Investigation of CO2 Adsorption on Avocado Stone-Derived Activated Carbon Obtained through NaOH Treatment
Abstract
1. Introduction
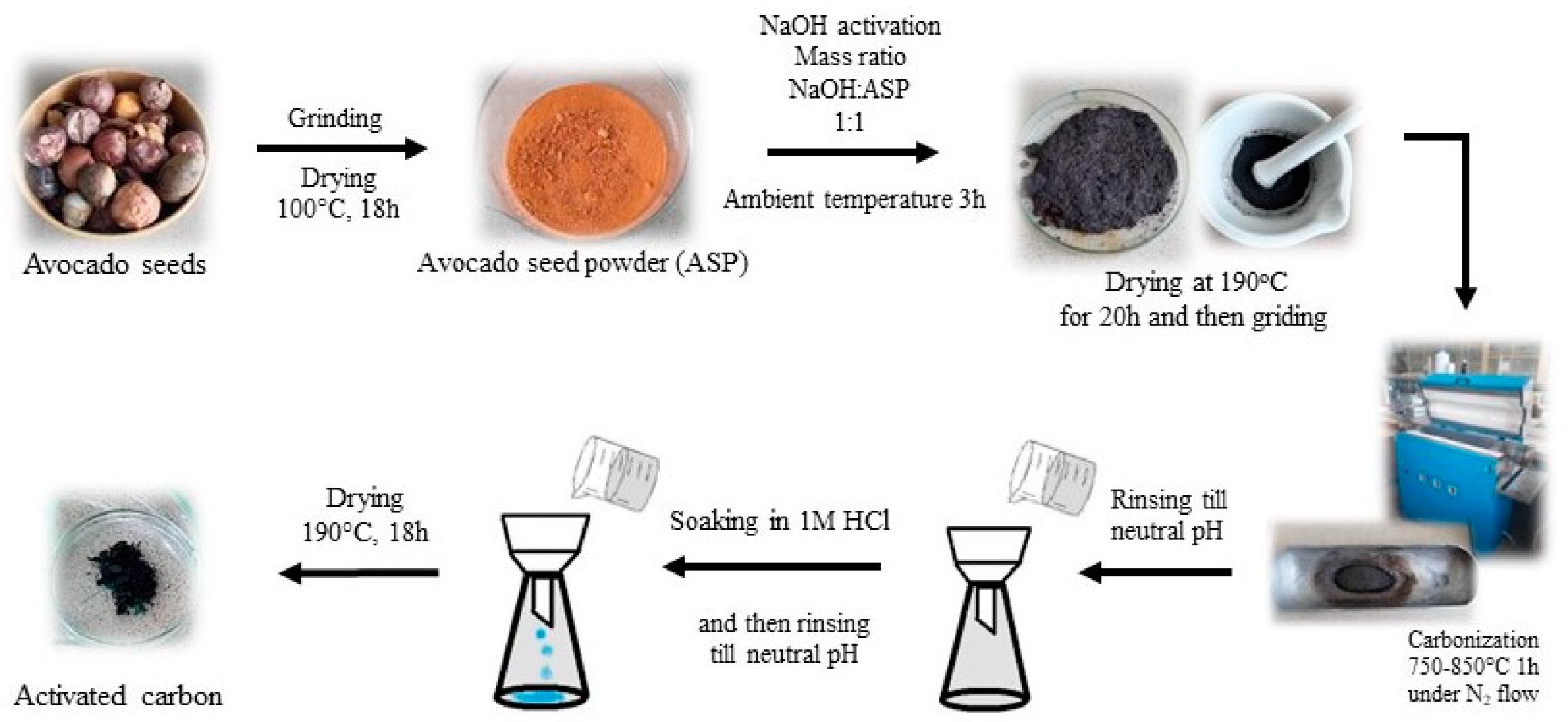
2. Materials and Methods
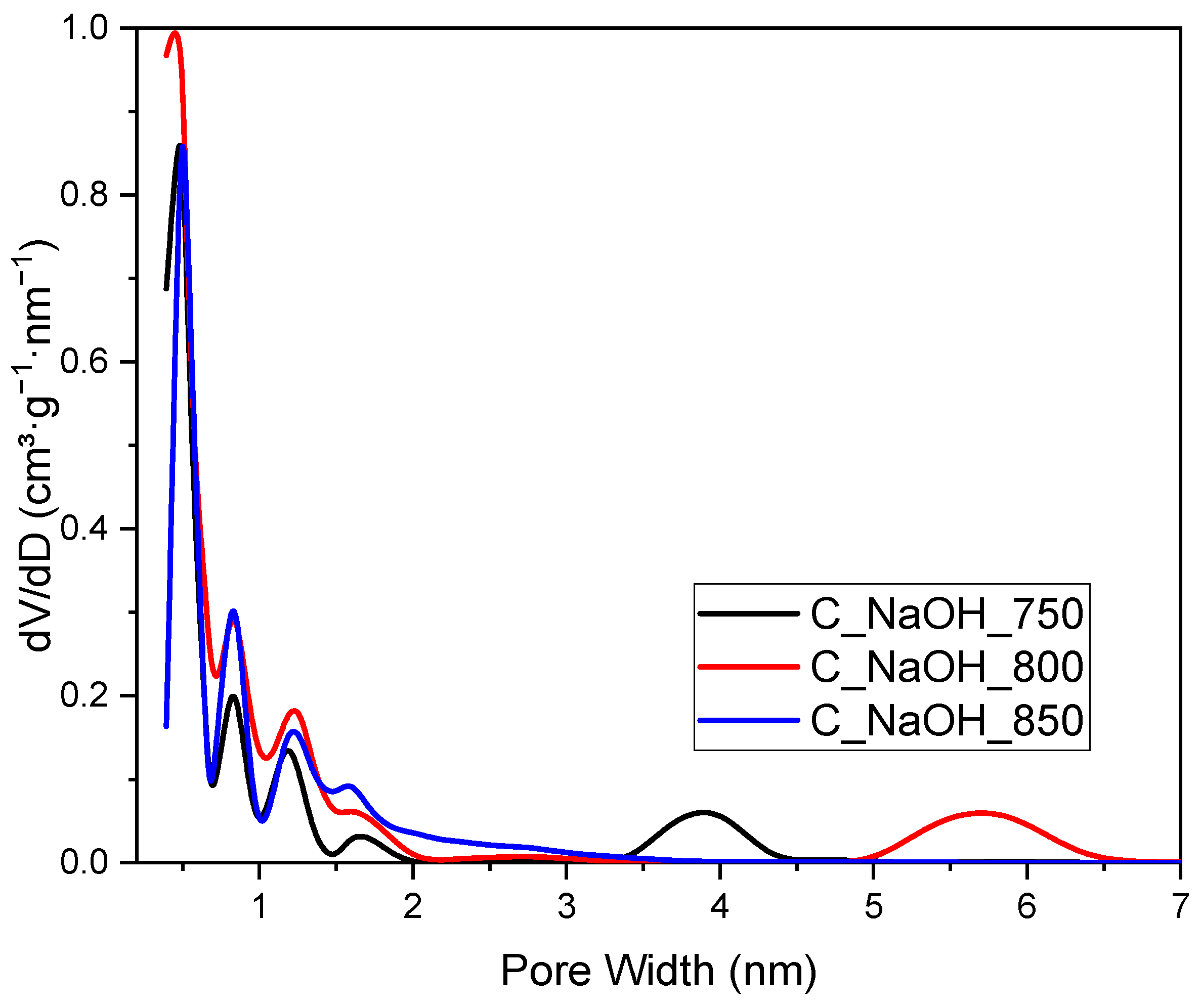
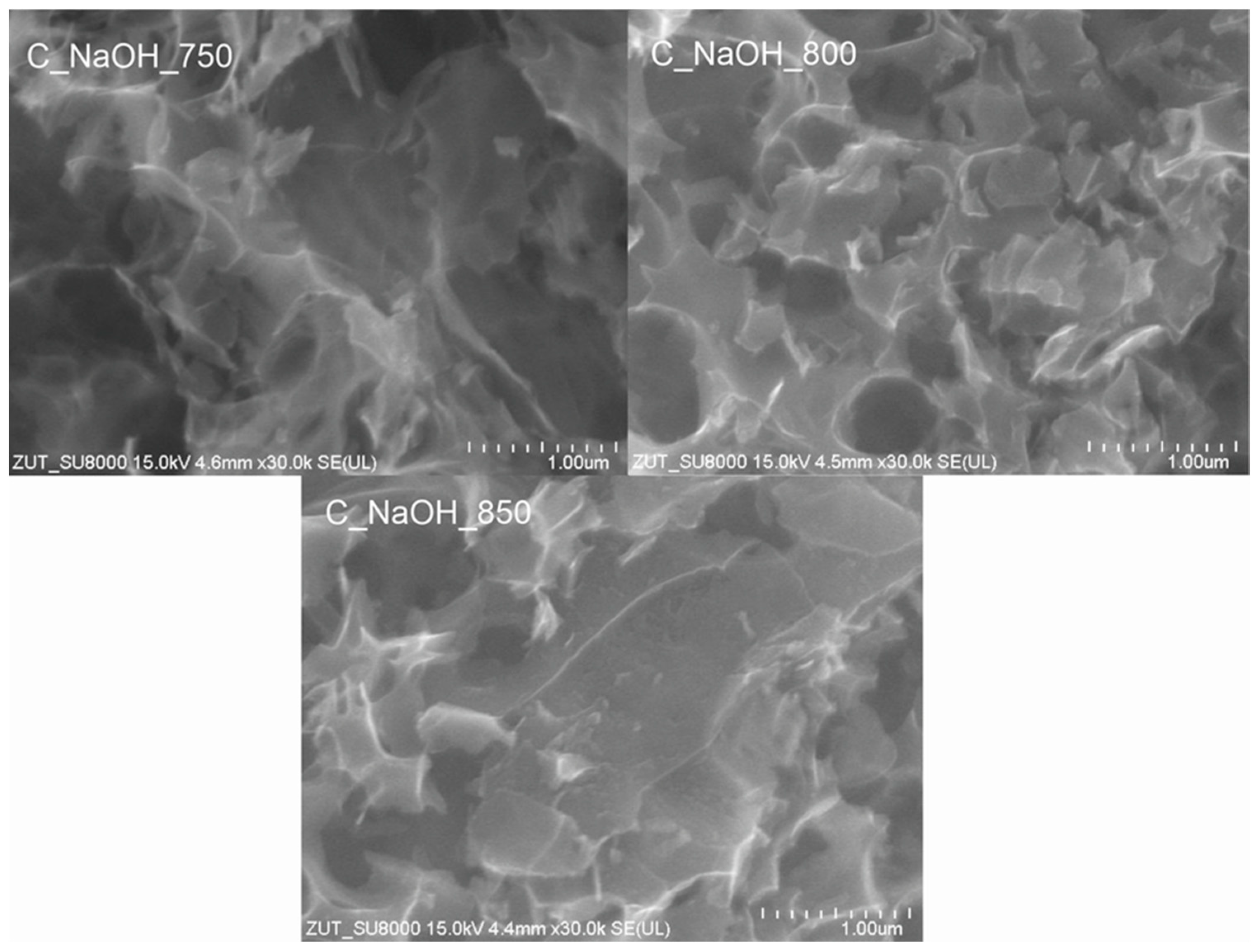
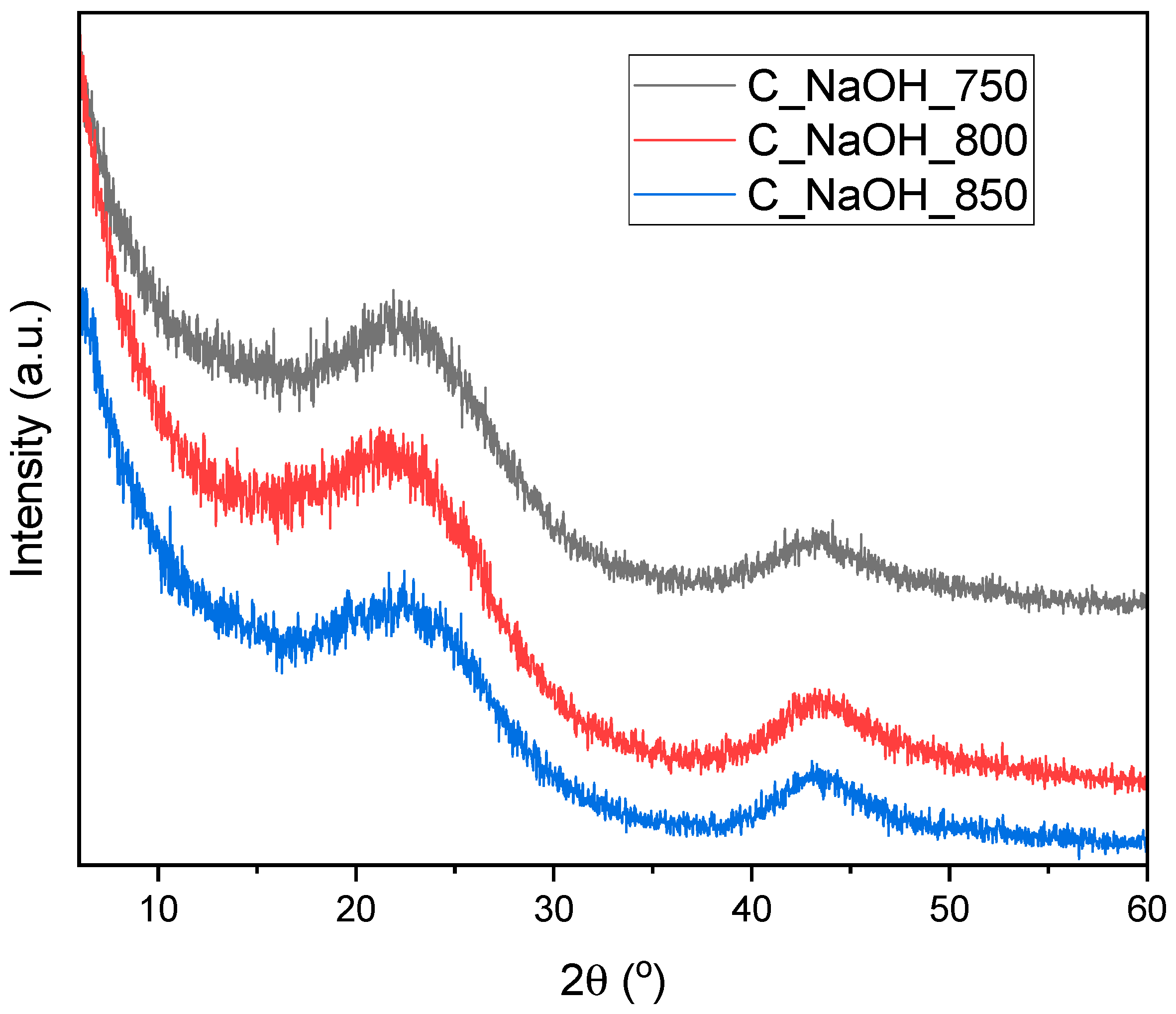
3. Results and Discussion
- q—gas equilibrium adsorption at pressure p;
- p—equilibrium pressure;
- qm—saturation capacity;
- b—equilibrium constant;
- n—exponential parameter representing the heterogeneity of the material.
- qe,o—theoretical adsorption capacity calculated from the model;
- qe,z—adsorption capacity determined experimentally;
- N—total number of measurements.
- xg1 (xg2)—the molar fractions of gas 1/gas 2 (g1/g2) in the adsorbed phase;
- yg1, (yg2)—the molar fractions of gas 1/gas 2 (g1/g2) in the bulk phase.
- -
- the changes in the gas absorption values (and the saturation capacity) with the increase in the temperature;
- -
- the isosteric heat of adsorption value.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Agriculture Data. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 5 June 2023).
