Modeling and Optimizing the Crystal Violet Dye Adsorption on Kaolinite Mixed with Cellulose Waste Red Bean Peels: Insights into the Kinetic, Isothermal, Thermodynamic, and Mechanistic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material, Methods, and Instruments
2.2. Pretreatment of Raw Clay
2.3. Extracting Cellulose from Red Bean Peels (RBPs)
2.4. Preparation of Kaol/Cel Composite
2.5. Experimental Design
2.6. Batch Adsorption Studies
3. Results and Discussion
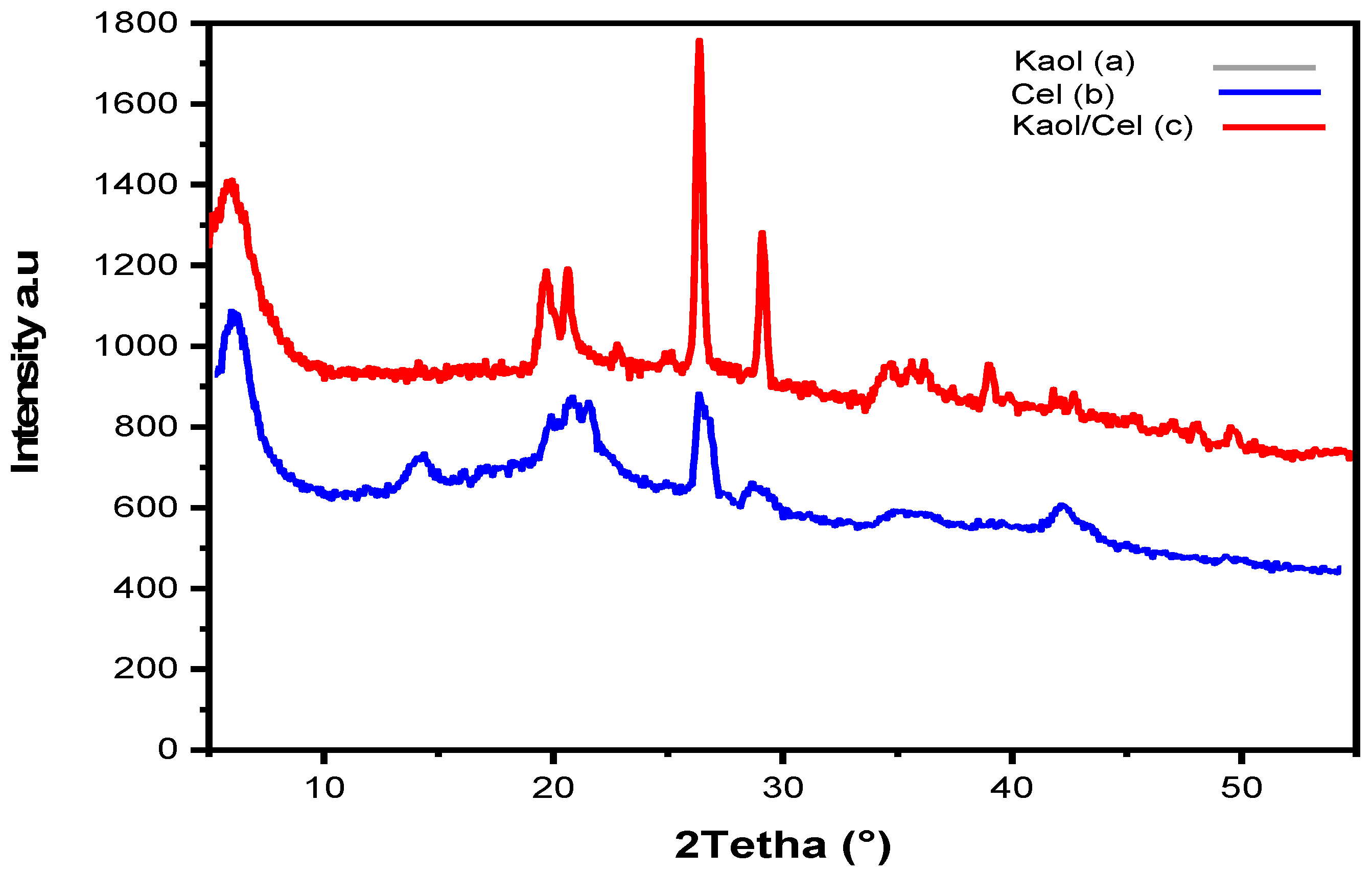
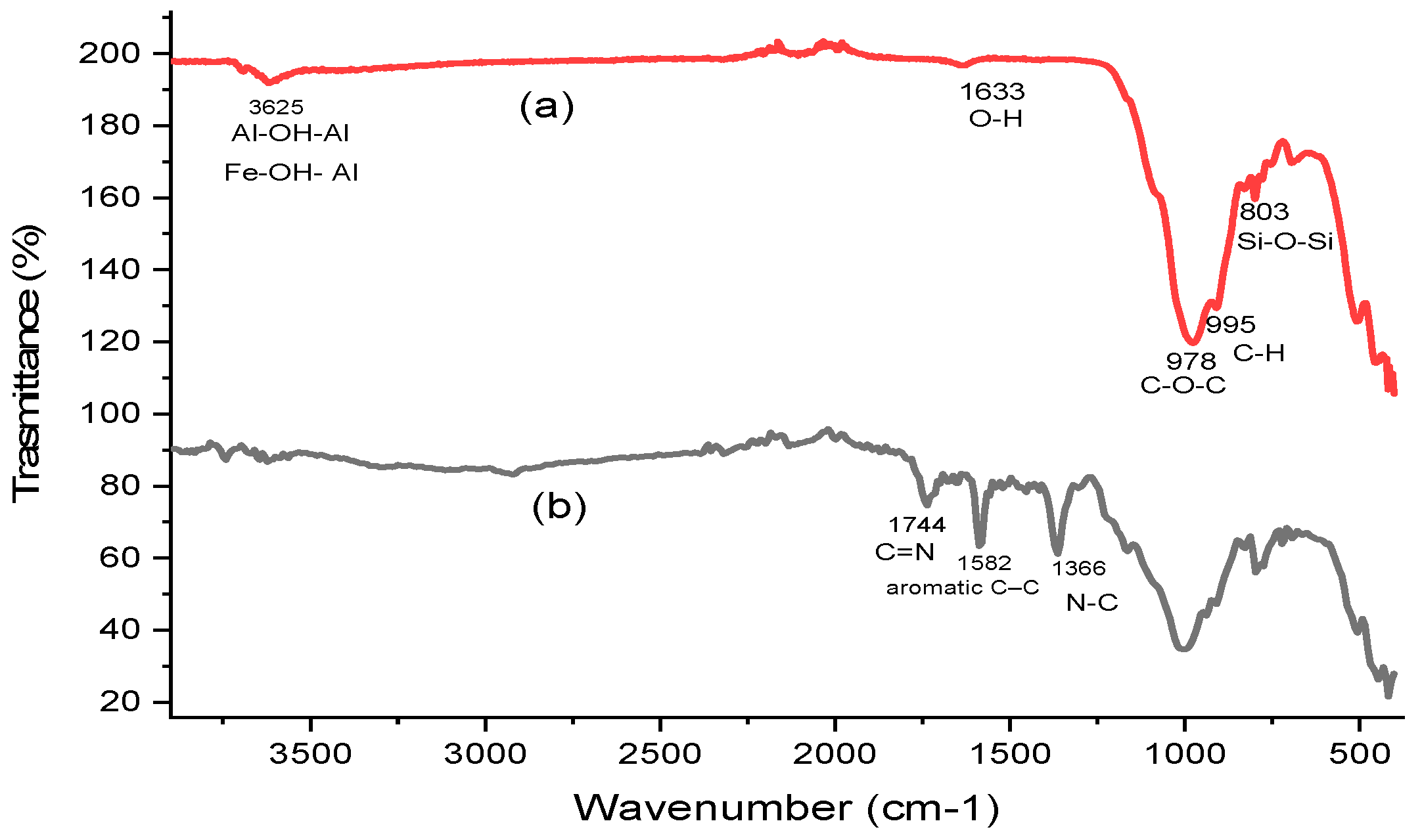
3.1. Adsorbent Characterization
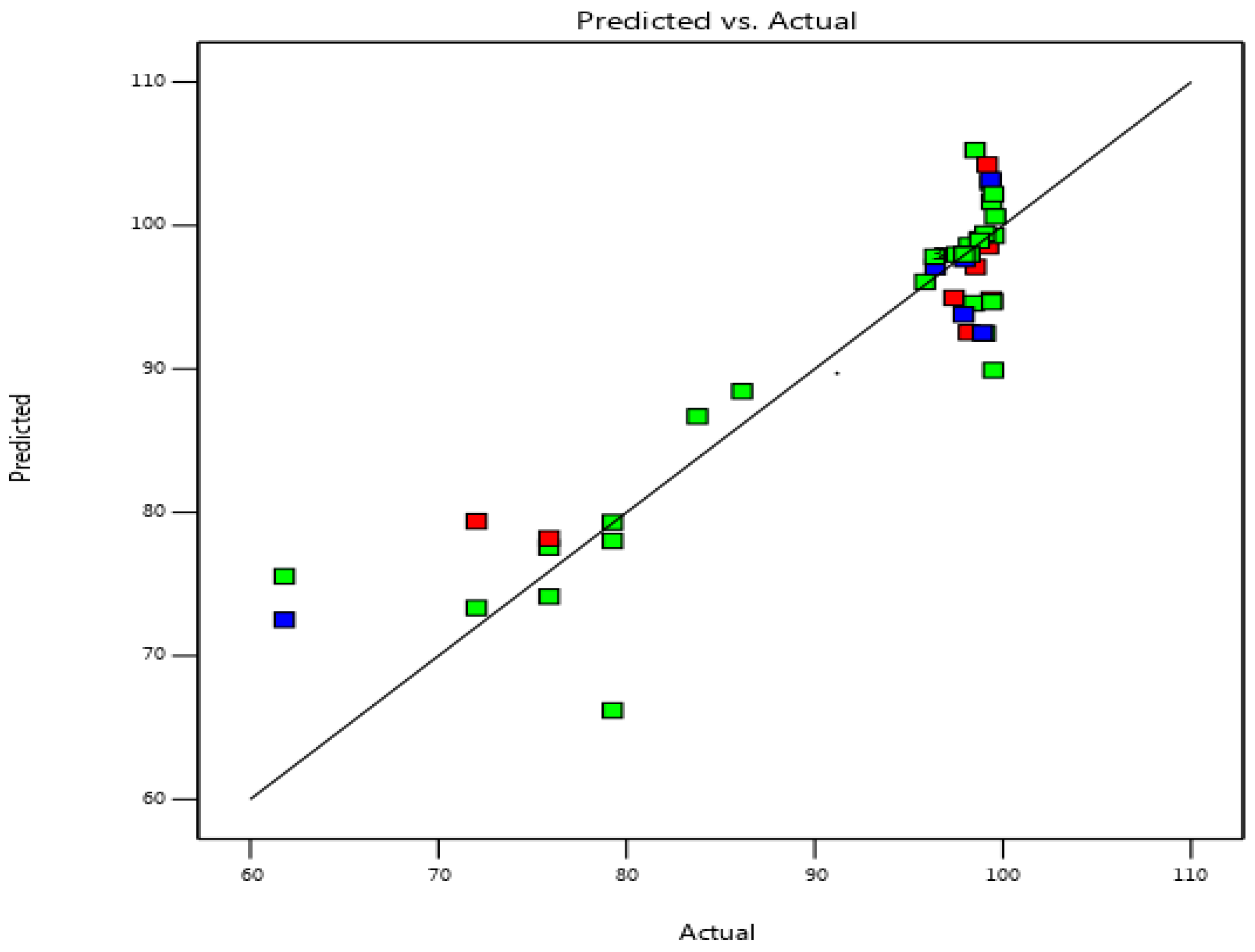
3.2. Fitting the Process Models
| Source | Sum of Squares | df | MeanSquare | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 4461.30 | 20 | 223.07 | 5.66 | <0.0001 | Significant |
| A: Cel loading | 2242.11 | 1 | 2242.11 | 56.92 | <0.0001 | Significant |
| B: Adsorbent dose | 555.69 | 1 | 555.69 | 14.11 | 0.0009 | Significant |
| C-pH | 106.31 | 1 | 106.31 | 2.70 | 0.1130 | Insignificant |
| D-Temp. | 7.19 | 1 | 7.19 | 0.1826 | 0.6728 | Insignificant |
| E-Time | 2.57 | 1 | 2.57 | 0.0652 | 0.8006 | Insignificant |
| AB | 0.0015 | 1 | 0.0015 | 0.0000 | 0.9951 | Insignificant |
| AC | 3.04 | 1 | 3.04 | 0.0772 | 0.7834 | Insignificant |
| AD | 49.21 | 1 | 49.21 | 1.25 | 0.2743 | Insignificant |
| AE | 26.74 | 1 | 26.74 | 0.6787 | 0.4178 | Insignificant |
| BC | 321.01 | 1 | 321.01 | 8.15 | 0.0085 | Significant |
| BD | 170.74 | 1 | 170.74 | 4.33 | 0.0477 | Significant |
| BE | 0.9046 | 1 | 0.9046 | 0.0230 | 0.8808 | Insignificant |
| CD | 0.7121 | 1 | 0.7121 | 0.0181 | 0.8941 | Insignificant |
| CE | 0.8080 | 1 | 0.8080 | 0.0205 | 0.8873 | Insignificant |
| DE | 1.85 | 1 | 1.85 | 0.0469 | 0.8303 | Insignificant |
| A2 | 814.98 | 1 | 814.98 | 20.69 | 0.0001 | Significant |
| B2 | 166.50 | 1 | 166.50 | 4.23 | 0.0504 | Insignificant |
| C2 | 3.48 | 1 | 3.48 | 0.0882 | 0.7689 | Insignificant |
| D2 | 11.12 | 1 | 11.12 | 0.2823 | 0.5999 | Insignificant |
| E2 | 0.1796 | 1 | 0.1796 | 0.0046 | 0.9467 | Insignificant |
| Residual | 984.81 | 25 | 39.39 | |||
| Cor Total | 5446.11 | 45 |
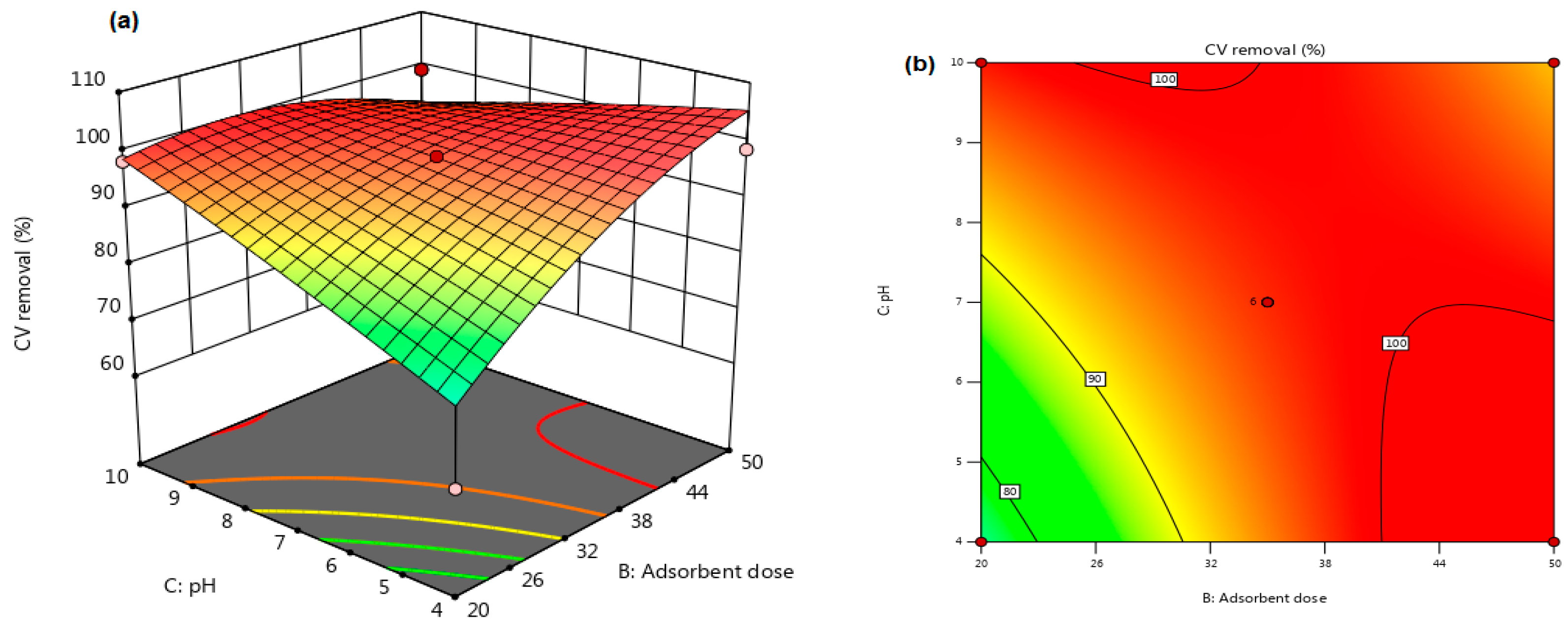
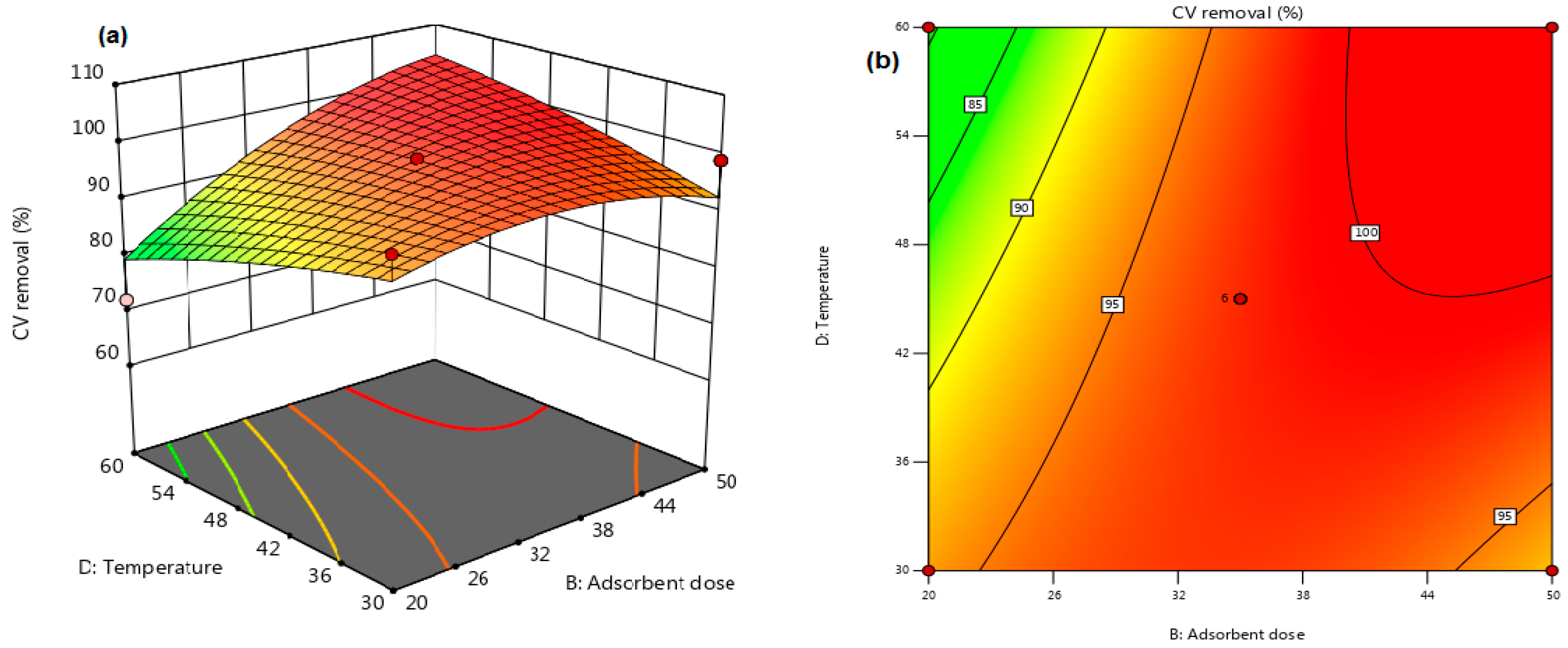
3.3. Interactions Significant for Crystal Violet (CV) Dye Removal

3.4. Adsorption Studies
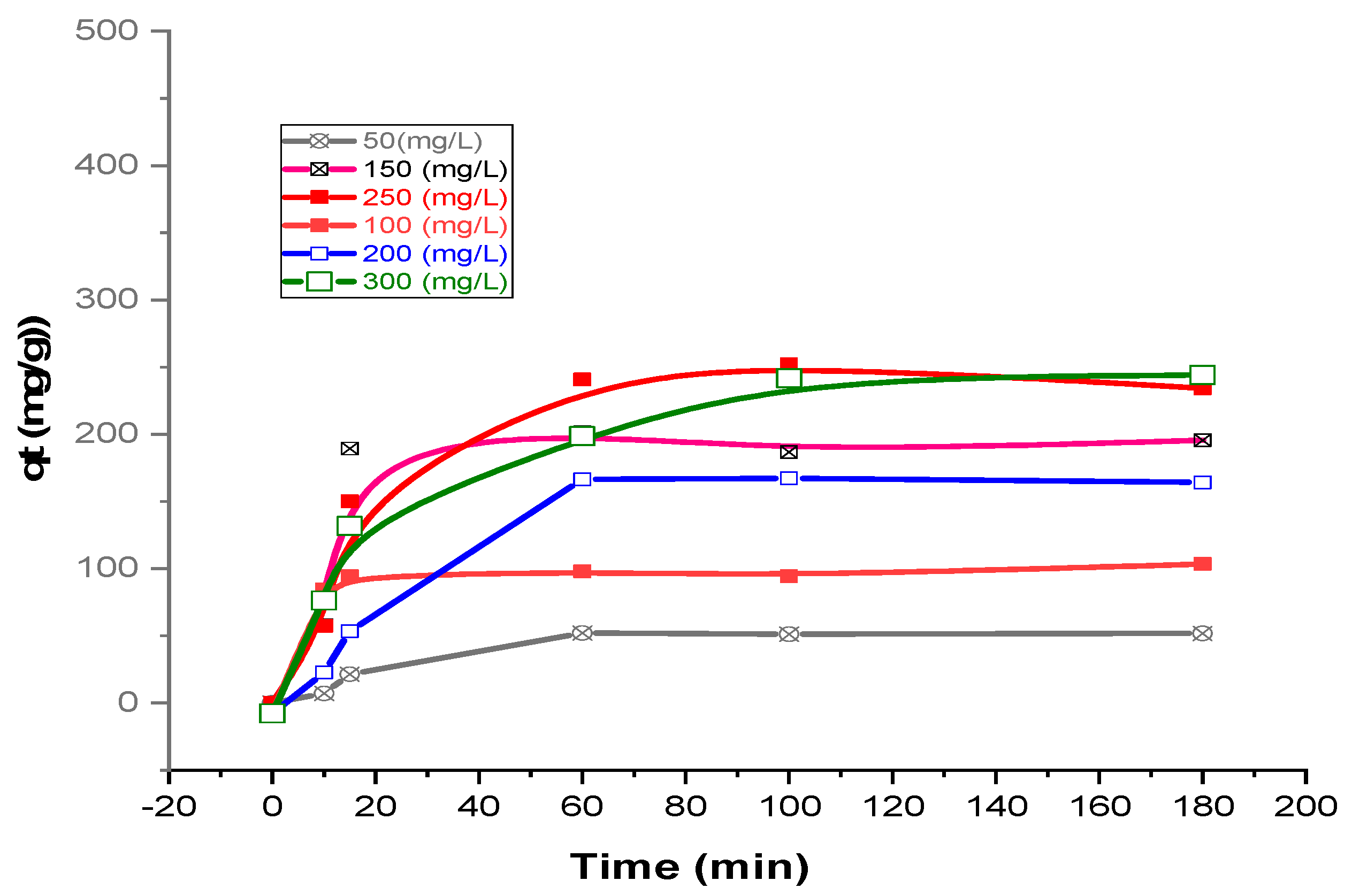
3.5. Kinetic Modeling
3.6. Isotherms for Adsorption
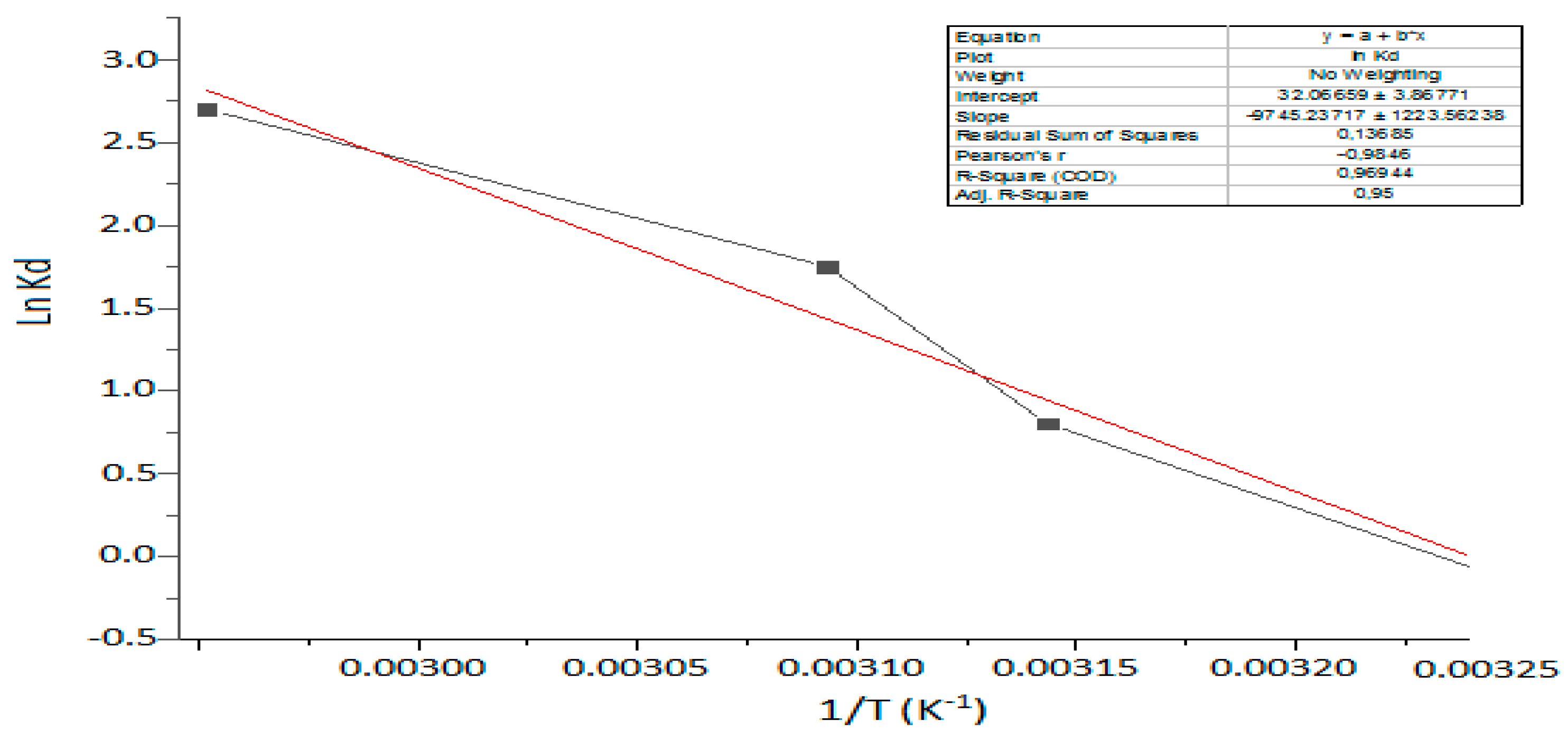
3.7. Thermodynamic Functions Results
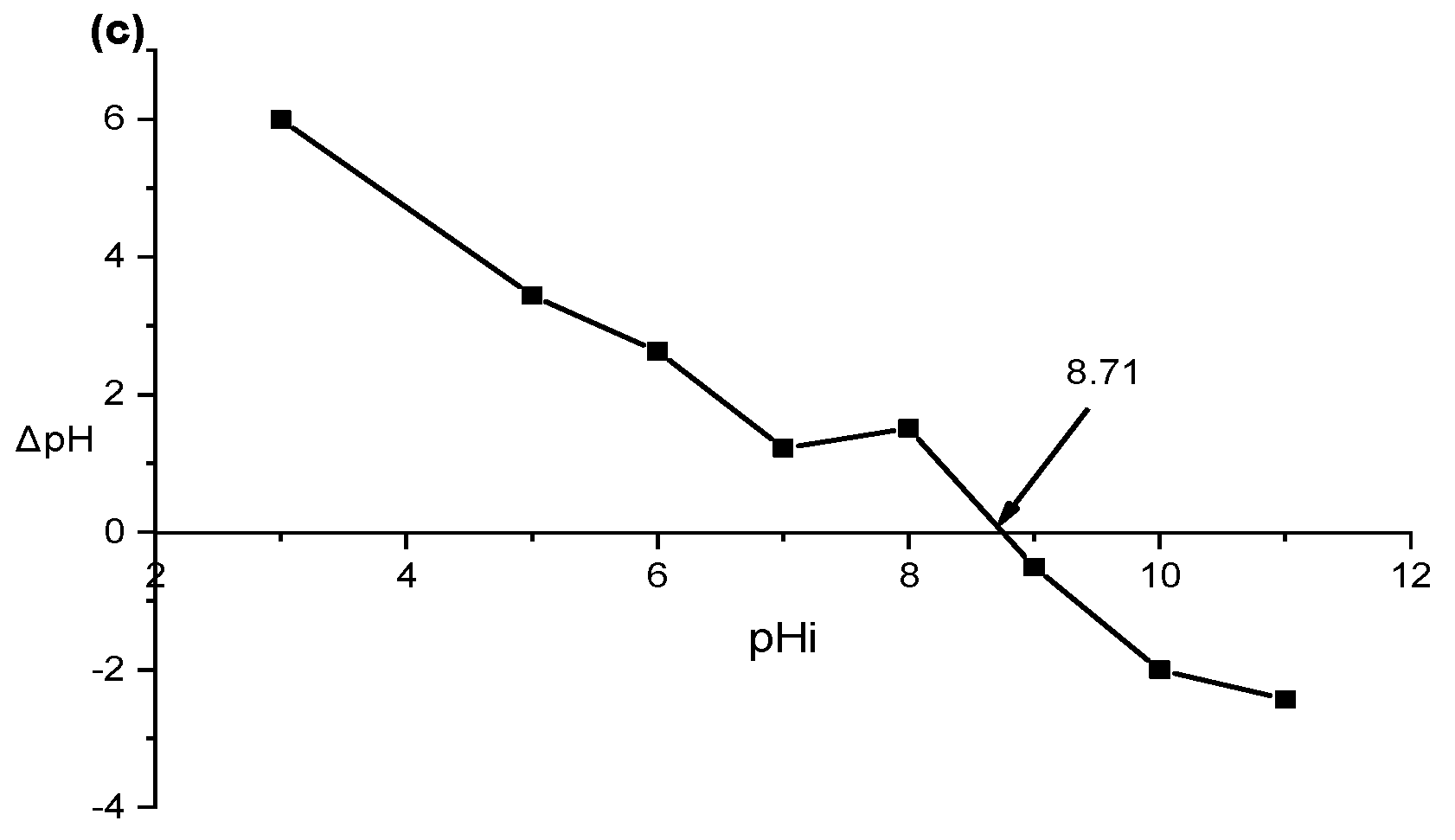
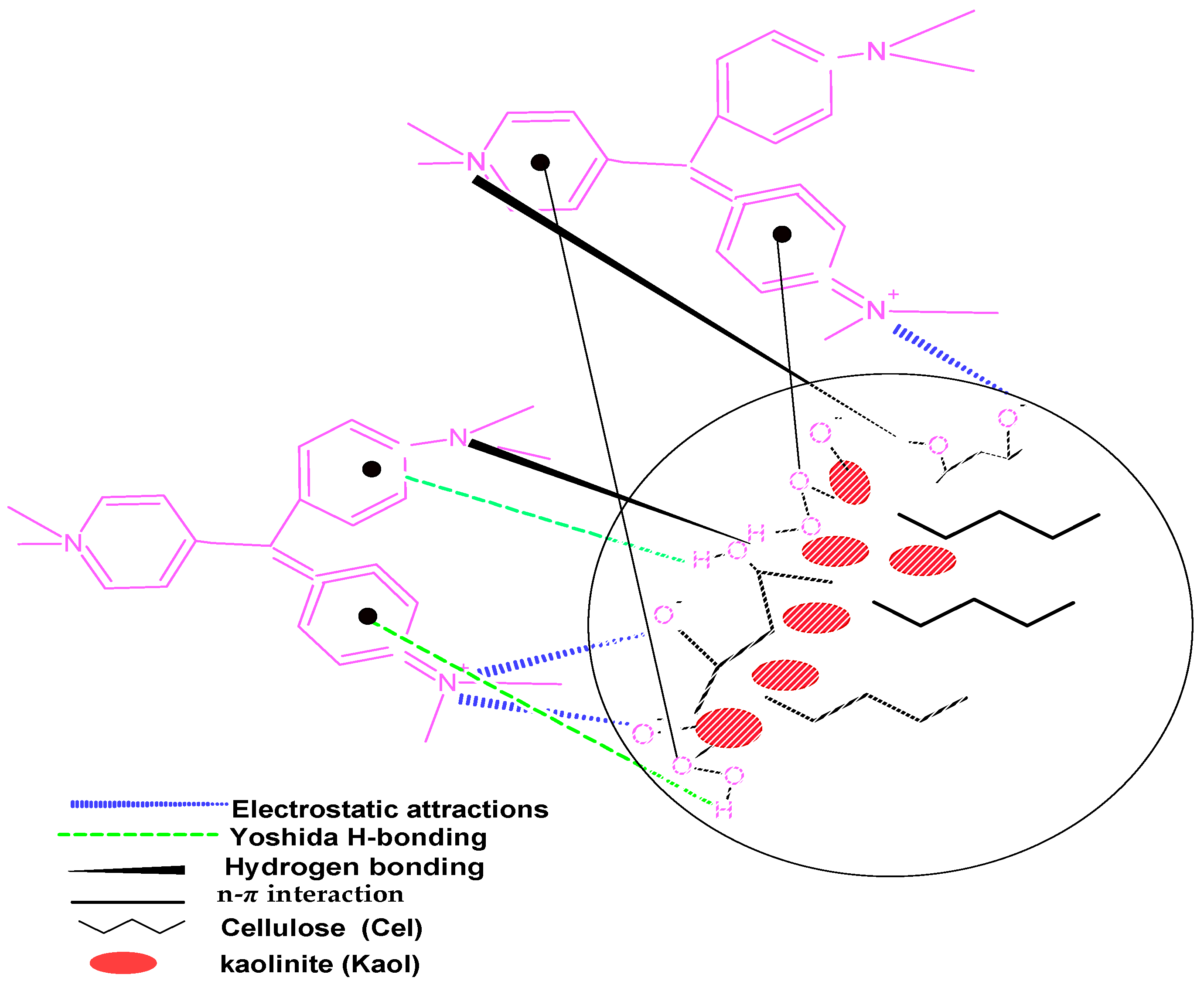
