Pulsed TIG Cladding of a Highly Carbon-, Chromium-, Molybdenum-, Niobium-, Tungsten- and Vanadium-Alloyed Flux-Cored Wire Electrode on Duplex Stainless Steel X2CrNiMoN 22-5-3
Abstract
1. Introduction
2. Experimental Procedure
- -
- Base metal—X2CrNiMoN22-5-3 (AISI 2205);
- -
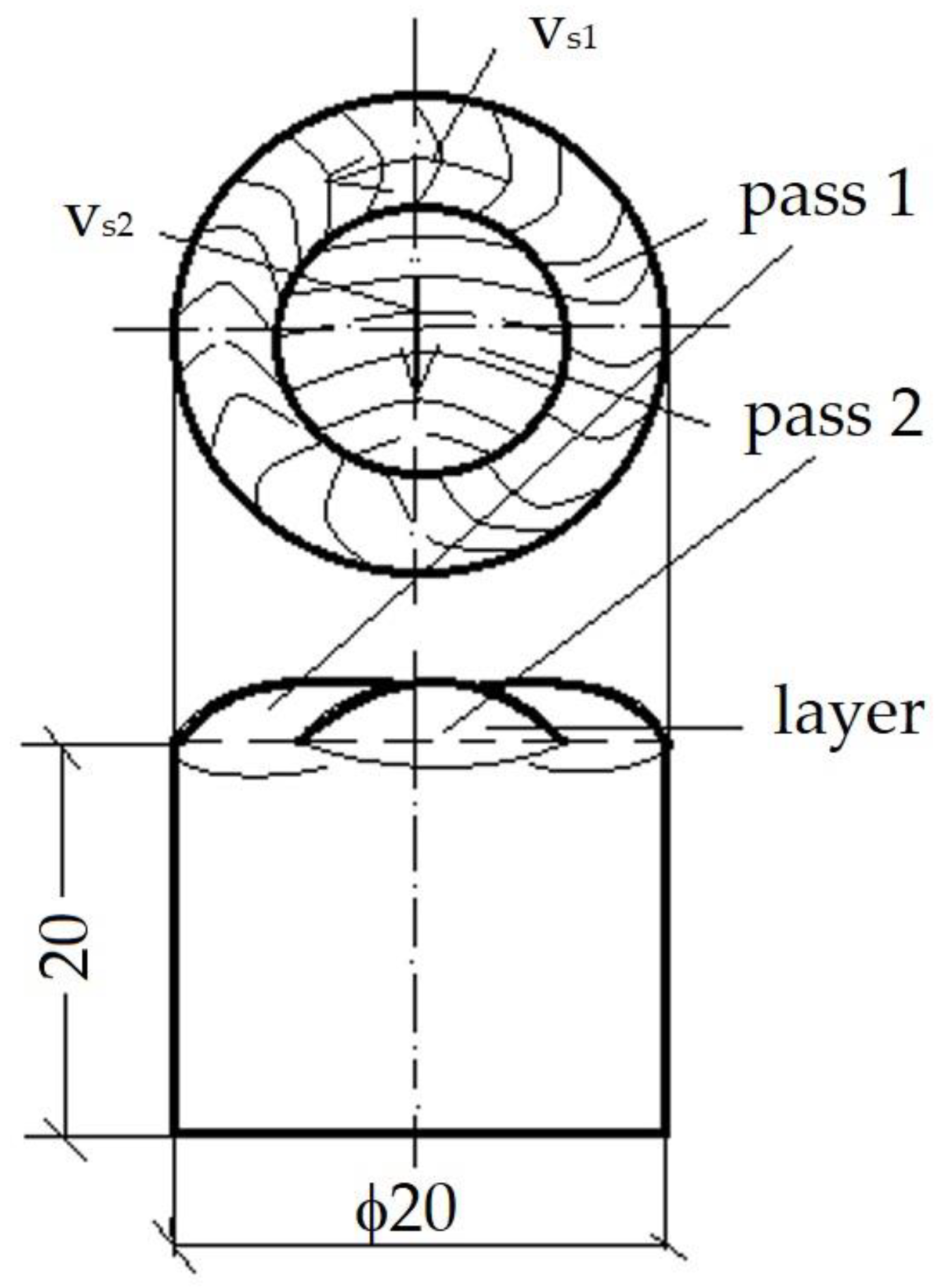
- Sample sizes—ø20 × 20 mm;
- -
- Filler material—Corodur 65 (Corthal 65), DIN EN 14700;
- -
- Type of filler material—tubular wire with self-protection;
- -
- Wire diameter—1.6 mm;
- -
- Shielding gas—Ar 100%, I1, EN 14,175 (Ar 4.8—LINDE);
- -
- Shielding gas flow rate—10 l/min;
- -
- Gas nozzle no. 7 (inner diameter 11.2 mm);
- -
- Non-fusible electrode—EWC20 EN 26,848;
- -
- Electrode diameter—2.4 mm;
- -
- How to arrange the passes—on the frontal surface of the sample;
- -
- Pass number—2 per layer, from outside to inside, as shown in Figure 3;
