Radionuclides’ Recovery from Seawater Using FIC and FIC A Sorbents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
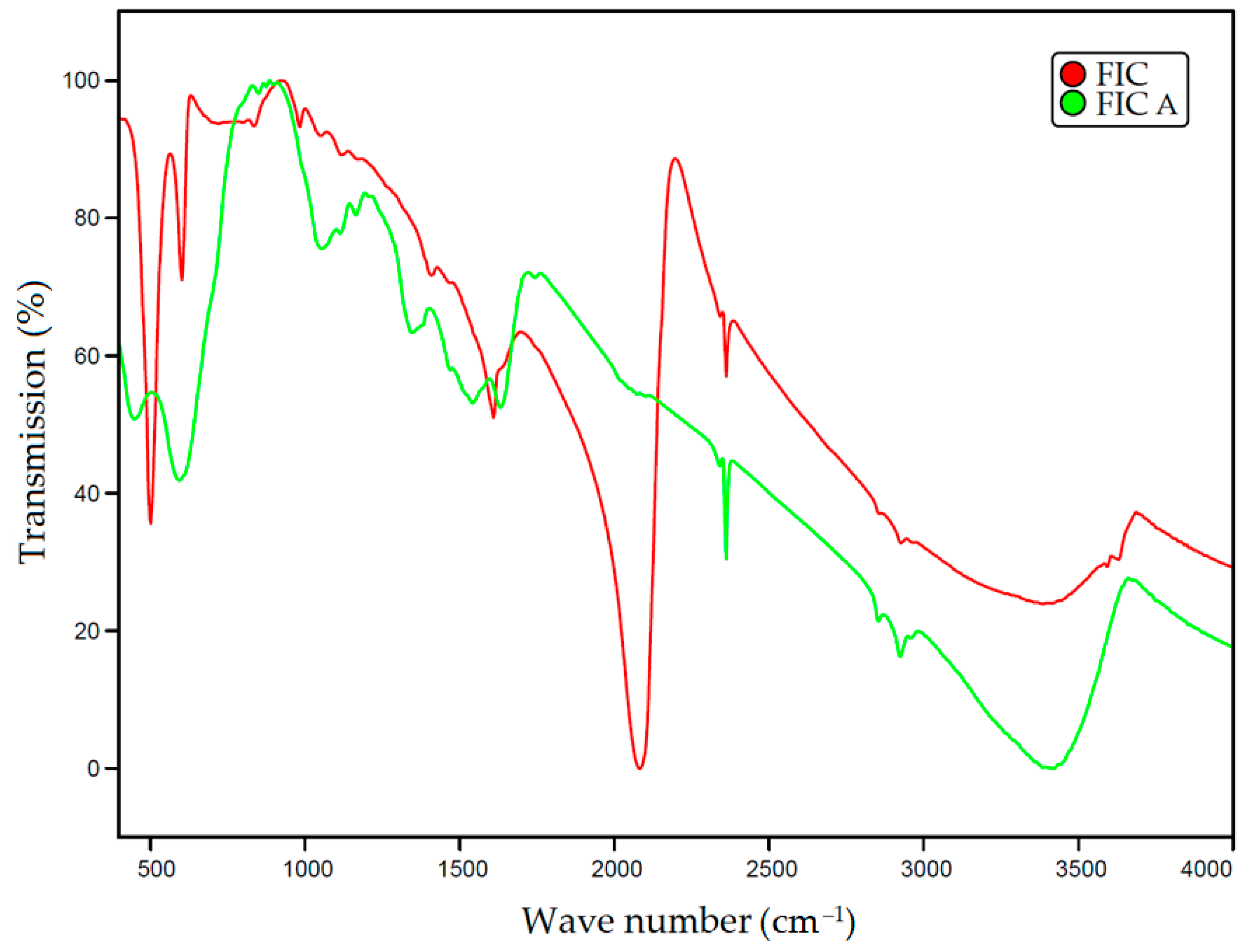
2.2. IR Spectroscopy of Sorbents
2.3. Sorption Laboratory Research
2.4. Determination of the Cesium, Phosphorus, and Beryllium Concentration in Solution and Quantitative Parameters of Sorption
2.5. Evaluation of the Sorption Efficiency
3. Results and Discussion
3.1. Distribution Coefficients of Cesium, Phosphorus, and Beryllium
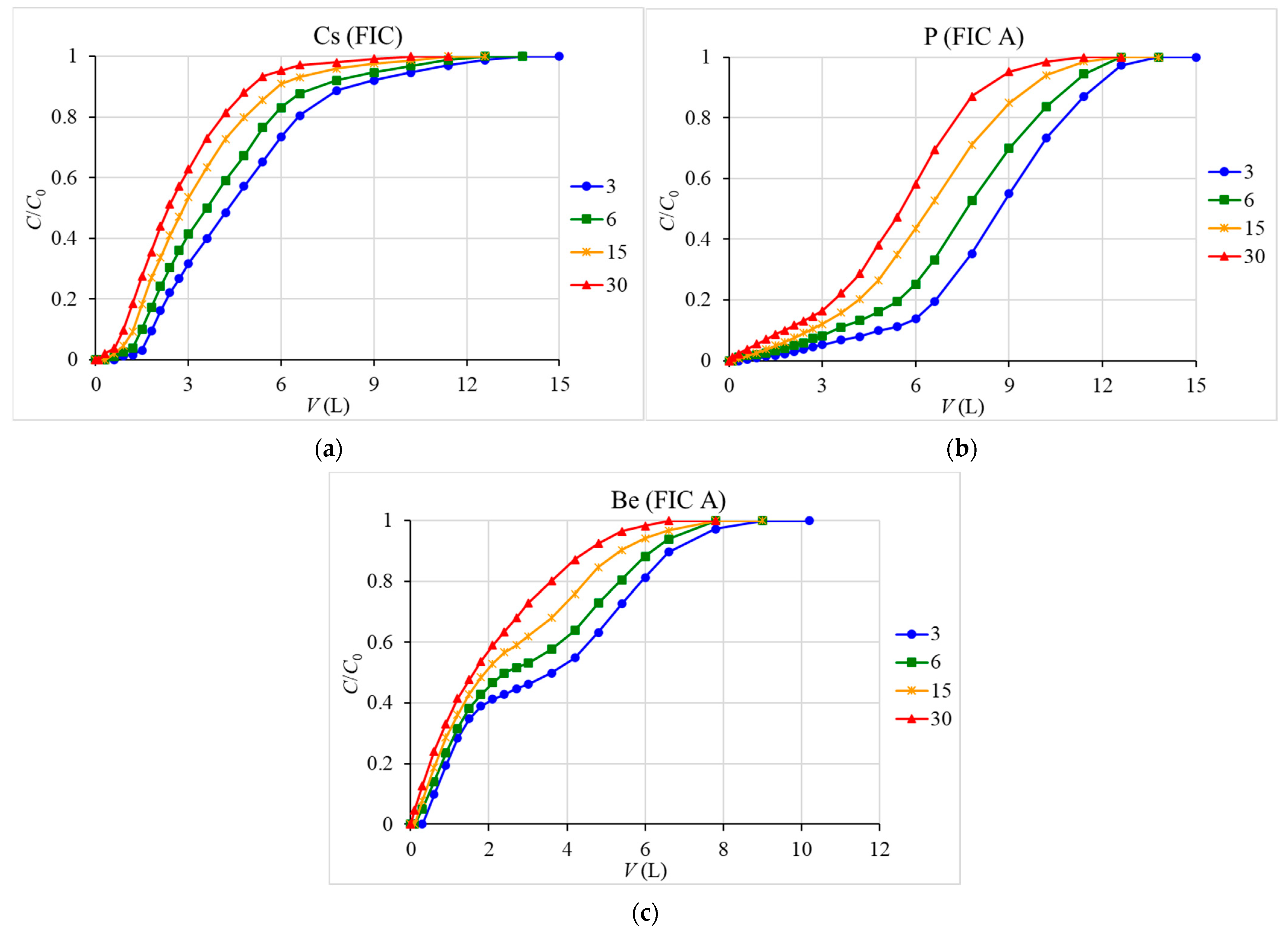
3.2. Sorption Dynamics
3.3. Sorption Kinetics
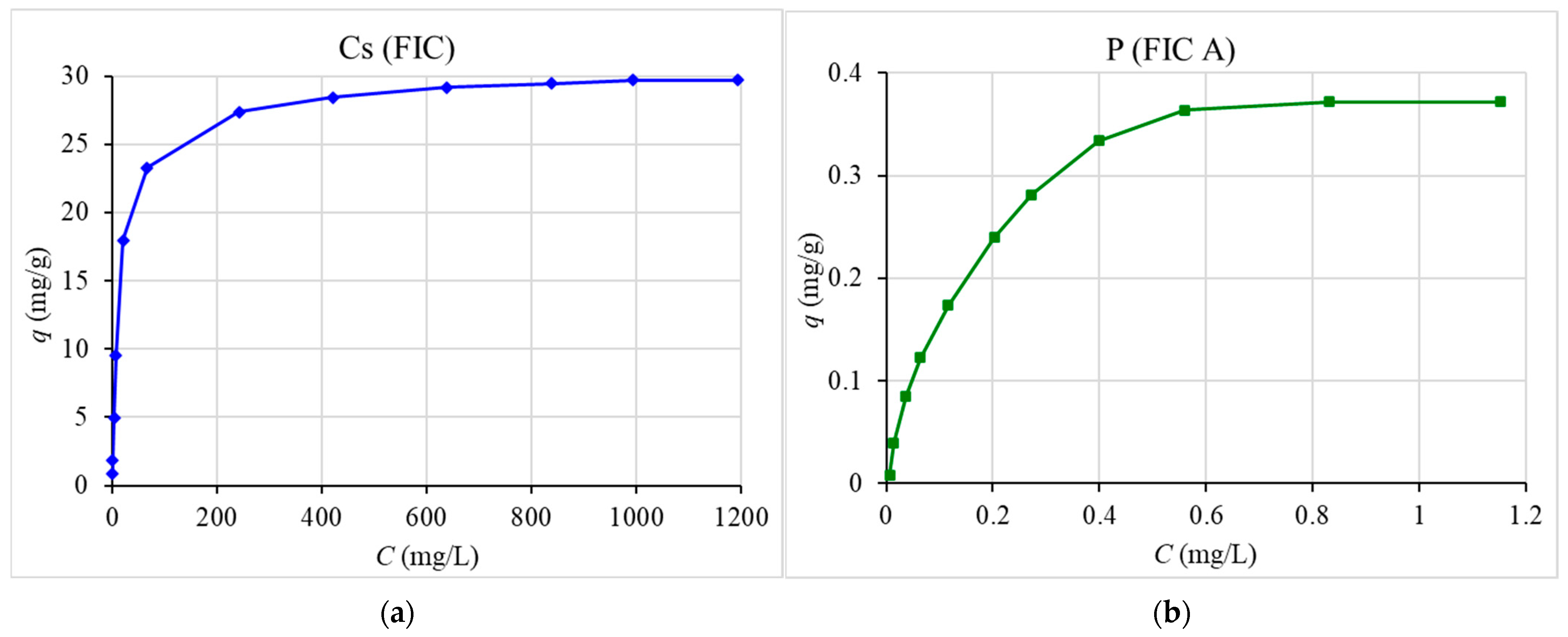
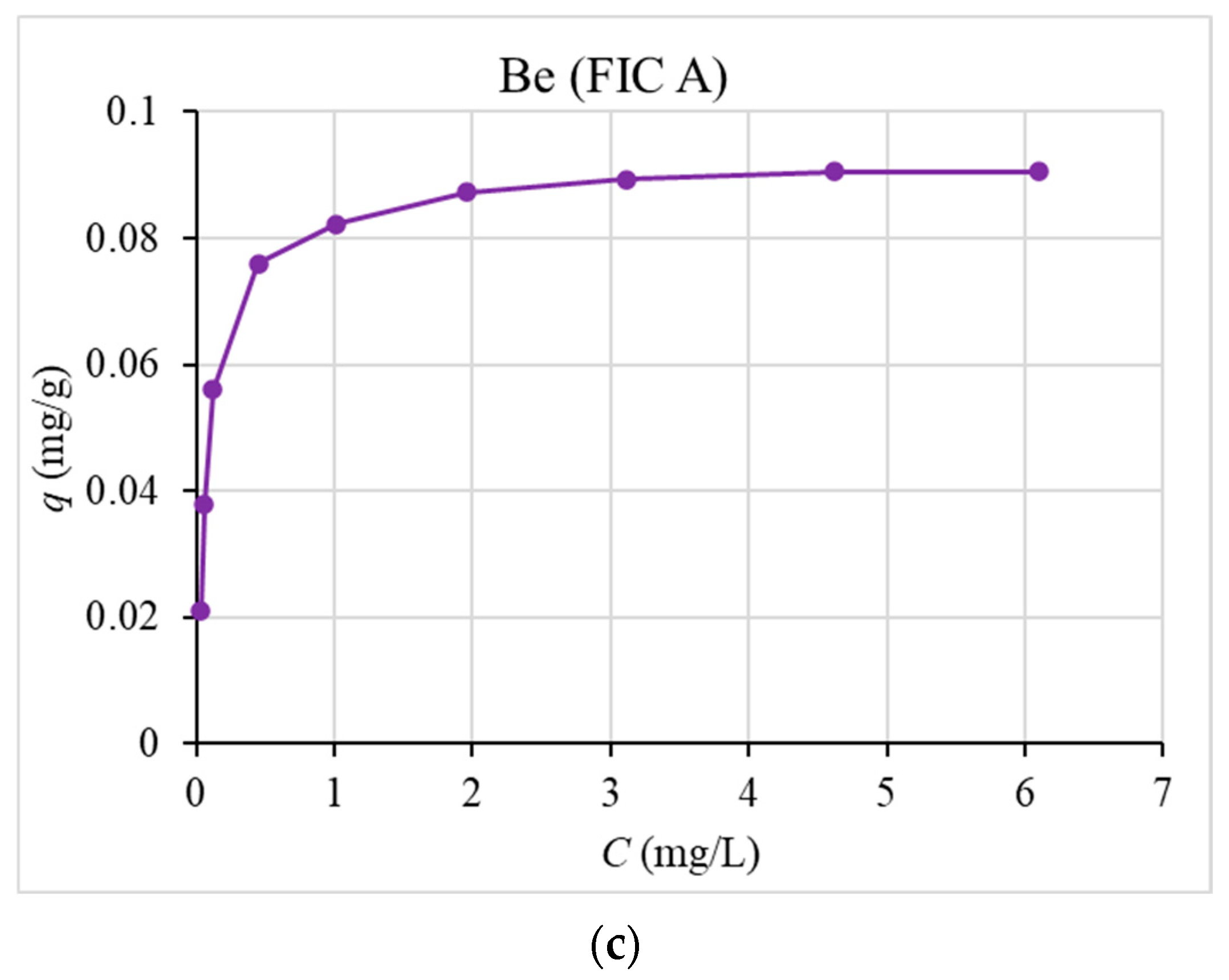
3.4. Sorption Isotherm
3.5. Sorption Efficiency
- Cesium on FIC sorbent by reaction Equation (2):
- Beryllium or lead on FIC A sorbent by reaction Equation (3):
- Thorium on FIC A sorbent by reaction Equation (4):
- Phosphorus or lead on FIC A sorbent by reaction Equation (5):
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMP | ammonium phosphomolybdate |
| DEC | dynamic exchange capacity |
| Fe-H | Fe-Hydrolyzed (sorbent based on Fe(OH)3 was obtained using pre-hydrolyzed PAN with precipitation of Fe(OH)3 with ammonia) |
| Fe-NH | Fe-Non-Hydrolyzed (sorbent based on Fe(OH)3 was obtained using non-hydrolyzed PAN and precipitation of Fe(OH)3 with ammonia) |
| Fe-SF | Fe-Sodium Ferrate (sorbent based on Fe(OH)3 was obtained using the prepared sodium ferrate) |
| FIC | activated carbon modified with iron(III) ferrocyanide |
| FIC A | activated FIC (activated carbon modified with iron(III) hydroxide, obtained by FIC sorbent treatment with sodium hydroxide solution) |
| FSS | ferrocyanide-silicate sorbent |
| PAN | polyacrylonitrile |
| RaDeCC | Radium Delayed Coincidence Counter |
| TDEC | total dynamic exchange capacity |
References
- Mansoorianfar, M.; Nabipour, H.; Pahlevani, F.; Zhao, Y.; Hussain, Z.; Hojjati-Najafabadi, A.; Hoang, H.Y.; Pei, R. Recent progress on adsorption of cadmium ions from water systems using metal-organic frameworks (MOFs) as an efficient class of porous materials. Environ. Res. 2022, 214, 114113. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Shahin, K.; Hojjati–Najafabadi, A.; Pei, R. MXene–laden bacteriophage: A new antibacterial candidate to control bacterial contamination in water. Chemosphere 2022, 290, 133383. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Yarusova, S.B.; Ivanets, A.I.; Papynov, E.K.; Belov, A.A.; Azon, S.A.; Buravlev, I.Y.; Panasenko, A.E.; Zadorozhny, P.A.; Mayorov, V.Y.; et al. Synthesis and spark plasma sintering of solid-state matrices based on calcium silicate for 60Co immobilization. J. Alloy Compd. 2022, 912, 165233. [Google Scholar] [CrossRef]
