Core–Shell Spheroid Structure TiO2/CdS Composites with Enhanced Photocathodic Protection Performance
Abstract
:1. Introduction
2. Materials and Methods
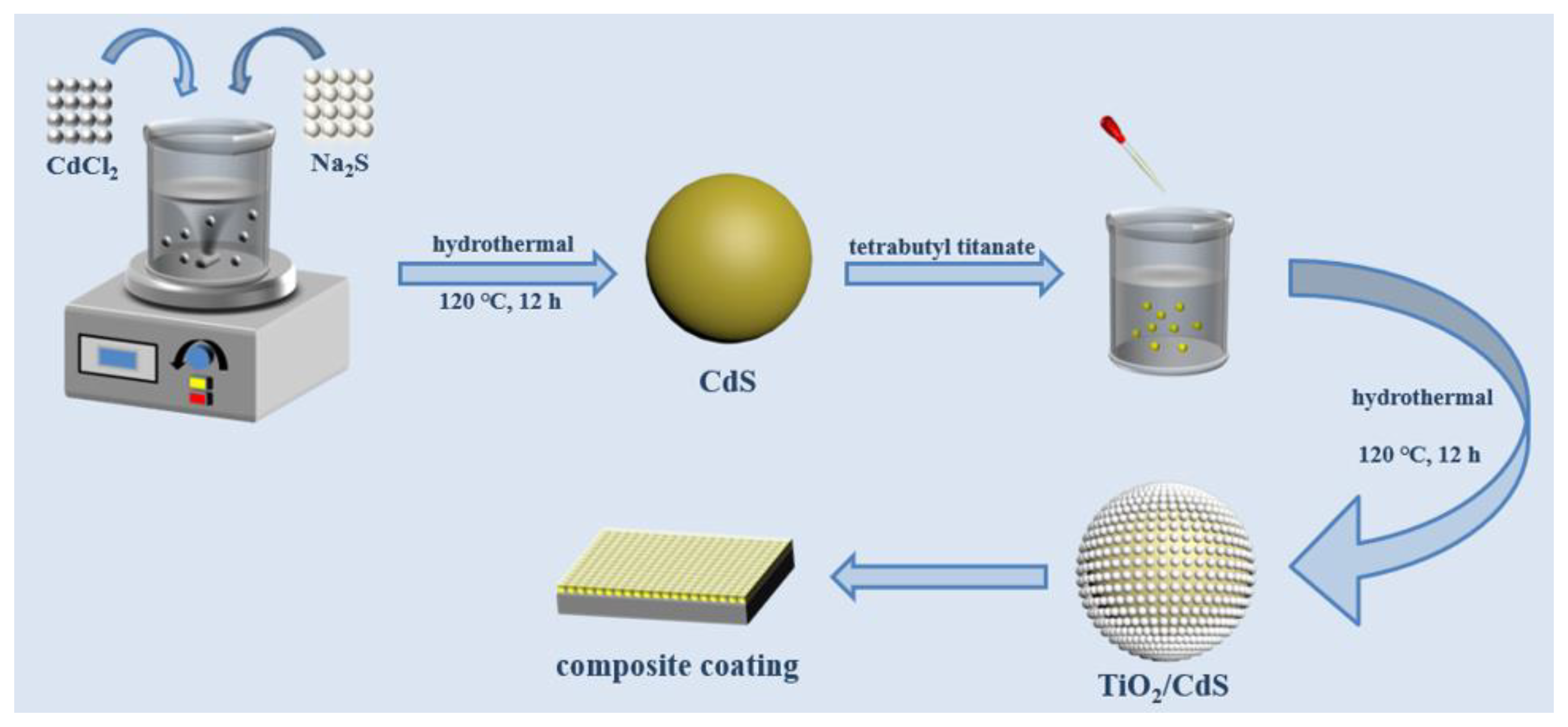
2.1. Preparation of CdS Nanomaterials
2.2. Preparation of TiO2/CdS Composites
2.3. Preparation and Application of TiO2/CdS-Modified Epoxy Resin Composite Coatings
2.4. Characterizations
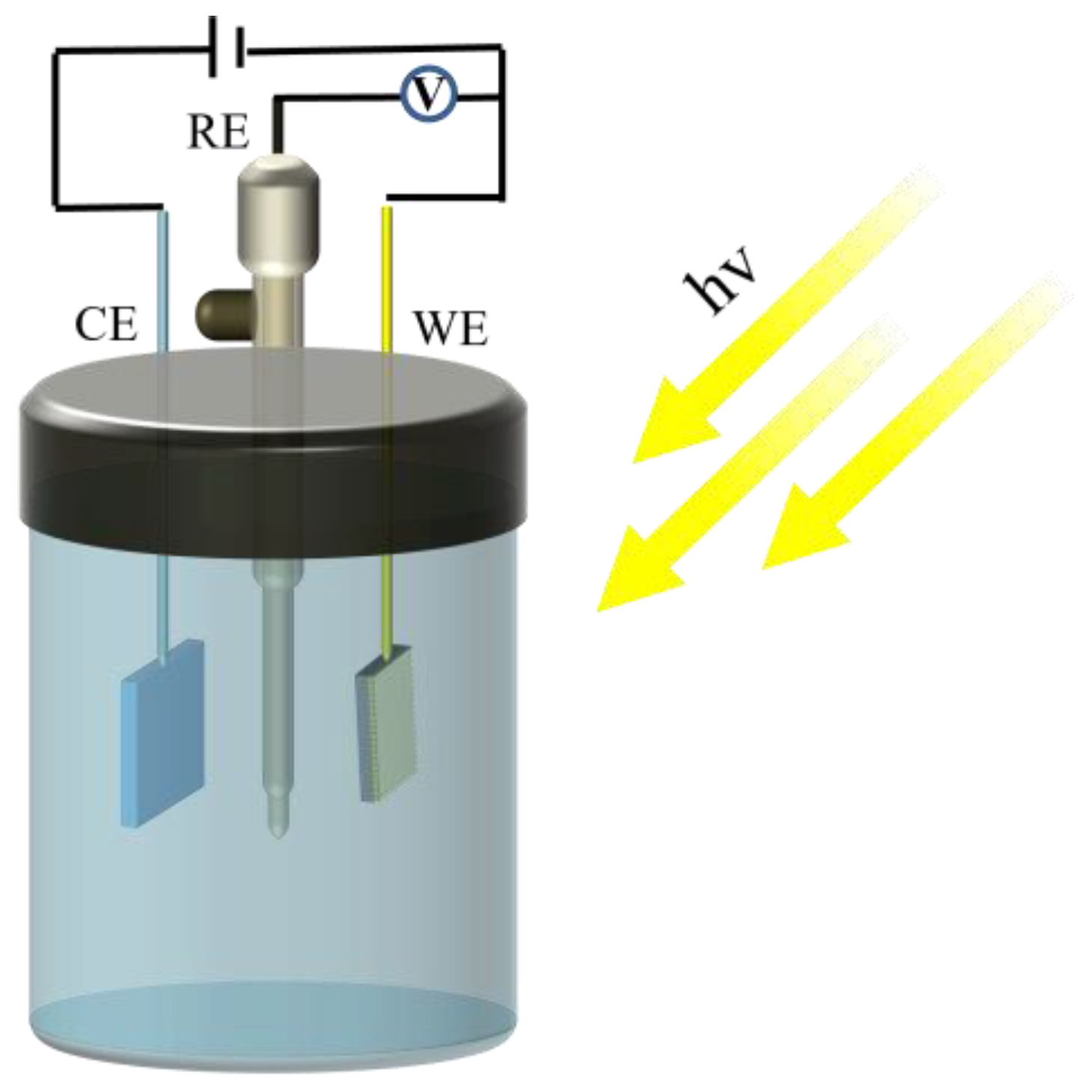
2.5. Photoelectric Performance Test
3. Results
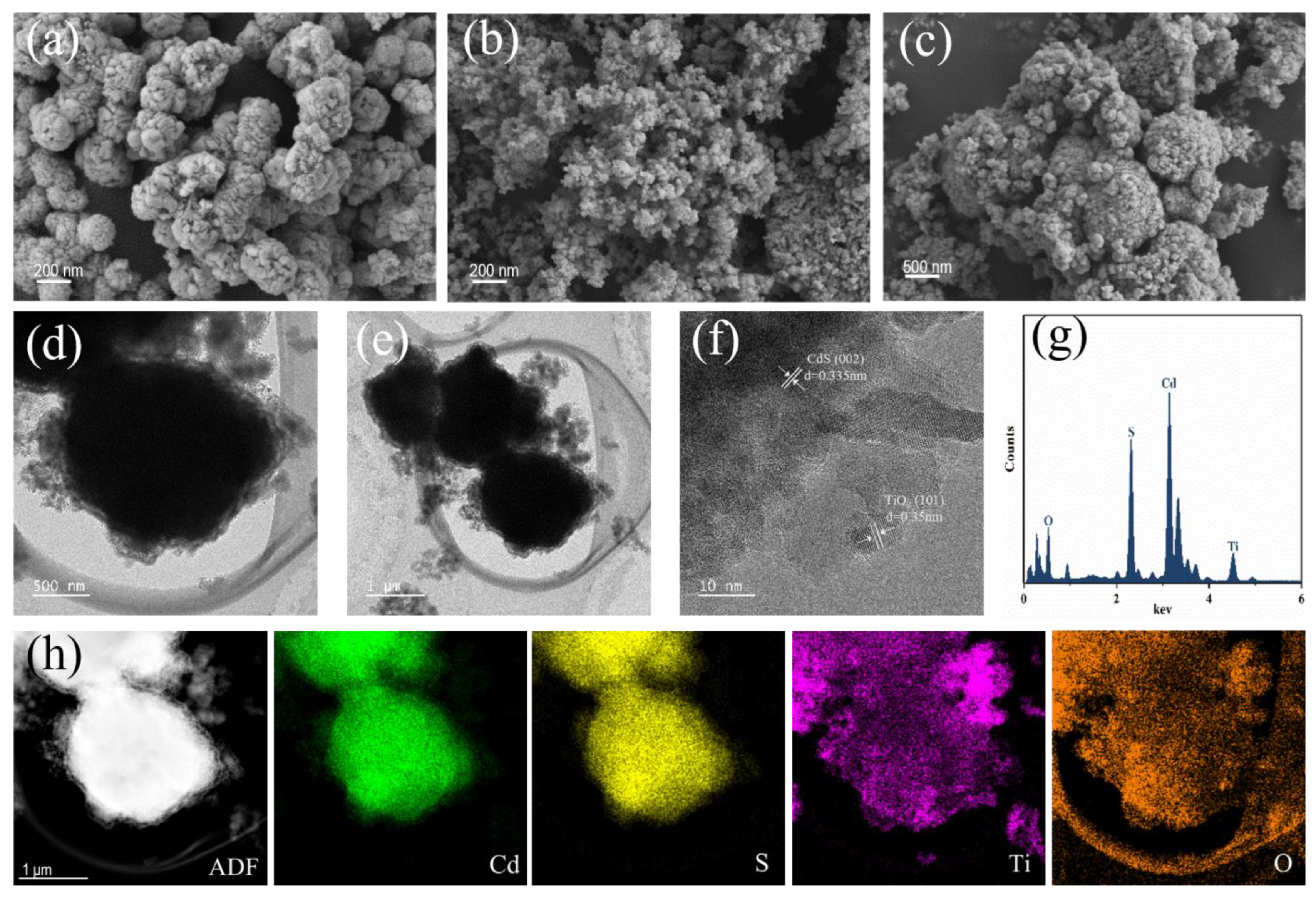
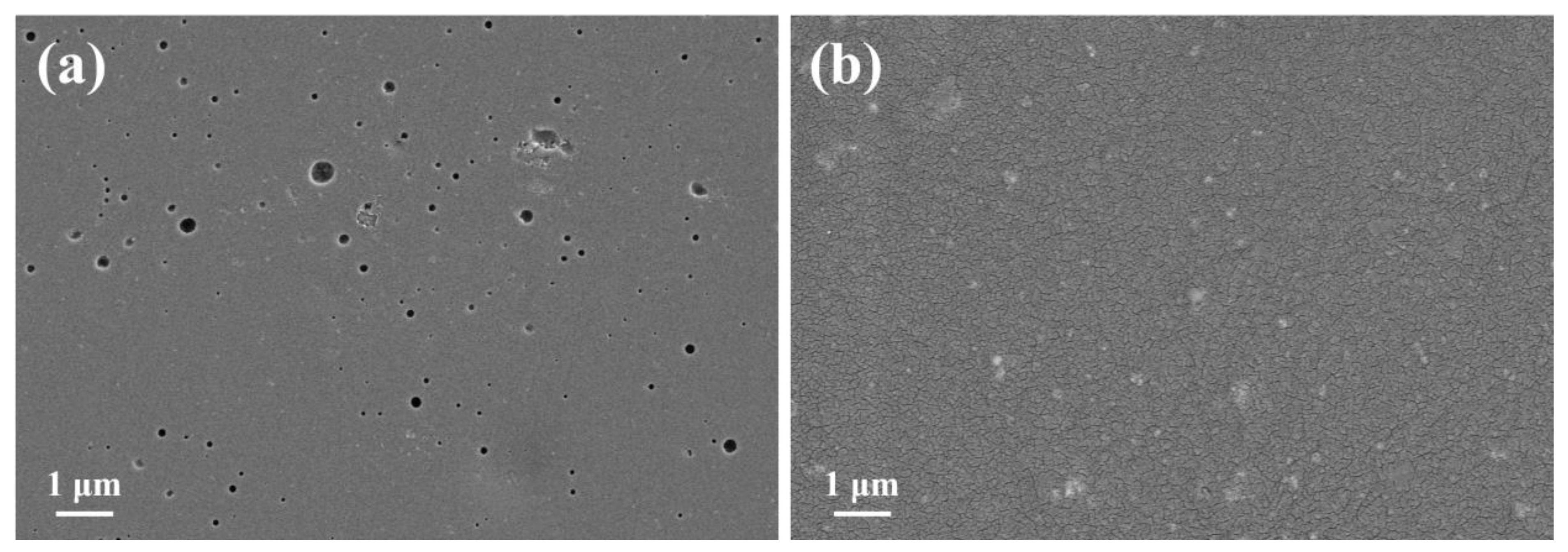
3.1. Microstructure Characterization
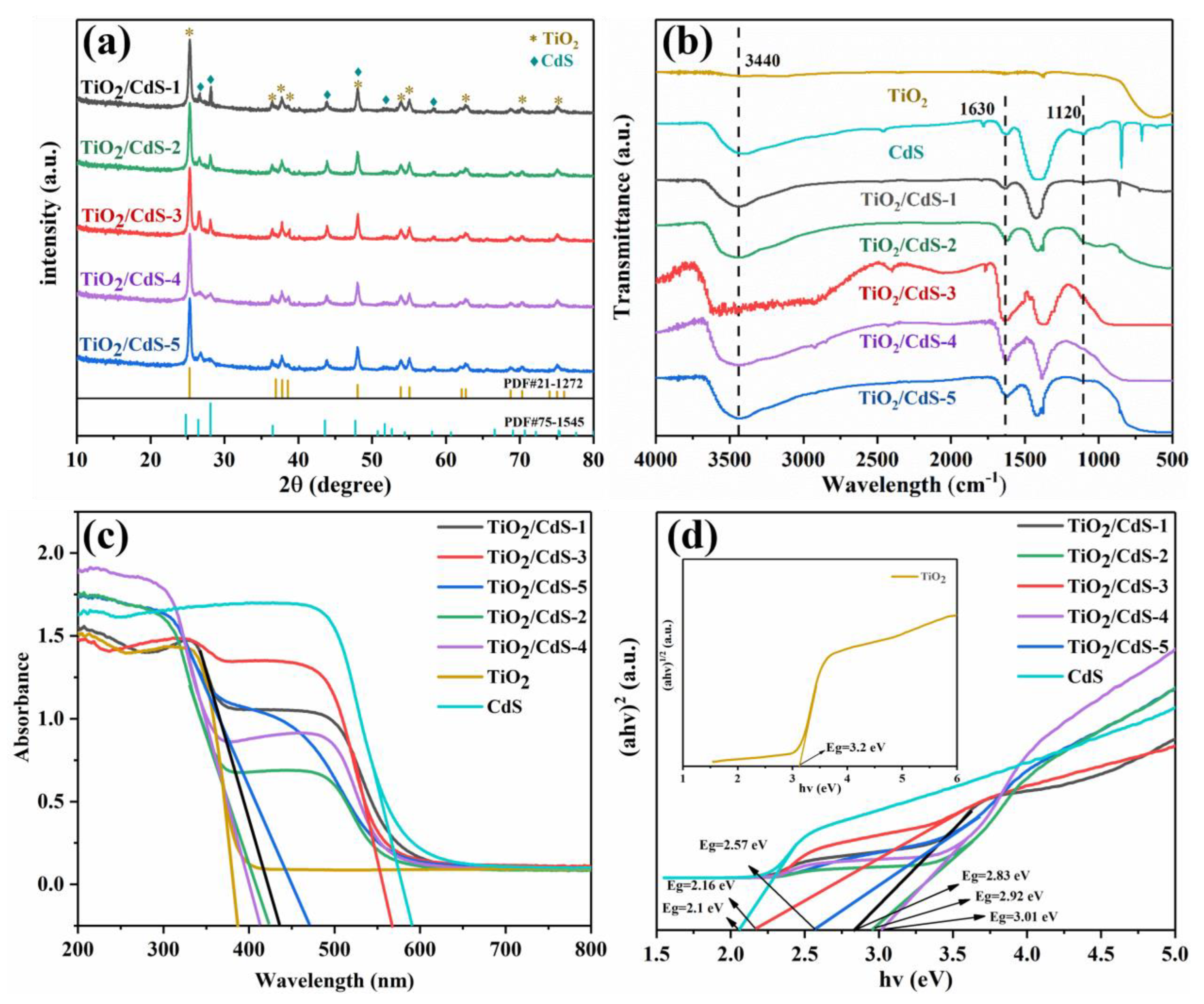
3.2. Physicochemical Characterization of TiO2/CdS Composites
3.3. Photoelectrochemical Performance
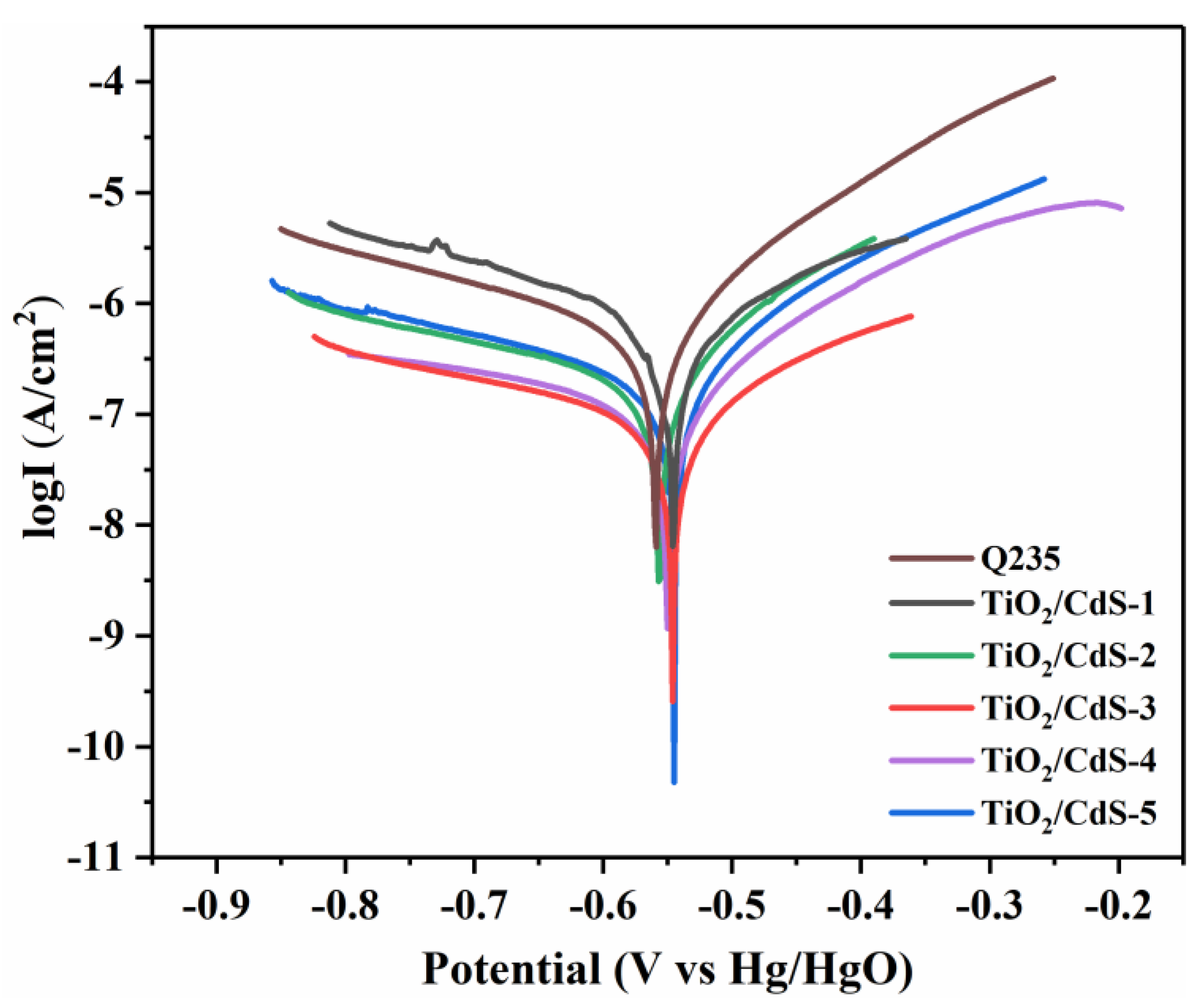
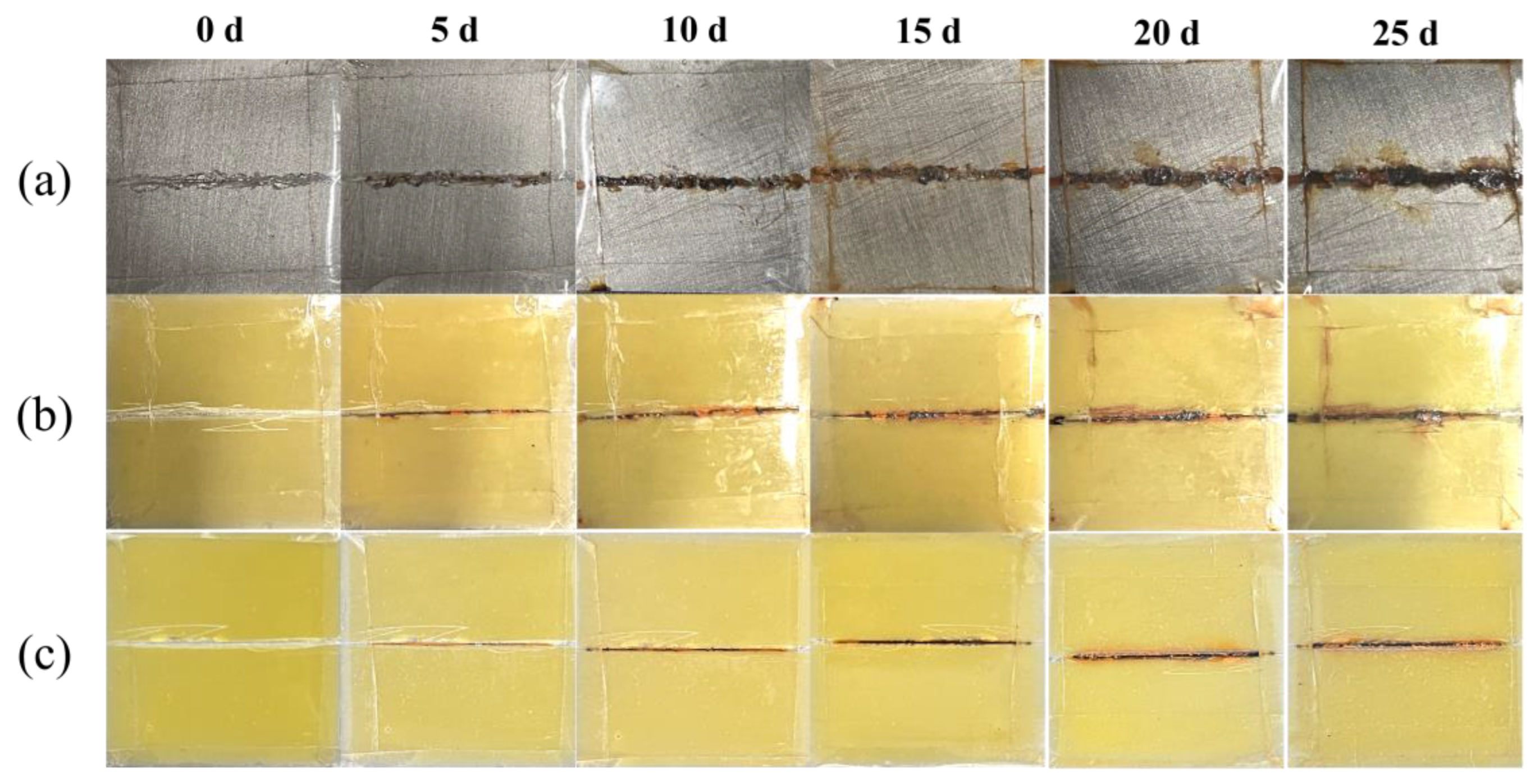
3.4. Anti-Corrosive Properties of Coatings
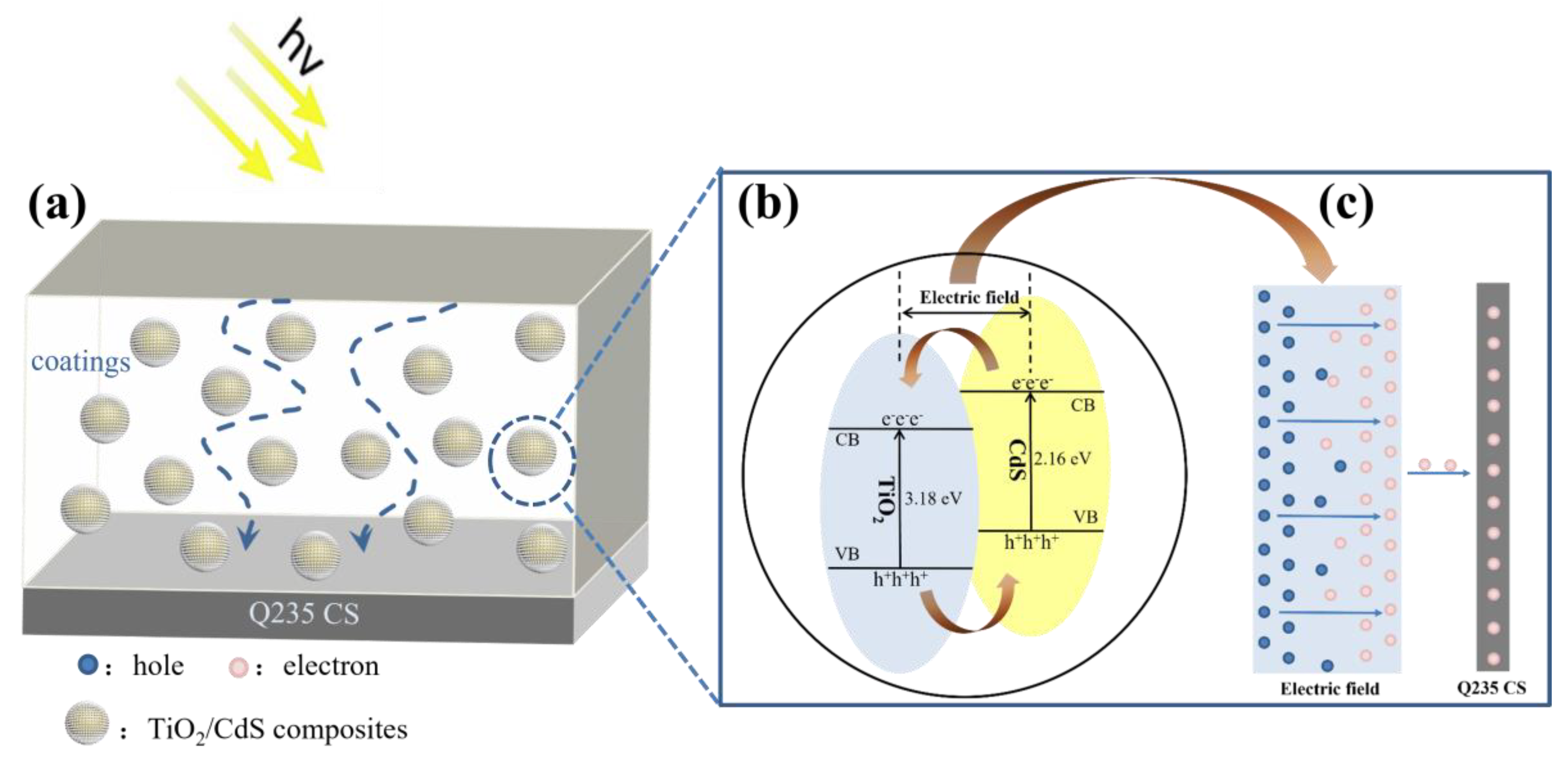
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Y.; Zhao, Y.; Ma, X.; Zhou, K.; Chen, Y. Influence of environmental factors on atmospheric corrosion in dynamic environment. Corros. Sci. 2018, 137, 163–175. [Google Scholar] [CrossRef]
- Yuan, J.; Tsujikawa, S. Characterization of Sol-Gel-Derived TiO2 Coatings and Their Photoeffects on Copper Substrates. J. Electrochem. Soc. 1995, 142, 3444–3450. [Google Scholar] [CrossRef]
- Xu, D.; Liu, Y.; Liu, Y.; Chen, F.; Zhang, C.; Liu, B. A review on recent progress in the development of photoelectrodes for photocathodic protection: Design, properties, and prospects. Mater. Des. 2021, 197, 109235. [Google Scholar] [CrossRef]
- Guan, Z.-C.; Jin, P.; Liu, Q.; Wang, X.; Chen, L.-F.; Xu, H.; Song, G.-L.; Du, R.-G. Carbon quantum dots/Ag sensitized TiO2 nanotube film for applications in photocathodic protection. J. Alloys Compd. 2019, 797, 912–921. [Google Scholar] [CrossRef]
- Techapiesancharoenkij, R.; Sripianem, W.; Tongpul, K.; Peamjharean, C.; Wichean, T.N.; Meesak, T.; Eiamchai, P. Investigation of the photocathodic protection of a transparent ZnO coating on an AISI type 304 stainless steel in a 3% NaCl solution. Surf. Coat. Technol. 2017, 320, 97–102. [Google Scholar] [CrossRef]
- Wang, X.-T.; Wei, Q.-Y.; Li, J.-R.; Li, H.; Zhang, Q.-X.; Ge, S.-S. Preparation of NiSe2/TiO2 nanocomposite for photocathodic protection of stainless steel. Mater. Lett. 2016, 185, 443–446. [Google Scholar] [CrossRef]
