Investigation of the Effect of Volumetric Hydrophobization on the Kinetics of Mass Transfer Processes Occurring in Cement Concretes during Corrosion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method of Determining the Grade of Concrete by Water Resistance
- –
- for concrete with a large aggregate: M = 6 + 300;
- –
- for fine-grained concrete: M = 12 + 540.
2.3. Determination of Density, Water Absorption, and Porosity of Concrete
2.4. Determination of the Compressive Strength of Cement Stone
2.5. Differential Thermal Analysis of Cement Stone
2.6. Quantitative Analysis of Calcium Ions in a Liquid Medium by the Method of Complexometry
2.7. Mathematical Model of the Second Type of Corrosion of Cement Concretes
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jedidi, M.; Benjeddou, O. Chemical Causes of Concrete Degradation. MOJ Civ. Eng. 2018, 4, 00095. [Google Scholar] [CrossRef]
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Redaelli, E.; Polder, R.B. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2013; 434p. [Google Scholar]
- Asamoto, S.; Sato, J.; Okazaki, S.; Chun, P.-J.; Sahamitmongkol, R.; Nguyen, G.H. The Cover Depth Effect on Corrosion-Induced Deterioration of Reinforced Concrete Focusing on Water Penetration: Field Survey and Laboratory Study. Materials 2021, 14, 3478. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Rumyantseva, V.E.; Krasilnikov, I.V.; Krasilnikova, I.A. Research of physical and chemical processes in the system “cement concrete—Liquid aggressive environment”. ChemChemTech 2022, 65, 61–70. [Google Scholar]
- Ortega, N.F.; Moro, J.M.; Meneses, R. Corrosion in Concrete Structures with Permanent Deformation in Marine Environment. Open Constr. Build. Technol. J. 2017, 11, 14–24. [Google Scholar] [CrossRef]
- Claisse, P.A. Transport Properties of Concrete: Modelling the Durability of Structures, 2nd ed.; Woodhead Publishing: Sawston, UK, 2020; 328p. [Google Scholar]
- Ding, X.; Liang, X.; Zhang, Y.; Fang, Y.; Zhou, J.; Kang, T. Capillary Water Absorption and Micro Pore Connectivity of Concrete with Fractal Analysis. Crystals 2020, 10, 892. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Ma, R.; Yang, L.; Hu, Y.; Zhang, J. Water-repellent additive that increases concrete cracking resistance in dry curing environments. Constr. Build. Mater. 2020, 249, 118704. [Google Scholar] [CrossRef]
- Tuskaeva, Z.; Karyaev, S. Influence of various additives on properties of concrete. E3S Web Conf. 2020, 164, 14007. [Google Scholar] [CrossRef]
- Okere, C.E.; Nwankwo, E.I.; Arinze, B.C.; Osukalu, E.J. Waterproof Concrete Additives and Their Effects on Concrete Properties. J. Multidiscip. Eng. Sci. Technol. JMEST 2017, 4, 7621–7623. [Google Scholar]
- Tian, Y.; Wang, P.; Zhao, T.; Ma, Z.; Jin, Z.; Zhao, H. Influence of Water-Repellent Treatment with Silicon Resin on Properties of Concrete. Adv. Mater. Sci. Eng. 2019, 2019, 5743636. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Concrete Admixtures Handbook. Properties, Science and Technology; William Andrew: Norwich, NY, USA, 1996; 1184p. [Google Scholar]
- Cappellesso, V.G.; dos Santos Petry, N.; Dal Molin, D.C.C.; Masuero, A.B. Use of crystalline waterproofing to reduce capillary porosity in concrete. J. Build. Pathol. Rehabil. 2016, 1, 9. [Google Scholar] [CrossRef]
- Medeiros, M.H.F.; Castro-Borges, P.; Aleixo, D.M.; Quarcioni, V.A.; Marcondes, C.G.N.; Helene, P. Reducing Water and Chloride Penetration Through Silicate Treatments for Concrete as a Mean to Control Corrosion Kinetics. Int. J. Electrochem. Sci. 2012, 7, 9682–9696. [Google Scholar]
- Jaskulski, R.; Jóźwiak-Niedźwiedzka, D.; Yakymechko, Y. Calcined Clay as Supplementary Cementitious Material. Materials 2020, 13, 4734. [Google Scholar] [CrossRef]
- Singh, N.B. Clays and Clay Minerals in the Construction Industry. Minerals 2022, 12, 301. [Google Scholar] [CrossRef]
- Geng, Y.; Li, S.; Hou, D.; Chen, X.; Jin, Z. Effect of SiO2 Sol/Silane Emulsion in Reducing Water and Chloride Ion Penetration in Concrete. Coatings 2020, 10, 682. [Google Scholar] [CrossRef]
- Ayad, A.; Said, A. Using Colloidal Nano Silica to Enhance the Performance of Cementitious Mortars. Open J. Civ. Eng. 2018, 8, 82–90. [Google Scholar] [CrossRef]
- Breilly, D.; Fadlallah, S.; Froidevaux, V.; Colas, A.; Allais, F. Origin and industrial applications of lignosulfonates with a focus on their use as superplasticizers in concrete. Constr. Build. Mater. 2021, 301, 124065. [Google Scholar] [CrossRef]
- Dvorkin, L.I. The influence of polyfunctional modifier additives on properties of cement-ash fine-grained concrete. Mag. Civ. Eng. 2020, 93, 121–133. [Google Scholar]
- Qin, Y.L.; Bai, M.X.; Zhang, Z.M.; Yang, D.J. Adsorption Behavior of Naphthalene Sulfonate Formaldehyde Condensate with Different Molecular Weights on the Cement Particle Surface. Adv. Mater. Res. 2012, 557–559, 870–876. [Google Scholar] [CrossRef]