- Dran’kov, A.; Shichalin, O.; Papynov, E.; Nomerovskii, A.; Mayorov, V.; Pechnikov, V.; Ivanets, A.; Buravlev, I.; Yarusova, S.; Zavjalov, A.; et al. Hydrothermal synthesis, structure and sorption performance to cesium and strontium ions of nanostructured magnetic zeolite composites. Nucl. Eng. Technol. 2022, 54, 1991–2003. [Google Scholar] [CrossRef]
- Panasenko, A.E.; Shichalin, O.O.; Yarusova, S.B.; Ivanets, A.I.; Belov, A.A.; Dran’kov, A.N.; Azon, S.A.; Fedorets, A.N.; Buravlev, I.Y.; Mayorov, V.Y.; et al. A novel approach for rice straw agricultural waste utilization: Synthesis of solid aluminosilicate matrices for cesium immobilization. Nucl. Eng. Technol. 2022, 54, 3250–3259. [Google Scholar] [CrossRef]
- Takata, H. Environmental recovery from 137Cs contamination in Japanese coastal waters shown by comparison of temporal distributions with European seas. J. Environ. Radioact. 2022, 251–252, 106961. [Google Scholar] [CrossRef]
- Matishov, G.G.; Ilyin, G.V. Strontium-90 in Seawater and Bottom Sediments of the Barents Sea Shelf (2000–2019). Dokl. Earth Sci. 2022, 505, 565–571. [Google Scholar] [CrossRef]
- Schulz, K.; Kadko, D.; Mohrholz, V.; Stephens, M.; Fer, I. Winter vertical diffusion rates in the Arctic Ocean, estimated from 7Be measurements and dissipation rate profiles. J. Geophys. Res. Oceans 2023, 128, e2022JC019197. [Google Scholar] [CrossRef]
- Lee, T.; Barg, E.; Lal, D. Studies of vertical mixing in the Southern California Bight with cosmogenic radionuclides 32P and 7Be. Limnol. Oceanogr. 1991, 36, 1044–1052. [Google Scholar] [CrossRef]
- Chen, M.; Chen, M.; Zheng, M.; Qiu, Y.; Chen, Q.; Li, Q. 210Po/210Pb disequilibria influenced by production and remineralization of particulate organic matter around Prydz Bay, Antarctica. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2021, 191–192, 104961. [Google Scholar] [CrossRef]
- Hong, Q.; Peng, S.; Zhao, D.; Cai, P. Cross-shelf export of particulate organic carbon in the northern South China Sea: Insights from a 234Th mass balance. Prog. Oceanogr. 2021, 193, 102532. [Google Scholar] [CrossRef]
- Frolova, M.A.; Bezhin, N.A.; Slizchenko, E.V.; Kozlovskaia, O.N.; Tananaev, I.G. Assessment of Seasonal Variability in Phosphorus Biodynamics by Cosmogenic Isotopes 32P, 33P around Balaklava Coast. Materials 2023, 16, 1791. [Google Scholar] [CrossRef]
- Duque, C.; Knee, K.L.; Russoniello, C.J.; Sherif, M.; Abu Risha, U.A.; Sturchio, N.C.; Michael, H.A. Submarine groundwater discharge data at meter scale (223Ra, 224Ra, 226Ra, 228Ra and 222Rn) in Indian River Bay (Delaware, US). Data Brief 2019, 27, 104728. [Google Scholar] [CrossRef] [PubMed]