- Guo, H.; Li, L.; Su, C.; Yu, D.; Liu, Z. Effective photocathodic protection for 304 stainless steel by PbS quantum dots modified TiO2 nanotubes. Mater. Chem. Phys. 2021, 258, 123914. [Google Scholar] [CrossRef]
- Ning, X.-B.; Wang, X.-T.; Shao, Q.; Ge, S.-S.; Chi, L.-F.; Nan, Y.-B.; Liang, H.; Hou, B.-R. ZnPc/TiO2 composites for photocathodic protection of 304 stainless steel. J. Electroanal. Chem. 2020, 858, 113802. [Google Scholar] [CrossRef]
- Wang, H.-P.; Guan, Z.-C.; Shi, H.-Y.; Wang, X.; Jin, P.; Song, G.-L.; Du, R.-G. Ag/SnO2/TiO2 nanotube composite film used in photocathodic protection for stainless steel. J. Photochem. Photobiol. A Chem. 2021, 417, 113353. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhong, B.; Li, F.; Wu, H.; Liu, J.; Yang, H.; Zhao, L.; Sun, Y.; Zhang, P.; Gao, L. Surface phosphorization for the enhanced photoelectrochemical performance of an Fe2O3/Si photocathode. Nanoscale 2022, 14, 11261–11269. [Google Scholar] [CrossRef]
- Wei, N.; Lin, Y.; Li, Z.; Sun, W.; Zhang, G.; Wang, M.; Cui, H. One-dimensional Ag2S/ZnS/ZnO nanorod array films for photocathodic protection for 304 stainless steel. J. Mater. Sci. Technol. 2020, 42, 156–162. [Google Scholar] [CrossRef]
- Cao, D.; Nasori, N.; Wang, Z.; Mi, Y.; Wen, L.; Yang, Y.; Qu, S.; Wang, Z.; Lei, Y. p-Type CuBi2O4: An easily accessible photocathodic material for high-efficiency water splitting. J. Mater. Chem. A 2016, 4, 8995–9001. [Google Scholar] [CrossRef]
- Xu, Y.; Jian, J.; Li, F.; Liu, W.; Jia, L.; Wang, H. Porous CuBi2O4 photocathodes with rationally engineered morphology and composition towards high-efficiency photoelectrochemical performance. J. Mater. Chem. A 2019, 7, 21997–22004. [Google Scholar] [CrossRef]
- Yang, W.; Lee, S.; Kwon, H.C.; Tan, J.; Lee, H.; Park, J.; Oh, Y.; Choi, H.; Moon, J. Time-Resolved Observations of Photo-Generated Charge-Carrier Dynamics in Sb2Se3 Photocathodes for Photoelectrochemical Water Splitting. ACS Nano 2018, 12, 11088–11097. [Google Scholar] [CrossRef]
- Dorraj, M.; Goh, B.T.; Sairi, N.A.; Woi, P.M.; Basirun, W.J. Improved visible-light photocatalytic activity of TiO2 co-doped with copper and iodine. Appl. Surf. Sci. 2018, 439, 999–1009. [Google Scholar] [CrossRef]
