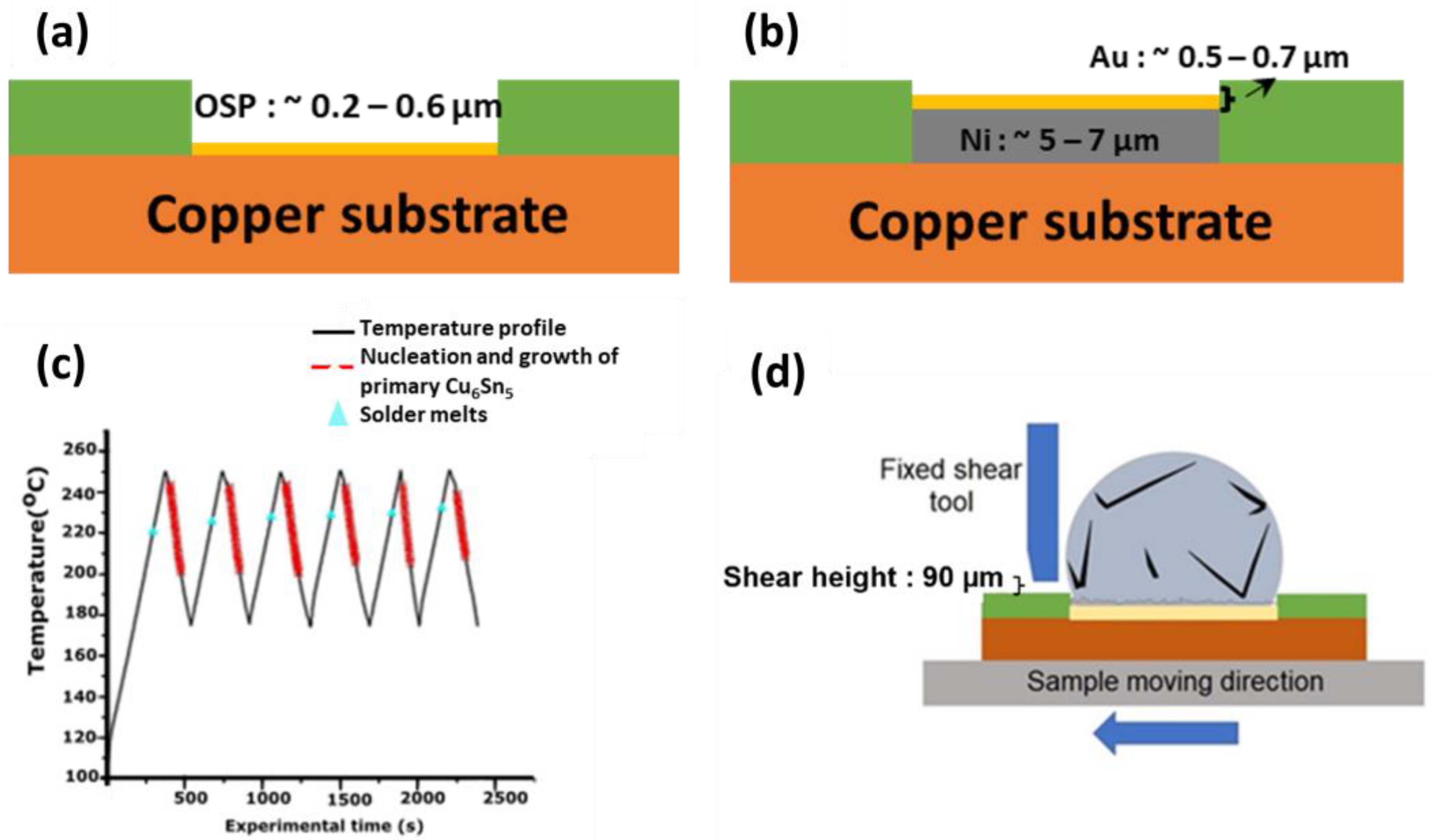
3.1. In Situ Imaging of Primary Intermetallic Growth during Multiple Reflows
In situ synchrotron X-ray imaging with a peak temperature of 250 °C was used to observe the nucleation and growth of the primary crystals in the solder of Sn-3.5Ag soldered on Cu-OSP and ENIG surface finish during multiple reflows.
