The Effect of 10% Carbamide Peroxide Dental Bleaching on the Physical Properties of Invisalign Aligners: An In Vitro Study
Abstract
1. Introduction
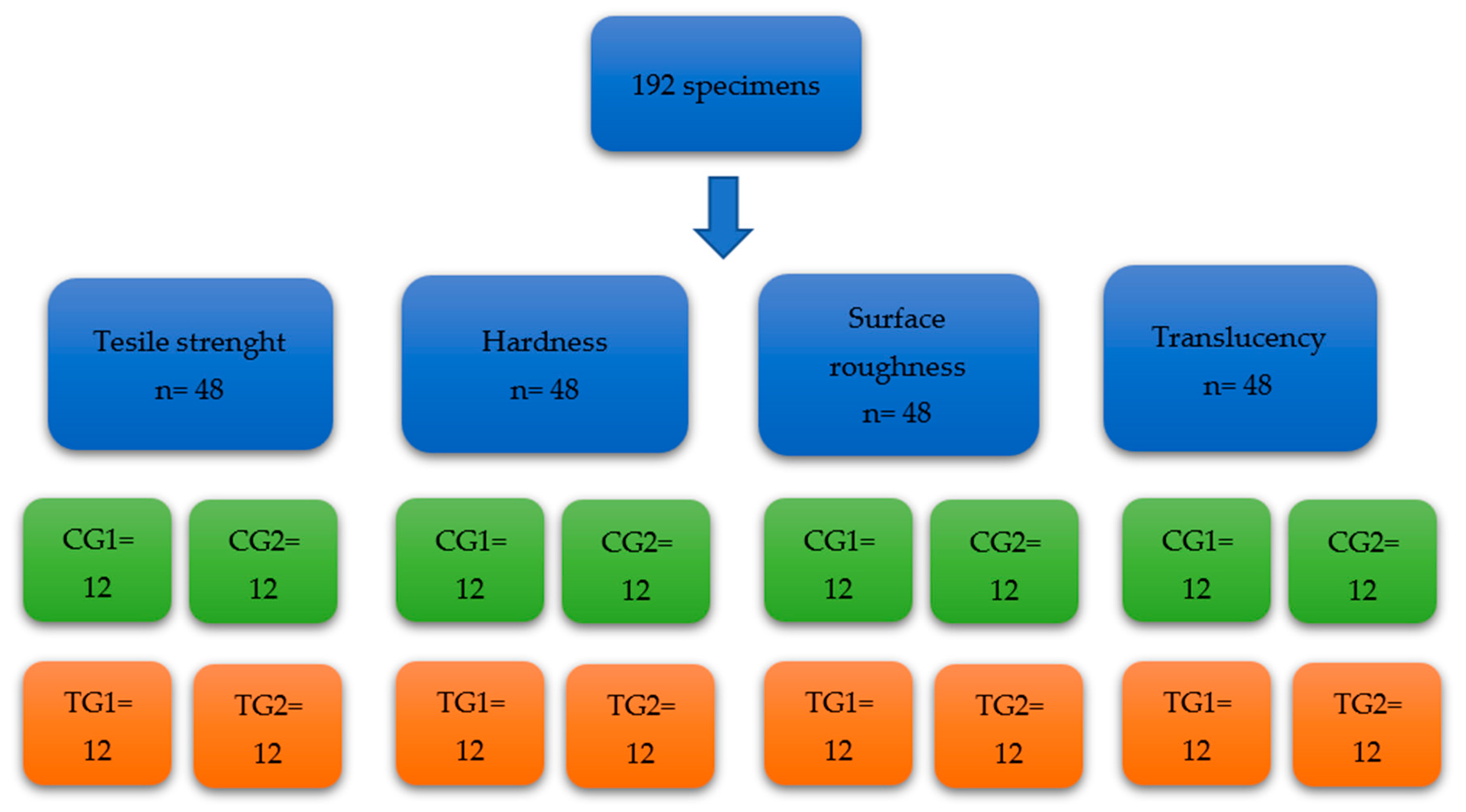
2. Materials and Methods
2.1. Protocol
2.1.1. Tensile Strength Testing
2.1.2. Hardness Testing
2.1.3. Surface Roughness Testing
2.1.4. Translucency Testing
2.2. Statistical Analysis
3. Results
3.1. Tensile Strength
3.2. Hardness
3.3. Surface Roughness
3.4. Translucency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putrino, A.; Barbato, E.; Galluccio, G. Clear Aligners: Between evolution and efficiency—A scoping review. Int. J. Environ. Res. Public Health 2021, 18, 2870. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, G.M.; Mapelli, A.; Maspero, C.; Santaniello, T.; Serafin, M.; Farronato, M.; Caprioglio, A. Direct 3D printing of clear orthodontic aligners: Current state and future possibilities. Materials 2021, 14, 1799. [Google Scholar] [CrossRef] [PubMed]
- Rossini, G.; Parrini, S.; Castroflorio, T.; Deregibus, A.; Debernardi, C.L. Efficacy of clear aligners in controlling orthodontic tooth movement: A systematic review. Angle Orthod. 2015, 85, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Weir, T. Clear aligners in orthodontic treatment. Aust. Dent. J. 2017, 62 (Suppl. 1), 58–62. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.T. Orthodontic clear aligner treatment. Semin. Orthod. 2017, 23, 83–89. [Google Scholar] [CrossRef]
- Kuncio, D.A. Invisalign: Current guidelines for effective treatment. N. Y. State Dent. J. 2014, 80, 11–14. [Google Scholar]
- Borda, A.F.; Garfinkle, J.S.; Covell, D.A.; Wang, M.; Doyle, L.; Sedgley, C.M. Outcome assessment of orthodontic clear aligner vs fixed appliance treatment in a teenage population with mild malocclusions. Angle Orthod. 2020, 90, 485–490. [Google Scholar] [CrossRef]
- Miller, K.B.; McGorray, S.P.; Womack, R.; Quintero, J.C.; Perelmuter, M.; Gibson, J.; Dolan, T.A.; Wheeler, T.T. A comparison of treatment impacts between Invisalign aligner and fixed appliance therapy during the first week of treatment. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 302.e1–302.e9. [Google Scholar] [CrossRef]
- Pacheco-Pereira, C.; Brandelli, J.; Flores-Mir, C. Patient satisfaction and quality of life changes after Invisalign treatment. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Alajmi, S.; Shaban, A.; Al-Azemi, R. Comparison of Short-Term Oral Impacts Experienced by Patients Treated with Invisalign or Conventional Fixed Orthodontic Appliances. Med. Princ. Pract. 2020, 29, 382–388. [Google Scholar] [CrossRef]
