Graphene-Based Materials for the Separator Functionalization of Lithium-Ion/Metal/Sulfur Batteries
Abstract
1. Introduction
2. Preparation Methods of Graphene-Based Materials
2.1. CVD
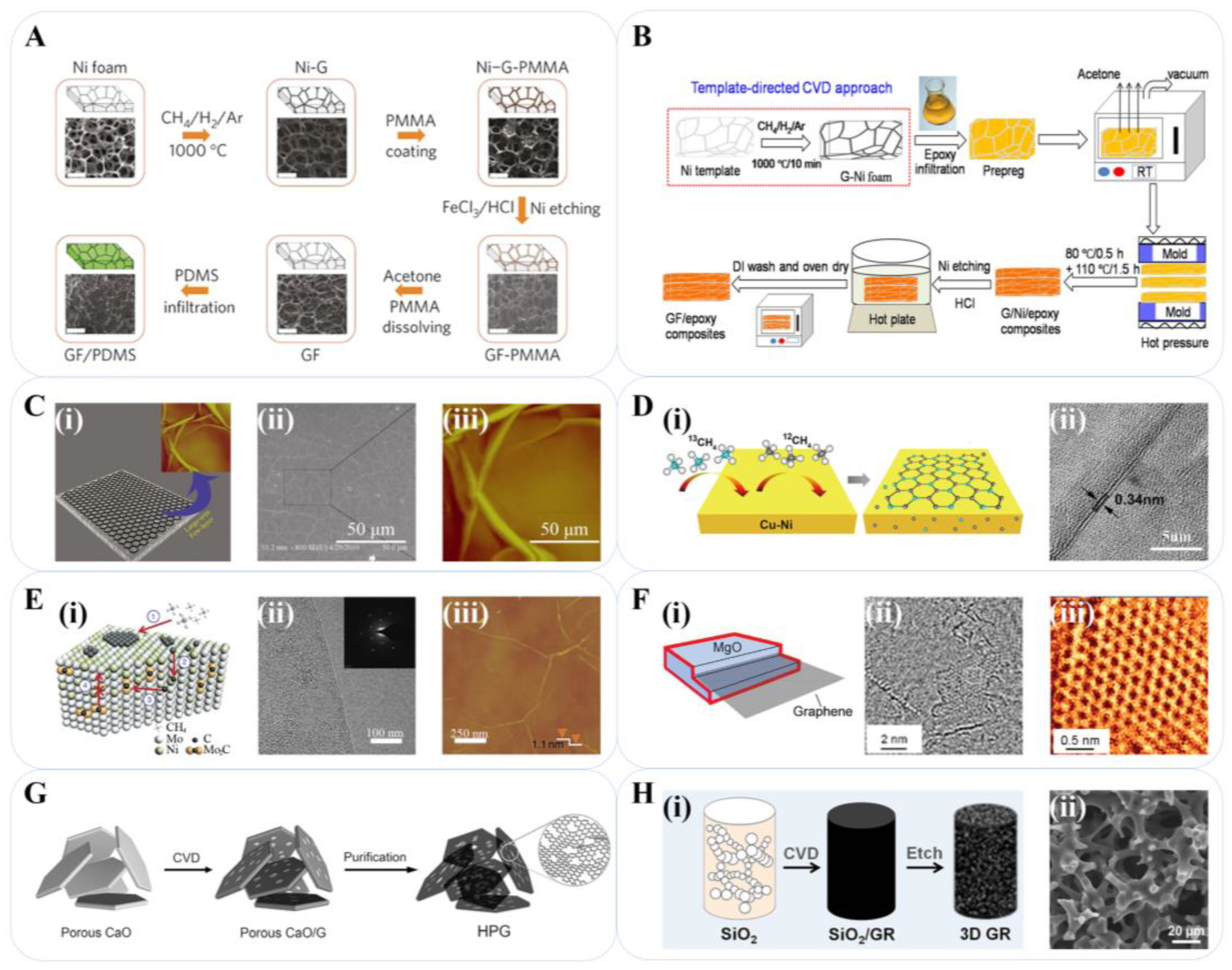
2.2. Pyrolysis

2.3. Others
2.3.1. Micromechanical Cleavage
2.3.2. Liquid Phase Exfoliation
2.3.3. Graphene-Oxide Reduction
2.3.4. Arc Discharge
2.3.5. Vacuum Filtration and Liquid-Air Interface Self-Assembly

3. Applications of Graphene-Based Materials for the Separator Functionalization in Lithium-Ion/Metal/Sulfur Batteries
3.1. The Use of Graphene-Based Materials for the Separator of a Lithium-Ion Battery
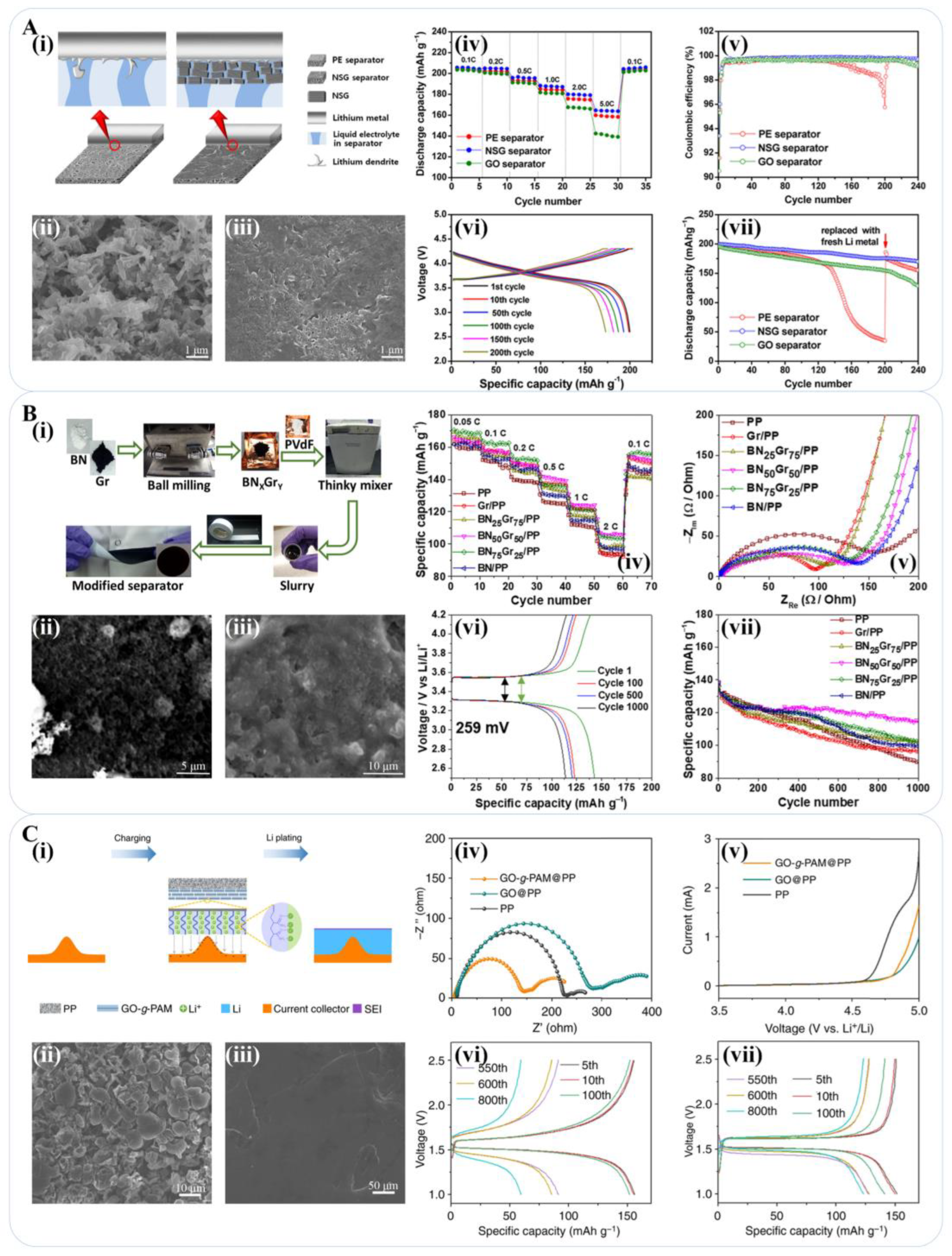
3.2. The Use of Graphene-Based Materials for the Separator of a Lithium-Metal Battery
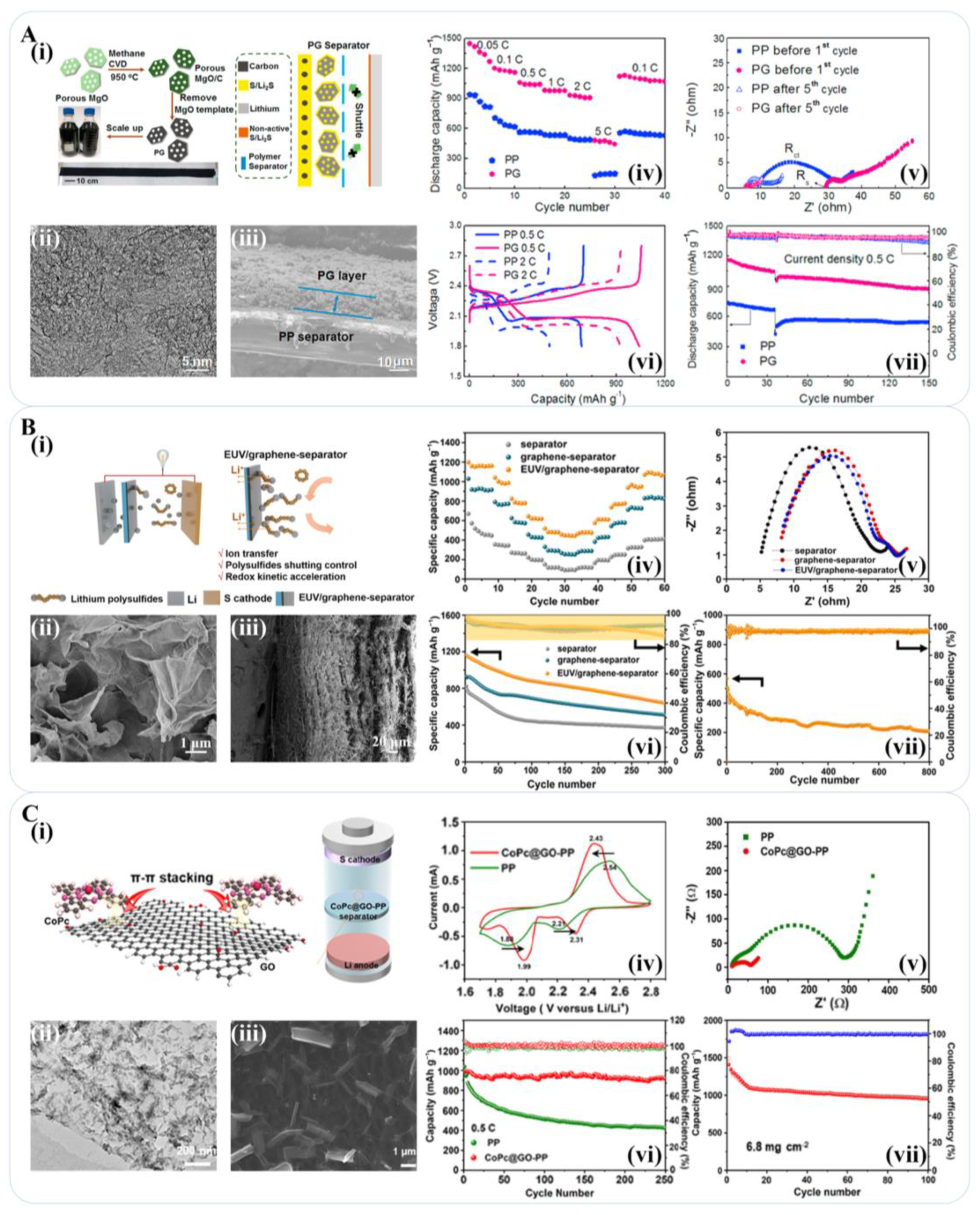
3.3. The Use of Graphene-Based Materials for the Separator of a Lithium-Sulfur Battery
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ma, G.Q.; Wen, Z.Y.; Wang, Q.S.; Shen, C.; Peng, P.; Jin, J.; Wu, X.W. Enhanced performance of lithium sulfur battery with self-assembly polypyrrole nanotube film as the functional interlayer. J. Power Sources 2015, 273, 511–516. [Google Scholar] [CrossRef]
- Su, Y.S.; Manthiram, A. Lithium-sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef]
- Ye, W.; Zhu, J.; Liao, X.J.; Jiang, S.H.; Li, Y.H.; Fang, H.; Hou, H.Q. Hierarchical three-dimensional micro/nano-architecture of polyaniline nanowires wrapped-on polyimide nanofibers for high performance lithium-ion battery separators. J. Power Sources 2015, 299, 417–424. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.C.; Lai, Y.Q.; Li, J.; Zhang, Z.Y.; Chen, W. Nitrogen-doped porous hollow carbon sphere-decorated separators for advanced lithium-sulfur batteries. J. Power Sources 2015, 300, 157–163. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, K.P.; Manesh, K.M.; Santhosh, P. Poly(vinylidene fluoride)-polydiphenylamine composite electrospun membrane as high-performance polymer electrolyte for lithium batteries. J. Membr. Sci. 2008, 318, 422–428. [Google Scholar] [CrossRef]
- Tabatabaei, S.H.; Carreau, P.J.; Ajji, A. Microporous membranes obtained from PP/HDPE multilayer films by stretching. J. Membr. Sci. 2009, 345, 148–159. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Croce, F.; Focarete, M.L.; Hassoun, J.; Meschini, I.; Scrosati, B. A safe, high-rate and high-energy polymer lithium-ion battery based on gelled membranes prepared by electrospinning. Energy Environ. Sci. 2011, 4, 921–927. [Google Scholar] [CrossRef]
- Guo, S.J.; Dong, S.J. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef]
- Chang, H.H.; Ho, T.H.; Su, Y.S. Graphene-Enhanced Battery Components in Rechargeable Lithium-Ion and Lithium Metal Batteries. C-J. Carbon Res. 2021, 7, 65. [Google Scholar] [CrossRef]
- Wang, X.B.; Zhang, Y.J.; Zhi, C.Y.; Wang, X.; Tang, D.M.; Xu, Y.B.; Weng, Q.H.; Jiang, X.F.; Mitome, M.; Golberg, D.; et al. Three-dimensional strutted graphene grown by substrate-free sugar blowing for high-power-density supercapacitors. Nat. Commun. 2013, 4, 2905. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Ren, W.C.; Gao, L.B.; Liu, B.L.; Pei, S.F.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.J.; Kang, J.L.; Liu, P.; Hirata, A.; Fujita, T.; Chen, M.W. Fabrication of large-scale nanoporous nickel with a tunable pore size for energy storage. J. Power Sources 2014, 247, 896–905. [Google Scholar] [CrossRef]
- Bae, S.H.; Lee, Y.B.; Sharma, B.K.; Lee, H.J.; Kim, J.H.; Ahn, J.H. Graphene-based transparent strain sensor. Carbon 2013, 51, 236–242. [Google Scholar] [CrossRef]
- Wang, X.N.; Qiu, Y.F.; Cao, W.W.; Hu, P.A. Highly Stretchable and Conductive Core-Sheath Chemical Vapor Deposition Graphene Fibers and Their Applications in Safe Strain Sensors. Chem. Mater. 2015, 27, 6969–6975. [Google Scholar] [CrossRef]
- Jia, J.J.; Sun, X.Y.; Lin, X.Y.; Shen, X.; Mai, Y.W.; Kim, J.K. Exceptional Electrical Conductivity and Fracture Resistance of 3D Interconnected Graphene Foam/Epoxy Composites. ACS Nano 2014, 8, 5774–5783. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Kong, T.; Wang, W.; Song, Q.; Zhang, D.; Ma, Q.Q.; Cheng, G.S. Interconnected Graphene Networks with Uniform Geometry for Flexible Conductors. Adv. Funct. Mater. 2015, 25, 6165–6172. [Google Scholar] [CrossRef]
- Li, Z.C.; Wu, P.; Wang, C.X.; Fan, X.D.; Zhang, W.H.; Zhai, X.F.; Zeng, C.G.; Li, Z.Y.; Yang, J.L.; Hou, J.G. Low-Temperature Growth of Graphene by Chemical Vapor Deposition Using Solid and Liquid Carbon Sources. ACS Nano 2011, 5, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Jia, X.T.; Ho, J.; Nezich, D.; Son, H.B.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Li, X.S.; Cai, W.W.; An, J.; Kim, S.; Nah, J.; Yang, D.X.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.Z.; Wu, B.; Guo, Y.L.; Huang, L.P.; Jiang, L.; Chen, J.Y.; Geng, D.C.; Liu, Y.Q.; Hu, W.P.; Yu, G. Synthesis of large-area, few-layer graphene on iron foil by chemical vapor deposition. Nano Res. 2011, 4, 1208–1214. [Google Scholar] [CrossRef]
- Wu, Y.P.; Chou, H.; Ji, H.X.; Wu, Q.Z.; Chen, S.S.; Jiang, W.; Hao, Y.F.; Kang, J.Y.; Ren, Y.J.; Piner, R.D.; et al. Growth Mechanism and Controlled Synthesis of AB-Stacked Bilayer Graphene on Cu-Ni Alloy Foils. ACS Nano 2012, 6, 7731–7738. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Liu, S.; Xie, X.M.; Ding, G.Q.; Wang, Y.Q.; Chu, P.K.; Gao, H.; Ren, W.; Yuan, Q.H.; et al. Synthesis of Layer-Tunable Graphene: A Combined Kinetic Implantation and Thermal Ejection Approach. Adv. Funct. Mater. 2015, 25, 3666–3675. [Google Scholar] [CrossRef]
- Dai, B.Y.; Fu, L.; Zou, Z.Y.; Wang, M.; Xu, H.T.; Wang, S.; Liu, Z.F. Rational design of a binary metal alloy for chemical vapour deposition growth of uniform single-layer graphene. Nat. Commun. 2011, 2, 522. [Google Scholar] [CrossRef]
