Study on the Interface Microstructure of TaC/GCr15 Steel Surface Reinforced Layer Formed by In-Situ Reaction
Abstract
1. Introduction
2. Materials and Methods
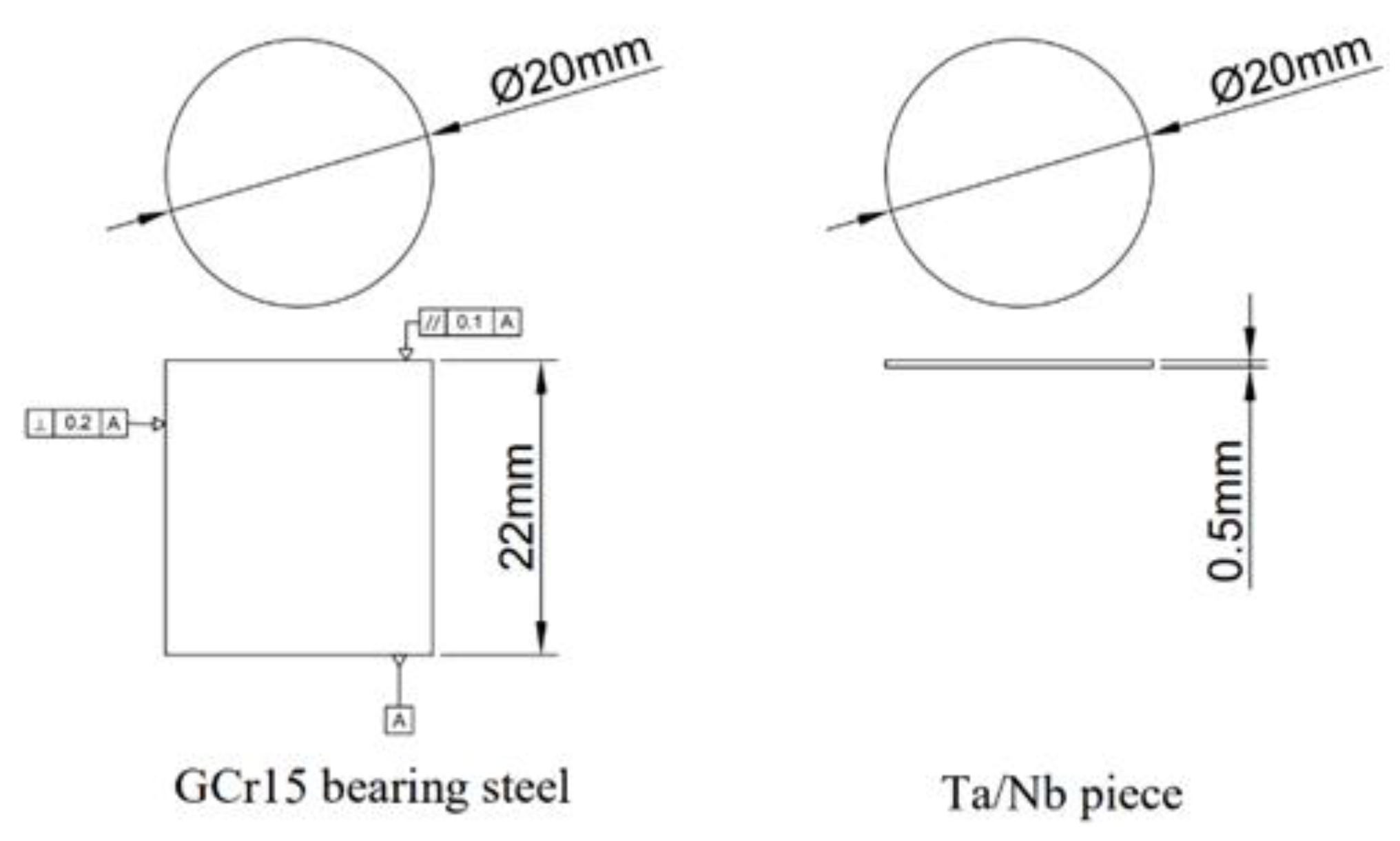
2.1. Raw Materials Preparation
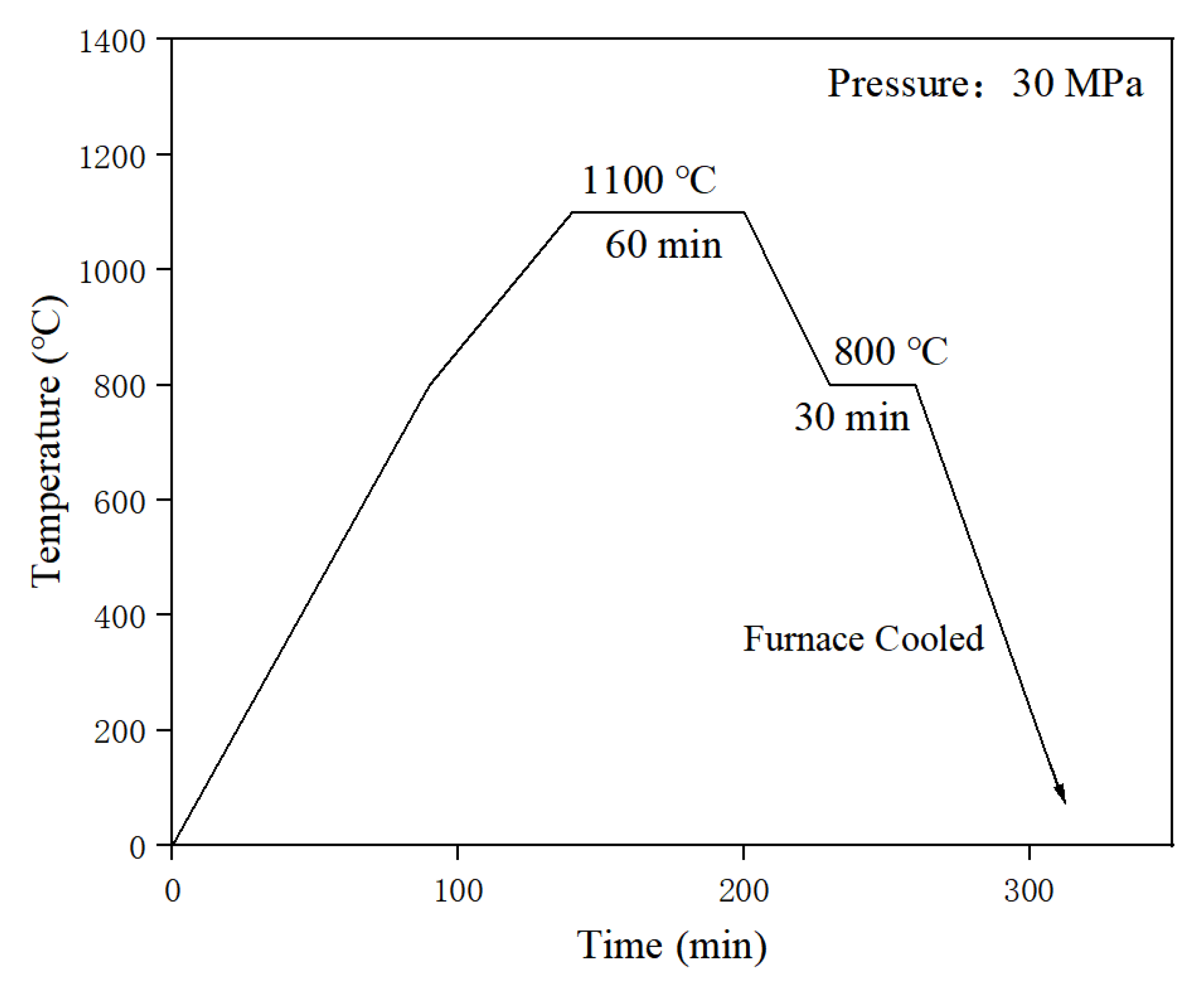
2.2. Preparation Methods
2.3. Experimental Instruments and Equipment
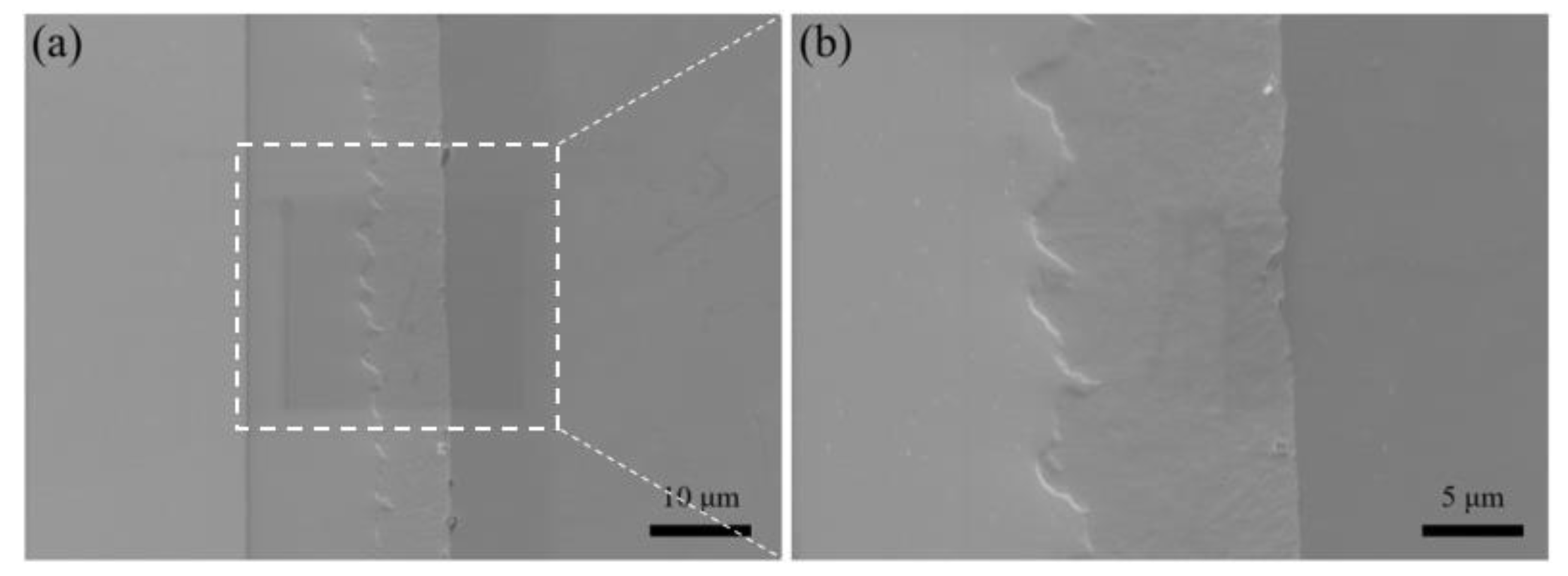
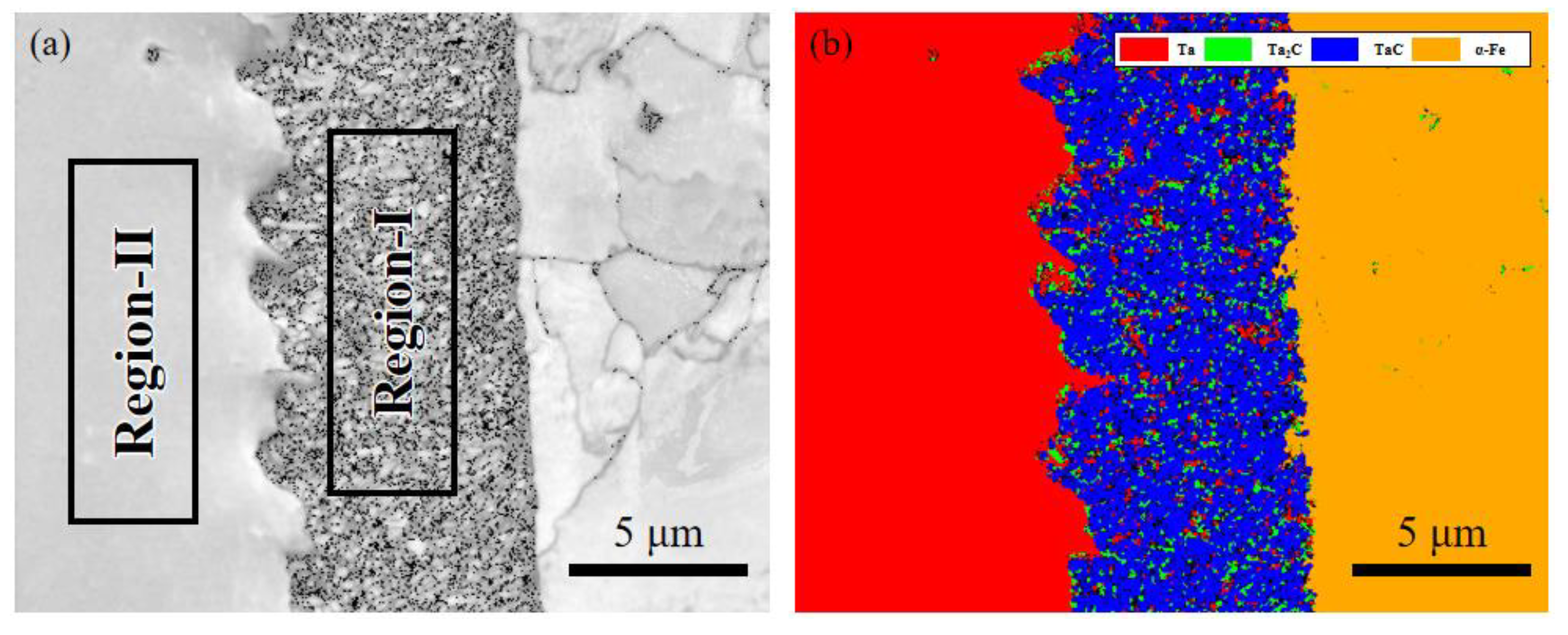
3. Microstructure and Microstructure Analysis
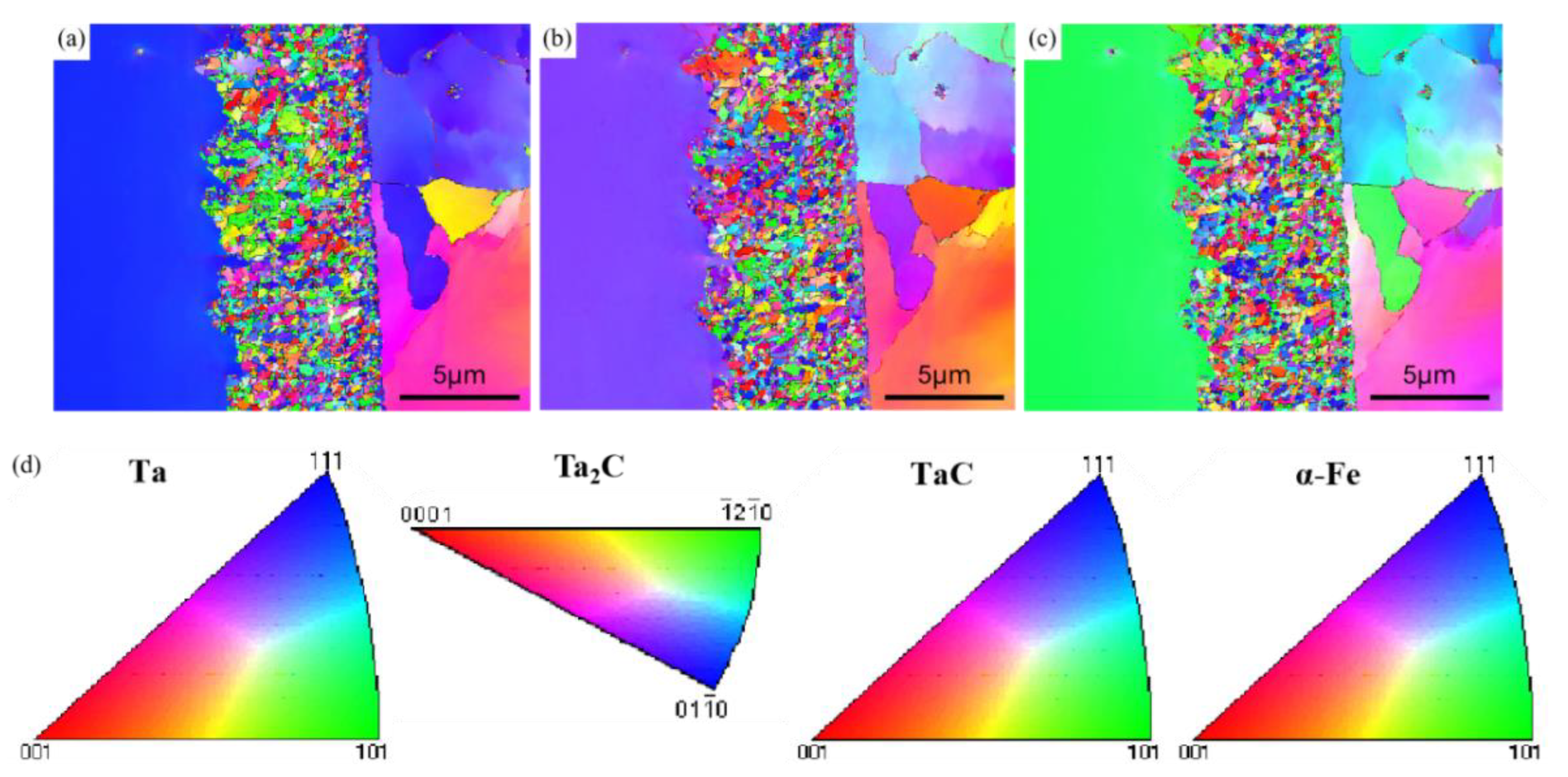
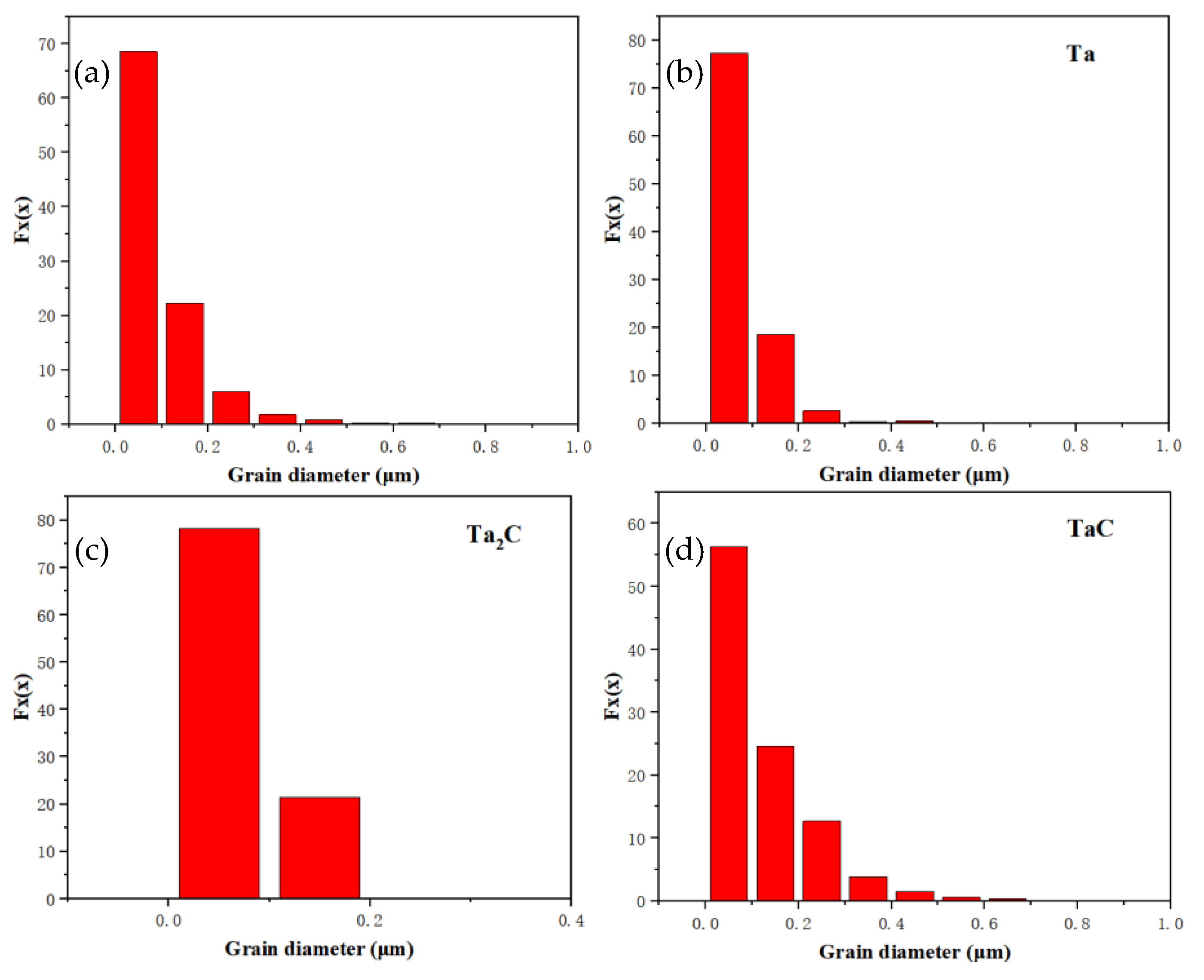
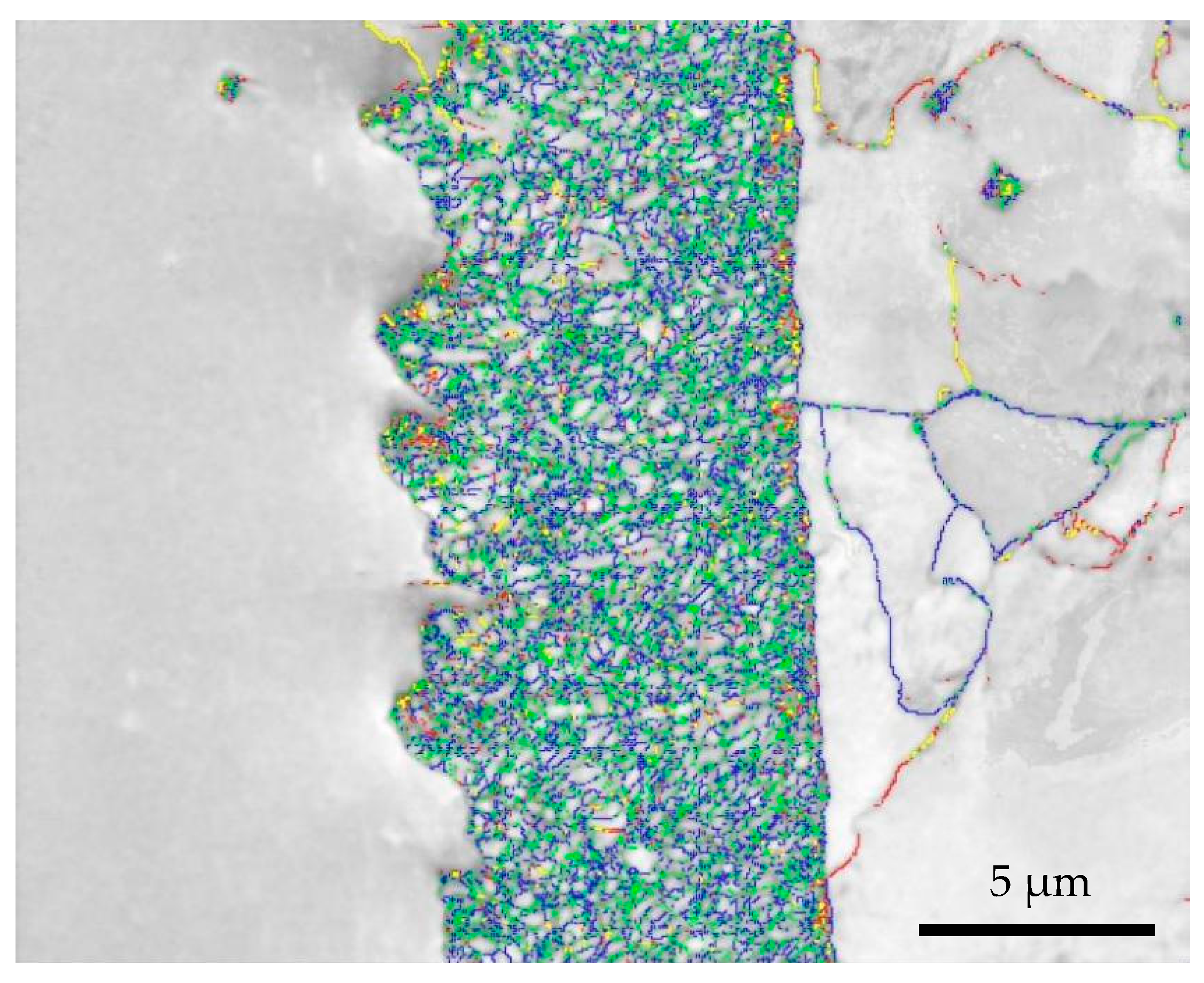
3.1. EBSD Crystal Plane Orientation Analysis
