A Laboratory and Field Assessment of the Performance of Rebar Coatings
Abstract
:1. Introduction
2. Research Methodology
2.1. Materials and Concrete Mix
2.2. Laboratory Investigation
2.2.1. Casting and Curing of Cylindrical Concrete Specimens
2.2.2. Testing of Cylindrical Concrete Specimens
2.2.3. Preparation and Testing of the Steel Panels
2.3. Field Investigations
2.3.1. Preparation of Field Specimens
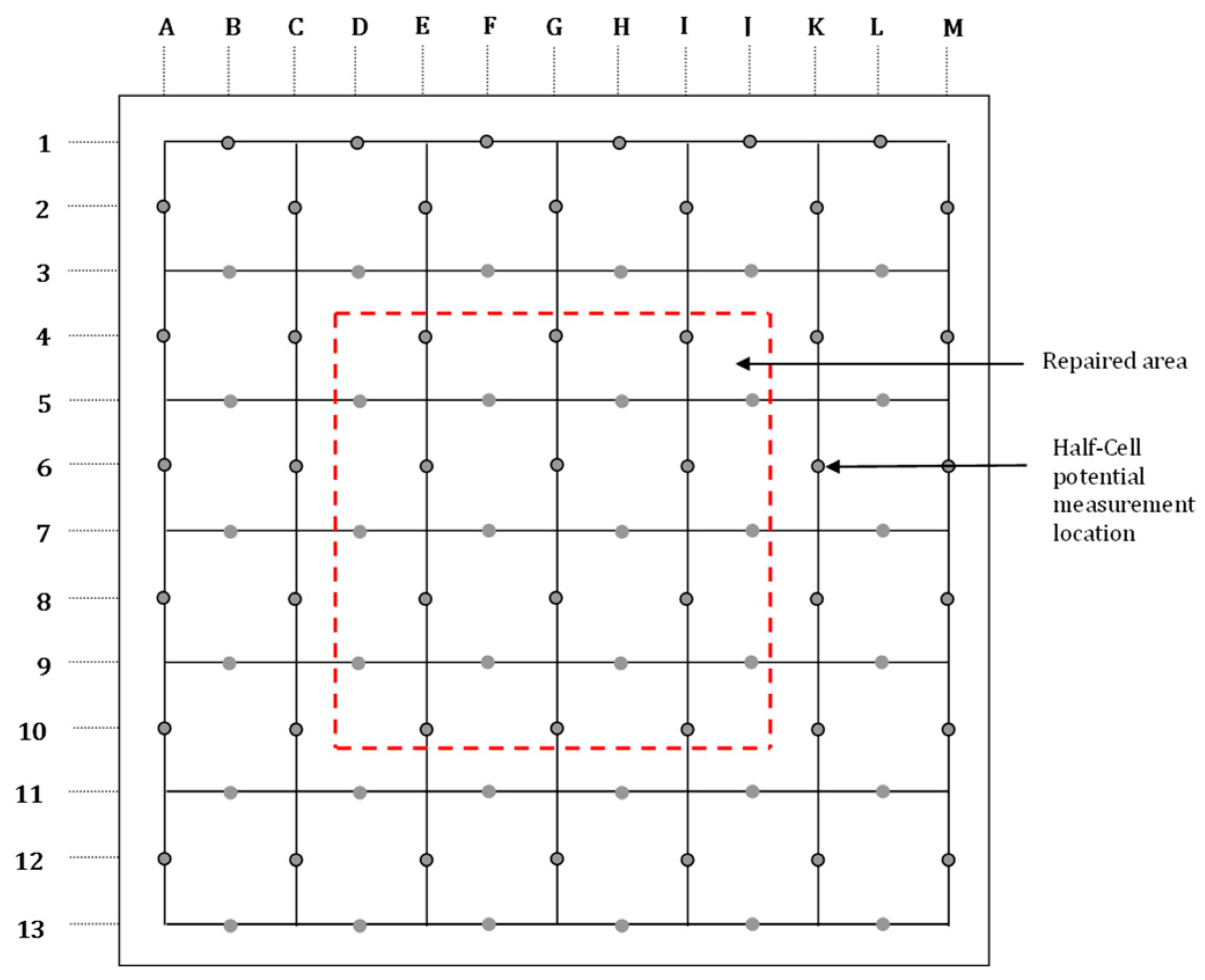
2.3.2. Exposure, Monitoring, and Testing of the Field Specimens
3. Results and Discussion
3.1. Analysis of Laboratory Investigations
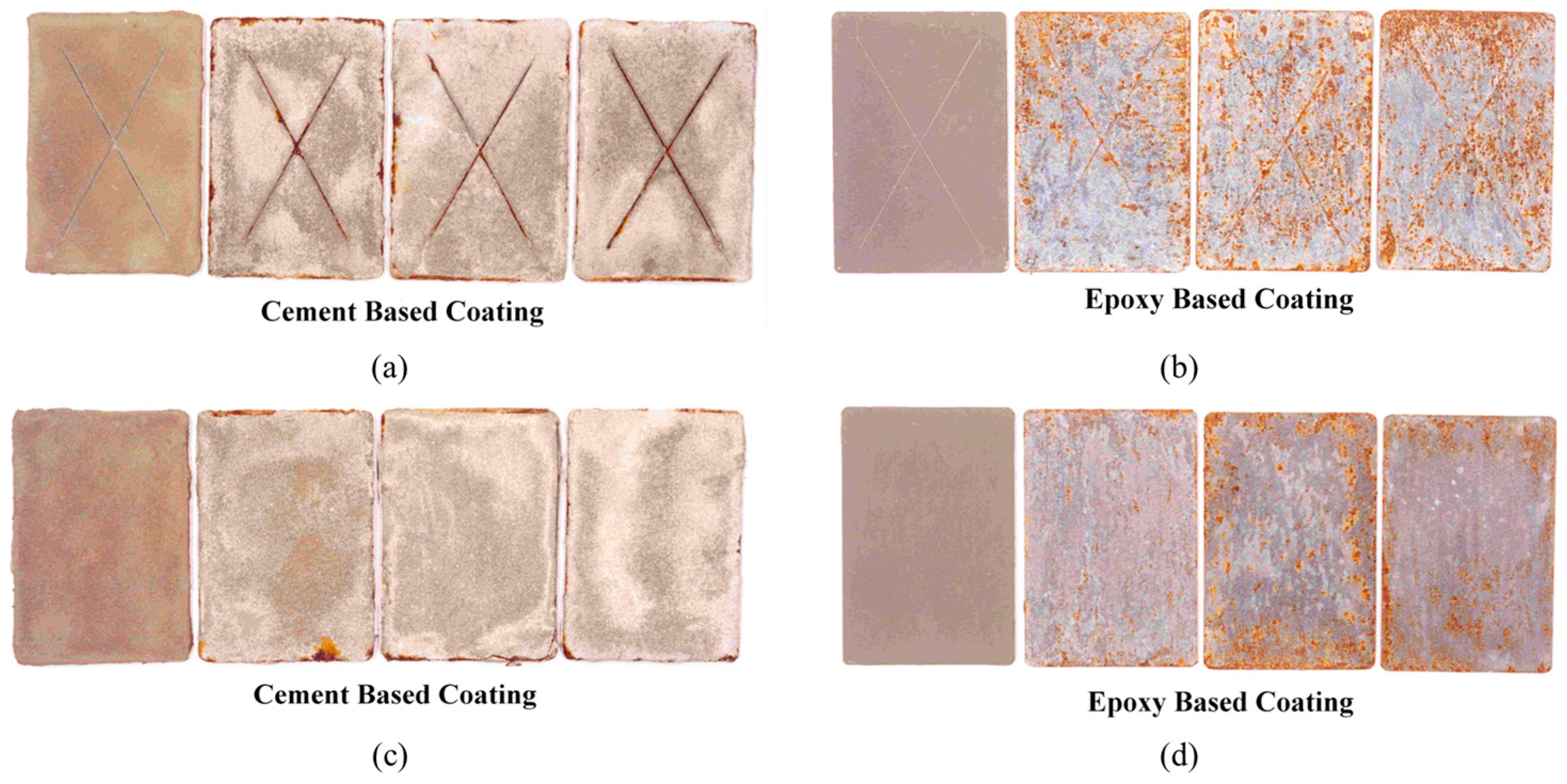
3.1.1. Salt Spray Results on Coated Panels
| Panel | ASTM Standard | Rating |
|---|---|---|