- Lin, T.Q.; Huang, F.Q.; Wan, D.Y.; Bi, H.; Xie, X.M.; Jiang, M.H. Self-regulating homogenous growth of high-quality graphene on Co-Cu composite substrate for layer control. Nanoscale 2013, 5, 5847–5853. [Google Scholar] [CrossRef] [PubMed]
- Weatherup, R.S.; Bayer, B.C.; Blume, R.; Ducati, C.; Baehtz, C.; Schlogl, R.; Hofmann, S. In Situ Characterization of Alloy Catalysts for Low-Temperature Graphene Growth. Nano Lett. 2011, 11, 4154–4160. [Google Scholar] [CrossRef]
- Wang, G.; Chen, D.; Lu, Z.T.; Guo, Q.L.; Ye, L.; Wei, X.; Ding, G.Q.; Zhang, M.; Di, Z.F.; Liu, S. Growth of homogeneous single-layer graphene on Ni-Ge binary substrate. Appl. Phys. Lett. 2014, 104, 666. [Google Scholar] [CrossRef]
- Jiang, X.F.; Wang, X.B.; Shen, L.M.; Wu, Q.; Wang, Y.N.; Ma, Y.W.; Wang, X.Z.; Hu, Z. High-performance Pt catalysts supported on hierarchical nitrogen-doped carbon nanocages for methanol electrooxidation. Chin. J. Catal. 2016, 37, 1149–1155. [Google Scholar] [CrossRef]
- Rummeli, M.H.; Bachmatiuk, A.; Scott, A.; Borrnert, F.; Warner, J.H.; Hoffman, V.; Lin, J.H.; Cuniberti, G.; Buchner, B. Direct Low-Temperature Nanographene CVD Synthesis over a Dielectric Insulator. ACS Nano 2010, 4, 4206–4210. [Google Scholar] [CrossRef]
- Tang, C.; Li, B.Q.; Zhang, Q.; Zhu, L.; Wang, H.F.; Shi, J.L.; Wei, F. CaO-Templated Growth of Hierarchical Porous Graphene for High-Power Lithium-Sulfur Battery Applications. Adv. Funct. Mater. 2016, 26, 577–585. [Google Scholar] [CrossRef]
- Shi, L.R.; Chen, K.; Du, R.; Bachmatiuk, A.; Rummeli, M.H.; Xie, K.W.; Huang, Y.Y.; Zhang, Y.F.; Liu, Z.F. Scalable Seashell-Based Chemical Vapor Deposition Growth of Three-Dimensional Graphene Foams for Oil-Water Separation. J. Am. Chem. Soc. 2016, 138, 6360–6363. [Google Scholar] [CrossRef]
- Mecklenburg, M.; Schuchardt, A.; Mishra, Y.K.; Kaps, S.; Adelung, R.; Lotnyk, A.; Kienle, L.; Schulte, K. Aerographite: Ultra Lightweight, Flexible Nanowall, Carbon Microtube Material with Outstanding Mechanical Performance. Adv. Mater. 2012, 24, 3486–3490. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Q.; Zhang, Q.; Huang, J.Q.; Tian, G.L.; Nie, J.Q.; Peng, H.J.; Wei, F. Unstacked double-layer templated graphene for high-rate lithium-sulphur batteries. Nat. Commun. 2014, 5, 3410. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Zhi, L.Q.; Liu, K.Q.; Dang, L.Q.; Liu, Z.H.; Lei, Z.B.; Yu, C.; Qiu, J.S. Thin-Sheet Carbon Nanomesh with an Excellent Electrocapacitive Performance. Adv. Funct. Mater. 2015, 25, 5420–5427. [Google Scholar] [CrossRef]
- Chen, K.; Li, C.; Shi, L.R.; Gao, T.; Song, X.J.; Bachmatiuk, A.; Zou, Z.Y.; Deng, B.; Ji, Q.Q.; Ma, D.L.; et al. Growing three-dimensional biomorphic graphene powders using naturally abundant diatomite templates towards high solution processability. Nat. Commun. 2016, 7, 13440. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Lin, T.Q.; Xu, F.; Tang, Y.F.; Liu, Z.Q.; Huang, F.Q. New Graphene Form of Nanoporous Monolith for Excellent Energy Storage. Nano Lett. 2016, 16, 349–354. [Google Scholar] [CrossRef]
- Xu, X.; Guan, C.; Xu, L.; Tan, Y.H.; Zhang, D.W.; Wang, Y.Q.; Zhang, H.; Blackwood, D.J.; Wang, J.; Li, M.; et al. Three Dimensionally Free-Formable Graphene Foam with Designed Structures for Energy and Environmental Applications. ACS Nano 2020, 14, 937–947. [Google Scholar] [CrossRef]
- Wei, N.; Yu, L.H.; Sun, Z.T.; Song, Y.Z.; Wang, M.L.; Tian, Z.N.; Xia, Y.; Cai, J.S.; Li, Y.Y.; Zhao, L.; et al. Scalable Salt-Templated Synthesis of Nitrogen-Doped Graphene Nanosheets toward Printable Energy Storage. ACS Nano 2019, 13, 7517–7526. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, D.X.; Cheng, X.; Guan, R.F.; Zhou, C.J. Amide salt pyrolysis fabrication of graphene nanosheets with multi-excitation single color emission. J. Colloid Interface Sci. 2022, 627, 671–680. [Google Scholar] [CrossRef]
- Xu, D.W.; Yang, S.; Chen, P.; Yu, Q.; Xiong, X.H.; Wang, J. Synthesis of magnetic graphene aerogels for microwave absorption by a in-situ pyrolysis. Carbon 2019, 146, 301–302. [Google Scholar] [CrossRef]
- Hojati-Talemi, P.; Simon, G.P. Preparation of graphene nanowalls by a simple microwave-based method. Carbon 2010, 48, 3993–4000. [Google Scholar] [CrossRef]
- Zeng, J.J.; Xu, C.Y.; Gao, T.; Jiang, X.F.; Wang, X.B. Porous monoliths of 3D graphene for electric double-layer supercapacitors. Carbon Energy 2021, 3, 32. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Y.F.; Fan, H.; Liu, M.; Zhuo, O.; Wang, X.Z.; Wu, Q.; Yang, L.; Ma, Y.W.; Hu, Z. Porous 3D Few-Layer Graphene-like Carbon for Ultrahigh-Power Supercapacitors with Well-Defined Structure–Performance Relationship. Adv. Mater. 2017, 29, 1604569. [Google Scholar] [CrossRef]
- Bo, X.J.; Li, M.; Han, C.; Zhang, Y.F.; Nsabimana, A.; Guo, L.P. Noble metal-free electrocatalysts for the oxygen reduction reaction based on iron and nitrogen-doped porous graphene. J. Mater. Chem. A 2015, 3, 1058–1067. [Google Scholar] [CrossRef]
- Yoon, S.M.; Choi, W.M.; Baik, H.; Shin, H.J.; Song, I.; Kwon, M.S.; Bae, J.J.; Kim, H.; Lee, Y.H.; Choi, J.-Y. Synthesis of Multilayer Graphene Balls by Carbon Segregation from Nickel Nanoparticles. ACS Nano 2012, 6, 6803–6811. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Guo, G.N.; Sheng, H.Y.; Qin, S.L.; Wang, B.W.; Han, D.D.; Li, T.T.; Yang, D.; Dong, A.G. Free-Standing, Ordered Mesoporous Few-Layer Graphene Framework Films Derived from Nanocrystal Superlattices Self-Assembled at the Solid- or Liquid-Air Interface. Chem. Mater. 2016, 28, 3823–3830. [Google Scholar] [CrossRef]
