; Ince, M.; Coutsolelos, A.G. Porphyrins and phthalocyanines as biomimetic tools for photocatalytic H2 production and CO2 reduction. Chem. Soc. Rev. 2022, 51, 6965–7045. [Google Scholar] [CrossRef]
- Andrei, V.; Ucoski, G.M.; Pornrungroj, C.; Uswachoke, C.; Wang, Q.; Achilleos, D.S.; Kasap, H.; Sokol, K.P.; Jagt, J.A.; Lu, H.; et al. Floating perovskite-BiVO4 devices for scalable solar fuel production. Nature 2022, 608, 518–522. [Google Scholar] [CrossRef]
- Fang, Y.; Hou, Y.; Fu, X.; Wang, X. Semiconducting polymers for oxygen evolution reaction under light illumination. Chem. Rev. 2022, 122, 4204–4256. [Google Scholar] [CrossRef]
- Kosco, J.; Gonzalez-Carrero, S.; Howells, C.T.; Fei, T.; Dong, Y.; Sougrat, R.; Harrison, G.T.; Firdaus, Y.; Sheelamanthula, R.; Purushothaman, B.; et al. Generation of long-lived charges in organic semiconductor heterojunction nanoparticles for efficient photocatalytic hydrogen evolution. Nat. Energy 2022, 7, 340–351. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Wrede, S.; Liu, A.; Brnovic, A.; Sicong Wang, S.; Axelssona, M.; Tian, H. Preparation, characterization, evaluation and mechanistic study of organic polymer nano-photocatalysts for solar fuel production. Chem. Soc. Rev. 2022, 51, 6909–6935. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent advances and perspectives for solar-driven water splitting using particulate photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.C.; Bayarkhuu, B.; Zhang, K.A.I.; Lee, D.K.; Byun, J. Solar-driven H2O2 production via cooperative auto-and photocatalytic oxidation in fine-tuned reaction media. Energy Environ. Sci. 2022, 15, 5082–5092. [Google Scholar] [CrossRef]
- Song, L.; Wang, W.; Yue, J.P.; Jiang, Y.X.; Wei, M.K.; Zhang, H.P.; Yan, S.S.; Liao, L.L.; Yu, D.G. Visible-light photocatalytic di-and hydro-carboxylation of unactivated alkenes with CO2. Nat. Catal. 2022, 5, 832–838. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Dolete, G.; Croitoru, A.-M.; Surdu, V.-A.; Trușcă, R.-D.; Motelica, L.; Oprea, O.-C.; Ficai, D.; Ficai, A.; et al. Preparation and Characterization of Chitosan/TiO2 Composite Membranes as Adsorbent Materials for Water Purification. Membranes 2022, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shao, W.; Li, X.; Jiao, X.; Zhu, J.; Sun, Y.; Xie, Y. Asymmetric triple-atom sites confined in ternary oxide enabling selective CO2 photothermal reduction to acetate. J. Am. Chem. Soc. 2021, 143, 18233–18241. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, S.; Fischer, K.; Schulze, A.; Schäfer, A.I. Photocatalytic degradation of steroid hormone micropollutants by TiO2-coated polyethersulfone membranes in a continuous flow-through process. Nat. Nanotechnol. 2022, 17, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, J.; Li, Y.; Cao, H. Highly efficient UV-visible-infrared light-driven photothermocatalytic steam biomass reforming to H2 on Ni nanoparticles loaded on mesoporous silica. Energy Environ. Sci. 2022, 15, 3041–3050. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, G.; Wang, S.; Li, Y.; Guo, Y.; Luan, D.; Gu, X.; Lou, X.W. Implanting CoOx Clusters on Ordered Macroporous ZnO Nanoreactors for Efficient CO2 Photoreduction. Adv. Mater. 2022, 34, 2204865. [Google Scholar] [CrossRef]
- Yoshino, S.; Iwase, A.; Yamaguchi, Y.; Suzuki, T.M.; Morikawa, T.; Kudo, A. Photocatalytic CO2 reduction using water as an electron donor under visible light irradiation by Z-scheme and photoelectrochemical systems over (CuGa)0.5ZnS2 in the presence of basic additives. J. Am. Chem. Soc. 2022, 144, 2323–2332. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Miao, Y.; Zhou, C.; Zhang, L.P.; Wu, L.Z.; Zhang, T. Accelerating Electron-Transfer Dynamics by TiO2-Immobilized Reversible Single-Atom Copper for Enhanced Artificial Photosynthesis of Urea. Adv. Mater. 2022, 34, 2207793. [Google Scholar] [CrossRef]