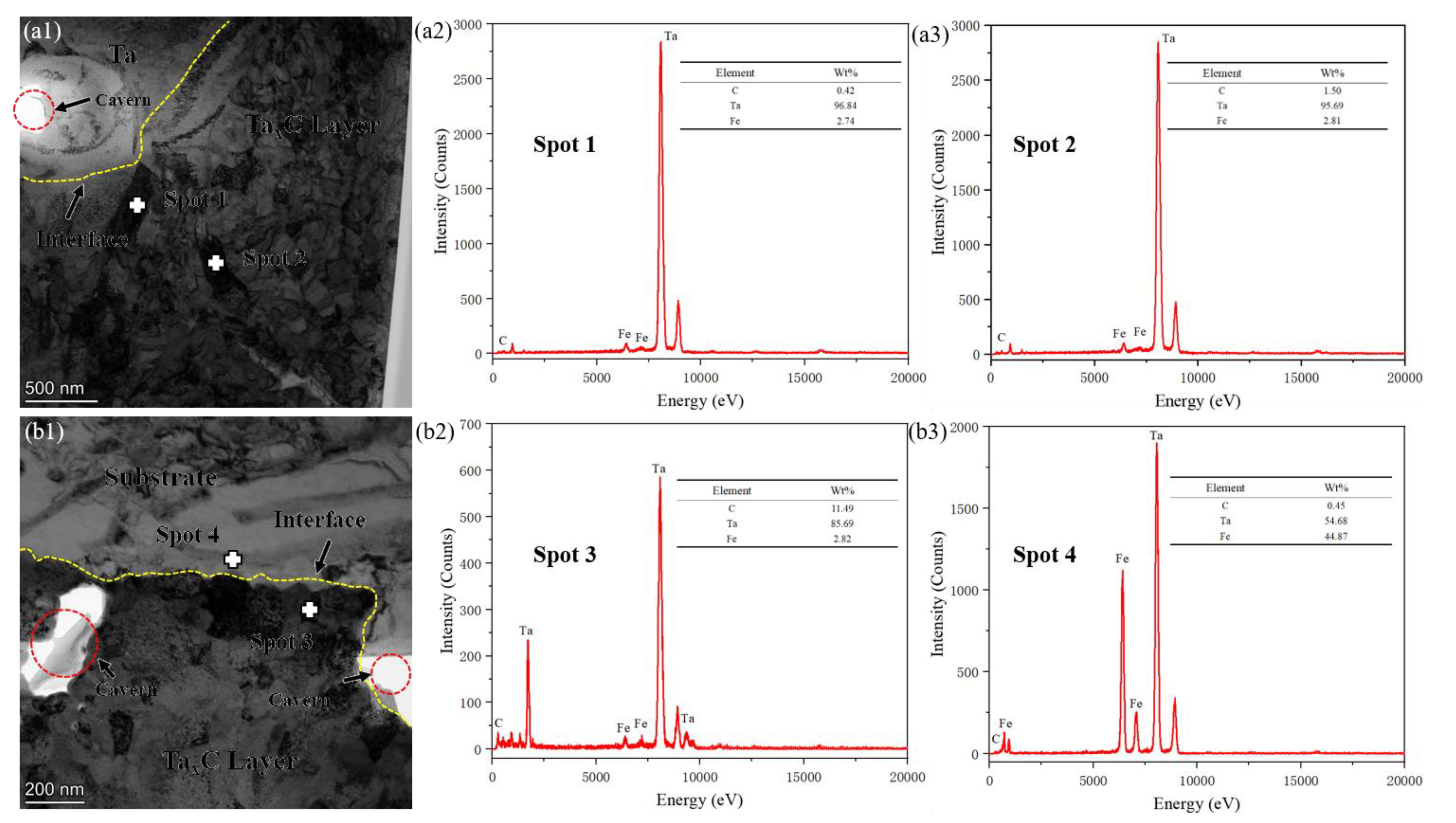
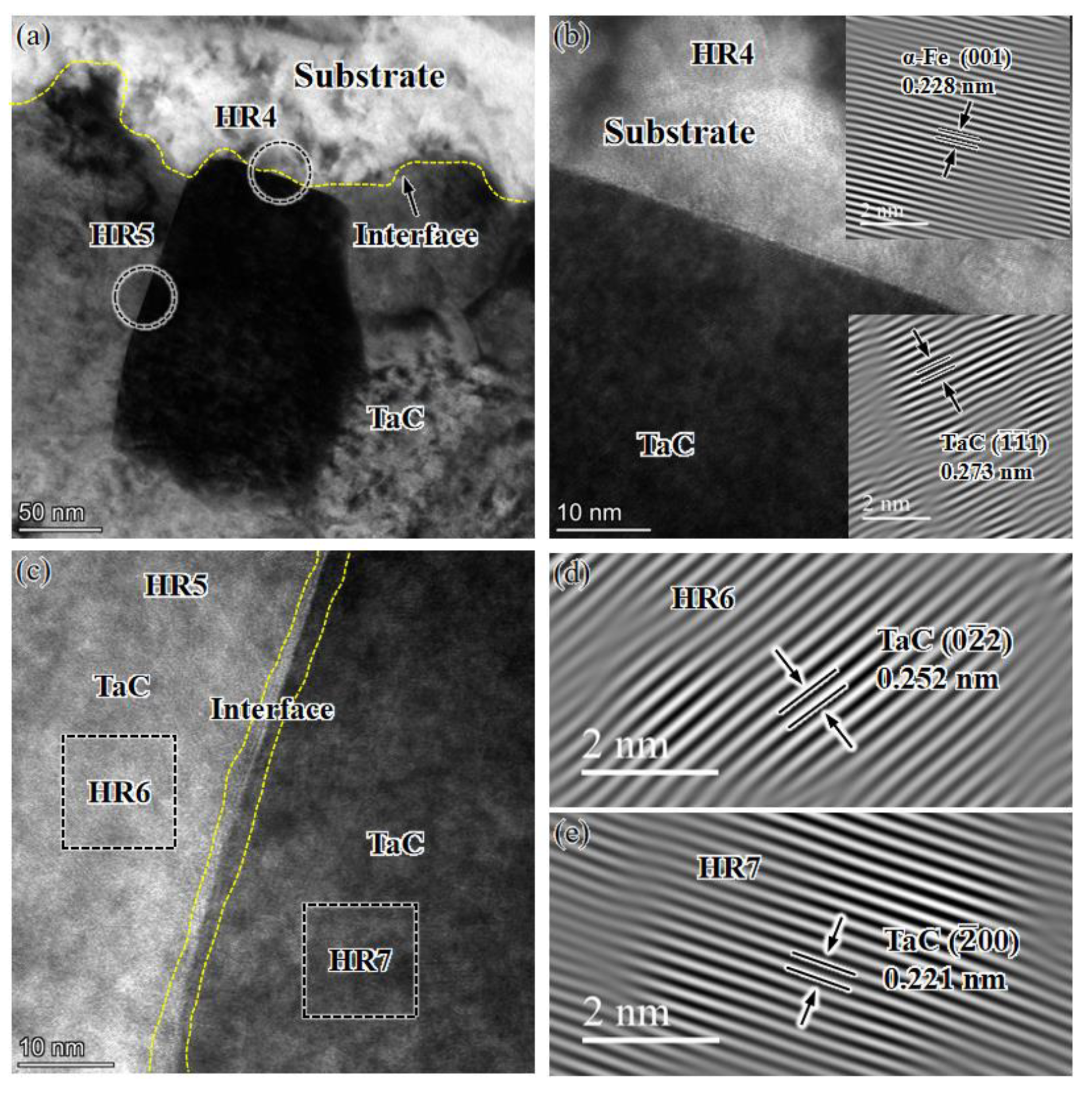
3.2. Analysis of TEM Microstructure and SAED Diffraction Pattern
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, L. Preparation and Microstructure and Properties of Cu2O-Cu Ceramic [D] Harbin; Harbin Institute of Technology: Harbin, China, 2009. [Google Scholar]
- Tjong, S.C.; Ma, Z.Y. Microstructural and mechanical characteristics of in situ metal matrix composites. Mater. Sci. Eng. R Rep. 2000, 29, 49–113. [Google Scholar] [CrossRef]
- Wu, R. Recent Development of Metal Matrix Composites. Acta Mater. Compos. Sin. 1987, 4, 1–9. [Google Scholar]