- -
- Number of layers:
- ▪
- Sample 1—1 layer;
- ▪
- Sample 2—2 layers;
- ▪
- Sample 3—3 layers.
- -
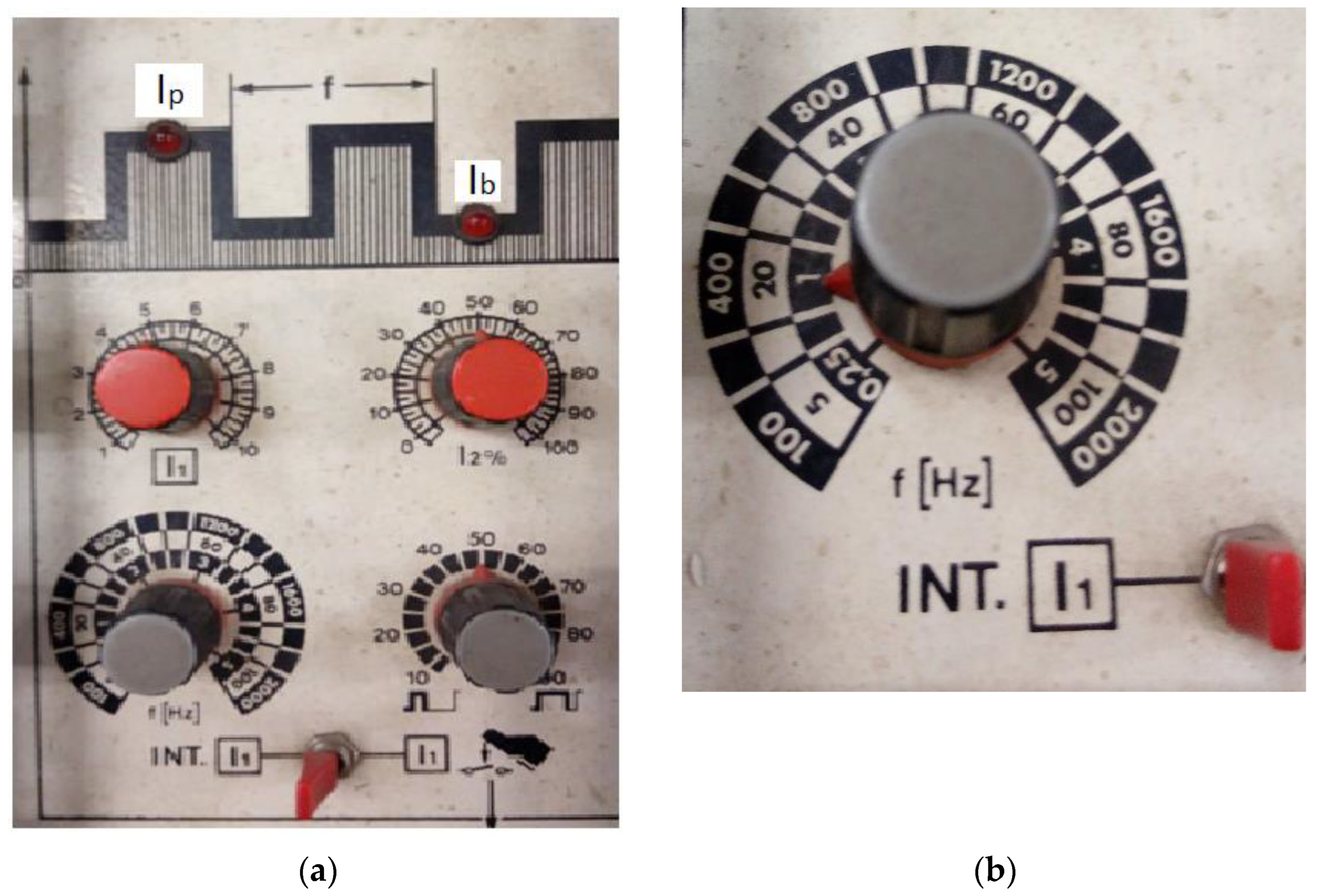
- Impulse current—130 A;
- -
- Base current—45 A;
- -
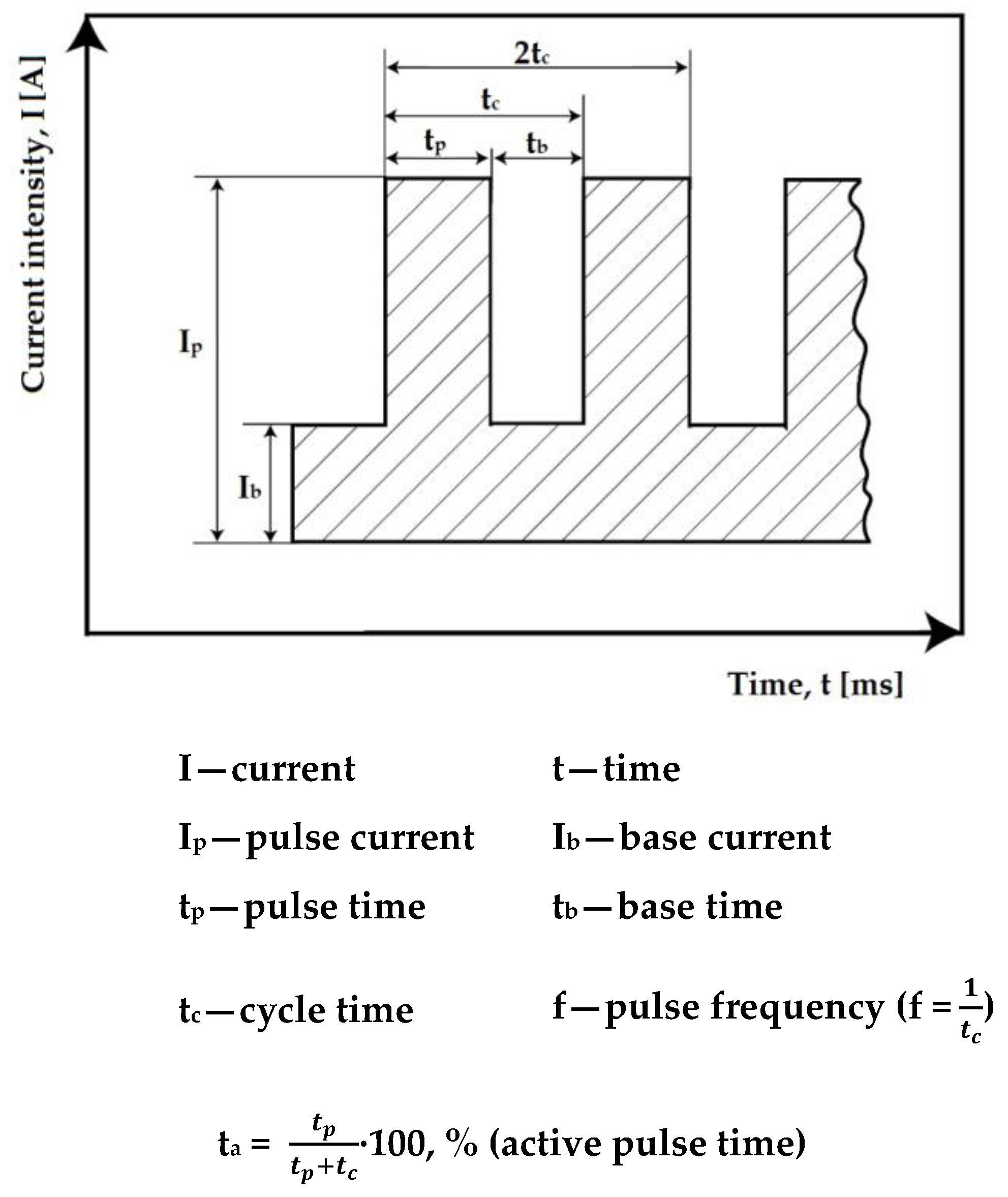
- Average welding current—49 A; [Im = (Ip·tp + Ib·tb)/(tp + tb)]—rectangular wave;
- -
- Arc voltage—11 V;
- -
- Pulse time—0.3 s;
- -
- Base time—0.6 s (tb = 2 tp);
- -
- Pulse frequency—f = 1 Hz;
- -
- Arc length—larc = 2.0–2.5 mm;
- ▪
- Layer I, pass 1:
- Welding time—10 s;
- Weld length—43 mm;
- Welding speed—26 cm/min;
- Linear energy—2540 J/cm.
- ▪
- Layer I, pass 2:
- Welding time—5 s;
- Weld length—10 mm;
- Welding speed—12 cm/min;
- Linear energy—5500 J/cm.
- ▪
- Layer II, pass 1:
- Welding time—8 s;
- Weld length—40 mm;
- Welding speed—30 cm/min;
- Linear energy—2200 J/cm.
