Atomic Layer Deposition of HfO2 Films Using TDMAH and Water or Ammonia Water
Abstract
1. Introduction
2. Materials and Methods
2.1. ALD Preparation of HfO2 Films
2.2. Materials Characterization
3. Results and Discussion
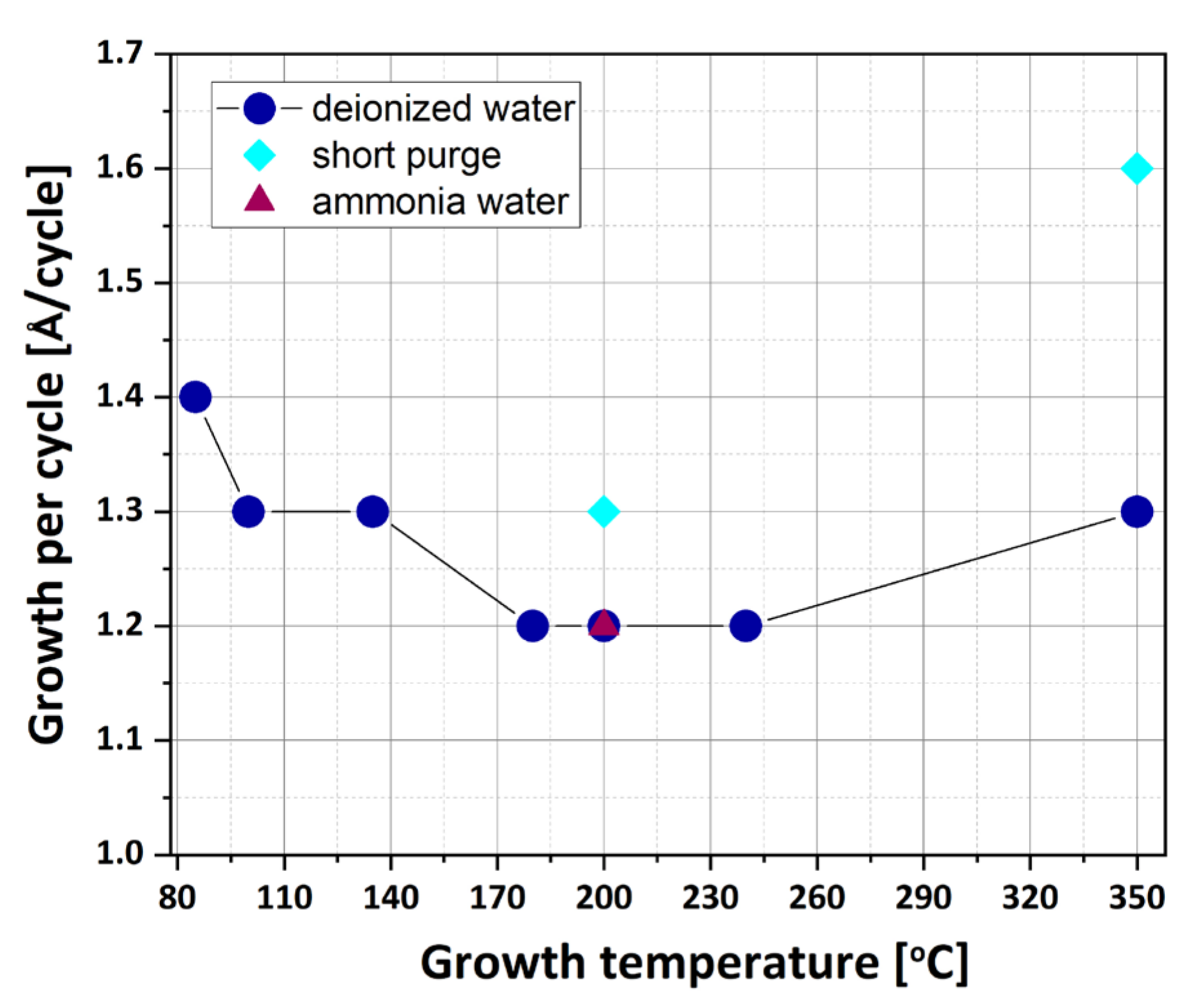
3.1. Film Thickness and Growth per Cycle
3.2. Chemical Component
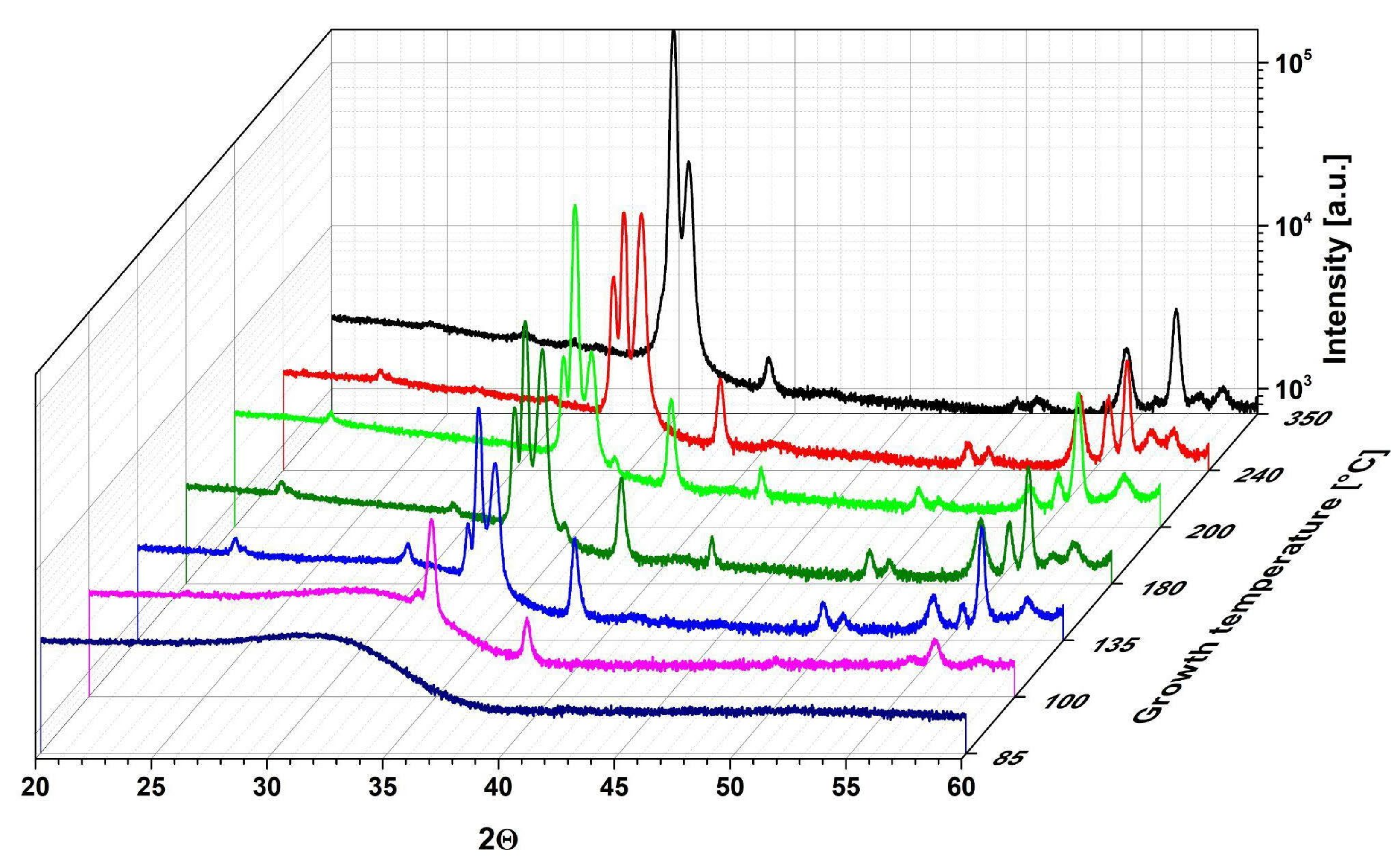
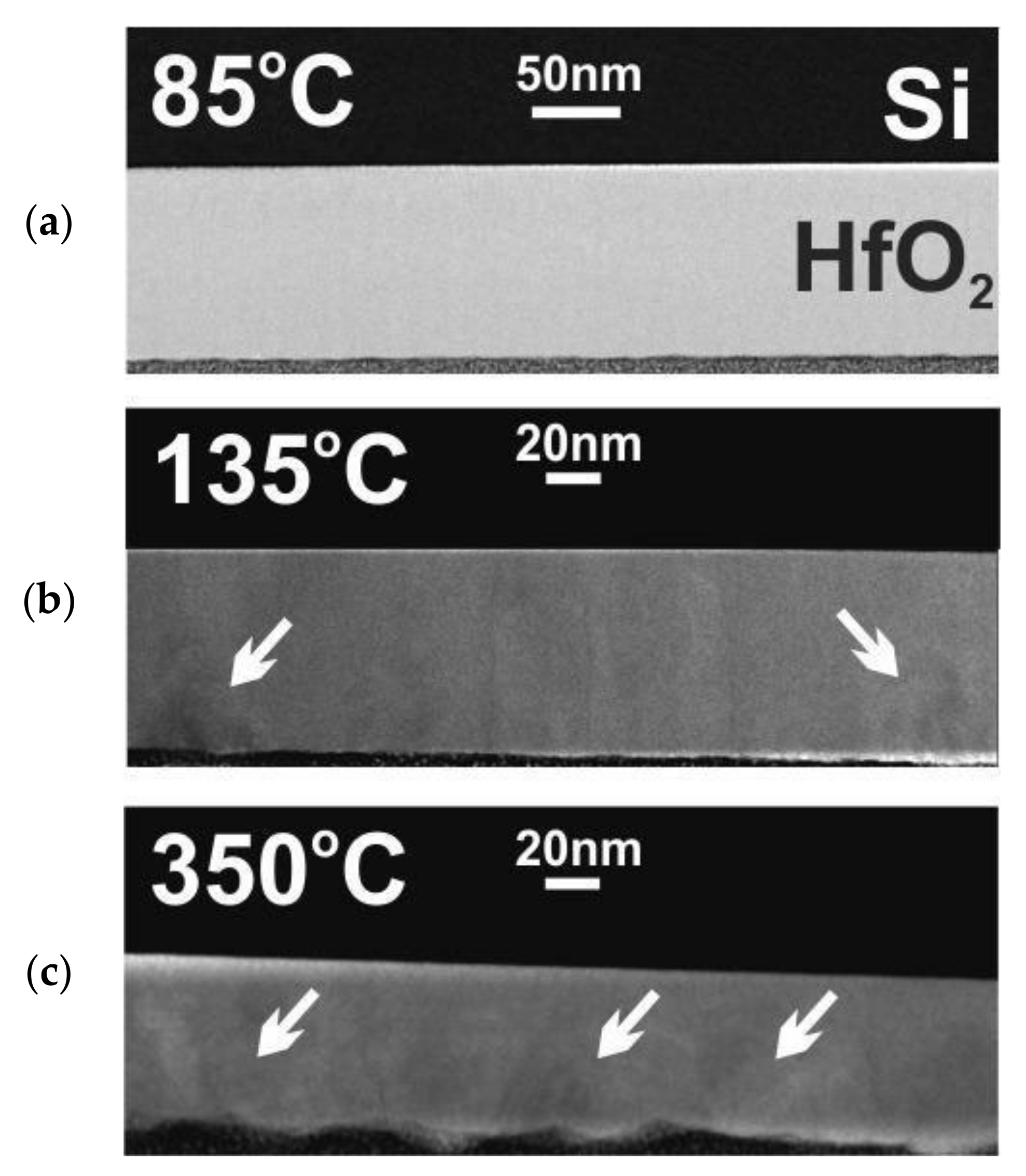
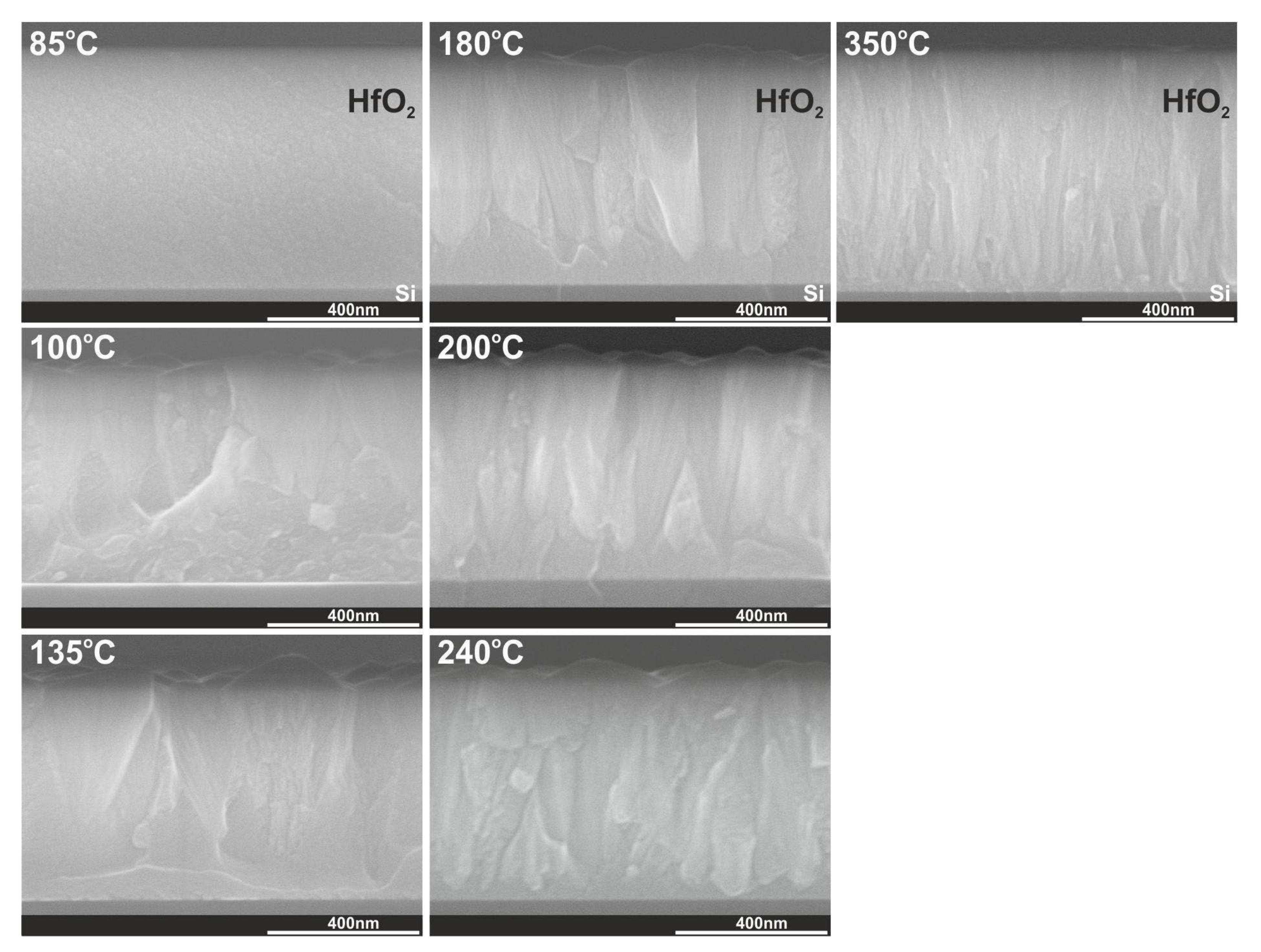
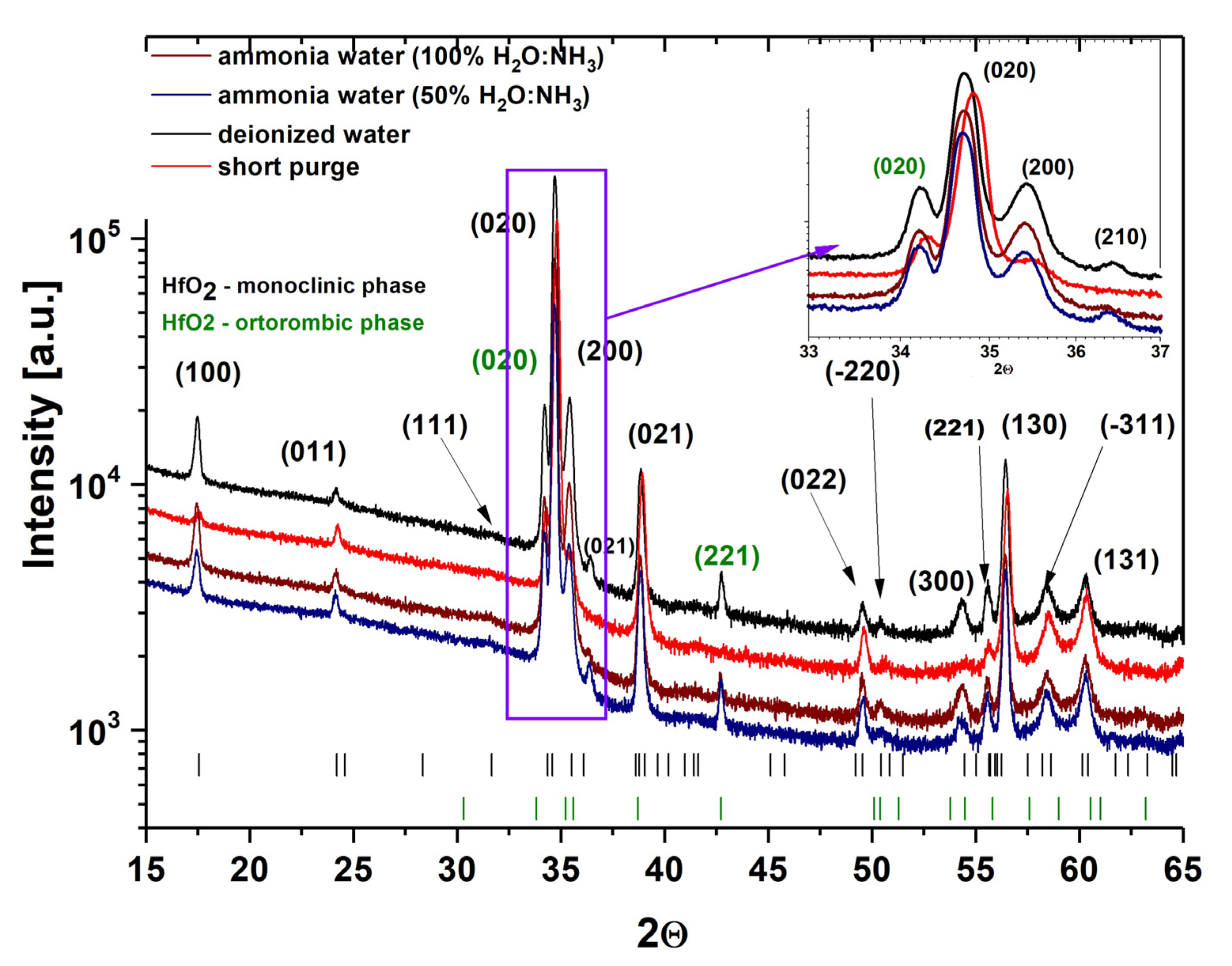
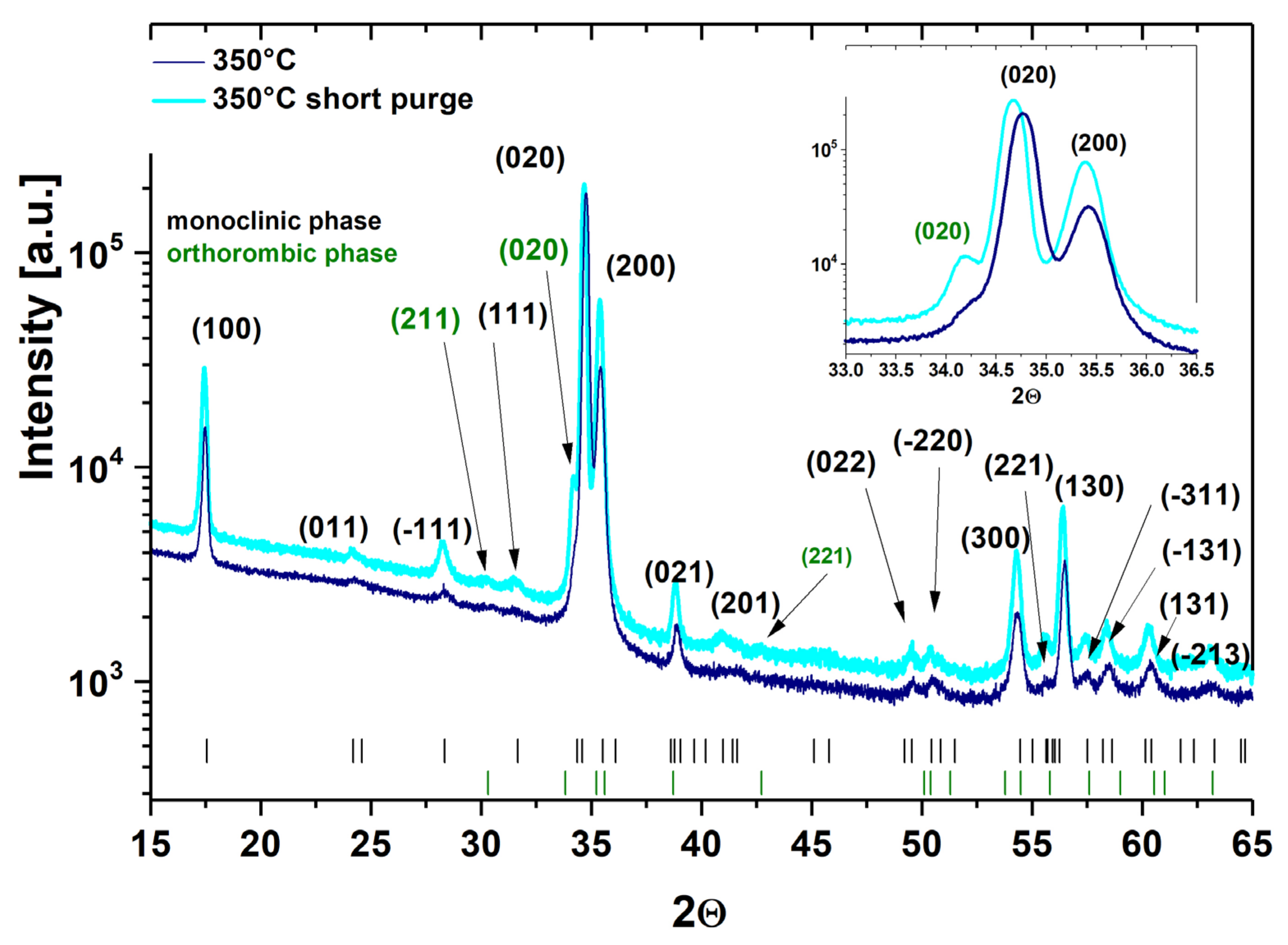
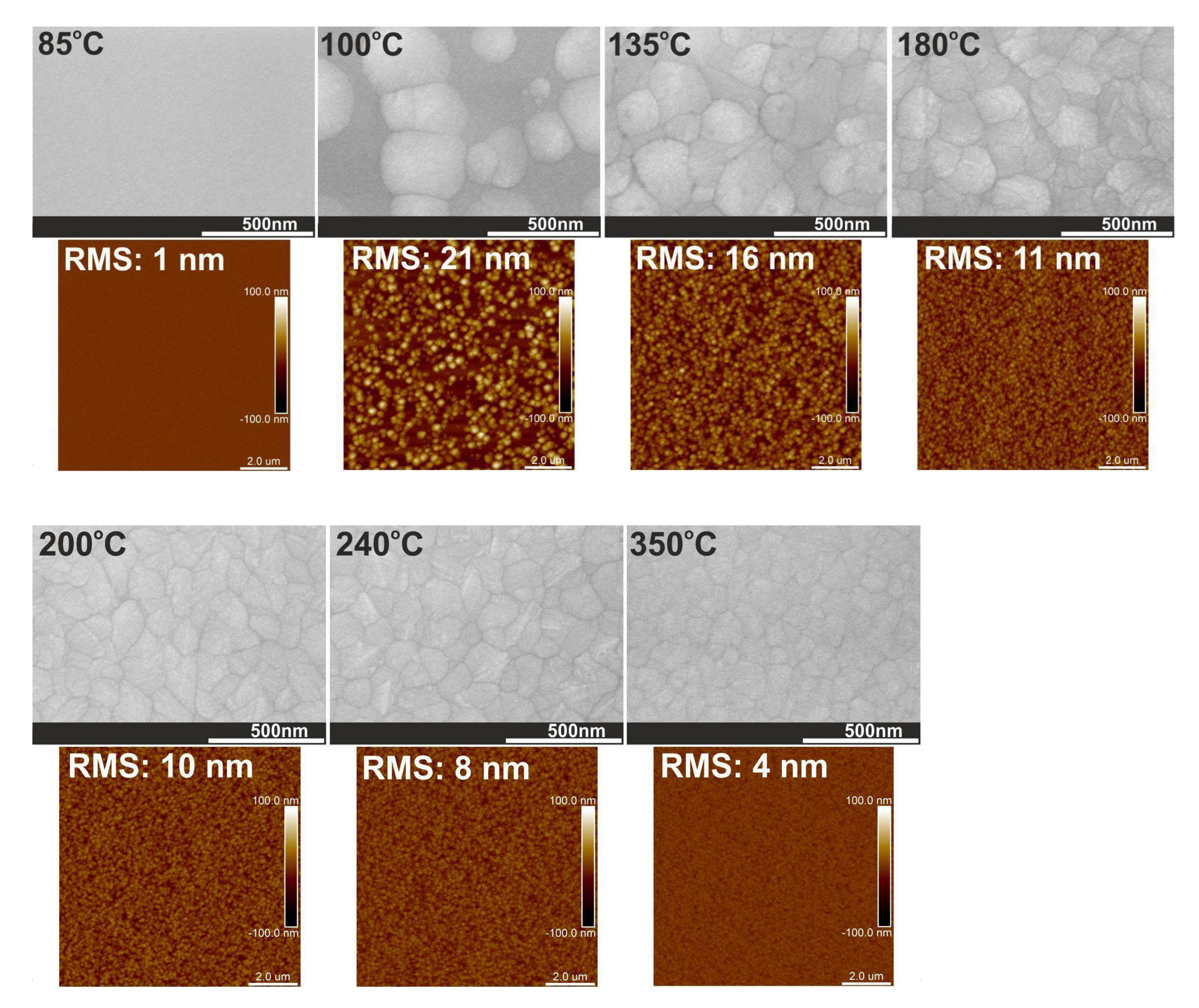
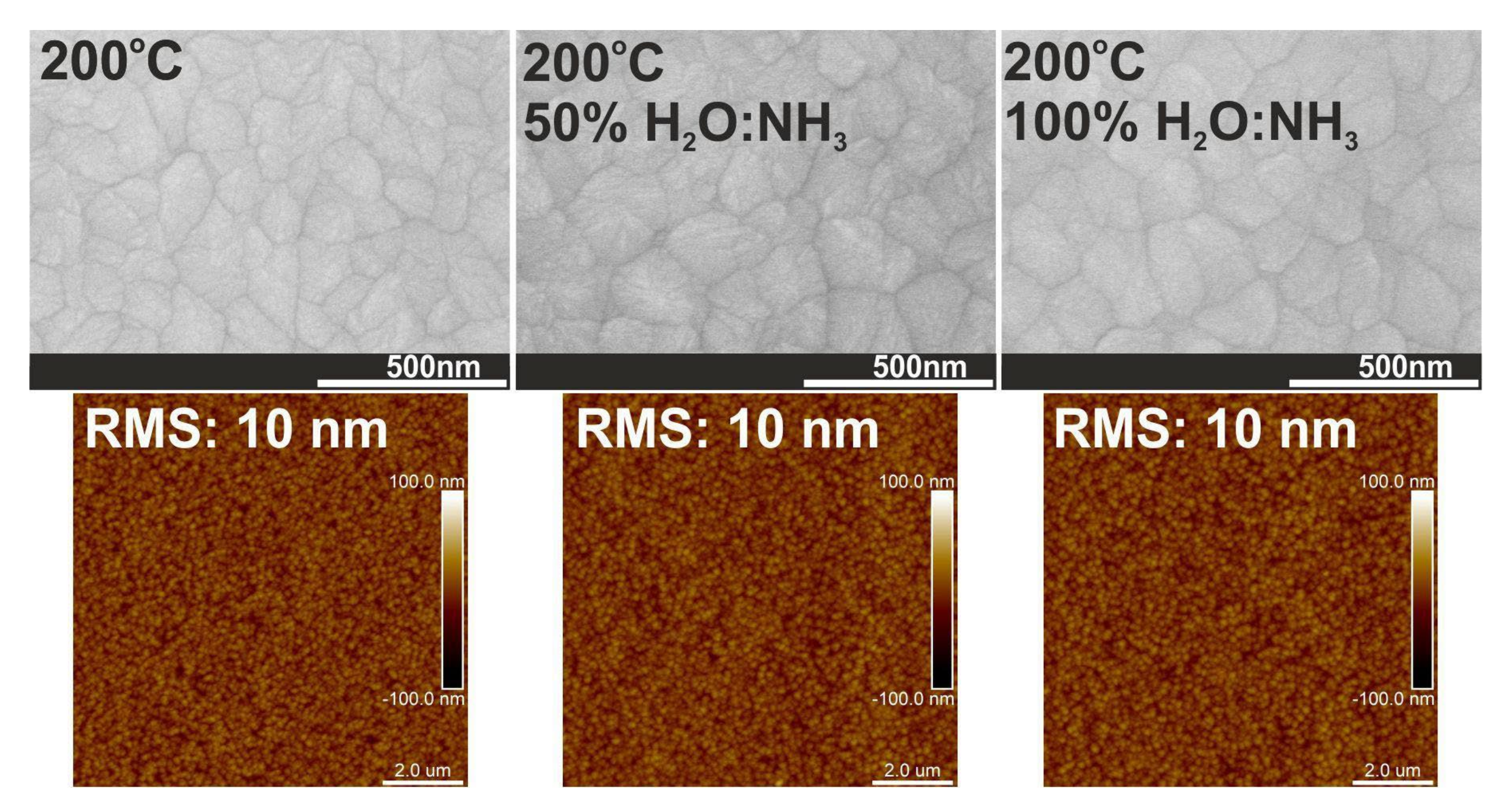
3.3. Crystal Structure and Surface Morphology
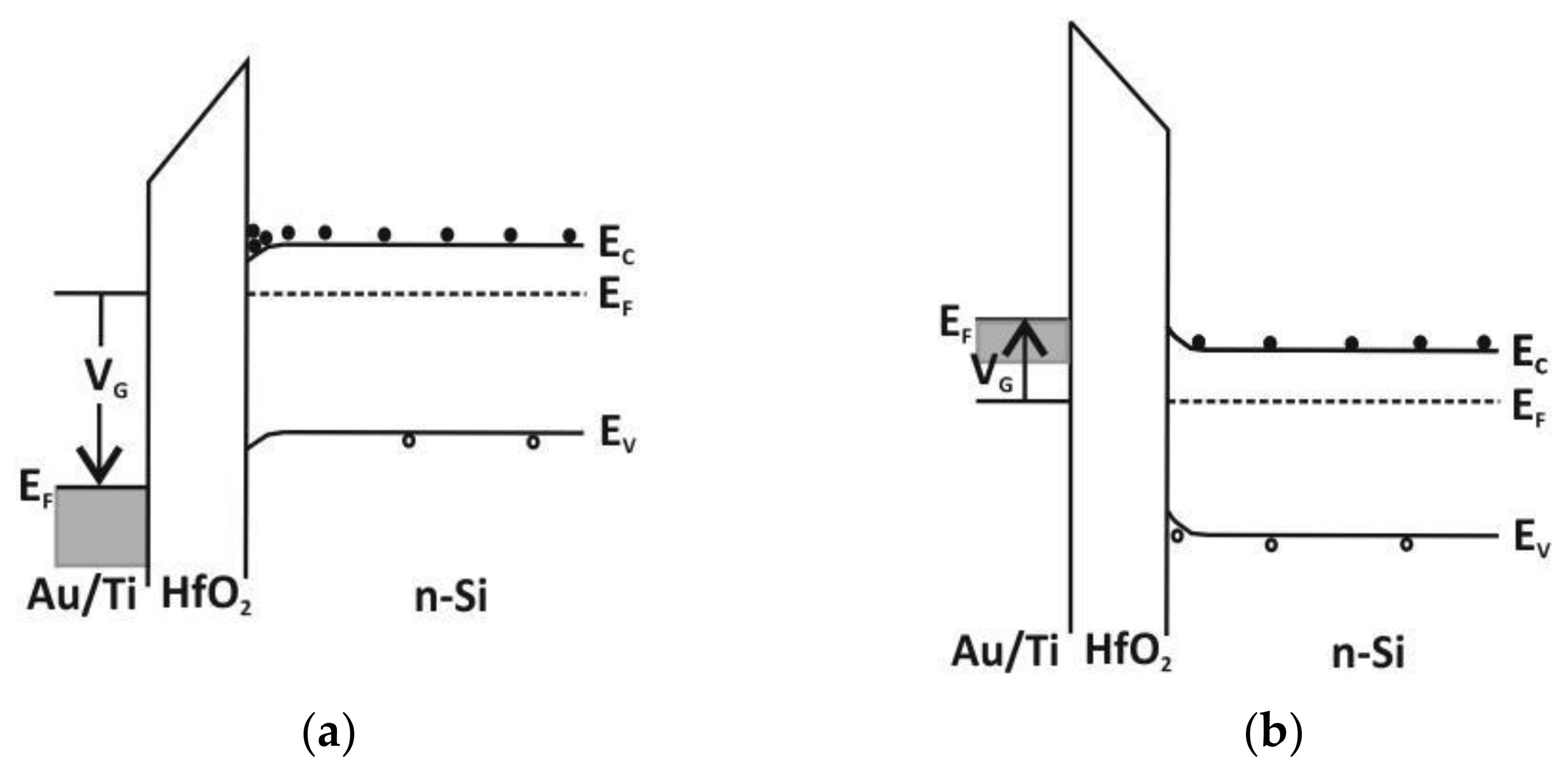
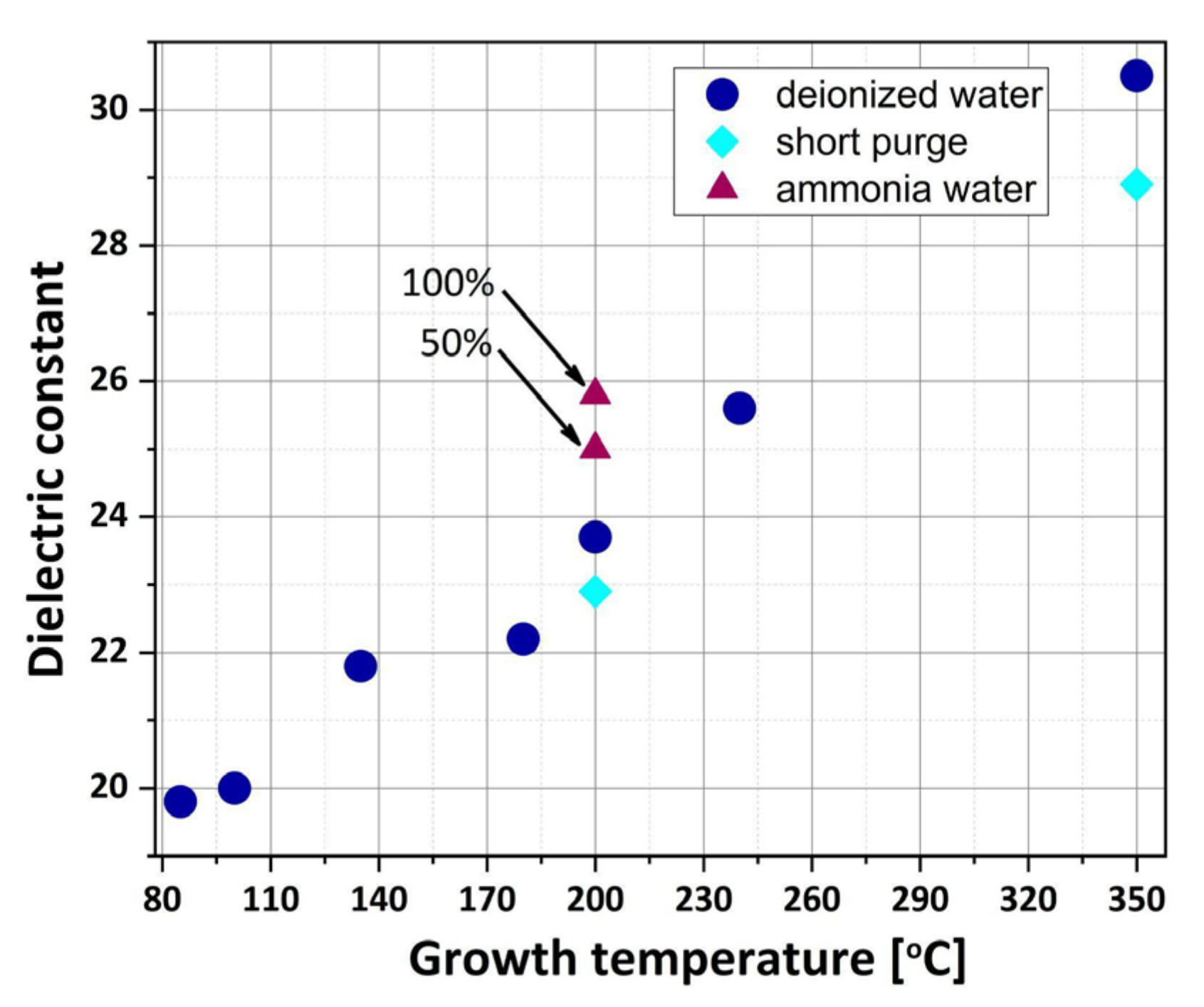
3.4. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robertson, J. High dielectric constant oxides. Eur. Phys. J. Appl. Phys. 2004, 28, 265. [Google Scholar] [CrossRef]
- Robertson, J. Band offsets of wide-band-gap oxides and implications for future electronic devices. J. Vac. Sci. Technol. 2000, B18, 1785. [Google Scholar] [CrossRef]
- Weber, M.J. Handbook of Optical Materials; CRC: Boca Raton, FL, USA, 2003; ISBN 0-8493-3512-4. [Google Scholar]
- Lee, B.-H.; Kang, L.; Nieh, R.; Lee, J.C. Thermal stability and electrical characteristics of ultrathin hafnium oxide gate dielectric reoxidized with rapid thermal annealing. Appl. Phys. Lett. 2000, 76, 1926. [Google Scholar] [CrossRef]
- Abromavičius, G.; Kičas, S.; Buzelis, R. High temperature annealing effects on spectral, microstructural and laser damage resistance properties of sputtered HfO2 and HfO2-SiO2 mixture-based UV mirrors. Opt. Mater. 2019, 95, 109245. [Google Scholar] [CrossRef]
- Ibègazéne, H.; Aplérine, S.; Diot, C. Yttria-stabilized hafnia-zirconia thermal barrier coatings: The influence of hafnia addition on TBC structure and high-temperature behaviour. J. Mater. Sci. 1995, 30, 938. [Google Scholar] [CrossRef]