3.8. Mechanisms of Adsorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Barasarathi, J.; Abdullah, P.S.; Uche, E.C. Application of magnetic carbon nanocomposite from agro-waste for the removal of pollutants from water and wastewater. Chemosphere 2022, 305, 135384. [Google Scholar] [CrossRef] [PubMed]
- Gurr, E. Synthetic Dyes in Biology, Medicine and Chemistry; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Nohynek, G.J.; Fautz, R.; Benech-Kieffer, F.; Toutain, H. Toxicity and human health risk of hair dyes. Food Chem. Toxicol. 2004, 42, 517–543. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Sultana, N.; Sayem, A.S.M.; Smriti, S.A. Sustainable adsorbents from plant-derived agricultural wastes for anionic dye removal: A review. Sustainability 2022, 14, 11098. [Google Scholar] [CrossRef]
- Zobeidi, A.; Bebba, A. Seasonal variations of physical, chemical parameters in a wastewater treatment plant by aerated lagoons at Southern-East of Algeria. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1097–1102. [Google Scholar]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Hussain, D.; Khan, S.A.; Alharthi, S.S.; Khan, T.A. Insight into the performance of novel kaolinite-cellulose/cobalt oxide nanocomposite as green adsorbent for liquid phase abatement of heavy metal ions: Modelling and mechanism. Arab. J. Chem. 2022, 15, 103925. [Google Scholar] [CrossRef]
- Deliyanni, E.; Peleka, E.; Lazaridis, N. Comparative study of phosphates removal from aqueous solutions by nanocrystalline akaganéite and hybrid surfactant-akaganéite. Sep. Purif. Technol. 2007, 52, 478–486. [Google Scholar] [CrossRef]
- Shakya, A.; Agarwal, T. Removal of Cr(VI) from water using pineapple peel derived biochars: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Rojas, O.J.; Wang, X.; Naebe, M. Kinetics and equilibrium adsorption of methylene blue onto cotton gin trash bioadsorbents. Cellulose 2020, 27, 6485–6504. [Google Scholar] [CrossRef]
- Mabrouk, S.; Bebba, A.A.; Kamarchou, A.; Zobeidi, A. Adsorption capacity of pollutants by using local clay mineral from urban wastewater Touggourt (South-East Algeria). Asian J. Res. Chem. 2020, 13, 85–90. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, P.; Liu, D.; Annabi-Bergaya, F.; Zhou, J.; Chen, F.; Liu, Z. Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors. Appl. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Narayana Saibaba, K.V. Kaolinite-Chitosan based Nano-Composites and Applications. Adv. Appl. Micro Nano Clay Biopolym. Based Compos. 2022, 125, 87–102. [Google Scholar]
- Atia, D.; Bebba, A.A.; Haddad, L.; Zobeidi, A. Elimination of organic pollutants from urban wastewater by illite-kaolinite local clay from south-east of Algeria. Cienc. Tecn. Vitivinic. 2018, 33, 17–28. [Google Scholar]