- Domínguez, M.P.; Araus, K.; Bonert, P.; Sánchez, F.; San Miguel, G.; Toledo, M. The Avocado and Its Waste: An Approach of Fuel Potential/Application; Springer: Cham, Switzerland, 2014; pp. 199–223. [Google Scholar]
- Perea-Moreno, A.-J.; Aguilera-Ureña, M.-J.; Manzano-Agugliaro, F. Fuel Properties of Avocado Stone. Fuel 2016, 186, 358–364. [Google Scholar] [CrossRef]
- Elizalde-González, M.P.; Mattusch, J.; Peláez-Cid, A.A.; Wennrich, R. Characterization of Adsorbent Materials Prepared from Avocado Kernel Seeds: Natural, Activated and Carbonized Forms. J. Anal. Appl. Pyrolysis 2007, 78, 185–193. [Google Scholar] [CrossRef]
- Bhaumik, M.; Choi, H.J.; Seopela, M.P.; McCrindle, R.I.; Maity, A. Highly Effective Removal of Toxic Cr(VI) from Wastewater Using Sulfuric Acid-Modified Avocado Seed. Ind. Eng. Chem. Res. 2014, 53, 1214–1224. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; da Silva, M.L.C.P.; Alvarez-Mendes, M.O.; dos Reis Coutinho, A.; Thim, G.P. Phenol Removal from Aqueous Solution by Activated Carbon Produced from Avocado Kernel Seeds. Chem. Eng. J. 2011, 174, 49–57. [Google Scholar] [CrossRef]
- Leite, A.J.; Sophia, A.C.; Thue, P.S.; dos Reis, G.S.; Dias, S.L.; Lima, E.C.; Vaghetti, J.C.P.; Pavan, F.A.; de Alencar, W.S. Activated Carbon from Avocado Seeds for the Removal of Phenolic Compounds from Aqueous Solutions. Desalin. Water Treat. 2017, 71, 168–181. [Google Scholar] [CrossRef]
- Salomón-Negrete, M.Á.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Duran-Valle, C.J. Water Defluoridation with Avocado-Based Adsorbents: Synthesis, Physicochemical Characterization and Thermodynamic Studies. J. Mol. Liq. 2018, 254, 188–197. [Google Scholar] [CrossRef]
- Serafin, J.; Kiełbasa, K.; Michalkiewicz, B. The New Tailored Nanoporous Carbons from the Common Polypody (Polypodium vulgare): The Role of Textural Properties for Enhanced CO2 Adsorption. Chem. Eng. J. 2022, 429, 131751. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Effect of the Porous Structure in Carbon Materials for CO2 Capture at Atmospheric and High-Pressure. Carbon 2014, 67, 230–235. [Google Scholar] [CrossRef]
- Deng, S.; Hu, B.; Chen, T.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Activated Carbons Prepared from Peanut Shell and Sunflower Seed Shell for High CO2 Adsorption. Adsorption 2015, 21, 125–133. [Google Scholar] [CrossRef]