- Song, S.; Qu, J.; Han, P.; Hülsey, M.J.; Zhang, G.; Wang, Y.; Wang, S.; Chen, D.; Lu, J.; Yan, N. Visible-light-driven amino acids production from biomass-based feedstocks over ultrathin CdS nanosheets. Nat. Commun. 2020, 11, 4899. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Wang, H.; Xiao, B.; Zhang, W.; Zhao, X.; Lv, T.; Thangamuthu, M.; Zhang, J.; Guo, Y.; et al. Single-atom Cu anchored catalysts for photocatalytic renewable H2 production with a quantum efficiency of 56%. Nat. Commun. 2022, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, M.; Wang, F. Oxygen-controlled photo-reforming of biopolyols to CO over Z-scheme CdS@g-C3N4. Chem 2022, 8, 465–479. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zhang, Q.; Yang, H.; Kato, K.; Yang, W.; Lu, Y.-R.; Liu, S.; Wang, C.; Yamakata, A.; Su, C.; et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 2021, 4, 374–384. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Z.; Gao, X.; Hong, X.; Zhu, J.; Wang, G.; Wu, Y.; Zeng, J.; Zheng, X. Synergy between palladium single atoms and nanoparticles via hydrogen spillover for enhancing CO2 photoreduction to CH4. Adv. Mater. 2022, 34, 2200057. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, K.; Liu, X.H.; Zhang, X.; Huang, H. P-Mediated Cu-N4 Sites in Carbon Nitride Realizing CO2 Photoreduction to C2H4 with Selectivity Modulation. Adv. Mater. 2023, 35, 2208132. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, Y.; Liu, Y.; Vequizo, J.J.M.; Sun, H.; Yang, C.; Yamakata, A.; Fan, F.; Lin, W.; Wang, X.; et al. Heteroatom dopants promote two-electron O2 reduction for photocatalytic production of H2O2 on polymeric carbon nitride. Angew. Chem. Int. Ed. 2020, 132, 16343–16351. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, W.; Fang, J.; Liu, Z.; Chen, D.; Pan, T.; Yu, Y.; Fang, Z. Highly dispersed bismuth oxide quantum dots/graphite carbon nitride nanosheets heterojunctions for visible light photocatalytic redox degradation of environmental pollutants. Appl. Catal. B Environ. 2021, 295, 120279. [Google Scholar] [CrossRef]
- Wang, X.; Hai, G.; Li, B.; Luan, Q.; Dong, W.; Wang, G. Construction of dual-Z-scheme WS2-WO3·H2O/g-C3N4 catalyst for photocatalytic H2 evolution under visible light. Chem. Eng. J. 2021, 426, 130822. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Q.; Song, M.; Li, L.; Li, Q.; Shang, J.K. Directing photocatalytic pathway to exceedingly high antibacterial activity in water by functionalizing holey ultrathin nanosheets of graphitic carbon nitride. Water Res. 2021, 198, 117125. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fan, M.; Yang, W.; Xiao, Y.; Zeng, L.; Wu, X.; Xu, Q.; Su, C.; He, Q. Homogeneous carbon/potassium-incorporation strategy for synthesizing red polymeric carbon nitride capable of near-infrared photocatalytic H2 production. Adv. Mater. 2021, 33, 2101455. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Li, L. Photocatalytic overall water splitting of carbon nitride by band-structure modulation. Matter 2021, 4, 1765–1767. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Wu, H.; Xue, J.; Ding, L.; Wang, R.; Wang, H. Fast hydrogen purification through graphitic carbon nitride nanosheet membranes. Nat. Commun. 2022, 13, 5852. [Google Scholar] [CrossRef]
- Chen, L.; Liang, X.; Wang, H.; Xiao, Q.; Qiu, X. Ultra-thin carbon nitride nanosheets for efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 442, 136115. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.; Wang, C.; Gan, L.; Chen, X.; Zhang, P.; Wang, Y.; Li, H.; Wang, L.; Zhou, X.; et al. Unraveling the dual defect sites in graphite carbon nitride for ultra-high photocatalytic H2O2 evolution. Energy Environ. Sci. 2022, 15, 830–842. [Google Scholar] [CrossRef]
- Zhang, J.; Lang, J.; Wei, Y.; Zheng, Q.; Liu, L.; Hu, Y.H.; Zhou, B.; Yuan, C.; Long, M. Efficient photocatalytic H2O2 production from oxygen and pure water over graphitic carbon nitride decorated by oxidative red phosphorus. Appl. Catal. B Environ. 2021, 298, 120522. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Chu, C.; Miao, W.; Li, Q.; Wang, D.; Liu, Y.; Mao, S. Highly efficient photocatalytic H2O2 production with cyano and SnO2 co-modified g-C3N4. Chem. Eng. J. 2022, 428, 132531. [Google Scholar] [CrossRef]
- Xu, L.; Tian, B.; Wang, T.; Yu, Y.; Wu, Y.; Cui, J.; Cao, Z.; Wu, J.; Zhang, W.; Zhang, P.; et al. Direct Z-scheme polymeric heterojunction boosts photocatalytic hydrogen production via a rebuilt extended π-delocalized network. Energy Environ. Sci. 2022, 15, 5059–5068. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Ma, T.; Zhang, Y.; Huang, H. 2D graphitic carbon nitride for energy conversion and storage. Adv. Funct. Mater. 2021, 31, 2102540. [Google Scholar] [CrossRef]
- Xie, P.; Ding, J.; Yao, Z.; Pu, T.; Zhang, P.; Huang, Z.; Wang, C.; Zhang, J.; Zecher-Freeman, N.; Zong, H.; et al. Oxo dicopper anchored on carbon nitride for selective oxidation of methane. Nat. Commun. 2022, 13, 1375. [Google Scholar] [CrossRef]