- Khan, T.A.; Dahiya, S.; Khan, E.A. Removal of direct red 81 from aqueous solution by adsorption onto magnesium oxide-coated kaolinite: Isotherm, dynamics and thermodynamic studies. Environ. Prog. Sustain. Energy 2017, 36, 45–58. [Google Scholar] [CrossRef]
- del Mar Orta, M.; Martín, J.; Santos, J.L.; Aparicio, I.; Medina-Carrasco, S.; Alonso, E. Biopolymer-clay nanocomposites as novel and ecofriendly adsorbents for environmental remediation. Appl. Clay Sci. 2020, 198, 105838. [Google Scholar] [CrossRef]
- Putro, J.N.; Santoso, S.P.; Ismadji, S.; Ju, Y.-H. Investigation of heavy metal adsorption in binary system by nanocrystalline cellulose–bentonite nanocomposite: Improvement on extended Langmuir isotherm model. Microporous Mesoporous Mater. 2017, 246, 166–177. [Google Scholar] [CrossRef]
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical activation of biochar for energy and environmental applications: A comprehensive review. Rev. Chem. Eng. 2019, 35, 777–815. [Google Scholar] [CrossRef]
- Jamshaid, A.; Hamid, A.; Muhammad, N.; Naseer, A.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Shah, N.S. Cellulose-based Materials for the Removal of Heavy Metals from Wastewater—An Overview. ChemBioEng Rev. 2017, 4, 240–256. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.F. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Tedjani, C.F.; Mya, O.B.; Rebiai, A. Isolation and characterization of cellulose from date palm tree spathe sheath. Sustain. Chem. Pharm. 2020, 17, 100307. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, H.; Liu, H.; Cai, P.; Xiao, H. Novel cellulose/montmorillonite mesoporous composite beads for dye removal in single and binary systems. Bioresour. Technol. 2019, 286, 121366. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Chen, L. A green composite hydrogel based on cellulose and clay as efficient absorbent of colored organic effluent. Carbohydr. Polym. 2019, 210, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.S.; Ghanta, S. Kaolinite–Cellulose based Nano–Composites and Applications. Adv. Appl. Micro Nano Clay Biopolym. Based Compos. 2022, 125, 275–301. [Google Scholar]
- Cai, J.; Lei, M.; Zhang, Q.; He, J.-R.; Chen, T.; Liu, S.; Fu, S.-H.; Li, T.-T.; Liu, G.; Fei, P. Electrospun composite nanofiber mats of Cellulose@ Organically modified montmorillonite for heavy metal ion removal: Design, characterization, evaluation of absorption performance. Compos. Part A Appl. Sci. Manuf. 2017, 92, 10–16. [Google Scholar] [CrossRef]
- Ashraf, M.-T.; AlHammadi, A.A.; El-Sherbeeny, A.M.; Alhammadi, S.; Al Zoubi, W.; Ko, Y.G.; Abukhadra, M.R. Synthesis of cellulose fibers/Zeolite-A nanocomposite as an environmental adsorbent for organic and inorganic selenium ions; Characterization and advanced equilibrium studies. J. Mol. Liq. 2022, 360, 119573. [Google Scholar] [CrossRef]