Figure 4 and Figure 6 demonstrate the solidification of the Cu
6Sn
5 and Ni
3Sn
4, and the (Cu, Ni)
6Sn
5 primary crystals for Sn-3.5Ag/Cu-OSP and Sn-3.5Ag/ENIG solder joints for each reflow cycle. The dark rods in the solder ball are the primary IMCs, the grey part is the Sn liquid solder, and the brighter colors are the shallow bubbles at the central solder. Apart from that, the interface between the solder Sn-3.5Ag and substrate are flux voids. The formation of bubbles was due to the flux outgassing process, where some gases are trapped at the solder interface during the soldering process. The size of the bubble increased gradually with the increasing temperature. As can be observed from
Figure 4a–d, there are “multi-bubbles” located at the interface during the whole soldering process that remained even after the fourth time reflow with increased volume. The “multi-bubbles” were obtained from an interaction of small bubbles with each other and expanded to a larger bubble volume. After reaching a specific time, some bubbles, including the “multi-bubbles,” disappeared due to the high-pressure content. Nevertheless, these will not be discussed further in this study.
As depicted in
Figure 4, it can be seen that the Cu
6Sn
5 primary intermetallics formed in various morphologies that consist of a mixture of a hexagonal rod, hollow faceted hexagonal rods, and “in-plane” branched form. Several studies reported that the different morphologies of Cu
6Sn
5 could be influenced by different factors, including the Cu content, the undercooling, and the cooling rate [
13,
14,
15]. In this research, it is observed that the formation of primary Cu
6Sn
5 crystals in a joint of Sn-3.5Ag/Cu-OSP can be categorized into three solidified locations based on reflow cycles: (i) first reflow, (ii) from second to fourth reflow, and (iii) from fifth to sixth cycle; these will be discussed later. The first category, shown in
Figure 4a, represents the solidification of the as-reflowed joints. Note that many primary Cu
6Sn
5 intermetallics with the hexagonal rod shape began nucleating and growing at the interface area and large faceted hexagonal rods with hollow areas marked as A, B, and C. During the early nucleation, the Cu
6Sn
5 crystal (A, B, and C) grew as filled rods and, after a few seconds, the hollowness started to widen perpendicularly to the growth tip along the rods. Additionally, the “in-plane” branched type of primary Cu
6Sn
5 nucleated in the Sn-3.5Ag/Cu-OSP was observed, marked D, E, F, G, and H. The growth kinetics and the apparent number of primary Cu
6Sn
5 in the Sn-3.5Ag/Cu-OSP were measured and plotted in a graph shown in
Figure 5.
After the first reflow cooled to ~173 °C at a rate of 0.33 °C/s, the solder was reflowed again for up to six cycles with the similar solder joint and temperature profile used at the first cycle. For the second category, it can be seen that the solidification of the primary Cu
6Sn
5 in the Sn-3.5Ag/Cu-OSP joint after the second reflow has a preferred nucleation point up to the fourth reflow cycle, where the primary intermetallic formed was quite similar, as presented in
Figure 4b–d. However, as can be seen, the growth location of Cu
6Sn
5 primary during solidification for the second cycle was different from the as-reflowed joints in
Figure 4a. Similar to the as-reflowed joints, the morphology of the Cu
6Sn
5 that formed after the second reflow is in the mixture of hexagonal rods, faceted hollow rods, and “in-plane” branched but is different in the number of crystals formed. It can be concluded that the number of primary Cu
6Sn
5 crystals increased considerably with the increasing reflow cycle due to the increment of Cu diffused from the substrate during the soldering process, as plotted in
Figure 5. Additionally, increasing the number of reflows, the average growth rate of the formation of Cu
6Sn
5 primary found became slower from 16.67 µm/s (first cycle) to 0.86 µm/s (sixth cycle) due to the diffusion barrier caused by the formation of IMC denser at the interface area and the saturation of the solder. Moreover, some transition of the primary Cu
6Sn
5 crystals’ morphologies occurs from the hexagonal rods to the in-plane branched due to the increased Cu diffused after multiple reflow cycles.