- Cui, H.J.; Zheng, J.F.; Zhu, Y.Y.; Wang, Z.J.; Jia, S.P.; Zhu, Z.P. Graphene frameworks synthetized with Na2CO3 as a renewable water-soluble substrate and their high rate capability for supercapacitors. J. Power Sources 2015, 293, 143–150. [Google Scholar] [CrossRef]
- Ma, H.L.; Qi, X.J.; Peng, D.Q.; Chen, Y.X.; Wei, D.C.; Ju, Z.C.; Zhuang, Q.C. Novel Fabrication Of N/S Co-doped Hierarchically Porous Carbon For Potassium-Ion Batteries. Chemistryselect 2019, 4, 11488–11495. [Google Scholar] [CrossRef]
- Hou, X.W.; Wang, W.J.; Gao, X.H.; Ran, K.; Huang, Y.L.; Zhang, Z.D.; Fang, Y.; Wang, S.; He, D.X.; Ye, W.P.; et al. Salt template assisted synthesis of Fe@graphene for high-performance electromagnetic wave absorption. Carbon 2022, 199, 268–278. [Google Scholar] [CrossRef]
- Wang, C.; Kang, J.; Sun, H.Q.; Ang, H.M.; Tade, M.O.; Wang, S.B. One-pot synthesis of N-doped graphene for metal-free advanced oxidation processes. Carbon 2016, 102, 279–287. [Google Scholar] [CrossRef]
- Wu, X.; Lam, C.W.K.; Wu, N.Q.; Pang, S.S.; Xing, Z.; Zhang, W.; Ju, Z.C. Multiple templates fabrication of hierarchical porous carbon for enhanced rate capability in potassium-ion batteries. Mater. Today Energy 2019, 11, 182–191. [Google Scholar] [CrossRef]
- Cao, J.Y.; Zhuang, H.; Guo, M.W.; Wang, H.N.; Xu, J.; Chen, Z.D. Facile preparation of mesoporous graphenes by the sacrificial template approach for direct methanol fuel cell application. J. Mater. Chem. A 2014, 2, 19914–19919. [Google Scholar] [CrossRef]
- Liu, H.H.; Zhang, H.L.; Xu, H.B.; Lou, T.P.; Sui, Z.T.; Zhang, Y. In situ self-sacrificed template synthesis of vanadium nitride/nitrogen-doped graphene nanocomposites for electrochemical capacitors. Nanoscale 2018, 10, 5246–5253. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, P.; Lu, W.T.; Wang, C.Y.; Li, Y.K.; Ding, C.; Gu, J.J.; Zheng, X.S.; Cao, F.F. Co Nanoparticles/Co, N, S Tri-doped Graphene Templated from In Situ -Formed Co, S Co-doped g-C3N4 as an Active Bifunctional Electrocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 28566–28576. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.F.; Li, R.Q.; Hu, M.; Hu, Z.; Golberg, D.; Bando, Y.; Wang, X.B. Zinc-Tiered Synthesis of 3D Graphene for Monolithic Electrodes. Adv. Mater. 2019, 31, 1901186. [Google Scholar] [CrossRef]
- Gao, T.; Xu, C.Y.; Li, R.Q.; Zhang, R.; Wang, B.L.; Jiang, X.F.; Hu, M.; Bando, Y.; Kong, D.S.; Dai, P.C.; et al. Biomass-Derived Carbon Paper to Sandwich Magnetite Anode for Long-Life Li-Ion Battery. ACS Nano 2019, 13, 11901–11911. [Google Scholar] [CrossRef]
- Wyss, K.M.; Beckham, J.L.; Chen, W.Y.; Luong, D.X.; Hundi, P.; Raghuraman, S.; Shahsavari, R.; Tour, J.M. Converting plastic waste pyrolysis ash into flash graphene. Carbon 2021, 174, 430–438. [Google Scholar] [CrossRef]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A.; Stanford, M.G.; Kittrell, C.; Chen, W.; Salvatierra, R.V.; Ren, M.Q.; McHugh, E.A.; Advincula, P.A.; et al. Gram-scale bottom-up flash graphene synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef]
- Cho, M.H.; Shin, J.S.; Roh, Y.S.; Lyo, I.W.; Jeong, K.; Whang, C.N.; Lee, J.S.; Yoo, J.Y.; Lee, N.I.; Fujihara, K.; et al. Characteristics of ultrathin SiO2 films using dry rapid thermal oxidation and Pt catalyzed wet oxidation. J. Vac. Sci. Technol. A 2003, 21, 1004–1008. [Google Scholar] [CrossRef]
- Ramachandran, V.; Brady, M.F.; Smith, A.R.; Feenstra, R.M.; Greve, D.W. Preparation of atomically flat surfaces on silicon carbide using hydrogen etching. J. Electron. Mater. 1998, 27, 308–312. [Google Scholar] [CrossRef]
- Forbeaux, I.; Themlin, J.M.; Charrier, A.; Thibaudau, F.; Debever, J.M. Solid-state graphitization mechanisms of silicon carbide 6H-SiC polar faces. Appl. Surf. Sci. 2000, 162, 406–412. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.M.; Li, T.B.; Li, X.B.; Ogbazghi, A.Y.; Feng, R.; Dai, Z.T.; Marchenkov, A.N.; Conrad, E.H.; First, P.N.; et al. Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J. Phys. Chem. B 2004, 108, 19912–19916. [Google Scholar] [CrossRef]
- Pakdehi, D.M.; Aprojanz, J.; Sinterhauf, A.; Pierz, K.; Kruskopf, M.; Willke, P.; Baringhaus, J.; Stockmann, J.P.; Traeger, G.A.; Hohls, F.; et al. Minimum Resistance Anisotropy of Epitaxial Graphene on SiC. ACS Appl. Mater. Interfaces 2018, 10, 6039–6045. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.F.; Norimatsu, W.; Iwata, H.; Matsuda, K.; Ito, T.; Kusunoki, M. Synthesis of Freestanding Graphene on SiC by a Rapid-Cooling Technique. Phys. Rev. Lett. 2016, 117, 205501. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Young, R.J.; Backes, C.; Zhao, W.; Zhang, X.; Zhukov, A.A.; Tillotson, E.; Conlan, A.P.; Ding, F.; Haigh, S.J.; et al. Mechanisms of Liquid-Phase Exfoliation for the Production of Graphene. ACS Nano 2020, 14, 10976–10985. [Google Scholar] [CrossRef]
- Gu, X.G.; Zhao, Y.; Sun, K.; Vieira, C.L.Z.; Jia, Z.J.; Cui, C.; Wang, Z.J.; Walsh, A.; Huang, S.D. Method of ultrasound-assisted liquid-phase exfoliation to prepare graphene. Ultrason. Sonochem. 2019, 58, 104630. [Google Scholar] [CrossRef]
- Khan, U.; O’Neill, A.; Lotya, M.; De, S.; Coleman, J.N. High-Concentration Solvent Exfoliation of Graphene. Small 2010, 6, 864–871. [Google Scholar] [CrossRef]
- Tung, T.T.; Yock, J.; Alotaibi, F.K.; Nine, M.J.; Karunagaran, R.; Krebsz, M.; Nguyen, G.T.; Tran, D.N.H.; Feller, J.F.; Losic, D. Graphene Oxide-Assisted Liquid Phase Exfoliation of Graphite into Graphene for Highly Conductive Film and Electromechanical Sensors. ACS Appl. Mater. Interfaces 2016, 8, 16521–16532. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Lynch-Branzoi, J.K.; Ashraf, A.; Tewatia, A.; Taghon, M.; Wooding, J.; Hendrix, J.; Kear, B.H.; Nosker, T.J. Shear exfoliation of graphite into graphene nanoflakes directly within polyetheretherketone and a spectroscopic study of this high modulus, lightweight nanocomposite. Compos. Part B-Eng. 2020, 188, 107842. [Google Scholar] [CrossRef]