- Jang, H.M.; Yoo, S.; Choi, Y.-K.; Park, S.; Kan, E. Adsorption Isotherm, Kinetic Modeling and Mechanism of Tetracycline on Pinus Taeda-Derived Activated Biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Hsu, L.-Y. High-Porosity Carbons Prepared from Bituminous Coal with Potassium Hydroxide Activation. Ind. Eng. Chem. Res. 1999, 38, 2947–2953. [Google Scholar] [CrossRef]
- Li, P.; Xing, C.; Qu, S.; Li, B.; Shen, W. Carbon Dioxide Capturing by Nitrogen-Doping Microporous Carbon. ACS Sustain. Chem. Eng. 2015, 3, 1434–1442. [Google Scholar] [CrossRef]
- Silvestre-Albero, A.; Silvestre-Albero, J.; Martínez-Escandell, M.; Rodríguez-Reinoso, F. Micro/Mesoporous Activated Carbons Derived from Polyaniline: Promising Candidates for CO2 Adsorption. Ind. Eng. Chem. Res. 2014, 53, 15398–15405. [Google Scholar] [CrossRef]
- De Souza, L.K.C.; Wickramaratne, N.P.; Ello, A.S.; Costa, M.J.F.; da Costa, C.E.F.; Jaroniec, M. Enhancement of CO2 Adsorption on Phenolic Resin-Based Mesoporous Carbons by KOH Activation. Carbon 2013, 65, 334–340. [Google Scholar] [CrossRef]
- Zhu, Y.; Kolar, P. Investigation of Adsorption of p-Cresol on Coconut Shell-Derived Activated Carbon. J. Taiwan Inst. Chem. Eng. 2016, 68, 138–146. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-Activated Carbon of High Surface Area Produced from Coconut Shell: Kinetics and Equilibrium Studies from the Methylene Blue Adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of Tetracycline by NaOH-Activated Carbon Produced from Macadamia Nut Shells: Kinetic and Equilibrium Studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Tan, Y.L.; Islam, M.A.; Asif, M.; Hameed, B.H. Adsorption of Carbon Dioxide by Sodium Hydroxide-Modified Granular Coconut Shell Activated Carbon in a Fixed Bed. Energy 2014, 77, 926–931. [Google Scholar] [CrossRef]
- He, S.; Chen, G.; Xiao, H.; Shi, G.; Ruan, C.; Ma, Y.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Facile Preparation of N-Doped Activated Carbon Produced from Rice Husk for CO2 Capture. J. Colloid Interface Sci. 2021, 582, 90–101. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, G.; Xu, Y.; Zhao, P. One Step N-Doping and Activation of Biomass Carbon at Low Temperature through NaNH2: An Effective Approach to CO2 Adsorbents. J. CO2 Util. 2019, 33, 320–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Z.; Liu, X.; Liu, F.; Yan, Z.; Zhou, S.; Wang, J.; Deng, S. Synthesis of Palm Sheath Derived-Porous Carbon for Selective CO2 Adsorption. RSC Adv. 2022, 12, 8592–8599. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Cruz Junior, O.F.; Sreńscek-Nazzal, J. Preparation of Low-Cost Activated Carbons from Amazonian Nutshells for CO2 Storage. Biomass Bioenergy 2021, 144, 105925. