The Components’ Roles in Thermal Stability and Flammability of Cork Powder
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Particle Size Distribution, Density and Morphology of Cork Powder
3.2. Chemical Composition and Characterization of Cork Components
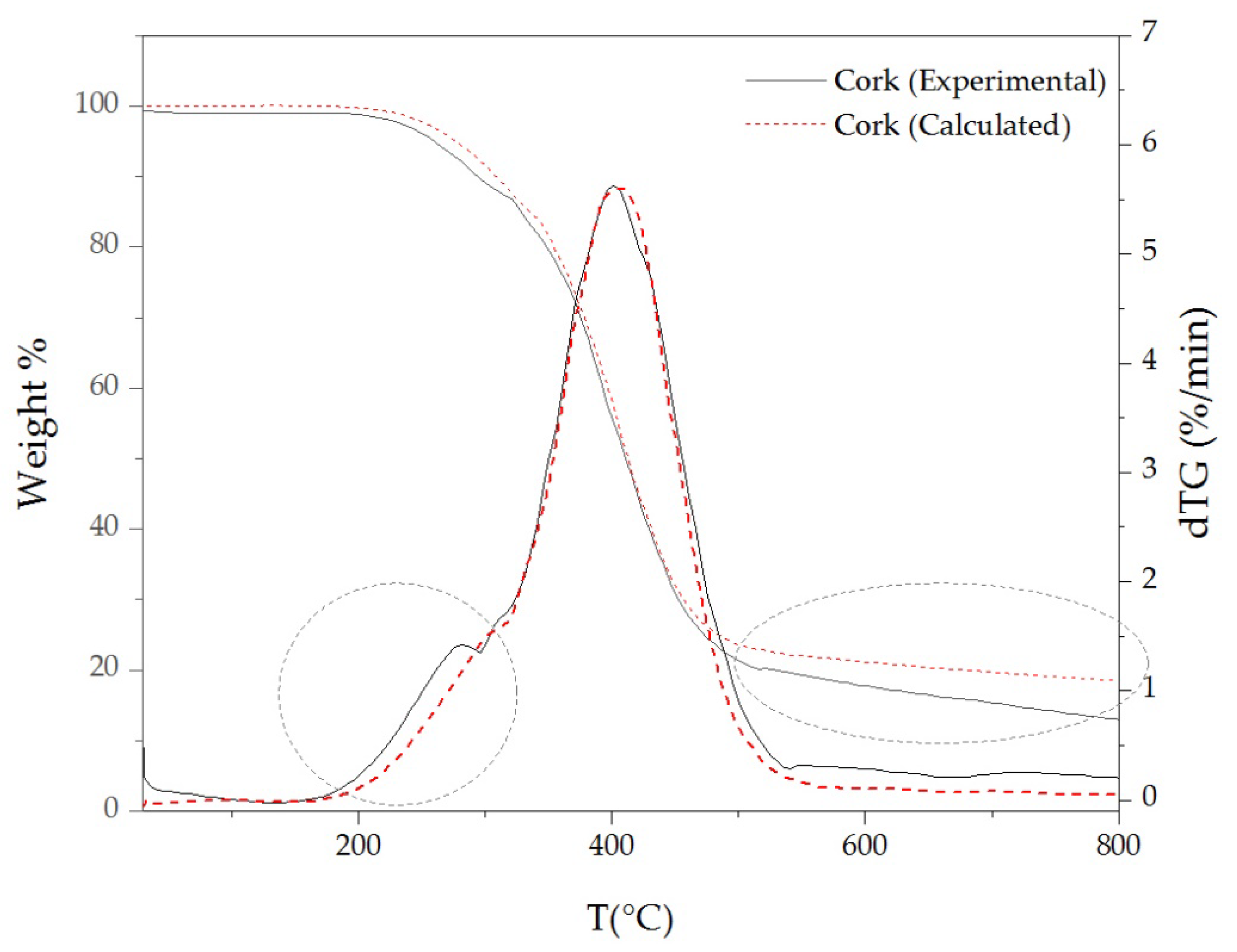
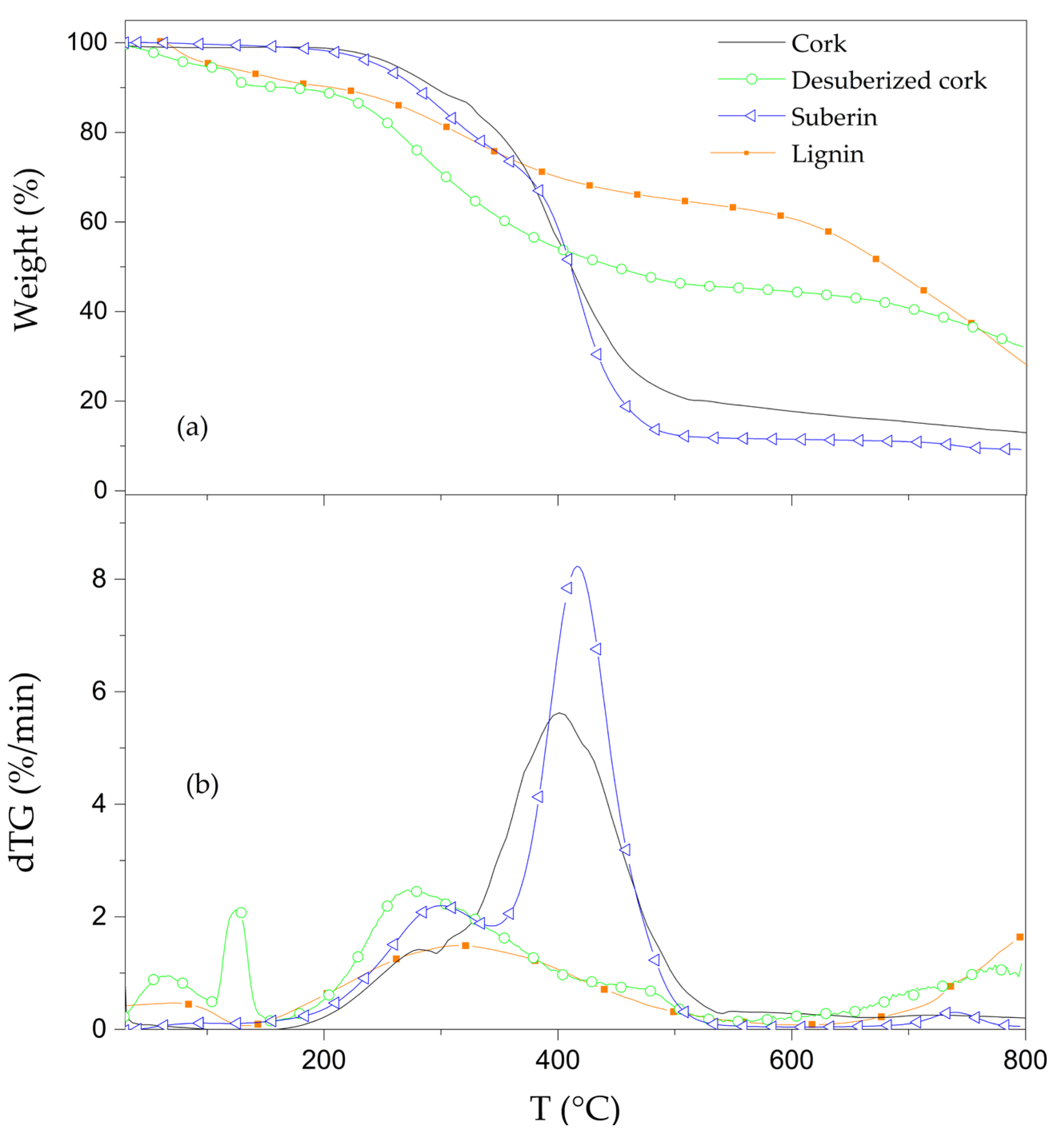
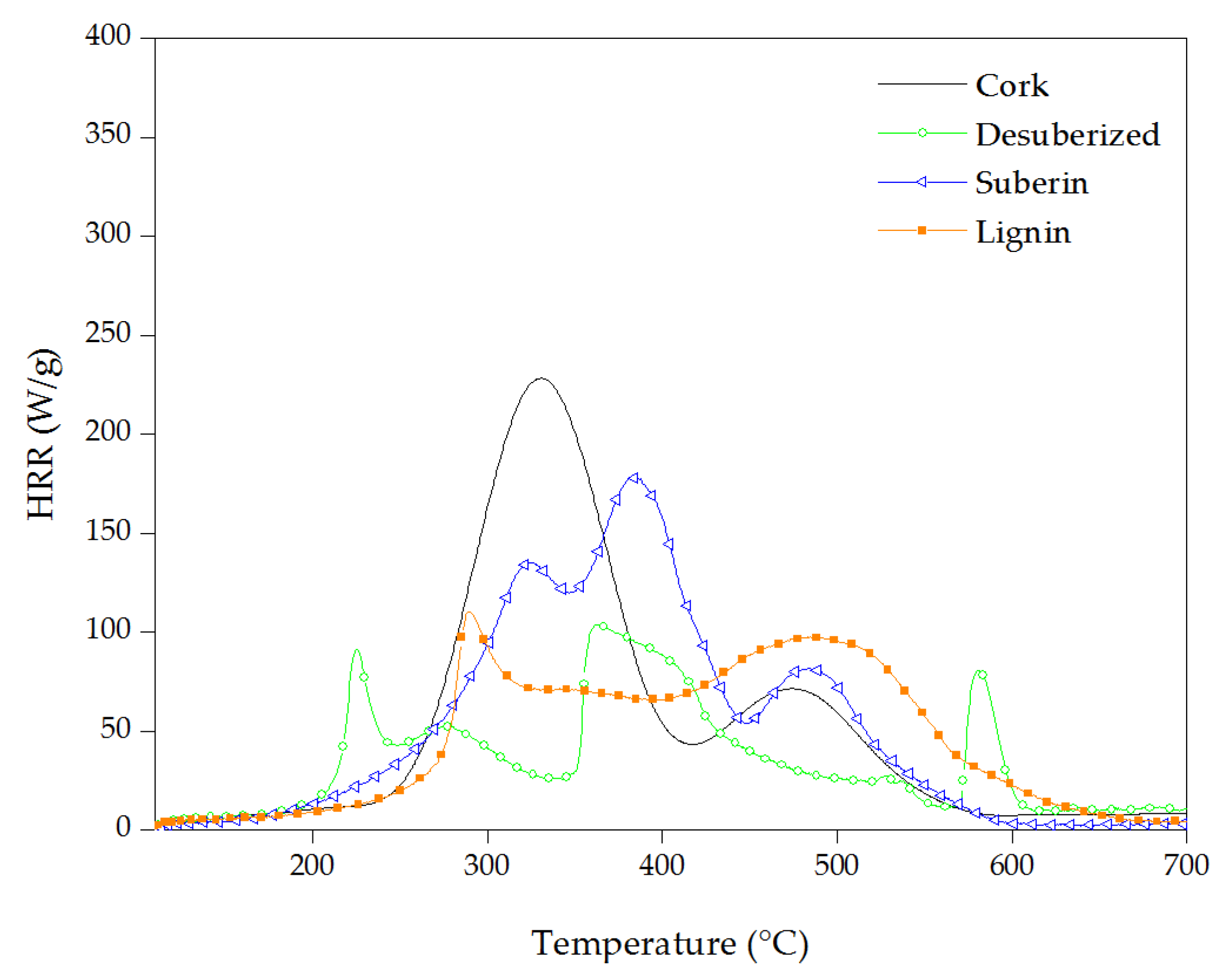
3.3. Thermal Stability of Cork Powder and Its Main Components
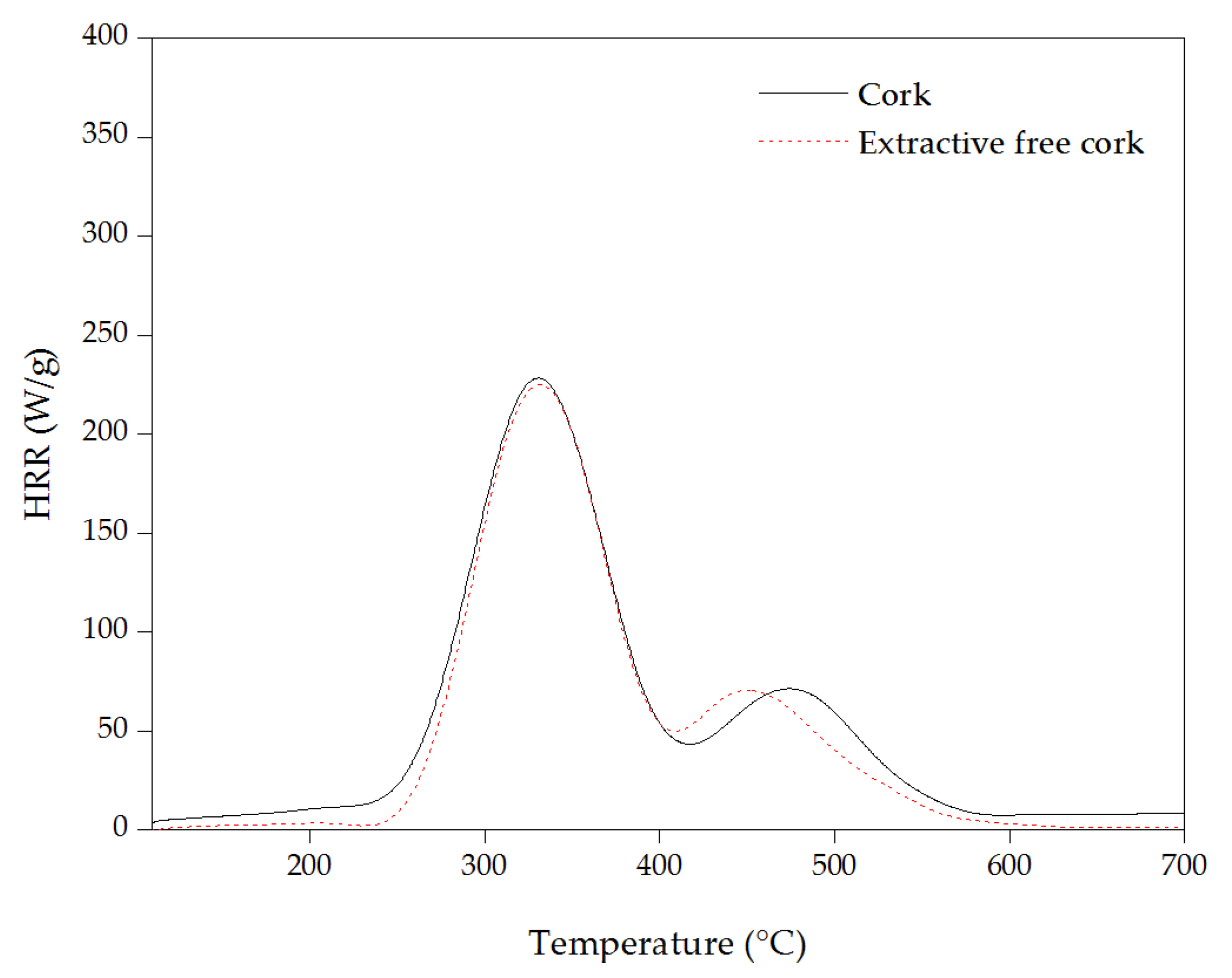
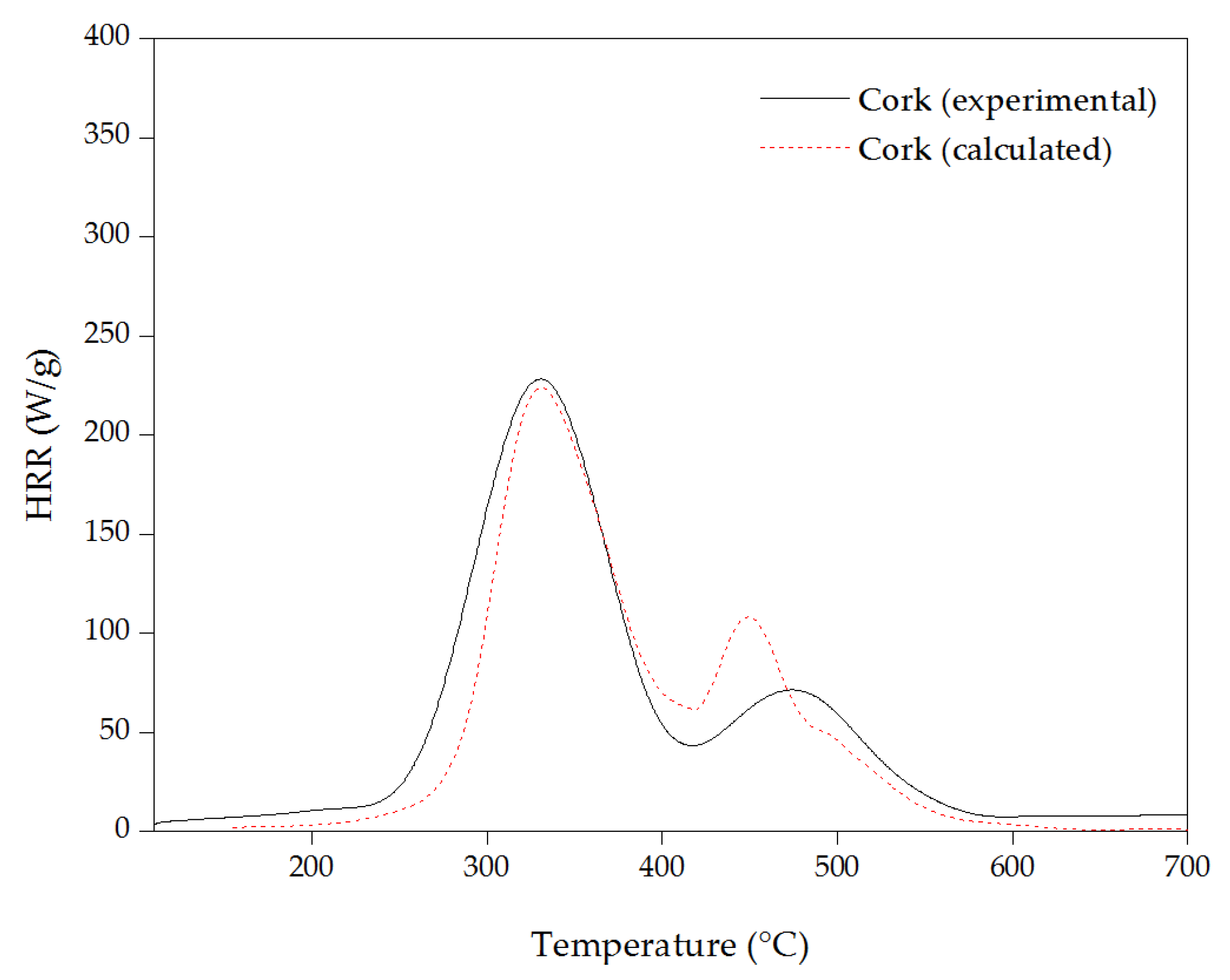
3.4. Micro-Scale Combustion Calorimeter (MCC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Gallo, E.; Schartel, B.; Acierno, D.; Russo, P. Flame retardant biocomposites: Synergism between phosphinate and nanometric metal oxides. Eur. Polym. J. 2011, 47, 1390–1401. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T.; Selke, S.E.; Harte, B.R.; Hinrichsen, G. Natural fibers, biopolymers, and biocomposites: An introduction. In Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005; pp. 1–36. [Google Scholar]
- Gil, L. Cork Composites: A Review. Materials 2009, 2, 776–789. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Ben Cheikh, R.; Baklouti, M.; Denchev, Z.; Cunha, A.M. Effect of surface treatment in cork reinforced composites. J. Polym. Res. 2010, 17, 519–528. [Google Scholar] [CrossRef]
- Fernandes, E.M.; Correlo, V.M.; Chagas, J.A.M.; Mano, J.F.; Reis, R.L. Properties of new cork-polymer composites: Advantages and drawbacks as compared with commercially available fibreboard materials. Compos. Struct. 2011, 93, 3120–3129. [Google Scholar] [CrossRef]
- Sen, A.; Van den Bulcke, J.; Defoirdt, N.; Van Acker, J.; Pereira, H. Thermal behaviour of cork and cork components. Thermochim. Acta 2014, 582, 94–100. [Google Scholar] [CrossRef]
- Fernandes, E.M.; Mano, J.F.; Reis, R.L. Hybrid cork-polymer composites containing sisal fibre: Morphology, effect of the fibre treatment on the mechanical properties and tensile failure prediction. Compos. Struct. 2013, 105, 153–162. [Google Scholar] [CrossRef]