- ▪
- Layer II, pass 2:
- Welding time—4 s;
- Weld length—10 mm;
- Welding speed—15 cm/min;
- Linear energy—4400 J/cm.
- ▪
- Layer 3, pass 1:
- Welding time—8 s;
- Weld length—40 mm;
- Welding speed—30 cm/min;
- Linear energy—2420 J/cm.
- ▪
- Layer III, pass 2:
- Welding time—3 s;
- Weld length—10 mm;
- Welding speed—20 cm/min;
- Linear energy—3300 J/cm.
3. Evaluation of the Experimental Data
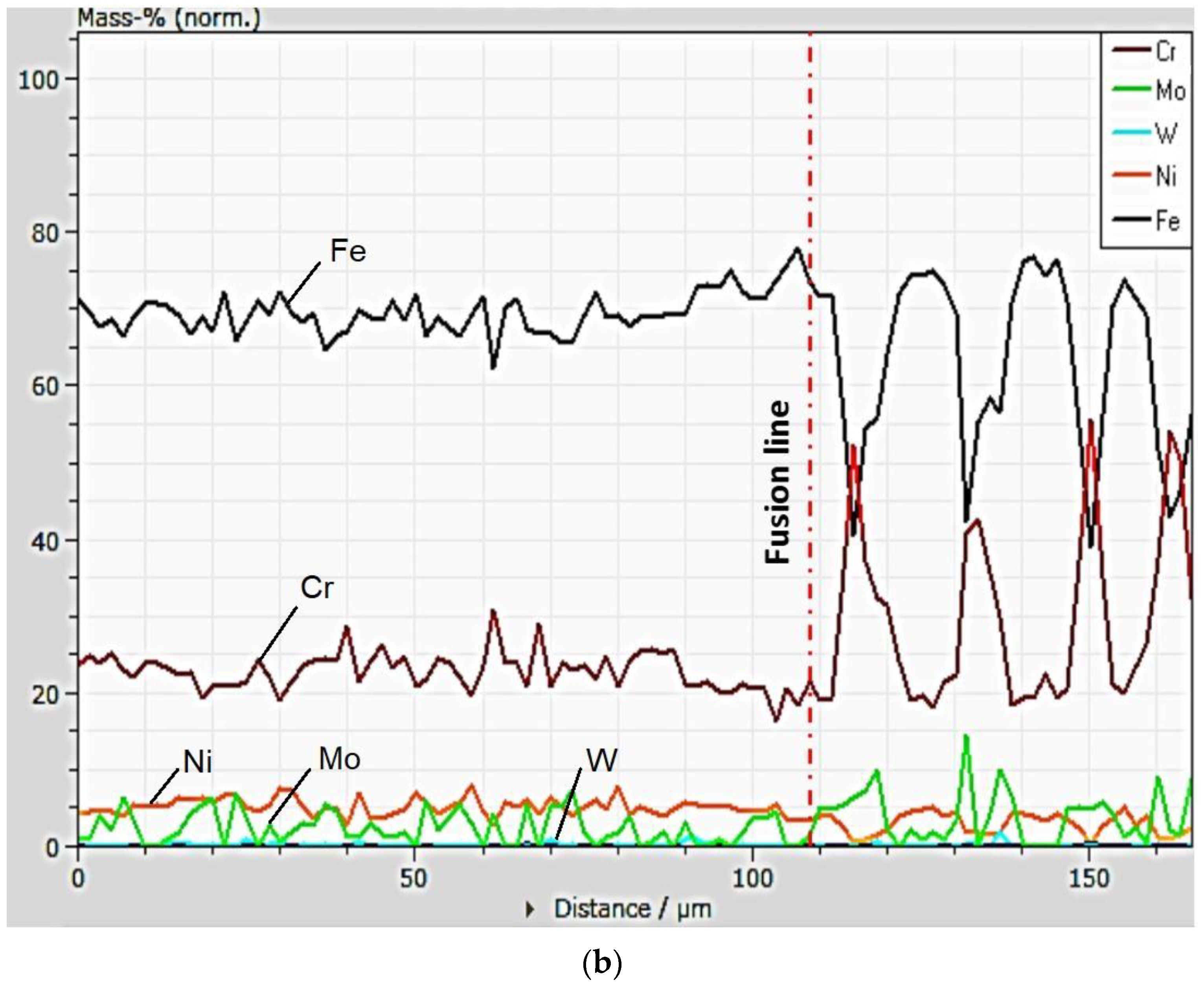
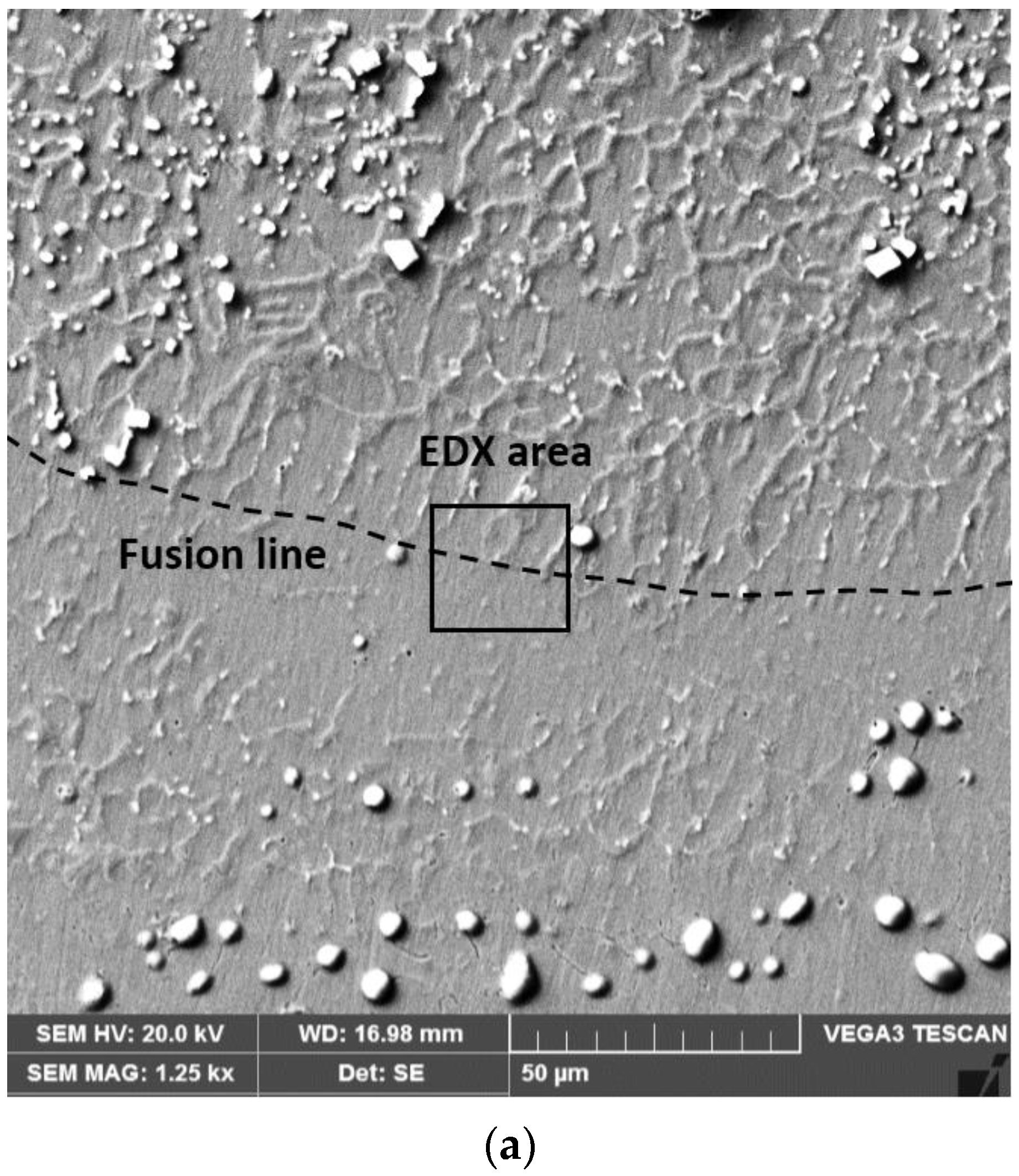
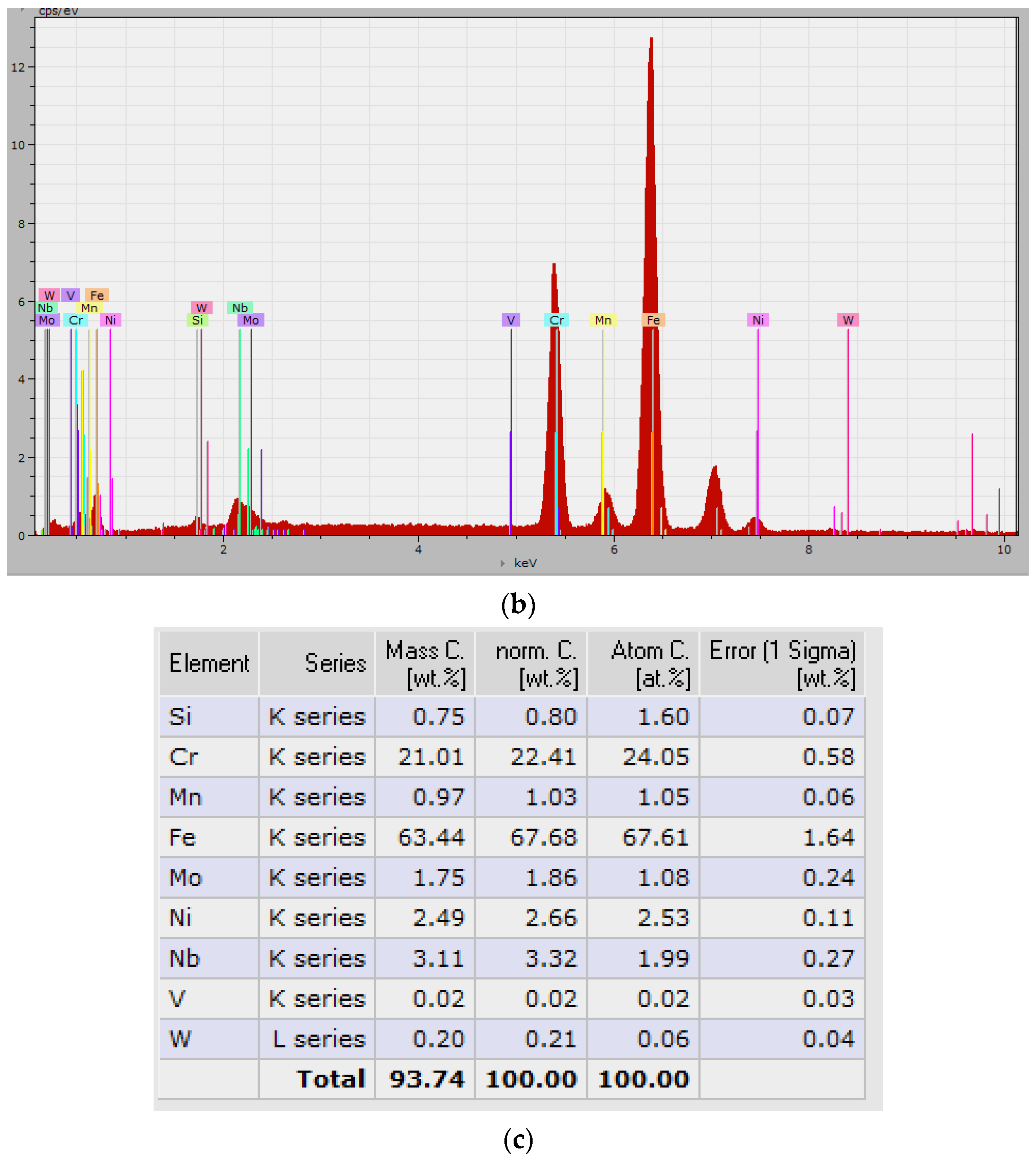
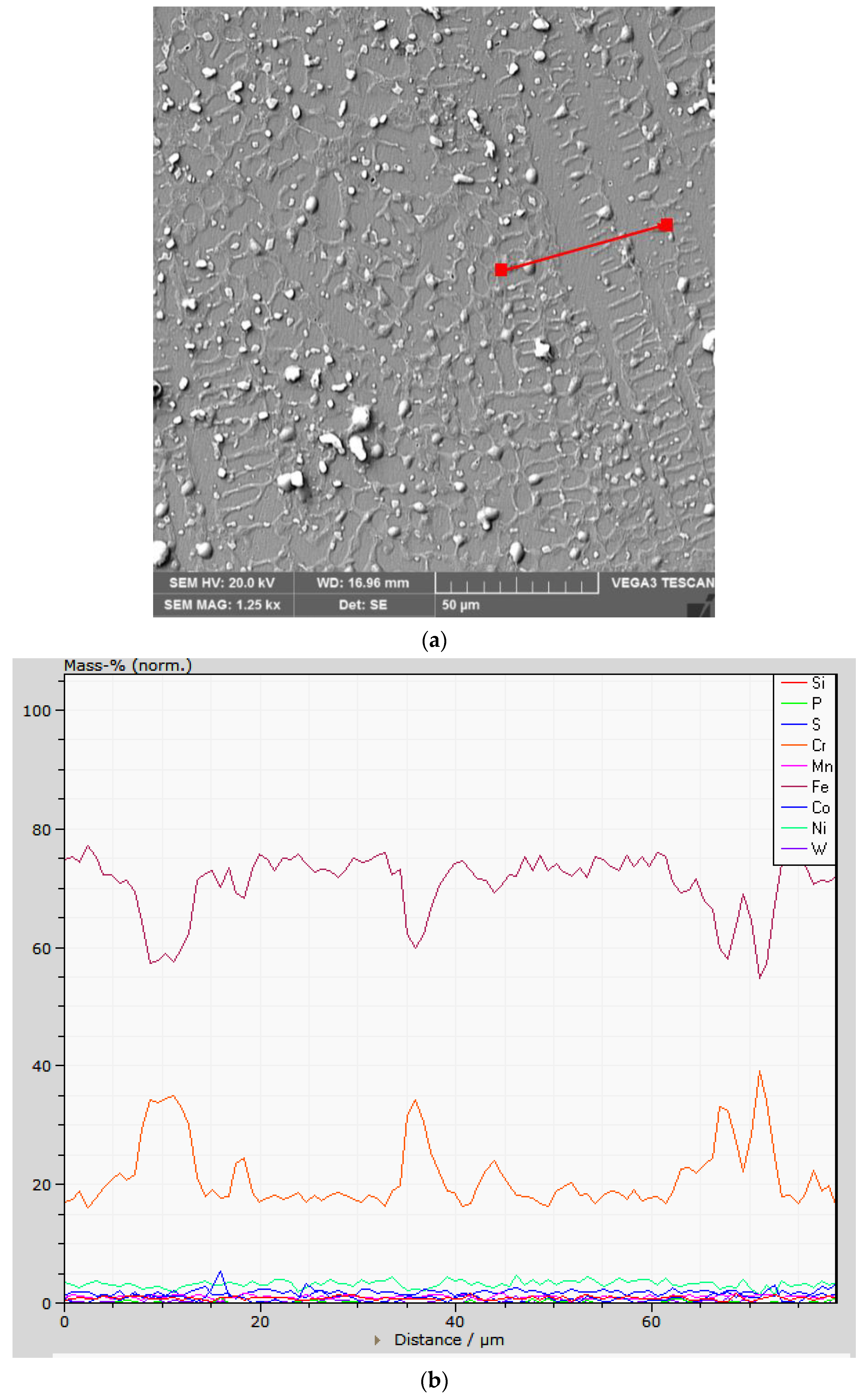
3.1. Chemical Heterogeneities and Degree of Dilution
- Physical processes—volatilization of alloying elements;
- Chemical reactions between the components of the alloy;
- Reactions between the molten metal and the environment;
- Dilution;
- The fluctuation of the solidification speed.
