Low Temperature Thermal Treatment of Incineration Fly Ash under Different Atmospheres and Its Recovery as Cement Admixture
Abstract
:1. Introduction
2. Materials and Experiment
2.1. Materials
2.2. Experiment
2.2.1. Low Temperature Thermal Treatment of the Water-Washed MSWI FA
2.2.2. Preparation of Cement with Different Admixture
2.2.3. Determination of Dioxin Content
2.2.4. Determination of the Contents of Heavy Metals
2.2.5. Leaching Characteristics of Heavy Metals
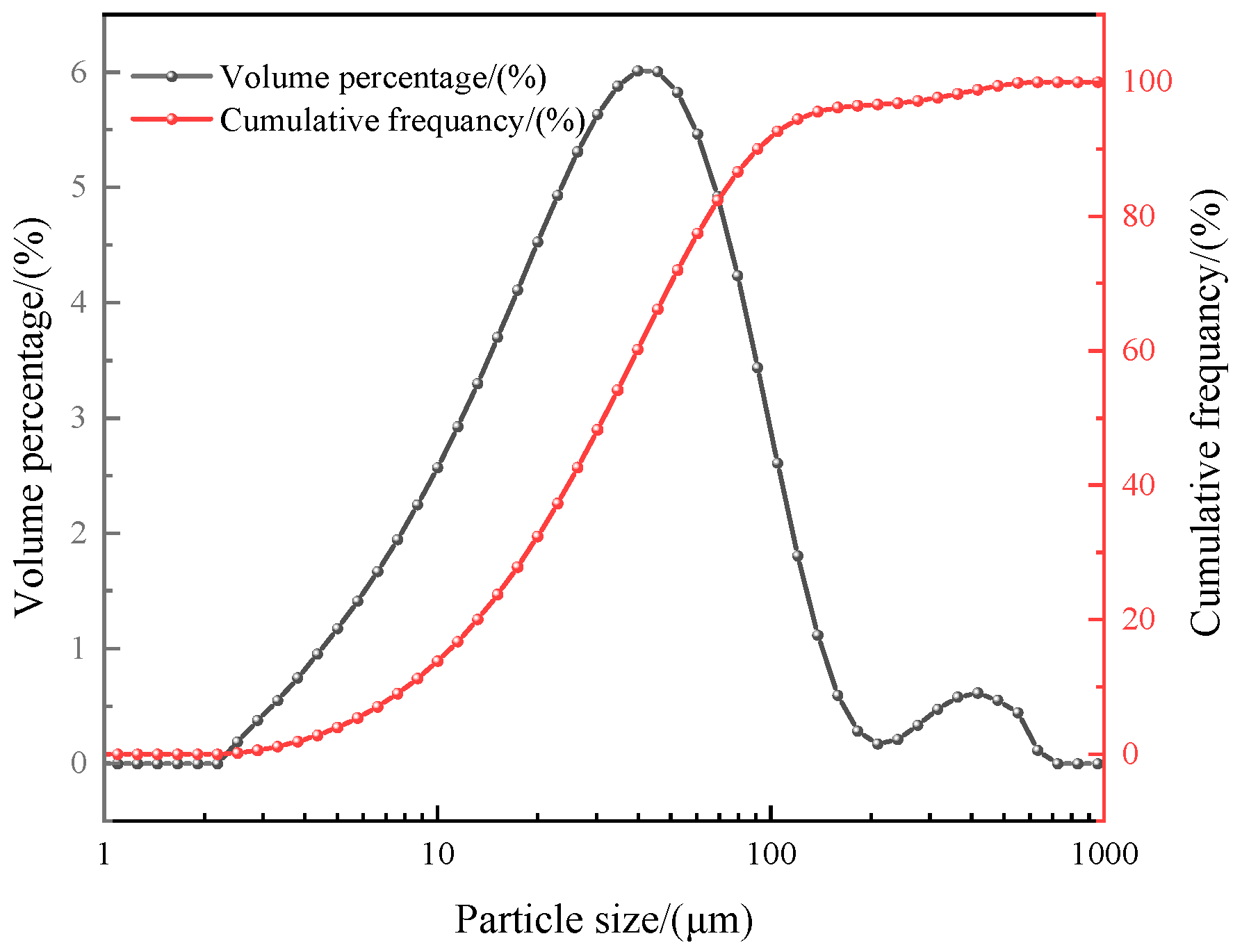
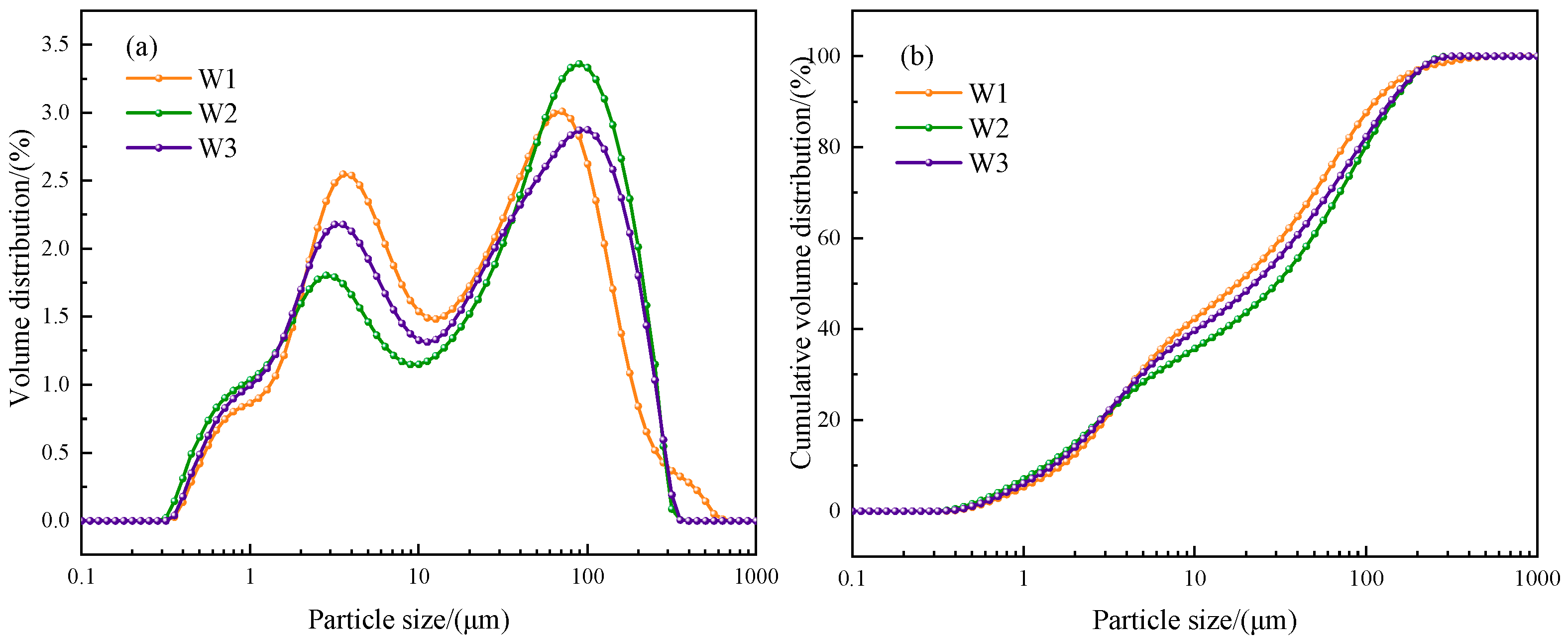