- Sellathurai, A.J.; Mypati, S.; Kontopoulou, M.; Barz, D.P.J. High yields of graphene nanoplatelets by liquid phase exfoliation using graphene oxide as a stabilizer. Chem. Eng. J. 2023, 451, 138365. [Google Scholar] [CrossRef]
- Hadi, A.; Zahirifar, J.; Karimi-Sabet, J.; Dastbaz, A. Graphene nanosheets preparation using magnetic nanoparticle assisted liquid phase exfoliation of graphite: The coupled effect of ultrasound and wedging nanoparticles. Ultrason. Sonochem. 2018, 44, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. Ser. 1827, 117, 355–388. [Google Scholar]
- Staudenmaier, L. Method for the preparation of graphitic acid. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 208, 1334–1339. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Lombardo, A.; Hasan, T.; Sun, Z.P.; Colombo, L.; Ferrari, A.C. Production and processing of graphene and 2d crystals. Mater. Today 2012, 15, 564–589. [Google Scholar] [CrossRef]
- Li, X.Z.; Li, J.; Tang, J.L.; Kang, J.; Zhang, Y.H. Study of influence of metal ions on CdTe/H2O2 chemiluminescence. J. Lumin. 2008, 128, 1229–1234. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabo, T.; Szeri, A.; Dekany, I. Graphite oxide: Chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Pei, S.F.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Steurer, P.; Wissert, R.; Thomann, R.; Mulhaupt, R. Functionalized Graphenes and Thermoplastic Nanocomposites Based upon Expanded Graphite Oxide. Macromol. Rapid Commun. 2009, 30, 316–327. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bardhan, N.M.; Chen, G.Y.; Li, Z.Y.; Belcher, A.M.; Grossman, J.C. New insights into the thermal reduction of graphene oxide: Impact of oxygen clustering. Carbon 2016, 100, 90–98. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Simple Method of Preparing Graphene Flakes by an Arc-Discharge Method. J. Phys. Chem. C 2009, 113, 4257–4259. [Google Scholar] [CrossRef]
- Pham, T.V.; Kim, J.G.; Jung, J.Y.; Kim, J.H.; Cho, H.; Seo, T.H.; Lee, H.; Kim, N.D.; Kim, M.J. High Areal Capacitance of N-Doped Graphene Synthesized by Arc Discharge. Adv. Funct. Mater. 2019, 29, 1905511. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, T.F.; Chen, H.H.; Ma, Y.F. The growth mechanism of few-layer graphene in the arc discharge process. Carbon 2016, 102, 494–498. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.L.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Zhang, L.; Chena, H.L.; Gaoab, C.J. A cross-linking graphene oxide-polyethyleneimine hybrid film containing ciprofloxacin: One-step preparation, controlled drug release and antibacterial performance. J. Mater. Chem. B 2015, 3, 1605–1611. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.S.; Yan, T.T.; Wen, X.R.; Zhang, J.P.; Shi, L.Y.; Zhong, Q.D. Three-dimensional macroporous graphene architectures as high performance electrodes for capacitive deionization. J. Mater. Chem. A 2013, 1, 11778–11789. [Google Scholar] [CrossRef]
- Pham, D.T.; Lee, T.H.; Luong, D.H.; Yao, F.; Ghosh, A.; Le, V.T.; Kim, T.H.; Li, B.; Chang, J.; Lee, Y.H. Carbon Nanotube-Bridged Graphene 3D Building Blocks for Ultrafast Compact Supercapacitors. ACS Nano 2015, 9, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Yang, Q.H.; Yang, Y.G.; Lv, W.; Wen, Y.F.; Hou, P.X.; Wang, M.Z.; Cheng, H.M. Self-Assembled Free-Standing Graphite Oxide Membrane. Adv. Mater. 2009, 21, 3007–3011. [Google Scholar] [CrossRef]
- Chen, W.J.; Gui, X.C.; Li, S.S.; Yang, L.L.; Liang, B.H.; Zhu, H.; She, J.C.; Tang, Z.K. Fabrication of wrinkled graphene based on thermal-enhanced Rayleigh-Benard convection for field electron emission. Carbon 2018, 129, 646–652. [Google Scholar] [CrossRef]
- Wei, W.; Lu, W.; Yang, Q.H. High-concentration graphene aqueous suspension and a membrane self-assembled at the liquid-air interface. New Carbon Mater. 2011, 26, 36–40. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X.W. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Xiang, Y.Y.; Li, J.S.; Lei, J.H.; Liu, D.; Xie, Z.Z.; Qu, D.Y.; Li, K.; Deng, T.F.; Tang, H.L. Advanced Separators for Lithium-Ion and Lithium-Sulfur Batteries: A Review of Recent Progress. ChemSusChem 2016, 9, 3023–3039. [Google Scholar] [CrossRef]
- Han, D.Z.; Wang, X.W.; Zhou, Y.N.; Zhang, J.Y.; Liu, Z.X.; Xiao, Z.C.; Zhou, J.Q.; Wang, Z.; Zheng, J.F.; Jia, Z.H.; et al. A Graphene-Coated Thermal Conductive Separator to Eliminate the Dendrite-Induced Local Hotspots for Stable Lithium Cycling. Adv. Energy Mater. 2022, 12, 2201190. [Google Scholar] [CrossRef]
- Liu, X.; Song, K.D.; Lu, C.; Huang, Y.T.; Duan, X.L.; Li, S.; Ding, Y.H. Electrospun PU@GO separators for advanced lithium ion batteries. J. Membr. Sci. 2018, 555, 1–6. [Google Scholar] [CrossRef]
- Song, K.D.; Huang, Y.T.; Liu, X.; Jiang, Y.H.; Zhang, P.; Ding, Y.H. Electrospun PI@GO separators for Li-ion batteries: A possible solution for high-temperature operation. J. Sol-Gel Sci. Technol. 2020, 94, 109–117. [Google Scholar] [CrossRef]
- Liao, H.Y.; Zhang, H.Y.; Qin, G.; Li, Z.H.; Li, L.Q.; Hong, H.Q. A macro-porous graphene oxide-based membrane as a separator with enhanced thermal stability for high-safety lithium-ion batteries. RSC Adv. 2017, 7, 22112–22120. [Google Scholar] [CrossRef]
- Zhu, G.L.; Jing, X.P.; Chen, D.J.; He, W.D. Novel composite separator for high power density lithium-ion battery. Int. J. Hydrogen Energy 2019, 45, 2917–2924. [Google Scholar] [CrossRef]
- Bu, A.X.; Tan, Y.; Fang, R.P.; Li, F.; Pei, S.F.; Ren, W.C. A graphene/PVDF/PP multilayer composite separator for long-life and high power lithium-ion batteries. New Carbon Mater. 2017, 32, 63–70. [Google Scholar] [CrossRef]