- Kashir, A.; Oh, S.; Hwang, H. Defect engineering to achieve wake-up free HfO2-based ferroelectrics. Adv. Eng. Mater. 2021, 23, 2000791. [Google Scholar] [CrossRef]
- Waldorf, A.J.; Dobrowolski, J.A.; Sullican, B.T.; Plante, L.M. Optical coatings deposited by reactive ion plating. Appl. Opt. 1993, 32, 5583. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yang, D.; Yang, W.; Chen, X.; Zhao, H.; Hou, J.; Yang, P. Structure and optical properties of HfO2 films on Si (100) substrates prepared by ALD at different temperatures. Phys. B Condens. Matter 2020, 584, 412065. [Google Scholar] [CrossRef]
- Panchanan, S.; Maity, R.; Baishya, S.; Maity, N.P. Modeling, simulation and analysis of surface potential and threshold voltage: Application to high-K material HfO2 based FinFET. Silicon 2021, 13, 3271–3289. [Google Scholar] [CrossRef]
- Lange, S.; Kiisk, V.; Aarik, J.; Kirm, M.; Sildos, I. Luminescence of ZrO2 and HfO2 thin films implanted with Eu and Er ions. Phys. Status Solidi C 2007, 4, 938–941. [Google Scholar] [CrossRef]
- Wiatrowski, A.; Obstarczyk, A.; Mazur, M.; Kaczmarek, D.; Wojcieszak, D. Characterization of HfO2 optical coatings deposited by MF magnetron sputtering. Coatings 2019, 9, 106. [Google Scholar] [CrossRef]
- Fadel, M.; Azim, M.O.A.; Omer, O.A.; Basily, R.R. A study of some optical properties of hafnium dioxide (HfO2) thin films and their applications. Appl. Phys. A 1998, 66, 335–343. [Google Scholar] [CrossRef]
- Yadav, S.; Gedam, A.; Tirkey, S. A dielectric modulated biosensor for SARS-CoV-2. IEEE Sens. J. 2020, 21, 14483–14490. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition chemistry: Recent developments and future challenges. Angew. Chem. Int. Ed. 2003, 42, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 2. [Google Scholar] [CrossRef]