- Idusuyi, N.; Olayinka, J.I. Dry sliding wear characteristics of aluminum metal matrix composites: A brief overview. J. Mater. Res. Technol. 2019, 8, 3338–3346. [Google Scholar] [CrossRef]
- Hill, H.; Weber, S.; Huth, S.; Niederhofer, P.; Theisen, W. The impact of processing on microstructure, single-phase properties and wear resistance of MMCs. Wear 2011, 271, 1895–1902. [Google Scholar] [CrossRef]
- Pezzato, L.; Brunelli, K.; Dolcet, P.; Dabalà, M. Plasma electrolytic oxidation coating produced on 39NiCrMo3 steel. Surf. Coat. Technol. 2016, 307, 73–80. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Gou, J.; Zhang, Q.; Zhou, F.; Wang, Y.; Chu, R. Study on the wear resistance of graphene-modified nanostructured Al2O3/TiO2 coatings. Appl. Surf. Sci. 2019, 492, 272–279. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, R.; Zhao, X.; Chen, C.; Pan, R. Study on the surface microstructure evolution and wear property of bainitic rail steel under dry sliding wear. Wear 2020, 448, 203–217. [Google Scholar] [CrossRef]
- Ge, W.; Li, X.; Cheng, X.; Zhang, Q. Research Progress of Preparation and Surface-treatment Technology of Metal-Ceramic Composite Coatings. Dev. Appl. Mater. 2011, 26, 95–99. [Google Scholar]
