Preparation and Application of pH-Sensitive Film Containing Anthocyanins Extracted from Lycium ruthenicum Murr.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Anthocyanins
2.3. Preparation of Films
2.4. Mechanical Properties
2.5. Barrier Properties
2.6. Determination of Total Volatile Basic Nitrogen (TVB-N)
2.7. Colorimetric Analysis
2.8. Fourier Transform Infrared (FT-IR) Spectra
2.9. Scanning Electron Microscopy (SEM)
2.10. XRD Measurement
2.11. Statistics Analysis
3. Results and Discussion
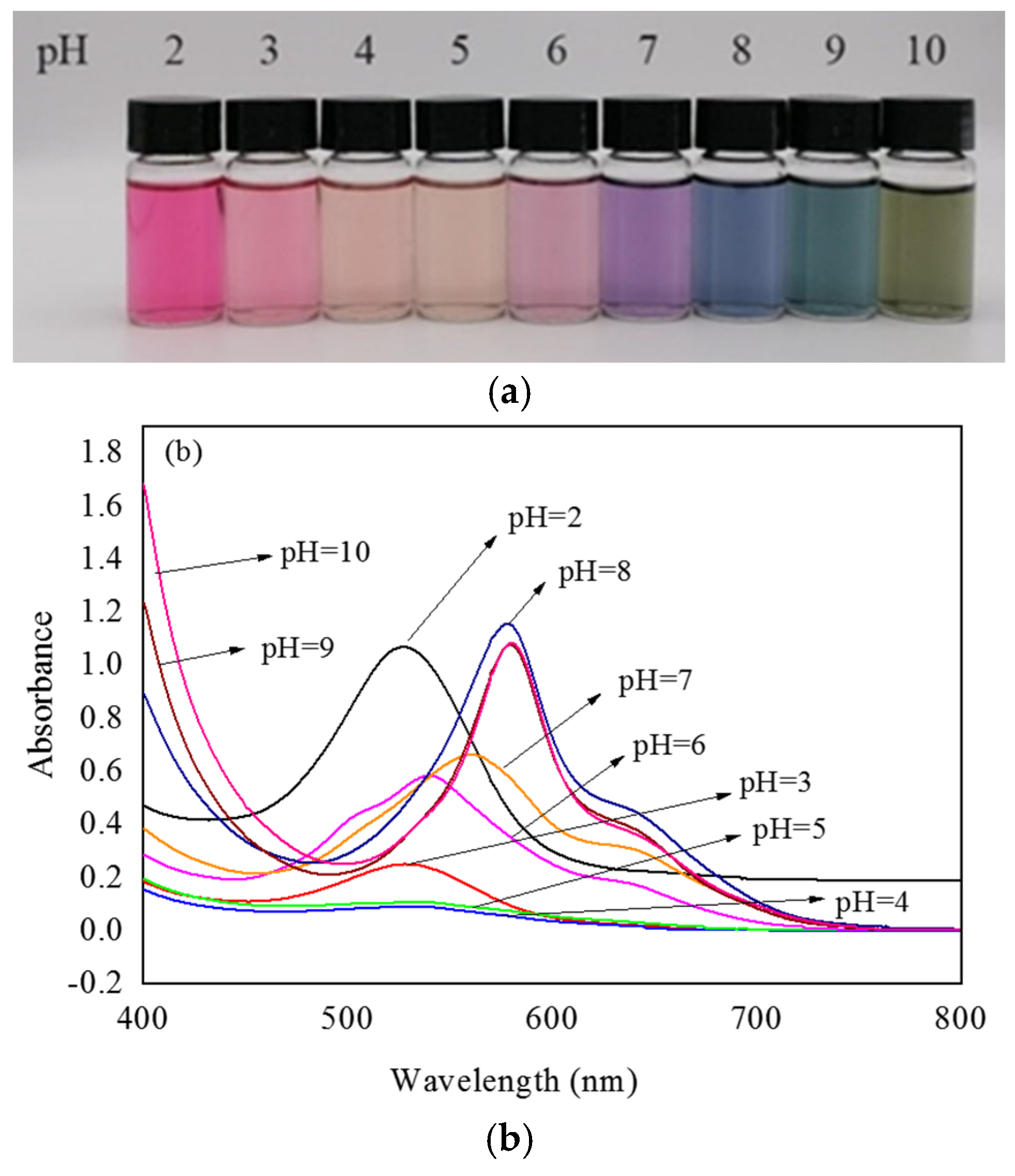
3.1. UV–Vis Spectra of Lycium ruthenicum Murr. Anthocyanin (LRMA) Solution
3.2. Mechanical and Barrier Properties
3.3. Color Response Efficiency and Colorimetric Analysis of pH-Sensitive Films
3.3.1. pH Response in Different Buffer Solutions
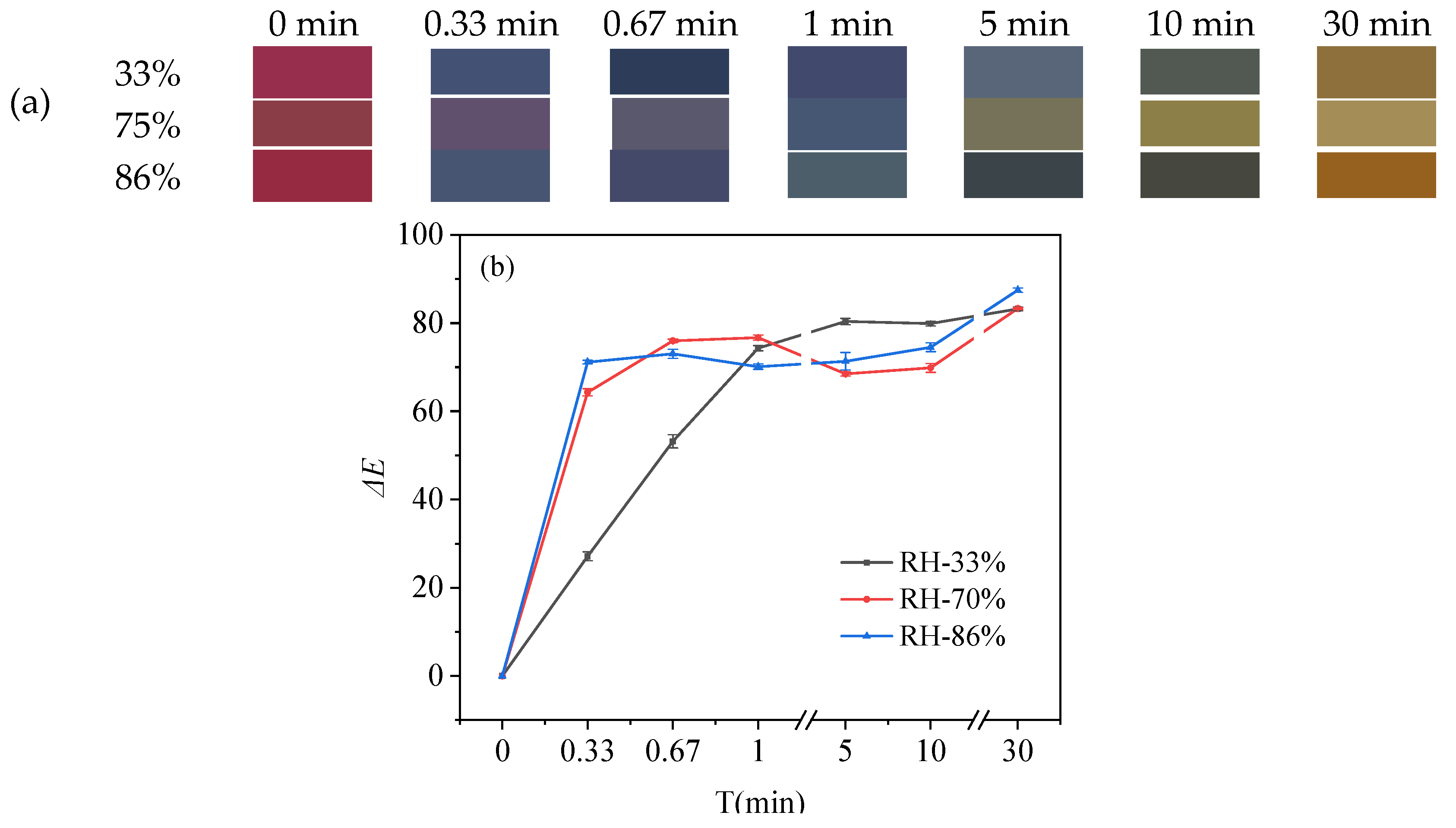
3.3.2. Visual Color Response of pH-Sensitive Film
3.3.3. Color Response in NH3 Atmosphere
3.4. XRD Patterns of ASKG/SPI and LRMA-Containing ASKG/SPI-Blended Film
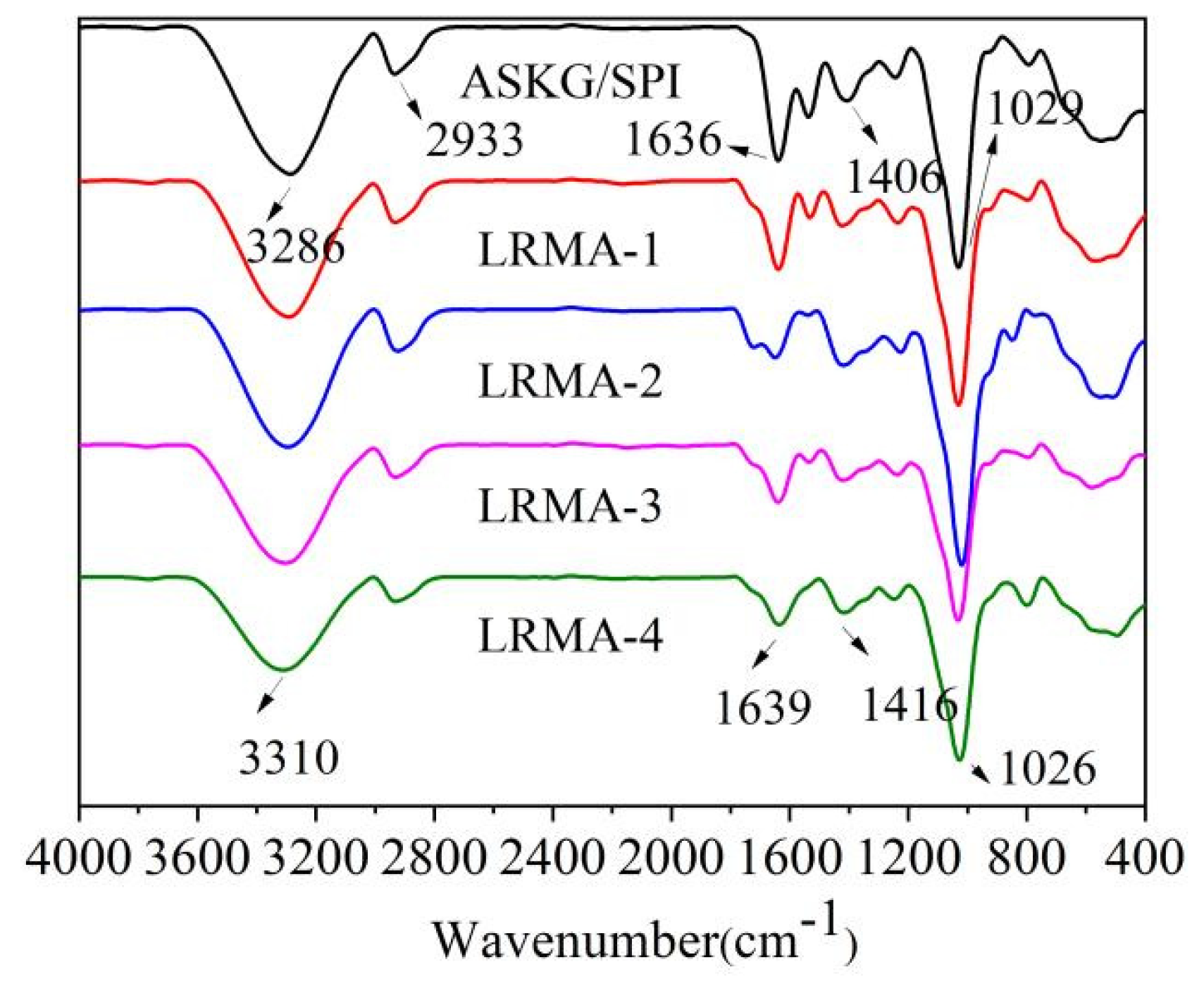
3.5. FT-IR Spectra of pH-Sensitive Film
3.6. Scanning Electron Microscopy (SEM)
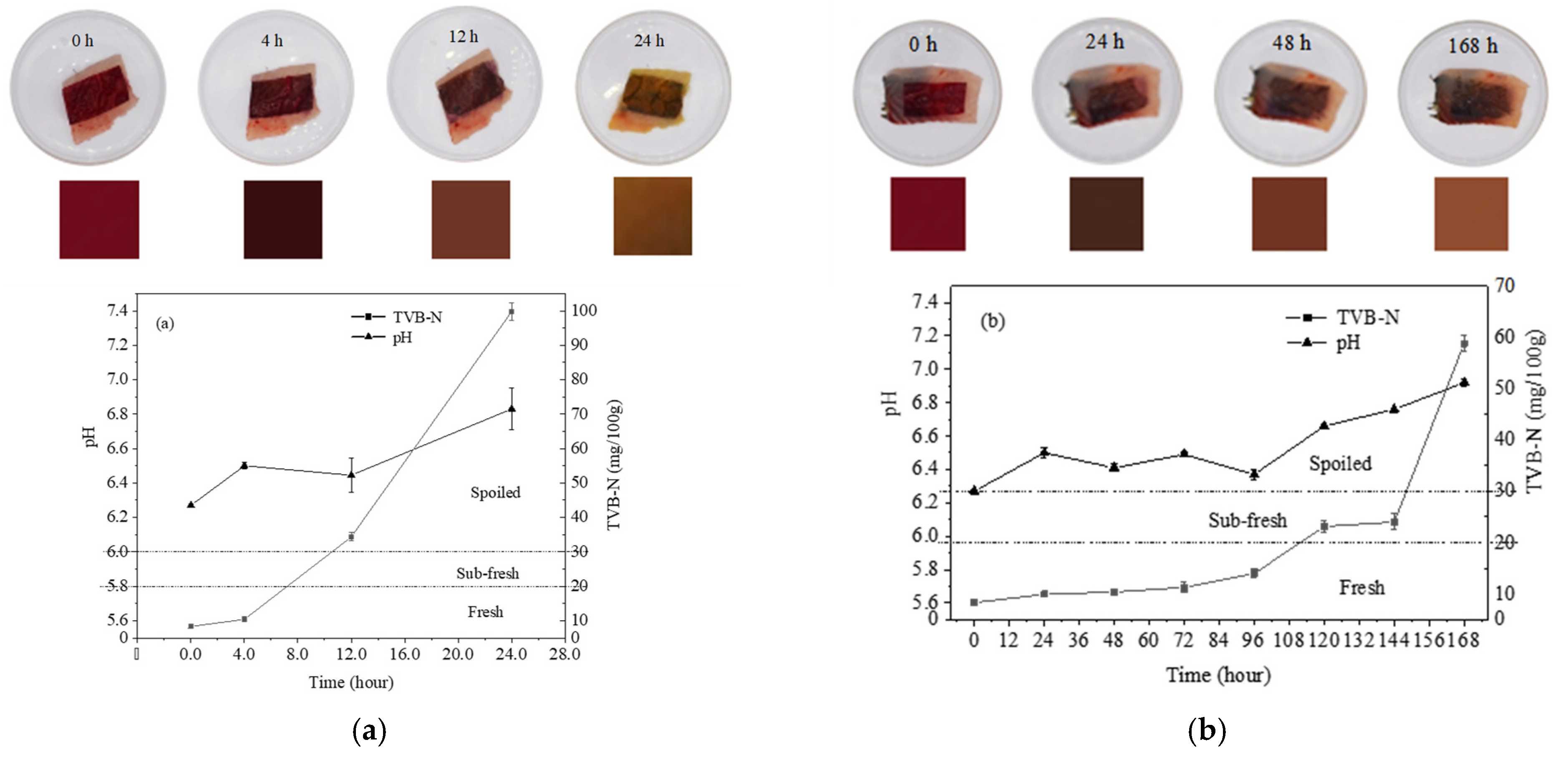
3.7. Correlation Study between Meat Spoilage and Colorimetric Change of pH-Sensitive Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as time–temperature indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; McClements, D.J.; Chen, L.; Jiao, A.Q.; Tian, Y.Q.; Ji, Z.Y. Recent advances in intelligent food packaging materials:Principles, preparation and applications. Food Chem. 2021, 375, 131738. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yu, X.Z.; Xie, F.; Fan, Y.Q.; Xu, X.L.; Qi, J.; Zhang, F.Y. pH-responsive double-layer indicator films based on konjac glucomannan/camellia oil and carrageenan/anthocyanin/curcumin for monitoring meat freshness. Food Hydrocoll. 2021, 118, 106695. [Google Scholar] [CrossRef]
- Ala, M.A.N.; Shahbazi, Y. The effects of novel bioactive carboxymethyl cellulose coatings on food-borne pathogenic bacteria and shelf life extension of fresh and sauced chicken breast fillets. LWT Food Sci. Technol. 2019, 111, 602–611. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 208–302. [Google Scholar] [CrossRef]
- Kong, J.L.; Ge, X.H.; Sun, Y.T.; Mao, M.R.; Yu, H.R.; Chu, R.X.; Wang, Y. Multi-functional pH-sensitive active and intelligent packaging based on highly cross-linked zein for the monitoring of pork freshness. Food Chem. 2022, 404, 134754. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2020, 166, 741–750. [Google Scholar] [CrossRef]