The third category refers to the fifth and sixth cycles of the Sn-3.5Ag/Cu-OSP solder joint, with a similar pattern in the nucleation location and growth of primary Cu
6Sn
5 that formed as displayed in
Figure 4e,f. During the fifth reflow process, a continuous layer of multi-bubbles suddenly burst and disappeared due to the higher gas pressure inside the bubbles [
16]. Henceforth, it is seen that the Cu
6Sn
5 primary began nucleating and growing from the interface area, as shown in
Figure 4e. It may be inferred that the formation of bubbles and interfacial voids at the interface can affect the growth of the IMC. As studied by Kunwar et al., the formation of bubbles at the interface could hinder the diffusion of Cu at the area of the bubbles and the resulting poor strength of the solder joints [
17]. As per
Figure 4e,f, most primary Cu
6Sn
5 formed are the “in-plane” branched type, and some of the primary crystals that start nucleates near the interface area are formed in the faceted hollow rods. Other than that, the development of the “in-plane” branched Cu
6Sn
5 in Sn-3.5Ag/Cu-OSP after the fifth cycle was added to by the continuous Cu diffusion from the substrate into the solder during multiple reflows, resulting in a higher concentration of Cu in the matrix of the solder. During the sixth reflow, the solder and primary IMC only partially melted due to the saturation limit. As can be observed, no new primary IMC can be formed since the β-Sn nucleates faster due to the saturation limit of the solder. Only a few pre-existing primary Cu
6Sn
5 partially melt during the heating and continue to grow until they are solidified.
Figure 6 illustrates a synchrotron radiograph of the solder joint solidification of Sn-3.5Ag/ENIG after multiple reflows six times. As seen in
Figure 6, there is a trapped interfacial void form at the interface; it continually expanded and remained until the sixth cycle. The increase in the radius void can be explained due to the heating process where the solder joint is subjected to multiple reflows. After being reflowed, a small amount of Ni
3Sn
4 was formed at the interface area because the Ni atoms diffused from the substrate and reacted with the Sn solder alloy. Apart from that, the formation of Ni
3Sn
4 was observed to be concentrated at the bottom area near the solder joint and remained after prolonged reflow of six numbers. No visible significant primary phases were observed during the multiple reflows up to the third cycle. However, the nucleation and growth of the small size of the primary (Cu, Ni)
6Sn
5 were observed at the bulk solder area after five reflows. This was found due to the limited amount of Ni content in a thin layer of ENIG surface finish (~5–7 µm), where some of the Ni atoms were already consumed at the previous reflow cycles to form Ni
3Sn
4 at the interfacial. Moreover, the (Cu, Ni)
6Sn
5 that formed in the Sn-3.5Ag/ENIG solder joint could be due to the Cu dissolution from the substrate during the process of soldering after the fifth reflow cycle.
It can be confirmed via the EDS analysis of the synchrotron sample after the sixth reflow, as shown in
Figure 7b; small (Cu, Ni)
6Sn
5 intermetallics started forming at solidified at the fifth cycle. Additionally, by using the EDS analysis as per
Figure 7b at point 3, a small Ag
3Sn was detected at the interfacial area after the third reflows, whereas, in the Sn-3.5Ag/Cu-OSP samples, the growth rate of (Cu, Ni)
6Sn
5 also decelerated from 1.82 µm/s at the fifth cycle to 0.48 µm/s due to the concentration at the interface area. The slower growth rate of IMC with respect to the multiple reflows could be attributed to the changing diffusion path of the atoms during the solid–liquid–solid interaction. Additionally, the initial IMC layers at the interface formed after the as-reflowed joint might retard the dissolution during the subsequent reflow.