- Gao, N.; Wan, T.; Xu, Z.; Ma, L.; Ramakrishna, S.; Liu, Y. Nitrogen doped TiO2/Graphene nanofibers as DSSCs photoanode. Mater. Chem. Phys. 2020, 255, 123542. [Google Scholar] [CrossRef]
- Xu, H.; Liu, W.; Cao, L.; Su, G.; Duan, R. Preparation of porous TiO2/ZnO composite film and its photocathodic protection properties for 304 stainless steel. Appl. Surf. Sci. 2014, 301, 508–514. [Google Scholar] [CrossRef]
- Ning, X.-b.; Ge, S.-s.; Wang, X.-t.; Li, H.; Li, X.-r.; Liu, X.-q.; Huang, Y.-l. Preparation and photocathodic protection property of Ag2S-TiO2 composites. J. Alloys Compd. 2017, 719, 15–21. [Google Scholar] [CrossRef]
- Lu, X.; Liu, L.; Ge, J.; Cui, Y.; Wang, F. Morphology controlled synthesis of Co(OH)2/TiO2 p-n heterojunction photoelectrodes for efficient photocathodic protection of 304 stainless steel. Appl. Surf. Sci. 2021, 537, 148002. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Wang, X.; Hou, B. 3D ZnIn2S4 nanosheets/TiO2 nanotubes as photoanodes for photocathodic protection of Q235 CS with high efficiency under visible light. J. Alloys Compd. 2019, 771, 892–899. [Google Scholar] [CrossRef]
- Lei, J.; Shao, Q.; Wang, X.; Wei, Q.; Yang, L.; Li, H.; Huang, Y.; Hou, B. ZnFe2O4/TiO2 nanocomposite films for photocathodic protection of 304 stainless steel under visible light. Mater. Res. Bull. 2017, 95, 253–260. [Google Scholar] [CrossRef]
- Han, C.; Shao, Q.; Lei, J.; Zhu, Y.; Ge, S. Preparation of NiO/TiO2 p-n heterojunction composites and its photocathodic protection properties for 304 stainless steel under simulated solar light. J. Alloys Compd. 2017, 703, 530–537. [Google Scholar] [CrossRef]
- Liu, W.; Yin, K.; He, F.; Ru, Q.; Zuo, S.; Yao, C. A highly efficient reduced graphene oxide/SnO2/TiO2 composite as photoanode for photocathodic protection of 304 stainless steel. Mater. Res. Bull. 2019, 113, 6–13. [Google Scholar] [CrossRef]
- Liu, W.; Du, T.; Ru, Q.; Zuo, S.; Cai, Y.; Yao, C. Preparation of graphene/WO3/TiO2 composite and its photocathodic protection performance for 304 stainless steel. Mater. Res. Bull. 2018, 102, 399–405. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhang, L.; Hou, B. CdTe and graphene co-sensitized TiO2 nanotube array photoanodes for protection of 304SS under visible light. Nanotechnology 2015, 26, 155704. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, W.; Cao, L.; Su, G.; Xu, H.; Liu, B. Graphene incorporated nanocrystalline TiO2 films for the photocathodic protection of 304 stainless steel. Appl. Surf. Sci. 2013, 283, 498–504. [Google Scholar] [CrossRef]