- Khudhair, M.H.R.; Elyoubi, M.S.; Elharfi, A. Study of the influence of water reducing and setting retarder admixtures of polycarboxylate «superplasticizers» on physical and mechanical properties of mortar and concrete. J. Mater. Environ. Sci. 2018, 9, 56–65. [Google Scholar]
- Ramachandran, V.S.; Lowery, M.S.; Malhotra, V.M. Behaviour of ASTM Type V cement hydrated in the presence of sulfonated melamine formaldehyde. Mater. Struct. 1995, 28, 133–138. [Google Scholar] [CrossRef]
- Paktiawal, A.; Alam, M. Effect of polycarboxylate ether-based superplasticizer dosage on fresh and hardened properties of cement concrete. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1166, 012013. [Google Scholar] [CrossRef]
- Masanaga, M.; Hirata, T.; Kawakami, H.; Morinaga, Y.; Nawa, T.; Elakneswaran, Y. Effects of a New Type of Shrinkage-Reducing Agent on Concrete Properties. Materials 2020, 13, 3018. [Google Scholar] [CrossRef]
- Özen, S.; Gökhan Altun, M.; Mardani-Aghabaglou, A. Effect of the polycarboxylate based water reducing admixture structure on self-compacting concrete properties: Main chain length. Constr. Build. Mater. 2020, 255, 119360. [Google Scholar] [CrossRef]
- Li, W.; Wittmann, F.H.; Jiang, R.; Zhao, T.; Wolfseher, R. Integral Water Repellent Concrete Produced by Addition of Metal Soaps. Restor. Build. Monum. 2012, 18, 41–48. [Google Scholar] [CrossRef]
- Misnikov, O.S.; Chertkova, E.Y. Hydrophobic modification of mineral binders by additives produced from peat. Eurasian Min. 2014, 1, 63–68. [Google Scholar]
- Badikova, A.D.; Sakhibgareev, S.R.; Fedina, R.A.; Rakhimov, M.N.; Tsadkin, M.A. Effective mineral additive on the basis of wastes of petrochemical plants for a concrete structural mix. Nanotechnol. Constr. 2020, 12, 34–40. [Google Scholar] [CrossRef]
- Teshaboeva, N.D. Basic issues of the theory of hydrophobization of cement systems by additives of products of petrochemical synthesis. Orient. Renaiss. Innov. Educ. Nat. Soc. Sci. 2021, 1, 475–479. [Google Scholar]
- Albayrak, A.T.; Yasar, M.; Gurkaynak, M.A.; Gurgey, I. Investigation of the effects of fatty acids on the compressive strength of the concrete and the grindability of the cement. Cem. Concr. Res. 2005, 35, 400–404. [Google Scholar] [CrossRef]
- Nemati Chari, M.; Naseroleslami, R.; Shekarchi, M. The impact of calcium stearate on characteristics of concrete. Asian J. Civ. Eng. 2019, 20, 1007–1020. [Google Scholar] [CrossRef]
- Lanzón, M.; Martínez, E.; Mestre, M.; Madrid, J.A. Use of zinc stearate to produce highly-hydrophobic adobe materials with extended durability to water and acid-rain. Constr. Build. Mater. 2017, 139, 114–122. [Google Scholar] [CrossRef]
- Ogurtsova, Y.N.; Strokova, V.V.; Labuzova, M.V. Hydrophobization of Concrete Using Granular Nanostructured Aggregate. IOP Conf. Ser. Mater. Sci. Eng. 2017, 262, 012013. [Google Scholar] [CrossRef]
- Maryoto, A.; Setijadi, R.; Widyaningrum, A.; Waluyo, S. Drying Shrinkage of Concrete Containing Calcium Stearate, (Ca(C18H35O2)2), with Ordinary Portland Cement (OPC) as a Binder: Experimental and Modelling Studies. Molecules 2020, 25, 4880. [Google Scholar] [CrossRef]
- Cellat, K.; Tezcan, F.; Kardaş, G.; Paksoy, H. Comprehensive investigation of butyl stearate as a multifunctional smart concrete additive for energy-efficient buildings. Int. J. Energy Res. 2019, 43, 7146–7158. [Google Scholar] [CrossRef]
- Kurdi, A.; Almoatham, N.; Mirza, M.; Ballweg, T.; Alkahlan, B. Potential Phase Change Materials in Building Wall Construction—A Review. Materials 2021, 14, 5328. [Google Scholar] [CrossRef]
- Liu, X.; Song, X.; Wang, Z.; Xia, C.; Li, T.; Li, X.; Xu, Q.; Cui, S.; Qian, S. Polymer for Internal Hydrophobization of Cement-Based Materials: Design, Synthesis, and Properties. Polymers 2021, 13, 3069. [Google Scholar] [CrossRef]
- Luginina, A.A.; Kuznetsov, S.V.; Voronov, V.V.; Yapryntsev, A.D.; Ivanov, V.K.; Petukhov, D.I.; Lyapin, A.A.; Ermakov, A.S.; Pominova, D.V.; Chernova, E.V.; et al. Hydrophobization of up-conversion luminescent films based on nanocellulose/MF2:Ho particles (M = Sr, Ca) by acrylic resin. Nanosyst. Phys. Chem. Math. 2019, 10, 585–598. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, P. Effect of hydrophobic agent in cement and concrete: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1116, 012175. [Google Scholar] [CrossRef]
- Sanjay Kumar, R.; Selvaraj, R.; Kumutha, R. Development of Superhydrophobic Coatings on Concrete Surfaces. Int. J. Sci. Res. (IJSR) 2017, 6, 363–368. [Google Scholar]
- Zhang, P.; Shang, H.; Hou, D.; Guo, S.; Zhao, T. The Effect of Water Repellent Surface Impregnation on Durability of Cement-Based Materials. Adv. Mater. Sci. Eng. 2017, 2017, 8260103. [Google Scholar] [CrossRef]