- Constitutional subordination;
- Dendritic segregation;
- Pollution;
- Irregular gas protection.
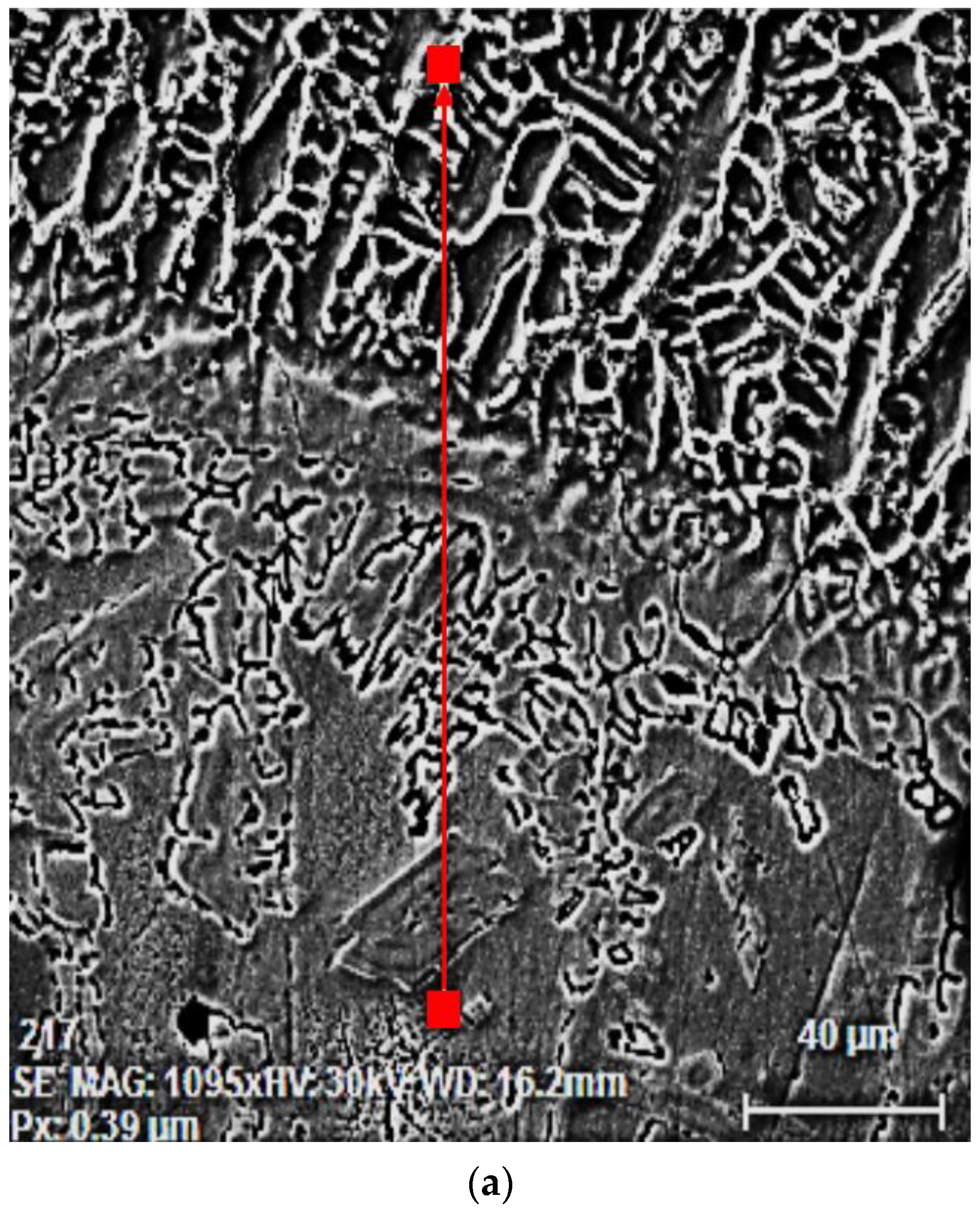
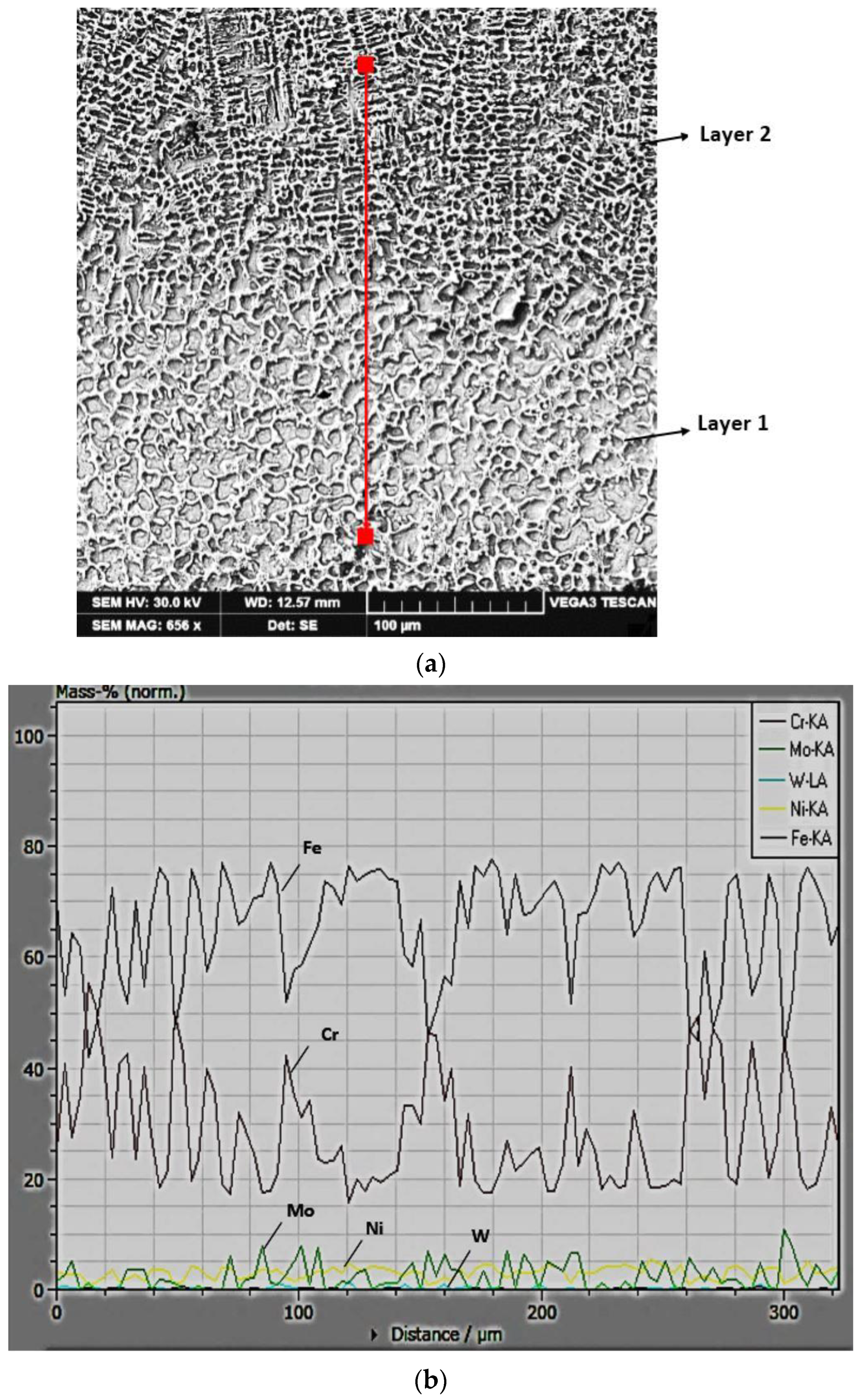
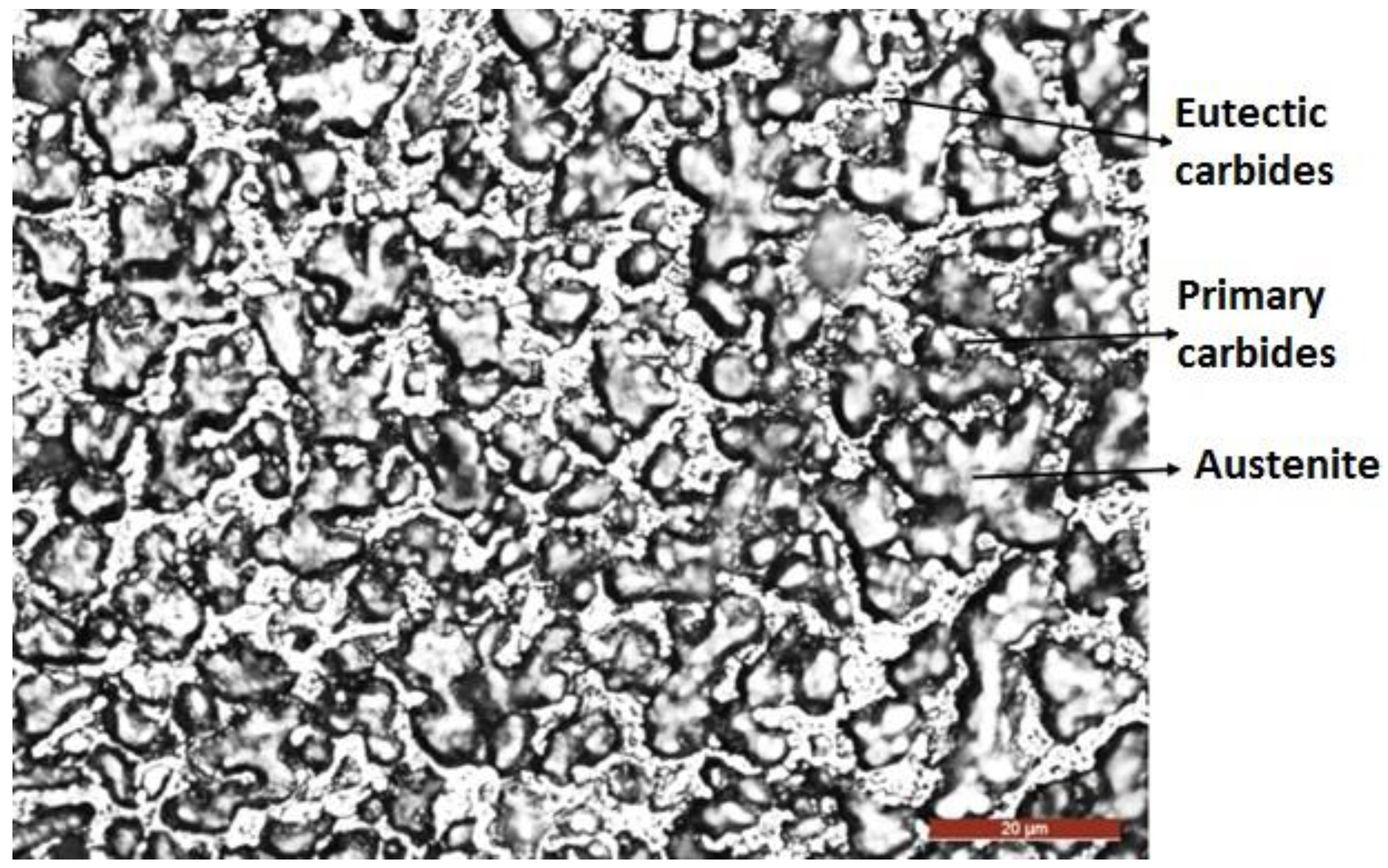
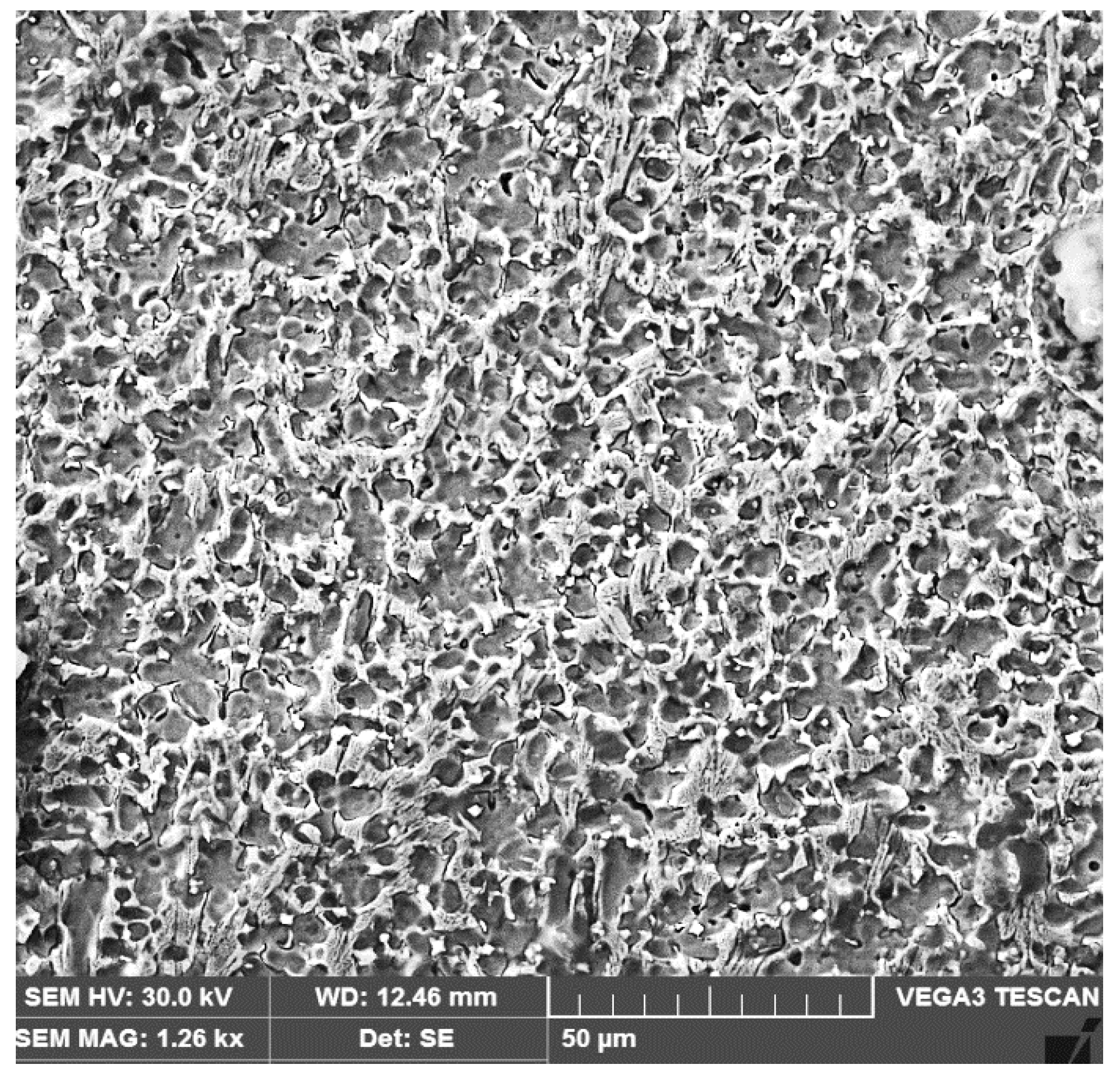
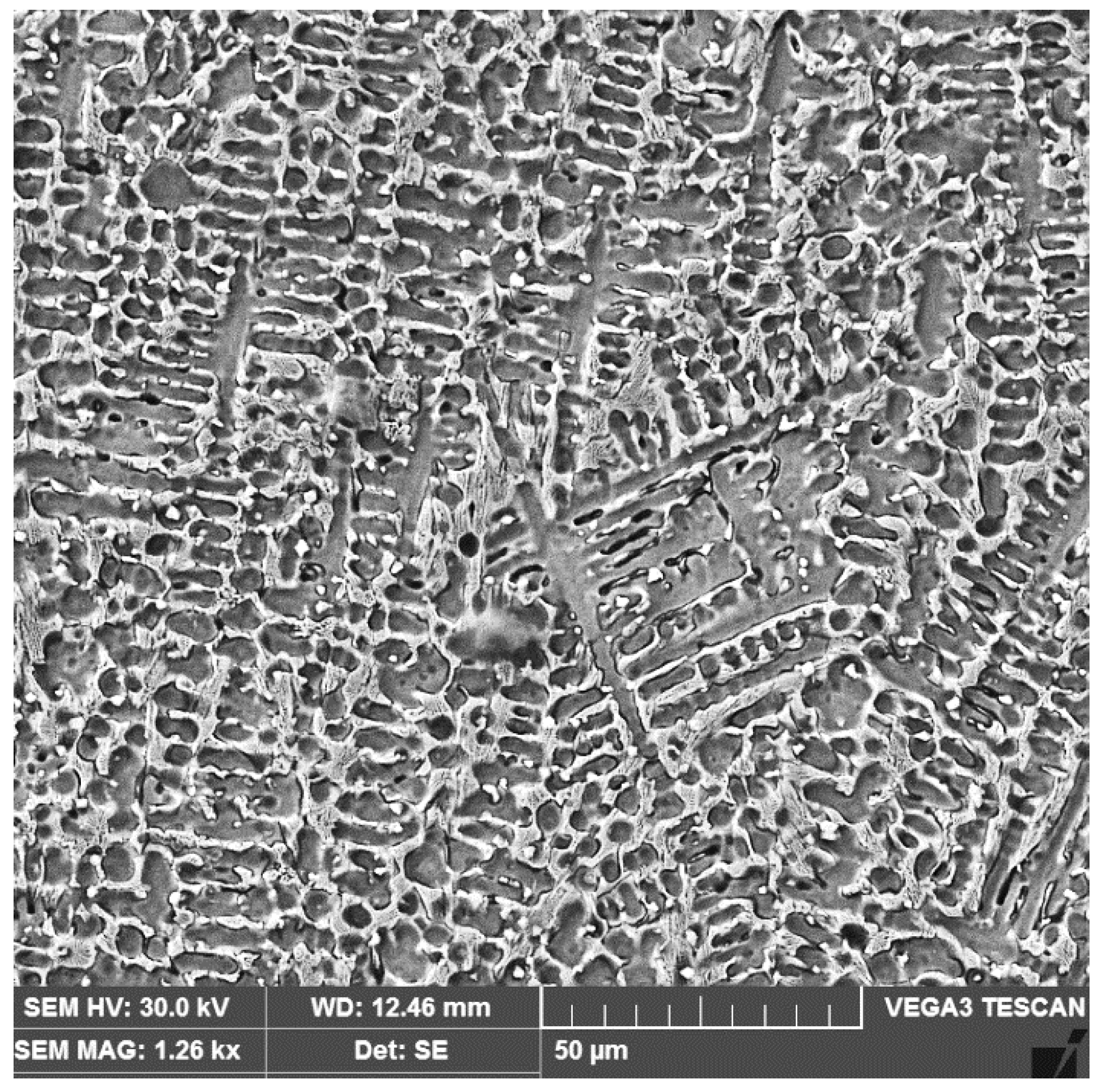
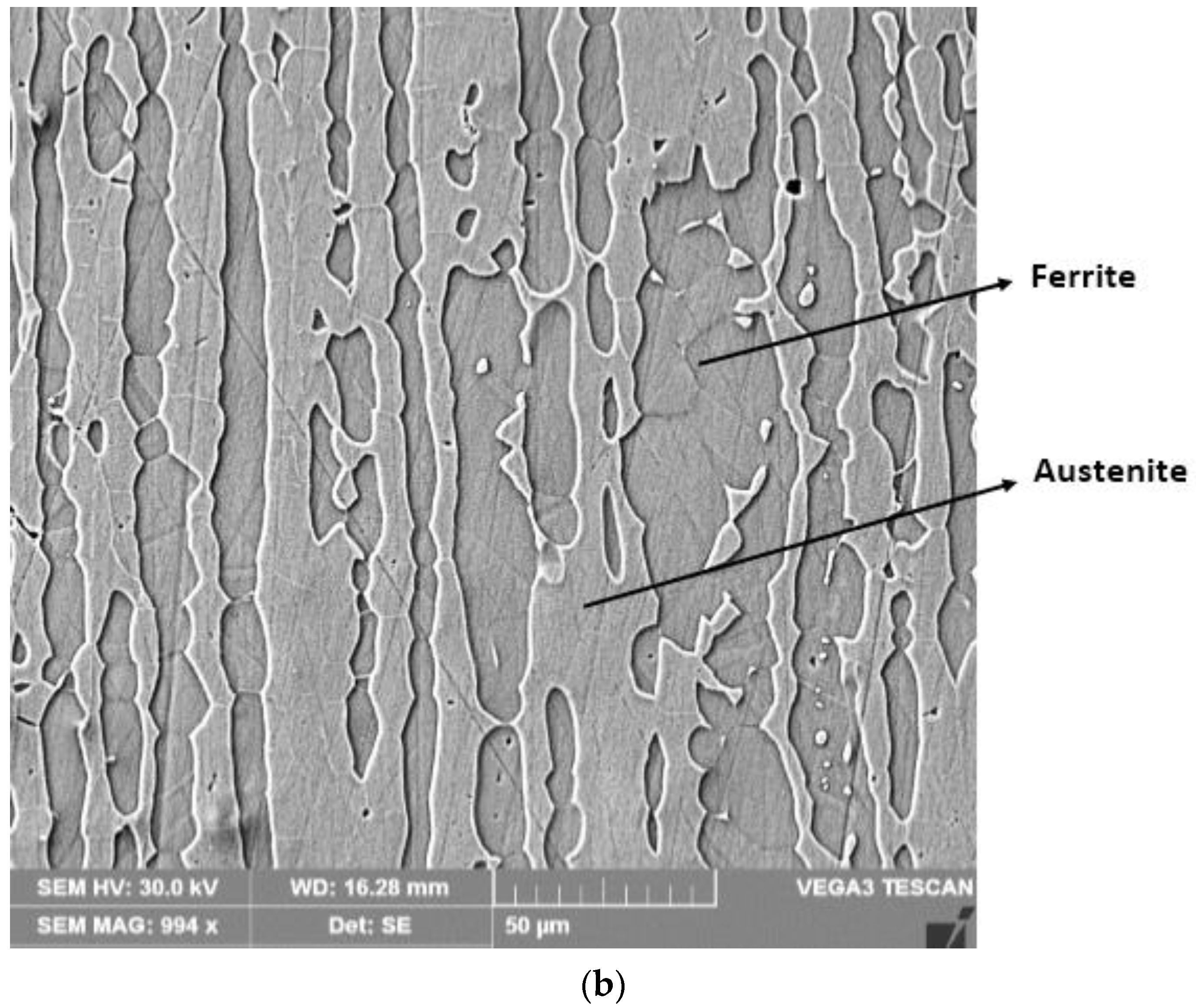
3.2. The Microstructure of the Layer–Substrate System
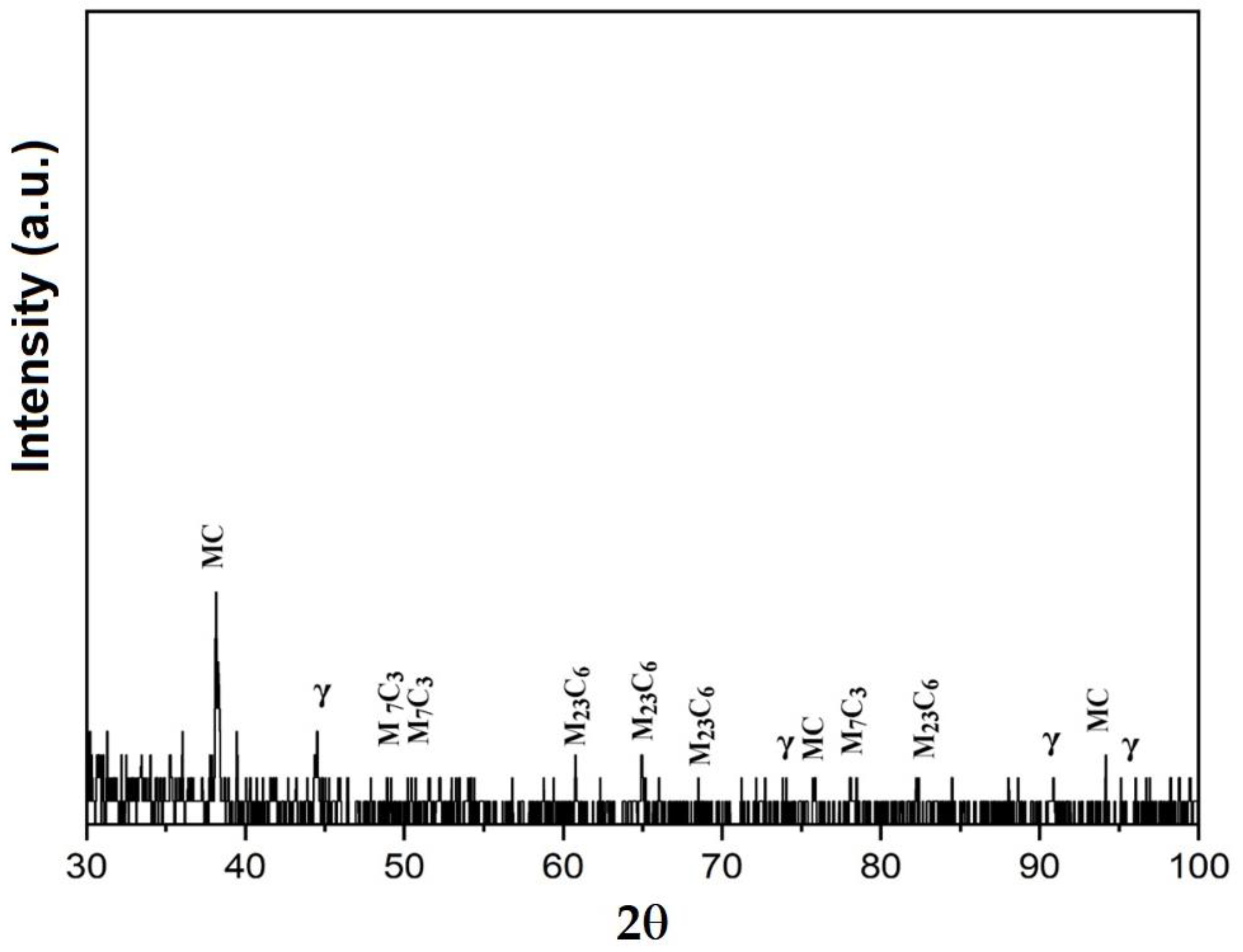
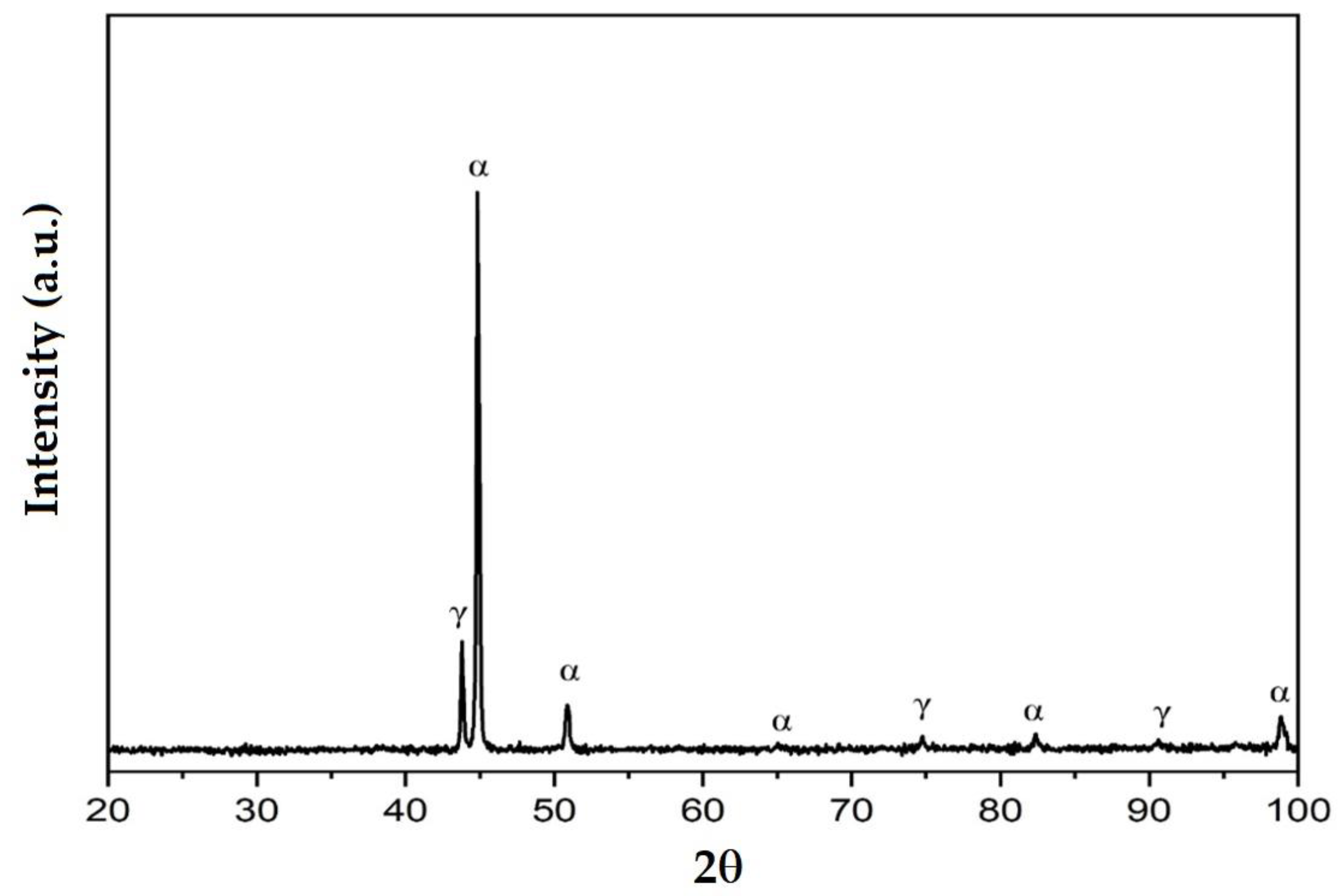