- Balu, S.; Chen, Y.L.; Chen, S.W.; Yang, T.C.K. Rational synthesis of BixFe1−xVO4 heterostructures impregnated sulfur-doped g-C3N4: A visible-light-driven type-II heterojunction photo (electro) catalyst for efficient photodegradation of roxarsone and photoelectrochemical OER reactions. Appl. Catal. B Environ. 2022, 304, 120852. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, P.; Wang, L.; Wang, S.; Shi, J.; Lan, X. Electronegativity Assisted Synthesis of Magnetically Recyclable Ni/NiO/g-C3N4 for Significant Boosting H2 Evolution. Materials 2021, 14, 2894. [Google Scholar] [CrossRef] [PubMed]
- Kowalkińska, M.; Fiszka Borzyszkowska, A.; Grzegórska, A.; Karczewski, J.; Głuchowski, P.; Łapiński, M.; Sawczak, M.; Zielińska-Jurek, A. Pilot-scale studies of WO3/S-doped g-C3N4 heterojunction toward photocatalytic NOx removal. Materials 2022, 15, 633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Cheng, Z.E.; Cheng, Z.; Tahir, A.A.; Luo, W.; Wang, C. Au surface plasmon resonance promoted charge transfer in Z-scheme system enables exceptional photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2022, 310, 121322. [Google Scholar] [CrossRef]
- Lin, F.; Zhou, S.; Wang, G.; Wang, J.; Gao, T.; Su, Y.; Wong, C.P. Electrostatic self-assembly combined with microwave hydrothermal strategy: Construction of 1D/1D carbon nanofibers/crystalline g-C3N4 heterojunction for boosting photocatalytic hydrogen production. Nano Energy 2022, 99, 107432. [Google Scholar] [CrossRef]
- Shi, H.; Li, Y.; Wang, X.; Yu, H.; Yu, J. Selective modification of ultra-thin g-C3N4 nanosheets on the (110) facet of Au/BiVO4 for boosting photocatalytic H2O2 production. Appl. Catal. B Environ. 2021, 297, 120414. [Google Scholar] [CrossRef]
- Yang, T.; Shao, Y.; Hu, J.; Qu, J.; Yang, X.; Yang, F.; Li, C.M. Ultrathin layered 2D/2D heterojunction of ReS2/high-crystalline g-C3N4 for significantly improved photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 448, 137613. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.; Bi, Z.; Ni, W.; Gurzadyan, G.; Zhu, Y.; Zhang, Z. Plasmonic active “Hot Spots”-confined photocatalytic CO2 reduction with high selectivity for CH4 production. Adv. Mater. 2022, 34, 2109330. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Q.; Ng, S.F.; Mohamed, A.R.; Ong, W.J. Point-to-face contact heterojunctions: Interfacial design of 0D nanomaterials on 2D g-C3N4 towards photocatalytic energy applications. Carbon Energy 2022, 4, 665–730. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, S.; Xing, Y.; Qin, H.; Zheng, Q.; Zhang, P.; Zhang, S.; Xu, X. Sandwich-like P-doped h-BN/ZnIn2S4 nanocomposite with direct Z-scheme heterojunction for efficient photocatalytic H2 and H2O2 evolution. Chem. Eng. J. 2022, 442, 136151. [Google Scholar] [CrossRef]
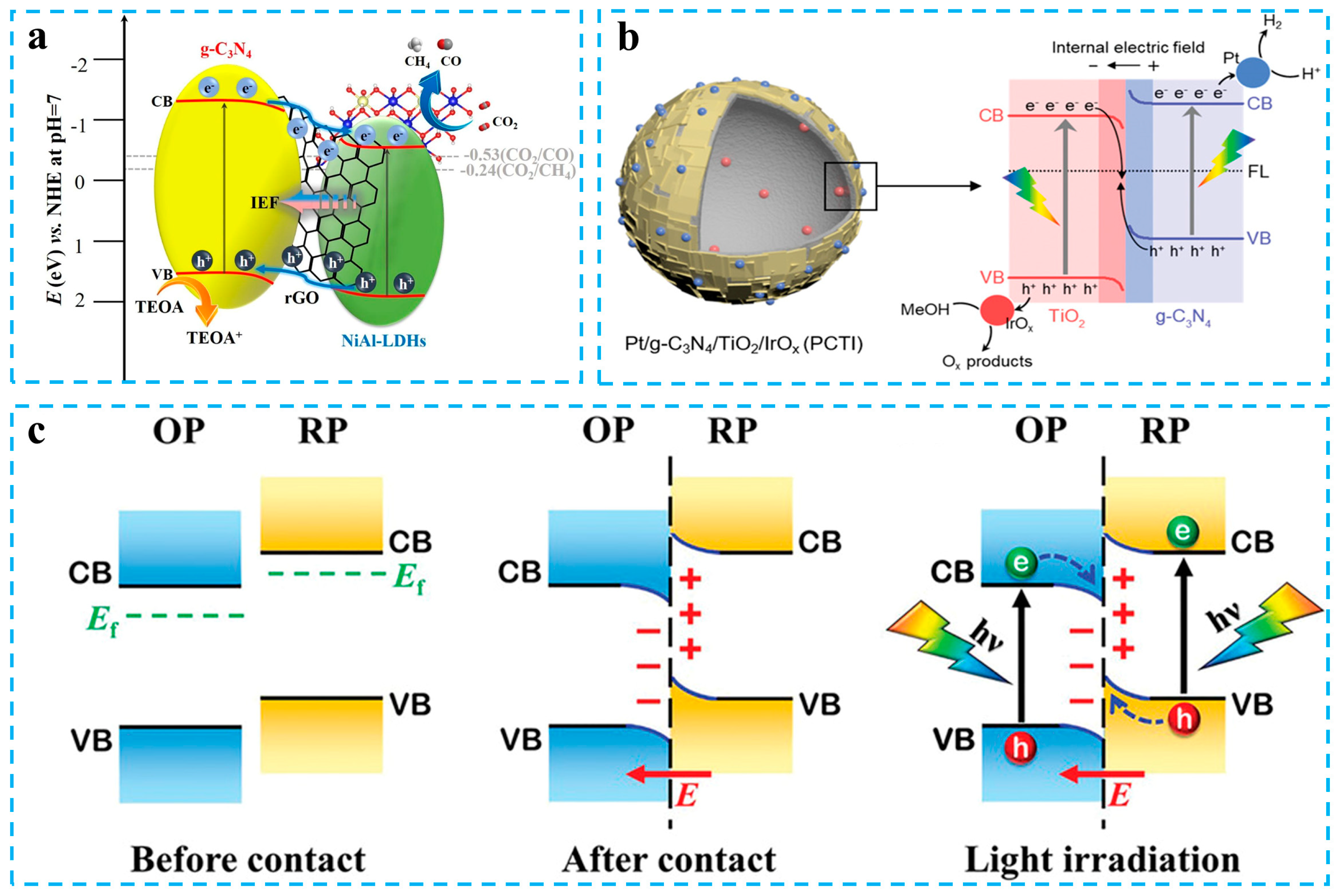
- Zhou, D.; Zhang, J.; Jin, Z.; Di, T.; Wang, T. Reduced graphene oxide assisted g-C3N4/rGO/NiAl-LDHs type II heterostructure with high performance photocatalytic CO2 reduction. Chem. Eng. J. 2022, 450, 138108. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, M.; Chen, H.; Weng, Y.X.; Zhong, J.; Li, Y.; Wang, S.; Chen, Z.; Xia, J.; Li, H. Interfacial chemical bond modulated Bi19S27Br3/g-C3N4 Z-scheme heterojunction for enhanced photocatalytic CO2 conversion. Appl. Catal. B Environ. 2022, 307, 121162. [Google Scholar] [CrossRef]
- Liu, C.; Dai, H.; Tan, C.; Pan, Q.; Hu, F.; Peng, X. Photo-Fenton degradation of tetracycline over Z-scheme Fe-g-C3N4/Bi2WO6 heterojunctions: Mechanism insight, degradation pathways and DFT calculation. Appl. Catal. B Environ. 2022, 310, 121326. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Z.; Wang, C.; Pan, J.; Luo, X. Facile Synthesis with TiO2 Xerogel and Urea Enhanced Aniline Aerofloat Degradation Performance of Direct Z-Scheme Heterojunction TiO2/g-C3N4 Composite. Materials 2022, 15, 3613. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Chai, Y.; Zhang, Z.; Zhu, Y. Efficient photocatalytic overall water splitting induced by the giant internal electric field of ag-C3N4/rGO/PDIP Z-scheme heterojunction. Adv. Mater. 2021, 33, 2007479. [Google Scholar] [CrossRef]