- Kamenık, J.; Dulaiova, H.; Sebesta, F.; St’astna, K. Fast concentration of dissolved forms of cesium radioisotopes from large seawater samples. J. Radioanal. Nucl. Chem. 2013, 296, 841–846. [Google Scholar] [CrossRef]
- Dovhyi, I.I.; Kremenchutskii, D.A.; Kozlovskaia, O.N.; Bezhin, N.A.; Milyutin, V.V.; Kozlitin, E.A. Distribution of 137Cs in the Surface Mixed Layer of the Black Sea in summer 2017. Phys. Oceanol. 2020, 36, 387–396. [Google Scholar] [CrossRef]
- Terada, K.; Hayakawa, H.; Sawada, K.; Kiba, T. Silica gel as a support for inorganic ion-exchangers for the determination of caesium-137 in natural waters. Talanta 1970, 17, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Wilson, T. A simple and precise analytical method for determining cesium-137 in seawater. ICES J. Mar. Sci. 1974, 36, 87–89. [Google Scholar] [CrossRef]
- Remez, V.P.; Zheltonozhko, E.V.; Sapozhnikov, Y.A. The experience of using ANFEZH sorbent for recovery of radioactive caesium from sea water. Radiat. Prot. Dosim. 1998, 75, 77–78. [Google Scholar] [CrossRef]
- Tokar’, E.; Zemskova, L.; Tutov, M.; Tananaev, I.; Dovhyi, I.; Egorin, A. Development and practical evaluation of the scheme for 137Cs concentrating from seawater using chitosan and mixed ferrocyanides of Zn-K and Ni-K. J. Radioanal. Nucl. Chem. 2020, 325, 567–575. [Google Scholar] [CrossRef]
- Voronina, A.V.; Noskova, A.Y.; Semenishchev, V.S.; Gupta, D.K. Decontamination of seawater from 137Cs and 90Sr radionuclides using inorganic sorbents. J. Environ. Radioact. 2020, 217, 106210. [Google Scholar] [CrossRef]
- Vincent, T.; Vincent, C.; Barré, Y.; Guari, Y.; Le Saout, G.; Guibal, E. Immobilization of metal hexacyanoferrates in chitin beads for cesium sorption: Synthesis and characterization. J. Mater. Chem. A 2014, 2, 10007–10021. [Google Scholar] [CrossRef]
- Vincent, T.; Vincent, C.; Guibal, E. Immobilization of Metal Hexacyanoferrate Ion-Exchangers for the Synthesis of Metal Ion Sorbents—A Mini-Review. Molecules 2015, 20, 20582–20613. [Google Scholar] [CrossRef] [PubMed]
- Towler, P.H.; Smith, J.D.; Dixon, D.R. Magnetic recovery of radium, lead and polonium from seawater samples after preconcentration on a magnetic adsorbent of manganese dioxide coated magnetite. Anal. Chim. Acta 1996, 328, 53–59. [Google Scholar] [CrossRef]
- Athon, M.T.; Fryxell, G.E.; Chuang, C.-Y.; Santschi, P.H. Sorption of selected radionuclides on different MnO2 phases. Environ. Chem. 2017, 14, 207–214. [Google Scholar] [CrossRef]