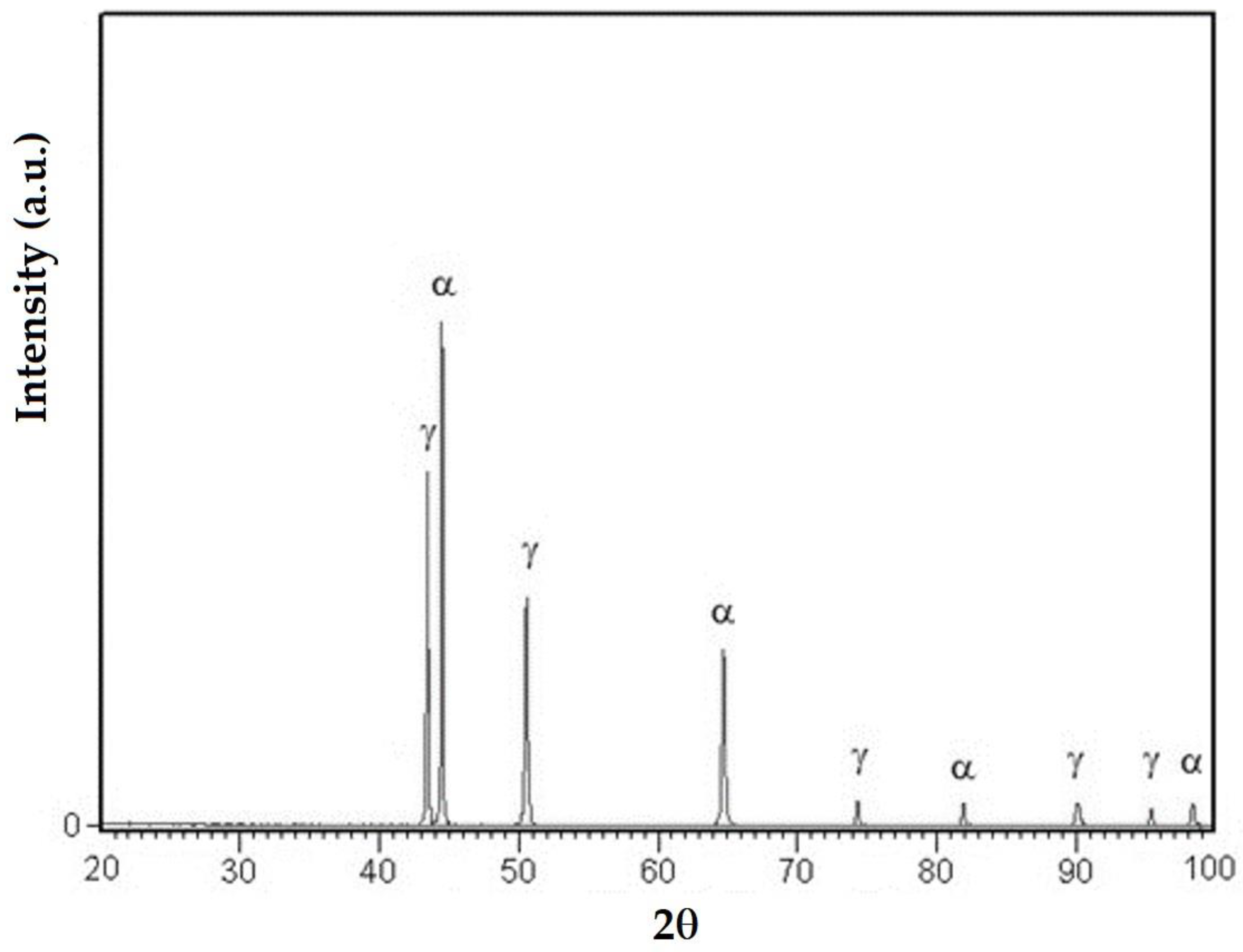
3.3. X-ray Diffraction Analyses
- The structural phases detected in the deposited metal are the particles of complex carbides dispersed in the solid solution γ with the fcc crystalline lattice.
- The high temperatures reached in the area adjacent to the fusion line promote, in the HAZ of the base metal, a predominantly ferritic microstructure with a reduced proportion of austenite resulting from the transformation into a solid state, initiated in the cooling stage of the deposited metal bath.