- Magalhaes da Silva, S.P.; Lima, P.S.; Oliveira, J.M. Non-isothermal crystallization kinetics of cork-polymer composites for injection molding. J. Appl. Polym. Sci. 2016, 133, 44124. [Google Scholar] [CrossRef]
- De Oliveira, H.; Yoon, B.; Michaud, V.; Nam, J.-D.; Suhr, J. All natural cork composites with suberin-based polyester and lignocellulosic residue. Ind. Crops Prod. 2017, 109, 843–849. [Google Scholar] [CrossRef]
- Reis, L.; Silva, A. Mechanical behavior of sandwich structures using natural cork agglomerates as core materials. J. Sandw. Struct. Mater. 2009, 11, 487–500. [Google Scholar] [CrossRef]
- Xie, Z.; Luo, E.Z.; Xu, J.B.; Wilson, I.H.; Zhao, L.H.; Zhang, X.X. Construction and characterization of a heating stage for a scanning probe microscope up to 215 degrees C. Rev. Sci. Instrum. 2000, 71, 2100–2103. [Google Scholar] [CrossRef]
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Lei, Y.; Jia, T.; Song, X. Chemical composition of cork from Quercus variablis. Wood Fiber Sci. 2012, 44, 214–219. [Google Scholar]
- Pereira, H. The rationale behind cork properties: A review of structure and chemistry. Bioresources 2015, 10, 6207–6229. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Structural differences between wood species: Evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. J. Appl. Polym. Sci. 2012, 126, E336–E343. [Google Scholar] [CrossRef]
- Cordeiro, N.; Belgacem, M.N.; Gandini, A.; Neto, C.P. Urethanes and polyurethanes from suberin 2: Synthesis and characterization. Ind. Crops Prod. 1999, 10, 1–10. [Google Scholar] [CrossRef]
- Sousa, A.F.; Gandini, A.; Silvestre, A.J.D.; Neto, C.P. Synthesis and characterization of novel biopolyesters from suberin and model comonomers. Chemsuschem 2008, 1, 1020–1025. [Google Scholar] [CrossRef]
- Sousa, A.F.; Gandini, A.; Silvestre, A.J.D.; Pascoal Neto, C.; Cruz Pinto, J.J.C.; Eckerman, C.; Holmbom, B. Novel suberin-based biopolyesters: From synthesis to properties. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2281–2291. [Google Scholar] [CrossRef]