- Feng, M.; Liu, Y.; Zhang, S.; Liu, Y.; Luo, N.; Wang, D. Carbon quantum dots (CQDs) modified TiO2 nanorods photoelectrode for enhanced photocathodic protection of Q235 carbon steel. Corros. Sci. 2020, 176, 108919. [Google Scholar] [CrossRef]
- Ren, J.; Qian, B.; Li, J.; Song, Z.; Hao, L.; Shi, J. Highly efficient polypyrrole sensitized TiO2 nanotube films for photocathodic protection of Q235 carbon steel. Corros. Sci. 2016, 111, 596–601. [Google Scholar] [CrossRef]
- Yang, L.; Yu, Y.; Zhang, J.; Chen, F.; Meng, X.; Qiu, Y.; Dan, Y.; Jiang, L. In-situ fabrication of diketopyrrolopyrrole-carbazole-based conjugated polymer/TiO2 heterojunction for enhanced visible light photocatalysis. Appl. Surf. Sci. 2018, 434, 796–805. [Google Scholar] [CrossRef]
- Ikhmayies, S.J.; Ahmad-Bitar, R.N. A study of the optical bandgap energy and Urbach tail of spray-deposited CdS:In thin films. J. Mater. Res. Technol. 2013, 2, 221–227. [Google Scholar] [CrossRef]
- Sasikala, G.; Thilakan, P.; Subramanian, C. Modification in the Chemical Bath Deposition Apparatus, Growth and Characterization of CdS Semiconducting Thin Films for Photovoltaic Applications. Solid State Phenom. 1999, 67, 291–296. [Google Scholar] [CrossRef]
- Huang, M.; Liu, C.; Cui, P.; Wu, T.; Feng, X.; Huang, H.; Zhou, J.; Wang, Y. Facet-Dependent Photoinduced Transformation of Cadmium Sulfide (CdS) Nanoparticles. Environ. Sci. Technol. 2021, 55, 13132–13141. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Zafar, M.; Kim, S.; Kim, D.H.; Jeon, C.W.; Lee, J.; Yong, K. Enhancing Durability and Photoelectrochemical Performance of the Earth Abundant Ni–Mo/TiO2/CdS/CIGS Photocathode under Various pH Conditions. ChemSusChem 2018, 11, 3679–3688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, R.-G.; Lin, Z.-Q.; Zhu, Y.-F.; Guo, Y.; Qi, H.-Q.; Xu, L.; Lin, C.-J. Highly efficient CdSe/CdS co-sensitized TiO2 nanotube films for photocathodic protection of stainless steel. Electrochim. Acta 2012, 83, 59–64. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Y.; Li, C.; Li, A.; Chang, X.; Liu, W.; Sun, Y.; Wang, T.; Gong, J. Spatial control of cocatalysts and elimination of interfacial defects towards efficient and robust CIGS photocathodes for solar water splitting. Energy Environ. Sci. 2018, 11, 2025–2034. [Google Scholar] [CrossRef]
- Cai, M.; Fan, X.; Yan, H.; Li, Y.; Song, S.; Li, W.; Li, H.; Lu, Z.; Zhu, M. In situ assemble Ti3C2Tx MXene@MgAl-LDH heterostructure towards anticorrosion and antiwear application. Chem. Eng. J. 2021, 419, 130050. [Google Scholar] [CrossRef]
- Cai, M.; Yan, H.; Song, S.; He, D.; Lin, Q.; Li, W.; Fan, X.; Zhu, M. State-of-the-art progresses for Ti3C2Tx MXene reinforced polymer composites in corrosion and tribology aspects. Adv. Colloid Interface Sci. 2022, 309, 102790. [Google Scholar] [CrossRef]
- Cai, M.; Feng, P.; Yan, H.; Li, Y.; Song, S.; Li, W.; Li, H.; Fan, X.; Zhu, M. Hierarchical Ti3C2Tx@MoS2 heterostructures: A first principles calculation and application in corrosion/wear protection. J. Mater. Sci. Technol. 2022, 116, 151–160. [Google Scholar] [CrossRef]
- Yan, H.; Fan, X.; Cai, M.; Song, S.; Zhu, M. Amino-functionalized Ti3C2Tx loading ZIF-8 nanocontainer@benzotriazole as multifunctional composite filler towards self-healing epoxy coating. J. Colloid Interface Sci. 2021, 602, 131–145. [Google Scholar] [CrossRef]
- Fan, X.; Yan, H.; Cai, M.; Song, S.; Huang, Y.; Zhu, M. Achieving parallelly-arranged Ti3C2Tx in epoxy coating for anti-corrosive/wear high-efficiency protection. Compos. Part B Eng. 2022, 231, 109581. [Google Scholar] [CrossRef]
- Hao, L.; Tang, S.; Yan, J.; Cheng, L.; Guan, S.; Zhao, Q.; Zhu, Z.; Lu, Y. Solar-responsive photocatalytic activity of amorphous TiO2 nanotube-array films. Mater. Sci. Semicond. Process. 2019, 89, 161–169. [Google Scholar] [CrossRef]
- Rahman, M.F.; Hossain, J.; Kuddus, A.; Tabassum, S.; Rubel, M.H.K.; Shirai, H.; Ismail, A.B.M. A novel synthesis and characterization of transparent CdS thin films for CdTe/CdS solar cells. Appl. Phys. A 2020, 126, 145. [Google Scholar] [CrossRef]
- Shinde, P.S.; Go, G.H.; Lee, W.J. Facile growth of hierarchical hematite (α-Fe2O3) nanopetals on FTO by pulse reverse electrodeposition for photoelectrochemical water splitting. J. Mater. Chem. 2012, 22, 10469. [Google Scholar] [CrossRef]