- The base metal in the solution treatment state has a microstructure made up of solid solutions, ferrite and austenite.
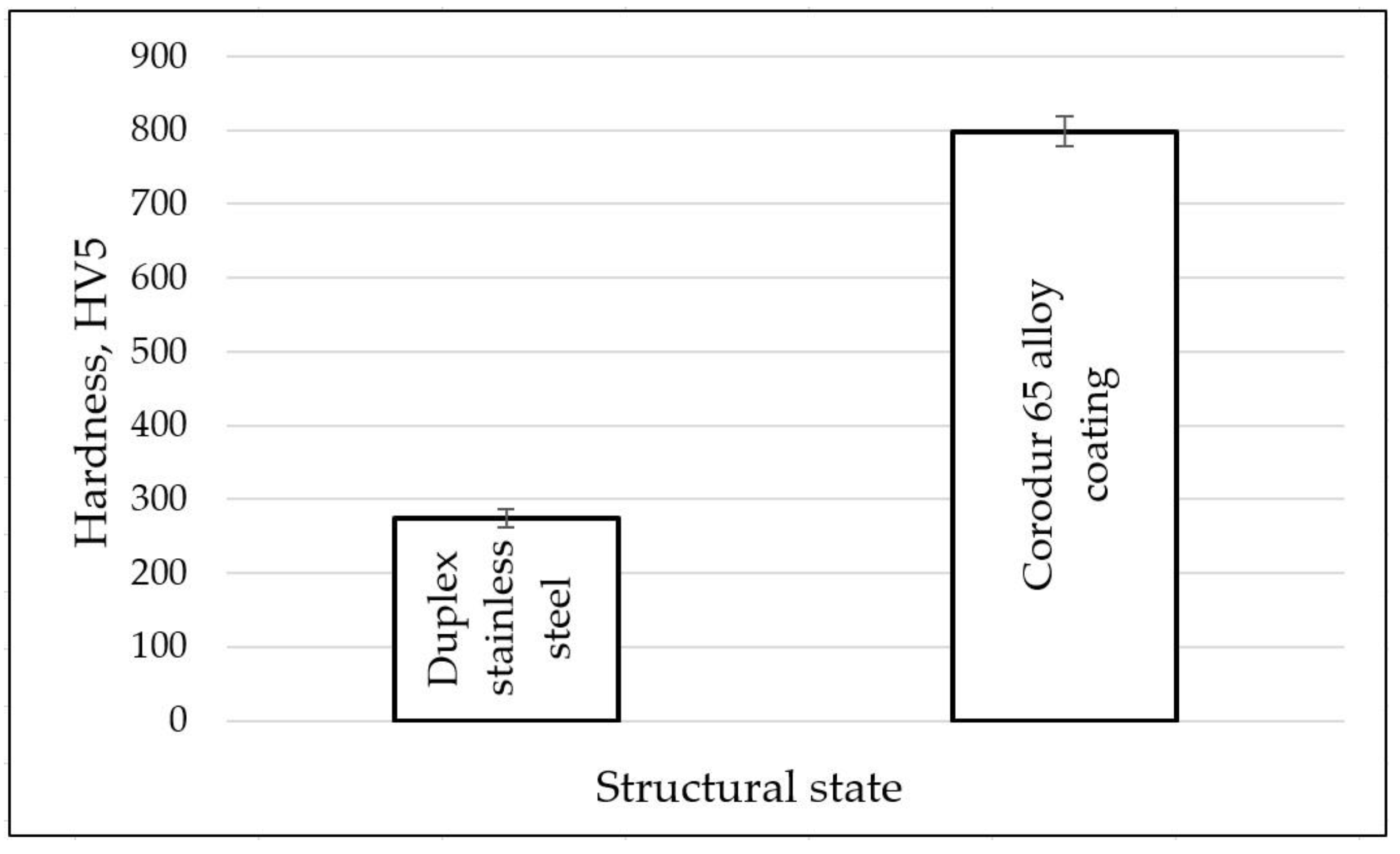
3.4. Hardness Measurements
4. Conclusions
- The most important process parameter is the welding current, as it influences the linear energy and the melting of the materials selected for the layer and for the substrate.
- The base metal was covered with three layers of the Corodur alloy—single, double and triple layer. The obtained hardness is remarkable, at approx. 810 HV5. Due to the low degree of dilution, there were no differences in the hardness between the deposited metal layers.
- The hardfacing process promotes, in the deposited layer, precipitation reactions of complex carbides finely dispersed in a tenacious austenite matrix.
- The precipitation of the carbide phases takes place both in the intercellular and interdendritic regions from the fusion zone located in close proximity to the base metal.