- Fernandes, E.M.; Aroso, I.M.; Mano, J.F.; Covas, J.A.; Reis, R.L. Functionalized cork-polymer composites (CPC) by reactive extrusion using suberin and lignin from cork as coupling agents. Compos. Part B Eng. 2014, 67, 371–380. [Google Scholar] [CrossRef]
- Cordeiro, N.; Blayo, A.; Belgacem, N.M.; Gandini, A.; Neto, C.P.; LeNest, J.F. Cork suberin as an additive in offset lithographic printing inks. Ind. Crops Prod. 2000, 11, 63–71. [Google Scholar] [CrossRef]
- Aroso, I.M.; Araujo, A.R.; Pires, R.A.; Reis, R.L. Cork: Current technological developments and future perspectives for this natural, renewable, and sustainable material. ACS Sustain. Chem. Eng. 2017, 5, 11130–11146. [Google Scholar] [CrossRef]
- Heinamaki, J.; Pirttimaa, M.M.; Alakurtti, S.; Pitkanen, H.P.; Kanerva, H.; Hulkko, J.; Paaver, U.; Aruvali, J.; Yliruusi, J.; Kogermann, K. Suberin fatty acids from outer birch bark: Isolation and physical material characterization. J. Nat. Prod. 2017, 80, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, P.; Collins, M.N.; Jesionowski, T.; Klapiszewski, L. The role of lignin and lignin-based materials in sustainable construction-A comprehensive review. Int. J. Biol. Macromol. 2021, 187, 624–650. [Google Scholar] [CrossRef]
- Graupner, N. Application of lignin as natural adhesion promoter in cotton fibre-reinforced poly(lactic acid) (PLA) composites. J. Mater. Sci. 2008, 43, 5222–5229. [Google Scholar] [CrossRef]
- Hilburg, S.L.; Elder, A.N.; Chung, H.; Ferebee, R.L.; Bockstaller, M.R.; Washburn, N.R. A universal route towards thermoplastic lignin composites with improved mechanical properties. Polymer 2014, 55, 995–1003. [Google Scholar] [CrossRef]
- Simkovic, I. Unexplored possibilities of all-polysaccharide composites. Carbohydr. Polym. 2013, 95, 697–715. [Google Scholar] [CrossRef]
- Lucenius, J.; Valle-Delgado, J.J.; Parikka, K.; Osterberg, M. Understanding hemicellulose-cellulose interactions in cellulose nanofibril-based composites. J. Colloid Interface Sci. 2019, 555, 104–114. [Google Scholar] [CrossRef]
- Matos, A.M.; Nunes, S.; Sousa-Coutinho, J. Cork waste in cement based materials. Mater. Des. 2015, 85, 230–239. [Google Scholar] [CrossRef]