- Kukli, K.; Aarik, J.; Uustare, T.; Lu, J.; Ritala, M.; Aidla, A.; Pung, L.; Hårsta, A.; Leskelä, M.; Kikas, A.; et al. Engineering structure and properties of hafnium oxide films by atomic layer deposition temperature. Thin Solid Film. 2005, 479, 1–11. [Google Scholar] [CrossRef]
- Kukli, K.; Ritala, M.; Sajavaara, T.; Keinonen, J.; Leskelä, M. Comparison of hafnium oxide films grown by atomic layer deposition from iodide and chloride precursors. Thin Solid Film. 2002, 416, 72–79. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Kiisler, A.A.; Uustare, T.; Sammelselg, V. Influence of substrate temperature on atomic layer growth and properties of HfO2 thin films. Thin Solid Film. 1999, 340, 110–116. [Google Scholar] [CrossRef]
- Aarik, L.; Arroval, T.; Mändar, H.; Rammula, R.; Aarik, J. Influence of oxygen precursors on atomic layer deposition of HfO2 and hafnium-titanium oxide films: Comparison of O3-and H2O-based processes. Appl. Surf. Sci. 2020, 530, 147229. [Google Scholar] [CrossRef]
- Alles, H.; Aarik, J.; Aidla, A.; Fay, A.; Kozlova, J.; Niilisk, A.; Pärs, M.; Rähn, M.; Wiesner, M.; Hakonen, P.; et al. Atomic layer deposition of HfO2 on graphene from HfCl4 and H2O. Open Phys. 2011, 9, 319–324. [Google Scholar] [CrossRef]
- Spiga, S.; Wiemer, C.; Tallarida, G.; Scarel, G.; Ferrari, S.; Seguini, G.; Fanciulli, M. Effects of the oxygen precursor on the electrical and structural properties of HfO2 films grown by atomic layer deposition on Ge. Appl. Phys. Lett. 2005, 87, 112904. [Google Scholar] [CrossRef]
- Mändar, H.; Rammula, R.; Aidla, A.; Aarik, J. Atomic layer deposition of epitaxial HfO2 thin films on r-cut sapphire. J. Mater. Res. 2013, 28, 1680–1686. [Google Scholar] [CrossRef]