- Butakova, M.D.; Saribekyan, S.S.; Mikhaylov, A.V. Influence of Silicon-Containing Additives on Concrete Waterproofness Property. IOP Conf. Ser. Mater. Sci. Eng. 2017, 262, 012006. [Google Scholar] [CrossRef]
- Grabowska, K.; Koniorczyk, M. Internal hydrophobization of cement mortar by addition of siloxanes. MATEC Web Conf. 2019, 282, 02030. [Google Scholar] [CrossRef]
- Medeiros, M.; Helene, P. Efficacy of surface hydrophobic agents in reducing water and chloride ion penetration in concrete. Mater. Struct. 2008, 41, 59–71. [Google Scholar] [CrossRef]
- Maryoto, A.; Sthenly Gan, B.; Intang Setyo Hermanto, N.; Setijadi, R. Effect of Calcium Stearate in the Mechanical and Physical Properties of Concrete with PCC and Fly Ash as Binders. Materials 2020, 13, 1394. [Google Scholar] [CrossRef]
- Azad, A.; Mousavi, S.F.; Karami, H.; Farzin, S. Application of Talc as an Eco-Friendly Additive to Improve the Structural Behavior of Porous Concrete. Iran. J. Sci. Technol. Trans. Civ. Eng. 2019, 43, 443–453. [Google Scholar] [CrossRef]
- Lima-Guerra, D.J.; Mello, I.; Resende, R.; Silva, R. Use of Bentonite and Organobentonite as Alternatives of Partial Substitution of Cement in Concrete Manufacturing. Int. J. Concr. Struct. Mater. 2014, 8, 15–26. [Google Scholar] [CrossRef]
- Raheem, S.A.; Saheb, M.A.; Moula, H.H.; Maula, B.H.; Alshreefi, R.A.; Bahnam, Q.M. Improve Light Weight Concrete Characteristics by Adding Paraffin Wax as Moisture Proof. Mater. Sci. Forum 2019, 972, 16–25. [Google Scholar] [CrossRef]
- Li, Z.; Sun, W.; Liu, S.; Li, Z.; Deng, B.; Wu, J.; Ding, Y.; Jiao, K.; Jin, X.; Lu, P.; et al. Pore Preservation and Failure Mechanism of Sinian Dengying Formation Carbonate Reservoirs: A Case Study of Two Ultradeep Wells in the Sichuan Basin, Western China. Geofluids 2021, 2021, 8387748. [Google Scholar] [CrossRef]
- Anikina, N.A.; Smirnov, V.F.; Smirnova, O.N.; Zaharova, E.A. Protection of construction materials based on acrylates from biodeterioration. Mag. Civ. Eng. 2018, 81, 116–124. [Google Scholar]
- Yao, S.Y.; Ge, Y. Effect of Styrene Butadiene Rubber Latex on Mortar and Concrete Properties. Adv. Eng. Forum 2012, 5, 283–288. [Google Scholar] [CrossRef]
- Abdo, S.; Galishnikova, V.V.; Fawzy, A.M. Properties of recycled aggregate pervious concrete modified with Styrene Butadiene Rubber Latex. Mag. Civ. Eng. 2021, 108, 10805. [Google Scholar]
- Kim, M.J.; Park, E.S.; Hwang, W.I.; Cho, W.J. Effect of FNS Incorporation on the Properties of Ternary Blended Cement Containing Blast Furnace Slag and Fly Ash. Adv. Mater. Sci. Eng. 2022, 2022, 1047648. [Google Scholar] [CrossRef]
- Sychova, A.M.; Svatovskaya, L.B.; Starchukov, D.S.; Soloviova, V.Y.; Gravit, M.V. The improving of the concrete quality in a monolithic clip. Mag. Civ. Eng. 2018, 80, 3–14. [Google Scholar]
- Cardenas, H.; Kupwade-Patil, K.; Eklund, S. Corrosion Mitigation in Mature Reinforced Concrete Using Nanoscale Pozzolan Deposition. J. Mater. Civ. Eng. 2011, 23, 752–760. [Google Scholar] [CrossRef]
- de Souza Oliveira, A.; Gomes, O.F.M.; Ferrara, L.; Fairbairn, E.M.R.; Filho, R.D.T. An overview of a twofold effect of crystalline admixtures in cement-based materials: From permeability-reducers to self-healing stimulators. J. Build. Eng. 2021, 41, 102400. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Roumyantseva, V.E.; Krasilnikov, I.V.; Konovalova, V.S.; Evsyakov, A.S. Monitoring of the Penetration of Chloride Ions to the Reinforcement Surface through a Concrete Coating during Liquid Corrosion. IOP Conf. Ser. Mater. Sci. Eng. 2018, 463, 042048. [Google Scholar] [CrossRef]
- Basheer, P.A.M.; Basheer, L.; Cleland, D.J.; Long, A.E. Surface Treatments for Concrete: Assessment Methods and Reported Performance. Constr. Build. Mater. 1997, 11, 413–429. [Google Scholar] [CrossRef]
- Suchorab, Z.; Barnat-Hunek, D.; Franus, M.; Łagód, G. Mechanical and Physical Properties of Hydrophobized Lightweight Aggregate Concrete with Sewage Sludge. Materials 2016, 9, 317. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, P.; Zhang, G. Effect of hydrophobic agent on efflorescence of portland cement-based decorative mortar. Jianzhu Cailiao Xuebao/J. Build. Mater. 2014, 17, 882–886. [Google Scholar]
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Effect of hydrophobic surface-modified fine aggregates on efflorescence control in geopolymer. Cem. Concr. Compos. 2022, 126, 104337. [Google Scholar] [CrossRef]
- Mussato, B.T.; Gepraegs, O.K.; Farnden, G. Relative Effects of Sodium Chloride and Magnesium Chloride on Reinforced Concrete: The State of Art. Transp. Res. Rec. J. Transp. Res. Board 2004, 1866, 59–66. [Google Scholar] [CrossRef]
- Markov, A.I. Way Determining Waterproofness of Cement Materials. Patent RU2187804, 20 August 2002. [Google Scholar]
- MI 2625-2000; Recommended Practice. National Measurement Standards. Cement Materials. Accelerated Method for Measuring Water Impermeability. VNIIFTRI GP: Moscow, Russia, 2000.
