Catalytic Oxidation Activity of NO over Mullite-Supported Amorphous Manganese Oxide Catalyst
Abstract
:1. Introduction
2. Materials and Methods
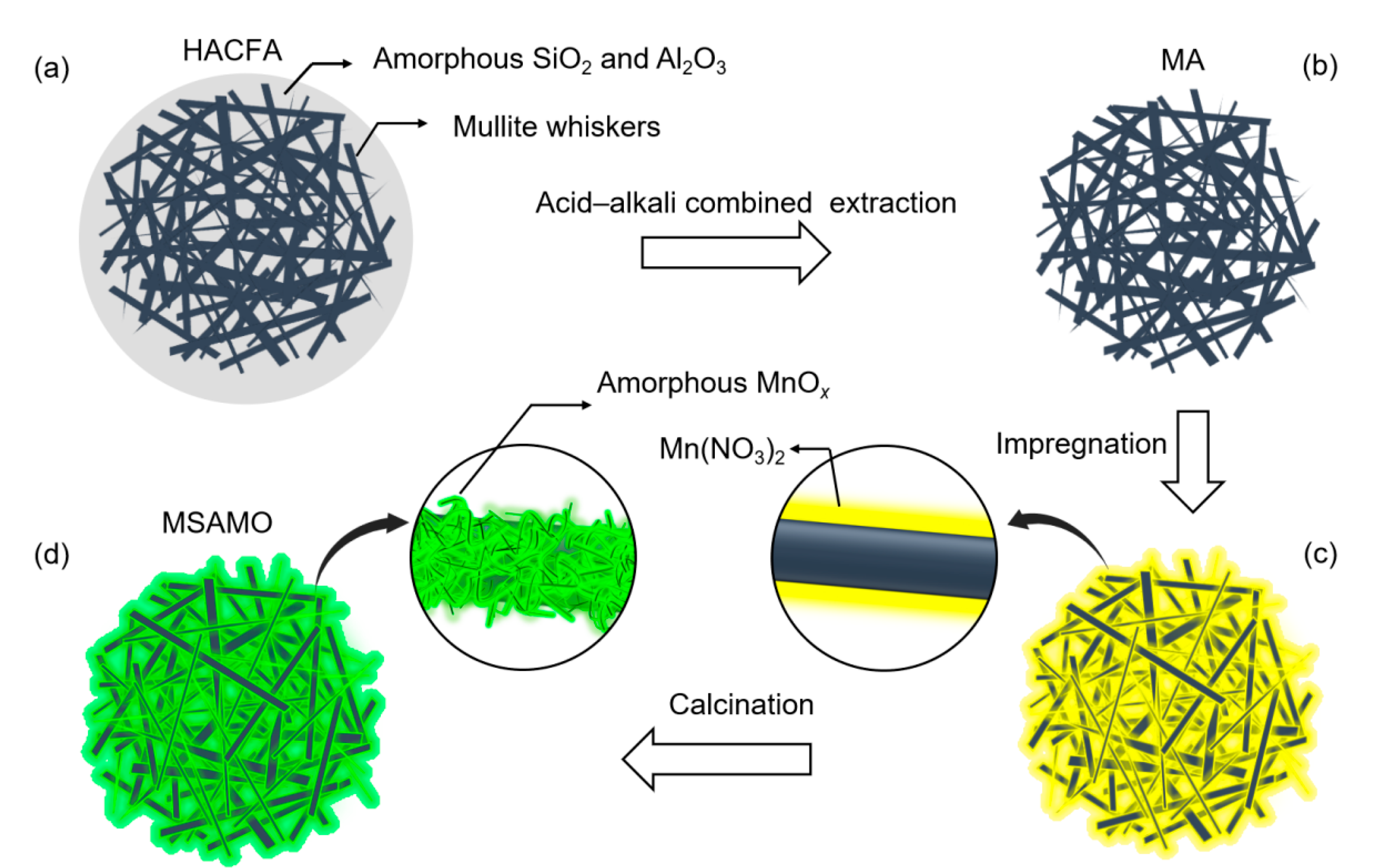
2.1. Catalyst Preparation
2.2. Catalyst Characterization
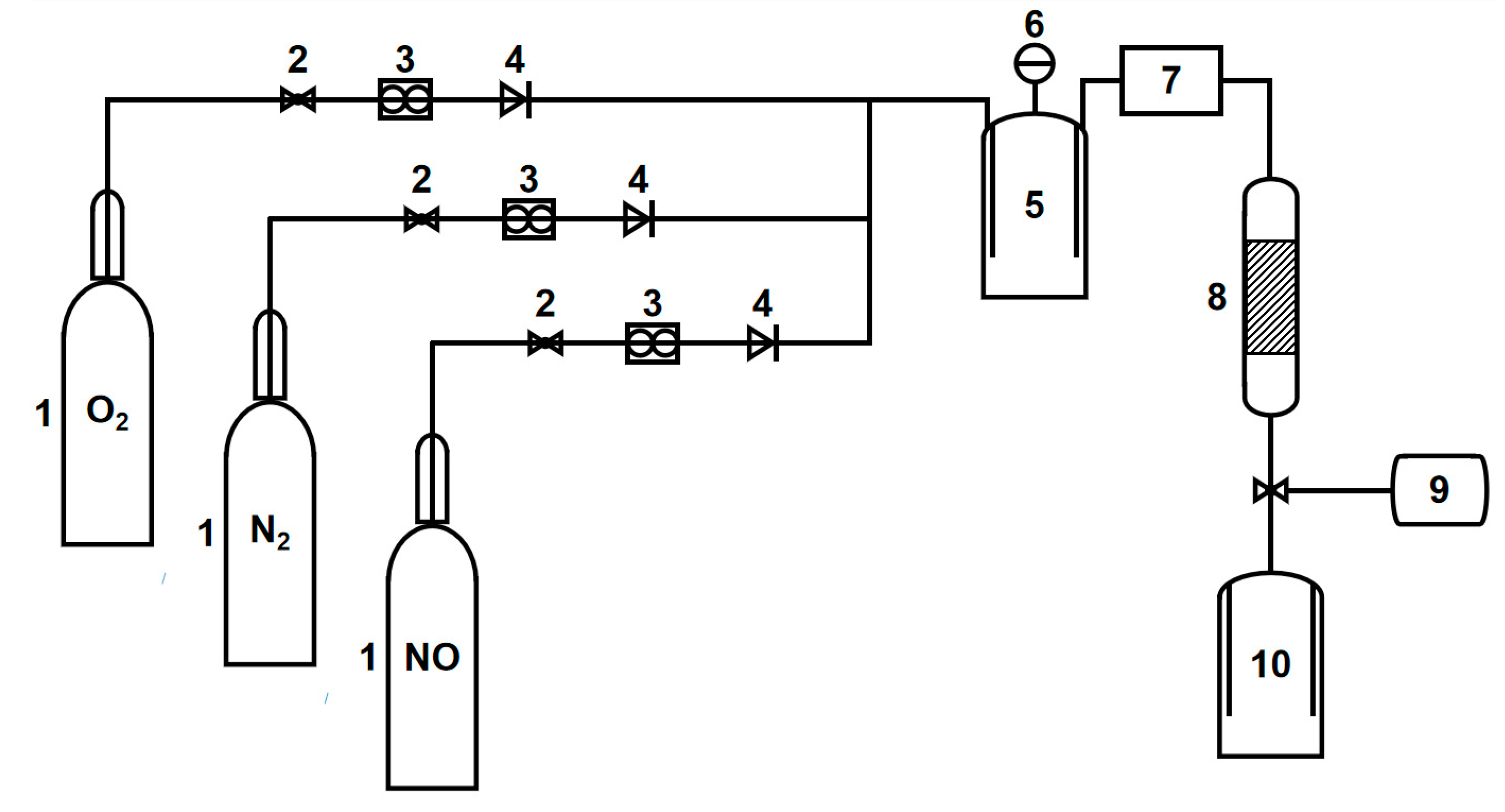
2.3. Evaluation Method of Catalyst Activity
3. Results and Discussion
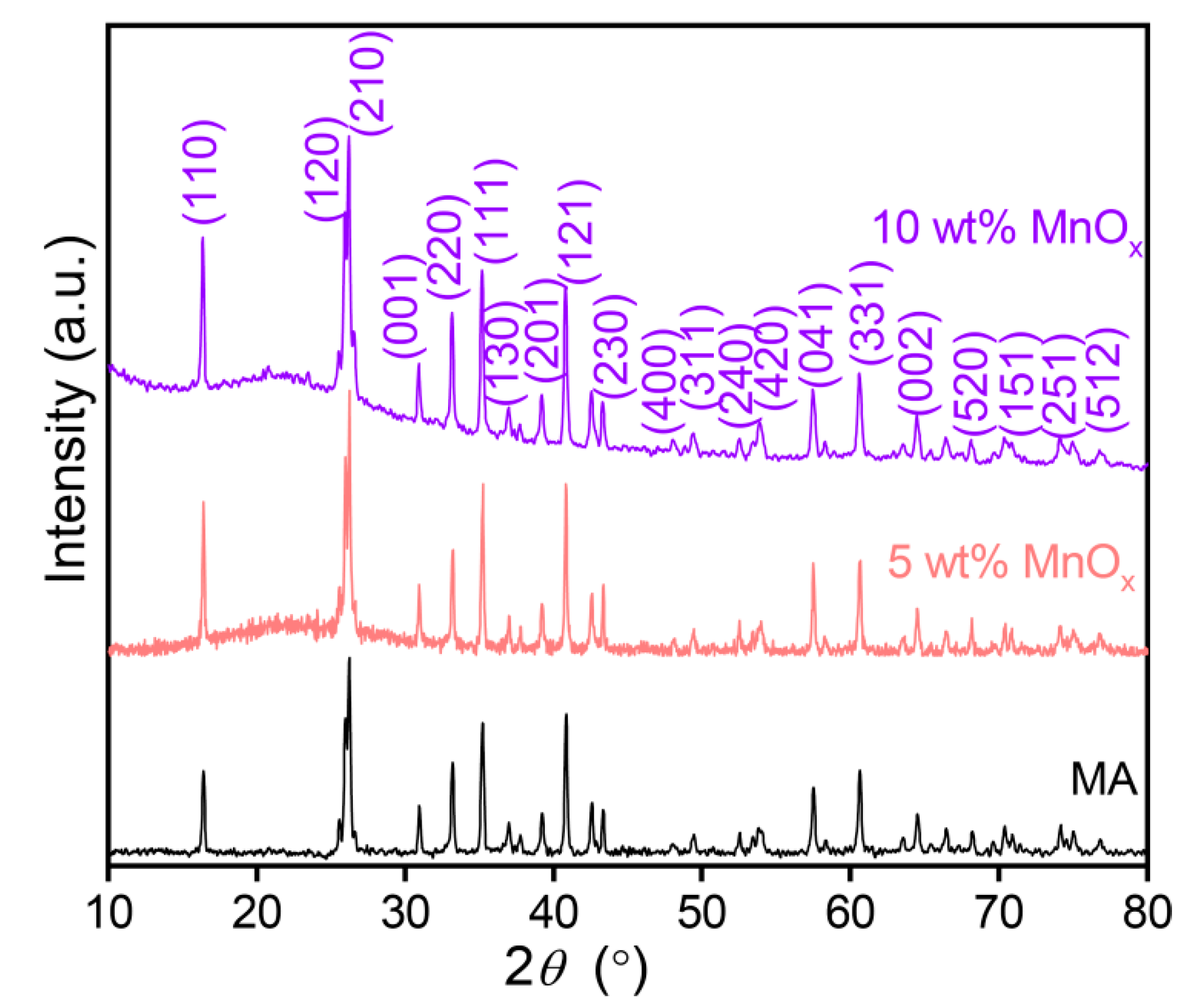
3.1. Phase Analysis
3.2. Microscopic Morphology Analysis
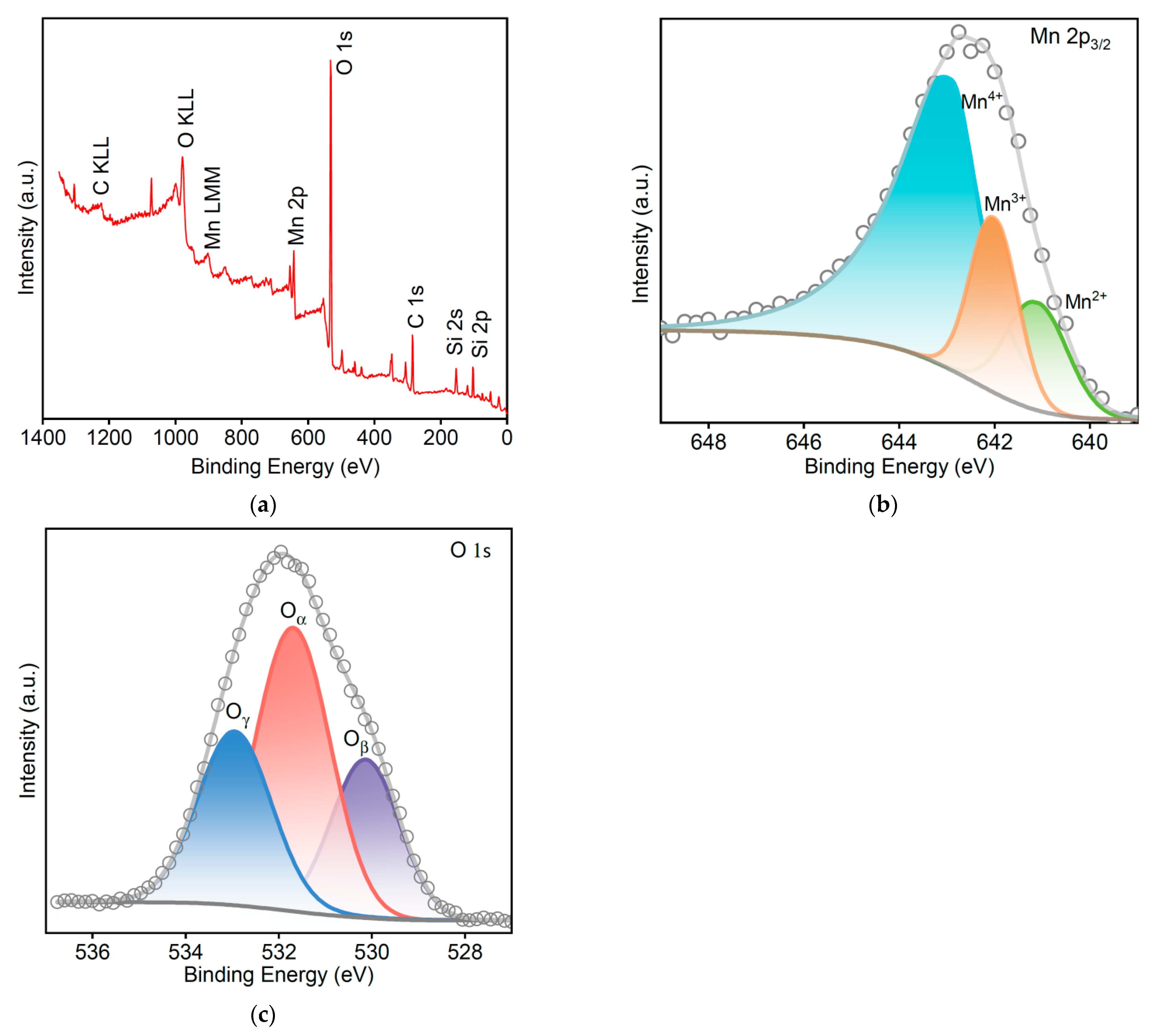
3.3. XPS Analysis of MSAMO Catalyst
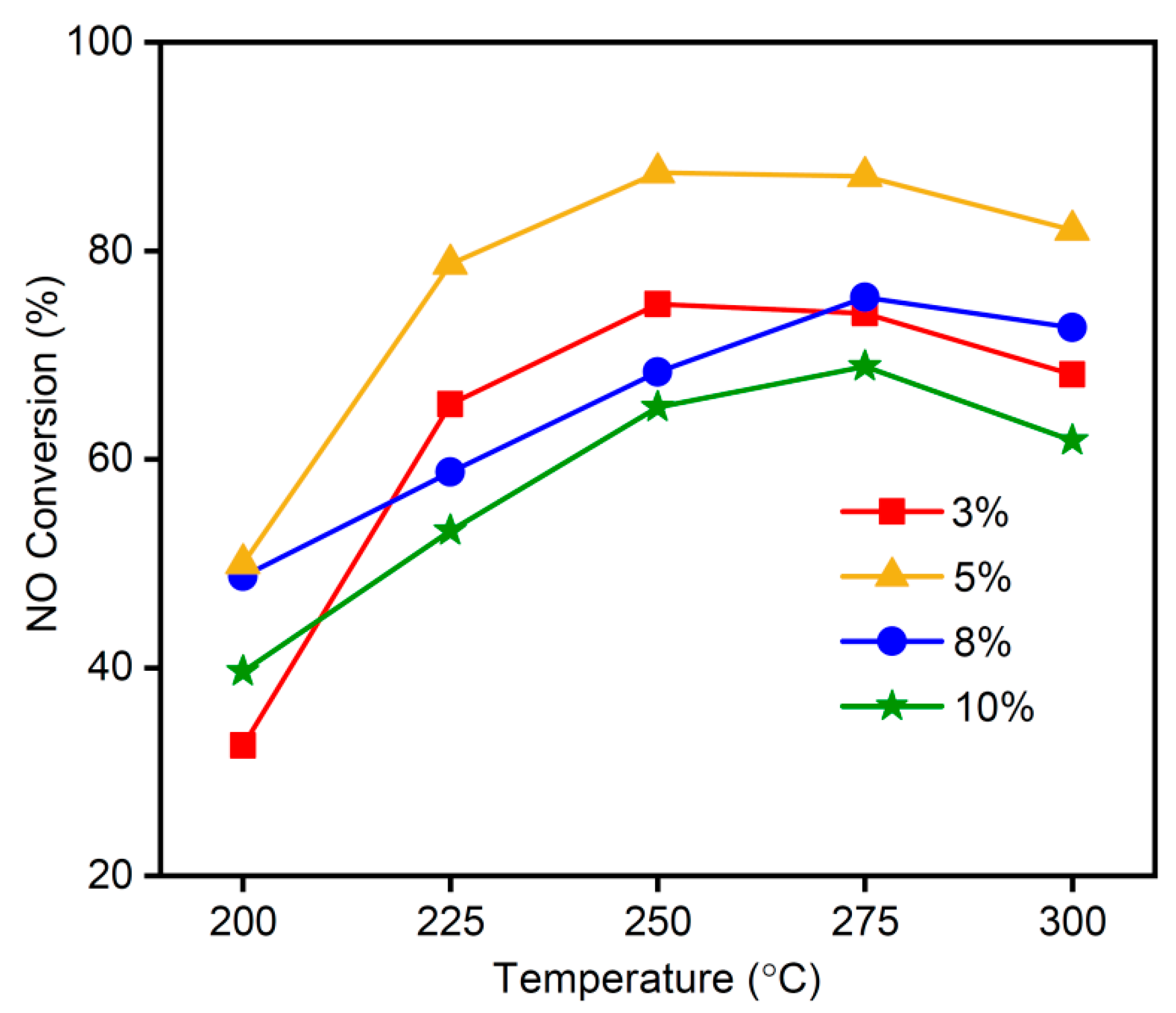
3.4. Effect of MnOx Quantity on NO Conversion Rate
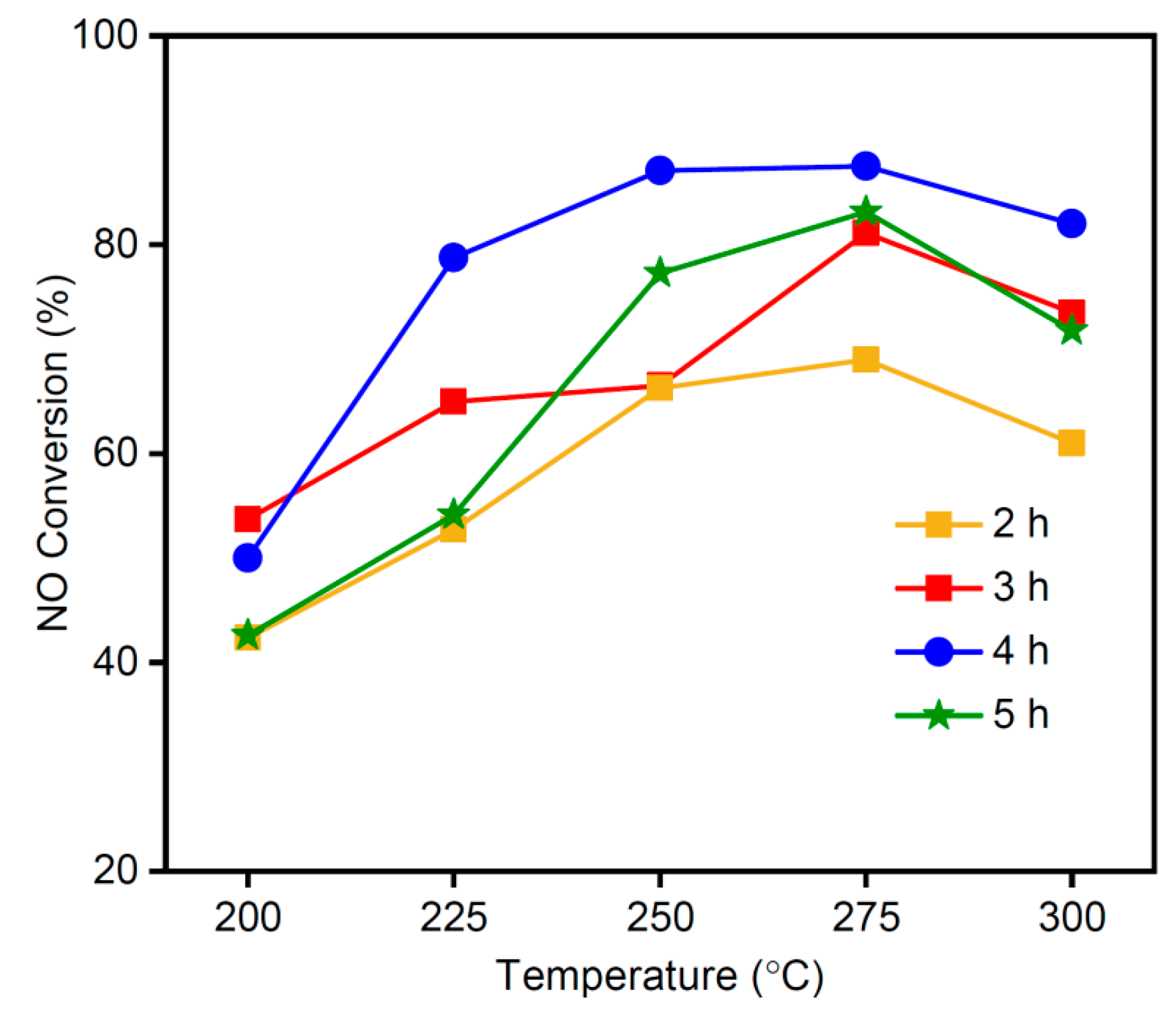
3.5. Effect of Calcination Time on NO Conversion Rate
3.6. Effect of Oxygen Content on NO Conversion Rate
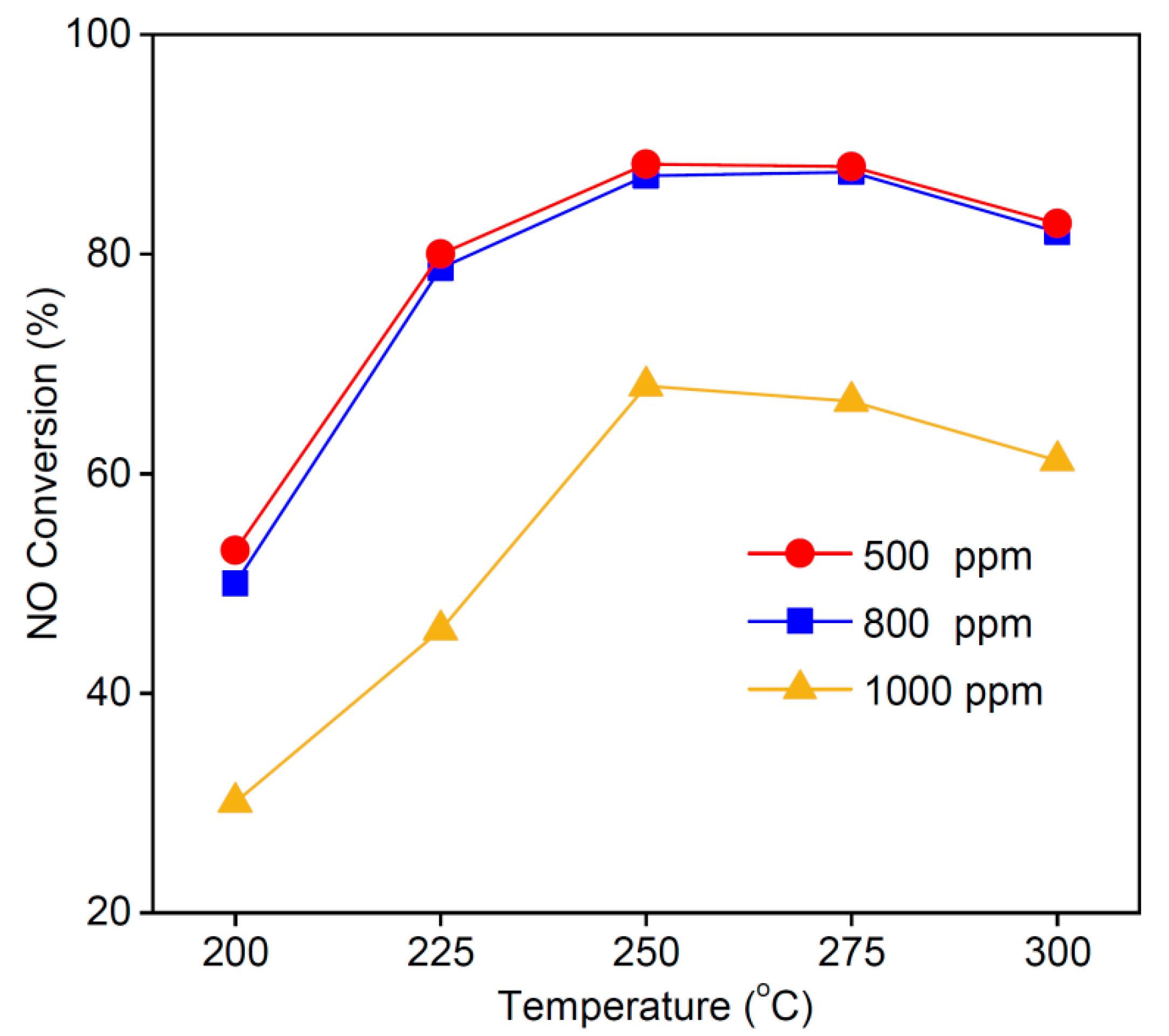
3.7. Effect of NO Concentration on NO Conversion Rate
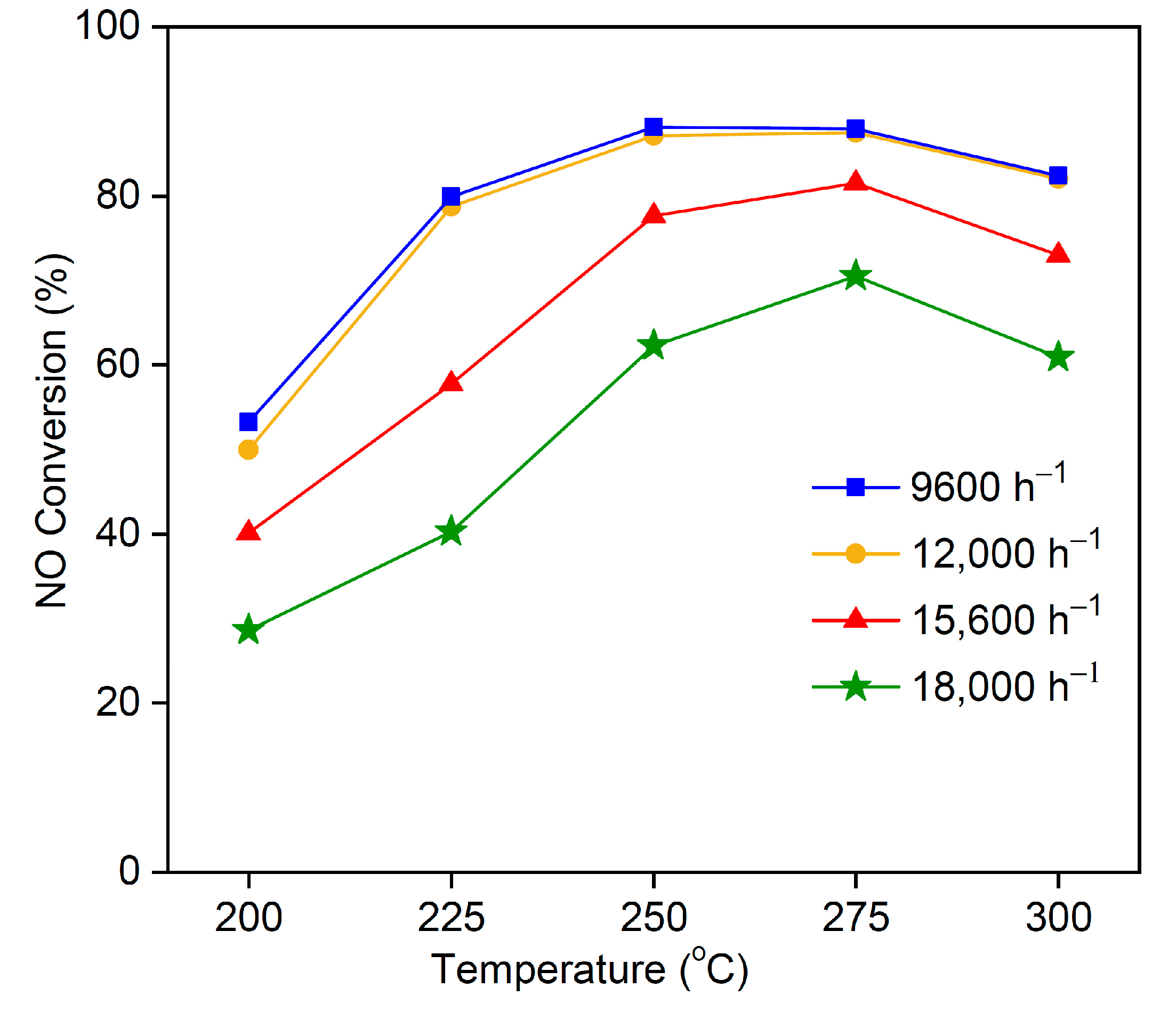
3.8. Effect of Space Velocity on NO Conversion Rate
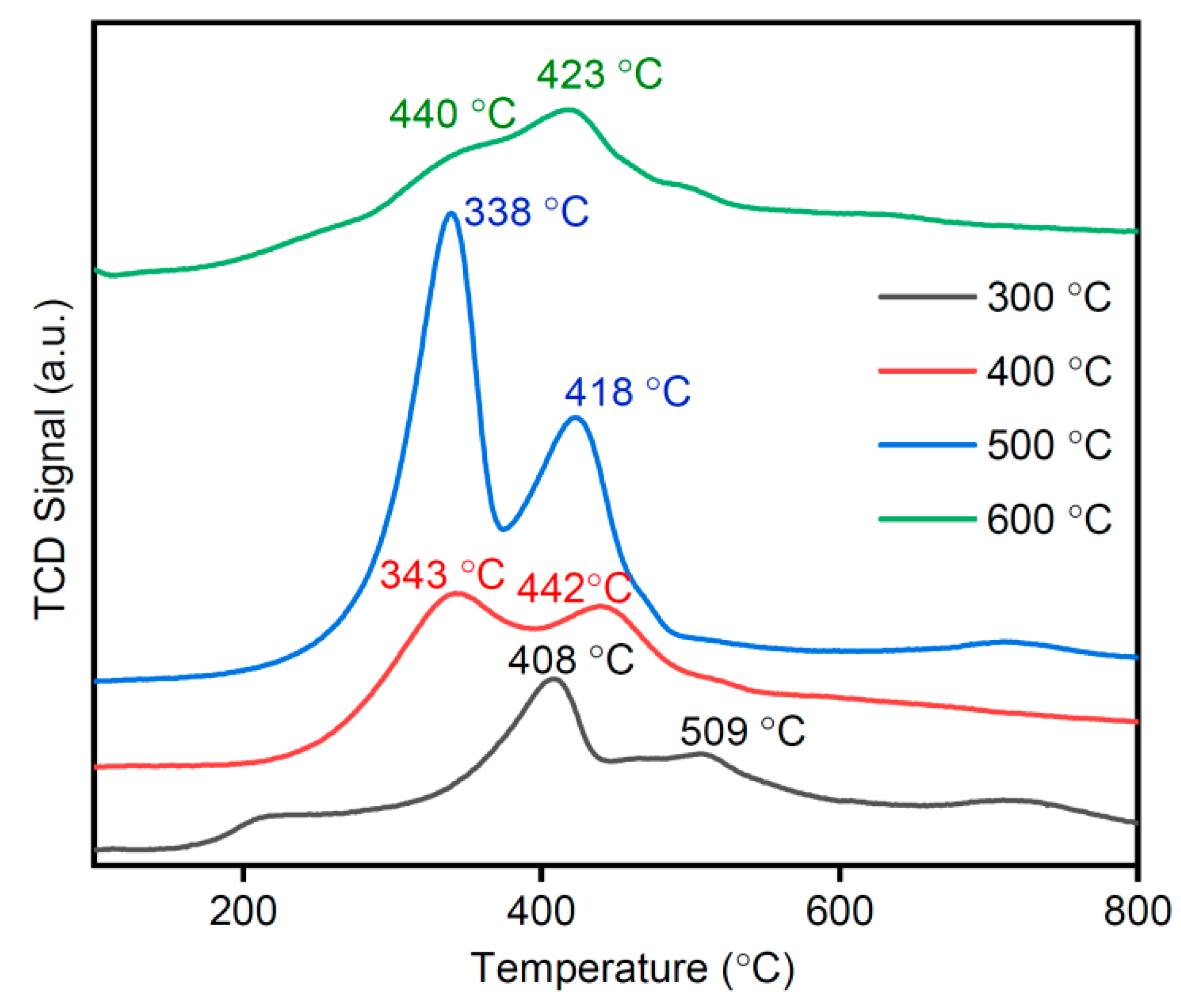
3.9. H2-TPR Analysis
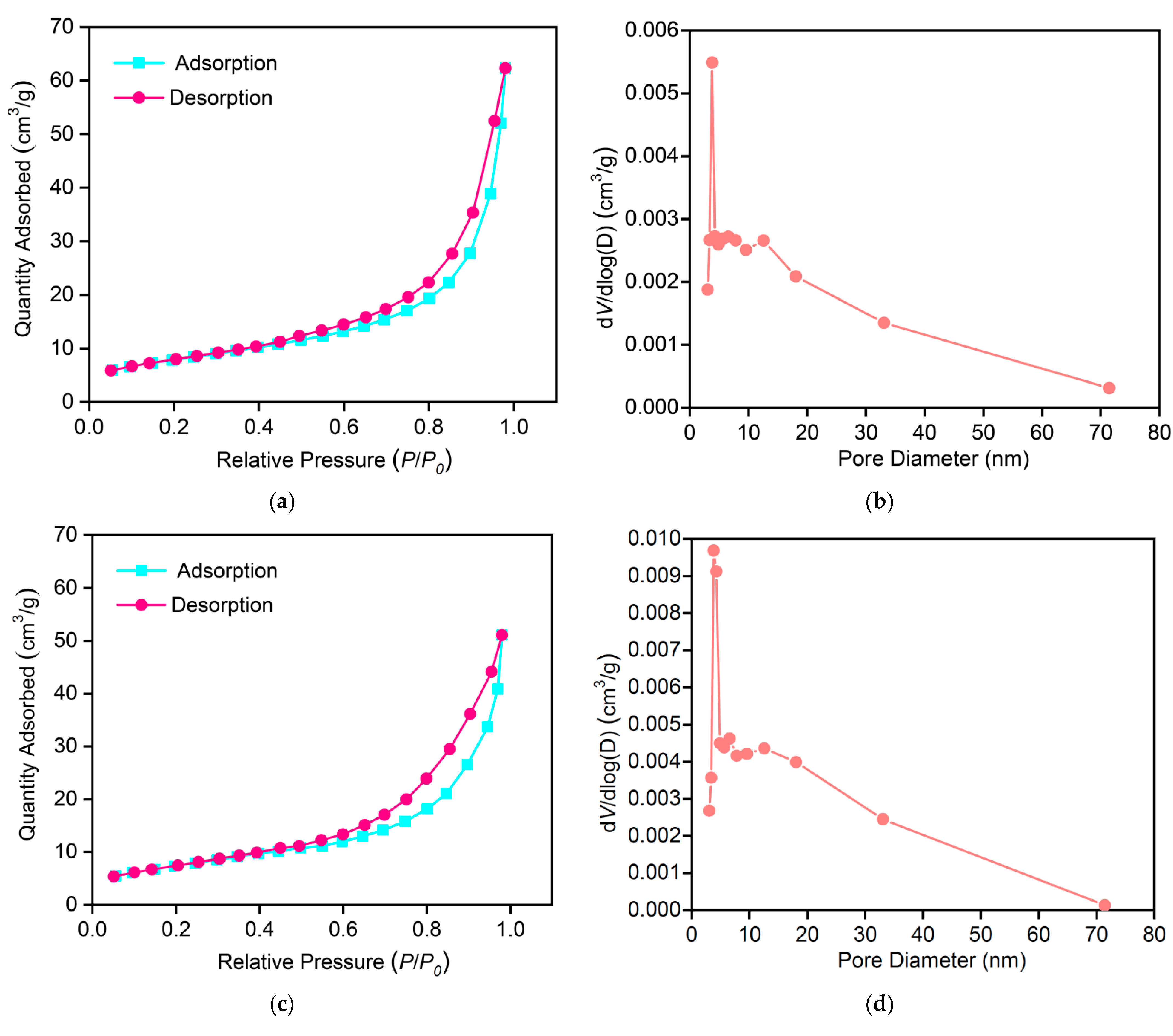
3.10. BET Measurement
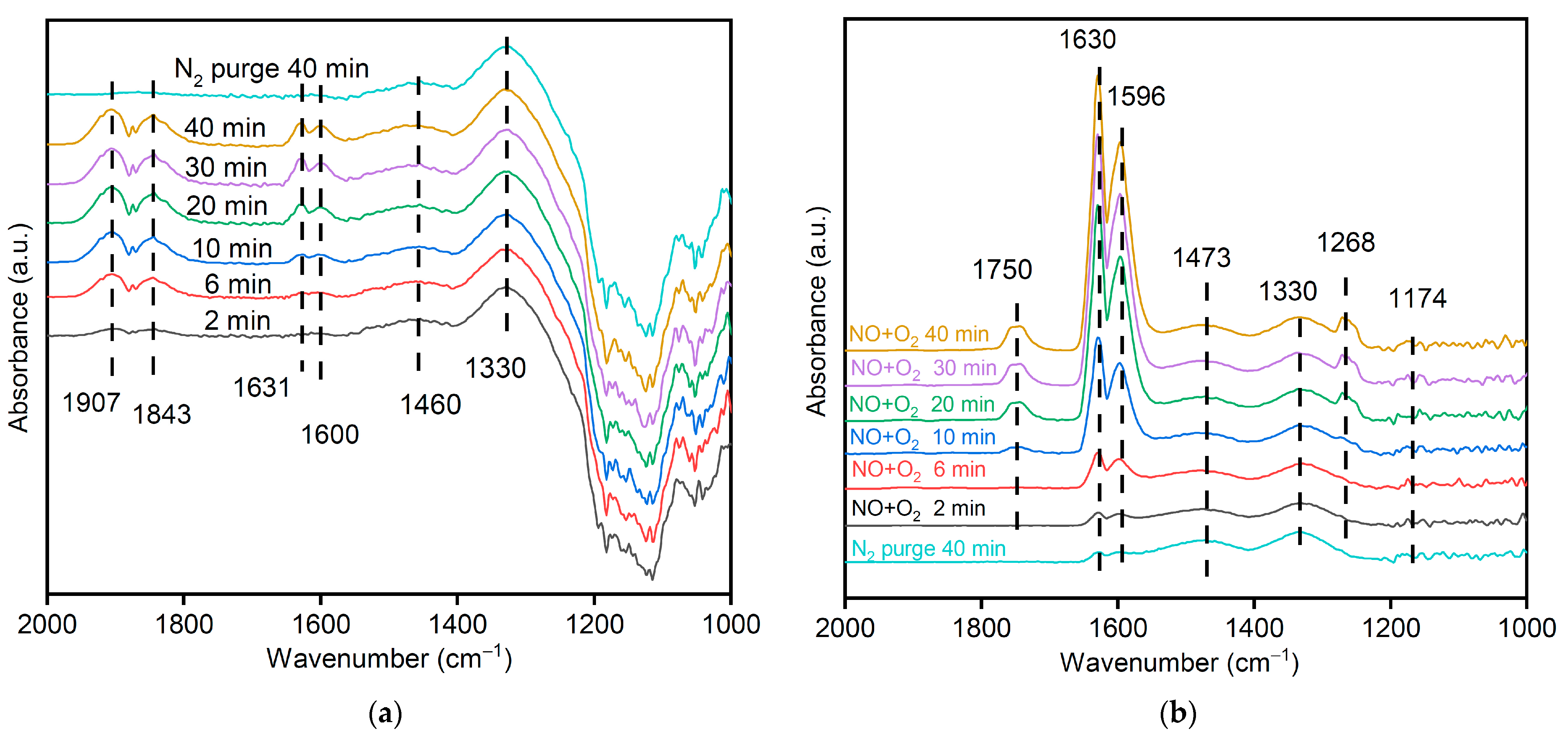
3.11. Mechanism of NO Catalytic Oxidation with MSAMO Catalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, L.; Yang, Q.; Peng, L.; Xie, C.; Luo, K.; Zhou, L. Molecular engineering-based a dual-responsive fluorescent sensor for sulfur dioxide and nitric oxide detecting in acid rain and its imaging studies in biosystems. J. Hazard. Mater. 