- Kuswandi, B.; Jayus; Restyana, A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Khazaei, N.; Esmaiili, M.; Djomeh, Z.E.; Ghasemiou, M.; Jouki, M. Characterization of new biodegradable edible film made from basil seed (Ocimum basilicum L.) gum. Carbohyd. Polym. 2014, 102, 199–206. [Google Scholar] [CrossRef]
- Yang, L.; Paulson, A.T. Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Res. Int. 2000, 33, 571–578. [Google Scholar] [CrossRef]
- Fan, Y.L.; Yang, J.; Duan, A.B.; Li, X.J. Pectin/sodium alginate/xanthan gum edible composite films as the fresh-cut package. Int.J. Biol. Macromol. 2021, 181, 1003–1009. [Google Scholar] [CrossRef]
- Li, T.; Xia, N.; Xu, L.; Zhang, H.; Zhang, H.J.; Chi, Y.J.; Zhang, Y.L.; Li, L.L.; Li, H.Y. Preparation, characterization and application of SPI-based blend film with antioxidant activity. Food Packag. Shelf 2021, 27, 100614. [Google Scholar] [CrossRef]
- Bersaneti, G.T.; Prudencio, S.H.; Mali, S.; Pedrine Colabone Celligoi, M.A. Assessment of a new edible film biodegradable based on starch-nystose to increase quality and the shelf life of blackberries. Food Biosci. 2021, 42, 101173. [Google Scholar] [CrossRef]
- Porta, R.; Pierro, P.D.; Roviello, V.; Sabbah, M. Tuning the Functional Properties of Bitter Vetch (Vicia ervilia) Protein Films Grafted with Spermidine. Int. J. Mol. Sci. 2017, 18, 2658. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.Y.; Zhang, Z.P.; Ren, F.; Miao, M.; Tian, Y.Q.; Jin, Z.Y. Starch-based biodegradable packaging materials: A review of their preparation, characterization and diverse applications in the food industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Cao, L.L.; Ge, T.T.; Meng, F.S.; Xu, S.Y.; Li, J.; Wang, L.J. An edible oil packaging film with improved barrier properties and heat sealability from cassia gum incorporating carboxylated cellulose nano crystal whisker. Food Hydrocoll. 2020, 98, 105251. [Google Scholar] [CrossRef]
- Kumar, R.; Rai, B.; Kumar, K. A Simple Approach for the Synthesis of Cellulose Nanofiber Reinforced Chitosan/PVP Bio Nanocomposite Film for Packaging. J. Polym. Environ. 2019, 27, 2963–2973. [Google Scholar] [CrossRef]
- Caner, C.; Vergano, P.J.; Wiles, J.L. Chitosan Film Mechanical and Permeation Properties as Affected by Acid, Plasticizer, and Storage. J. Food Sci. 1998, 63, 1049–1053. [Google Scholar] [CrossRef]
- Cao, L.L.; Liu, W.B.; Wang, L.J. Developing a green and edible film from Cassia gum: The effects of glycerol and sorbitol. J. Clean. Prod. 2018, 175, 276–282. [Google Scholar] [CrossRef]