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Biomass Derived Low-Cost Microporous Adsorbents for Efficient CO2 Capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Mumtaz, H.; Farhan, M.; Amjad, M.; Riaz, F.; Kazim, A.H.; Sultan, M.; Farooq, M.; Mujtaba, M.A.; Hussain, I.; Imran, M.; et al. Biomass Waste Utilization for Adsorbent Preparation in CO2 Capture and Sustainable Environment Applications. Sustain. Energy Technol. Assess. 2021, 46, 101288. [Google Scholar] [CrossRef]
- Kishibayev, K.K.; Serafin, J.; Tokpayev, R.R.; Khavaza, T.N.; Atchabarova, A.A.; Abduakhytova, D.A.; Ibraimov, Z.T.; Sreńscek-Nazzal, J. Physical and Chemical Properties of Activated Carbon Synthesized from Plant Wastes and Shungite for CO2 Capture. J. Environ. Chem. Eng. 2021, 9, 106798. [Google Scholar] [CrossRef]
- Yang, J.; Yue, L.; Hu, X.; Wang, L.; Zhao, Y.; Lin, Y.; Sun, Y.; DaCosta, H.; Guo, L. Efficient CO2 Capture by Porous Carbons Derived from Coconut Shell. Energy Fuels 2017, 31, 4287–4293. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Wang, D.; Liu, S.; Fang, G.; Geng, G.; Ma, J. From Trash to Treasure: Direct Transformation of Onion Husks into Three-Dimensional Interconnected Porous Carbon Frameworks for High-Performance Supercapacitors in Organic Electrolyte. Electrochim. Acta 2016, 216, 405–411. [Google Scholar] [CrossRef]
- Wu, Z.; Tian, K.; Huang, T.; Hu, W.; Xie, F.; Wang, J.; Su, M.; Li, L. Hierarchically Porous Carbons Derived from Biomasses with Excellent Microwave Absorption Performance. ACS Appl. Mater. Interfaces 2018, 10, 11108–11115. [Google Scholar] [CrossRef]
- Zhang, R.; Qiao, J.; Zhang, X.; Yang, Y.; Zheng, S.; Li, B.; Liu, W.; Liu, J.; Zeng, Z. Biomass-Derived Porous Carbon for Microwave Absorption. Mater. Chem. Phys. 2022, 289, 126437. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Leng, W.; Li, J.; Zhang, J.; Cai, Z.; Hassan, E. Carbon Nanostructure of Kraft Lignin Thermally Treated at 500 to 1000 °C. Materials. 2017, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Elsayed, I.; Song, X.; Shmulsky, R.; Hassan, E.B. Microporous Carbon Nanoflakes Derived from Biomass Cork Waste for CO2 Capture. Sci. Total Environ. 2020, 748, 142465. [Google Scholar] [CrossRef] [PubMed]
- Serafin, J.; Sreńscek-Nazzal, J.; Kamińska, A.; Paszkiewicz, O.; Michalkiewicz, B. Management of Surgical Mask Waste to Activated Carbons for CO2 Capture. J. CO2 Util. 2022, 59, 101970. [Google Scholar] [CrossRef]