2022, 435, 128947. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, H.; Jackiewicz-Rek, W.; Jarosławski, J.; Chilmon, K.; Szkop, A. Ozone formation during photocatalytic oxidation of nitric oxides under UV irradiation with the use of commercial TiO2 photocatalytic powders. Materials 2022, 15, 5905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, C.; Zhang, L.; He, H.; Luo, M.; Jia, Y.; Li, Y. Application of plasma treatment in preparation of soybean oil factory sludge catalyst and its application in selective catalytic oxidation (SCO) denitration. Materials 2018, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, Z.; Jia, Y.; Han, Y. Preparation of MnO2@CeO2 core–shell catalyst and its application in SCO denitration performance. Appl. Phys. A Mater. Sci. Process. 2022, 128, 568. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, J.; Zhao, Z.; Chen, Y.; Xu, C.; Duan, A.; Jiang, G.; He, H. Highly active catalysts of gold nanoparticles supported on three-dimensionally ordered macroporous LaFeO3 for soot oxidation. Angew. Chem. Int. Ed. 2011, 50, 2326–2329. [Google Scholar] [CrossRef]
- Feng, H.; Li, H.; Liu, X.; Huang, Y.; Pan, Q.; Peng, R.; Du, R.; Zheng, X.; Yin, Z.; Li, S.; et al. Porphyrin-based Ti-MOFs conferred with single-atom Pt for enhanced photocatalytic hydrogen evolution and NO removal. Chem. Eng. J. 2022, 428, 132045. [Google Scholar] [CrossRef]