- Ferreira, J.; Girotto, E.M. Optical pH sensitive material based on bromophenol blue-doped polypyrrole. Sens. Actuat. B Chem. 2009, 137, 426–431. [Google Scholar] [CrossRef]
- Dong, S.Y.; Luo, M.; Peng, G.D.; Cheng, W.H. Broad range pH sensor based on sol–gel entrapped indicators on fibre optic. Sensor. Actuat. B Chem. 2007, 129, 94–98. [Google Scholar] [CrossRef]
- Golasz, L.B.; da Silva, J.; da Silva, S.B. Film with anthocyanins as an indicator of chilled pork deterioration. Food Sci. Technol. 2013, 33, 155–162. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Faseha, A.; Benjakul, S.; Kaewprachu, P. Application of anthocyanin as a color indicator in gelatin films. Food Biosci. 2020, 36, 100603. [Google Scholar] [CrossRef]
- Duan, A.B.; Yang, J.; Wu, L.Y.; Wang, T.; Liu, Q.Y.; Liu, Y.P. Preparation, physicochemical and application evaluation of raspberry anthocyanin and curcumin based on chitosan/starch/gelatin film. Int. J. Biol. Macromol. 2022, 220, 147–158. [Google Scholar] [CrossRef]
- Chayavanich, K.; Thiraphibundet, P.; Imyim, A. Biocompatible film sensors containing red radish extract for meat spoilage observation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117601. [Google Scholar] [CrossRef]
- Hashim, S.B.H.; Tahir, H.E.; Liu, L.; Zhang, J.J.; Zhai, X.D.; Mahdi, A.A.; Awad, F.N.; Hassan, M.M.; Zou, X.B.; Shi, J.Y. Intelligent colorimetric pH sensoring packaging films based on sugarcane wax/agar integrated with butterfly pea flower extract for optical tracking of shrimp freshness. Food Chem. 2022, 373 Pt B, 131514. [Google Scholar] [CrossRef]
- Terra, A.L.M.; Moreira, J.B.; Costa, J.A.V.; Morais, M.G.D. Development of pH indicators from nanofibers containing microalgal pigment for monitoring of food quality. Food Biosci. 2021, 44 Pt A, 101387. [Google Scholar] [CrossRef]
- Zhao, M.N.; Nuerjiang, M.; Bai, X.; Feng, J.; Kong, B.H.; Sun, F.D.; Li, Y.; Xia, X.F. Monitoring dynamic changes in chicken freshness at 4 °C and 25 °C using pH-sensitive intelligent films based on sodium alginate and purple sweet potato peel extracts. Int. J. Biol. Macromol. 2022, 216, 361–373. [Google Scholar] [CrossRef]
- Chen, S.L.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S.F. Development of pH indicator and antimicrobial cellulose nanofibre packaging film based on purple sweet potato anthocyanin and oregano essential oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Liang, J.Y.; Hu, Z.A.; Wang, Y.P.; Zhang, S.T. Chemical characterization of Artemisia seed polysaccharide. Carbohyd. Polym. 2007, 67, 213–218. [Google Scholar] [CrossRef]