- Moon, H.S.; Hsiao, K.C.; Wu, M.C.; Yun, Y.; Hsu, Y.J.; Yong, K. Spatial separation of cocatalysts on Z-scheme organic/inorganic heterostructure hollow spheres for enhanced photocatalytic H2 evolution and In-depth analysis of the charge-transfer mechanism. Adv. Mater. 2023, 35, 2200172. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Sun, J.; Wu, X.; Liang, H.; Qu, Y.; Jing, L. BiFeO3/Bi2Fe4O9 S-scheme heterojunction hollow nanospheres for high-efficiency photocatalytic o-chlorophenol degradation. Appl. Catal. B Environ. 2022, 319, 121893. [Google Scholar] [CrossRef]
- Liu, C.; Niu, H.; Wang, D.; Gao, C.; Said, A.; Liu, Y.; Wang, G.; Tung, C.H.; Wang, Y. S-scheme Bi-oxide/Ti-oxide molecular hybrid for photocatalytic cycloaddition of carbon dioxide to epoxides. ACS Catal. 2022, 12, 8202–8213. [Google Scholar] [CrossRef]
- Chen, K.; Shi, Y.; Shu, P.; Luo, Z.; Shi, W.; Guo, F. Construction of core–shell FeS2@ZnIn2S4 hollow hierarchical structure S-scheme heterojunction for boosted photothermal-assisted photocatalytic H2 production. Chem. Eng. J. 2023, 454, 140053. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Huo, P.; Yan, Y.; Liao, G.; Ding, G.; Liu, C. Boosting H2 production over C60-mediated NH2-MIL-125(Ti)/Zn0.5Cd0.5S S-scheme heterojunction via enhanced interfacial carrier separation. Small 2021, 17, 2102539. [Google Scholar] [CrossRef]
- Han, X.; Lu, B.; Huang, X.; Liu, C.; Chen, S.; Chen, J.; Zeng, Z.; Deng, S.; Wang, J. Novel p-and n-type S-scheme heterojunction photocatalyst for boosted CO2 photoreduction activity. Appl. Catal. B Environ. 2022, 316, 121587. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Wang, Q.; Zhang, Y.; Meng, L.; Wang, L. In situ construction of S-scheme AgBr/BiOBr heterojunction with surface oxygen vacancy for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B Environ. 2022, 301, 120802. [Google Scholar] [CrossRef]
- Xia, B.; He, B.; Zhang, J.; Li, L.; Zhang, Y.; Yu, J.; Ran, J.; Qiao, S.Z. TiO2/FePS3 S-scheme heterojunction for greatly raised photocatalytic hydrogen evolution. Adv. Energy Mater. 2022, 12, 2201449. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Cheng, B.; Yu, J.; Fan, J. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation. Chin. J. Catal. 2021, 42, 56–68. [Google Scholar] [CrossRef]
- Truong, H.B.; Huy, B.T.; Lee, Y.I.; Nguyen, H.T.; Cho, J.; Hur, J. Magnetic visible-light activated photocatalyst CuFe2O4/Bi2WO6/mpg-C3N4 for the treatment of natural organic matter. Chem. Eng. J. 2023, 453, 139777. [Google Scholar] [CrossRef]
- Pan, T.; Chen, D.; Xu, W.; Fang, J.; Wu, S.; Liu, Z.; Wu, K.; Fang, Z. Anionic polyacrylamide-assisted construction of thin 2D-2D WO3/g-C3N4 step-scheme heterojunction for enhanced tetracycline degradation under visible light irradiation. J. Hazard. Mater. 2020, 393, 122366. [Google Scholar] [CrossRef]
- Kang, L.; Xu, L.; Han, Z.; Yu, H.; Wu, Q.; Wu, M.; He, Z.; Wang, L.; Yang, H. Strategy to enhance photocatalytic performance of heterojunctional composite by dimensionality modulating: Insights into the scheme in interfacial charge migration and mass transfer. Chem. Eng. J. 2022, 429, 132355. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, A.; Cheng, T.; Pan, J.; Song, J.; Zhang, L.; Guan, R.; Xi, X.; Zhang, J. 2D/2D S-scheme heterojunction with a covalent organic framework and g-C3N4 nanosheets for highly efficient photocatalytic H2 evolution. Chin. J. Catal. 2022, 43, 2592–2605. [Google Scholar] [CrossRef]
- Jia, X.; Hu, C.; Sun, H.; Cao, J.; Lin, H.; Li, X.; Chen, S. A dual defect co-modified S-scheme heterojunction for boosting photocatalytic CO2 reduction coupled with tetracycline oxidation. Appl. Catal. B Environ. 2023, 324, 122232. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, D.; Yan, J.; Lee, L.Y.S. Photocatalytic CO2 reduction enabled by interfacial S-scheme heterojunction between ultrasmall copper phosphosulfide and g-C3N4. ACS Appl. Mater. Interfaces 2021, 13, 9762–9770. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhen, Y.; Sun, Y.; Li, L.; Ding, D. ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium (VI) removal. Chem. Eng. J. 2021, 415, 129002. [Google Scholar] [CrossRef]
- Le, S.; Zhu, C.; Cao, Y.; Wang, P.; Liu, Q.; Zhou, H.; Chen, C.; Wang, S.; Duan, S. V2O5 nanodot-decorated laminar C3N4 for sustainable photodegradation of amoxicillin under solar light. Appl. Catal. B Environ. 2022, 303, 120903. [Google Scholar] [CrossRef]
- Shen, X.; Song, B.; Shen, X.; Shen, C.; Shan, S.; Xue, Q.; Chen, X.; Li, S. Rationally designed S-scheme heterojunction of C3N4/Bi2MoO6/carbon fiber cloth as a recyclable, macroscopic and efficient photocatalyst for wastewater treatment. Chem. Eng. J. 2022, 445, 136703. [Google Scholar] [CrossRef]