- Biswakarma, N.; Sarma, P.; Baruah, S.; Gour, N.; Deka, R. Catalytic oxidation of NO on [Au-M]- (M = Pd and Pt) bimetallic dimers: An insight from density functional theory approach. J. Phys. Chem. C. 2020, 124, 3059–3068. [Google Scholar] [CrossRef]
- Adjimi, S.; García-Vargas, J.; Díaz, J.; Retailleau, L.; Gil, S.; Pera-Titus, M.; Guo, Y.; Giroir-Fendler, A. Highly efficient and stable Ru/K-OMS-2 catalyst for NO oxidation. Appl. Catal. B Environ. 2017, 219, 459–466. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, X.; Dai, J.; Zhao, M.; Zhong, L.; Wang, J.; Chen, Y. Active oxygen-promoted NO catalytic on monolithic Pt-based diesel oxidation catalyst modified with Ce. Catal. Today 2019, 327, 64–72. [Google Scholar] [CrossRef]
- Tang, N.; Liu, Y.; Wang, H.; Wu, Z. Mechanism study of NO catalytic oxidation over MnOx/TiO2 catalysts. J. Phys. Chem. C 2011, 115, 8214–8220. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Y.; Yang, L.; Yang, M.; Zhang, Y.; Ma, C.; Meng, X.; Xu, J.; Wang, J.; Qiao, W. Understanding structure-performance relationships of CoOx/CeO2 catalysts for NO catalytic oxidation: Facet tailoring and bimetallic interface designing. J. Hazard. Mater. 2023, 451, 131144. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Hu, Y.; Cheng, T.; Jiang, Z.; Wang, Z.; Zhu, C. Low-temperature selective catalytic reduction of NO with NH3 over an FeOx/β-MnO2 composite. RSC Adv. 2023, 13, 6378–6388. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Sun, Y.; Shen, Q.; Zhang, X.; Zhang, S. Insight into structure defects and catalytic mechanism for NO oxidation over Ce0.6Mn0.4Ox solid solutions catalysts: Effect of manganese precursors. Chemosphere 2020, 243, 125406. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, H.; Wang, Y.; Lyu, Y. Performance and mechanism comparison of manganese oxides at different valence states for catalytic oxidation of NO. Chem. Eng. J. 2019, 361, 1161–1172. [Google Scholar] [CrossRef]
- Qi, G.; Li, W. NO oxidation to NO2 over manganese-cerium mixed oxides. Catal. Today 2015, 258, 205–213. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Lyu, Y. In-situ DRIFTS for reaction mechanism and SO2 poisoning mechanism of NO oxidation over γ-MnO2 with good low-temperature activity. Catal. Lett. 2018, 149, 753–765. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Chu, C.; Li, N.; Li, J.; Zhao, S. In-situ DRIFTS for the mechanistic studies of NO oxidation over α-MnO2, β-MnO2 and γ-MnO2 catalysts. Chem. Eng. J. 2017, 322, 525–537. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Lv, Y. Catalytic oxidation of NO over MnO2 with different crystal structures. RSC Adv. 2016, 6, 54032–54040. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.; Dai, Q.; Li, D. Effect of Ce and La on the structure and activity of MnOx catalyst in catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2012, 111–112, 141–149. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Z.; Cui, C.; Wang, B.; Liu, W.; Liu, W.; Wang, L. Amorphous manganese oxide as highly active catalyst for soot oxidation. Environ. Sci. Pollut. Res. 2020, 27, 13488–13500. [Google Scholar] [CrossRef]
- Schmitz, P.; Kudla, R.; Drews, A.; Chen, A.; Lowe-Ma, C.; McCabe, R.; Schneider, W.; Goralski, C., Jr. NO oxidation over supported Pt: Impact of precursor, support, loading, and processing conditions evaluated via high throughput experimentation. Appl. Catal. B Environ. 2006, 67, 246–256. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Xian, G.; Zhang, N.; Li, J. A high-efficiency CuO/CeO2 catalyst for diclofenac degradation in Fenton-like system. Front. Chem. 2019, 7, 796. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Ding, X.; Zhao, Y.; Deng, X.; Wang, D.; Jin, X.; Ran, S. Preparation of mullite whiskers from high alumina fly ash and its reinforced porous structure. J. Mater. Res. Technol. 2023, 24, 3323–3333. [Google Scholar] [CrossRef]
- Ji, H.; Mi, X.; Tian, Q.; Liu, C.; Yao, J.; Ma, S.; Zeng, G. Recycling of mullite from high-alumina coal fly ash by a mechanochemical activation method: Effect of particle size and mechanism research. Sci. Total Environ. 2021, 784, 147100. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, L.; Ma, S.; Wang, X.; Wang, Y. Study on preparation and performance of mullite-corundum catalyst carrier. Chin. J. Process. Eng. 2022, 22, 79–88. [Google Scholar] [CrossRef]