- Cao, X.; Jin, J.F.; Cao, J.Y.; Zong, Y. Wear Resistance of Iron Matrix Composites Reinforced by Mixed-type Particles. J. Mater. Eng. 2017, 45, 62–67. [Google Scholar]
- Mo, J.; Tu, X.; Zheng, B.; Li, W. Fabrication and Abrasion Resistance of ZTA Particle Reinforced High Chromium Cast Iron Matrix Composites. Hot Work. Technol. 2020, 49, 57–61. [Google Scholar]
- Javdani, A.; Daei-Sorkhabi, A.H. Mechanical behavior of blended powder semisolid formed Al7075/B4C composites under different experimental conditions. Trans. Nonferrous Met. Soc. China 2018, 28, 1298–1310. [Google Scholar] [CrossRef]
- Hassan, S.F.; Gupta, M. Development of a novel magnesium-Ni composite with improved mechanical properties. J. Alloys Compd. 2002, 335, L10–L15. [Google Scholar] [CrossRef]
- Perez, P.; Garces, G.; Adeva, P. Mechanical properties of an Mg-10(vol.%)Ti composite. Compos. Sci. Technol. 2004, 64, 145–151. [Google Scholar]
- Matin, M.A.; Lu, L.; Gupta, M. Investigation of the reactions between boron and titanium compounds with magnesium. Scr. Mater. 2001, 45, 479–486. [Google Scholar] [CrossRef]
- Premkumar, M.K.; Chu, M.G. Al-TiC particulate composite produced by a liquid state in-situ process. Mater. Sci. Eng. A 1995, 202, 172–178. [Google Scholar] [CrossRef]
- Zhang, E.; Zeng, S.; Li, Q.; Bo, Y.; Ma, M. A study on the kinetic process of reaction synthesis of TiC: Part I. Experimental research and theoretical model. Metall. Mater. Trans. A 1999, 30, 1147–1151. [Google Scholar] [CrossRef]
- Jia, H.; Liu, J.; Sun, Z. Effect of introduction of tantalum carbide on preparation and properties of titano-silicon-carbon ceramics. Sci. Technol. Innov. 2020, 3, 73–74. [Google Scholar]
- Jianfeng, H. Research on Wear Resistance of Surface Microstructure of GCr15 Bearing Steel Based on Strengthened Grinding Processing. Ph.D. Thesis, Guangzhou University, Guangzhou, China.