- The profile of the chemical composition in a direction transverse to that of cellular and dendritic growth indicates an increase in the Cr concentration and a decrease in the Fe concentration in the intercellular region.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madadi, F.; Ashrafizadeh, F.; Shamanian, M. Optimization of pulsed TIG cladding process for stellite alloy on carbon steel using RSM F. J. Alloys Compd. 2012, 510, 71–77. [Google Scholar] [CrossRef]
- Rajeev, G.P.; Kamaraj, M.; Srinivasa, R. Bakshi. Hardfacing of AISI H13 tool steel with Stellite 21 alloy using cold metal transfer welding process. Surf. Coat. Technol. 2017, 326, 63–71. [Google Scholar]
- Messler, R.W., Jr. Principles of Welding—Processes, Physics, Chemistry, and Metallurgy; John Wiley & Sons.: Hoboken, NJ, USA, 2004; pp. 49–154. [Google Scholar]
- Dobrzański, L.A.; Mikuła, J. Structure and properties of PVD and CVD coated Al2O3 + TiC mixed oxide tool ceramics for dry on high speed cutting processes. J. Mater. Process. Technol. 2005, 15, 822–831. [Google Scholar] [CrossRef]
- Koseki, S.; Inoue, K.; Morito, S.; Ohba, T.; Usuki, H. Comparison of TiN-coated tools using CVD and PVD processes during continuous cutting of Ni-based superalloys. Surf. Coat. Technol. 2015, 283, 353–363. [Google Scholar] [CrossRef]
- Thakur, A.; Gangopadhyay, S. Influence of tribological properties on the performance of uncoated, CVD and PVD coated tools in machining of Incoloy 825. Tribol. Int. 2016, 102, 198–212. [Google Scholar] [CrossRef]
- Niranatlumpong, P.; Koiprasert, H. Phase transformation of NiCrBSi–WC and NiBSi–WC arc sprayed coatings. Surf. Coat. Technol. 2011, 206, 440–445. [Google Scholar] [CrossRef]
- Daram, P.; Banjongprasert, C. The influence of post treatments on the microstructure and corrosion behavior of thermally sprayed NiCrMoAl alloy coating. Surf. Coat. Technol. 2020, 384, 125166. [Google Scholar] [CrossRef]
- Suraj, R. Hardfacing and its effect on wear and corrossion performance of various ferrous welded mild steels. Mater. Today Proc. 2021, 42, 842–850. [Google Scholar] [CrossRef]
- Özen, F.; Onar, V.; Çil, G.; Gel, M. Wear resistance and microstructural evaluation of a hardfacing welded S355J2 steel pipe piles. Mater. Test. 2022, 64, 800–808. [Google Scholar] [CrossRef]
- Sönmez, M.; Topuz, P. Effect of TIG welding parameters in joining grade 2 pure titanium. Mater. Test. 2021, 63, 420–425. [Google Scholar] [CrossRef]
- Di Schino, A.; Testani, C. Corrosion behavior and mechanical properties of AISI 316 stainless steel clad Q235 plate. Metals 2020, 10, 552. [Google Scholar] [CrossRef]
- Balaguru, S.; Vela, M.; Chellapandi, P. Measurement of the residual stresses and investigation of their effects on a hardfaced grid plate due to thermal cycling in a pool type sodium-cooled fast reactor. Sci. Technol. Nucl. Install. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Xi, B.; Liu, B.; Li, S.; Wang, D.; Zhang, Y.; Szakálos, P.; Ejenstam, J.; Wallenius, J.; He, G.; Zhang, W. Influence of TIG and Laser Welding Processes of Fe-10Cr-4Al-RE Alloy Cracks Overlayed on 316L Steel Plate. Materials 2022, 15, 3541. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Lim, L.C.; Chen, Z.D. Laser Cladding of nickel-based hardfacing alloys. Surf. Coat. Technol. 1998, 106, 174–182. [Google Scholar]
- Rojacz, H.; Katsic, C.; Kirchgaßner, M.; Kirchmayer, R.; Badisch, E. Impact-abrasive wear of martensitic steels and complex iron-based hardfacing alloys. Wear 2022, 492–493, 204183. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Goel, P.; Singh, H. Analysis of wear and hardness during surface hardfacing of alloy steel by thermal spraying, electric arc and TIG welding. Mater. Proc. 2022, 50, 1599–1605. [Google Scholar] [CrossRef]
- Liu, W.; Gao, D. Microstructure and wear of Ni-WC hardfacing used for steel-body PDC bits. Int. J. Refract. Met. Hard Mater. 2021, 101, 105683. [Google Scholar] [CrossRef]
- Balaguru, S.; Mohammad, A.; Gupta, M. Investigations on different hardfacing processes for High temperature applications of Ni-Cr-B-Si alloy hardfaced on austenitic stainless steel components. J. Mater. Res. Technol. 2020, 9, 10062–10072. [Google Scholar] [CrossRef]
- Bembenek, M.; Prysyazhnyuk, P.; Shihab, T.; Machnik, R.; Ivanov, O.; Ropyak, L. Microstructure and Wear Characterization of the Fe-Mo-B-C-Based Hardfacing Alloys Deposited by Flux-Cored Arc Welding. Materials 2022, 15, 5074. [Google Scholar] [CrossRef]
- Gurumoorthy, K.; Kamaraj, M.; Prasad Rao, K.; Sambasiva Rao, A.; Venugopal, S. Microstructural aspects of plasma transferred arc surfaced Ni-based hardfacing alloy. Mater. Sciece Eng. A 2007, 456, 11–19. [Google Scholar] [CrossRef]
- Hemmati, I.; Ocelik, V.; De Hosson, J.T.M. Dilution effects in laser cladding of Ni-Cr-B-Si-C hardfacing alloys. Mater. Lett. 2012, 84, 69–72. [Google Scholar] [CrossRef]
- Silva, C.C.; Miranda, E.C.; Motta, M.F.; Miranda, H.C.; Farias, J.P. Influence of arc length on dilution and weld bead geometry of Ni-based alloy using GTAW process with cold wire feed. In Proceedings, 20th International Congress of Mechanical Engineering; Brazilian Society of Mechanical Science and Engineering: Gramado, Brazil, 2009. [Google Scholar]
- Miranda, E.C.; Silva, C.C.; Miranda, H.C.; Motta, M.F.; Farias, J.P. Adjustment of the wire feeder parameters in GTAW process for coatings applications. In Proceeding, XXXV CONSOLDA—National Welding Congress; Brazilian Welding Society: São Paulo, Brazil, 2009. [Google Scholar]
- Anik, S.; Dorn, L. Schweißeignung Metallischer Werkstoffe; Deutscher Verlag fuer Schweisstechnik, DVS-Verlag: Frankfurt am Main, Germany, November 1995; pp. 303–311. [Google Scholar]
- Mitelea, I.; Budau, V. Materiale si Tratamente Termice Pentru Structuri Sudate (Materials and Heat Treatments for Welded Structures); West Publishing House Timisoara: Timisoara, Romania, 1992; pp. 225–236. [Google Scholar]
- Klimpel, A. Industrial surfacing and hardfacing technology, fundamentals and applications. Weld. Technol. Rev. 2020, 91, 33–42. [Google Scholar] [CrossRef]
- Pelz, A. Microstructure and Properties of New Developed Fe-Cr-C-B Powders for Wear-Protection Purposes. In Proceedings of the Thermal Spray 2009: Proceedings from the International Thermal Spray Conference, Las Vegas, NV, USA, 4–7 May 2009; pp. 849–854. [Google Scholar]



| C% | Si% | Mn % | Cr% | Mo% | Nb% | V% | W% | Fe% |
|---|---|---|---|---|---|---|---|---|
| 5.08 | 0.94 | 0.42 | 21.1 | 6.94 | 6.72 | 1.02 | 1.88 | Rest |
| C% | Si% | Mn% | Cr% | Ni% | Mo% | P% | S% | N% | Fe% |
|---|---|---|---|---|---|---|---|---|---|
| 0.017 | 0.72 | 1.80 | 22.08 | 5.02 | 2.90 | 0.021 | 0.012 | 0.16 | Rest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutașcu, D.; Karancsi, O.; Mitelea, I.; Crăciunescu, C.M.; Buzdugan, D.; Uțu, I.-D. Pulsed TIG Cladding of a Highly Carbon-, Chromium-, Molybdenum-, Niobium-, Tungsten- and Vanadium-Alloyed Flux-Cored Wire Electrode on Duplex Stainless Steel X2CrNiMoN 22-5-3. Materials 2023, 16, 4557. https://doi.org/10.3390/ma16134557
Mutașcu D, Karancsi O, Mitelea I, Crăciunescu CM, Buzdugan D, Uțu I-D. Pulsed TIG Cladding of a Highly Carbon-, Chromium-, Molybdenum-, Niobium-, Tungsten- and Vanadium-Alloyed Flux-Cored Wire Electrode on Duplex Stainless Steel X2CrNiMoN 22-5-3. Materials. 2023; 16(13):4557. https://doi.org/10.3390/ma16134557
Chicago/Turabian StyleMutașcu, Daniel, Olimpiu Karancsi, Ion Mitelea, Corneliu Marius Crăciunescu, Dragoș Buzdugan, and Ion-Dragoș Uțu. 2023. "Pulsed TIG Cladding of a Highly Carbon-, Chromium-, Molybdenum-, Niobium-, Tungsten- and Vanadium-Alloyed Flux-Cored Wire Electrode on Duplex Stainless Steel X2CrNiMoN 22-5-3" Materials 16, no. 13: 4557. https://doi.org/10.3390/ma16134557
APA StyleMutașcu, D., Karancsi, O., Mitelea, I., Crăciunescu, C. M., Buzdugan, D., & Uțu, I.-D. (2023). Pulsed TIG Cladding of a Highly Carbon-, Chromium-, Molybdenum-, Niobium-, Tungsten- and Vanadium-Alloyed Flux-Cored Wire Electrode on Duplex Stainless Steel X2CrNiMoN 22-5-3. Materials, 16(13), 4557. https://doi.org/10.3390/ma16134557