- Fernandes, E.M.; Correlo, V.M.; Chagas, J.A.M.; Mano, J.F.; Reis, R.L. Cork based composites using polyolefin’s as matrix: Morphology and mechanical performance. Compos. Sci. Technol. 2010, 70, 2310–2318. [Google Scholar] [CrossRef]
- Gil, L. Cork powder waste: An overview. Biomass Bioenergy 1997, 13, 59–61. [Google Scholar] [CrossRef]
- Hobbs, C.E. Recent advances in bio-based flame retardant additives for synthetic polymeric materials. Polymers 2019, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, M.N.; Shah, A.U.R.; Song, J.-I. Improved flame-retardant and tensile properties of thermoplastic starch/flax fabric green composites. Carbohydr. Polym. 2017, 168, 201–211. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Yang, J.; Hu, Y. Preparation of lignin-silica hybrids and its application in intumescent flame-retardant poly(lactic acid) system. High Perform. Polym. 2012, 24, 738–746. [Google Scholar] [CrossRef]
- Shi, T.; Zhang, S.; Shi, X. Oxidized regenerated celluloses to fabricate high fire safety for epoxy resin with super expansion char layer. Cellulose 2021, 28, 2995–3015. [Google Scholar] [CrossRef]
- Jove, P.; Angels Olivella, M.; Cano, L. Study of the variability in chemical composition of bark layers of cork from different production areas. Bioresources 2011, 6, 1806–1815. [Google Scholar] [CrossRef]
- Sen, A.; Miranda, I.; Esteves, B.; Pereira, H. Chemical characterization, bioactive and fuel properties of waste cork and phloem fractions from Quercus cerris L. bark. Ind. Crops Prod. 2020, 157, 112909. [Google Scholar] [CrossRef]
- Pereira, H. Chemical composition and variability of cork from Quercus suber L. Wood Sci. Technol. 1988, 22, 211–218. [Google Scholar] [CrossRef]
- Shangguan, W.; Chen, Z.; Zhao, J.; Song, X. Thermogravimetric analysis of cork and cork components from Quercus variabilis. Wood Sci. Technol. 2018, 52, 181–192. [Google Scholar] [CrossRef]
- Wise, L.E.; Murphy, M.; Daddieco, A.A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and studies on the hemicelluloses. Tech. Assoc. Pap. 1946, 29, 210–218. [Google Scholar]