- Xu, D.; Yang, M.; Liu, Y.; Zhu, R.; Lv, X.; Zhang, C.; Liu, B. Fabrication of an innovative designed TiO2 nanosheets/CdSe/polyaniline/graphene quaternary composite and its application as in-situ photocathodic protection coatings on 304SS. J. Alloys Compd. 2020, 822, 153685. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, K.; Yin, Y.; Du, X.; Zhou, Q. TiN/TiO2/MXene ternary composite with photocathodic protection for 304 stainless steel. Chem. Eng. J. 2022, 442, 114110. [Google Scholar] [CrossRef]
- Boonserm, A.; Kruehong, C.; Seithtanabutara, V.; Artnaseaw, A.; Kwakhong, P. Photoelectrochemical response and corrosion behavior of CdS/TiO2 nanocomposite films in an aerated 0.5 M NaCl solution. Appl. Surf. Sci. 2017, 419, 933–941. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Zhao, Q.; Ge, C.; Hou, B.; Hu, Y.; Ning, Y. Photogenerated cathodic protection properties of Ag/Ag3PO4/TiO2 nanocomposites. J. Photochem. Photobiol. A Chem. 2022, 432, 114110. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, K.; Du, L.; Gong, D.; Lu, G.; Qiu, P. Zn3In2S6/TiO2 Nanocomposites for Highly Efficient Photocathodic Protection to Carbon Steel. ACS Appl. Nano Mater. 2022, 5, 18297–18306. [Google Scholar] [CrossRef]
- Liu, J.; Wang, N.; Zheng, F.; Wang, C.; Wang, J.; Hou, B.; Zhao, Q.; Ning, Y.; Hu, Y. CuInS2/TiO2 heterojunction with elevated photo-electrochemical performance for cathodic protection. J. Mater. Sci. Technol. 2022, 122, 211–218. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Cui, X.; Zhu, J.; Li, Y.; Zhang, P.; Li, J. Direct Z-scheme nanoporous BiVO4/CdS quantum dots heterojunction composites as photoanodes for photocathodic protection of 316 stainless steel under visible light. Appl. Surf. Sci. 2022, 603, 154394. [Google Scholar] [CrossRef]
- Sharma, H.K.; Sharma, S.K.; Vemula, K.; Koirala, A.R.; Yadav, H.M.; Singh, B.P. CNT facilitated interfacial charge transfer of TiO2 nanocomposite for controlling the electron-hole recombination. Solid State Sci. 2021, 112, 106492. [Google Scholar] [CrossRef]
- Guan, Z.-C.; Wang, X.; Jin, P.; Tang, Y.-Y.; Wang, H.-P.; Song, G.-L.; Du, R.-G. Enhanced photoelectrochemical performances of ZnS-Bi2S3/TiO2/WO3 composite film for photocathodic protection. Corros. Sci. 2018, 143, 31–38. [Google Scholar] [CrossRef]