- Colley, S.; Thomson, J. Particulate/solution analysis of 226Ra, 230Th and 210Pb in sea water sampled by in-situ large volume filtration and sorption by manganese oxyhydroxide. Sci. Total Environ. 1994, 155, 273–283. [Google Scholar] [CrossRef]
- Suriyanarayanan, S.; Brahmanandhan, G.M.; Samivel, K.; Ravikumar, S.; Shahul Hameed, P. Assessment of 210Po and 210Pb in marine biota of the Mallipattinam ecosystem of Tamil Nadu, India. J. Environ. Radioact. 2010, 101, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Bezhin, N.A.; Frolova, M.A.; Dovhyi, I.I.; Kozlovskaia, O.N.; Slizchenko, E.V.; Shibetskaia, I.G.; Khlystov, V.A.; Tokar’, E.A.; Tananaev, I.G. The Sorbents Based on Acrylic Fiber Impregnated by Iron Hydroxide (III): Production Methods, Properties, Application in Oceanographic Research. Water 2022, 14, 2303. [Google Scholar] [CrossRef]
- Villa-Alfageme, M.; Mas, J.L.; Hurtado-Bermudez, S.; Masqué, P. Rapid determination of 210Pb and 210Po in water and application to marine samples. Talanta 2016, 160, 28–35. [Google Scholar] [CrossRef]
- Biggin, C.D.; Cook, G.T.; MacKenzie, A.B.; Pates, J.M. A time efficient method for the determination of 210Pb, 210Bi and 210Po activities in seawater using liquid scintillation spectrometry. Anal. Chem. 2002, 74, 671–677. [Google Scholar] [CrossRef]
- Dovhyi, I.I.; Bezhin, N.A.; Kapranov, S.V.; Lyapunov, A.Y. Lead sorption by extraction chromatographic resins on the base Di-(tert-butylcyclohexano)-18-crown-6 and its application for analysis of marine samples. J. Radioanal. Nucl. Chem. 2020, 324, 1189–1201. [Google Scholar] [CrossRef]
- Surbeck, H. In Alpha Spectrometry Sample Preparation Using Selectively Adsorbing Thin Films. In Proceedings of the ICRM Conference on Low Level Radioactivity Measurement Techniques, Mol, Belgium, 18–22 October 1999; pp. 97–100. [Google Scholar]
- Henderson, P.B.; Morris, P.J.; Moore, W.S.; Charette, M.A. Methodological advances for measur-ing low-level radium isotopes in seawater. J. Radioanal. Nucl. Chem. 2013, 296, 357–362. [Google Scholar] [CrossRef]
- Moon, D.S.; Burnett, W.C.; Nour, S.; Horwitz, P.; Bond, A. Preconcentration of radium isotopes from natural waters using MnO2 resin. Appl. Radiat. Isot. 2003, 59, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Silker, W.B.; Robertson, D.E.; Rieck, H.G., Jr.; Perkins, R.W.; Prospero, J.M. Beryllium-7 in Ocean Water. Sci. New Ser. 1968, 161, 879–880. Available online: https://www.jstor.org/stable/1724874?seq=1#fndtn-page_scan_tab_contents (accessed on 29 April 2023). [CrossRef]