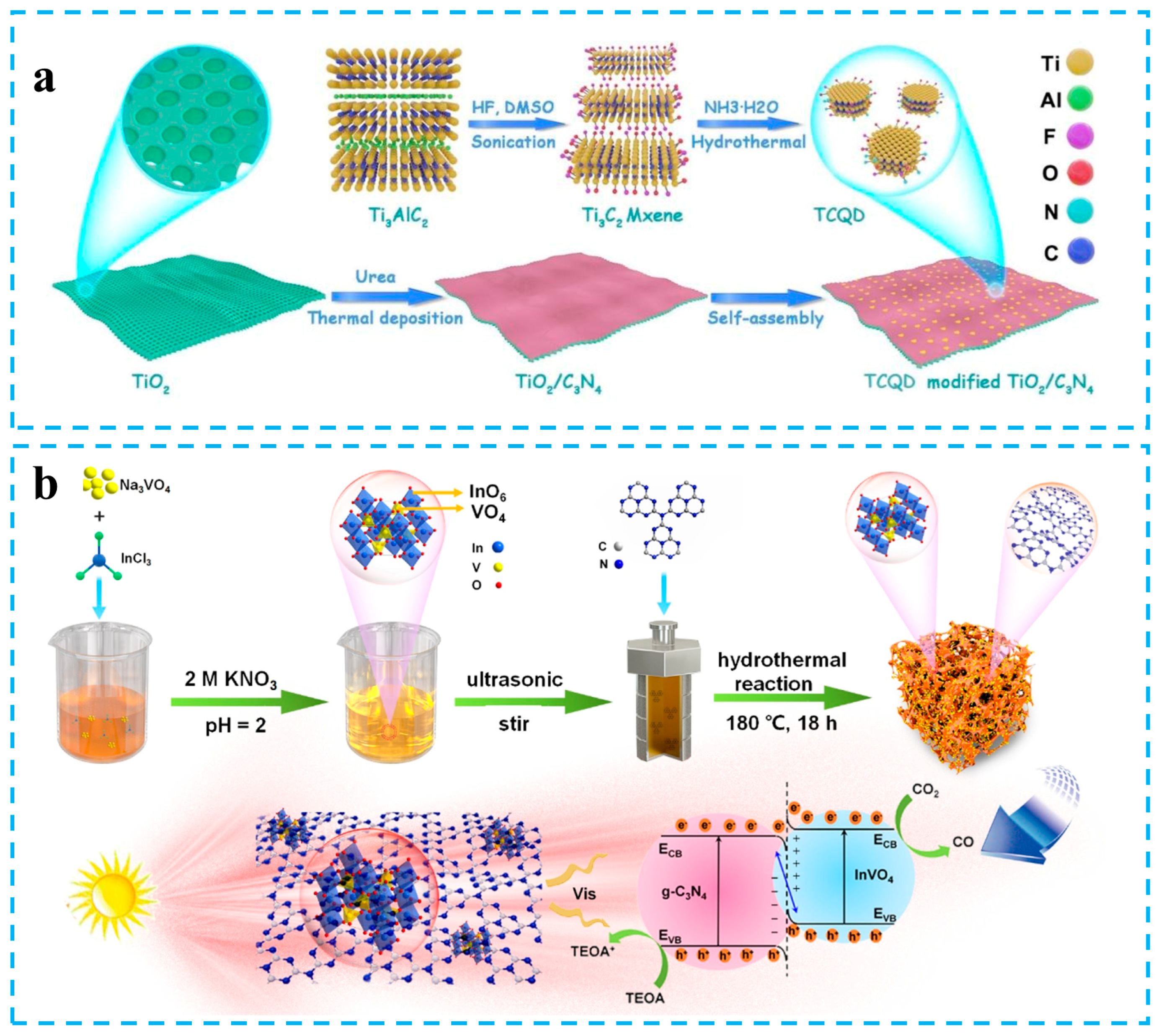
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Gong, S.; Teng, X.; Niu, Y.; Liu, X.; Xu, M.; Xu, C.; Ji, L.; Chen, Z. Construction of S-scheme 0D/2D heterostructures for enhanced visible-light-driven CO2 reduction. Appl. Catal. B Environ. 2021, 298, 120521. [Google Scholar] [CrossRef]
- Ruan, X.; Huang, C.; Cheng, H.; Zhang, Z.; Cui, Y.; Li, Z.; Xie, T.; Ba, K.; Zhang, H.; Zhang, L.; et al. A Twin S-scheme artificial photosynthetic system with self-assembled heterojunctions yields superior photocatalytic hydrogen evolution rate. Adv. Mater. 2023, 35, 2209141. [Google Scholar] [CrossRef]
- Chen, Y.; Su, F.; Xie, H.; Wang, R.; Ding, C.; Huang, J.; Xu, Y.; Ye, L. One-step construction of S-scheme heterojunctions of N-doped MoS2 and S-doped g-C3N4 for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 404, 126498. [Google Scholar] [CrossRef]
- Cheng, C.; He, B.; Fan, J.; Cheng, B.; Cao, S.; Yu, J. An inorganic/organic S-scheme heterojunction H2-production photocatalyst and its charge transfer mechanism. Adv. Mater. 2021, 33, 2100317. [Google Scholar] [CrossRef] [PubMed]
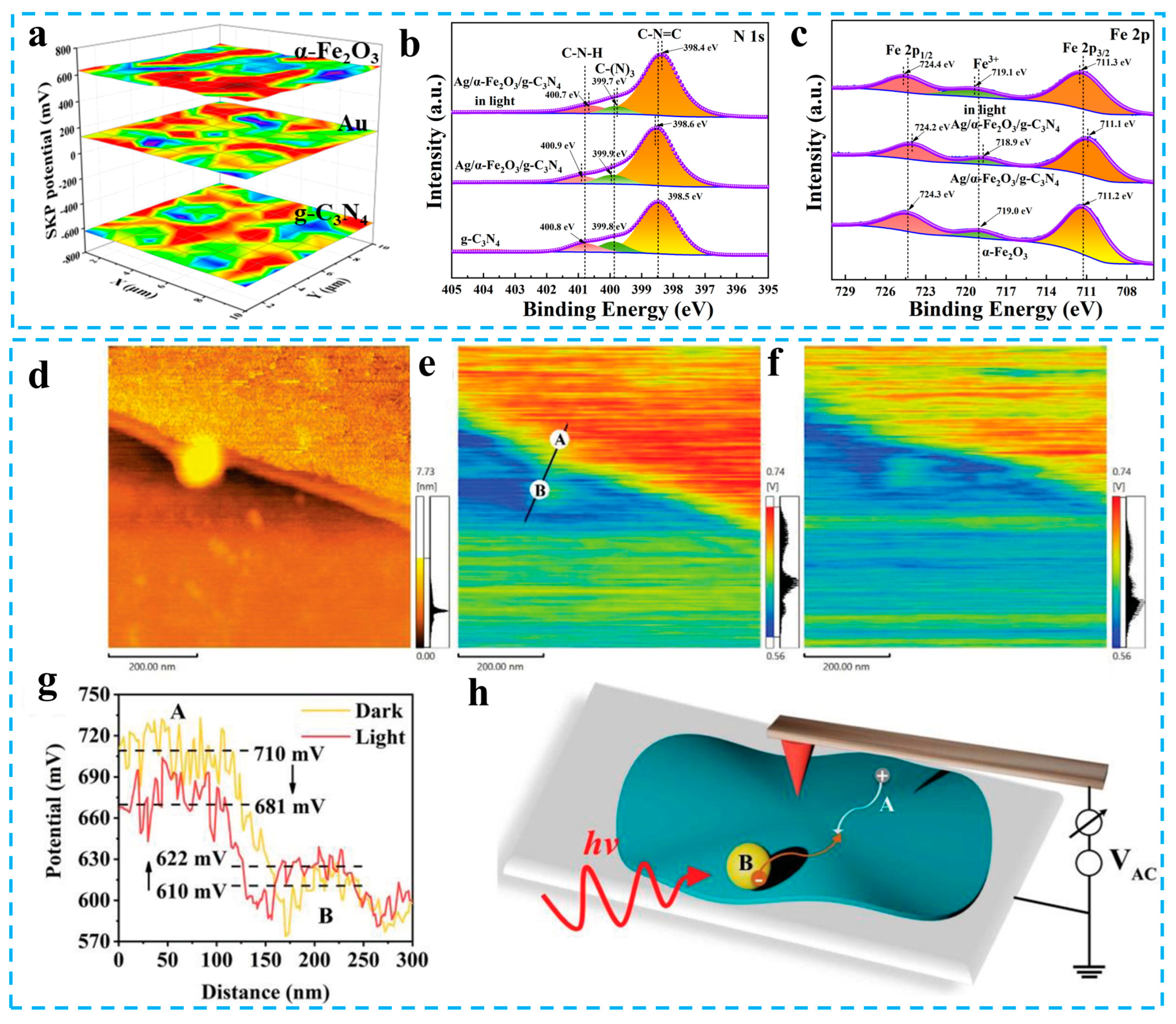
- Xiao, Y.; Yao, B.; Wang, Z.; Chen, T.; Xiao, X.; Wang, Y. Plasma Ag-modified α-Fe2O3/g-C3N4 self-assembled S-scheme heterojunctions with enhanced photothermal-photocatalytic-fenton performances. Nanomaterials 2022, 12, 4212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, J.; Yang, T.; Yang, X.; Qu, J.; Li, C.M. Efficient photocatalytic H2-evolution coupled with valuable furfural-production on exquisite 2D/2D LaVO4/g-C3N4 heterostructure. Nano Energy 2022, 92, 106714. [Google Scholar] [CrossRef]
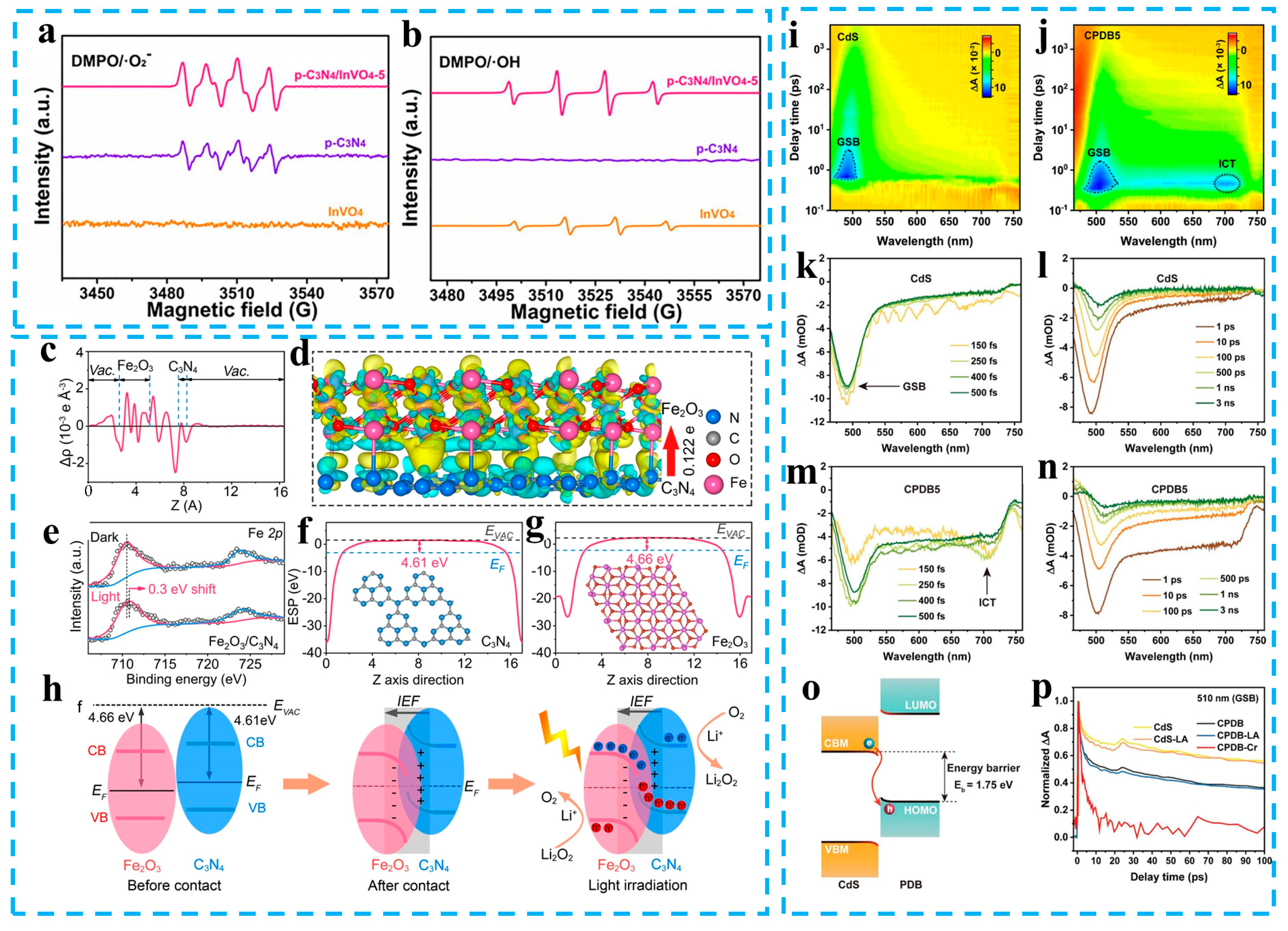
- Wang, L.; Chen, D.; Miao, S.; Chen, F.; Guo, C.; Ye, P.; Ning, J.; Zhong, Y.; Hu, Y. Nitric acid-assisted growth of InVO4 nanobelts on protonated ultrathin C3N4 nanosheets as an S-scheme photocatalyst with tunable oxygen vacancies for boosting CO2 conversion. Chem. Eng. J. 2022, 434, 133867. [Google Scholar] [CrossRef]
- Zhu, Z.; Lv, Q.; Ni, Y.; Gao, S.; Geng, J.; Liang, J.; Li, F. Internal electric field and interfacial bonding engineered step-scheme junction for a visible-light-involved lithium-oxygen battery. Angew. Chem. Int. Ed. 2022, 61, e202116699. [Google Scholar]
- Cheng, C.; Zhang, J.; Zhu, B.; Liang, G.; Zhang, L.; Yu, J. Verifying the charge-transfer mechanism in S-scheme heterojunctions using femtosecond transient absorption spectroscopy. Angew. Chem. Int. Ed. 2023, 135, e202218688. [Google Scholar] [CrossRef]
- Wang, M.; Kang, J.; Li, S.; Zhang, J.; Tang, Y.; Liu, S.; Liu, J.; Tang, P. Electro-assisted heterogeneous activation of peroxymonosulfate by g-C3N4 under visible light irradiation for tetracycline degradation and its mechanism. Chem. Eng. J. 2022, 436, 135278. [Google Scholar] [CrossRef]
- Huo, T.; Ba, G.; Deng, Q.; Yu, F.; Wang, G.; Li, H.; Hou, W. A dual strategy for synthesizing carbon/defect comodified polymeric carbon nitride porous nanotubes with boosted photocatalytic hydrogen evolution and synchronous contaminant degradation. Appl. Catal. B Environ. 2021, 287, 119995. [Google Scholar] [CrossRef]
- Hu, C.; Chen, F.; Wang, Y.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Exceptional cocatalyst-free photo-enhanced piezocatalytic hydrogen evolution of carbon nitride nanosheets from strong in-plane polarization. Adv. Mater. 2021, 33, 2101751. [Google Scholar] [CrossRef]
- Shen, R.; Zhang, L.; Chen, X.; Jaroniec, M.; Li, N.; Li, X. Integrating 2D/2D CdS/α-Fe2O3 ultrathin bilayer Z-scheme heterojunction with metallic β-NiS nanosheet-based ohmic-junction for efficient photocatalytic H2 evolution. Appl. Catal. B Environ. 2020, 266, 118619. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, T.; Yu, C.; Lu, R. Ultrafast interlayer charge separation, enhanced visible-light absorption, and tunable overpotential in twisted graphitic carbon nitride bilayers for water splitting. Adv. Mater. 2021, 33, 2104695. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Ran, Y.; Cui, Y.; Zhang, Y.; Fang, Y.; Zhang, W.; Yu, X.; Lan, H.; An, X. Assembly-synthesis of puff pastry-like g-C3N4/CdS heterostructure as S-junctions for efficient photocatalytic water splitting. Chem. Eng. J. 2022, 431, 133348. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Huo, Y.; Dai, K.; Li, S.; Chen, S. Two-dimensional sulfur-and chlorine-codoped g-C3N4/CdSe-amine heterostructures nanocomposite with effective interfacial charge transfer and mechanism insight. Appl. Catal. B Environ. 2021, 280, 119452. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Mei, F.; Li, Z.; Dai, K.; Zhang, J.; Liang, C. Step-scheme porous g-C3N4/Zn0.2Cd0.8S-DETA composites for efficient and stable photocatalytic H2 production. Chin. J. Catal. 2020, 41, 41–49. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Zhang, Y.; Zhang, M.; Huang, W.; Yu, C.; Sun, J.; Feng, M.J.; Dong, S.; Sun, J. Porous g-C3N4/TiO2 S-scheme heterojunction photocatalyst for visible-light driven H2 production and simultaneous wastewater purification. Int. J. Hydrogen Energy 2021, 46, 32413–32424. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Liu, E.; Fan, J. Novel S-scheme 2D/2D BiOBr/g-C3N4 heterojunctions with enhanced photocatalytic activity. Chin. J. Catal. 2021, 42, 1519–1529. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Fan, J.; Sun, W.; Liu, E. S-scheme bimetallic sulfide ZnCo2S4/g-C3N4 heterojunction for photocatalytic H2 evolution. Ceram. Int. 2021, 47, 30194–30202. [Google Scholar] [CrossRef]
- Tang, S.; Yang, S.; Chen, Y.; Yang, Y.; Li, Z.; Zi, L.; Liu, Y.; Wang, Y.; Li, Z.; Fu, Z.; et al. Ionothermally synthesized S-scheme isotype heterojunction of carbon nitride with significantly enhanced photocatalytic performance for hydrogen evolution and carbon dioxide reduction. Carbon 2023, 201, 815–828. [Google Scholar] [CrossRef]
- Wang, L.; Yang, T.; Peng, L.; Zhang, Q.; She, X.; Tang, H.; Liu, Q. Dual transfer channels of photo-carriers in 2D/2D/2D sandwich-like ZnIn2S4/g-C3N4/Ti3C2 MXene S-scheme/Schottky heterojunction for boosting photocatalytic H2 evolution. Chin. J. Catal. 2022, 43, 2720–2731. [Google Scholar] [CrossRef]
- Bi, F.; Su, Y.; Zhang, Y.; Chen, M.; Darr, J.A.; Weng, X.; Wu, Z. Vacancy-defect semiconductor quantum dots induced an S-scheme charge transfer pathway in 0D/2D structures under visible-light irradiation. Appl. Catal. B Environ. 2022, 306, 121109. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Z.; Wang, H.; Liao, G.; Bai, S.; Zou, J.; Wu, P.; Zhang, P.; Li, X. Sulfur-doped g-C3N4/g-C3N4 isotype step-scheme heterojunction for photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 118, 15–24. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, W.; Shi, Y.; Xu, Z.; Shi, W.; Shi, Y.; Sun, H.; Li, L.; Guo, F.; Wen, H. Carbon dots as solid-state electron mediator and electron acceptor in S-scheme heterojunction for boosted photocatalytic hydrogen evolution. Appl. Surf. Sci. 2022, 595, 153482. [Google Scholar] [CrossRef]
- Lei, Z.; Cao, X.; Fan, J.; Hu, X.; Hu, J.; Li, N.; Sun, T.; Liu, E. Efficient photocatalytic H2 generation over In2.77S4/NiS2/g-C3N4 S-scheme heterojunction using NiS2 as electron-bridge. Chem. Eng. J. 2023, 457, 141249. [Google Scholar] [CrossRef]
- Wang, W.; Deng, C.; Xie, S.; Li, Y.; Zhang, W.; Sheng, H.; Chen, C.; Zhao, J. Photocatalytic C–C coupling from carbon dioxide reduction on copper oxide with mixed-valence copper (I)/copper (II). J. Am. Chem. Soc. 2021, 143, 2984–2993. [Google Scholar] [CrossRef]
- Wang, F.; Fang, R.; Zhao, X.; Kong, X.P.; Hou, T.; Shen, K.; Li, Y. Ultrathin nanosheet assembled multishelled superstructures for photocatalytic CO2 reduction. ACS Nano 2022, 16, 4517–4527. [Google Scholar] [CrossRef]
- Shangguan, W.; Liu, Q.; Wang, Y.; Sun, N.; Liu, Y.; Zhao, R.; Li, Y.; Wang, C.; Zhao, J. Molecular-level insight into photocatalytic CO2 reduction with H2O over Au nanoparticles by interband transitions. Nat. Commun. 2022, 13, 3894. [Google Scholar] [CrossRef]
- Yi, J.D.; Xie, R.; Xie, Z.L.; Chai, G.L.; Liu, T.F.; Chen, R.P.; Huang, Y.B.; Cao, R. Highly selective CO2 electroreduction to CH4 by in situ generated Cu2O single-type sites on a conductive MOF: Stabilizing key intermediates with hydrogen bonding. Angew. Chem. Int. Ed. 2020, 59, 23641–23648. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, C.; Cui, P.; Li, C.; Zhang, B.; Gan, L.; Zhang, S.; Zhang, X.; Zhou, X.; Sun, Z.; et al. Ultrahigh photocatalytic CO2 reduction efficiency and selectivity manipulation by single-tungsten-atom oxide at the atomic step of TiO2. Adv. Mater. 2022, 34, 2109074. [Google Scholar] [CrossRef] [PubMed]