3.2. Interfacial Intermetallic Layer Formation and Growth after Multiple
A continuous intermetallic layer can provide reliable interconnection bonding between the solder and substrate with an appropriate thickness in the solder joint. However, excessive growth of the IMC’s layer will degrade the solder joint reliability.
Figure 8 and
Figure 9 depict the SEM cross-sectional images of the solder joints in Sn-3.5Ag/Cu-OSP and Sn-3.5Ag/ENIG focusing on the interfacial IMC after multiple reflows up to six cycles, respectively. These samples were prepared separately from the in situ synchrotron imaging experiments. As shown in
Figure 8, the Cu-Sn IMC layer is generated at an interface between the solder and substrate for Sn-3.5Ag/Cu-OSP solder joint. Using the EDS, the Cu
6Sn
5 phase was detected where it was composed of Cu 53.61 at.% and Sn 43.46 at.%. The Cu
6Sn
5 layer forms due to the heterogenous nucleation and the Cu diffusion from the substrate into the solder at the interface reaction between the Sn and Cu. The Ag
3Sn also formed in small sizes that were finely dispersed into the eutectic region. At the as-reflowed joint of the Sn-3.5Ag/Cu-OSP solder joint, a scallop-liked Cu
6Sn
5 IMC layer was observed at the interface. It is worth noting that the scallop-liked Cu
6Sn
5 grew and became thicker, transforming into planar-liked morphology after a prolonged reflow of sixth cycles. The changes could be attributed to the higher diffusion paths at the channels between the scallops that allow the Cu from the substrate to diffuse faster through it and also owing to the ripe flux of the Cu
6Sn
5 layer growth [
18]. Additionally, fewer cracks also occur in the Cu
6Sn
5 layer parallel to the substrate after the fifth reflow cycle in
Figure 8e. These cracks’ formation is caused by the stress generated in the IMCs during the multiple reflow process.
Figure 9a–f represents the Sn-3.5Ag/ENIG solder joint’s micrograph after being reflowed six times. It can be observed that, after the Sn-3.5Ag solder reacts with the Ni-containing surface finish, a loose-like thin Ni
3Sn
4 intermetallic layer was formed at the interface. It also can be seen that there is a dark Ni-P-rich layer present underneath the Ni
3Sn
4, marked as a red dash line in
Figure 9a. With the help of EDS analysis, it was confirmed that the interfacial microstructure in Sn-3.5Ag/ENIG joints consists of Ni
3Sn
4 and a Ni-P rich IMC layer composed of Ni (43.28 at.%), Sn (56.72 at.%), Ni (81.40 at.%), and P (11.07 at.%), respectively. As stated by Kumar et al., the P element was produced during the electroless Ni plating process and then reacted with the Ni layer substrate to form the Ni
3Sn
4 [
19]. This observation aligns with previous studies that pointed out that the Ni
3Sn
4 formed at the interface between Sn-3.5Ag and the Ni substrate [
5]. Even though the Ni
3Sn
4 IMC formed as a spallation on the Sn-3.5Ag/ENIG solder joints, it was discovered to have no effect on the strength of the solder joints. Note that a thin layer of (gold) Au appeared to have fully dissolved into the solder matrix after being reflowed. Nevertheless, no Au element detected in the interface area might be due to the detection limit of EDS [
20]. Additionally, no large Cu-Sn-Ni IMC was found in the Sn-3.5Ag/ENIG solder joint due to the Ni layer’s capability to suppress the Cu dissolution from the substrate up to the third cycle reflow. However, there was a Cu-Sn-Ni IMC formation detected at the sixth reflow cycles, as found in
Figure 7b point 4 with the IMC composed of Ni (11.23 at.%), Sn (74.92 at.%), and Cu (13.64 at.%), since the dissolution of Cu can occur after the fourth reflow cycle. Similar to the Sn-3.5Ag/Cu-OSP joints, as the number of reflow cycles increased, the size and thickness of the Ni
3Sn
4 interface layer increase as well, as depicted in
Figure 9f.