- GOST 12730.5-2018; Concretes. Methods for determination of water tightness. Standartinform: Moscow, Russia, 2018.
- GOST 10180-2012; Concretes. Methods for strength determination using reference specimens. Standartinform: Moscow, Russia, 2012.
- Fedosov, S.V.; Roumyantseva, V.E.; Krasilnikov, I.V.; Konovalova, V.S. Physical and mathematical modelling of the mass transfer process in heterogeneous systems under corrosion destruction of reinforced concrete structures. IOP Conf. Ser. Mater. Sci. Eng. 2018, 456, 012039. [Google Scholar] [CrossRef]
- Fic, S.; Szewczak, A.; Barnat-Hunek, D.; Łagód, G. Processes of Fatigue Destruction in Nanopolymer-Hydrophobised Ceramic Bricks. Materials 2017, 10, 44. [Google Scholar] [CrossRef]
- Tsoy, V.M.; Turgayev, J.A.; Narbaev, U.I. Studying the structure and properties of cement stone with a complex of modifying additives. ISJ Theor. Appl. Sci. 2023, 2, 281–288. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Rahman, M.M.; Chamberlain, D.A. Development of hydrophobic concrete by adding dual-crystalline admixture at mixing stage. Struct. Concr. 2018, 19, 1504–1511. [Google Scholar] [CrossRef]
- Klisińska-Kopacz, A.; Tišlova, R. Effect of hydrophobization treatment on the hydration of repair Roman cement mortars. Constr. Build. Mater. 2012, 35, 735–740. [Google Scholar] [CrossRef]
- Bogdanov, R.R.; Ibragimov, R.A. Process of hydration and structure formation of the modified self-compacting concrete. Mag. Civ. Eng. 2017, 5, 14–24. [Google Scholar]
- Zhang, H.; Zhou, Y.; Mu, S.; Cai, J.; Hong, J.; Liu, J.; Zhao, Y. Pore Structure and Permeability of Cementitious Materials Containing a Carboxylic Acid Type Hydrophobic Agent. Front. Mater. 2022, 9, 907638. [Google Scholar] [CrossRef]
- Trofimova, I.; Shpyrko, N. Investigating the influence of volumetric hydrophobization on the formation of phase composition of cement stone and its physical-mechanical properties. East.-Eur. J. Enterp. Technol. 2019, 4, 32–38. [Google Scholar] [CrossRef]