- Papadimitriou, A.; Mousoulea, S.; Gkantidis, N.; Kloukos, D. Clinical effectiveness of invisalign® orthodontic treatment: A systematic review. Prog. Orthod. 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Kankam, H.; Madari, S.; Sawh-Martinez, R.; Bruckman, K.C.; Steinbacher, D.M. Comparing outcomes in orthognathic surgery using clear aligners versus conventional fixed appliances. J. Craniofacial Surg. 2019, 30, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Sword, R.J.; Haywood, V.B. Teeth bleaching efficacy during clear aligner orthodontic treatment. Compend. Contin. Educ. Dent. 2020, 41, e11–e16. [Google Scholar] [PubMed]
- Slack, M.E.; Swift, E.J.; Rossouw, P.E.; Phillips, C. Tooth whitening in the orthodontic practice: A survey of orthodontists. Am. J. Orthod. Dentofac. Orthop. 2013, 143 (Suppl. 4), S64–S71. [Google Scholar] [CrossRef]
- Haywood, V.B.; Heymann, H.O. Nightguard vital bleaching. Quintessence Int. 1989, 20, 173–176. [Google Scholar]
- Mounika, A.; Mandava, J.; Roopesh, B.; Karri, G. Clinical evaluation of color change and tooth sensitivity with in-office and home bleaching treatments. Indian J. Dent. Res. 2018, 29, 423–427. [Google Scholar] [CrossRef]
- Chemin, K.; Rezende, M.; Milan, F.M.; Dantas, T.B.; Gomes, K.D.N.; Kossatz, S. Clinical evaluation of 10% hydrogen peroxide on tooth sensitivity and effectiveness in at home dental bleaching. J. Contemp. Dent. Pract. 2018, 19, 1376–1380. [Google Scholar] [CrossRef]
- Sutil, E.; da Silva, K.L.; Terra, R.M.O.; Burey, A.; Rezende, M.; Reis, A.; Loguercio, A.D. Effectiveness and adverse effects of at-home dental bleaching with 37% versus 10% carbamide peroxide: A randomized, blind clinical trial. J. Esthet. Restor. Dent. 2022, 34, 313–321. [Google Scholar] [CrossRef]
- Oliverio, T.; Cremonini, F.; Lombardo, L.; Siciliani, G. Tooth whitening in association with clear aligner treatment. J. Clin. Orthod. 2019, 53, 508–517. [Google Scholar]
- Levrini, L.; Paracchini, L.; Bakaj, R.; Diaconu, A.; Cortese, S. Dental bleaching during orthodontic treatment with aligners. Int. J. Esthet. Dent. 2020, 15, 44–54. [Google Scholar]
- Seleem, D.; Dadjoo, S.; Ha, A.; Santos, C.; Mirfarsi, S.; Matsumura-Lem, K.; Lazarchik, D. Effect of 10% carbamide peroxide on tooth shade, plaque index and gingival index during invisalign treatment. Dent. J. 2021, 9, 48. [Google Scholar] [CrossRef]
- Dos Santos, P.R.; Meneghim, M.C.; Ambrosano, G.M.B.; Filho, M.V.; Vedovello, S.A.S. Influence of quality of life, self-perception, and self-esteem on orthodontic treatment need. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 143–147. [Google Scholar] [CrossRef]
- Martina, S.; Rongo, R.; Bucci, R.; Razionale, A.V.; Valletta, R.; D’Antò, V. In vitro cytotoxicity of different thermoplastic materials for clear aligners. Angle Orthod. 2019, 89, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Basting, R.; Júnior, A.L.R.; Serra, M.C. The effect of 10% carbamide peroxide bleaching material on microhardness of sound and demineralized enamel and dentin in situ. Oper Dent. 2001, 26, 531–539. [Google Scholar] [PubMed]