The quantitative measurement of the thickness of each solder joint was conducted after the sixth reflow cycle by dividing the total area of the IMC layer and the total length of the IMC. The mean thickness layer for each cycle was plotted in
Figure 10a to compare the different thickness growths over multiple reflow cycles on both solder joints. The results indicate that the mean thickness of the Cu
6Sn
5 layer at the Sn-3.5Ag/Cu-OSP interface increased from ~4.98 μm after the first reflow to a maximum of ~11.28 after the sixth cycle. For the Sn-3.5Ag/ENIG solder joints, the mean thickness of the Ni
3Sn
4 layer grew from ~2.03 μm after being reflowed to ~4.25 μm after the sixth reflow. Both the thicknesses of Cu
6Sn
5 and Ni
3Sn
4 IMC’s layers increased linearly as the joints were subjected to multiple reflows. However, the Cu
6Sn
5 IMC layer on Sn-3.5Ag/Cu-OSP was ~3 times thicker relative to the Ni
3Sn
4. This can be attributed to the rapid growth of the interfacial layer from the dissolution/diffusion of Cu from the substrate.
Additionally, the thin layer of Ni
3Sn
4 in the Sn-3.5Ag/ENIG solder joint that appears after performing multiple reflows could be due to the Ni layer’s effectiveness in acting as a diffusion barrier. Besides that, the formation of this thin layer was due to the formation of a pre-existing IMC layer at the initial reflow that helps to inhibit the diffusion between Cu from the substrate and solder. These results agreed with the study by Zhong et al. [
3]. According to the literature, a Cu
3Sn IMC layer may be present at the interface between the Cu
6Sn
5 and Cu substrate after exposure to high-temperature aging. Nevertheless, there are no Cu
3Sn phases detected on the Sn-3.5Ag/Cu-OSP interface due to the limitation of the characterization used.
It is known that the kinetic growth of the IMC layer can be either diffusion controlled or interfacial reaction controlled. The relationship between the thickness of the interfacial layer with respect to multiple reflows can be expressed according to the power–law equation as follows:
where
W is the average thickness of the interfacial layer,
k is the growth rate constant,
t is the reflow time above the liquidus temperature during reflow soldering, and
n is the time exponent. By plotting the graph of the interfacial thickness against the reflow time (
Figure 10b) and against the square root of the reflow time (
Figure 10c), both the
k and
n values are obtained from the slope of the linear regression curve, respectively. The IMC growth mechanism is controlled by grain boundary diffusion if the
n is 0.33. When the time exponent time
n is 0.5 or 1, the growth mechanism of the interfacial layer is controlled by a bulk diffusion-controlled or an interface reaction rate-controlled process, respectively [
4]. Note that the value of the time exponent
n in this study for Cu
6Sn
5 in Sn-3.5Ag/Cu-OSP and Ni
3Sn
4 in Sn-3.5Ag/ENIG joint was 0.47 and 0.42, respectively. The exponent time of the Cu
6Sn
5 and Ni
4Sn
4 values obtained was close to 0.5, indicating that a bulk diffusion-controlled mechanism governed the growth of the interfacial IMC layers. Thus, increasing the IMC layer during multiple reflows followed the square root time law, where
W = kt0.5 for both solder joints. Based on the
k value, the results present that the Sn-3.5Ag reflowed on the Cu-OSP substrate had a slightly higher growth rate constant relative to the ENIG with values of 0.58 and 0.21 µm/s, respectively. Therefore, it can be concluded that the rapid growth of the interfacial layer occurs at the Cu interface linearly with the square root of the reflow time. Additionally, it can be inferred that the Ni layer from the ENIG surface finish can be regarded as an effective diffusion barrier that hindered the formation of Cu
6Sn
5 intermetallics by suppressing the Cu dissolution from the substrate during the soldering process.