- Barbosa, A.Q.; da Silva, L.F.M.; Oechsner, A.; Abenojar, J.; del Real, J.C. Influence of the size and amount of cork particles on the impact toughness of a structural adhesive. J. Adhes. 2012, 88, 452–470. [Google Scholar] [CrossRef]
- Flores, M.; Rosa, M.E.; Barlow, C.Y.; Fortes, M.A.; Ashby, M.F. Properties and uses of consolidated cork dust. J. Mater. Sci. 1992, 27, 5629–5634. [Google Scholar] [CrossRef]
- Gibson, L.J.; Easterling, K.E.; Ashby, M.F. The structure and mechanics of cork. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1981, 377, 99–117. [Google Scholar] [CrossRef]
- Marques, A.V.; Pereira, H.; Meier, D.; Faix, O. Quantitative analysis of cork (Quercus suber L.) and milled cork lignin by FTIR spectroscopy, analytical pyrolysis, and total hydrolysis. Holzforschung 1994, 48, 43–50. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Pereira, H. Cellular structure and chemical composition of cork from the Chinese cork oak (Quercus variabilis). J. Wood Sci. 2013, 59, 1–9. [Google Scholar] [CrossRef]
- Lopes, M.H.; Barros, A.S.; Neto, C.P.; Rutledge, D.; Delgadillo, I.; Gil, A.M. Variability of cork from Portuguese Quercus suber studied by solid-state C-13-NMR and FTIR spectroscopies. Biopolymers 2001, 62, 268–277. [Google Scholar] [CrossRef]
- Gandolfi, S.; Ottolina, G.; Riva, S.; Fantoni, G.P.; Patel, I. Complete chemical analysis of carmagnola hemp hurds and structural features of its components. Bioresources 2013, 8, 2641–2656. [Google Scholar] [CrossRef]
- Neto, C.P.; Rocha, J.; Gil, A.; Cordeiro, N.; Esculcas, A.P.; Rocha, S.; Delgadillo, I.; Dejesus, J.D.P.; Correia, A.J.F. 13C Solid-state nuclear magnetic resonance and Fourier transform infrared studies of the thermal decomposition of cork. Solid State Nucl. Magn. Reson. 1995, 4, 143–151. [Google Scholar] [CrossRef]
- Ferreira, R.; Garcia, H.; Sousa, A.F.; Freire, C.S.R.; Silvestre, A.J.D.; Rebelo, L.P.N.; Pereira, C.S. Isolation of suberin from birch outer bark and cork using ionic liquids: A new source of macromonomers. Ind. Crops Prod. 2013, 44, 520–527. [Google Scholar] [CrossRef]