- Agnieszka, G. Pressureless sintering of single-phase tantalum carbide and niobium carbide. Eur. Ceram. Soc. 2013, 33, 2391–2398. [Google Scholar]
- Hung, F.Y.; Yan, Z.Y.; Chen, L.H. Microstructural characteristics of PTA-overlayed NbC on pure Ti. Surf. Coat. Technol. 2006, 200, 6881–6887. [Google Scholar] [CrossRef]
- Barzilai, S.; Raveh, A.; Frage, N. Inter-diffusion of carbon into niobium coatings deposited on graphite. Thin Solid Film. 2006, 496, 450–456. [Google Scholar] [CrossRef]
- Mesquita, R.A.; Schuh, C.A. Tool steel coatings based on niobium carbide and carbon nitride compounds. Surf. Coat. Technol. 2012, 207, 472–479. [Google Scholar] [CrossRef]
- Yang, Y.; Wen, S.; Wei, Q.; Li, W.; Liu, J.; Shi, Y. Effect of scan line spacing on texture, phase and nano hardness of TiAl/TiB2 metal matrix composites fabricated by selective laser melting. J. Alloys Compd. 2017, 728, 803–814. [Google Scholar] [CrossRef]





| Materials | C | Si | Mn | Cr | Ni | Mo | Cu | Fe | Ta |
|---|---|---|---|---|---|---|---|---|---|
| GCr15 | 0.93 | 0.22 | 0.61 | 1.70 | 0.17 | 0.22 | 0.19 | Bal. | - |
| Ta plate | - | - | - | - | - | - | - | - | 99.9 |
| Atomic Radius (Ǻ) | Molecular Weight | Space Group | Maximum Solubility in α-Fe wt. (%) | Maximum Solubility in γ-Fe wt. (%) | Crystal Structure | ||
|---|---|---|---|---|---|---|---|
| Type | Cell Parameter | ||||||
| Ta | 2.09 | - | Im-3m (229) | Finite | Finite | bcc | a = b = c = 330.58 pm α = β = γ = 90° |
| Ta2C | - | 374 | P-3ml (164) | - | - | hcp | a = b = 310.46 pm c = 494.44 pm α = β = 90° γ = 120° |
| TaC | - | 193 | Fm-3m (225) | - | 0.5–1.0 (1250 °C) | fcc | a = b = c = 445.47 pm α = β = γ = 90° |
| C | 0.91 | - | - | 0.0218 | 2.11 | - | - |
| α-Fe | 1.430 | - | Im-3m (229) | - | - | bcc | a = b = c = 286.60 pm α = β = γ = 90° |
| γ-Fe | 1.425 | - | Fm-3m (225) | - | - | fcc | a = b = c = 365.99 pm α = β = γ = 90° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, R.; Xu, Y.; Liu, Z.; Chen, L.; Zhu, Y. Study on the Interface Microstructure of TaC/GCr15 Steel Surface Reinforced Layer Formed by In-Situ Reaction. Materials 2023, 16, 3790. https://doi.org/10.3390/ma16103790
Li J, Li R, Xu Y, Liu Z, Chen L, Zhu Y. Study on the Interface Microstructure of TaC/GCr15 Steel Surface Reinforced Layer Formed by In-Situ Reaction. Materials. 2023; 16(10):3790. https://doi.org/10.3390/ma16103790
Chicago/Turabian StyleLi, Jilin, Ruixue Li, Yunhua Xu, Zhuolin Liu, Le Chen, and Yao Zhu. 2023. "Study on the Interface Microstructure of TaC/GCr15 Steel Surface Reinforced Layer Formed by In-Situ Reaction" Materials 16, no. 10: 3790. https://doi.org/10.3390/ma16103790
APA StyleLi, J., Li, R., Xu, Y., Liu, Z., Chen, L., & Zhu, Y. (2023). Study on the Interface Microstructure of TaC/GCr15 Steel Surface Reinforced Layer Formed by In-Situ Reaction. Materials, 16(10), 3790. https://doi.org/10.3390/ma16103790