- Hausmann, D.M.; Kim, E.; Becker, J.; Gordon, R.G. Atomic layer deposition of hafnium and zirconium oxides using metal amide precursors. Chem. Mater. 2002, 14, 4350–4358. [Google Scholar] [CrossRef]
- Bradley, D.C. Thomas. IMJ Chem. Soc. 1960, 225, 3857. [Google Scholar] [CrossRef]
- Tsyganova, E.I.; Dyagileva, L.M.; Aleksandrov, Y.A. Thermal stability of zirconium organic derivatives with ligands of different nature. Russ. J. Chem. 1999, 69, 1532. [Google Scholar]
- Cardin, D.J.; Lappert, M.F.; Raston, C.L. Chemistry of Organo-Zirconium and-Hafnium Compounds; Ellis Horwood Limited: West Sussex, UK, 1986. [Google Scholar]
- Gieraltowska, S.; Wachnicki, L.; Witkowski, B.S.; Godlewski, M.; Guziewicz, E. Atomic layer deposition grown composite dielectric oxides and ZnO for transparent electronic applications. Thin Solid Film. 2012, 520, 4694–4697. [Google Scholar] [CrossRef]
- Gieraltowska, S.; Wachnicki, L.; Witkowski, B.S.; Mroczynski, R.; Dluzewski, P.; Godlewski, M. Characterization of dielectric layers grown at low temperature by atomic layer deposition. Thin Solid Film. 2015, 577, 97–102. [Google Scholar] [CrossRef]
- Gieraltowska, S.; Wachnicki, L.; Witkowski, B.S.; Guziewicz, E.; Godlewski, M. Thin Films of High-k Oxides and ZnO for Transparent Electronic Devices. Chem. Vap. Depos. 2013, 19, 213–220. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Film. 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Shang, G.; Peacock, P.W.; Robertson, J. Stability and band offsets of nitrogenated high-dielectric-constant gate oxides. Appl. Phys. Lett. 2004, 84, 106–108. [Google Scholar] [CrossRef]
- Wachnicki, L.; Gieraltowska, S.; Witkowski, B.S.; Godlewski, M.; Guziewicz, E. Growth and characterization of Ti-based films obtained from two selected precursors: H2O, TiCl4, Ti(N(CH3)2)4 or Al2(CH3)6 by the ALD method. Mater. Sci. Semicond. Process. 2022, 148, 106792. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Publishing: Boston, MA, USA, 1956. [Google Scholar]