- El-Shater, R.; Shimy, H.; Saafan, S.; Darwish, M.; Zhou, D.; Trukhanov, A.; Trukhanov, S.; Fakhry, F. Synthesis, characterization, and magnetic properties of Mn nanoferrites. J. Alloys Compd. 2022, 928, 166954. [Google Scholar] [CrossRef]
- Ming, S.; Wang, P.; Liu, P.; Duan, J.; Mei, F.; Pang, L.; Chen, Z.; Li, T. Promotional effect of metal cations doping on OMS-2 catalysts for NH3-SCR reaction. Chem. Eng. J. 2020, 379, 122287. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, B.; Liu, Y.; Liu, J.; Hu, L.; Tang, Y.; Wang, M. In-situ regulation of acid sites on Mn-based perovskite@mullite composite for promoting catalytic oxidation of chlorobenzene. J. Colloid Interface Sci. 2022, 606, 1866–1873. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Qu, L.; Fu, M.; Zeng, G.; Fan, C.; Ma, J.; Zhan, F. Catalytic oxidation of NO with O2 over FeMnOx/TiO2: Effect of iron and manganese oxides loading sequences and the catalytic mechanism study. Appl. Surf. Sci. 2014, 300, 58–65. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, N.; Xiao, L.; Liu, Y.; Wang, H. MnOx/TiO2 composite nanoxides synthesized by deposition-precipitation method as a superior catalyst for NO oxidation. J Colloid Interf. Sci. 2010, 352, 143–148. [Google Scholar] [CrossRef]