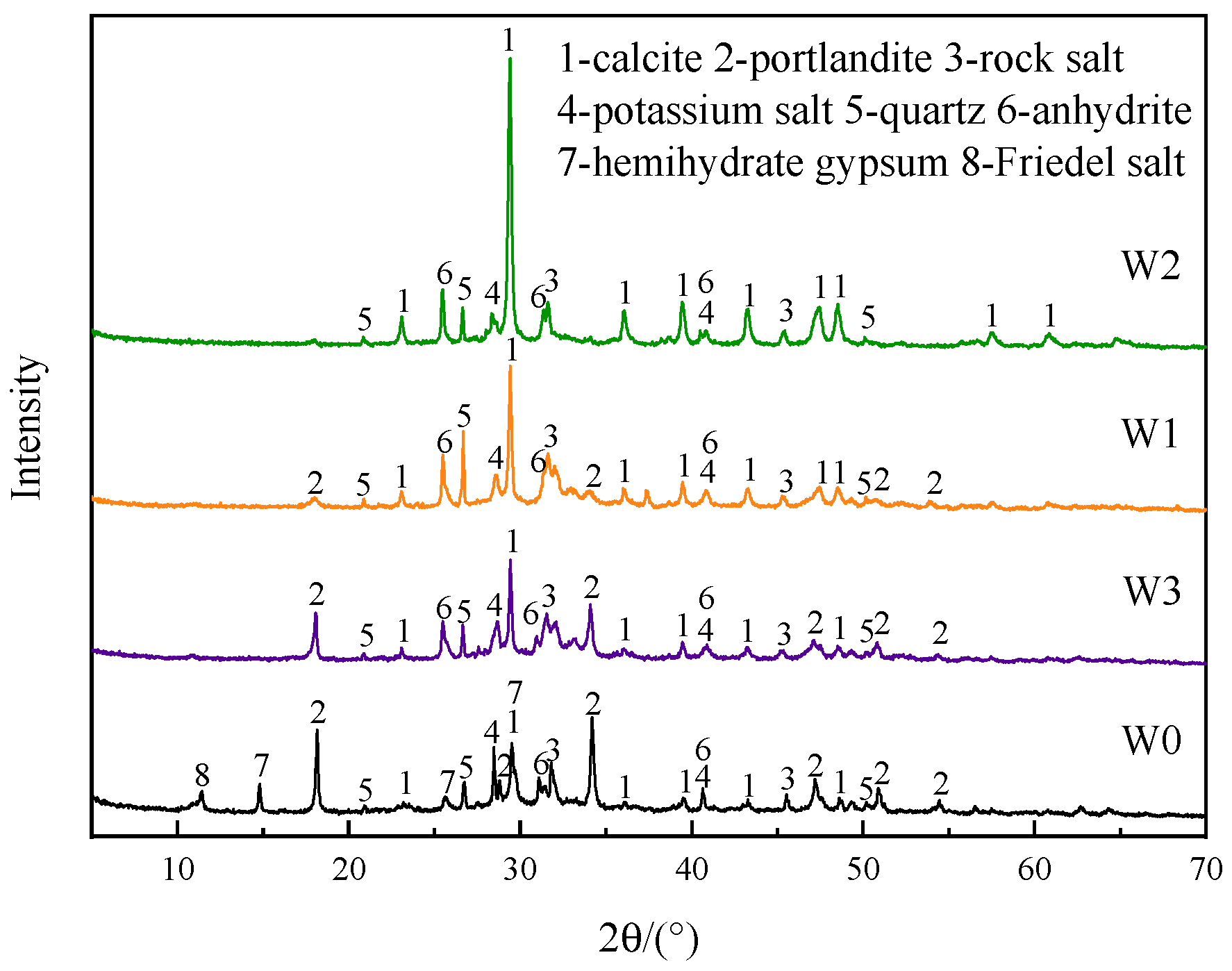
2.2.6. Particle Size Distribution, XRD, and SEM
2.2.7. Cement Workability and Mortar Fluidity
2.2.8. Mechanical Properties of Cement
2.2.9. Preparation of Cement Hydration Products for XRD Analysis Samples
3. Results and Discussion
3.1. Effect of Thermal Treatment Atmosphere on Physicochemical Properties of the Water-Washed MSWI FA
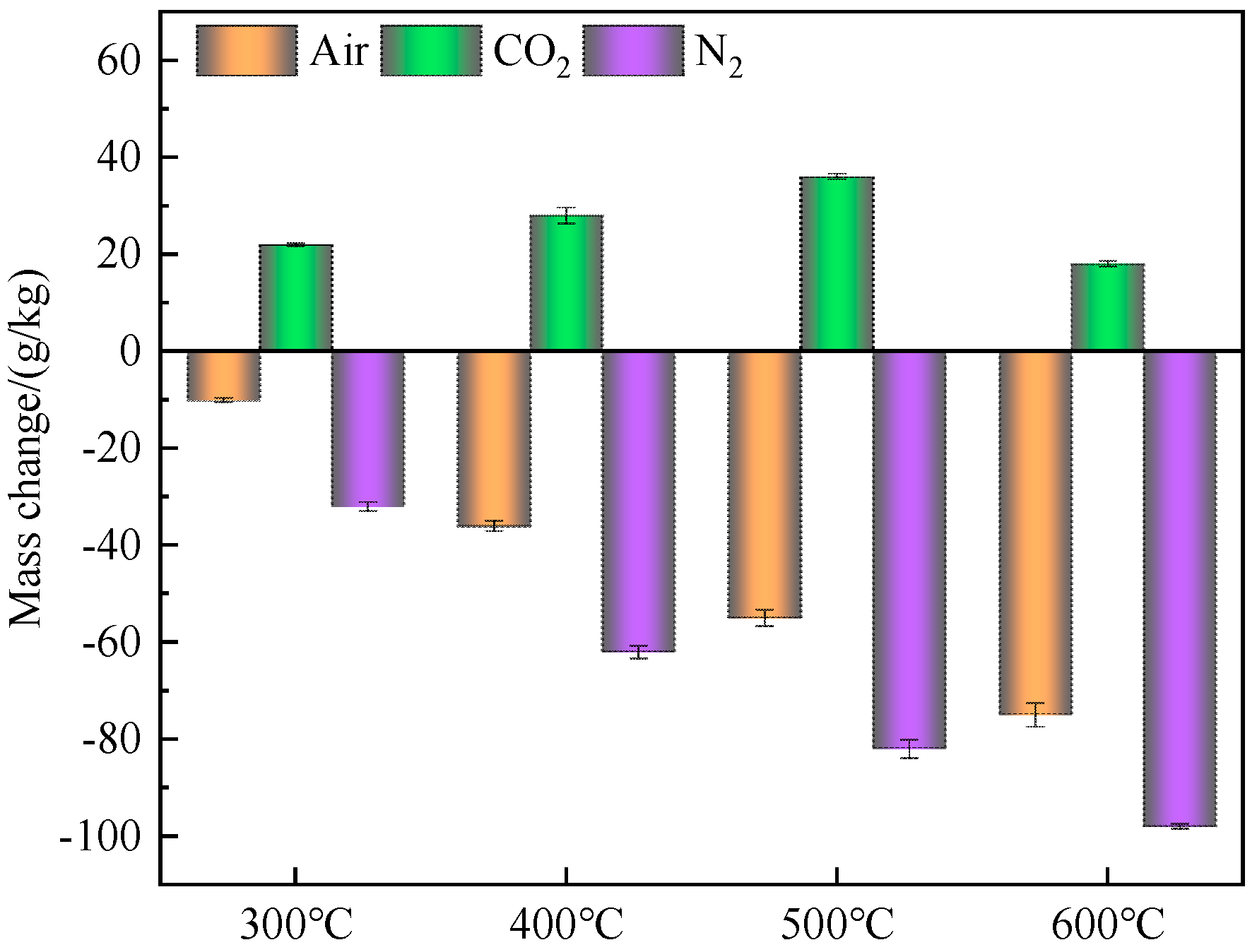
3.1.1. The Mass Change
3.1.2. Particle Size Distribution
3.1.3. Phase Composition
3.1.4. Microstructure
3.1.5. Heavy Metal Leaching Characteristics
3.1.6. Dioxin Content