- Bai, S.N.; Yong, T.W.; Yun, X.F. Survey and prospect of the studies on the extraction of oil and glue from Artemisia. Packag. Food Mach. 2000, 18, 17–23. [Google Scholar]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based eible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Denavi, G.; Tapia-Blácido, D.R.; Añón, M.C.; Sobral, P.J.A.; Mauri, A.N.; Menegalli, F.C. Effects of drying conditions on some physical properties of soy protein films. J. Food Eng. 2009, 90, 341–349. [Google Scholar] [CrossRef]
- Tibor, F.; Francis, F.J. Quantitative Methods for Anthocyanins. Extraction and Determination of Total Anthocyanin in Cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Shi, Q.L.; Zhang, Y.X.; Liu, J.; Wu, X.T.; Fang, Z.X. Development and characterization of active and pH-sensitive films based on psyllium seed gum incorporated with free and microencapsulated mulberry pomace extracts. Food Chem. 2021, 352, 129333. [Google Scholar] [CrossRef]
- Wu, H.J.; Lei, Y.L.; Zhu, R.; Zhao, M.J.; Lu, J.Y.; Xiao, D.; Jiao, C.; Zhang, Z.Q.; Shen, G.H.; Li, S.S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]
- Ruan, C.C.; Zhang, Y.M.; Sun, Y.; Gao, X.L.; Xiong, G.Y.; Liang, J. Effect of sodium alginate and carboxymethyl cellulose edible coating with epigallocatechin gallate on quality and shelf life of fresh pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar] [CrossRef]
- Cheng, M.; Cui, Y.J.; Yan, X.R.; Zhang, R.F.; Wang, J.; Wang, X.Y. Effect of dual-modified cassava starches on intelligent packaging films containing red cabbage extracts. Food Hydrocoll. 2022, 124 Pt A, 107225. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zhang, X.M.; Li, C.C.; Qin, Y.; Xiao, L.X.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2019, 147, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, L.L.; Wang, Y.J.; Chen, Z.Q.; Zhang, M.; Chen, H.X. Characterization and functional properties of a pectin/tara gum based edible film with ellagitannins from the unripe fruits of Rubus chingii Hu. Food Chem. 2020, 325, 126964. [Google Scholar] [CrossRef] [PubMed]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Molar absorptivities (ε) and spectral and colorimetric characteristics of purple sweet potato anthocyanins. Food Chem. 2019, 271, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohyd. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]