- Yan, H.H.; Bie, Y.H.; Cui, X.Y.; Xiong, G.P.; Chen, L. A computational investigation of thermal effect on lithium dendrite growth. Energy Convers. Manag. 2018, 161, 193–204. [Google Scholar] [CrossRef]
- Li, L.; Basu, S.; Wang, Y.P.; Chen, Z.Z.; Hundekar, P.; Wang, B.W.; Shi, J.; Shi, Y.F.; Narayanan, S.; Koratkar, N. Self-heating-induced healing of lithium dendrites. Science 2018, 359, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Wang, A.N.; Hong, W.W.; Yang, L.; Tian, Y.; Qiu, X.J.; Zou, G.Q.; Hou, H.S.; Ji, X.B. Bi-Based Electrode Materials for Alkali Metal-Ion Batteries. Small 2020, 16, 2004022. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, H.M.; Liu, X.; Zhou, C.; Li, G.R.; Liu, S.; Gao, X.P. A dimensionally stable lithium alloy based composite electrode for lithium metal batteries. Chem. Eng. J. 2022, 450, 138074. [Google Scholar] [CrossRef]
- Morag, A.; Yu, M.H. Layered electrode materials for non-aqueous multivalent metal batteries. J. Mater. Chem. A 2021, 9, 19317–19345. [Google Scholar] [CrossRef]
- Piao, N.; Ji, X.; Xu, H.; Fan, X.L.; Chen, L.; Liu, S.F.; Garaga, M.N.; Greenbaum, S.C.; Wang, L.; Wang, C.S.; et al. Countersolvent Electrolytes for Lithium-Metal Batteries. Adv. Energy Mater. 2020, 10, 1903568. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, T.H.; Jeurgens, L.P.H.; Kong, X.; Choi, J.W.; Coskun, A. Electrolyte engineering for highly inorganic solid electrolyte interphase in high-performance lithium metal batteries. Chem 2023, 9, 682–697. [Google Scholar] [CrossRef]
- Wang, H.S.; Yu, Z.; Kong, X.; Kim, S.C.; Boyle, D.T.; Qin, J.; Bao, Z.N.; Cui, Y. Liquid electrolyte: The nexus of practical lithium metal batteries. Joule 2022, 6, 588–616. [Google Scholar] [CrossRef]
- Yang, H.C.; Li, J.; Sun, Z.H.; Fang, R.P.; Wang, D.W.; He, K.; Cheng, H.M.; Li, F. Reliable liquid electrolytes for lithium metal batteries. Energy Storage Mater. 2020, 30, 113–129. [Google Scholar] [CrossRef]
- Fu, K.K.; Gong, Y.H.; Liu, B.Y.; Zhu, Y.Z.; Xu, S.M.; Yao, Y.G.; Luo, W.; Wang, C.W.; Lacey, S.D.; Dai, J.Q.; et al. Toward garnet electrolyte-based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Sci. Adv. 2017, 3, 1601659. [Google Scholar] [CrossRef]
- Liu, S.F.; Ji, X.; Yue, J.; Hou, S.; Wang, P.F.; Cui, C.Y.; Chen, J.; Shao, B.W.; Li, J.R.; Han, F.D.; et al. High Interfacial-Energy Interphase Promoting Safe Lithium Metal Batteries. J. Am. Chem. Soc. 2020, 142, 2438–2447. [Google Scholar] [CrossRef]
- Popovic, J. The importance of electrode interfaces and interphases for rechargeable metal batteries. Nat. Commun. 2021, 12, 6240. [Google Scholar] [CrossRef]
- Yang, M.H.; Mo, Y.F. Interfacial Defect of Lithium Metal in Solid-State Batteries. Angew. Chem. Int. Ed. 2021, 60, 21494–21501. [Google Scholar] [CrossRef]
- Luo, Y.S.; Mou, P.Z.; Yuan, W.L.; Li, L.P.; Fan, Y.Z.; Chen, Y.; Chen, X.M.; Shu, J.; Zhang, L.Y. Anti-liquid metal permeation separator for stretchable potassium metal batteries. Chem. Eng. J. 2023, 452, 139157. [Google Scholar] [CrossRef]
- Huo, H.Y.; Li, X.N.; Chen, Y.; Liang, J.N.; Deng, S.X.; Gao, X.J.; Doyle-Davis, K.; Li, R.Y.; Guo, X.X.; Shen, Y.; et al. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Mater. 2020, 29, 361–366. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Z.L.; Xu, J.; Lv, Y.P.; Jin, Y. Composite separator based on PI film for advanced lithium metal batteries. J. Mater. Sci. Technol. 2022, 102, 264–271. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Wang, X.M.; Li, B.Q.; Shi, P.; Huang, J.Q.; Chen, A.B.; Zhang, Q. Crosstalk shielding of transition metal ions for long cycling lithium-metal batteries. J. Mater. Chem. A 2020, 8, 4283–4289. [Google Scholar] [CrossRef]
- Shin, W.K.; Kannan, A.G.; Kim, D.W. Effective Suppression of Dendritic Lithium Growth Using an Ultrathin Coating of Nitrogen and Sulfur Codoped Graphene Nanosheets on Polymer Separator for Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2015, 7, 23700–23707. [Google Scholar] [CrossRef]
- Ye, M.H.; Gao, J.; Xiao, Y.K.; Xu, T.; Zhao, Y.; Qu, L.T. Metal/graphene oxide batteries. Carbon 2017, 125, 299–307. [Google Scholar] [CrossRef]
- Gong, Y.J.; Heo, J.W.; Lee, H.; Kim, H.; Cho, J.; Pyo, S.; Yun, H.; Kim, H.; Park, S.Y.; Yoo, J.; et al. Nonwoven rGO Fiber-Aramid Separator for High-Speed Charging and Discharging of Li Metal Anode. Adv. Energy Mater. 2020, 10, 2001479. [Google Scholar] [CrossRef]
- Kim, P.J.; Pol, V.G. High Performance Lithium Metal Batteries Enabled by Surface Tailoring of Polypropylene Separator with a Polydopamine/Graphene Layer. Adv. Energy Mater. 2018, 8, 1802665. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; Kim, P.J.; Kim, K.; Qi, Z.M.; Wang, H.Y.; Pol, V.G. Engineered heat dissipation and current distribution boron nitride-graphene layer coated on polypropylene separator for high performance lithium metal battery. J. Colloid Interface Sci. 2021, 583, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Chen, Y.F.; Yu, B.; Wang, B.; Wang, X.Q.; Zhang, W.L.; Yang, D.X.; He, J.R. Lithiophilic 3D VN@N-rGO as a Multifunctional Interlayer for Dendrite-Free and Ultrastable Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 20125–20136. [Google Scholar] [CrossRef]
- Zhang, X.J.; Ma, F.; Srinivas, K.; Yu, B.; Chen, X.; Wang, B.; Wang, X.Q.; Liu, D.W.; Zhang, Z.H.; He, J.R.; et al. Fe3N@N-doped graphene as a lithiophilic interlayer for highly stable lithium metal batteries. Energy Storage Mater. 2022, 45, 656–666. [Google Scholar] [CrossRef]
- Zhang, X.J.; Chen, Y.F.; Ma, F.; Chen, X.; Wang, B.; Wu, Q.; Zhang, Z.H.; Liu, D.W.; Zhang, W.L.; He, J.R.; et al. Regulating Li uniform deposition by lithiophilic interlayer as Li-ion redistributor for highly stable lithium metal batteries. Chem. Eng. J. 2022, 436, 134945. [Google Scholar] [CrossRef]