| Coated and scribed | D1654 [40] Procedure A | 10 |
| Coated and scribed | D714 [39] | No blistering or delamination of the coating and no corrosion spots |
| Coated and scribed | D610 [43] | Rust grade: 2 (0% of surface rusted) |
| Coated and unscribed | D1654 [40] Procedure B | 10 |
| Coated and unscribed | D714 [39] | No blistering or delamination of coating and no corrosion spots |
| Coated and unscribed | D610 [43] | Rust grade: 2 (0% of surface rusted) |
| Panel | ASTM Standard | Rating |
|---|---|---|
| Coated and scribed | D1654 [40] Procedure A | 10 |
| Coated and scribed | D714 [39] | No blistering or delamination of coating but lots of corrosion spots |
| Coated and scribed | D610 [43] | Rust grade: 2 (33% of surface rusted) |
| Coated and unscribed | D1654 [40] Procedure B | 10 |
| Coated and unscribed | D714 [39] | No blistering or delamination of coating but lots of corrosion spots |
| Coated and unscribed | D610 [43] | Rust grade: 2 (33% of surface rusted) |
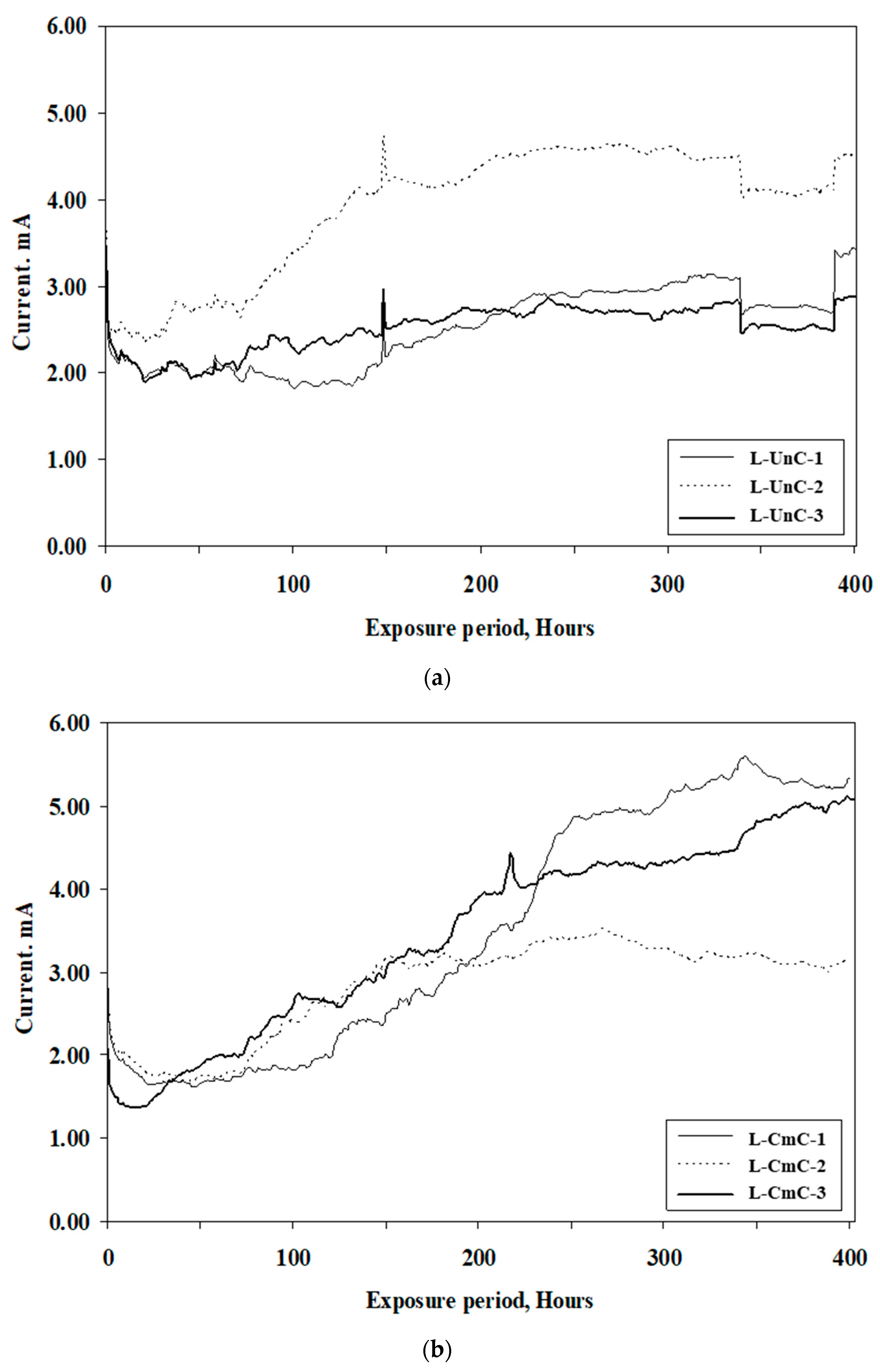
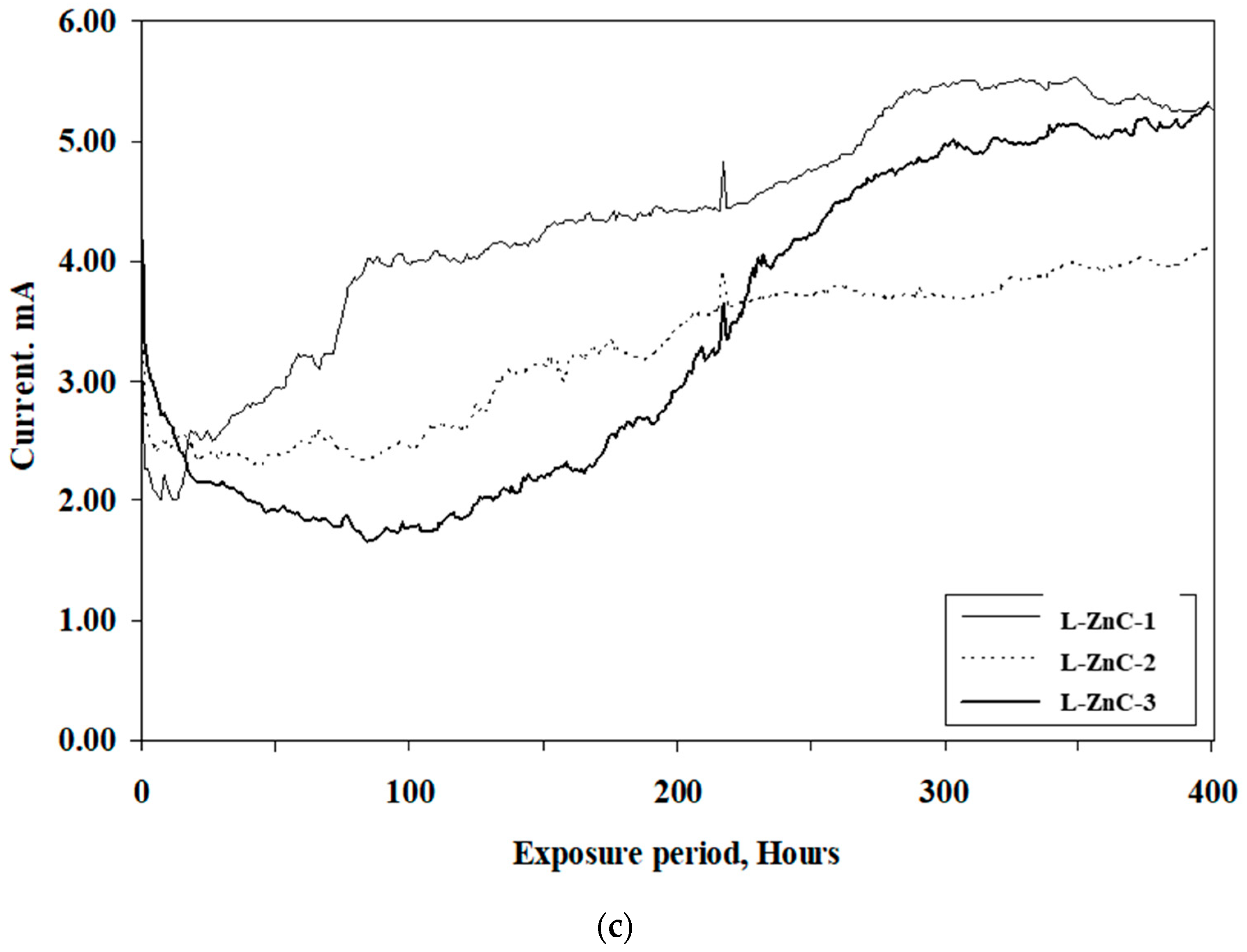
3.1.2. Results of Accelerated Corrosion Tests
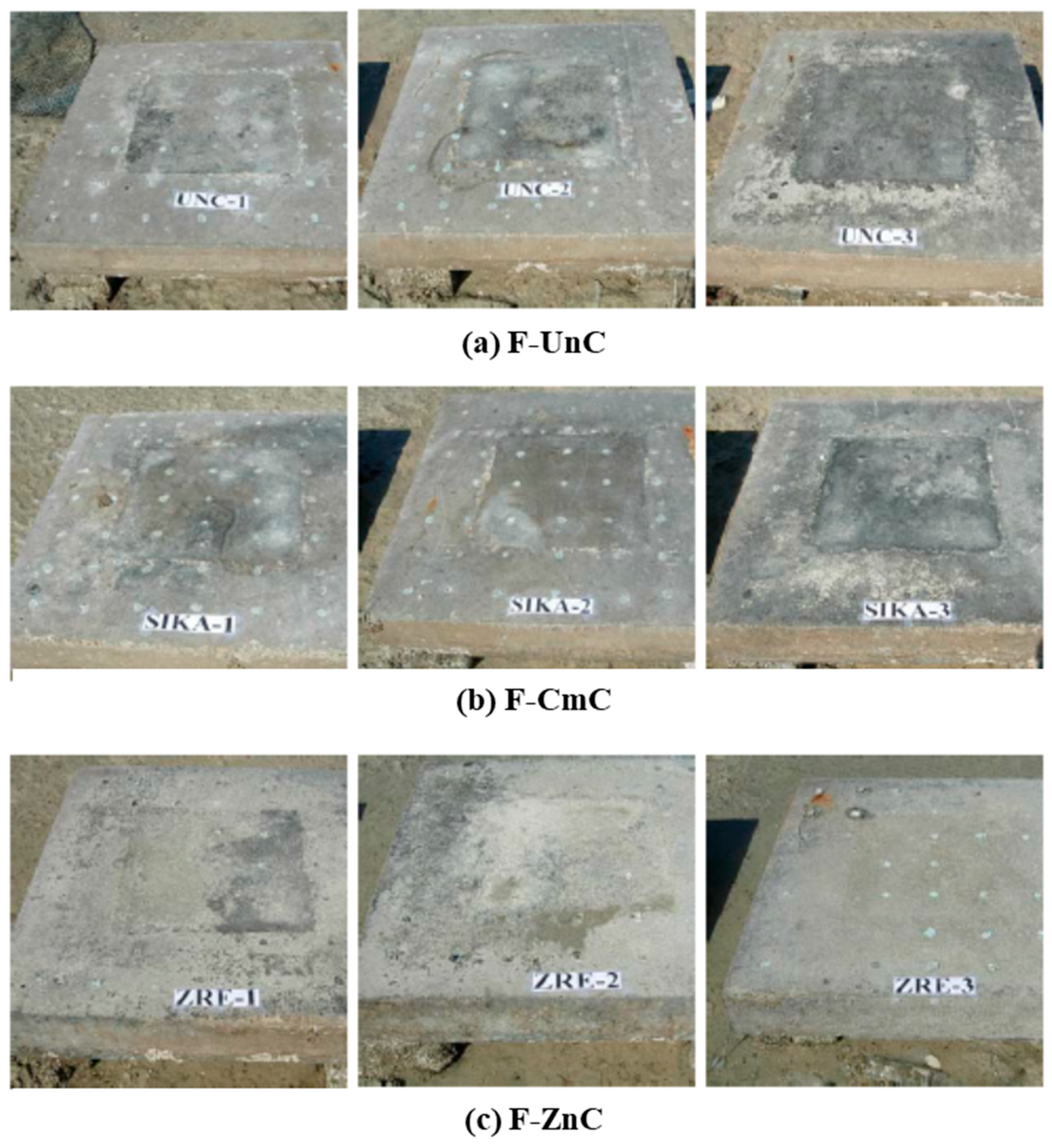
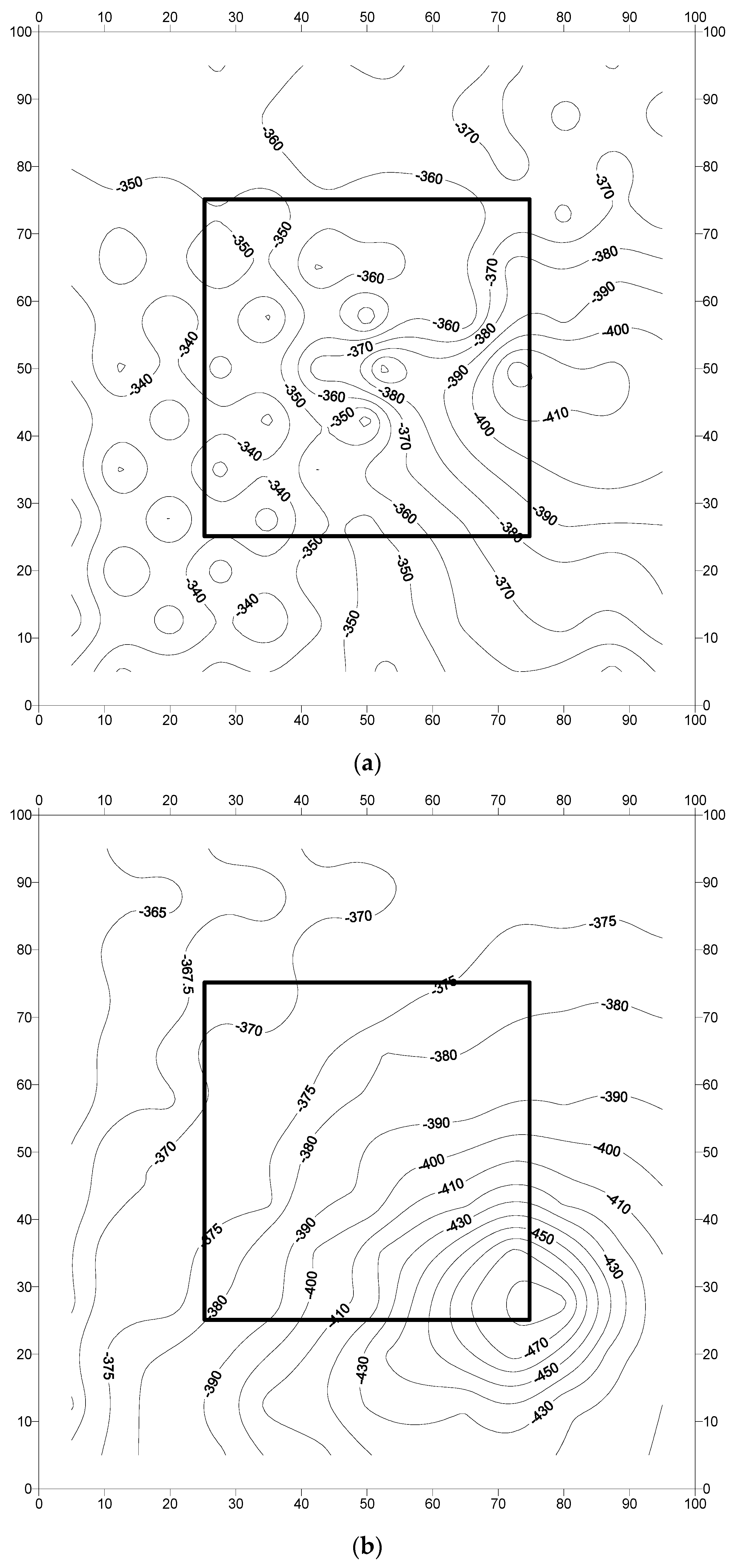
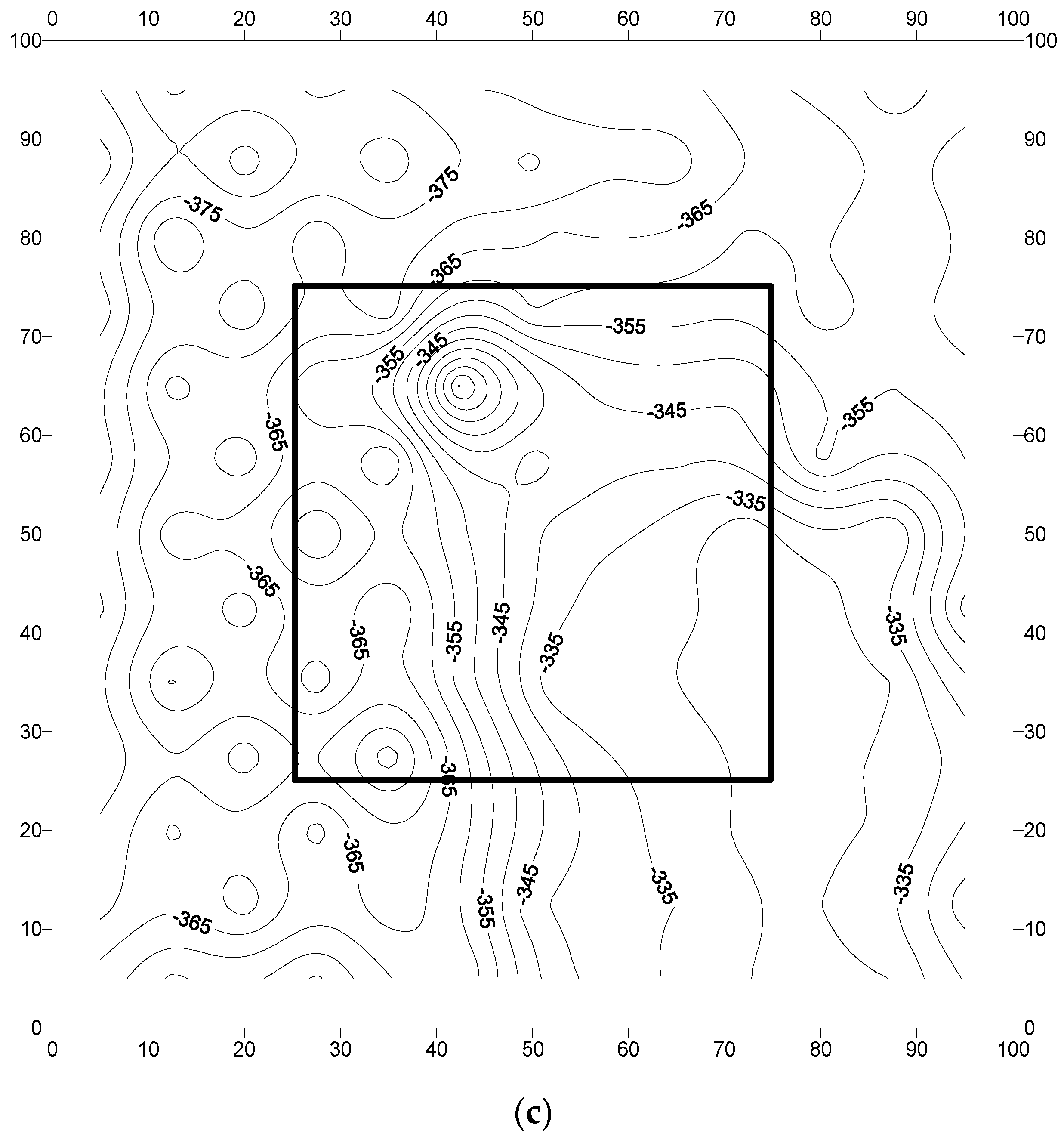
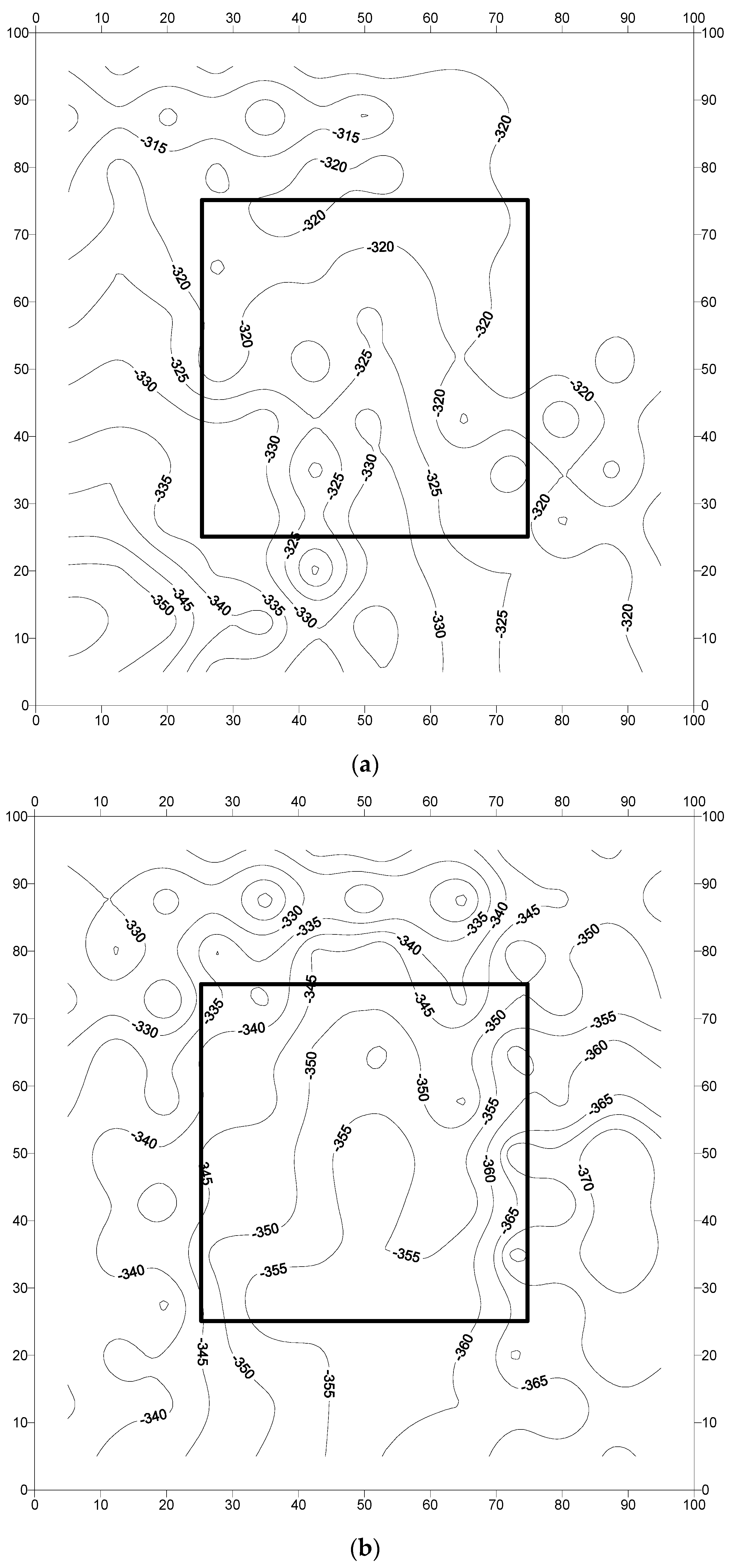
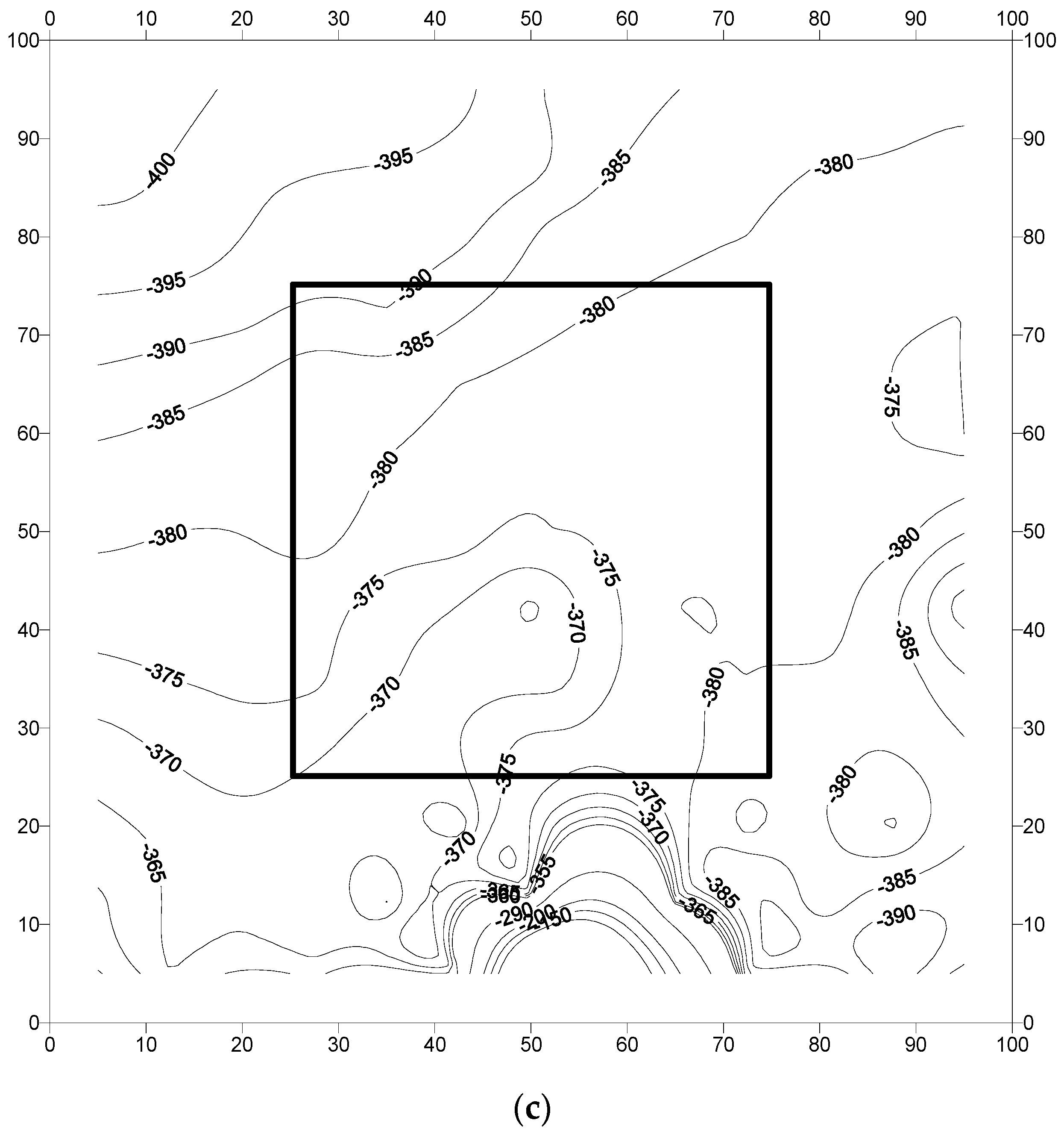
3.2. Analysis of Field Results
4. Conclusions
- i.
- There was no blistering and/or delamination of the coating on any of the coated steel panels exposed to the salt spray