- Nakanishi, T.; Kusakabe, M.; Aono, T.; Yamada, M. Simultaneous measurements of cosmogenic radionuclides 32P, 33P and 7Be in dissolved and particulate forms in the upper ocean. J. Radioanal. Nucl. Chem. 2009, 279, 769–776. [Google Scholar] [CrossRef]
- Kadko, D. Upwelling and primary production during the U.S. GEOTRACES East Pacific Zonal Transect. Glob. Biogeochem. Cycles 2017, 31, 218–232. [Google Scholar] [CrossRef]
- Dovhyi, I.I.; Bezhin, N.A.; Tananaev, I.G. Sorption methods in marine radiochemistry. Rus. Chem. Rev. 2021, 90, 1544–1565. [Google Scholar] [CrossRef]
- Andrews, J.E.; Hartin, C.; Buesseler, K.O. 7Be analyses in seawater by low background gamma-spectroscopy. J. Radioanal. Nucl. Chem. 2008, 277, 253–259. [Google Scholar] [CrossRef]
- Hirose, K.; Aoyama, M.; Igarashi, Y.; Komura, K. Improvement of 137Cs analysis in small volume seawater samples using the Ogoya underground facility. J. Radioanal. Nucl. Chem. 2008, 276, 795–798. [Google Scholar] [CrossRef]
- Yücel, M.; Moore, W.S.; Butler, I.B.; Boyce, A.; Luther, G.W. Recent sedimentation in the Black Sea: New insights from radionuclide distributions and sulfur isotopes. Deep Sea Res. Part I Oceanogr. Res. Pap. 2012, 66, 103–113. [Google Scholar] [CrossRef]
- Dulaiova, H.; Burnett, W.C. An efficient method for γ-spectrometric determination of radium-226,228 via manganese fibers. Limnol. Oceanogr. Methods 2004, 2, 256–261. [Google Scholar] [CrossRef]
- Dovhyi, I.I.; Kremenchutskii, D.A.; Bezhin, N.A.; Shibetskaya, Y.G.; Tovarchii, Y.Y.; Egorin, A.M.; Tokar, E.A.; Tananaev, I.G. MnO2 fiber as a sorbent for radionuclides in oceanographic investigations. J. Radioanal. Nucl. Chem. 2020, 323, 539–547. [Google Scholar] [CrossRef]
- Chouyyok, W.; Warner, C.L.; Mackie, K.E.; Warner, M.G.; Gill, G.; Addleman, R.S. Nanostructured Metal Oxide Sorbents for the Collection and Recovery of Uranium from Seawater. Ind. Eng. Chem. Res. 2016, 55, 4195–4207. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Liu, S.; Zhou, Y.; Liu, D.; Fu, C.; Ye, L. Efficient extraction of UO22+ from seawater by polyethylenimine functionalized activated carbon (PEI-AC): Adsorption performance and mechanism. J. Radioanal. Nucl. Chem. 2022, 331, 4635–4648. [Google Scholar] [CrossRef]
- Avramenko, V.A.; Burkov, I.S.; Zheleznov, V.V.; Khokhlov, K.A.; Lysenko, N.I. Sorption-reagent reprocessing of liquid radioactive wastes from salvaged nuclear powered submarines. At. Energy 2002, 92, 488–492. [Google Scholar] [CrossRef]