- Sen, A.; Marques, A.V.; Gominho, J.; Pereira, H. Study of thermochemical treatments of cork in the 150-400 degrees C range using colour analysis and FTIR spectroscopy. Ind. Crops Prod. 2012, 38, 132–138. [Google Scholar] [CrossRef]
- Esteves, B.; Graca, J.; Pereira, H. Extractive composition and summative chemical analysis of thermally treated eucalypt wood. Holzforschung 2008, 62, 344–351. [Google Scholar] [CrossRef]
- Sartori, C.J.; Mota, G.S.; Miranda, I.; Mori, F.A.; Pereira, H. Tannin extraction and characterization of polar extracts from the barks of two eucalyptus urophylla hybrids. Bioresources 2018, 13, 4820–4831. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Li, X.; Shupe, T.F.; Hse, C.Y. Chinese tallow tree (Sapium sebiferum) utilization: Characterization of extractives and cell-wall chemistry. Wood Fiber Sci. 2007, 39, 319–324. [Google Scholar]
- Mburu, F.; Sirmah, P.; Muisu, F.; Dumarcay, S.; Gerardin, P. Selected wood properties of Prunus africana (Hook) grown in Kenya as possible reasons for its high natural durability. Drv. Ind. 2013, 64, 19–24. [Google Scholar] [CrossRef]
- Mattos, B.D.; Lourencon, T.V.; Gatto, D.A.; Serrano, L.; Labidi, J. Chemical characterization of wood and extractives of fast-growing Schizolobium parahyba and Pinus taeda. Wood Mater. Sci. Eng. 2016, 11, 209–216. [Google Scholar] [CrossRef]
- Shebani, A.N.; van Reenen, A.J.; Meincken, M. The effect of wood extractives on the thermal stability of different wood-LLDPE composites. Thermochim. Acta 2009, 481, 52–56. [Google Scholar] [CrossRef]
- Shebani, A.N.; van Reenen, A.J.; Meincken, M. The effect of wood extractives on the thermal stability of different wood species. Thermochim. Acta 2008, 471, 43–50. [Google Scholar] [CrossRef]
- Jusner, P.; Barbini, S.; Schiehser, S.; Bacher, M.; Schwaiger, E.; Potthast, A.; Rosenau, T. Impact of residual extractives on the thermal stability of softwood Kraft pulp. Cellulose 2022, 29, 8797–8810. [Google Scholar] [CrossRef]
- Mohammed, J.K.; Mahdi, A.A.; Ahmed, M.I.; Ma, M.; Wang, H. Preparation, deproteinization, characterization, and antioxidant activity of polysaccharide from Medemia argun fruit. Int. J. Biol. Macromol. 2020, 155, 919–926. [Google Scholar] [CrossRef]