- Hu, C.; Li, T.; Yin, H.; Hu, L.; Tang, J.; Ren, K. Preparation and corrosion protection of three different acids doped polyaniline/epoxy resin composite coatings on carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126069. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Huang, T.-C.; Yeh, T.-C.; Huang, H.-Y.; Ji, W.-F.; Chou, Y.-C.; Hung, W.-I.; Yeh, J.-M.; Tsai, M.-H. Electrochemical studies on aniline-pentamer-based electroactive polyimide coating: Corrosion protection and electrochromic properties. Electrochim. Acta 2011, 56, 10151–10158. [Google Scholar] [CrossRef]
- Abu Bakar, N.H.; Ali, G.A.M.; Ismail, J.; Algarni, H.; Chong, K.F. Size-dependent corrosion behavior of graphene oxide coating. Prog. Org. Coat. 2019, 134, 272–280. [Google Scholar] [CrossRef]
- Wan, S.; Chen, H.; Ma, X.; Chen, L.; Lei, K.; Liao, B.; Dong, Z.; Guo, X. Anticorrosive reinforcement of waterborne epoxy coating on Q235 steel using NZ/BNNS nanocomposites. Prog. Org. Coat. 2021, 159, 106410. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Zhang, W.; Lin, C.; Wang, Z.; Wang, X.; Xu, H.; Feng, J.; Hou, B.; Yan, W.; et al. Efficient photocathodic protection enabled by a multi-dimensional quaternary hybrid superstructure. Chem. Eng. J. 2021, 421, 127858. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, Y.; Mu, W.; Liu, J.; He, J.; Zhou, X. Magnesium phosphate cement prepared with electric furnace ferronickel slag: Properties and its hydration mechanism. Constr. Build. Mater. 2021, 300, 123991. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Wang, X.; Xu, H.; Wang, H.; Zhao, X.; Feng, J.; Yan, W.; Ren, Z. Preparation of PPy/TiO2 core-shell nanorods film and its photocathodic protection for 304 stainless steel under visible light. Mater. Res. Bull. 2020, 124, 110751. [Google Scholar] [CrossRef]




| Systems | Metal | Current Density | OCP Drop | Ref. |
|---|---|---|---|---|
| ZnO | 304 SS | 26 μA/cm2 | −0.38 V | [5] |
| NiSe2/TiO2 | 304 SS | 283 μA/cm2 | - | [6] |
| PbS/TiO2 | 304 SS | 3.2 mA/cm2 | −720 mV | [7] |
| ZnPc/TiO2 | 304 SS | 90 μA/cm2 | −700 mV | [8] |
| Ag/SnO2/TiO2 | 403 SS | 90 μA/cm2 | −415 mV | [9] |