- Song, Y.K.; Chew, I.M.L.; Choong, T.S.Y.; Tan, J.; Tan, K.W. Isolation of Nanocrystalline Cellulose from oil palm empty fruit bunch—A response surface methodology study. MATEC Web Conf. 2016, 60, 04009. [Google Scholar] [CrossRef]
- Kaouah, F.; Boumaza, S.; Berrama, T.; Trari, M.; Bendjama, Z. Preparation and characterization of activated carbon from wild olive cores (oleaster) by H3PO4 for the removal of Basic Red 46. J. Clean. Prod. 2013, 54, 296–306. [Google Scholar] [CrossRef]
- Al Arni, S.; Mahmoud, E.; Converti, A.; Mhamed, B.; Alsamani, A.M.S.; Saad, G.; Nadir, A. Application of Date Palm Surface Fiber as an Efficient Biosorbent for Wastewater Treatment. ChemBioEng Rev 2023, 10, 55–64. [Google Scholar] [CrossRef]
- Taj, R.; El Askary, M.; Saad, N.; Basyoni, M. Mineralogical investigation and some sedimentary phenomena of Ubhur Formation, north Jeddah, Saudi Arabia. J. King Abdulaziz Univ. Mar. Sci. 2002, 13, 93–110. [Google Scholar] [CrossRef]
- Atia, D.; Zobeidi, A. An investigation into the mineralogical and physicochemical characterization of El-Oued (Algeria) clay. Neuroquantology 2022, 20, 1048–1058. [Google Scholar]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S.C. Isolation and characterization of cellulose nanofibers from culinary banana peel using high-intensity ultrasonication combined with chemical treatment. Carbohydr. Polym. 2016, 137, 608–616. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; AlHammadi, A.; El-Sherbeeny, A.M.; Salam, M.A.; El-Meligy, M.A.; Awwad, E.M.; Luqman, M. Enhancing the removal of organic and inorganic selenium ions using an exfoliated kaolinite/cellulose fibres nanocomposite. Carbohydr. Polym. 2021, 252, 117163. [Google Scholar] [CrossRef]
- Mohadi, R. Adsorption of congo red using kaolinite-cellulose adsorbent. Sci. Technol. Indones. 2017, 2, 29–36. [Google Scholar]
- Özer, A.; Gürbüz, G.; Çalimli, A.; Körbahti, B.K. Biosorption of copper(II) ions on Enteromorpha prolifera: Application of response surface methodology (RSM). Chem. Eng. J. 2009, 146, 377–387. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, R.; Wang, J.; Wang, J.-F.; Li, X.-M. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport 2014, 25, 1151–1155. [Google Scholar] [CrossRef]
- Boushehrian, M.M.; Esmaeili, H.; Foroutan, R. Ultrasonic assisted synthesis of Kaolin/CuFe2O4 nanocomposite for removing cationic dyes from aqueous media. J. Environ. Chem. Eng. 2020, 8, 103869. [Google Scholar] [CrossRef]