- Varma, C.A.K.; Kumar, K.J. Structural, functional and pH sensitive release characteristics of water-soluble polysaccharide from the seeds of Albizia lebbeck L. Carbohydr. Polym. 2017, 175, 502–508. [Google Scholar] [CrossRef]
- Yuan, T.-Q.; Xu, F.; He, J.; Sun, R.-C. Structural and physico-chemical characterization of hemicelluloses from ultrasound-assisted extractions of partially delignified fast-growing poplar wood through organic solvent and alkaline solutions. Biotechnol. Adv. 2010, 28, 583–593. [Google Scholar] [CrossRef]
- Teixeira, M.; Sonnier, R.; Otazaghine, B.; Ferry, L.; Aubert, M.; Tirri, T.; Wilen, C.-E.; Rouif, S. Radiation-grafting of flame retardants on flax fabrics—A comparison between different flame retardant structures. Radiat. Phys. Chem. 2018, 145, 135–142. [Google Scholar] [CrossRef]
- Hajj, R.; El Hage, R.; Sonnier, R.; Otazaghine, B.; Gallard, B.; Rouif, S.; Nakhl, M.; Lopez-Cuesta, J.-M. Grafting of phosphorus flame retardants on flax fabrics: Comparison between two routes. Polym. Degrad. Stab. 2018, 147, 25–34. [Google Scholar] [CrossRef]
- Dorez, G.; Ferry, L.; Sonnier, R.; Taguet, A.; Lopez-Cuesta, J.M. Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J. Anal. Appl. Pyrolysis 2014, 107, 323–331. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J.M. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Renner, J.S.; Mensah, R.A.; Jiang, L.; Xu, Q. A critical assessment of the fire properties of different wood species and bark from small- and bench-scale fire experiments. J. Therm. Anal. Calorim. 2022, 148, 1423–1434. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Pereira, H. The thermochemical degradation of cork. Wood Sci. Technol. 1992, 26, 259–269. [Google Scholar] [CrossRef]

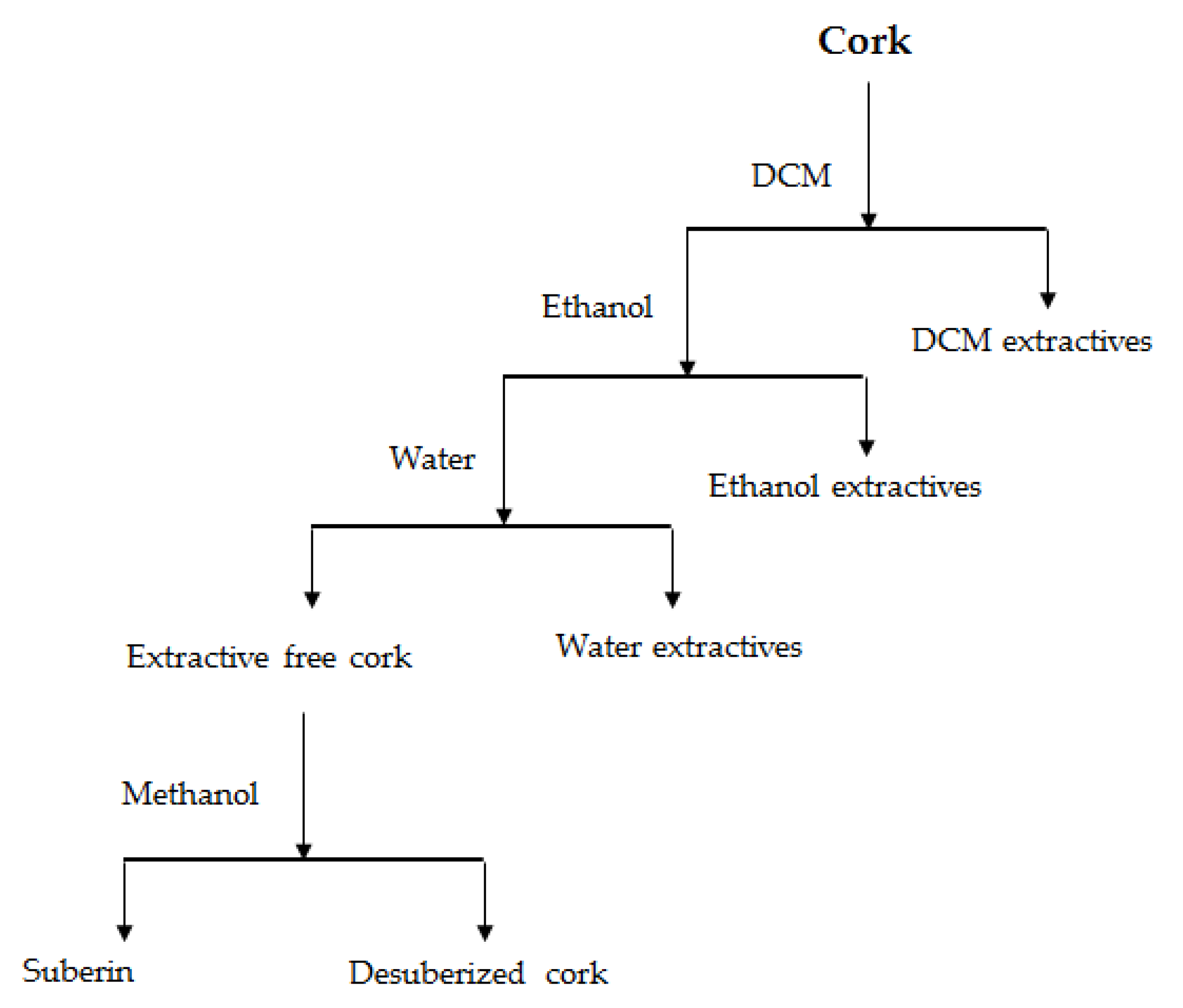
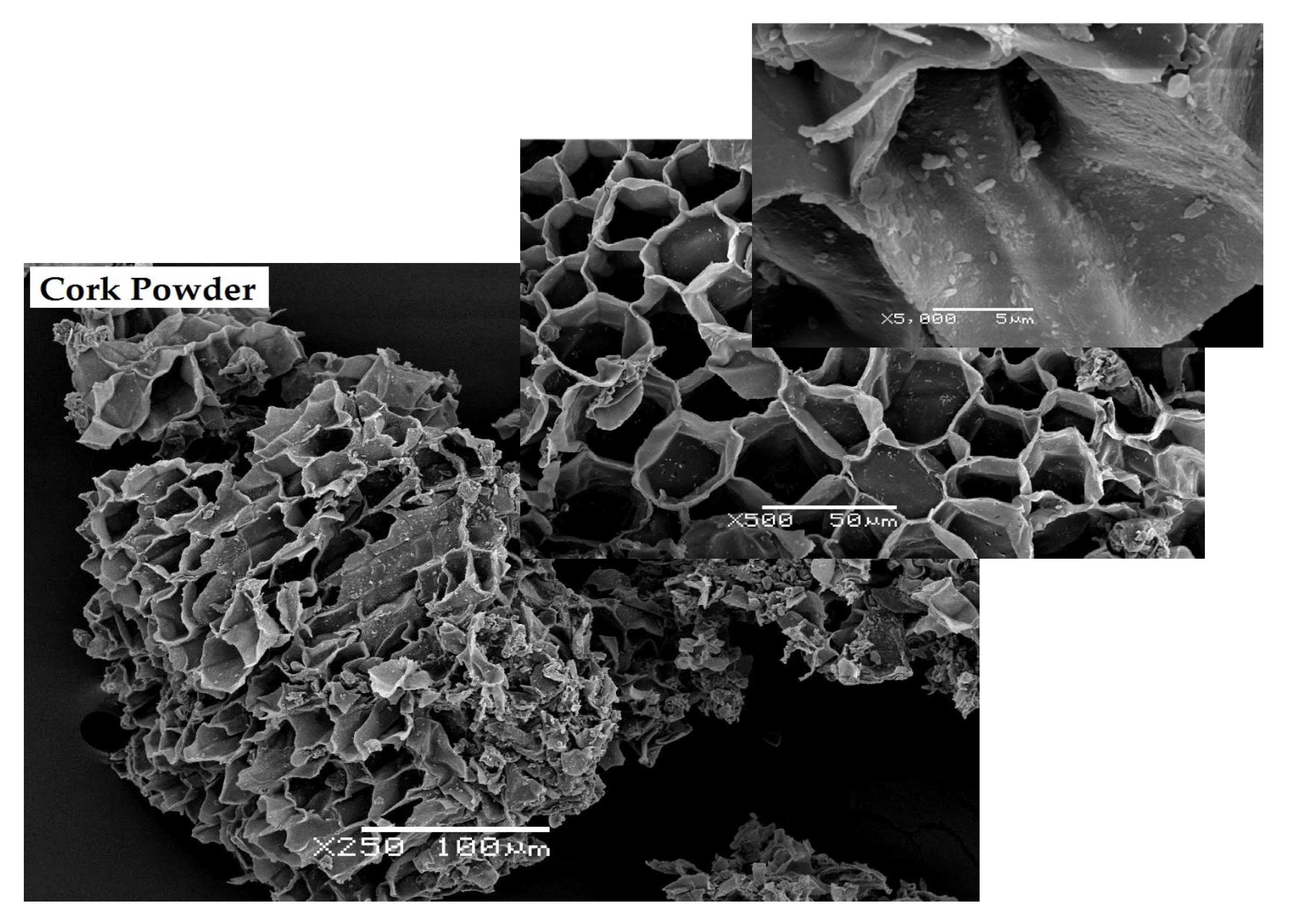

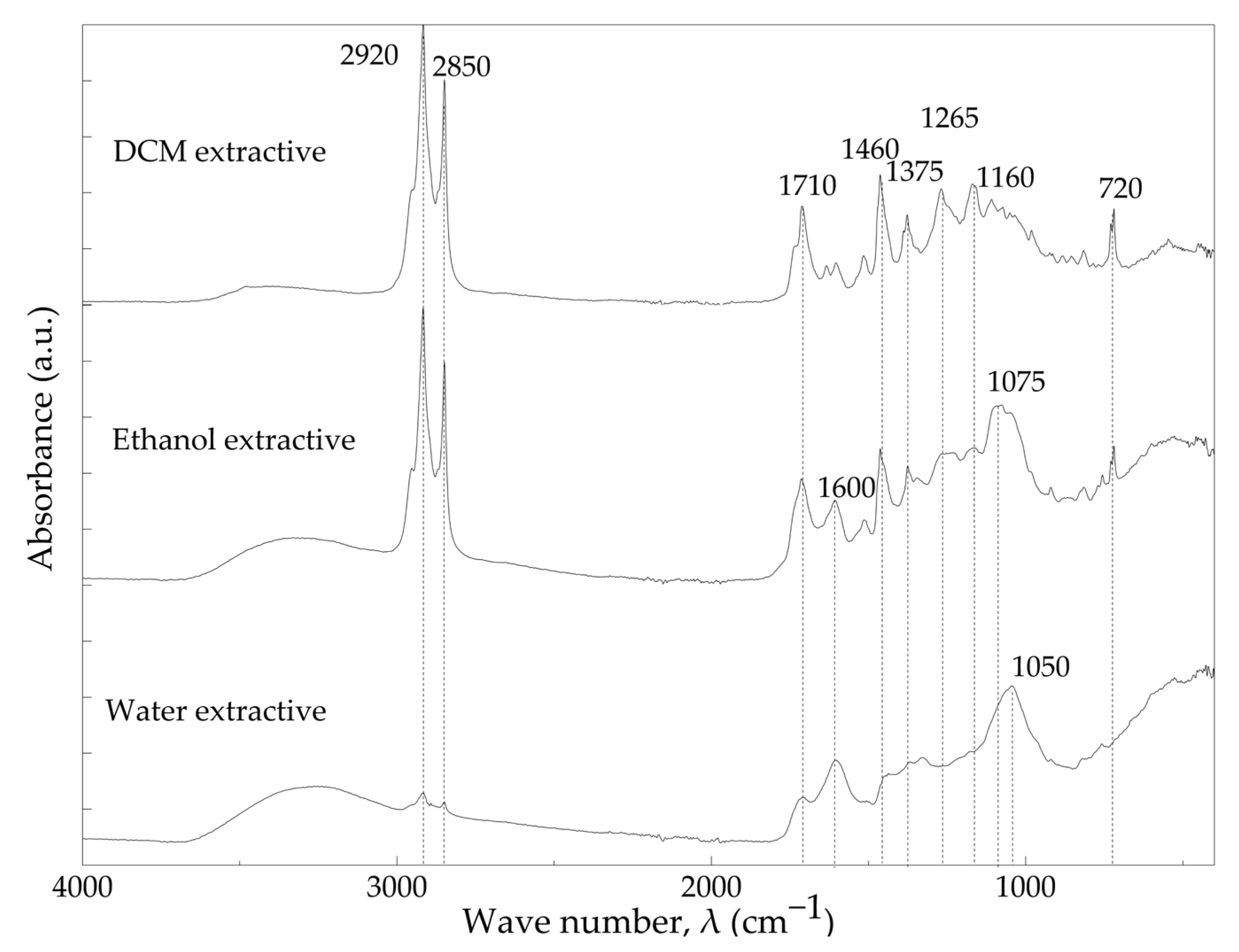

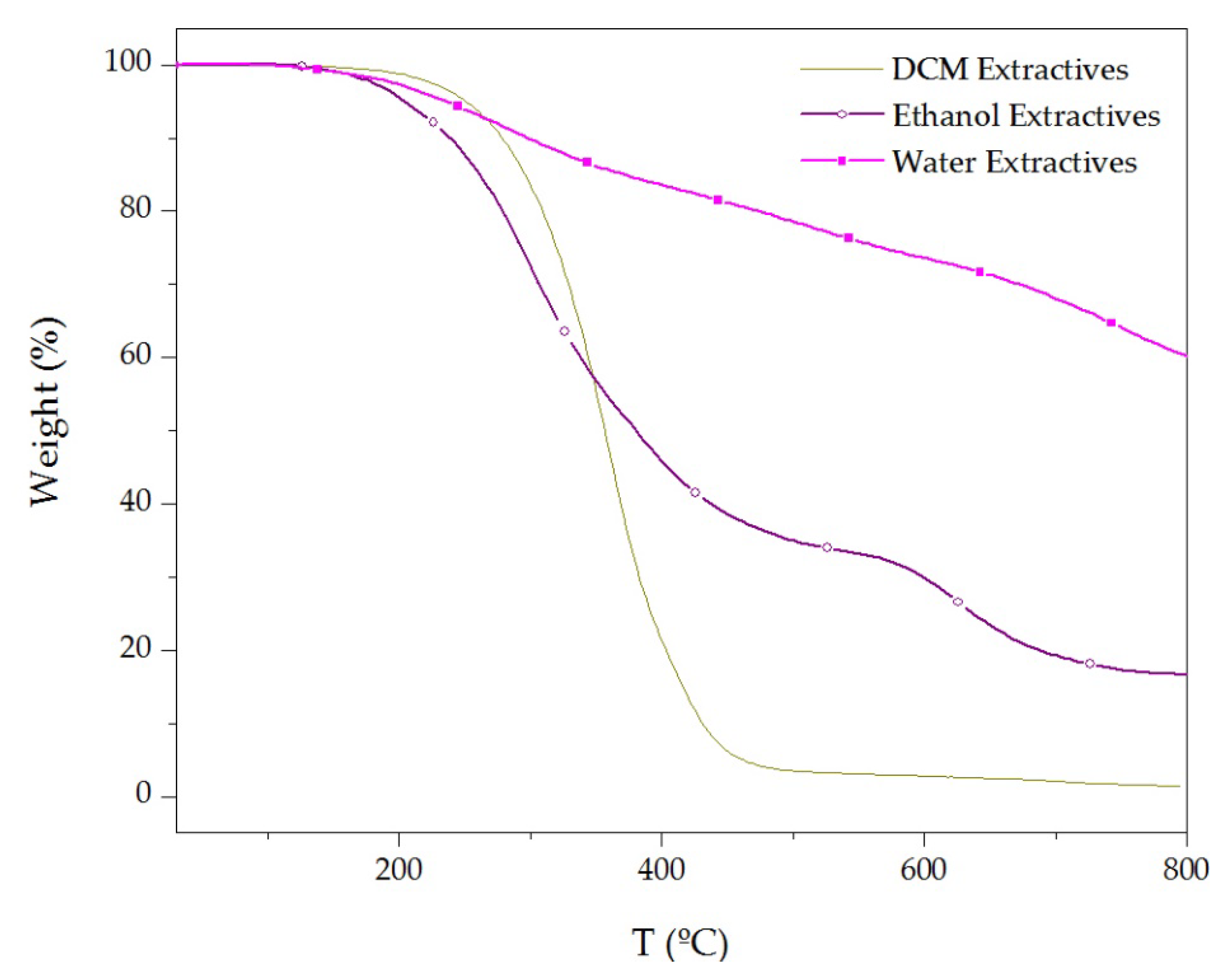

| Chemical Component | Wt% * |
|---|---|
| Suberin | 40 |
| Lignin | 24 |
| Polysaccharides | 19 |