- Wilk, G.D.; Wallace, R.M.; Anthony, J. High-κ gate dielectrics: Current status and materials properties considerations. J. Appl. Phys. 2001, 89, 5243–5275. [Google Scholar] [CrossRef]
- Sze, S.M.; Li, Y.; Ng, K.K. Physics of Semiconductor Devices, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Chapter 4. [Google Scholar]
- Jang, W.; Kim, H.; Kweon, Y.; Jung, C.; Cho, H.; Shin, S.; Kim, H.; Lim, K.; Jeon, H.; Lim, H. Remote plasma atomic layer deposition of silicon nitride with bis (dimethylaminomethyl-silyl) trimethylsilyl amine and N2 plasma for gate spacer. J. Vac. Sci. Technol. A Vac. Surf. Film. 2018, 36, 031514. [Google Scholar] [CrossRef]
- Kim, B.; Lee, N.; Park, S.; Park, T.; Song, J.; Han, S.; Park, H.; Lee, D.; Kim, H.; Jeon, H. Atomic layer deposition of titanium dioxide films using a metal organic precursor (C12H23N3Ti) and H2O (DI water). J. Alloys Compd. 2021, 857, 157931. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-temperature Al2O3 atomic layer deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Juppo, M.; Rahtu, A.; Ritala, M.; Leskelä, M. In situ mass spectrometry study on surface reactions in atomic layer deposition of Al2O3 thin films from trimethylaluminum and water. Langmuir 2000, 16, 4034–4039. [Google Scholar] [CrossRef]
- Kirm, M.; Aarik, J.; Jürgens, M.; Sildos, I. Thin films of HfO2 and ZrO2 as potential scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A 2005, 537, 251–255. [Google Scholar] [CrossRef]
- Gritsenko, V.A.; Islamov, D.R.; Perevalov, T.V.; Aliev, V.S.; Yelisseyev, A.P.; Lomonova, E.E.; Pustovarov, V.A.; Chin, A. Oxygen vacancy in hafnia as a blue luminescence center and a trap of charge carriers. J. Phys. Chem. C 2016, 120, 19980–19986. [Google Scholar] [CrossRef]