- Młodzik, J.; Sreńscek-Nazzal, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Activated Carbons from Molasses as CO2 Sorbents. Acta Phys. Pol. A 2016, 129, 402–404. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Activated Carbon Spheres for CO2 Adsorption. ACS Appl. Mater. Interfaces 2013, 5, 1849–1855. [Google Scholar] [CrossRef]
- Deng, S.; Wei, H.; Chen, T.; Wang, B.; Huang, J.; Yu, G. Superior CO2 Adsorption on Pine Nut Shell-Derived Activated Carbons and the Effective Micropores at Different Temperatures. Chem. Eng. J. 2014, 253, 46–54. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular Bamboo-Derived Activated Carbon for High CO2 Adsorption: The Dominant Role of Narrow Micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.-H.; Gogotsi, Y. Effect of Pore Size on Carbon Dioxide Sorption by Carbide Derived Carbon. Energy Environ. Sci. 2011, 4, 3059. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Cruz Junior, O.F.; Sreńscek-Nazzal, J. Design of Highly Microporous Activated Carbons Based on Walnut Shell Biomass for H2 and CO2 Storage. Carbon 2023, 201, 633–647. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of Mixed-Gas Adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Herm, Z.R.; Swisher, J.A.; Smit, B.; Krishna, R.; Long, J.R. Metal−Organic Frameworks as Adsorbents for Hydrogen Purification and Precombustion Carbon Dioxide Capture. J. Am. Chem. Soc. 2011, 133, 5664–5667. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yuan, D.; Sculley, J.; Zhao, D.; Krishna, R.; Zhou, H.-C. Sulfonate-Grafted Porous Polymer Networks for Preferential CO2 Adsorption at Low Pressure. J. Am. Chem. Soc. 2011, 133, 18126–18129. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-C.; Xu, X.-K.; Chen, Q.; Zhuang, Z.-Z.; Bu, X.-H. Nitrogen-Rich Diaminotriazine-Based Porous Organic Polymers for Small Gas Storage and Selective Uptake. Polym. Chem. 2013, 4, 4690. [Google Scholar] [CrossRef]
- Wang, J.; Krishna, R.; Yang, J.; Dandamudi, K.P.R.; Deng, S. Nitrogen-Doped Porous Carbons for Highly Selective CO2 Capture from Flue Gases and Natural Gas Upgrading. Mater. Today Commun. 2015, 4, 156–165. [Google Scholar] [CrossRef]
- Yue, L.; Xia, Q.; Wang, L.; Wang, L.; DaCosta, H.; Yang, J.; Hu, X. CO2 Adsorption at Nitrogen-Doped Carbons Prepared by K2CO3 Activation of Urea-Modified Coconut Shell. J. Colloid Interface Sci. 2018, 511, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Serafin, J.; Baca, M.; Biegun, M.; Mijowska, E.; Kaleńczuk, R.J.; Sreńscek-Nazzal, J.; Michalkiewicz, B. Direct Conversion of Biomass to Nanoporous Activated Biocarbons for High CO2 Adsorption and Supercapacitor Applications. Appl. Surf. Sci. 2019, 497, 143722. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Kou, J.; Sun, L.-B. Fabrication of Nitrogen-Doped Porous Carbons for Highly Efficient CO2 Capture: Rational Choice of a Polymer Precursor. J. Mater. Chem. A 2016, 4, 17299–17307. [Google Scholar] [CrossRef]