3.2. The Effect of Water-Washed Incineration Fly Ash before and after Heat Treatment as Admixture on Cement Properties
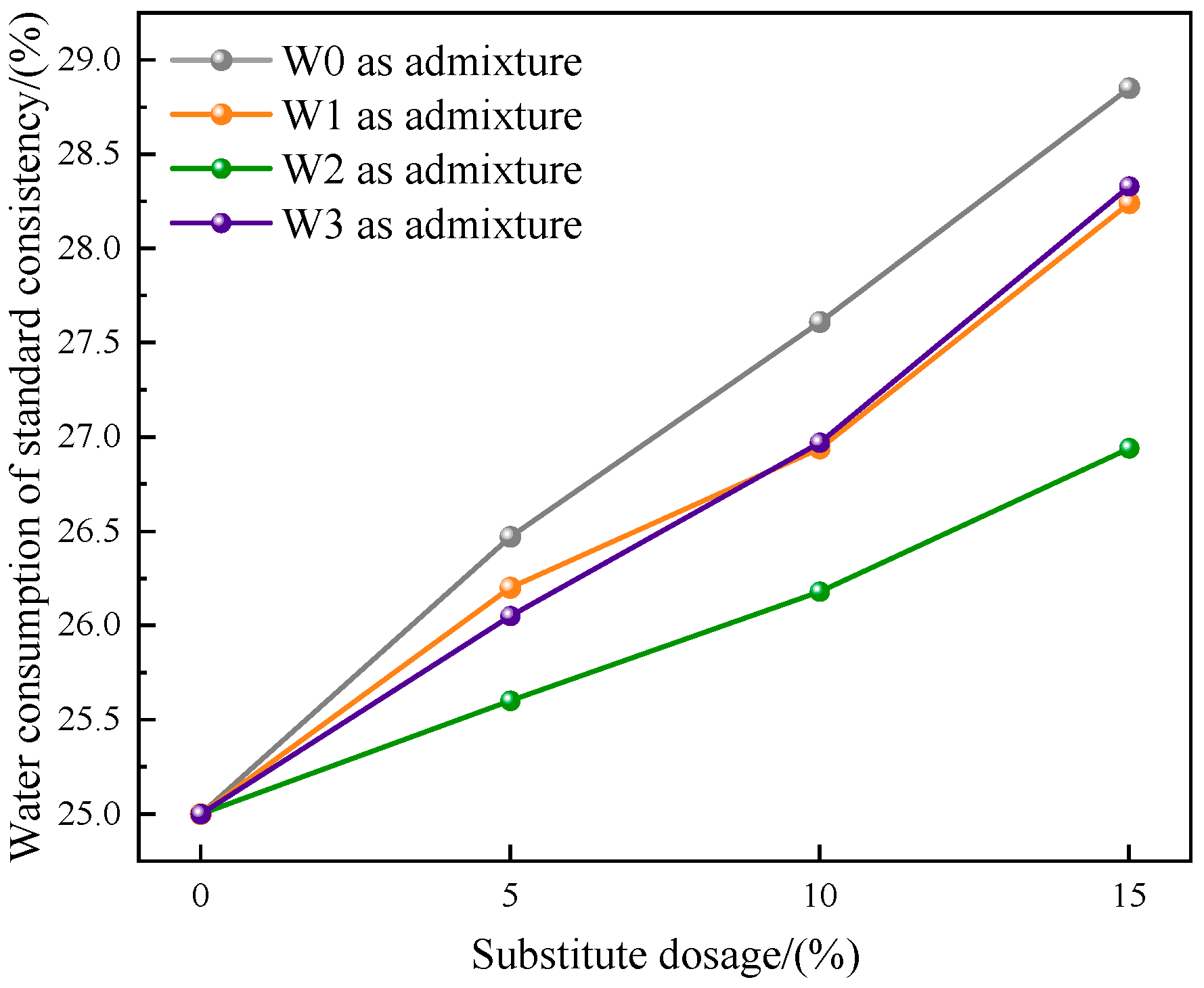
3.2.1. Water Consumption of Standard Consistency
3.2.2. Setting Time
3.2.3. Mortar Fluidity
3.2.4. Strength
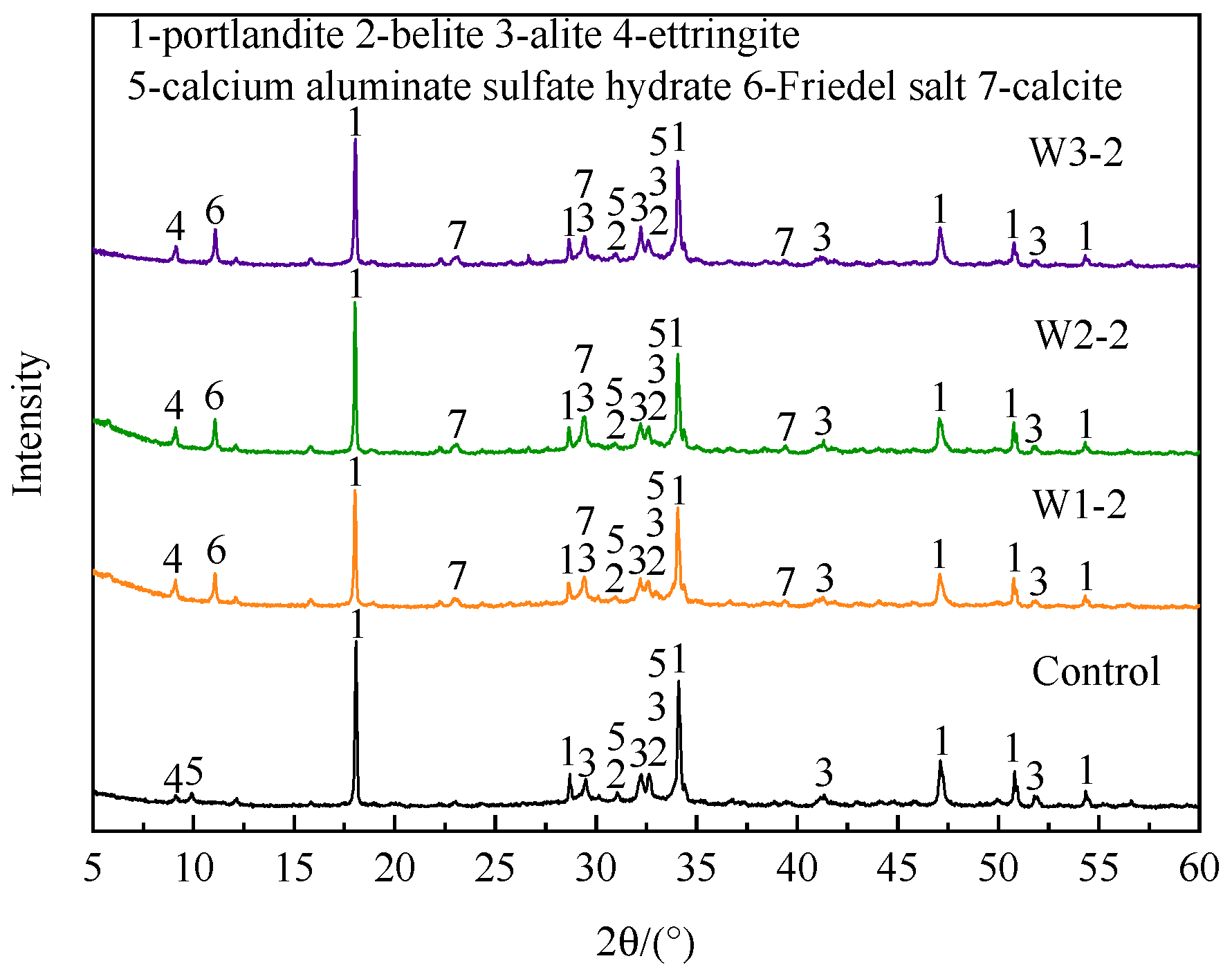
3.2.5. Hydration Products
3.2.6. Leaching Characteristics of Heavy Metals in Cements
4. Conclusions
- The mass of fly ash increased after heat treatment in CO2 atmosphere. When the temperature was 500 °C, the weight gain reached the maximum, and almost all Ca(OH)2 in fly ash was carbonized into CaCO3. It could also be observed in SEM that the surface of fly ash formed tightly packed CaCO3 crystals. This proved that fly ash had the ability to store CO2.
- The toxic equivalent quantities of dioxins in fly ash after heat treatment (500 °C + 1 h) in air, CO2, and N2 atmospheres were 17.12 ng TEQ/kg, 0.25 ng TEQ/kg, and 0.14 ng TEQ/kg, respectively, and the degradation rates were 69.95%, 99.56%, and 99.75%, respectively.
- Compared with the control group without admixture, the water consumption of cement standard consistency increased, and the fluidity of mortar decreased after adding fly ash that was heat-treated in three atmospheres, but the changes of fly ash after heat treatment in CO2 atmosphere were the smallest.
- Compared with the untreated fly ash used as admixture, under the same dosage, when fly ash was used as admixture after heat treatment, the water consumption of cement standard consistency was reduced, the fluidity of mortar was increased, and the strength of 28 d mortar was obviously improved. The changes of fly ash after heat treatment in CO2 atmosphere were the most obvious.