- Lopes, G.C.; Bonissoni, L.; Baratieri, L.N.; Vieira, L.C.C.; Monteiro, S. Effect of bleaching agents on the hardness and morphology of enamel. J. Esthet. Restor. Dent. 2002, 14, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Furlan, I.S.; Bridi, E.C.; Amaral, F.L.B.D.; França, F.M.G.; Turssi, C.P.; Basting, R. Effect of high- or low-concentration bleaching agents containing calcium and/or fluoride on enamel microhardness. Gen. Dent. 2017, 65, 66–70. [Google Scholar]
- Karimi, Z.; Saoui, H.; Sakout, M.; Abdallaoui, F. Effect of Vital Bleaching on Micromorphology of Enamel Surface: An in Vitro Study. Prim. Dent. J. 2021, 10, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Zanolla, J.; Marques, A.; Costa, D.; Souza, A.; Coutinho, M. Influence of tooth bleaching on dental enamel microhardness: A systematic review and meta-analysis. Aust. Dent. J. 2017, 62, 276–282. [Google Scholar] [CrossRef]
- Bradley, T.G.; Teske, L.; Eliades, G.; Zinelis, S.; Eliades, T. Do the mechanical and chemical properties of InvisalignTM appliances change after use? A retrieval analysis. Eur. J. Orthod. 2016, 38, 27–31. [Google Scholar] [CrossRef]
- Condo, R.; Pazzini, L.; Cerroni, L.; Pasquantonio, G.; Lagana, G.; Pecora, A.; Mussi, V.; Rinaldi, A.; Mecheri, B.; Licoccia, S.; et al. Mechanical properties of “two generations” of teeth aligners: Change analysis during oral permanence. Dent. Mater J. 2018, 37, 835–842. [Google Scholar] [CrossRef]
- Fang, D.; Li, F.; Zhang, Y.; Bai, Y.; Wu, B.M. Changes in mechanical properties, surface morphology, structure, and composition of Invisalign material in the oral environment. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Westrich, R.M. Use of the Scanning Electron Microscope in Microhardness Testing of High-Hardness Materials; American Society for Testing and Materials International: West Conshohocken, PA, USA, 1985. [Google Scholar]
- Shahdad, S.A.; McCabe, J.F.; Bull, S.; Rusby, S.; Wassell, R.W. Hardness measured with traditional Vickers and Martens hardness methods. Dent. Mater. 2007, 23, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Iliadi, A.; Enzler, V.; Polychronis, G.; Peltomaki, T.; Zinelis, S.; Eliades, T. Effect of cleansers on the composition and mechanical properties of orthodontic aligners in vitro. Prog. Orthod. 2022, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.K.; Cantele, A.; Polychronis, G.; Zinelis, S.; Eliades, T. Changes in Roughness and Mechanical Properties of Invisalign® Appliances after One- and Two-Weeks Use. Materials 2019, 12, 2406. [Google Scholar] [CrossRef] [PubMed]
- Daniele, V.; Macera, L.; Taglieri, G.; Spera, L.; Marzo, G.; Quinzi, V. Color Stability, Chemico-Physical and Optical Features of the Most Common PETG and PU Based Orthodontic Aligners for Clear Aligner Therapy. Polymers 2021, 14, 14. [Google Scholar] [CrossRef]
- Matis, B.A.; Cochran, M.A.; Eckert, G. Review of the effectiveness of various tooth whitening systems. Oper Dent. 2009, 34, 230–235. [Google Scholar] [CrossRef]