- Liang, T.Q.; Wang, L.J. Preparation, Characterization and application of a low water-sensitive artemisia sphaerocephala krasch. gum intelligent film incorporated with anionic cellulose nanofiber as a reinforcing component. Polymers 2020, 12, 247. [Google Scholar] [CrossRef]
- Mei, L.X.; Nafchi, A.M.; Ghasemipour, F.; Easa, A.M.; Jafarzadeh, S.; Al-Hassan, A.A. Characterization of pH sensitive sago starch films enriched with anthocyanin-rich torch ginger extract. Int. J. Biol. Macromol. 2020, 164, 4603–4612. [Google Scholar] [CrossRef]
- You, P.Q.; Wang, L.; Zhou, N.; Yang, Y.; Pang, J. A pH-intelligent response fish packaging film: Konjac glucomannan/carboxymethyl cellulose/blackcurrant anthocyanin antibacterial composite film. Int. J. Biol. Macromol. 2022, 204, 386–396. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.; René, N.; Jamshidian, M.; Akhtar, M.J.; Arab-Tehrany, E.; Jacquot, M.; Desobry, S. Microstructure and physico-chemical evaluation of nano-emulsion-based antimicrobial peptides embedded in bioactive packaging films. Food Hydrocoll. 2012, 29, 407–419. [Google Scholar] [CrossRef]
- Yan, Y.S.; Duan, S.Q.; Zhang, H.L.; Liu, Y.T.; Li, C.; Hu, B.; Liu, A.P.; Wu, D.T.; He, J.L.; Wu, W.J. Preparation and characterization of Konjac glucomannan and pullulan composite films for strawberry preservation. Carbohydr. Polym. 2020, 243, 116446. [Google Scholar] [CrossRef]
- Li, C.C.; Yun, D.W.; Wang, Z.Y.; Xu, F.F.; Tang, C.; Liu, J. Development of Shrimp Freshness Indicating Films by Embedding Anthocyanins-Rich Rhododendron simsii Flower Extract in Locust Bean Gum/Polyvinyl Alcohol Matrix. Materials 2022, 15, 7557. [Google Scholar] [CrossRef]
- Liang, T.Q.; Wang, L.J. Preparation and characterization of a novel edible film based on Artemisia sphaerocephala Krasch. gum: Effects of type and concentration of plasticizers. Food Hydrocoll. 2018, 77, 502–508. [Google Scholar] [CrossRef]
- Liang, T.Q.; Sun, G.H.; Gao, L.L.; Li, J.; Wang, L.J. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocoll. 2019, 87, 858–868. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.P.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of pH sensitive film based on starch/carbon nano dots incorporating anthocyanin for monitoring spoilage of pork. Food Control 2021, 126, 108039. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Fang, C.Q.; Liu, D.H.; Zhou, X.; Wang, D.; Zhang, W. Preparation and characterization of the protein edible film extracted from the migratory locust (Locusta migratoria). Food Packag. Shelf Life 2022, 33, 100899. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Zhou, X.; Fang, C.Q.; Wang, D. Characterization of the Antimicrobial Edible Film Based on Grasshopper Protein/Soy Protein Isolate/Cinnamaldehyde Blend Crosslinked with Xylose. Front. Nutr. 2022, 9, 796356. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.J.; Liu, S.J.; Lu, K.K.; Tang, K.Y.; Liu, J. Heat sealable soluble soybean polysaccharide/gelatin blend edible films for food packaging applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Fang, C.Q.; Zhang, W.; Lei, W.Q.; Wang, D.; Zhou, X. Novel grasshopper protein/soy protein isolate/pullulan ternary blend with hesperidin derivative for antimicrobial edible film. Arab. J. Chem. 2023, 16, 104563. [Google Scholar] [CrossRef]
- Wu, Y.L.; Li, C.W. A double-layer smart film based on gellan gum/modified anthocyanin and sodium carboxymethyl cellulose/starch/Nisin for application in chicken breast. Int. J. Biol. Macromol. 2023, 232, 123464. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Du, J.; Zhou, H.M.; Zhou, S.Y.; Lv, Y.N.; Cheng, Y.; Tao, Y.H.; Lu, J.; Wang, H.S. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocoll. 2022, 129, 107665. [Google Scholar] [CrossRef]
- Cheng, M.; Kong, R.Q.; Zhang, R.F.; Wang, X.Y.; Wang, J.; Chen, M.L. Effect of glyoxal concentration on the properties of corn starch/poly(vinyl alcohol)/carvacrol nanoemulsion active films. Ind. Crops Prod. 2021, 171, 113864. [Google Scholar] [CrossRef]
- Wei, Y.X.; Huang, Z.; Yu, Z.L.; Han, C.; Yang, C.R. Preparation and Properties of Fractionated Soybean Protein Isolate Films. Materials 2021, 14, 5436. [Google Scholar] [CrossRef] [PubMed]
- Eedem, B.G.; Kaya, S. Characterization and application of novel composite films based on soy protein isolate and sunflower oil produced using freeze drying method. Food Chem. 2022, 366, 130709. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Zhang, L.H.; Zhang, M.; Mujumdar, A.S.; Wang, D.Y.; Ma, Y.M. Novel multilayer chitosan/emulsion-loaded syringic acid grafted apple pectin film with sustained control release for active food packaging. Food Hydrocoll. 2023, 142, 108823. [Google Scholar] [CrossRef]
- Xu, H.Y.; Shi, Y.; Gao, L.; Shi, N. Preparation and characterization of PH-responsive polyvinyl alcohol/chitosan/anthocyanin films. Food Sci. Technol. 2023, 43, e98022. [Google Scholar] [CrossRef]