- Shaban, M.; Sayed, M.I.; Shahien, M.G.; Abukhadra, M.R.; Ahmed, Z.M. Adsorption behavior of inorganic-and organic-modified kaolinite for Congo red dye from water, kinetic modeling, and equilibrium studies. J. Sol-Gel Sci. Technol. 2018, 87, 427–441. [Google Scholar] [CrossRef]
- Chen, C.; Sun, K.; Wang, A.; Wang, S.; Jiang, J. Catalytic graphitization of cellulose using nickel as catalyst. BioResources 2018, 13, 3165–3176. [Google Scholar] [CrossRef]
- Sabna, V.; Thampi, S.G.; Chandrakaran, S. Adsorption of crystal violet onto functionalised multi-walled carbon nanotubes: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 2016, 134, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, A.S.; Mohammad, A.-T.; Jawad, A.H. Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. J. Clean. Prod. 2019, 232, 43–56. [Google Scholar] [CrossRef]
- Zain, Z.M.; Abdulhameed, A.S.; Jawad, A.H.; ALOthman, Z.A.; Yaseen, Z.M. A pH-sensitive surface of chitosan/sepiolite clay/algae biocomposite for the removal of malachite green and remazol brilliant blue R dyes: Optimization and adsorption mechanism study. J. Polym. Environ. 2023, 31, 501–518. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Al Arni, S.; Ghareba, S.; Solisio, C.; Palma, M.S.A.; Converti, A. Methods of reactive red 141 dye decolorization, treatment, and removal from industrial wastewaters: A critical review. Environ. Eng. Sci. 2021, 38, 577–591. [Google Scholar] [CrossRef]
- Temkin, M. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Coconut Husk Derived Activated Carbon via Microwave Induced Activation: Effects of Activation Agents, Preparation Parameters and Adsorption Performance. Chem. Eng. J. 2012, 184, 57–65. [Google Scholar] [CrossRef]
- El-Sayed, G.O. Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber. Desalination 2011, 272, 225–232. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Saha, P.D. Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Çoruh, S.; Geyikçi, F.; Ergun, O.N. Dye Removal from Aqueous Solution by Adsorption onto Fly Ash; ATINER’s Conference Paper Series ENV2012-0057; Athens Institute for Education and Research: Athens, Greece, 2012. [Google Scholar]
- El Naeem, G.A.; Abd-Elhamid, A.; Farahat, O.O.; El-Bardan, A.A.; Soliman, H.M.; Nayl, A. Adsorption of crystal violet and methylene blue dyes using a cellulose-based adsorbent from sugercane bagasse: Characterization, kinetic and isotherm studies. J. Mater. Res. Technol. 2022, 19, 3241–3254. [Google Scholar]
- Khan, S.A.; Rehman, T.U.; Shah, L.A.; Ullah, M. Magnetite graphene oxide-doped superadsorbent hydrogel for efficient removal of crystal violet from wastewater. Chem. Pap. 2023, 77, 2725–2735. [Google Scholar] [CrossRef]