- Bezhin, N.A.; Dovhyi, I.I.; Milyutin, V.V.; Kaptakov, V.O.; Kozlitin, E.A.; Egorin, A.M.; Tokar’, E.A.; Tananaev, I.G. Study of sorbents for analysis of radiocesium in seawater samples by one-column method. J. Radioanal. Nucl. Chem. 2021, 327, 1095–1103. [Google Scholar] [CrossRef]
- Andriukonis, E.; Ramanaviciene, A.; Ramanavicius, A. Synthesis of Polypyrrole Induced by [Fe(CN)6]3− and Redox Cycling of [Fe(CN)6]4−/[Fe(CN)6]3−. Polymers 2018, 10, 749. [Google Scholar] [CrossRef]
- Logacheva, V.A.; Afonin, N.N.; Lukin, A.N.; Nikitin, L.N.; Kiseleva, Y.A. IR spectroscopy of the Fe–TiO2 film system obtained by magnetron sputtering. Condens. Media Interphase Bound. 2017, 19, 239–247. (In Russian) [Google Scholar]
- Nekrasova, N.A.; Milyutin, V.V.; Kaptakov, V.O.; Kozlitin, E.A. Inorganic Sorbents for Wastewater Treatment from Radioactive Contaminants. Inorganics 2023, 11, 126. [Google Scholar] [CrossRef]
- El-Shazly, E.A.A.; Dakroury, G.A.; Someda, H.H. Kinetic and isotherm studies for the sorption of 134Cs and 60Co radionuclides onto supported titanium oxide. J. Radioanal. Nucl. Chem. 2021, 330, 127–139. [Google Scholar] [CrossRef]
- Korostelev, P.P. Photometric and Complexometric Analysis in Metallurgy; Metallurgy: Moscow, Russia, 1984. (In Russian) [Google Scholar]
- Mass Concentration of Phosphates in Sea Waters; Guidance Document 52.10.738-2010; Publishing Factory Offset Printing: Moscow, Russia, 2010; 27p. (In Russian)
- Bezhin, N.A.; Kremenchutskii, D.A.; Slizchenko, E.V.; Kozlovskaia, O.N.; Shibetskaia, I.G.; Milyutin, V.V.; Tananaev, I.G. Estimation of 137Cs Distribution and Recovery Using Various Types of Sorbents in the Black Sea Surface Layer. Processes 2023, 11, 603. [Google Scholar] [CrossRef]
- Mann, D.R.; Casso, S.A. In situ chemisorption of radiocesium from seawater. Mar. Chem. 1984, 14, 307–318. [Google Scholar] [CrossRef]
- Bezhin, N.A.; Dovhyi, I.I.; Tokar, E.A.; Tananaev, I.G. Physical and chemical regularities of cesium and strontium recovery from the seawater by sorbents of various types. J. Radioanal. Nucl. Chem. 2021, 330, 1101–1111. [Google Scholar] [CrossRef]
- Bezhin, N.A.; Frolova, M.A.; Kozlovskaya, O.N.; Slizchenko, E.V.; Shibetskaya, Y.G.; Tananaev, I.G. Physical and chemical regularities of phosphorus and beryllium recovery from the seawater by acrylate fiber based on iron(+3) hydroxide. Processes 2022, 10, 2010. [Google Scholar] [CrossRef]
- Plazinski, W.; Dziuba, J.; Rudzinski, W. Modeling of Sorption Kinetics: The Pseudo-Second Order Equation and the Sorbate Intraparticle Diffusivity. Adsorption 2013, 19, 1055–1064. [Google Scholar] [CrossRef]