- Yang, L.; Sheng, L.; Gao, X.X.; Xie, X.; Bai, Y.Z.; Liu, G.J.; Dong, H.Y.; Wang, T.; Huang, X.L.; He, J.P. rGO/Li-Al-LDH composite nanosheets modified commercial polypropylene (PP) separator to suppress lithium dendrites for lithium metal battery. Electrochim. Acta 2022, 430, 141073. [Google Scholar] [CrossRef]
- Chen, Y.F.; Li, J.H.; Ju, Y.; Cheng, R.B.; Zhai, Y.Y.; Sheng, J.L.; Liu, H.Q.; Li, L. Regulating Li-ion flux distribution via holey graphene oxide functionalized separator for dendrite-inhibited lithium metal battery. Appl. Surf. Sci. 2022, 592, 153222. [Google Scholar] [CrossRef]
- Li, C.F.; Liu, S.H.; Shi, C.G.; Liang, G.H.; Lu, Z.T.; Fu, R.W.; Wu, D.C. Two-dimensional molecular brush-functionalized porous bilayer composite separators toward ultrastable high-current density lithium metal anodes. Nat. Commun. 2019, 10, 1363. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L.Q. Nanostructured Metal Oxides and Sulfides for Lithium-Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef]
- Chien, Y.C.; Menon, A.S.; Brant, W.R.; Brandell, D.; Lacey, M.J. Simultaneous Monitoring of Crystalline Active Materials and Resistance Evolution in Lithium-Sulfur Batteries. J. Am. Chem. Soc. 2020, 142, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.Y.; Xiao, R.J.; Gu, L.; Guo, Y.G.; Wen, R.; Wan, L.J. Interfacial Mechanism in Lithium-Sulfur Batteries: How Salts Mediate the Structure Evolution and Dynamics. J. Am. Chem. Soc. 2018, 140, 8147–8155. [Google Scholar] [CrossRef]
- Song, J.X.; Yu, Z.X.; Gordin, M.L.; Wang, D.H. Advanced Sulfur Cathode Enabled by Highly Crumpled Nitrogen-Doped Graphene Sheets for High-Energy-Density Lithium-Sulfur Batteries. Nano Lett. 2016, 16, 864–870. [Google Scholar] [CrossRef]
- Hao, B.Y.; Li, H.; Lv, W.; Zhang, Y.B.; Niu, S.Z.; Qi, Q.; Xiao, S.J.; Li, J.; Kang, F.Y.; Yang, Q.H. Reviving catalytic activity of nitrides by the doping of the inert surface layer to promote polysulfide conversion in lithium-sulfur batteries. Nano Energy 2019, 60, 305–311. [Google Scholar] [CrossRef]
- Wang, P.Y.; Zeng, R.; You, L.; Tang, H.; Zhong, J.S.; Wang, S.Q.; Yang, T.; Liu, J.W. Graphene-Like Matrix Composites with Fe2O3 and Co3O4 as Cathode Materials for Lithium-Sulfur Batteries. ACS Appl. Nano Mater. 2020, 3, 1382–1390. [Google Scholar] [CrossRef]
- Seo, S.D.; Park, D.; Park, S.; Kim, D.W. “Brain-Coral-Like” Mesoporous Hollow CoS2@N-Doped Graphitic Carbon Nanoshells as Efficient Sulfur Reservoirs for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1903712. [Google Scholar] [CrossRef]
- Zhai, P.Y.; Peng, H.J.; Cheng, X.B.; Zhu, L.; Huang, J.Q.; Zhu, W.C.; Zhang, Q. Scaled-up fabrication of porous-graphene-modified separators for high-capacity lithium-sulfur batteries. Energy Storage Mater. 2017, 7, 56–63. [Google Scholar] [CrossRef]
- Ma, G.Q.; Huang, F.F.; Wen, Z.Y.; Wang, Q.S.; Hong, X.H.; Jin, J.; Wu, X.W. Enhanced performance of lithium sulfur batteries with conductive polymer modified separators. J. Mater. Chem. A 2016, 4, 16968–16974. [Google Scholar] [CrossRef]
- Peng, H.-J.; Wang, D.-W.; Huang, J.-Q.; Cheng, X.-B.; Yuan, Z.; Wei, F.; Zhang, Q. Janus Separator of Polypropylene-Supported Cellular Graphene Framework for Sulfur Cathodes with High Utilization in Lithium-Sulfur Batteries. Adv. Sci. 2016, 3, 1500268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.M.; Li, L.; Wang, D.W.; Shan, X.Y.; Pei, S.F.; Li, F.; Cheng, H.M. A Flexible Sulfur-Graphene-Polypropylene Separator Integrated Electrode for Advanced Li-S Batteries. Adv. Mater. 2015, 27, 641–647. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Bae, J.; Chung, S.H.; Zhang, W.K.; Manthiram, A.; Yu, G.H. Nanostructured Host Materials for Trapping Sulfur in Rechargeable Li-S Batteries: Structure Design and Interfacial Chemistry. Small Methods 2018, 2, 1700279. [Google Scholar] [CrossRef]
- Li, S.L.; Zhang, W.F.; Zheng, J.F.; Lv, M.Y.; Song, H.Y.; Du, L. Inhibition of Polysulfide Shuttles in Li-S Batteries: Modified Separators and Solid-State Electrolytes. Adv. Energy Mater. 2021, 11, 2000779. [Google Scholar] [CrossRef]
- Han, P.; Manthiram, A. Boron- and nitrogen-doped reduced graphene oxide coated separators for high-performance Li-S batteries. J. Power Sources 2017, 369, 87–94. [Google Scholar] [CrossRef]
- Qi, X.M.; Huang, L.W.; Luo, Y.T.; Chen, Q.H.; Chen, Y.G. Ni3Sn2/nitrogen-doped graphene composite with chemisorption and electrocatalysis as advanced separator modifying material for lithium sulfur batteries. J. Colloid Interface Sci. 2022, 628, 896–910. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Wang, Z.X.; Pan, P.; Mao, J.T.; Ni, C.K.; Zhang, M.M.; Chen, Q.; Zeng, Y.; Hu, Y. Excimer ultraviolet-irradiated graphene separator for suppressing polysulfide shuttling in Li-S batteries. J. Alloys Compd. 2022, 903, 163932. [Google Scholar] [CrossRef]
- Shaibani, M.; Akbari, A.; Sheath, P.; Easton, C.D.; Banerjee, P.C.; Konstas, K.; Fakhfouri, A.; Barghamadi, M.; Musameh, M.M.; Best, A.S.; et al. Suppressed Polysulfide Crossover in Li-S Batteries through a High-Flux Graphene Oxide Membrane Supported on a Sulfur Cathode. ACS Nano 2016, 10, 7768–7779. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.D.; Chen, C.; Lu, Y.; Zang, J.; Jiang, M.J.; Kim, D.; Zhang, X.W. Highly porous polyacrylonitrile/graphene oxide membrane separator exhibiting excellent anti-self-discharge feature for high-performance lithium-sulfur batteries. Carbon 2016, 101, 272–280. [Google Scholar] [CrossRef]
- Shen, C.L.; Li, Y.; Gong, M.J.; Zhou, C.; An, Q.Y.; Xu, X.; Mai, L.Q. Ultrathin Cobalt Phthalocyanine@Graphene Oxide Layer-Modified Separator for Stable Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 60046–60053. [Google Scholar] [CrossRef]