for 1000 h. However, many corrosion spots were noticed on the steel panels coated with the zinc-rich epoxy steel primer. A few corrosion spots were noted on the steel panels coated with the cement-based epoxy coating. However, the coating remained intact after exposure to the salt spray. A rating of 10 and a rust grade of 2 (0% rust) were assigned to the specimens coated with the cement-based epoxy coating, and the values of 10 and 2 (33% rust) were assigned to the specimens coated with the zinc-rich epoxy coating.
- ii.
- The time to crack of concrete specimens, due to the accelerated corrosion of steel bars coated with the cement-based coating, was longer than the specimens coated with the zinc-rich epoxy coating. The average time to crack in the concrete specimens with uncoated steel bars was 113 h, while it was 282 and 110 h, respectively, in the bars coated with cement-based and zinc-rich epoxy coatings.
- iii.
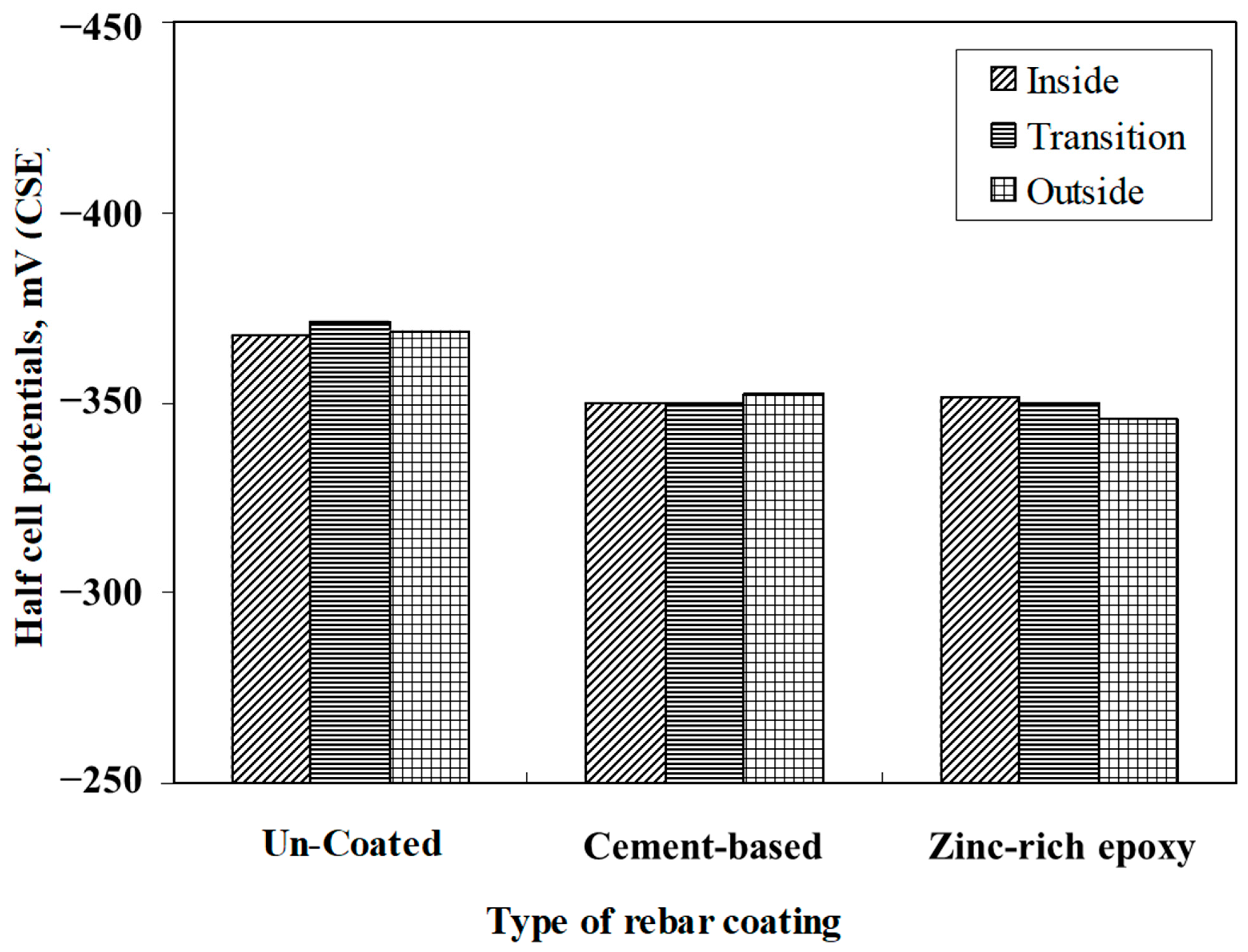
- The corrosion potentials on steel in the slab specimens did not indicate the formation of incipient anodes at the junction of old and new concrete. The average corrosion potential in the uncoated specimens was more negative than −350 mV CSE (ASTM C876 threshold value) compared to the average potentials on steel coated with the cement-based epoxy resin or zinc-rich epoxy. The corrosion potentials on steel coated with cement-based epoxy resins and zinc-rich epoxy coatings were almost similar.
- iv.