3.3. Solder Joint Strength
The strength of the Sn-3.5Ag/Cu-OSP and Sn-3.5Ag/ENIG solder joints after six reflows was evaluated at shear speeds of 100 mm/s and 2000 mm/s. Lower shear speeds were used to determine the strength of the solder joint at the solder matrix area, while higher shear speeds were used to determine the strength near the/at the interfacial layer of the joint.
Figure 11a represents the solder joint strength of Sn-3.5Ag reflowed on Cu-OSP and ENIG after the first, third, and sixth reflow cycles at 100 mm/s shear speeds. The result revealed that the Sn-3.5Ag/ENIG has a higher average shear strength at first reflowed with 57 N compared to the Sn-3.5Ag/Cu-OSP joint with an average shear strength of 40 N. When increasing the number of reflow cycles up to the sixth cycle, the Sn-3.5Ag/ENIG solder joint was observed to have slightly decreased its strength by 6% to 50 N, while, for the Sn-3.5Ag/Cu-OSP joints, this decreased by 20–35% to 25 N. Here, the joint strength decreased as the number of reflow cycles increased due to the increasing number of large brittle Cu
6Sn
5 primary intermetallics.
Figure 11b illustrates the average shear strength of Sn-3.5Ag/Cu-OSP and Sn-3.5Ag/ENIG solder joints when tested at a 2000 mm/s shear speed for the first, third, and sixth reflow cycles. Similar to the solder joint strength tested at 100 mm/s shear speed, the Sn-3.5Ag/ENIG solder joints showed a higher average shear strength than Sn-3.5Ag/Cu-OSP at a 2000 mm/s shear speed. However, the results indicate that the shear strength of Sn-3.5Ag/ENIG slightly decreased by ~8% from 66 N to 56 N, while, for Sn-3.5Ag/Cu-OSP, the joint strength decreased by ~16% from 45 N to 33 N after multiple reflows. The significant decrement of Sn-3.5Ag/Cu-OSP solder joint strength after shear testing at 2000 mm/s could be attributed to excessive Cu dissolution during multiple reflows. Therefore, this resulted in a thicker formation of the Cu
6Sn
5 intermetallic layer at the interfacial area and also formed large brittle Cu
6Sn
5 primary crystals at the solder bulk area relative to the Sn-3.5Ag/ENIG joints.
After the shear tests, the samples were inspected via SEM analysis to clarify each sheared solder joint’s failure modes.
Figure 12 demonstrates the fracture surfaces mode analysis of the Sn-3.5Ag/Cu-OSP and Sn-3.5Ag/ENIG solder joints after the shear test at 100 mm/s and 2000 mm/s shear speeds. Previous researchers reported that the crack location would generally form at the bulk solder area when sheared at the low shear speed [
21,
22,
23,
24]. At the same time, the crack location for higher shear speed will form near/at the interfacial IMC area. As depicted in
Figure 12a–f, it can be observed that all multiple reflow samples for both solder joints crack at the bulk solder after shear tests at 100 mm/s. From the fracture modes result, it was observed that both solder joints were in a quasi-ductile mode where more than 50% of the solder residue is left after shearing at 100 mm/s. Meanwhile,
Figure 12g–l shows the crack along the interface area for solder joints when tested with a shear speed of 2000 mm/s. However, after multiple reflows, the fracture surface for both solder joints revealed a quasi-brittle mode that was significantly affected by the thickness of the IMC layer. Here, the exposed brittle IMC area was more dominant than the retained solder area.
3.4. Finite Element Analysis
The current study chose the shear speed of 100 mm/s for the shear test condition in Sn-3.5Ag/Cu-OSP solder joint as the base case. The effect of Cu
6Sn
5 primary crystal on the shear strength of the solder joints was determined.