- Ratajczak, R.; Stonert, A.; Guziewicz, E.; Gierałtowska, S.; Krajewski, T.; Luka, G.; Wachnicki, L.; Witkowski, B.; Godlewski, M. RBS/channeling analysis of zinc oxide films grown at low temperature by atomic layer deposition. Acta Phys. Pol. A 2013, 123, 899–903. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Mändar, H.; Sammelselg, V.; Uustare, T. Texture development in nanocrystalline hafnium dioxide thin films grown by atomic layer deposition. J. Cryst. Growth 2000, 220, 105–113. [Google Scholar] [CrossRef]
- Jung, H.S.; Jeon, S.H.; Kim, H.K.; Yu, I.H.; Lee, S.Y.; Lee, J.; Chung, Y.J.; Cho, D.Y.; Lee, N.I.; Park, T.J. The Impact of Carbon Concentration on the Crystalline Phase and Dielectric Constant of Atomic Layer Deposited HfO2 Films on Ge Substrate. ECS J. Solid State Sci. Technol. 2012, 1, N33. [Google Scholar] [CrossRef]
- Bocquillon, G.; Susse, C.; Vodar, B. Allotropie de l’oxyde d’hafnium sous haute pression. Rev. Int. Hautes Temp. Refract 1968, 5, 247–251. [Google Scholar]
- Ramana, C.V.; Bharathi, K.K.; Garcia, A.; Campbell, A.L. Growth behavior, lattice expansion, strain, and surface morphology of nanocrystalline, monoclinic HfO2 thin films. J. Phys. Chem. C 2012, 116, 9955–9960. [Google Scholar] [CrossRef]
- Geller, S.; Corenzwit, E. Hafnium oxide, HfO2 (monoclinic). Anal. Chem. 1953, 25, 1774. [Google Scholar] [CrossRef]
- Kalidindi, N.R.; Manciu, F.S.; Ramana, C.V. Crystal structure, phase, and electrical conductivity of nanocrystalline W0.95Ti0.05O3 thin films. ACS Appl. Mater. Interfaces 2011, 3, 863–868. [Google Scholar] [CrossRef]
- Aguirre, B.; Vemuri, R.S.; Zubia, D.; Engelhard, M.H.; Shutthananadan, V.; Bharathi, K.K.; Ramana, C.V. Growth, microstructure and electrical properties of sputter-deposited hafnium oxide (HfO2) thin films grown using a HfO2 ceramic target. Appl. Surf. Sci. 2011, 257, 2197–2202. [Google Scholar] [CrossRef]