- Cui, M.; Li, Y.; Wang, X.; Wang, J.; Shen, M. Effect of preparation method on MnOx-CeO2 catalysts for NO oxidation. J. Rare Earth. 2013, 31, 572–576. [Google Scholar] [CrossRef]
- Wei, M.; Zhao, X.; Fu, L.; Zhang, S. Performance study and application of new coal-fired boiler flue gas heat recovery system. Appl. Energ. 2017, 188, 121–129. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, L.; Zhang, C.; He, H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B Environ. 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Yang, X.; Song, S.; Li, J.; Jing, F.; Chu, W. Enhanced catalytic performances of in situ-assembled LaMnO3/δ-MnO2 hetero-structures for toluene combustion. Catal. Today 2019, 327, 19–27. [Google Scholar] [CrossRef]
- Tang, R.; Hong, W.; Srinivasakannan, C.; Liu, X.; Wang, X.; Duan, X. A novel mesoporous Fe-silica aerogel composite with phenomenal adsorption capacity for malachite green. Sep. Purif. Technol. 2022, 281, 119950. [Google Scholar] [CrossRef]
- Keshri, K.; Bhattacharjee, S.; Singha, A.; Bhaumik, A.; Chowdhury, B. Synthesis of cyclic carbonates of different epoxides using CO2 as a C1 building block over Ag/TUD-1 mesoporous silica catalyst: A solvent free approach. Mol. Catal. 2022, 522, 112234. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, J.; Wang, H.; Zhu, T.; Li, P.; Jing, P. Catalytic oxidation activity of NO on TiO2-supported Mn-Co composite oxide catalysts. Acta Phys. Chim. Sin. 2013, 29, 385–390. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Lyu, Y. High catalytic activity of Mn-based catalyst in NO oxidation at low temperature and over a wide temperature span. Mol. Catal. 2018, 454, 21–29. [Google Scholar] [CrossRef]