- Bernardon, J.K.; Ferrari, P.; Baratieri, L.N.; Rauber, G.B. Comparison of treatment time versus patient satisfaction in at-home and in-office tooth bleaching therapy. J. Prosthet. Dent. 2015, 114, 826–830. [Google Scholar] [CrossRef]





| Testing | Tensile Strength CG1 | Tensile Strength CG2 | Tensile Strength TG1 | Tensile Strength TG2 |
| Mean (±S.D *) | 14.04 ± 1.83 | 13.26 ± 1.48 | 13.62 ± 1.9 | 12.64 ± 1.5 |
| Median | 14.14 | 13.28 | 12.89 | 12.59 |
| 95% Confidence interval (LB, UB) | 12.87, 15.20 | 12.31, 14.20 | 12.41, 14.83 | 11.68, 13.59 |
| Testing | Hardness CG1 | Hardness CG2 | Hardness TG1 | Hardness TG2 |
| Mean (±S.D) | 6.93 ± 1.92 | 5.91 ± 0.78 | 4.43 ± 0.86 | 2.2 ± 0.29 |
| Median | 6.13 | 5.8 | 4.45 | 2.13 |
| 95% Confidence interval (LB, UB) | 5.70, 8.15 | 5.41, 6.41 | 3.88, 4.99 | 2.01, 2.38 |
| Testing | Surface Roughness CG1 | Surface Roughness CG2 | Surface Roughness TG1 | Surface Roughness TG2 |
| Mean (±S.D) | 1.42 ± 0.39 | 1.71 ± 0.3 | 1.6 ± 0.32 | 1.93 ± 0.28 |
| Median | 1.45 | 1.67 | 1.7 | 1.93 |
| 95% Confidence interval (LB, UB) | 1.16, 1.67 | 1.52. 1.90 | 1.39. 1.81 | 1.75, 2.11 |
| Testing (External Surface) | Surface Roughness CG1 | Surface Roughness CG2 | Surface Roughness TG1 | Surface Roughness TG2 |
| Mean (±S.D) | 0.55 ± 0.14 | 0.63 ± 0.15 | 0.58 ± 0.12 | 0.68 ± 0.13 |
| Median | 0.51 | 0.57 | 0.55 | 0.65 |
| 95% Confidence interval (LB, UB) | 0.45, 0.64 | 0.53, 0.72 | 0.50, 0.65 | 0.59, 0.77 |
| Testing | Translucency CG1 | Translucency CG2 | Translucency TG1 | Translucency TG2 |
| Mean (±S.D) | 3.64 ± 1.39 | 3.64 ± 1.22 | 3.60 ± 1.53 | 3.8 ± 1.89 |
| Median | 3.62 | 3.55 | 3.71 | 3.78 |
| 95% Confidence interval (LB, UB) | 2.75, 4.52 | 2.87, 4.42 | 2.62, 4.57 | 2.60, 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khashashneh, M.; Ratnayake, J.; Choi, J.J.E.; Mei, L.; Lyons, K.; Brunton, P. The Effect of 10% Carbamide Peroxide Dental Bleaching on the Physical Properties of Invisalign Aligners: An In Vitro Study. Materials 2023, 16, 4125. https://doi.org/10.3390/ma16114125
Khashashneh M, Ratnayake J, Choi JJE, Mei L, Lyons K, Brunton P. The Effect of 10% Carbamide Peroxide Dental Bleaching on the Physical Properties of Invisalign Aligners: An In Vitro Study. Materials. 2023; 16(11):4125. https://doi.org/10.3390/ma16114125
Chicago/Turabian StyleKhashashneh, Majd, Jithendra Ratnayake, Joanne Jung Eun Choi, Li Mei, Karl Lyons, and Paul Brunton. 2023. "The Effect of 10% Carbamide Peroxide Dental Bleaching on the Physical Properties of Invisalign Aligners: An In Vitro Study" Materials 16, no. 11: 4125. https://doi.org/10.3390/ma16114125
APA StyleKhashashneh, M., Ratnayake, J., Choi, J. J. E., Mei, L., Lyons, K., & Brunton, P. (2023). The Effect of 10% Carbamide Peroxide Dental Bleaching on the Physical Properties of Invisalign Aligners: An In Vitro Study. Materials, 16(11), 4125. https://doi.org/10.3390/ma16114125