- Jing, W.T.; Zu, J.H.; Zou, K.Y.; Dai, X.; Song, Y.Y.; Han, J.Q.; Sun, J.J.; Tan, Q.; Chen, Y.Z.; Liu, Y.N. Sandwich-like strontium fluoride graphene-modified separator inhibits polysulfide shuttling and lithium dendrite growth in lithium-sulfur batteries. J. Mater. Chem. A 2022, 10, 4833–4844. [Google Scholar] [CrossRef]
- Zuo, X.T.; Zhen, M.M.; Wang, C. Ni@N-doped graphene nanosheets and CNTs hybrids modified separator as efficient polysulfide barrier for high-performance lithium sulfur batteries. Nano Res. 2019, 12, 829–836. [Google Scholar] [CrossRef]
- Li, H.P.; Sun, L.C.; Zhao, Y.; Tan, T.Z.; Zhang, Y.G. A novel CuS/graphene-coated separator for suppressing the shuttle effect of lithium/sulfur batteries. Appl. Surf. Sci. 2019, 466, 309–319. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wan, F.; Wang, X.Y.; Cao, H.M.; Dai, X.; Niu, Z.Q.; Wang, Y.J.; Chen, J. Dual-Functional Graphene Carbon as Polysulfide Trapper for High Performance Lithium Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 5594–5602. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.Y.; Chen, W.; Lv, W.Q.; Huang, J.W.; Zhu, J.; Chu, J.W.; Yan, C.Y.; Wu, C.Y.; Yan, Y.C.; He, W.D.; et al. Inhibiting Polysulfide Shuttling with a Graphene Composite Separator for Highly Robust Lithium-Sulfur Batteries. Joule 2018, 2, 2091–2104. [Google Scholar] [CrossRef]


| Separator | Thermal Shrinkage (%) | Electrolyte Uptake (%) | Anode /Cathode | Rate Performance | Cycle Performance | Main Functions | Ref. |
|---|---|---|---|---|---|---|---|
| Celgard 2400 | Great shrinkage | 77 | Li/LiFePO4 | 135, 113, 83, 30 mAh g−1 at 0.5, 1, 2, 5 C | 138 mAh g−1 (0.2 C, after 100 cycles) | (1) Improve wettability; (2) Provide abundant active sites; (3) Ensure excellent thermal/mechanical stability. | [101] |
| PU@GO | 20 (170 °C, 1 h) | 733 | Li/LiFePO4 | 147, 121, 93, 55 mAh g−1 at 0.5, 1, 2, 5 C | 154 mAh g−1 (0.2 C, after 100 cycles) | [101] | |
| PI@GO | 25 (280 °C, 1 h) | - | Li/LiFePO4 | 149, 137, 111, 49 mAh g−1 at 0.5, 1, 2, 5 C | - | [102] | |
| GO-g-HBPE | 0 (200 °C, 0.5 h) | 158 | Li/stainless steel | 135, 129, 118, 95 mAh g−1 at 0.5, 1, 2, 5 C | 122 mAh g−1 (0.2 C, after 200 cycles) | [103] | |
| PVDF-co-HFP/GO | 12 (160 °C, 1 h) | 498 | Li/LiFePO4 | 164, 139, 118, 100 mAh g−1 at 0.5, 2, 5, 10 C | 152 mAh g−1 (0.2 C, after 200 cycles) | [104] | |
| G/PVDF-PL | - | 327 | Li/LiFePO4 | 153, 148, 135, 111 mAh g−1 at 0.5, 2, 5, 10 C | 129 mAh g−1 (2 C, after 200 cycles) | [105] |
| Separator | Electrolyte | Anode /Cathode | Rate Performance | Cycle Performance | Main Functions | Ref. |
|---|---|---|---|---|---|---|
| PP | 1 M LiPF6 in DOL/DME (1:1, v/v) with 2% LiNO3 | Li/NCM811 | 197.5, 177, 102, 11 mAh g−1 at 0.2, 0.5, 1, 2 C | 13 mAh g−1 (1 C, after 200 cycles) | (1) Inhibit lithium dendrites growth; (2) Provide abundant active sites; (3) Ensure excellent thermal/mechanical stability. | [100] |
| graphene/PP | 1 M LiPF6 in DOL/DME (1:1, v/v) with 2% LiNO3 | Li/NCM811 | 199, 184, 115, 28 mAh g−1 at 0.2, 0.5, 1, 2 C | 120 mAh g−1 (1 C, after 200 cycles) | [100] | |
| PE/NSG | 1.15 M LiPF6 in EC/DEC (3:7, v:v) | Li/LiNi0.8Co0.15Al0.05O2 | 195, 187, 178, 162 mAh g−1 at 0.5, 1, 2, 5 C | 170 mAh g−1 (0.5 C, after 240 cycles) | [124] | |
| GO | 1 M LiPF6 dissolved in EC/DEC/DMC (1/1/1, w/w/w) | Li/GO | 245.6, 239.7, 227.5, 210.5 mAh g−1 at 0.5, 1, 2, 5 C | - | [125] | |
| rGOF-A | 1 M LiPF6 in EC/DEC/DMC (1/1/1, v/v/v) | Li/LiFePO4 | 147.49, 100.72, 78.83 mAh g−1 at 0.5, 1, 2 C | 144.63 mAh g−1 (5 C, after 1000 cycles) | [126] | |
| PDA/Gr-CMC | 1 M LiPF6 dissolved in (EC/EMC/DEM) (1/1/1, w/w/w) | Li/LiFePO4 | 89, 77, 65, 39 mAh g−1 at 0.5, 1, 2, 4 C | 130.21 mAh g−1 (1 C, after 1000 cycles) | [127] | |
| BN50Gr50/PP | 1.0 M LiPF6 in EC:DEC | Li/LiFePO4 | 139, 124 106 mAh g−1 at 0.5, 1, 2 C | 114 mAh g−1 (1 C, after 1000 cycles) | [128] | |
| VN@N-rGO/PP | 1 M LiPF6 in EC/DEC (1/1/1, v/v) | Li/LiFePO4 | 149.4, 134.1, 111.7, 101.9 mAh g−1 at 0.5, 1, 2, 3 C | 98.2 mAh g−1 (3 C, after 200 cycles) | [129] | |
| Fe3N@NG/PP | 1 M LiTFSI in EC/DEC (1:1) | Li/LiFePO4 | 149.4, 133.5, 114.6 mAh g−1 at 0.5, 1, 2 C | 88 mAh g−1 (2 C, after 350 cycles) | [130] | |
| WNG/PP | 1 M LiPF6 in EC/DEC (1/1, v/v) | Li/LiFePO4 | 135.9, 107.6, 92.4 mAh g−1 at 1, 2, 3 C | 111.6 mAh g−1 (1 C, after 300 cycles) | [131] | |
| HGO-PAN | - | Li/LiFePO4 | 157, 151, 141, 126 mAh g−1 at 0.5, 1, 2, 5 C | 127 mAh g−1 (2 C, after 900 cycles) | [132] | |
| GO-g-PAM@PP | 1 M LiPF6 in EC/DEC (1/1, w/w) | Li|Li4Ti5O12 | - | 123.2 mAh g−1 (3 C, after 800 cycles) | [133] |
| Separator | Sulfur Loading (mg cm−2) | Rate Performance | Cycle Performance | Main Functions | Ref. |
|---|---|---|---|---|---|
| PP | 1.8–2.0 | 564, 533, 490, 141 mAh g−1 at 0.2, 0.5, 1, 2 C | 540 mAh g−1 (0.5 C, after 150 cycles) | (1) Inhibit the transfer of polysulfide; (2) Buffer the volume change of electrodes; (3) Provide abundant active sites; (4) Ensure excellent thermal/mechanical stability. | [142] |
| PG | 1.8–2.0 | 1038, 975, 903, 440 mAh g−1 at 0.2, 0.5, 1, 2 C | 877 mAh g−1 (0.5 C, after 150 cycles) | [142] | |
| PPy nanotube | 2.5–3 | 195, 187, 178, 162 mAh g−1 at 0.5, 1, 2, 5 C | 801.6 mAh g−1 (0.5 C, after 300 cycles) | [143] | |
| CGF | 1.2 | 1096, 1029, 966 mAh g−1 at 0.5 1, 2 C | 779 mAh g−1 (0.5 C, after 300 cycles) | [144] | |
| S-G@PP | 1.5–2.1 | 1128, 980, 833, 670, 586 mAh g−1 at 0.75, 1.5, 3, 6, 9 A g−1 | 663 mAh g−1 (1.5 A g−1, after 500 cycles) | [145] | |
| N-rGO | 4.0 | 1060, 927, 779 mAh g−1 at 0.5, 1, 2 C | 758.3 mAh g−1 (1 C, after 400 cycles) | [148] | |
| Ni3Sn2/NG | 1.1–1.6 | 1280.5, 1060.2, 927.5, 778.8 mAh g−1 at 0.2, 0.5, 1, 2 C | 758.3 mAh g−1 (1 C, after 400 cycles) | [149] | |
| EUV/graphene | 1.55 | 824.4, 643.5, 518 and 456.3 mAh g−1 at 0.5, 1, 2, 2.5 C | 640.5 mAh g−1 (0.2 C, after 300 cycles) | [150] | |