- Tkacha, E.V.; Semenova, V.S.; Tkacha, S.A.; Rozovskayaa, T.A. Highly effective water-repellent concrete with improved physical and technical properties. Procedía Eng. 2015, 111, 763–769. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Roumyantseva, V.E.; Konovalova, V.S.; Evsyakov, A.S. The role of colmatation in liquid corrosion of hydrophobized concrete. IOP Conf. Ser. Mater. Sci. Eng. 2020, 896, 012096. [Google Scholar] [CrossRef]
- Rumyantseva, V.E.; Konovalova, V.S.; Narmaniya, B.E. Changes in the structural and phase composition and strength characteristics of concrete during liquid corrosion in chloride-containing media. J. Phys. Conf. Ser. 2021, 1926, 012057. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Rumyantseva, V.E.; Krasilnikov, I.V.; Konovalova, V.S.; Karavaev, I.V. Determination of safe service life of structures made of concrete containing hydrophobic additives. Izv. Vyss. Uchebnykh Zaved. Seriya Teknol. Tekst. Promyshlennosti 2017, 6, 268–276. [Google Scholar]
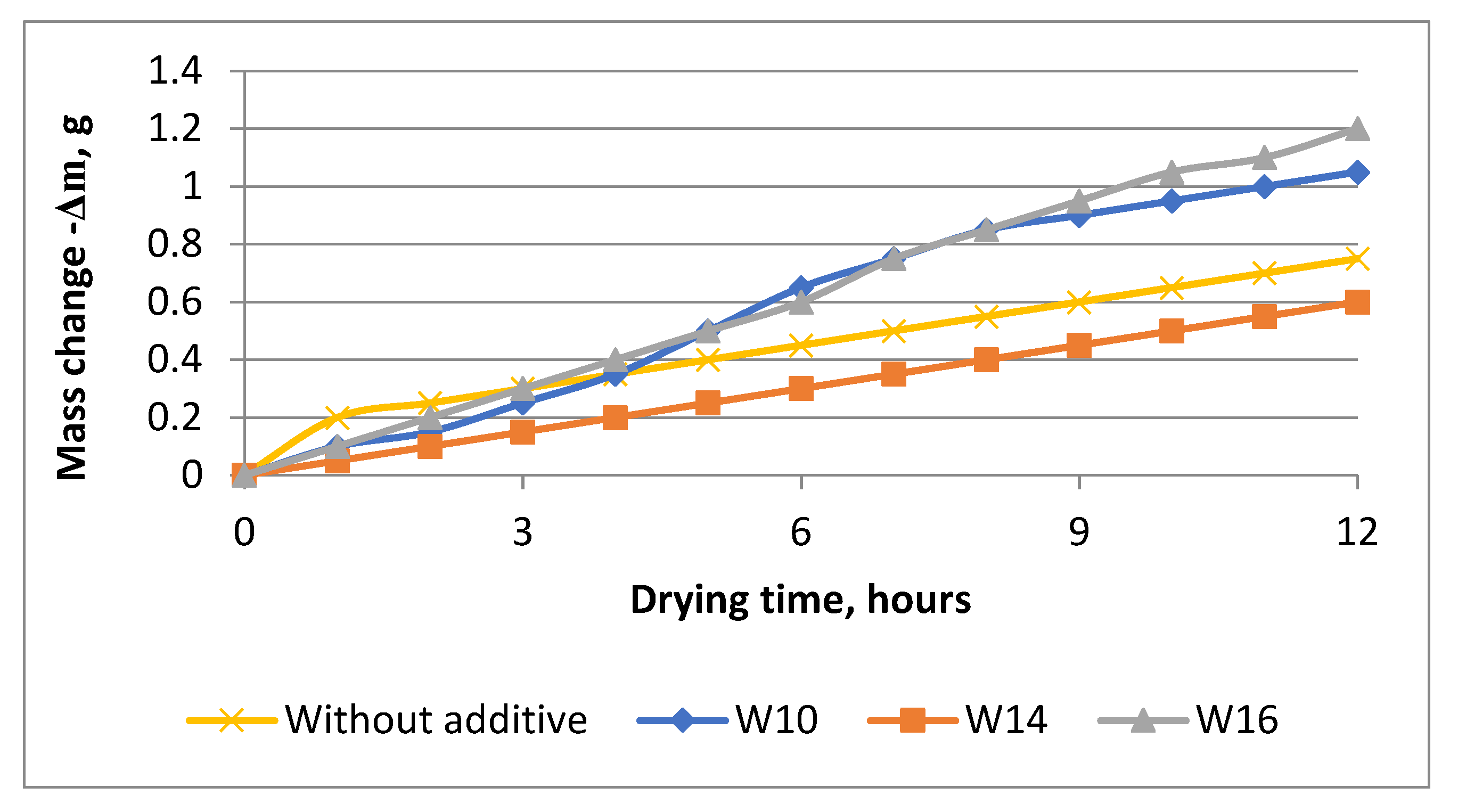
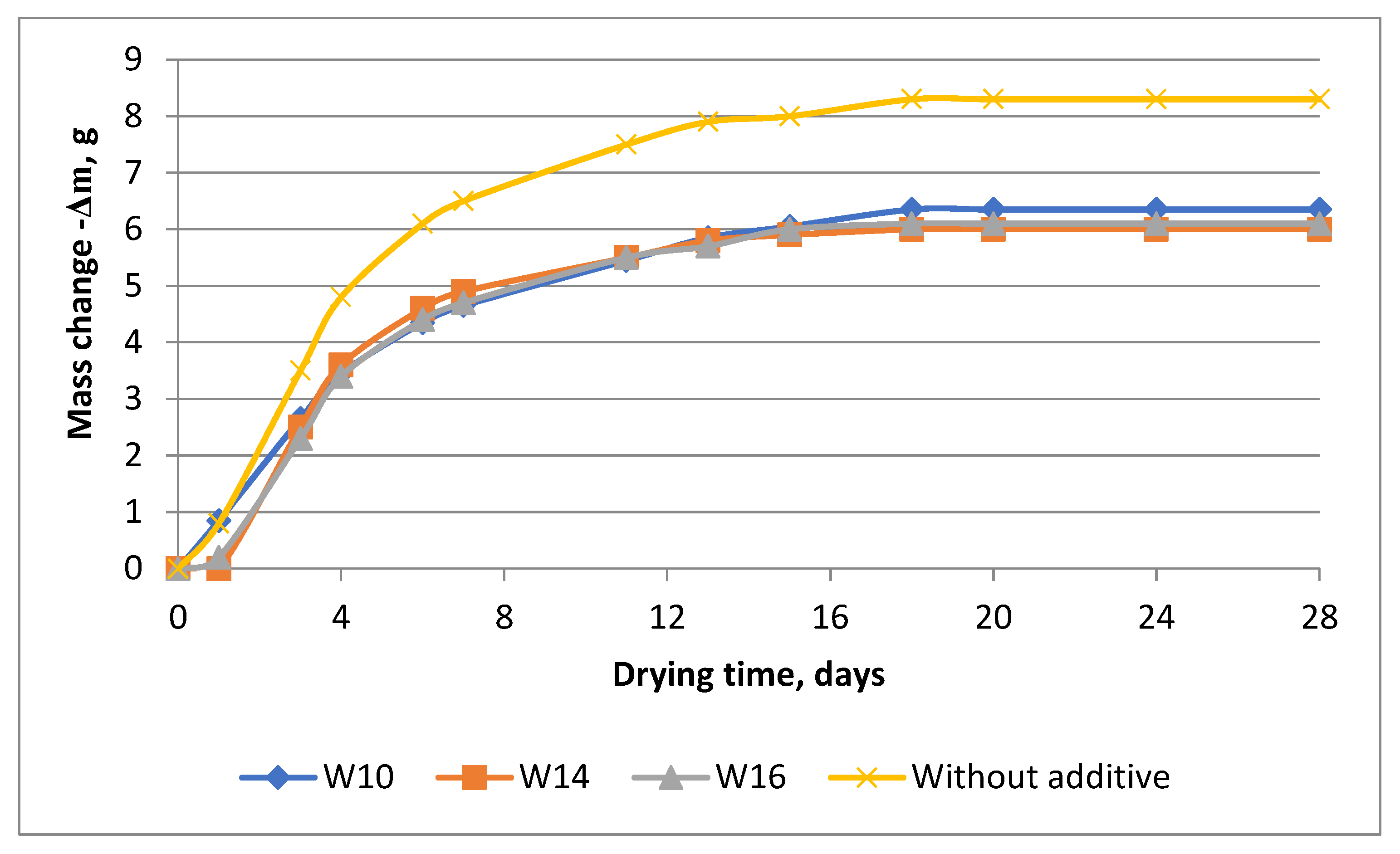

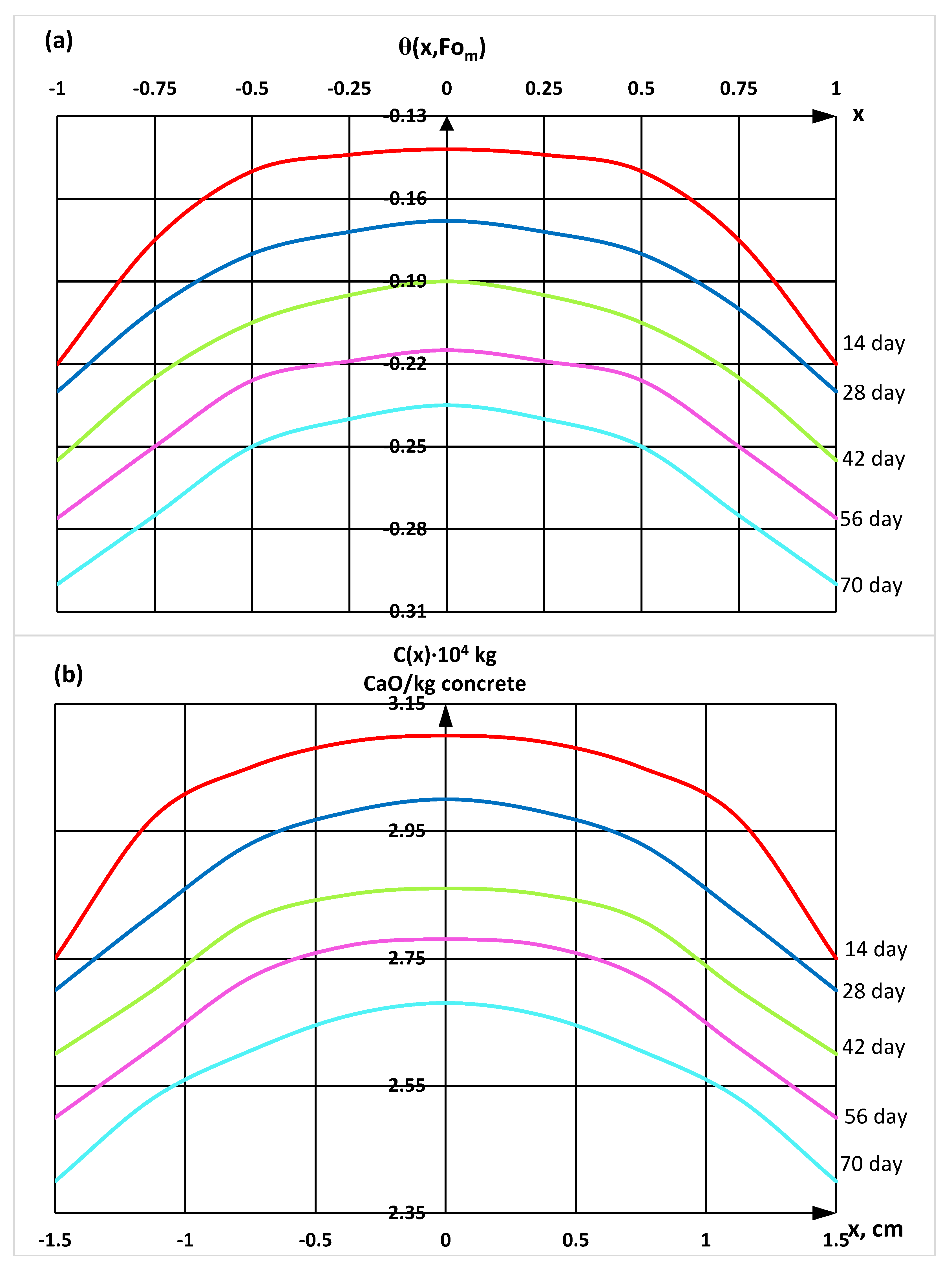
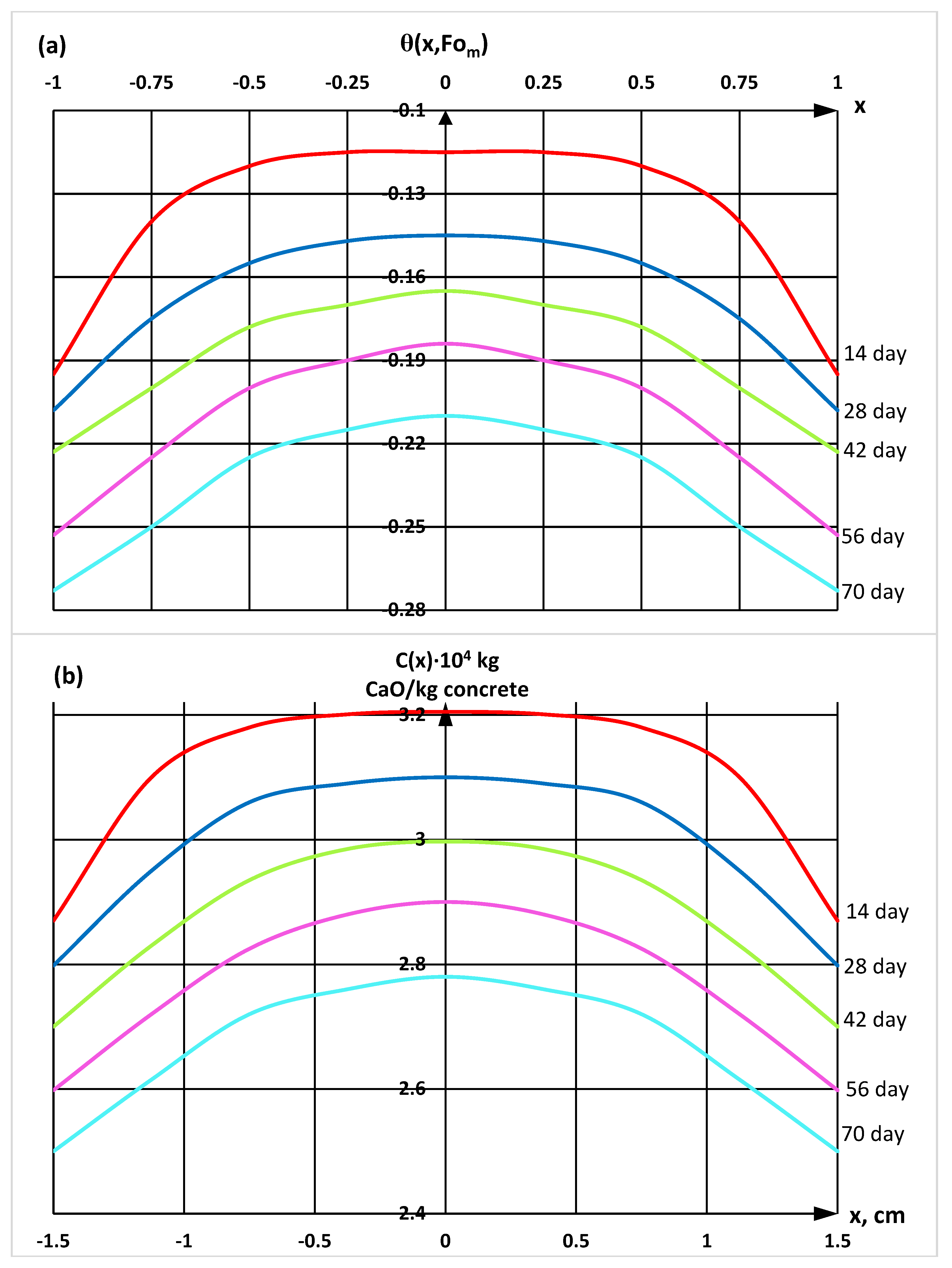
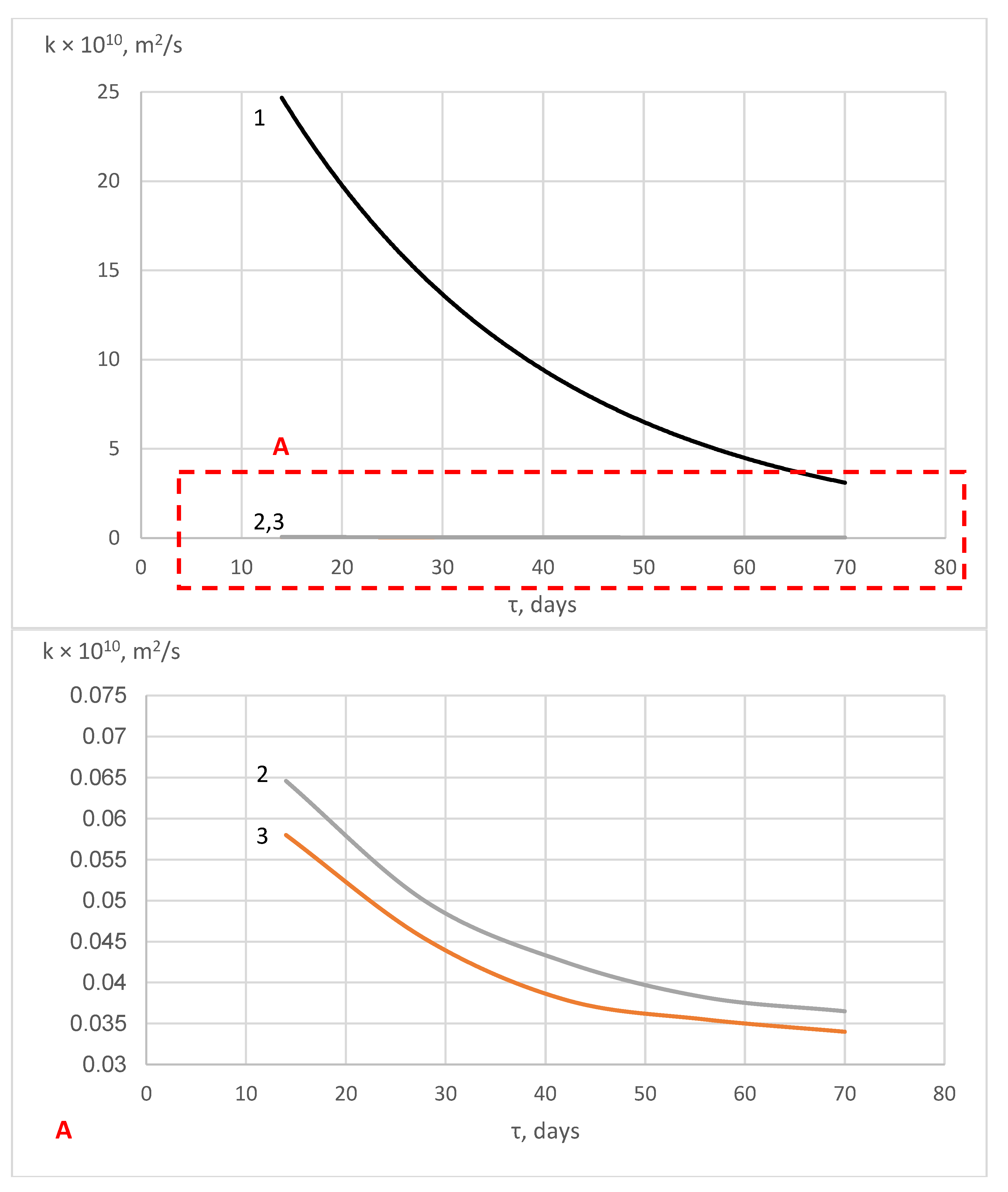
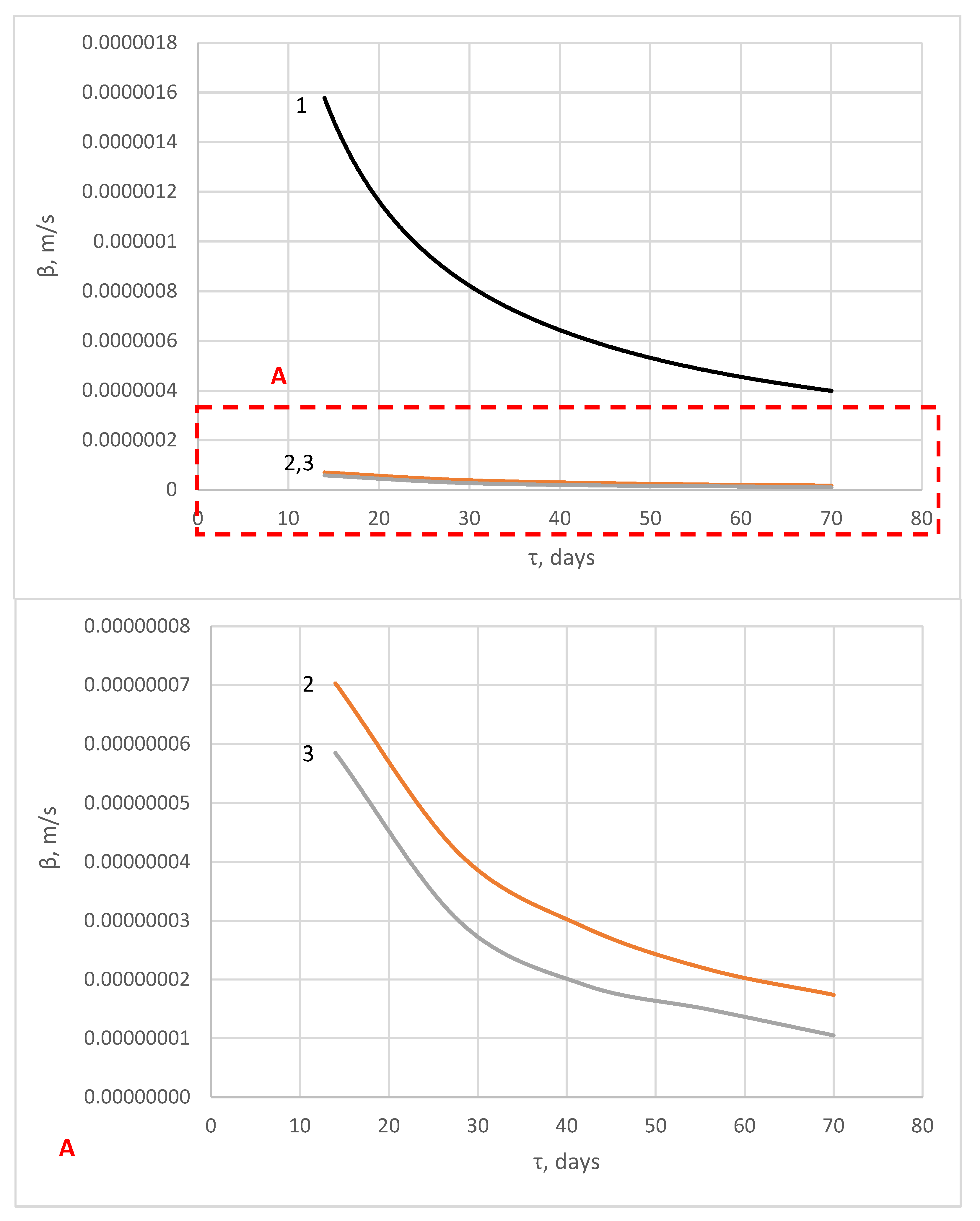
| Components | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | R2O |
|---|---|---|---|---|---|---|---|
| Amount [%] | 21.24 | 5.65 | 4.30 | 65.87 | 0.86 | 0.44 | 0.71 |
| Components | C3S | C2S | C3A | C3AF |
|---|---|---|---|---|
| Amount [%] | 61.40 | 14.60 | 7.69 | 13.01 |
| Characteristic | Concrete Grade for Water Resistance | ||
|---|---|---|---|
| W10 | W14 | W16 | |
| Density [kg/m3] | 2432.1 | 2568.2 | 2604.4 |
| Water absorption [%] | 4.0 | 3.7 | 3.5 |
| Porosity [%] | 6.8 | 6.0 | 5.7 |
| Strength [MPa] | 54.58 | 58.18 | 63.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konovalova, V.S. Investigation of the Effect of Volumetric Hydrophobization on the Kinetics of Mass Transfer Processes Occurring in Cement Concretes during Corrosion. Materials 2023, 16, 3827. https://doi.org/10.3390/ma16103827
Konovalova VS. Investigation of the Effect of Volumetric Hydrophobization on the Kinetics of Mass Transfer Processes Occurring in Cement Concretes during Corrosion. Materials. 2023; 16(10):3827. https://doi.org/10.3390/ma16103827
Chicago/Turabian StyleKonovalova, Viktoriya S. 2023. "Investigation of the Effect of Volumetric Hydrophobization on the Kinetics of Mass Transfer Processes Occurring in Cement Concretes during Corrosion" Materials 16, no. 10: 3827. https://doi.org/10.3390/ma16103827
APA StyleKonovalova, V. S. (2023). Investigation of the Effect of Volumetric Hydrophobization on the Kinetics of Mass Transfer Processes Occurring in Cement Concretes during Corrosion. Materials, 16(10), 3827. https://doi.org/10.3390/ma16103827