- Javadian, H. Application of kinetic, isotherm and thermodynamic models for the adsorption of Co(II) ions on polyamidine/polypyrrole copolymer nanofibers from aqueous solution. J. Ind. Eng. Chem. 2014, 20, 4233–4241. [Google Scholar] [CrossRef]
- Johnson, B.E.; Santschi, P.H.; Addleman, R.S.; Douglas, M.; Davidson, J.; Fryxell, G.E.; Schwantes, J.M. Optimization and Evaluation of Mixed-Bed Chemisorbents for Extracting Fission and Activation Products from Marine and Fresh Waters. Anal. Chim. Acta 2011, 708, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Aono, T.; Yamada, M.; Kusakabe, M. Temporal and spatial variations of 137Cs in the waters off a nuclear fuel reprocessing facility in Rokkasho, Aomori, Japan. J. Radioanal. Nucl. Chem. 2010, 283, 831–838. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the sol-id/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Gulin, S.B.; Egorov, V.N.; Duka, M.S.; Sidorov, I.G.; Proskurnin VYu Mirzoyeva NYu Bey, O.N.; Gulina, L.V. Deep-water profiling of 137Cs and 90Sr in the Black Sea: A further insight into dynamics of the post-Chernobyl radioactive contamination. J. Radioanal. Nucl. Chem. 2015, 304, 779–783. [Google Scholar] [CrossRef]
- Chukharev, A.M.; Pavlov, M.I. Model and Experimental Estimates of Vertical Mixing Intensity in the Sea Upper Homogeneous Layer. Phys. Oceanogr. 2021, 28, 309–325. [Google Scholar] [CrossRef]
- Orekhova, N.A. Nutrients Dynamics in the Surface Waters of the Black Sea. Phys. Oceanogr. 2021, 28, 660–676. [Google Scholar] [CrossRef]
- Roca-Martí, M.; Puigcorbé, V.; Rutgers van der Loeff, M.M.; Katlein, C.; Fernández-Méndez, M.; Peeken, I.; Masqué, P. Carbon export fluxes and export efficiency in the central Arctic during the record sea-ice minimum in 2012: A joint 234Th/238U and 210Po/210Pb study. J. Geophys. Res. Oceans 2016, 121, 5030–5049. [Google Scholar] [CrossRef]


| Sorbent | Manufacturer | Appearance | Particle Size, mm | Bulk Weight, g/cm3 | Sorbent Composition | |
|---|---|---|---|---|---|---|
| Support | Sorption-Active Phase: Content (Mass %) | |||||
| FIC | Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences | dark blue irregular granules | 0.1–1.0 | 0.25–0.4 | activated carbon | iron(III) ferrocyanide; not less than 10 |
| FIC A | black irregular granules | iron(III) hydroxide; not less than 10 | ||||
| Sorbent | FIC | FIC A | |
|---|---|---|---|
| Recovered element | Cs | P | Be |
| Kd, mL/g | (1.3 ± 0.2)∙104 | (3.6 ± 0.2)∙103 | 510 ± 45 |
| Sorbent | Recovered Element | Parameter | Flow Rate, mL/min | |||
|---|---|---|---|---|---|---|
| 3 | 6 | 15 | 30 | |||
| FIC | Cs | DEC, mg/g | 5.61 | 3.73 | 2.80 | 1.87 |
| TDEC, mg/g | 27.5 | 23.5 | 19.1 | 15.8 | ||