- Based on the results of the tested coatings in salt spray exposure and accelerated corrosion, it may be concluded that the cement-based coating performs better than the zinc-rich coating. There may be a decrease in the bond between steel and concrete. However, the decrease in the bond will be within the allowable value.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertolini, L.; Elsener, B.; Redaelli, E.; Pedeferri, P.; Polder, R. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair, 2nd ed.; Wiley: Weinheim, Germany, 2013. [Google Scholar]
- Li, S.; Jin, Z.; Pang, B.; Li, J. Durability performance of an RC beam under real marine all corrosion zones exposure for 7 years. Case Stud. Constr. Mater. 2022, 17, e01516. [Google Scholar] [CrossRef]
- Hou, L.; Guo, S.; Zhou, B.; Xu, S.; Chen, D. Bond-slip behavior of corroded rebar embedded in ultrahigh toughness cementitious composite. J. Mater. Civil Eng. 2018, 30, 4018132. [Google Scholar] [CrossRef]
- Yu, X.; Robuschi, S.; Fernandez, I.; Lundgren, K. Numerical assessment of bond-slip relationships for naturally corroded plain reinforcement bars in concrete beams. Eng. Struct. 2021, 239, 112309. [Google Scholar] [CrossRef]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International measures of prevention, application, and economics of corrosion technologies study. NACE Int. 2016, 216, 2–3. [Google Scholar]
- Byrne, A.; Holmes, N.; Norton, B. State-of-the-art review of cathodic protection for reinforced concrete structures. Mag. Concr. Res. 2016, 68, 664–677. [Google Scholar] [CrossRef] [Green Version]
- Pei, X.; Noël, M.; Green, M.; Fam, A.; Shier, G. Cementitious coatings for improved corrosion resistance of steel reinforcement. Surf. Coat. Technol. 2017, 315, 188–195. [Google Scholar] [CrossRef]
- Wang, F.; Xue, X.; Hua, J.; Chen, Z.; Huang, L.; Wang, N.; Jin, J. Non-uniform corrosion influences on mechanical performances of stainless-clad bimetallic steel bars. Mar. Struct. 2022, 86, 103276. [Google Scholar] [CrossRef]
- Hua, J.; Yang, Z.; Xue, X.; Huang, L.; Wang, N.; Chen, Z. Bond properties of bimetallic steel bar in seawater sea-sand concrete at different ages. Constr. Build. Mater. 2022, 323, 126539. [Google Scholar] [CrossRef]
- Hua, J.; Wang, F.; Xiang, Y.; Yang, Z.; Xue, X.; Huang, L.; Wang, N. Mechanical properties of stainless-clad bimetallic steel bars exposed to elevated temperatures. Fire Saf. J. 2022, 127, 103521. [Google Scholar] [CrossRef]
- Wang, F.; Hua, J.; Xue, X.; Ding, Z.; Lyu, Y.; Liu, Q. Low-cycle fatigue performance of bimetallic steel bar considering the effect of inelastic buckling. Constr. Build. Mater. 2022, 351, 128787. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U. Corrosion-resistance evaluation of coated and specialty bars. Eur. J. Environ. Civil Eng. 2022, 26, 5821–5842. [Google Scholar] [CrossRef]
- Jalili, M.M.; Moradian, S.; Hosseinpour, D. The use of inorganic conversion coatings to enhance the corrosion resistance of reinforcement and the bond strength at the rebar/concrete. Constr. Build. Mater. 2009, 23, 233–238. [Google Scholar] [CrossRef]
- Arya, E.K.; Ittyeipe, A.V.; Kamde, D.K.; Dhanya, B.S. Service life of reinforced concrete (RC) systems with cement-polymer-composite (CPC) coated steel rebars. Indian Concr. J. 2020, 94, 68–80. [Google Scholar]
- Al-Amoudi, O.S.B.; Maslehuddin, M.; Ibrahim, M. Long-term performance of fusion-bonded epoxy-coated steel bars in chloride-contaminated concrete. ACI Mater. J. 2004, 101, 303–309. [Google Scholar] [CrossRef]
- Elleithy, W.M.; Al-Amoudi, O.S.; Sharif, A.M.; Maslehuddin, M. Corrosion resistance and bond strength of epoxy-coated steel bars. Arab. J. Sci. Eng. 1998, 23, 775. [Google Scholar]
- Kamde, D.K.; Pillai, R.G. Corrosion initiation and its effect on bond characteristics and service life of reinforced concrete systems with Cement-Polymer-Composite coated steel rebars. Structures 2022, 44, 248–260. [Google Scholar] [CrossRef]
- Criado, M.; Sobrados, I.; Bastidas, J.M.; Sanz, J. Corrosion behaviour of coated steel rebars in carbonated and chloride-contaminated alkali-activated fly ash mortar. Prog. Org. Coat. 2016, 99, 11–22. [Google Scholar] [CrossRef]
- Tang, F.; Chen, G.; Volz, J.S.; Brow, R.K.; Koenigstein, M.L. Cement-modified enamel coating for enhanced corrosion resistance of steel reinforcing bars. Cem. Concr. Compos. 2013, 35, 171–180. [Google Scholar] [CrossRef]
- Venkatesan, P.; Palaniswamy, N.; Rajagopal, K. Corrosion performance of coated reinforcing bars embedded in concrete and exposed to natural marine environment. Prog. Org. Coat. 2006, 56, 8–12. [Google Scholar] [CrossRef]
- Kamde, D.K.; Pillai, R.G. Effect of surface preparation on corrosion of steel rebars coated with cement-polymer-composites (CPC) and embedded in concrete. Constr. Build. Mater. 2020, 237, 117616. [Google Scholar] [CrossRef]
- Ababneh, A.N.; Sheban, M.A.; Abu-Dalo, M.A. Effectiveness of benzotriazole as corrosion protection material for steel reinforcement in concrete. J. Mater. Civil Eng. 2012, 24, 141–151. [Google Scholar] [CrossRef]
- Cubides, Y.; Castaneda, H. Corrosion protection mechanisms of carbon nanotube and zinc-rich epoxy primers on carbon steel in simulated concrete pore solutions in the presence of chloride ions. Corros. Sci. 2016, 109, 145–161. [Google Scholar] [CrossRef] [Green Version]
- Ramezanzadeh, B.; Moghadam, M.H.M.; Shohani, N.; Mahdavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Li, H.Y.; Duan, J.Y.; Wei, D.D. Comparison on corrosion behaviour of arc sprayed and zinc-rich coatings. Surf. Coat. Technol. 2013, 235, 259–266. [Google Scholar] [CrossRef]
- Chen, M.; Wei, Y.; Zheng, H.; Yu, L.; Liu, Q.; Wang, Y.; Yuan, H.; Wang, C.; Li, W. Ca-LDH-modified cementitious coating to enhance corrosion resistance of steel bars. J. Build. Eng. 2022, 51, 104301. [Google Scholar] [CrossRef]
- Pei, X.; Noël, M.; Fam, A.; Green, M. Development length of steel reinforcement with corrosion protection cementitious coatings. Cem. Concr. Compos. 2015, 60, 34–43. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U.; Maslehuddin, M.; Al-Zahrani, M.M.; Al-Juraifani, E.A.; Al-Idi, S.H.; Al-Mehthel, M. Performance evaluation of cement-based surface coatings. ACI Spec. Publ. 2000, 193, 321–335. [Google Scholar]
- Wang, K.; Liu, Z.; Wang, Z.; Yang, W. Study on polymer modified cement-based coating with healing effect on rusty carbon steel. Int. J. Corros. 2014, 2014, 628191. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Lu, K.; Han, C.; Wu, Q. Study on anti-corrosion performance of silica fume modified magnesium potassium phosphate cement-based coating on steel. Case Stud. Constr. Mater. 2022, 17, e01467. [Google Scholar] [CrossRef]
- Zhang, F.; Myers, J.J.; Liao, W.; Hui, C.; Ma, H. Investigation of corrosion mechanism of ribbed mild steel bars coated with magnesium potassium phosphate cement paste. Constr. Build. Mater. 2023, 371, 130639. [Google Scholar] [CrossRef]
- Qureshi, T.; Wang, G.; Mukherjee, S.; Akibul Islam, M.; Filleter, T.; Singh, C.V.; Panesar, D.K. Graphene-based anti-corrosive coating on steel for reinforced concrete infrastructure applications: Challenges and potential. Constr. Build. Mater. 2022, 351, 128947. [Google Scholar] [CrossRef]
- Jorge, S.; Dias-da-Costa, D.; Júlio, E.N.B.S. Influence of anti-corrosive coatings on the bond of steel rebars to repair mortars. Eng. Struct. 2012, 36, 372–378. [Google Scholar] [CrossRef]
- Harilal, M.; George, R.P.; Albert, S.K.; Philip, J. A new ternary composite steel rebar coating for enhanced corrosion resistance in chloride environment. Constr. Build. Mater. 2022, 320, 126307. [Google Scholar] [CrossRef]
- ASTM C150/C150M-18; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- ASTM A615; Standard Specification for Deformed and Plain Billet-Steel Bars for Concrete. ASTM International: West Conshohocken, PA, USA, 2022; pp. 1–5.