- Niinistö, J.; Mäntymäki, M.; Kukli, K.; Costelle, L.; Puukilainen, E.; Ritala, M.; Leskelä, M. Growth and phase stabilization of HfO2 thin films by ALD using novel precursors. J. Cryst. Growth 2010, 312, 245–249. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Luo, X.; Li, Y.; Wuu, D.S.; He, K.; Feng, Z.C. Effects of growth temperature and thickness on structure and optical properties of Ga2O3 films grown by pulsed laser deposition. Superlattices Microstruct. 2019, 131, 21–29. [Google Scholar] [CrossRef]
- Fang, S.J.; Haplepete, S.; Chen, W.; Helms, C.R.; Edwards, H. Analyzing atomic force microscopy images using spectral methods. J. Appl. Phys. 1997, 82, 5891–5898. [Google Scholar] [CrossRef]
- Guziewicz, E.; Przezdziecka, E.; Snigurenko, D.; Jarosz, D.; Witkowski, B.S.; Dluzewski, P.; Paszkowicz, W. Abundant acceptor emission from nitrogen-doped ZnO films prepared by atomic layer deposition under oxygen-rich conditions. ACS Appl. Mater. Interfaces 2017, 9, 26143–26150. [Google Scholar] [CrossRef]
- Nayak, M.; Tseng, T.Y. Dielectric tunability of barium strontium titanate films prepared by a sol–gel method. Thin Solid Film. 2002, 408, 194–199. [Google Scholar] [CrossRef]
- Biercuk, M.J.; Monsma, D.J.; Marcus, C.M.; Becker, J.S.; Gordon, R.G. Low-temperature atomic-layer-deposition lift-off method for microelectronic and nanoelectronic applications. Appl. Phys. Lett. 2003, 83, 2405. [Google Scholar] [CrossRef]
- Bersuker, G.; Zeitzoff, P.; Brown, G.; Huff, H.R. Dielectrics for future transistors. Mater. Today 2004, 7, 26. [Google Scholar] [CrossRef]
- Wager, J.F. Transparent electronics. Science 2003, 300, 1245. [Google Scholar] [CrossRef] [PubMed]





| Growth Temperature [°C] | Changed Growth Condition * | O:Hf [atomic Ratio] | C [atom.%] | N [atom.%] |