| Extractives | 14 |
| -DCM Extractives | 7 |
| -Ethanol Extractives | 3 |
| -Water Extractives | 4 |
| Ash | 3 |
| Total mass | 100 |
| Materials | TD Step | Tpeak (°C) | ML (%) | R800°C (%) |
|---|---|---|---|---|
| Cork | 1 | 280 | 10.5 | 13.1 |
| 2 | 400 | 70.1 | ||
| Extractive-free cork | 1 | 300 | 8.7 | 17.1 |
| 2 | 400 | 71.4 | ||
| Desuberized cork | 1 | 65 | 5.1 | 32.6 |
| 2 | 125 | 4.9 | ||
| 3 | 270 | 49.8 | ||
| Suberin | 1 | 295 | 33.8 | 9.5 |
| 2 | 420 | 52.1 | ||
| 3 | 740 | 1.5 | ||
| Lignin | 1 | 60 | 6.7 | 29.4 |
| 2 | 350 | 32.5 | ||
| DCM extractives | 1 | 360 | 95.8 | 1.4 |
| Ethanol extractives | 1 | 310 | 66.1 | 16.7 |
| 2 | 645 | 16.9 | ||
| Water extractives | 1 | 280 | 16.3 | 60.1 |
| 2 | 520 | 12.1 | ||
| 3 | 745 | 11.4 |
| Samples | pHRR (W/g) | THR (kJ/g) | Temperature to pHRR (°C) |
|---|---|---|---|
| Cork | 230 ± 12 | 31.5 ± 0.3 | 330 ± 1 |
| 70 ± 5 | 475 ± 1 | ||
| Extractive-free cork | 225 ± 12 | 27.7 ± 0.9 | 330 ± 2 |
| 70 ± 5 | 455 ± 1 | ||
| Desuberized cork | 90 ± 2 | 24.1 ± 0.4 | 225 ± 1 |
| 105 ± 8 | 365 ± 1 | ||
| 80 ± 4 | 580 ± 0 | ||
| Suberin | 135 ± 17 | 27.2 ± 0.5 | 325 ± 1 |
| 175 ± 11 | 390 ± 1 | ||
| 80 ± 2 | 480 ± 0 | ||
| Lignin | 110 ± 12 | 26.8 ± 0.5 | 290 ± 1 |
| 95 ± 12 | 490 ± 1 | ||
| DCM extractive | 365 ± 4 | 36.6 ± 0.7 | 410 ± 2 |
| Ethanol extractive | 220 ± 10 | 30.1 ± 0.9 | 385 ± 0 |
| Water extractive | 35 ± 0 | 4.1 ± 0.1 | 310 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghonjizade-Samani, F.; Haurie, L.; Malet, R.; Realinho, V. The Components’ Roles in Thermal Stability and Flammability of Cork Powder. Materials 2023, 16, 3829. https://doi.org/10.3390/ma16103829
Ghonjizade-Samani F, Haurie L, Malet R, Realinho V. The Components’ Roles in Thermal Stability and Flammability of Cork Powder. Materials. 2023; 16(10):3829. https://doi.org/10.3390/ma16103829
Chicago/Turabian StyleGhonjizade-Samani, Farnaz, Laia Haurie, Ramón Malet, and Vera Realinho. 2023. "The Components’ Roles in Thermal Stability and Flammability of Cork Powder" Materials 16, no. 10: 3829. https://doi.org/10.3390/ma16103829