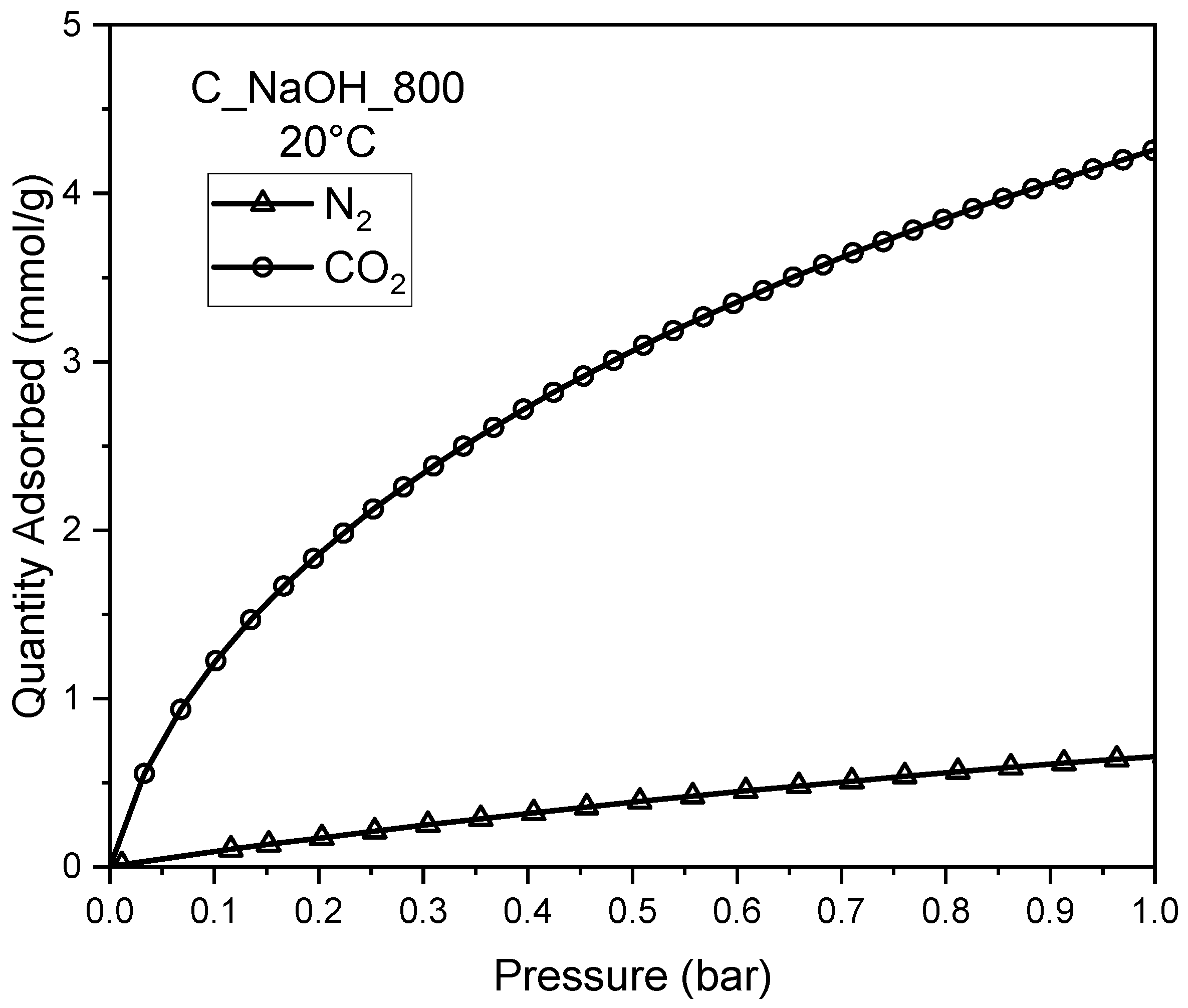
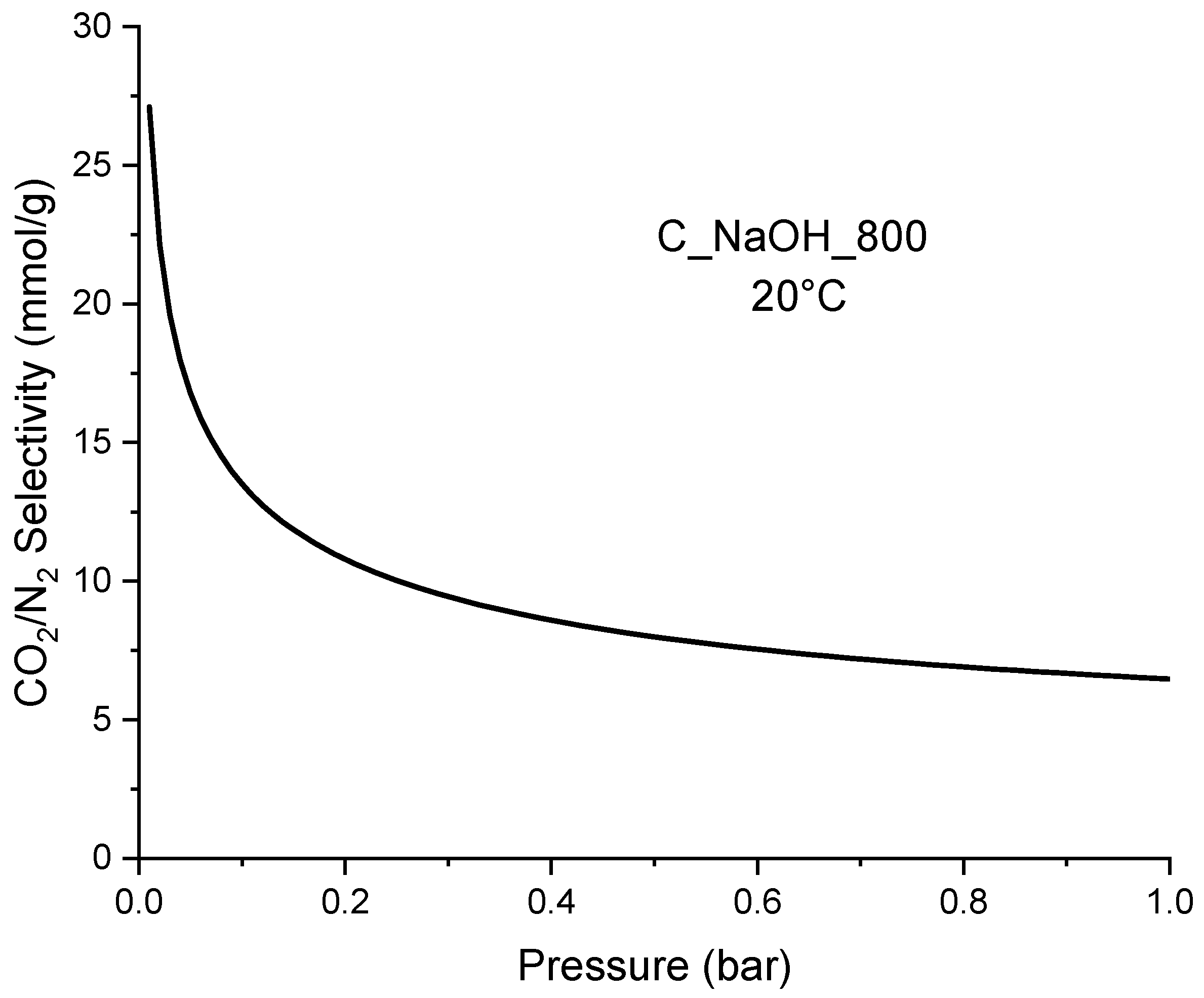
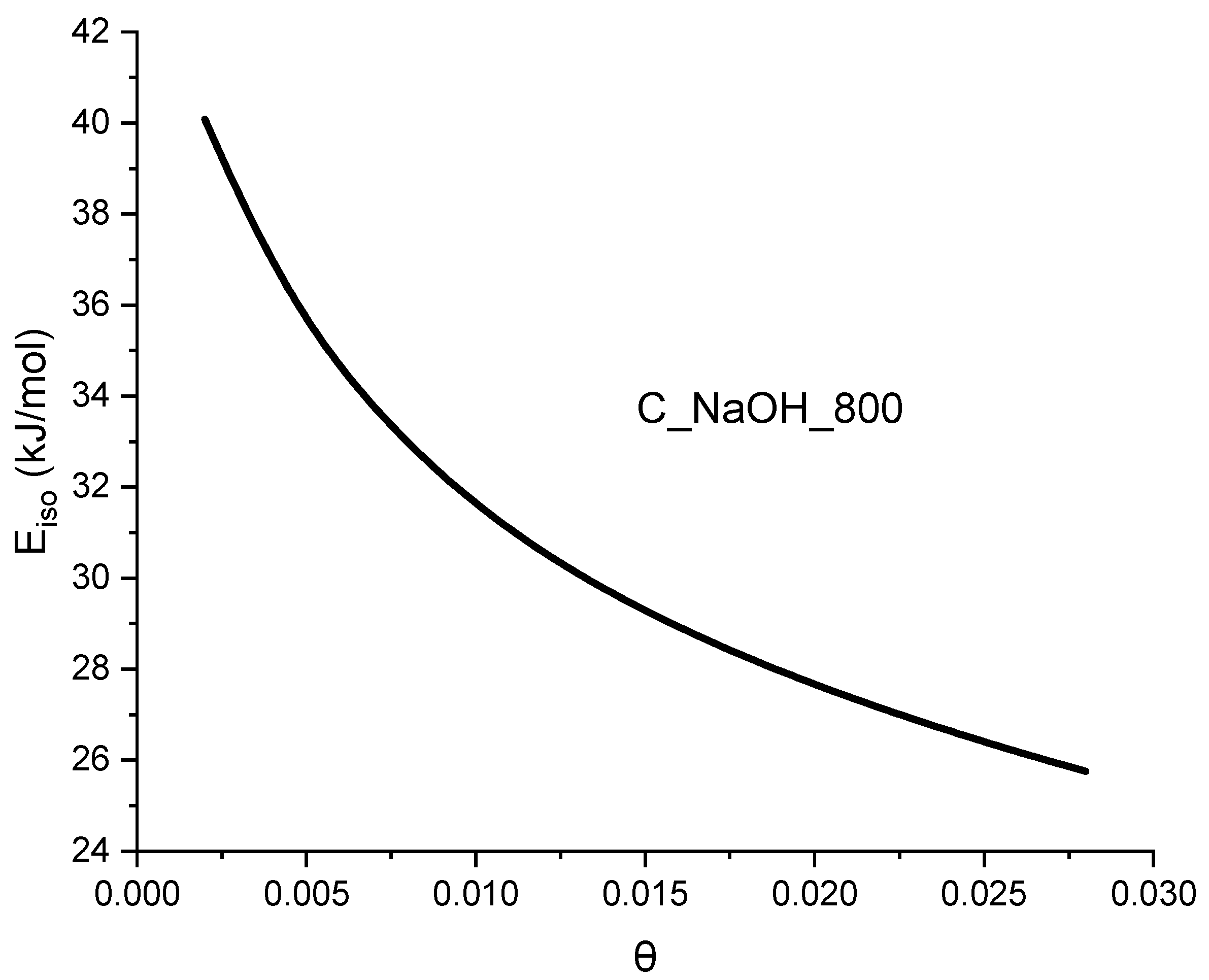
| Biomass | qCO2_0C | References |
|---|---|---|
| (mmol/g) | ||
| rice husk | 5.83 | [21] |
| walnut shell | 5.22 | [22] |
| palm sheath | 5.28 | [23] |
| Amazonian nutshells | 5.13 | [24] |
| lignocellulose | 5.20 | [25] |
| peanut shells | 5.20 | [26] |
| pomegranate peels | 3.90 | [26] |
| birch | 4.50 | [27] |
| coconut shell | 6.04 | [28] |
| corn cobs | 2.70 | [26] |
| AC | SSA | Vtot | Vmicro | maxD | qCO2_0C |
|---|---|---|---|---|---|
| [m2/g] | [cm3/g] | cm3/g | [nm] | [mmol/g] | |
| C_NaOH_750 | 817 | 0.538 | 0.259 | 297 | 4.6 |
| C_NaOH_800 | 1172 | 0.691 | 0.375 | 312 | 5.9 |
| C_NaOH_850 | 918 | 0.574 | 0.295 | 241 | 3.7 |
| AC | Temp. | qm | b | n | LSM |
|---|---|---|---|---|---|
| [°C] | [mmol/g] | [bar−1] | |||
| Carbon dioxide | |||||
| C_NaOH_750 | 0 | 10.26 | 0.84 | 0.62 | 4 × 10−3 |
| C_NaOH_800 | 0 | 14.46 | 0.69 | 0.64 | 7 × 10−3 |
| C_NaOH_850 | 0 | 12.21 | 0.43 | 0.65 | 4 × 10−3 |
| C_NaOH_800 | 20 | 10.59 | 0.67 | 0.72 | 15 × 10−3 |
| Nitrogen | |||||
| C_NaOH_800 | 20 | 2.34 | 0.39 | 0.99 | 3 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siemak, J.; Wróbel, R.J.; Pęksiński, J.; Michalkiewicz, B. Investigation of CO2 Adsorption on Avocado Stone-Derived Activated Carbon Obtained through NaOH Treatment. Materials 2023, 16, 4390. https://doi.org/10.3390/ma16124390
Siemak J, Wróbel RJ, Pęksiński J, Michalkiewicz B. Investigation of CO2 Adsorption on Avocado Stone-Derived Activated Carbon Obtained through NaOH Treatment. Materials. 2023; 16(12):4390. https://doi.org/10.3390/ma16124390
Chicago/Turabian StyleSiemak, Joanna, Rafał J. Wróbel, Jakub Pęksiński, and Beata Michalkiewicz. 2023. "Investigation of CO2 Adsorption on Avocado Stone-Derived Activated Carbon Obtained through NaOH Treatment" Materials 16, no. 12: 4390. https://doi.org/10.3390/ma16124390
APA StyleSiemak, J., Wróbel, R. J., Pęksiński, J., & Michalkiewicz, B. (2023). Investigation of CO2 Adsorption on Avocado Stone-Derived Activated Carbon Obtained through NaOH Treatment. Materials, 16(12), 4390. https://doi.org/10.3390/ma16124390