- The fly ash after heat treatment in CO2 atmosphere could be used as cement admixture for resource utilization. On the one hand, dioxins were effectively degraded, and the prepared cement had no risk of heavy metal leaching. On the other hand, when the dosage was 10% or less, the performance of the prepared cement met the requirements of P•O42.5 Portland cement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, W.; Shi, W.; Shi, Y.; Chen, D.; Liu, B.; Chu, C.; Li, D.; Li, Y.; Chen, G. Plasma vitrification and heavy metals solidification of MSW and sewage sludge incineration fly ash. J. Hazard. Mater. 2020, 408, 124809. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cheng, Y.; He, D.; Yang, E.-H. Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci. Total Environ. 2019, 668, 90–103. [Google Scholar] [CrossRef]
- Shapiro-Bengtsen, S.; Andersen, F.M.; Münster, M.; Zou, L. Municipal solid waste available to the Chinese energy sector—Provincial projections to 2050. Waste Manag. 2020, 112, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Shunda, l.; Jiang, X.; Zhao, Y.; Yan, J. Disposal technology and new progress for dioxins and heavy metals in fly ash from municipal solid waste incineration: A critical review. Environ. Pollut. 2022, 311, 119878. [Google Scholar] [CrossRef] [PubMed]
- Leckner, B. Process aspects in combustion and gasification Waste-to-Energy (WtE) units. Waste Manag. 2015, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Ghouleh, Z.; Shao, Y. Turning municipal solid waste incineration into a cleaner cement production. J. Clean. Prod. 2018, 195, 268–279. [Google Scholar] [CrossRef]
- Funari, V.; Braga, R.; Bokhari, S.N.H.; Dinelli, E.; Meisel, T. Solid residues from Italian municipal solid waste incinerators: A source for “critical” raw materials. Waste Manag. 2015, 45, 206–216. [Google Scholar] [CrossRef]
- Peng, Z.; Weber, R.; Ren, Y.; Wang, J.; Sun, Y.; Wang, L. Characterization of PCDD/Fs and heavy metal distribution from municipal solid waste incinerator fly ash sintering process. Waste Manag. 2020, 103, 260–267. [Google Scholar] [CrossRef]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the management of MSW incineration ashes from gas cleaning: New perspectives on recovery of secondary raw materials and circular economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef]
- Ferraro, A.; Farina, I.; Race, M.; Colangelo, F.; Cioffi, R.; Fabbricino, M. Pre-treatments of MSWI fly-ashes: A comprehensive review to determine optimal conditions for their reuse and/or environmentally sustainable disposal. Rev. Environ. Sci. Bio/Technol. 2019, 18, 453–471. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, M.; Wu, X.; Han, Y.; Geng, J.; Wang, T.; Wan, S.; Hou, H. Reductive solidification/stabilization of chromate in municipal solid waste incineration fly ash by ascorbic acid and blast furnace slag. Chemosphere 2017, 182, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Quina, M.J.; Bordado, J.C.; Quinta-Ferreira, R.M. Treatment and use of air pollution control residues from MSW incineration: An overview. Waste Manag. 2008, 28, 2097–2121. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, D.; Molin, C.; Hupa, M. Thermal treatment of solid residues from WtE units: A review. Waste Manag. 2015, 37, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yan, J.; Xie, Z. Detoxifying PCDD/Fs and heavy metals in fly ash from medical waste incinerators with a DC double arc plasma torch. J. Environ. Sci. 2013, 25, 1362–1367. [Google Scholar] [CrossRef]
- Sakai, S.-I.; Hiraoka, M. Municipal solid waste incinerator residue recycling by thermal processes. Waste Manag. 2000, 20, 249–258. [Google Scholar] [CrossRef]
- Fujimori, T.; Toda, A.; Mukai, K.; Takaoka, M. Incineration of carbon nanomaterials with sodium chloride as a potential source of PCDD/Fs and PCBs. J. Hazard. Mater. 2020, 382, 121030. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Lin, X.; Li, X. Decomposition and reformation pathways of PCDD/Fs during thermal treatment of municipal solid waste incineration fly ash. J. Hazard. Mater. 2020, 394, 122526. [Google Scholar] [CrossRef]