|---|---|---|---|---|
| 85 | - | 2.03 | 6.5 | 4.5 |
| 100 | - | 2.03 | 6 | 4.5 |
| 135 | - | 1.94 | 6 | 4.5 |
| 180 | - | 1.94 | 6 | 4.5 |
| 200 | - | 1.94 | 6 | 4.5 |
| short purge | 1.91 | 5.8 | 4 | |
| 50% H2O:NH3 | 1.94 | 6 | 5 | |
| 100% H2O:NH3 | 1.94 | 6 | 5 | |
| 240 | - | 1.94 | 6 | 4.5 |
| 350 | - | 2.00 | 6.5 | 4.8 |
| short purge | 1.86 | 6 | 4.5 |
| Growth Temperature [°C] | Changed Growth Condition * | Particle Size on Surface [nm] | Crystals Size ± 4 34° (020) [nm] |
|---|---|---|---|
| 85 | - | 2 | - |
| 100 | - | 83 | 29 |
| 135 | - | 65 | 38 |
| 180 | - | 43 | 38 |
| 200 | - | 33 | 39 |
| short purge | 34 | 37 | |
| 50% H2O:NH3 | 38 | 37 | |
| 100% H2O:NH3 | 38 | 38 | |
| 240 | - | 30 | 40 |
| 350 | - | 17 | 39 |
| short purge | 27 | 39.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gieraltowska, S.; Wachnicki, L.; Dluzewski, P.; Witkowski, B.S.; Godlewski, M.; Guziewicz, E. Atomic Layer Deposition of HfO2 Films Using TDMAH and Water or Ammonia Water. Materials 2023, 16, 4077. https://doi.org/10.3390/ma16114077
Gieraltowska S, Wachnicki L, Dluzewski P, Witkowski BS, Godlewski M, Guziewicz E. Atomic Layer Deposition of HfO2 Films Using TDMAH and Water or Ammonia Water. Materials. 2023; 16(11):4077. https://doi.org/10.3390/ma16114077
Chicago/Turabian StyleGieraltowska, Sylwia, Lukasz Wachnicki, Piotr Dluzewski, Bartlomiej S. Witkowski, Marek Godlewski, and Elzbieta Guziewicz. 2023. "Atomic Layer Deposition of HfO2 Films Using TDMAH and Water or Ammonia Water" Materials 16, no. 11: 4077. https://doi.org/10.3390/ma16114077
APA StyleGieraltowska, S., Wachnicki, L., Dluzewski, P., Witkowski, B. S., Godlewski, M., & Guziewicz, E. (2023). Atomic Layer Deposition of HfO2 Films Using TDMAH and Water or Ammonia Water. Materials, 16(11), 4077. https://doi.org/10.3390/ma16114077