Figure 13a depicts the shear force–displacement curves obtained via computational analysis, which were compared to the experimental data of the as-reflowed Sn-3.5Ag/Cu-OSP solder joints after the shear test. From
Figure 13a, the patterns of the curves from the FEA agree with the experimental data for the solder joints after being reflowed. The length of the primary crystal for FE modeling was constructed, followed by the experimental, in the range of 120–750 µm. Furthermore, the valid model compares the FEA with different sizes, amounts, and distributions of Cu
6Sn
5 primary intermetallics.
Figure 13b–d presents the contours of the equivalent von Mises stress, total deformation, and the equivalent elastic strain distribution of the solder joints in FEA. Note that the force (shear tools) was set to move from the right to the left sides in the
x-axis direction. The high total deformation in the solder joint was discovered to be at the tip of the solder ball after the strike with the shear ram. Additionally, the maximum contour of the von Mises stress and equivalent strain distribution occurred close to the junction of the solder ball as per
Figure 13b–d. Between
Figure 12a and
Figure 13b–d, the computational analysis result correlates well with the experimental solder joint, where both failure modes occurred near the interface. Even though the curve trend of the results obtained is similar, the result, such as the shear force values, is expected to vary from the simulation and experiment since many factors could influence the data accuracy. Other than that, the experimental error during sample preparation and some experimental factors are not included in the computational simulation. Nevertheless, the experimental results and the computational modeling under increasing the number of primary intermetallics show a similar decrease in the solder joint strength trends. Consequently, studying the effects of primary intermetallics using FEA is reliable.
The strength of the solder joint can be affected by many aspects, including forming primary intermetallics. As reported above, the formation of the primary will increase in numbers and sizes in the solder joints after performing the multiple reflows, resulting in the poor strength of the solder joints. Using the valid model in the current study (
Figure 13), the effects of the primary intermetallic were further investigated by manipulating the sizes, numbers, and distribution of the solder joints.
Figure 14 illustrates the shear force–displacement curves obtained from the simulation, where 16 primary Cu
6Sn
5 (followed by the validation model) with different sizes were constructed. The length of the small primary in
Figure 14a ranges between 50 and 120 µm, while the large primary in
Figure 14b is 130–450 µm. The finding shows that the smaller-sized Cu
6Sn
5 crystal that formed in the bulk Sn-3.5Ag solder joints slightly increased the solder joint strength compared to the larger sizes due to its brittle nature. More shear forces are required for a smaller size than the larger size of Cu
6Sn
5 crystal (
Figure 14c). These results aligned with our previous work, where the intermetallics formed in the SAC305 reflowed on the immersion tin (ImSn) were smaller. Note that they resulted in a higher solder joint strength than the immersion silver (ImAg) surface finish [
14].
Moreover, the numbers of primary intermetallic effects in the solder joints were also conducted using FEA, as shown in
Figure 15a. The primary crystals were designed in a 3D finite model using Solidworks, ranging between 130 µm and 750 µm, for 21 and 26 primaries. The results show that increased primary numbers resulted in a lower solder joint due to its brittleness. The total volume fraction was changed relatively when the Cu6Sn5 particles changed. Correspondingly, the distribution effect for each number from the data in
Figure 15a was investigated, and the results are presented in
Figure 15b–d. The distributions of the primary crystals were constructed into three categories: homogeneously, centerally, and peripherally distributed, as illustrated in
Figure 3. A similar length of the primary crystals was used to study the effect of the distributions, as in
Figure 15a.
Figure 15b–d shows the shear force–displacement curves for the different distributions obtained from the simulation. It can be seen from the curves graph simulations that the distribution of the Cu
6Sn
5 intermetallics formed in the solder joints slightly affected the strength of the solder joints. In this experimental and modeling simulation work, it can be concluded that the solder joint’s strength depended on the sizes and number of primary intermetallics formed. At the same time, the distributions of primary crystals did not significantly affect the solder joint’s strength.