- Uddin, M.K.; Abd Malek, N.N.; Jawad, A.H.; Sabar, S. Pyrolysis of rubber seed pericarp biomass treated with sulfuric acid for the adsorption of crystal violet and methylene green dyes: An optimized process. Int. J. Phytoremediat. 2022, 25, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, A.S.; Jawad, A.H.; Kashi, E.; Radzun, K.A.; ALOthman, Z.A.; Wilson, L.D. Insight into adsorption mechanism, modeling, and desirability function of crystal violet and methylene blue dyes by microalgae: Box-Behnken design application. Algal Res. 2022, 67, 102864. [Google Scholar] [CrossRef]
- Hanafi, N.A.M.; Abdulhameed, A.S.; Jawad, A.H.; ALOthman, Z.A.; Yousef, T.A.; Al Duaij, O.; Alsaiari, N.S. Optimized removal process and tailored adsorption mechanism of crystal violet and methylene blue dyes by activated carbon derived from mixed orange peel and watermelon rind using microwave-induced ZnCl2 activation. Biomass Convers. Biorefinery 2022, 1–13. [Google Scholar] [CrossRef]
- Jani, N.A.; Haddad, L.; Abdulhameed, A.S.; Jawad, A.H.; ALOthman, Z.A.; Yaseen, Z.M. Modeling and optimization of the adsorptive removal of crystal violet dye by durian (Durio zibethinus) seeds powder: Insight into kinetic, isotherm, thermodynamic, and adsorption mechanism. Biomass Convers. Biorefinery 2022, 1–14. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mubarak, N.S.A.; Abdulhameed, A.S. Hybrid crosslinked chitosan-epichlorohydrin/TiO2 nanocomposite for reactive red 120 dye adsorption: Kinetic, isotherm, thermodynamic, and mechanism study. J. Polym. Environ. 2020, 28, 624–637. [Google Scholar] [CrossRef]
- Chang, J.; Ma, J.; Ma, Q.; Zhang, D.; Qiao, N.; Hu, M.; Ma, H. Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl. Clay Sci. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Chebli, D.; Bouguettoucha, A.; Mekhalef, T.; Nacef, S.; Amrane, A. Valorization of an agricultural waste, Stipa tenassicima fibers, by biosorption of an anionic azo dye, Congo red. Desalination Water Treat. 2015, 54, 245–254. [Google Scholar] [CrossRef]
- Singh, S.K.; Das, A. The n → π* interaction: A rapidly emerging non-covalent interaction. Phys. Chem. Chem. Phys. 2015, 17, 9596–9612. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.L.; Hunt, A.J.; Budarin, V.L.; Shuttleworth, P.S.; Miller, K.L.; Clark, J.H. The importance of being porous: Polysaccharide-derived mesoporous materials for use in dye adsorption. RSC Adv. 2012, 2, 8992–8997. [Google Scholar] [CrossRef]


| Factors | Levels | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| A: Loading (%) | 0 | 25 | 50 |
| B: Adsorbent dose (g) | 0.02 | 0.035 | 0.05 |
| C: pH | 4 | 7 | 10 |
| D: Temperature (°C) | 30 | 45 | 60 |
| E: Contact time (min) | 5 | 65.5 | 120 |