- Rose, K.R.; Arunima, R.; Siji, K.M.; Pillai, P.S.; Joseph, S.; Laly, A.P. pH indicator films fabricated from soy protein isolate modified with chitin nanowhisker and Clitoria ternatea flower extract. Curr. Res. Food Sci. 2022, 5, 743–751. [Google Scholar] [CrossRef]
- Huang, Q.X.; Jiao, X.D.; Yan, B.W.; Zhang, N.N.; Huang, J.L.; Zhao, J.X.; Zhang, H.; Chen, W.; Fan, D.M. Changes in physicochemical properties of silver carp (Hypophthalmichthys molitrix) surimi during chilled storage: The roles of spoilage bacteria. Food Chem. 2022, 387, 132847. [Google Scholar] [CrossRef]
- Fahimeh, H.; Homa, B.; Abdorreza, M.N.; Marzieh, B. Preparation and characterization of an intelligent film based on fish gelatin and Coleus scutellarioides anthocyanin to monitor the freshness of rainbow trout fish fillet. Food Sci. Nutr. 2022, 11, 379–389. [Google Scholar] [CrossRef]
- He, H.M.; Song, Y.D.; Li, M.N.; Zhang, H.; Li, J.L.; Huang, H.; Li, Y.X. A novel anthocyanin electrospun film by caffeic acid co-pigmentation for real-time fish freshness monitoring. Anal. Methods Adv. Methods Appl. 2022, 15, 228–239. [Google Scholar] [CrossRef]
- Koshy, R.R.; Reghunadhan, A.; Mary, S.K.; Thomas, K.; Ajish, K.R.; Thomas, S.; Pothen, L.A. Intelligent pH-sensitive films from whole arrowroot powder and soy protein isolate incorporating red cabbage anthocyanin: Monitoring freshness of shrimps and ammonia in fish farming ponds. N. J. Chem. 2022, 19, 9036–9047. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, J.R.; Jia, Z.X.; Yang, X.T.; Zhou, Z.Y. Intelligent pH indicator films containing anthocyanins extracted from blueberry peel for monitoring tilapia fillet freshness. J. Sci. Food Agric. 2020, 101, 1800–1811. [Google Scholar] [CrossRef]



| Film | Thickness (mm) | Mechanical Properties | Barrier Properties | ||
|---|---|---|---|---|---|
| TS (MPa) | EB (%) | WVP (g/m2·24 h) | OP (cm3/m2·24 h·0.1 MPa) | ||
| ASKG/SPI | 0.056 ± 0.001 d | 1.543 ± 0.120 d | 202.292 ± 2.567 a | 560.22 ± 0.04 c | 1.024 ± 0.013 b |
| LRMA-1% | 0.081 ± 0.008 c | 4.874 ± 0.259 b | 12.395 ± 2.394 d | 746.41 ± 0.02 b | 0.156 ± 0.025 c |
| LRMA-2% | 0.092 ± 0.003 bc | 3.505 ± 0.122 c | 25.054 ± 2.843 c | 915.19 ± 0.01 a | 0.160 ± 0.032 c |
| LRMA-3% | 0.118 ± 0.001 a | 3.863 ± 0.476 c | 86.150 ± 2.609 b | 450.30 ± 0.01 d | 4.755 ± 0.017 a |
| LRMA-4% | 0.098 ± 0.010 ab | 8.147 ± 1.764 a | 5.560 ± 2.833 e | 446.36 ± 0.02 d | 4.716 ± 0.036 a |
| Sample | pH | L* | a* | b* | Photographs | |
|---|---|---|---|---|---|---|
| Before Sensing | After Sensing | |||||