| FIC A | P | DEC, mg/g | 0.027 | 0.018 | 0.009 | 0.0045 |
| TDEC, mg/g | 0.358 | 0.313 | 0.265 | 0.224 | ||
| Be | DEC, mg/g | 0.0132 | 0.0088 | 0.0044 | 0.0022 | |
| TDEC, mg/g | 0.0716 | 0.0616 | 0.0509 | 0.0409 | ||
| Sorbent | Recovered Element | Intraparticle Diffusion * | Pseudo-First-Order * | Pseudo-Second-Order * | Elovich Model * | qe exp, mg/g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KI, mg/(g∙h0.5) | c, mg/g | r2 | K1, h−1 | ge, mg/g | r2 | K2, g/(mg∙h) | ge, mg/g | r2 | α, g/(mg∙h) | β, g/mg | r2 | |||
| FIC | Cs | 0.129 | 1.14 | 0.648 | 0.128 | 0.349 | 0.810 | 1.87 | 1.86 | 1.00 | 178 | 5.45 | 0.899 | 1.84 |
| FIC A | P | 0.0030 | 0.0124 | 0.805 | 0.130 | 0.0147 | 0.959 | 34.8 | 0.0298 | 0.999 | 0.218 | 256 | 0.952 | 0.0293 |
| Be | 0.0008 | 0.0075 | 0.897 | 0.126 | 0.0047 | 0.985 | 109 | 0.0123 | 0.999 | 4.45 | 1000 | 0.987 | 0.0122 | |
| Sorbent | Recovered Element | Langmuir Isotherm * | Freindlich Isotherm * | Dubinin–Radushkevich Isotherm * | qm exp, MΓ/Γ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm, mg/g | KL, L/mg | r2 | KF, mg/g | n | r2 | qm, mg/g | β, mol2/kJ2 | E, kJ/mol | r2 | |||
| FIC | Cs | 29.9 | 0.067 | 0.999 | 4.18 | 3.18 | 0.901 | 25.4 | 0.011 | 6.65 | 0.959 | 29.7 |
| FIC A | P | 0.384 | 8.32 | 0.996 | 0.468 | 1.94 | 0.949 | 0.976 | 0.0096 | 7.22 | 0.957 | 0.372 |
| Be | 0.091 | 13.1 | 0.999 | 0.075 | 5.95 | 0.866 | 0.101 | 0.0068 | 8.57 | 0.940 | 0.091 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezhin, N.A.; Milyutin, V.V.; Kuzmenkova, N.V.; Shibetskaia, I.G.; Kozlovskaia, O.N.; Slizchenko, E.V.; Razina, V.A.; Tananaev, I.G. Radionuclides’ Recovery from Seawater Using FIC and FIC A Sorbents. Materials 2023, 16, 4181. https://doi.org/10.3390/ma16114181
Bezhin NA, Milyutin VV, Kuzmenkova NV, Shibetskaia IG, Kozlovskaia ON, Slizchenko EV, Razina VA, Tananaev IG. Radionuclides’ Recovery from Seawater Using FIC and FIC A Sorbents. Materials. 2023; 16(11):4181. https://doi.org/10.3390/ma16114181
Chicago/Turabian StyleBezhin, Nikolay A., Vitaliy V. Milyutin, Natalia V. Kuzmenkova, Iuliia G. Shibetskaia, Ol’ga N. Kozlovskaia, Evgeniy V. Slizchenko, Victoria A. Razina, and Ivan G. Tananaev. 2023. "Radionuclides’ Recovery from Seawater Using FIC and FIC A Sorbents" Materials 16, no. 11: 4181. https://doi.org/10.3390/ma16114181
APA StyleBezhin, N. A., Milyutin, V. V., Kuzmenkova, N. V., Shibetskaia, I. G., Kozlovskaia, O. N., Slizchenko, E. V., Razina, V. A., & Tananaev, I. G. (2023). Radionuclides’ Recovery from Seawater Using FIC and FIC A Sorbents. Materials, 16(11), 4181. https://doi.org/10.3390/ma16114181