| Run | A: Cel Loading (%) | B: Adsorbent Dose (g) | C: pH | D: Temperature (°C) | E: Contact Time (min) | Dye Removal (%) |
|---|---|---|---|---|---|---|
| 1 | 25 | 0.035 | 7 | 45 | 17.5 | 97.95 |
| 2 | 0 | 0.035 | 7 | 60 | 17.5 | 75.88 |
| 3 | 25 | 0.020 | 7 | 60 | 17.5 | 72.02 |
| 4 | 25 | 0.050 | 10 | 45 | 17.5 | 99.05 |
| 5 | 25 | 0.035 | 10 | 30 | 17.5 | 98.75 |
| 6 | 0 | 0.035 | 7 | 45 | 30 | 72.02 |
| 7 | 50 | 0.035 | 10 | 45 | 17.5 | 99.34 |
| 8 | 50 | 0.020 | 7 | 45 | 17.5 | 99.48 |
| 9 | 0 | 0.050 | 7 | 45 | 17.5 | 79.25 |
| 10 | 25 | 0.020 | 4 | 45 | 17.5 | 61.82 |
| 11 | 25 | 0.050 | 7 | 45 | 30 | 99.05 |
| 12 | 25 | 0.050 | 7 | 60 | 17.5 | 99.16 |
| 13 | 25 | 0.050 | 7 | 30 | 17.5 | 98.91 |
| 14 | 25 | 0.035 | 7 | 45 | 17.5 | 97.95 |
| 15 | 50 | 0.035 | 7 | 45 | 5 | 96.37 |
| 16 | 25 | 0.020 | 10 | 45 | 17.5 | 98.19 |
| 17 | 25 | 0.035 | 10 | 60 | 17.5 | 99.21 |
| 18 | 25 | 0.035 | 7 | 60 | 5 | 98.55 |
| 19 | 25 | 0.035 | 7 | 45 | 17.5 | 97.95 |
| 20 | 50 | 0.035 | 7 | 45 | 30 | 99.49 |
| 21 | 50 | 0.035 | 7 | 60 | 17.5 | 99.37 |
| 22 | 0 | 0.035 | 7 | 30 | 17.5 | 61.82 |
| 23 | 25 | 0.035 | 10 | 45 | 5 | 99.58 |
| 24 | 25 | 0.050 | 4 | 45 | 17.5 | 98.51 |
| 25 | 25 | 0.035 | 4 | 60 | 17.5 | 98.18 |
| 26 | 0 | 0.035 | 4 | 45 | 17.5 | 75.88 |
| 27 | 25 | 0.020 | 7 | 45 | 5 | 86.14 |
| 28 | 25 | 0.020 | 7 | 30 | 17.5 | 97.91 |
| 29 | 25 | 0.035 | 7 | 60 | 30 | 97.40 |
| 30 | 25 | 0.050 | 7 | 45 | 5 | 99.52 |
| 31 | 0 | 0.035 | 10 | 45 | 17.5 | 75.88 |
| 32 | 25 | 0.035 | 7 | 30 | 30 | 97.97 |
| 33 | 50 | 0.035 | 7 | 30 | 17.5 | 99.34 |
| 34 | 25 | 0.035 | 4 | 45 | 30 | 99.41 |
| 35 | 0 | 0.035 | 7 | 45 | 5 | 79.25 |
| 36 | 25 | 0.035 | 4 | 30 | 17.5 | 99.41 |
| 37 | 25 | 0.035 | 4 | 45 | 5 | 98.44 |
| 38 | 25 | 0.020 | 7 | 45 | 30 | 83.76 |
| 39 | 25 | 0.035 | 7 | 45 | 17.5 | 98.22 |
| 40 | 50 | 0.035 | 4 | 45 | 17.5 | 95.86 |
| 41 | 25 | 0.035 | 7 | 45 | 17.5 | 97.54 |
| 42 | 25 | 0.035 | 7 | 45 | 17.5 | 98.22 |
| 43 | 25 | 0.035 | 7 | 30 | 5 | 96.40 |
| 44 | 50 | 0.050 | 7 | 45 | 17.5 | 99.40 |
| 45 | 0 | 0.020 | 7 | 45 | 17.5 | 79.25 |
| 46 | 25 | 0.035 | 10 | 45 | 30 | 98.75 |
| Concentration (mg/L) | qe,exp (mg/g) | PFO | PSO | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg/g) | k1 (1/min) | R2 | qe,cal (mg/g) | k2 10−2 (g/mg. min) | R2 | ||
| 50 | 51.74 | 54.23 | 0.0459 | 0.963 | 59.52 | 0.074 | 0.994 |
| 100 | 131.5 | 130.53 | 0.0531 | 0.955 | 133.33 | 0.298 | 0.999 |
| 150 | 195.6 | 180.33 | 0.0277 | 0.994 | 198.33 | 0.042 | 1 |
| 200 | 203.2 | 182.66 | 0.0215 | 0.945 | 208.56 | 0.016 | 0.997 |
| 250 | 252.1 | 192.31 | 0.0318 | 0.938 | 294.12 | 0.014 | 0.995 |
| 300 | 297.7 | 274.82 | 0.0434 | 0.954 | 299.58 | 0.020 | 0.996 |
| Model | Parameters | Value |