- Qaraah, F.A.; Mahyoub, S.A.; Hezam, A.; Qaraah, A.; Xin, F.; Xiu, G. Synergistic effect of hierarchical structure and S-scheme heterojunction over O-doped g-C3N4/N-doped Nb2O5 for highly efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2022, 315, 121585. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Yu, Y.; Cui, J.; Li, X.; Zhang, Y.; Wang, C.; Yu, X.; Ye, J. Defective g-C3N4/covalent organic framework van der Waals heterojunction toward highly efficient S-scheme CO2 photoreduction. Appl. Catal. B Environ. 2022, 301, 120814. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M.; Mohamed, A.R. Constructing S-scheme heterojunction of carbon nitride nanorods (g-CNR) assisted trimetallic CoAlLa LDH nanosheets with electron and holes moderation for boosting photocatalytic CO2 reduction under solar energy. Chem. Eng. J. 2022, 433, 133693. [Google Scholar] [CrossRef]
- Xie, Q.; He, W.; Liu, S.; Li, C.; Zhang, J.; Wong, P.K. Bifunctional S-scheme g-C3N4/Bi/BiVO4 hybrid photocatalysts toward artificial carbon cycling. Chin. J. Catal. 2020, 41, 140–153. [Google Scholar] [CrossRef]
- Mei, F.; Zhang, J.; Liang, C.; Dai, K. Fabrication of novel CoO/porous graphitic carbon nitride S-scheme heterojunction for efficient CO2 photoreduction. Mater. Lett. 2021, 282, 128722. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B. Constructing S-scheme 2D/0D g-C3N4/TiO2 NPs/MPs heterojunction with 2D-Ti3AlC2 MAX cocatalyst for photocatalytic CO2 reduction to CO/CH4 in fixed-bed and monolith photoreactors. J. Mater. Sci. Technol. 2022, 106, 195–210. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, J.; Dai, K.; Liang, C. Amine-modified S-scheme porous g-C3N4/CdSe–diethylenetriamine composite with enhanced photocatalytic CO2 reduction activity. ACS Appl. Energy Mater. 2021, 4, 956–968. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, M.; Song, X.; Zhou, W.; Liu, X.; Hu, P. 3D Fe-MOF embedded into 2D thin layer carbon nitride to construct 3D/2D S-scheme heterojunction for enhanced photoreduction of CO2. Chin. J. Catal. 2022, 43, 2625–2636. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Shangguan, Y.; Wu, X.; Chen, H. Selective CO2 photoreduction to CH4 mediated by dimension-matched 2D/2D Bi3NbO7/g-C3N4 S-scheme heterojunction. Chin. J. Catal. 2022, 43, 246–254. [Google Scholar] [CrossRef]
- Zhao, T.; Li, D.; Zhang, Y.; Chen, G. Constructing built-in electric field within CsPbBr3/sulfur doped graphitic carbon nitride ultra-thin nanosheet step-scheme heterojunction for carbon dioxide photoreduction. J. Colloid Interface Sci. 2022, 628, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, D.; Xu, Q.; Huang, S. Constructing hierarchical ZnIn2S4/g-C3N4 S-scheme heterojunction for boosted CO2 photoreduction performance. Chem. Eng. J. 2022, 437, 135153. [Google Scholar] [CrossRef]
- Wang, K.; Peng, L.; Shao, X.; Cheng, Q.; Wang, J.; Li, K.; Wang, H. Nb–O–C charge transfer bridge in 2D/2D Nb2O5/g-C3N4 S-scheme heterojunction for boosting solar-driven CO2 reduction: In situ illuminated X-ray photoelectron spectroscopy investigation and mechanism insight. Solar RRL 2022, 6, 2200434. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Miao, C.; Xia, F.; Wang, Y.; Wang, Y.; Liu, C.; Che, G. g-C3N4/BiOI S-scheme heterojunction: A 2D/2D model platform for visible-light-driven photocatalytic CO2 reduction and pollutant degradation. J. Environ. Chem. Eng. 2022, 10, 108201. [Google Scholar] [CrossRef]
- Dai, B.; Zhao, W.; Wei, W.; Cao, J.; Yang, G.; Li, S.; Sun, C.; Leung, D.Y.C. Photocatalytic reduction of CO2 and degradation of Bisphenol-S by g-C3N4/Cu2O@Cu S-scheme heterojunction: Study on the photocatalytic performance and mechanism insight. Carbon 2022, 193, 272–284. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, J.; Macyk, W.; Wageh, S.; Al-Ghamdi, A.A.; Wang, L. C3N4/PDA S-scheme heterojunction with enhanced photocatalytic H2O2 production performance and its mechanism. Adv. Sust. Syst. 2023, 7, 2200113. [Google Scholar] [CrossRef]
- Deng, X.; Chen, R.; Wang, C.; Liang, Z.; Zhao, Z.; Shi, W.; Cui, F. Iron-tungsten oxides modified oxygen-rich carbon nitride with defects S-scheme heterojunction for boosting photo-Fenton like removal of pollutants. Chem. Eng. J. 2023, 451, 138629. [Google Scholar] [CrossRef]
- Xia, P.; Cao, S.; Zhu, B.; Liu, M.; Shi, M.; Yu, J.; Zhang, Y. Designing a 0D/2D S-scheme heterojunction over polymeric carbon nitride for visible-light photocatalytic inactivation of bacteria. Angew. Chem. Int. Ed. 2020, 59, 5218–5225. [Google Scholar] [CrossRef]