- Al-Zahrani, M.M.; Al-Dulaijan, S.U.; Ibrahim, M.; Saricimen, H.; Sharif, F.M. Effect of waterproofing coatings on steel reinforcement corrosion and physical properties of concrete. Cem. Concr. Compos. 2002, 24, 127–137. [Google Scholar] [CrossRef]
- ASTM B117; Standard Practice for Operating Salt Spray (FOG) Apparatus. ASTM International: West Conshohocken, PA, USA, 2003; Volume 3, pp. 1–5. [CrossRef]
- ASTM D714; Standard Test Method for Evaluating Degree of Blistering of Paints. ASTM International: West Conshohocken, PA, USA, 2006; pp. 1–6.
- ASTM D1654; Standard Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive Environments. ASTM International: West Conshohocken, PA, USA, 2012; Volume 8, pp. 5–12.
- Al-Tholaia, M.M.H.; Azad, A.K.; Ahmad, S.; Baluch, M.H. A comparative study of corrosion resistance of different coatings for mortar-embedded steel plates. Constr. Build. Mater. 2014, 56, 74–80. [Google Scholar] [CrossRef]
- Kalendová, A.; Sapurina, I.; Stejskal, J.; Veselý, D. Anticorrosion properties of polyaniline-coated pigments in organic coatings. Corros. Sci. 2008, 50, 3549–3560. [Google Scholar] [CrossRef]
- ASTM D610; Standard Test Method for Evaluating Degree of Rusting on Painted Steel Surfaces. ASTM International: West Conshohocken, PA, USA, 2001; Volume D610-01, pp. 1–6.
- Ding, L.; Poursaee, A. The impact of sandblasting as a surface modification method on the corrosion behavior of steels in simulated concrete pore solution. Constr. Build. Mater. 2017, 157, 591–599. [Google Scholar] [CrossRef]
- ASTM C876; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM International: West Conshohocken, PA, USA, 2015.
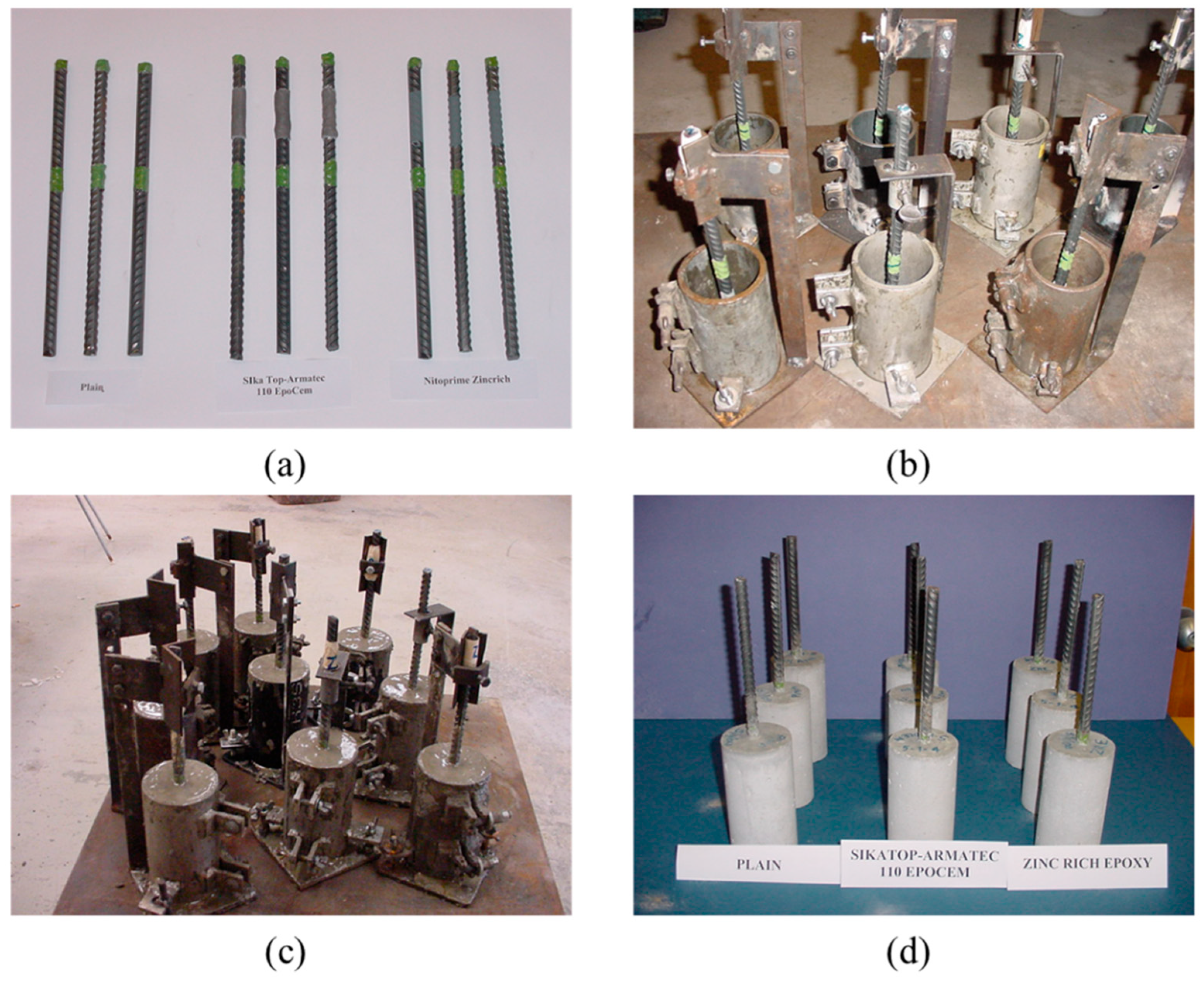


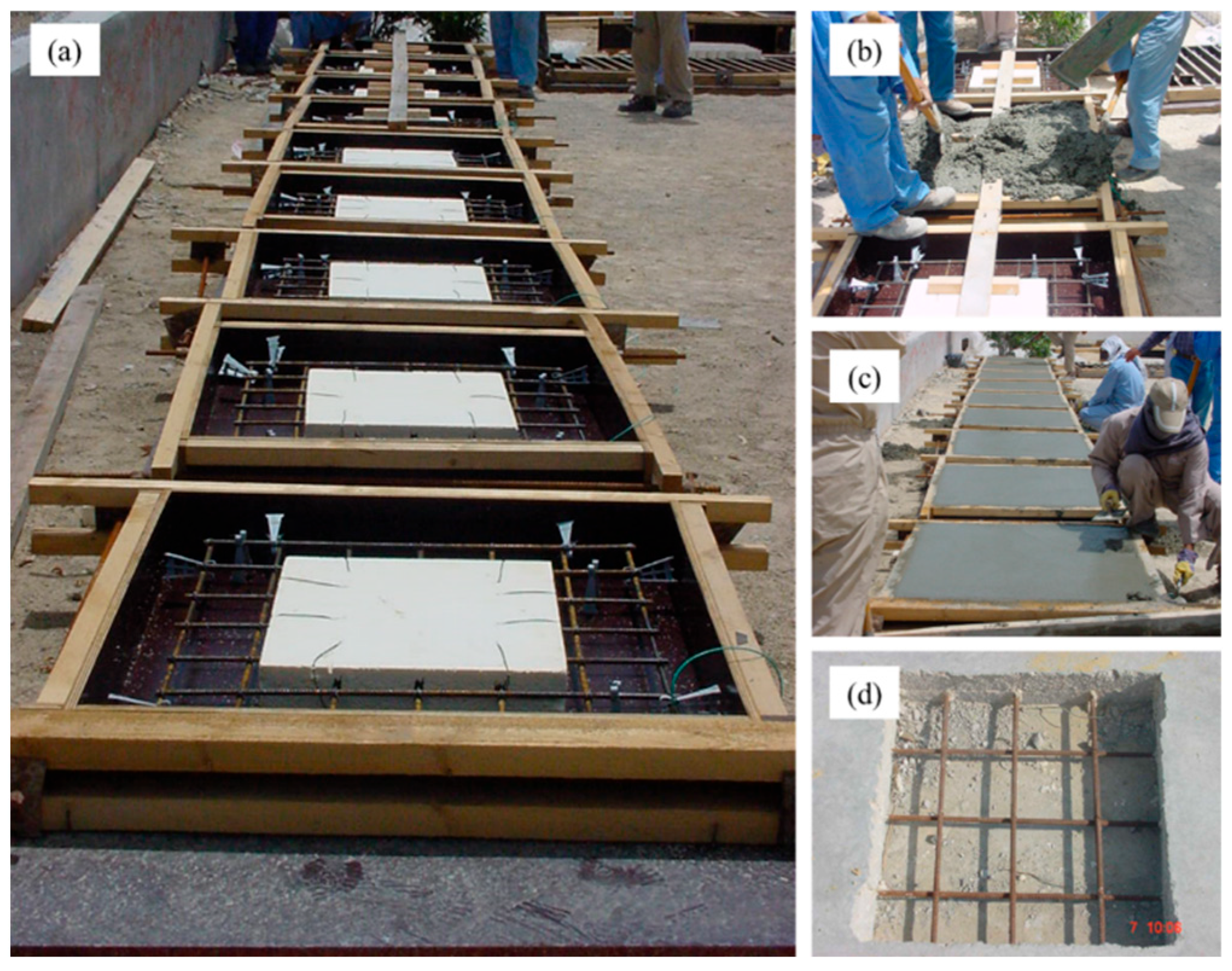
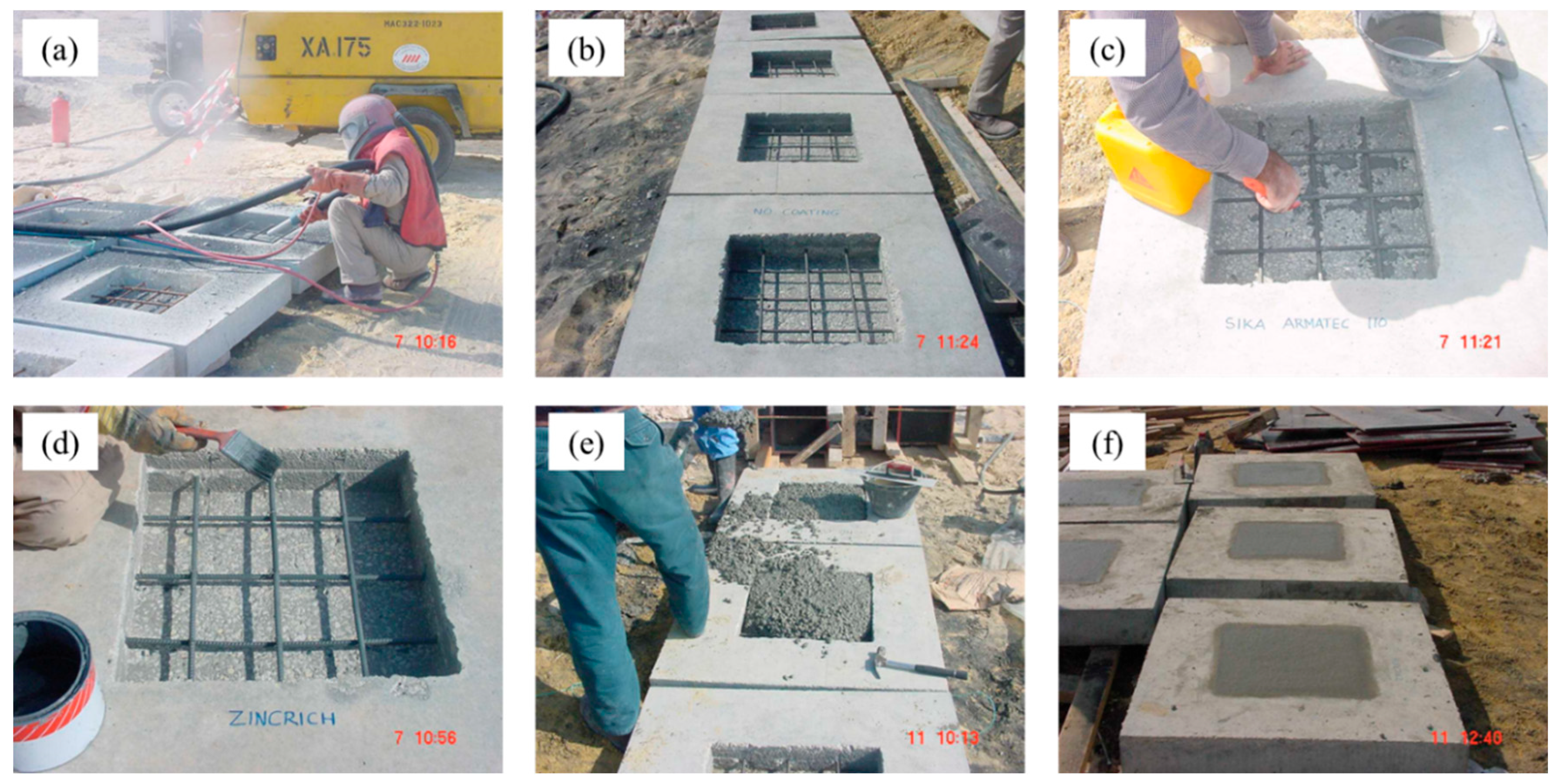


| Coating Type | Composition |
|---|---|