- Wu, H.-L.; Lu, S.-Y.; Yan, J.-H.; Li, X.-D.; Chen, T. Thermal removal of PCDD/Fs from medical waste incineration fly ash—Effect of temperature and nitrogen flow rate. Chemosphere 2011, 84, 361–367. [Google Scholar] [CrossRef]
- Weber, R.; Sakurai, T.; Hagenmaier, H. Formation and destruction of pcdd/pcdf during heat treatment of fly ash samples from fluidized bed incinerators. Chemosphere 1999, 38, 2633–2642. [Google Scholar] [CrossRef]
- Stach, J.; Pekárek, V.R.; Grabic, R.; Lojkásek, M.; Pacáková, V. Dechlorination of polychlorinated biphenyls, dibenzo-p-dioxins and dibenzofurans on fly ash. Chemosphere 2000, 41, 1881–1887. [Google Scholar] [CrossRef]
- Song, G.-J.; Kim, S.H.; Seo, Y.-C.; Kim, S.-C. Dechlorination and destruction of PCDDs/PCDFs in fly ashes from municipal solid waste incinerators by low temperature thermal treatment. Chemosphere 2008, 71, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bertos, M.F.; Simons, S.J.R. Kinetic study of accelerated carbonation of municipal solid waste incinerator air pollution control residues for sequestration of flue gas CO2. Energy Environ. Sci. 2008, 1, 370–377. [Google Scholar] [CrossRef]
- Baciocchi, R.; Polettini, A.; Pomi, R.; Prigiobbe, V.; Von Zedwitz, V.N.; Steinfeld, A. CO2 Sequestration by Direct Gas−Solid Carbonation of Air Pollution Control (APC) Residues. Energy Fuels 2006, 20, 1933–1940. [Google Scholar] [CrossRef]
- Ni, P.; Xiong, Z.; Tian, C.; Li, H.; Zhao, Y.; Zhang, J.; Zheng, C. Influence of carbonation under oxy-fuel combustion flue gas on the leachability of heavy metals in MSWI fly ash. Waste Manag. 2017, 67, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Jianguo, J.; Maozhe, C.; Yan, Z.; Xin, X. Pb stabilization in fresh fly ash from municipal solid waste incinerator using accelerated carbonation technology. J. Hazard. Mater. 2009, 161, 1046–1051. [Google Scholar] [CrossRef]
- Bie, R.; Chen, P.; Song, X.; Ji, X. Characteristics of municipal solid waste incineration fly ash with cement solidification treatment. J. Energy Inst. 2016, 89, 704–712. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J. Sequestration of flue gas CO(2) by direct gas-solid carbonation of air pollution control system residues. Environ. Sci. Technol. 2012, 46, 13545–13551. [Google Scholar] [CrossRef]
- Partanen, J.; Backman, P.; Backman, R.; Hupa, M. Absorption of HCl by limestone in hot flue gases. Part II: Importance of calcium hydroxychloride. Fuel 2005, 84, 1674–1684. [Google Scholar] [CrossRef]
- Zhu, F.; Takaoka, M.; Oshita, K.; Kitajima, Y.; Inada, Y.; Morisawa, S.; Tsuno, H. Chlorides behavior in raw fly ash washing experiments. J. Hazard. Mater. 2010, 178, 547–552. [Google Scholar] [CrossRef]
- Stieglitz, L. Selected Topics on the De Novo Synthesis of PCDD/PCDF on Fly Ash. Environ. Eng. Sci. 1998, 15, 5–18. [Google Scholar] [CrossRef]
- Thomas, J.J.; Allen, A.J.; Jennings, H.M. Hydration Kinetics and Microstructure Development of Normal and CaCl2-Accelerated Tricalcium Silicate Pastes. J. Phys. Chem. C 2009, 113, 19836–19844. [Google Scholar] [CrossRef]
- Balonis, M.; Lothenbach, B.; Le Saout, G.; Glasser, F.P. Impact of chloride on the mineralogy of hydrated Portland cement systems. Cem. Concr. Res. 2010, 40, 1009–1022. [Google Scholar] [CrossRef]
- Chen, L.; Nakamura, K.; Hama, T. Review on stabilization/solidification methods and mechanism of heavy metals based on OPC-based binders. J. Environ. Manag. 2023, 332, 117362. [Google Scholar] [CrossRef] [PubMed]




| Chemical Composition | Water-Washed MSWI FA |
|---|---|
| CaO | 48.21 |
| SO3 | 8.54 |
| SiO2 | 6.59 |
| Fe2O3 | 2.42 |
| K2O | 2.03 |
| MgO | 2.00 |
| Al2O3 | 1.81 |
| Na2O | 1.49 |
| ZnO | 0.61 |
| Others | 6.71 |
| LOI (950 °C) | 19.59 |
| Heavy Metal | Water-Washed MSWI FA |
|---|---|
| Zn | 3212.19 |
| Pb | 312.95 |
| Cd | 133.80 |
| Cu | 116.07 |
| As | 74.16 |
| Cr | 72.68 |
| Sb | 68.96 |
| Ni | 51.46 |
| V | 18.49 |
| Co | 14.78 |
| Mo | 13.85 |
| Tl | 4.87 |
| Be | 0.54 |
| Hg | 0.25 |
| Chemical Composition | Portland Cement Clinker | Natural Dihydrate Gypsum |
|---|---|---|
| CaO | 63.60 | 49.95 |
| SO3 | 0.29 | 45.58 |
| SiO2 | 23.02 | 1.56 |
| Al2O3 | 5.32 | 0.68 |
| Fe2O3 | 3.46 | 0.40 |
| K2O | 1.03 | 0.07 |
| MgO | 1.53 | 0.35 |
| Others | 0.85 | 1.41 |
| LOI (950 °C) | 0.90 | ND |