- Zhao, D.; Dong, C.L.; Wang, B.; Chen, C.; Huang, Y.C.; Diao, Z.; Li, S.; Guo, L.; Shen, S. Synergy of dopants and defects in graphitic carbon nitride with exceptionally modulated band structures for efficient photocatalytic oxygen evolution. Adv. Mater. 2019, 31, 1903545. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.L.; Huang, Y.C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Aschauer, U. Charge transfer observed in light-activated catalyst particles. Nature 2022, 610, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ren, Z.; Liang, Y.; Zhang, G.; Dittrich, T.; Liu, R.; Liu, Y.; Zhao, Y.; Pang, S.; An, H.; et al. Spatiotemporal imaging of charge transfer in photocatalyst particles. Nature 2022, 610, 296–301. [Google Scholar] [CrossRef] [PubMed]


| Photocatalysts | Synthesis Strategy | Characteristics | Ref. |
|---|---|---|---|
| S-g-C3N4/TiO2 | Electrospinning | Small diameter, uniform morphology, time and cost-consuming | [68] |
| CuFe2O4/Bi2WO6/mpg-C3N4 | Hydrothermal and solvothermal | High crystallinity, controllable morphology, low efficiency | [69] |
| WO3/g-C3N4 | Thermal polycondensation | Facile, highly efficient, uncontrollable morphology | [70] |
| TpPa-1-COF/g-C3N4 | Self-assembly | Uniform morphology, complicated preparation process | [72] |
| 70IB/CNx | Deposition-precipitation | Facile, highly efficient, easy aggregation | [73] |
| us-Cu3P|S/CN | Solid-state method | High-efficiency, simple, uncontrollable morphology | [74] |
| V2O5/C3N4 | Mechanical agitation | Facile, highly efficient, relatively poor interface contacts | [76] |
| TiO2/C3N4/Ti3C2 | Electrostatic self-assembly | High-efficiency, and low-cost, complex conditions | [78] |
| CdS/PT | In-situ growth | Uniform morphology, close contact, complicated preparation process | [82] |
| Photocatalysts | Auxiliary Conditions | Light Source | H2 Evolution (μmol h−1 g−1) | Ref. |
|---|---|---|---|---|
| S-pCN/WO2.72 | TEOA | Xe lamp (300 W, λ > 420 nm) | 786.0 | [96] |
| g-C3N4/Zn0.2Cd0.8S-DETA | Na2S, Na2SO3, H2PtCl6 | Xe lamp (300 W, λ > 420 nm) | 6690.0 | [97] |
| g-C3N4/TiO2 | TEOA, Pt | Xe lamp (300 W, λ > 420 nm) | 974.6 | [98] |
| BiOBr/g-C3N4 | TEOA | Xe lamp (300 W) | 106.63 | [99] |
| ZnCo2S4/g-C3N4 | TEOA | Xe lamp (300 W) | 6619.0 | [100] |
| ISCNMT | MeOH, K2PtCl6 | Xe lamp (110 mW cm−2, λ > 400 nm) | 9640.0 | [101] |
| ZnIn2S4/g-C3N4/Ti3C2 | TEOA | Xe lamp (300W, λ > 420 nm) | 2452.1 | [102] |
| TiO2-OV/g-C3N4 | TEOA, H2PtCl6 | Xe lamp (300 w, λ > 400 nm) | 6308.0 | [103] |
| S-g-C3N4-E | TEOA, Pt | Xe lamp (300 W, λ > 420 nm) | 5548.1 | [104] |
| ZnIn2S4/CD/g-C3N4 | TEOA, NaCl, H2PtCl6 | Xe lamp (300 W, λ > 420 nm) | 17580 | [105] |
| In2.77S4/NiS2/g-C3N4 | TEOA | Xe lamp (300 W) | 7481.7 | [106] |
| Photocatalysts | Light Source | CH4 (μmol h−1 g−1) | CO (μmol h−1 g−1) | Ref. |
|---|---|---|---|---|
| g-C3N4/Bi/BiVO4 | Xe lamp (300 W, λ > 420 nm) | - | 1.25 | [115] |
| CoO/PCN | Xe lamp (300 W, λ > 410 nm) | - | 40.31 | [116] |
| TiO2/Ti3AlC2/g-C3N4 | HID car lamp (35 W) | 2103.50 | 297.26 | [117] |
| g-C3N4/CdSe-DETA | Xe lamp (300 W, λ > 420 nm) | - | 25.87 | [118] |
| g-C3N4/Fe-MOF | Xe lamp (300 W, full spectrum) | - | 19.17 | [119] |
| Bi3NbO7/g-C3N4 | Xe lamp (300 W, λ > 420 nm) | 37.60 | 4.18 | [120] |
| CsPbBr3/S-g-C3N4 | Xe lamp (300 W, λ > 400 nm) | - | 83.60 | [121] |
| ZnIn2S4/g-C3N4 | Xe lamp (300 W, full spectrum) | - | 883.00 | [122] |
| Nb2O5/g-C3N4 | Xe lamp (300 W, full spectrum) | 20.89 | 171.98 | [123] |
| BiOI/g-C3N4 | Xe lamp (300 W, λ > 400 nm) | - | 3.11 | [124] |
| g-C3N4/Cu2O@Cu | Xe lamp (300 W, full spectrum) | 3.10 | 10.80 | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Tian, X.; Chen, Y.; Xiao, X.; Chen, T.; Wang, Y. Recent Advances in Carbon Nitride-Based S-scheme Photocatalysts for Solar Energy Conversion. Materials 2023, 16, 3745. https://doi.org/10.3390/ma16103745
Xiao Y, Tian X, Chen Y, Xiao X, Chen T, Wang Y. Recent Advances in Carbon Nitride-Based S-scheme Photocatalysts for Solar Energy Conversion. Materials. 2023; 16(10):3745. https://doi.org/10.3390/ma16103745
Chicago/Turabian StyleXiao, Yawei, Xu Tian, Yunhua Chen, Xuechun Xiao, Ting Chen, and Yude Wang. 2023. "Recent Advances in Carbon Nitride-Based S-scheme Photocatalysts for Solar Energy Conversion" Materials 16, no. 10: 3745. https://doi.org/10.3390/ma16103745