| Ag2S/ZnS/ZnO | 304 SS | 19 μA/cm2 | −0.50 V | [11] |
| Ag/Ag3PO4/TiO2 | 304 SS | 130 µA/cm2 | −900 mV | [48] |
| Zn3In2S6/TiO2 | Carbon steel | 1.91 mA/cm2 | −0.49 V | [49] |
| CuInS2/TiO2 | 304 SS | 118 μA/cm2 | −0.99 V | [50] |
| BiVO4/CdS | 316 SS | 304 μA/cm2 | −330 mV | [51] |
| Sample | Rct (ohm·cm2) | Rs (ohm·cm2) | Cdl (F/cm2) | n | ECC. Models |
|---|---|---|---|---|---|
| Q235 | 45,626 | 22.73 | 4.3992 × 10−5 | 0.41 | RQR |
| TiO2/CdS−1 | 25,632 | 24.18 | 1.0372 × 10−4 | 0.91 | R(Q(R(QR))) |
| TiO2/CdS−2 | 18,956 | 28.87 | 1.1107 × 10−4 | 0.86 | R(Q(R(QR))) |
| TiO2/CdS−3 | 12,216 | 25.27 | 8.9728 × 10−5 | 0.80 | R(Q(R(QR))) |
| TiO2/CdS−4 | 12,625 | 24.30 | 1.0882 × 10−4 | 0.92 | R(Q(R(QR))) |
| TiO2/CdS−5 | 14,899 | 23.93 | 1.1273 × 10−4 | 0.92 | R(Q(R(QR))) |
| Sample | Ecorr (V) | Icorr (A/cm2) | Rp (ohms/cm2) | CR (mm/Year) | PEF% |
|---|---|---|---|---|---|
| Q235 | −0.559 | 5.598 × 10−7 | 58,797.6 | 1.3 × 10−2 | / |
| TiO2/CdS-1 | −0.557 | 2.455 × 10−7 | 155,261.9 | 5.703 × 10−3 | 62.13% |
| TiO2/CdS-2 | −0.550 | 2.089 × 10−7 | 176,507.0 | 4.853 × 10−3 | 66.69% |
| TiO2/CdS-3 | −0.545 | 1.149 × 10−7 | 405,424.3 | 2.669 × 10−3 | 85.51% |
| TiO2/CdS-4 | −0.546 | 1.298 × 10−7 | 296,483.8 | 3.015 × 10−3 | 80.17% |
| TiO2/CdS-5 | −0.550 | 1.306 × 10−7 | 294,487.2 | 3.034 × 10−3 | 80.03% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Li, B.; Zhang, X.; Ke, X.; Xiao, R. Core–Shell Spheroid Structure TiO2/CdS Composites with Enhanced Photocathodic Protection Performance. Materials 2023, 16, 3927. https://doi.org/10.3390/ma16113927
Chen T, Li B, Zhang X, Ke X, Xiao R. Core–Shell Spheroid Structure TiO2/CdS Composites with Enhanced Photocathodic Protection Performance. Materials. 2023; 16(11):3927. https://doi.org/10.3390/ma16113927
Chicago/Turabian StyleChen, Tingting, Bo Li, Xiaolong Zhang, Xiang Ke, and Rengui Xiao. 2023. "Core–Shell Spheroid Structure TiO2/CdS Composites with Enhanced Photocathodic Protection Performance" Materials 16, no. 11: 3927. https://doi.org/10.3390/ma16113927
APA StyleChen, T., Li, B., Zhang, X., Ke, X., & Xiao, R. (2023). Core–Shell Spheroid Structure TiO2/CdS Composites with Enhanced Photocathodic Protection Performance. Materials, 16(11), 3927. https://doi.org/10.3390/ma16113927