|---|---|---|
| Langmuir | qmax (mg/g) | 294.12 |
| Ka (L/mg) | 0.03 | |
| R2 | 0.99 | |
| Freundlich | Kf (mg/g) (L/mg)1/n | 38.37 |
| n | 2.43 | |
| R2 | 0.98 | |
| Temkin | KT (L/mg) | 0.07 |
| bT (JHZ[J/mol]) | 22.40 | |
| R2 | 0.91 |
| Adsorbents | qmax (mg/g) | References |
|---|---|---|
| Palm kernel fiber | 78.9 | [52] |
| Rice husk NaOH-modified | 44.87 | [53] |
| Fly ash | 74.6 | [54] |
| Cellulose-based from sugercane bagasse | 107.5 | [55] |
| Magnetite graphene oxide-doped super adsorbent hydrogel | 88.78 | [56] |
| Rubber seed pericarp treated with sulfuric acid | 302.7 | [57] |
| Microalgae | 243.0 | [58] |
| Zeolite–montmorillonite | 150.52 | [59] |
| Durian seeds powder | 158 | [60] |
| Kaol/Cel–25 | 294.12 | This study |
| T (K) | Lnkd | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (kJ/mol K) |
|---|---|---|---|---|
| 303.15 | 0.0867 | −0.22 | −78.65 | −0.238 |
| 313.15 | 0.5931 | −1.54 | ||
| 318.15 | 1.5411 | −4.07 | ||
| 333.15 | 2.9099 | −8.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mecheri, R.; Zobeidi, A.; Atia, S.; Neghmouche Nacer, S.; Salih, A.A.M.; Benaissa, M.; Ghernaout, D.; Arni, S.A.; Ghareba, S.; Elboughdiri, N. Modeling and Optimizing the Crystal Violet Dye Adsorption on Kaolinite Mixed with Cellulose Waste Red Bean Peels: Insights into the Kinetic, Isothermal, Thermodynamic, and Mechanistic Study. Materials 2023, 16, 4082. https://doi.org/10.3390/ma16114082
Mecheri R, Zobeidi A, Atia S, Neghmouche Nacer S, Salih AAM, Benaissa M, Ghernaout D, Arni SA, Ghareba S, Elboughdiri N. Modeling and Optimizing the Crystal Violet Dye Adsorption on Kaolinite Mixed with Cellulose Waste Red Bean Peels: Insights into the Kinetic, Isothermal, Thermodynamic, and Mechanistic Study. Materials. 2023; 16(11):4082. https://doi.org/10.3390/ma16114082
Chicago/Turabian StyleMecheri, Razika, Ammar Zobeidi, Salem Atia, Salah Neghmouche Nacer, Alsamani A. M. Salih, Mhamed Benaissa, Djamel Ghernaout, Saleh Al Arni, Saad Ghareba, and Noureddine Elboughdiri. 2023. "Modeling and Optimizing the Crystal Violet Dye Adsorption on Kaolinite Mixed with Cellulose Waste Red Bean Peels: Insights into the Kinetic, Isothermal, Thermodynamic, and Mechanistic Study" Materials 16, no. 11: 4082. https://doi.org/10.3390/ma16114082
APA StyleMecheri, R., Zobeidi, A., Atia, S., Neghmouche Nacer, S., Salih, A. A. M., Benaissa, M., Ghernaout, D., Arni, S. A., Ghareba, S., & Elboughdiri, N. (2023). Modeling and Optimizing the Crystal Violet Dye Adsorption on Kaolinite Mixed with Cellulose Waste Red Bean Peels: Insights into the Kinetic, Isothermal, Thermodynamic, and Mechanistic Study. Materials, 16(11), 4082. https://doi.org/10.3390/ma16114082