| GO membrane | 1–1.2 | 1285, 1256, 870 mAh g−1 at 0.2, 0.5, 1 C | 835 mAh g−1 (0.5 C, after 100 cycles) | [151] | |
| PAN/GO | 0.7–1 | 591, 448, 337 mAh g−1 at 0.5 C, 1 C, 2 C | 597 mAh g−1 (0.2 C, after 100 cycles) | [152] | |
| CoPc@GO-PP | 6.8 | - | 919 mAh g−1 (0.5 C, after 250 cycles) | [153] | |
| SrF2-G/PP | 2.3 | 1131, 1083, 950, 878 mAh g−1 at 0.5, 1, 2, 5 C | 811 mAh g−1 (0.2 C, after 110 cycles) | [154] | |
| Ni@NG-CNTs-PP | 1–1.2 | 935, 822, 711, 545 mAh g−1 at 0.5, 1, 2, 5 C | 127 mAh g−1 (2 C, after 900 cycles) | [155] | |
| CuS/graphene-coated separator | 1.85 | 999, 864, 701, 523 mAh g−1 at 0.5, 1, 2, 5 C | 760 mAh g−1 (0.2 C, after 100 cycles) | [156] | |
| G@PP | 1.5–2.1 | 980, 833, 670, 586 mAh g−1 at 1.5, 3, 6, 9 A g−1 | 663 mAh g−1 (1.5 A g−1, after 500 cycles) | [145] | |
| ODC/rGO-Coated Separator | 0.5 | 969, 844, 710, 465 mAh g−1 at 0.5, 1, 2, 5 C | 592 mAh g−1 (1 C, after 600 cycles) | [157] | |
| rGO@SL/PP | 1.5 | 701, 603, 490, 465 mAh g−1 at 0.05, 0.1, 0.2 C | 523 mAh g−1 (2 C, after 1000 cycles) | [158] |
| Separator | Rate Performance | Cycle Performance | Main Functions | Ref. |
|---|---|---|---|---|
| Lithium-ion batteries | ||||
| Celgard 2400 | 135, 113, 83, 30 mAh g−1 at 0.5, 1, 2, 5 C | 138 mAh g−1 (0.2 C, after 100 cycles) | (1) Improve wettability; (2) Provide abundant active sites; (3) Ensure excellent thermal/mechanical stability. | [101] |
| PU@GO | 147, 121, 93, 55 mAh g−1 at 0.5, 1, 2, 5 C | 154 mAh g−1 (0.2 C, after 100 cycles) | [101] | |
| PI@GO | 149, 137, 111, 49 mAh g−1 at 0.5, 1, 2, 5 C | - | [102] | |
| GO-g-HBPE | 135, 129, 118, 95 mAh g−1 at 0.5, 1, 2, 5 C | 122 mAh g−1 (0.2 C, after 200 cycles) | [103] | |
| PVDF-co-HFP/GO | 164, 139, 118, 100 mAh g−1 at 0.5, 2, 5, 10 C | 152 mAh g−1 (0.2 C, after 200 cycles) | [104] | |
| G/PVDF-PL | 153, 148, 135, 111 mAh g−1 at 0.5, 2, 5, 10 C | 129 mAh g−1 (2 C, after 200 cycles) | [105] | |
| Lithium-metal batteries | ||||
| PP | 197.5, 177, 102, 11 mAh g−1 at 0.2, 0.5, 1, 2 C | 13 mAh g−1 (1 C, after 200 cycles) | (1) Inhibit lithium dendrites growth; (2) Provide abundant active sites; (3) Ensure excellent thermal/mechanical stability. | [100] |
| graphene/PP | 199, 184, 115, 28 mAh g−1 at 0.2, 0.5, 1, 2 C | 120 mAh g−1 (1 C, after 200 cycles) | [100] | |
| PE/NSG | 195, 187, 178, 162 mAh g−1 at 0.5, 1, 2, 5 C | 170 mAh g−1 (0.5 C, after 240 cycles) | [124] | |
| GO | 245.6, 239.7, 227.5, 210.5 mAh g−1 at 0.5, 1, 2, 5 C | - | [125] | |
| rGOF-A | 147.49, 100.72, 78.83 mAh g−1 at 0.5, 1, 2 C | 144.63 mAh g−1 (5 C, after 1000 cycles) | [126] | |
| PDA/Gr-CMC | 89, 77, 65, 39 mAh g−1 at 0.5, 1, 2, 4 C | 130.21 mAh g−1 (1 C, after 1000 cycles) | [127] | |
| Lithium-sulfur batteries | ||||
| PP | 564, 533, 490, 141 mAh g−1 at 0.2, 0.5, 1, 2 C | 540 mAh g−1 (0.5 C, after 150 cycles) | (1) Inhibit the transfer of polysulfide; (2) Buffer the volume change of electrodes; (3) Provide abundant active sites; (4) Ensure excellent thermal/mechanical stability. | [142] |
| PG | 1038, 975, 903, 440 mAh g−1 at 0.2, 0.5, 1, 2 C | 877 mAh g−1 (0.5 C, after 150 cycles) | [142] | |
| PPy nanotube | 195, 187, 178, 162 mAh g−1 at 0.5, 1, 2, 5 C | 801.6 mAh g−1 (0.5 C, after 300 cycles) | [143] | |
| CGF | 1096, 1029, 966 mAh g−1 at 0.5 1, 2 C | 779 mAh g−1 (0.5 C, after 300 cycles) | [144] | |
| S-G@PP | 1128, 980, 833, 670, 586 mAh g−1 at 0.75, 1.5, 3, 6, 9 A g−1 | 663 mAh g−1 (1.5 A g−1, after 500 cycles) | [145] | |
| N-rGO | 1060, 927, 779 mAh g−1 at 0.5, 1, 2 C | 758.3 mAh g−1 (1 C, after 400 cycles) | [148] | |
| Ni3Sn2/NG | 1280.5, 1060.2, 927.5, 778.8 mAh g−1 at 0.2, 0.5, 1, 2 C | 758.3 mAh g−1 (1 C, after 400 cycles) | [149] | |
| EUV/graphene | 824.4, 643.5, 518 and 456.3 mAh g−1 at 0.5, 1, 2, 2.5 C | 640.5 mAh g−1 (0.2 C, after 300 cycles) | [150] | |
| GO membrane | 1285, 1256, 870 mAh g−1 at 0.2, 0.5, 1 C | 835 mAh g−1 (0.5 C, after 100 cycles) | [151] | |
| PAN/GO | 591, 448, 337 mAh g−1 at 0.5 C, 1 C, 2 C | 597 mAh g−1 (0.2 C, after 100 cycles) | [152] | |
| CoPc@GO-PP | - | 919 mAh g−1 (0.5 C, after 250 cycles) | [153] | |
| SrF2-G/PP | 1131, 1083, 950, 878 mAh g−1 at 0.5, 1, 2, 5 C | 811 mAh g−1 (0.2 C, after 110 cycles) | [154] | |
| Ni@NG-CNTs-PP | 935, 822, 711, 545 mAh g−1 at 0.5, 1, 2, 5 C | 127 mAh g−1 (2 C, after 900 cycles) | [155] | |
| CuS/graphene-coated separator | 999, 864, 701, 523 mAh g−1 at 0.5, 1, 2, 5 C | 760 mAh g−1 (0.2 C, after 100 cycles) | [156] | |
| G@PP | 980, 833, 670, 586 mAh g−1 at 1.5, 3, 6, 9 A g−1 | 663 mAh g−1 (1.5 A g−1, after 500 cycles) | [145] | |
| ODC/rGO-Coated Separator | 969, 844, 710, 465 mAh g−1 at 0.5, 1, 2, 5 C | 592 mAh g−1 (1 C, after 600 cycles) | [157] | |
| rGO@SL/PP | 701, 603, 490, 465 mAh g−1 at 0.05, 0.1, 0.2 C | 523 mAh g−1 (2 C, after 1000 cycles) | [158] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Sun, W.; Sun, Z.; Ding, R.; Wang, X. Graphene-Based Materials for the Separator Functionalization of Lithium-Ion/Metal/Sulfur Batteries. Materials 2023, 16, 4449. https://doi.org/10.3390/ma16124449
Huang Z, Sun W, Sun Z, Ding R, Wang X. Graphene-Based Materials for the Separator Functionalization of Lithium-Ion/Metal/Sulfur Batteries. Materials. 2023; 16(12):4449. https://doi.org/10.3390/ma16124449
Chicago/Turabian StyleHuang, Zongle, Wenting Sun, Zhipeng Sun, Rui Ding, and Xuebin Wang. 2023. "Graphene-Based Materials for the Separator Functionalization of Lithium-Ion/Metal/Sulfur Batteries" Materials 16, no. 12: 4449. https://doi.org/10.3390/ma16124449
APA StyleHuang, Z., Sun, W., Sun, Z., Ding, R., & Wang, X. (2023). Graphene-Based Materials for the Separator Functionalization of Lithium-Ion/Metal/Sulfur Batteries. Materials, 16(12), 4449. https://doi.org/10.3390/ma16124449