- Jia, J.; Ran, R.; Guo, X.; Wu, X.; Chen, W.; Weng, D. Enhanced low-temperature NO oxidation by iron-modified MnO2 catalysts. Catal. Commun. 2019, 119, 139–143. [Google Scholar] [CrossRef]
- Mou, C.; Li, H.; Dong, N.; Hui, S.; Wang, D. Effect of Ce addition on adsorption and oxidation of NO over MnO/Al2O3. Adsorpt. Sci. Technol. 2021, 2021, 3131309. [Google Scholar] [CrossRef]



| Oxides | Al2O3 | SiO2 | CaO | Fe2O3 | TiO2 | LOI |
|---|---|---|---|---|---|---|
| Content | 46.62 | 42.81 | 3.44 | 2.75 | 2.04 | 2.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhao, L.; Zhou, T.; Ma, S.; Wang, X. Catalytic Oxidation Activity of NO over Mullite-Supported Amorphous Manganese Oxide Catalyst. Materials 2023, 16, 3821. https://doi.org/10.3390/ma16103821
Yang J, Zhao L, Zhou T, Ma S, Wang X. Catalytic Oxidation Activity of NO over Mullite-Supported Amorphous Manganese Oxide Catalyst. Materials. 2023; 16(10):3821. https://doi.org/10.3390/ma16103821
Chicago/Turabian StyleYang, Jianlin, Lu Zhao, Tianran Zhou, Shuhua Ma, and Xiaohui Wang. 2023. "Catalytic Oxidation Activity of NO over Mullite-Supported Amorphous Manganese Oxide Catalyst" Materials 16, no. 10: 3821. https://doi.org/10.3390/ma16103821