| Cement-based coating | Cementitious, three-component epoxy resin |
| Zinc-rich epoxy coating | Zinc primer, supplied as a single component grey-colored liquid based on metallic zinc and epoxy resin |
| Specimen ID | Details | ||
|---|---|---|---|
| L-UnC-1 | L-UnC-2 | L-UnC-3 | Lab cylindrical specimens with uncoated steel bars |
| F-UnC-1 | F-UnC-2 | F-UnC-3 | Field slab specimens with uncoated steel bars |
| L-CmC-1 | L-CmC-2 | L-CmC-3 | Lab cylindrical specimens with cement-based coating on the steel bars |
| F-CmC-1 | F-CmC-2 | F-CmC-3 | Field slab specimen with cement-based coating on the steel bars |
| L-ZnC-1 | L-ZnC-2 | L-ZnC-3 | Lab cylindrical specimens with zinc-rich epoxy coating on the steel bars |
| F-ZnC-1 | F-ZnC-2 | F-ZnC-3 | Field slab specimens with zinc-rich epoxy coating on the steel bars |
| Details | Value |
|---|---|
| Salt solution | 5% NaCl |
| pH of the salt solution | 6.8 to 7.2 |
| Air supply | 20 psi |
| Chamber temperature | 35 ± 1 °C |
| Period of exposure | 1000 h |
| Relative humidity | 80% |
| Specimen Designation | Time to Crack, h | Average Time to Crack, h |
|---|---|---|
| L-UnC-1 | 135 | 113 |
| L-UnC-2 | 130 | |
| L-UnC-3 | 75 | |
| L-CmC-1 | 240 | 282 |
| L-CmC-2 | 265 | |
| L-CmC-3 | 340 | |
| L-ZnC-1 | 75 | 110 |
| L-ZnC-2 | 125 | |
| L-ZnC-3 | 130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dulaijan, S.U. A Laboratory and Field Assessment of the Performance of Rebar Coatings. Materials 2023, 16, 4270. https://doi.org/10.3390/ma16124270
Al-Dulaijan SU. A Laboratory and Field Assessment of the Performance of Rebar Coatings. Materials. 2023; 16(12):4270. https://doi.org/10.3390/ma16124270
Chicago/Turabian StyleAl-Dulaijan, Salah U. 2023. "A Laboratory and Field Assessment of the Performance of Rebar Coatings" Materials 16, no. 12: 4270. https://doi.org/10.3390/ma16124270