| LRMA-1% | 2 3 4 5 6 7 8 9 10 | 86.31 ± 1.98 c 76.13 ± 2.14 a 82.72 ± 3.12 bc 88.09 ± 2.66 c 96.73 ± 4.95 d 77.34 ± 4.63 ab 83.58 ± 1.08 c 86.58 ± 2.67 c 94.85 ± 1.95 d | 11.86 ± 1.00 f 22.94 ± 0.96 g 8.38 ± 0.38 e 7.55 ± 0.25 e 0.78 ± 0.03 a 7.55 ± 0.51 e 4.85 ± 0.10 c 6.12 ± 0.27 d 2.17 ± 0.21 b | 1.48 ± 0.14 c −5.31 ± 0.25 a 3.12 ± 0.03 e 2.49 ± 0.15 d 1.40 ± 0.04 c 2.49 ± 0.18 d 3.58 ± 0.22 f 5.68 ± 0.27 g 0.06 ± 0.01 b |  |  |
| LRMA-2% | 2 3 4 5 6 7 8 9 10 | 68.66 ± 1.22 c 62.08 ± 1.37 b 79.57 ± 1.37 d 69.81 ± 3.13 c 89.42 ± 0.45 g 59.67 ± 1.08 a 61.56 ± 0.35 b 73.94 ± 2.11 e 83.47 ± 0.22 f | 33.09 ± 0.42 g 37.30 ± 0.11 h 11.73 ± 0.31 d 16.58 ± 076 d 3.69 ± 0.03 a 17.16 ± 1.09 ef 18.53 ± 0.72 f 8.67 ± 0.14 c 4.61 ± 0.16 b | −6.47 ± 0.49 b −12.39 ± 0.30 a 5.42 ± 0.11 de 6.60 ± 0.36 e 0.32 ± 0.09 c −11.47 ± 1.13 a −13.03 ± 0.81 a 3.71 ± 0.55 d −0.10 ± 0.07 c |  |  |
| LRMA-3% | 2 3 4 5 6 7 8 9 10 | 57.98 ± 0.69 d 54.96 ± 1.38 c 69.61 ± 1.62 e 75.50 ± 1.96 f 74.02 ± 2.20 f 35.04 ± 0.93 a 48.37 ± 0.76 b 58.15 ± 1.05 d 55.65 ± 1.28 c | 56.66 ± 1.77 h 37.34 ± 1.12 e 19.8 ± 0.08 b 19.28 ± 1.73 b 9.99 ± 1.01 a 22.27 ± 0.35 c 46.93 ± 0.99 f 28.69 ± 0.56 d 51.57 ± 1.57 g | −13.92 ± 0.38 a −9.01 ± 1.85 b 2.56 ± 1.25 d 1.00 ± 0.25 d 2.53 ± 0.28 d −7.54 ± 1.13 b −13.42 ± 0.74 a −4.25 ± 0.66 c −14.42 ± 1.07 a |  |  |
| LRMA-4% | 2 3 4 5 6 7 8 9 10 | 45.00 ± 1.95 b 47.10 ± 1.51 cd 62.24 ± 1.98 e 40.55 ± 1.26 a 45.51 ± 0.76 bc 47.49 ± 0.45 d 44.78 ± 1.35 b 63.32 ± 0.78 e 47.26 ± 1.29 cd | 65.24 ± 0.22 g 49.23 ± 0.36 d 24.83 ± 0.59 a 52.45 ± 0.36 e 55.45 ± 0.17 f 34.93 ± 1.14 b 50.27 ± 1.28 d 43.37 ± 0.46 c 53.67 ± 0.52 e | −4.05 ± 1.01 d −2.31 ± 1.02 e 0.80 ± 0.09 f −15.50 ± 2.35 a −13.45 ± 0.25 b −10.96 ± 0.06 c −11.03 ± 0.75 c −12.16 ± 0.88 bc −13.72 ± 0.73 b |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Gao, L.; Wang, J.; Xue, Z.; Zhang, M.; Ma, X.; Wang, G.; Lv, S. Preparation and Application of pH-Sensitive Film Containing Anthocyanins Extracted from Lycium ruthenicum Murr. Materials 2023, 16, 3828. https://doi.org/10.3390/ma16103828
Zhao Y, Gao L, Wang J, Xue Z, Zhang M, Ma X, Wang G, Lv S. Preparation and Application of pH-Sensitive Film Containing Anthocyanins Extracted from Lycium ruthenicum Murr. Materials. 2023; 16(10):3828. https://doi.org/10.3390/ma16103828
Chicago/Turabian StyleZhao, Yucong, Le Gao, Jing Wang, Ziyan Xue, Mengyao Zhang, Xueli Ma, Guohua Wang, and Shenghua Lv. 2023. "Preparation and Application of pH-Sensitive Film Containing Anthocyanins Extracted from Lycium ruthenicum Murr." Materials 16, no. 10: 3828. https://doi.org/10.3390/ma16103828