| Sequence Number | Water-Washed MSWI FA | Portland Cement Clinker | Natural Dihydrate Gypsum |
|---|---|---|---|
| Control | 0 | 95 | 5 |
| W0/W1/W2/W3-1 | 5 | 90 | 5 |
| W0/W1/W2/W3-2 | 10 | 85 | 5 |
| W0/W1/W2/W3-3 | 15 | 80 | 5 |
| Category | D10 (μm) | D50 (μm) | D90 (μm) | Dispersion |
|---|---|---|---|---|
| W1 | 1.66 | 17.78 | 112.81 | 6.25 |
| W2 | 1.35 | 30.00 | 144.10 | 4.76 |
| W3 | 1.48 | 22.21 | 138.74 | 6.18 |
| Heavy Metal | W0 | W1 | W2 | W3 | Landfill Limit |
|---|---|---|---|---|---|
| Cr | 0.2728 | 0.0763 | 0.0824 | 0.1255 | 4.5 |
| Ni | 0.0186 | 0.0102 | 0.0053 | 0.0108 | 0.5 |
| Cu | 0.0249 | 0.0581 | 0.0297 | 0.0345 | 40 |
| Zn | 0.2049 | 0.1434 | 0.1562 | 0.1862 | 100 |
| Cd | 0.0007 | 0.0002 | 0.0002 | 0.0004 | 0.15 |
| Pb | 0.0476 | 0.0866 | 0.0321 | 0.0847 | 0.25 |
| Heavy Metal | W0 | W1 | W2 | W3 | Landfill Limit |
|---|---|---|---|---|---|
| Cr | 0.4786 | 0.2877 | 0.3190 | 0.1310 | 4.5 |
| Ni | 0.0761 | 0.0444 | 0.0671 | 0.0410 | 0.5 |
| Cu | 0.1672 | 0.3160 | 0.2609 | 0.2754 | 40 |
| Zn | 11.3309 | 7.0022 | 6.0715 | 7.9853 | 100 |
| Cd | 0.0850 | 0.0469 | 0.0327 | 0.0565 | 0.15 |
| Pb | 0.3187 | 0.2879 | 0.1298 | 0.2658 | 0.25 |
| Isomer | TEF | W0 | W1 | W2 | W3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | TEQ | C | TEQ | C | TEQ | C | TEQ | ||
| 2,3,7,8-T4CDD | 1 | 650 | 650 | 440 | 440 | 100 | 100 | 32 | 32 |
| 1,2,3,7,8-P5CDD | 0.5 | 420 | 210 | 270 | 135 | 124 | 62 | 96 | 48 |
| 1,2,3,4,7,8-H6CDD | 0.1 | 11,000 | 1100 | 4550 | 455 | 30 | 3 | 25 | 2.50 |
| 1,2,3,6,7,8-H6CDD | 0.1 | 18,000 | 1800 | 8350 | 835 | 23 | 2.30 | 20 | 2 |
| 1,2,3,7,8,9-H6CDD | 0.1 | 12,000 | 1200 | 6380 | 638 | 21 | 2.10 | 20 | 2 |
| 1,2,3,4,6,7,8-H7CDD | 0.01 | 180,000 | 1800 | 66,700 | 667 | 60 | 0.60 | 41 | 0.41 |
| O8CDD | 0.001 | 470,000 | 470 | 135,000 | 135 | 4300 | 4.30 | 180 | 0.18 |
| 2,3,7,8-T4CDF | 0.1 | 28,000 | 2800 | 8000 | 800 | 120 | 12 | 100 | 10 |
| 1,2,3,7,8-P5CDF | 0.05 | 48,000 | 2400 | 8700 | 435 | 66 | 3.30 | 42 | 2.10 |
| 2,3,4,7,8-P5CDF | 0.5 | 56,000 | 28,000 | 15,844 | 7922 | 70 | 35 | 36 | 18 |
| 1,2,3,4,7,8-H6CDF | 0.1 | 38,000 | 3800 | 9400 | 940 | 55 | 5.50 | 42 | 4.20 |
| 1,2,3,6,7,8-H6CDF | 0.1 | 49,000 | 4900 | 11,240 | 1124 | 60 | 6 | 42 | 4.20 |
| 1,2,3,7,8,9-H6CDF | 0.1 | 13,000 | 1300 | 2670 | 267 | 80 | 8 | 65 | 6.50 |
| 2,3,4,6,7,8-H6CDF | 0.1 | 49,000 | 4900 | 18,770 | 1877 | 55 | 5.50 | 48 | 4.80 |
| 1,2,3,4,6,7,8-H7CDF | 0.01 | 140,000 | 1400 | 37,500 | 375 | 39 | 0.39 | 640 | 6.40 |
| 1,2,3,4,7,8,9-H7CDF | 0.01 | 17,000 | 170 | 4500 | 45 | 60 | 0.60 | 24 | 0.24 |
| O8CDF | 0.001 | 73,000 | 73 | 30,000 | 30 | 550 | 0.55 | 340 | 0.34 |
| TEQ | - | - | 56,973 | - | 17,120 | - | 251.14 | - | 143.87 |
| Heavy Metal | W0-3 | W1-3 | W2-3 | W3-3 | Landfill Limit |
|---|---|---|---|---|---|
| Cr | 0.0663 | 0.0255 | 0.0038 | ND | 4.5 |
| Ni | 0.0015 | 0.0045 | 0.0035 | 0.0042 | 0.5 |
| Cu | ND | ND | ND | ND | 40 |
| Zn | 0.0065 | ND | 0.0111 | 0.0031 | 100 |
| Cd | 0.0001 | 0.0001 | ND | ND | 0.15 |
| Pb | 0.0014 | 0.0018 | 0.0010 | 0.0026 | 0.25 |
| Heavy Metal | W0-3 | W1-3 | W2-3 | W3-3 | Landfill Limit |
|---|---|---|---|---|---|
| Cr | 0.0705 | 0.0449 | 0.0602 | 0.0450 | 4.5 |
| Ni | 0.0192 | 0.0134 | 0.0314 | 0.0143 | 0.5 |
| Cu | 0.0691 | 0.0592 | 0.0697 | 0.0787 | 40 |
| Zn | 0.7283 | 0.7137 | 0.5463 | 0.5767 | 100 |
| Cd | 0.0121 | 0.0199 | 0.0136 | 0.0149 | 0.15 |
| Pb | 0.0548 | 0.0808 | 0.0540 | 0.0450 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Li, J.; Ma, X.; Da, Y.; Yuan, H. Low Temperature Thermal Treatment of Incineration Fly Ash under Different Atmospheres and Its Recovery as Cement Admixture. Materials 2023, 16, 3923. https://doi.org/10.3390/ma16113923
He T, Li J, Ma X, Da Y, Yuan H. Low Temperature Thermal Treatment of Incineration Fly Ash under Different Atmospheres and Its Recovery as Cement Admixture. Materials. 2023; 16(11):3923. https://doi.org/10.3390/ma16113923
Chicago/Turabian StyleHe, Tingshu, Jiangbo Li, Xiaodong Ma, Yongqi Da, and Hudie Yuan. 2023. "Low Temperature Thermal Treatment of Incineration Fly Ash under Different Atmospheres and Its Recovery as Cement Admixture" Materials 16, no. 11: 3923. https://doi.org/10.3390/ma16113923





