Technological Processes for Increasing the Cavitation Erosion Resistance of Nimonic 80A Superalloys
Abstract
:1. Introduction
- -
- Chemical composition, respectively the nature and amount of alloying elements;
- -
- Semi-finished product development technology (cast, laminated, etc.);
- -
- Surface hardening treatment (thermal, thermomechanical, thermo-chemical, etc.);
- -
- The values of the mechanical properties (Rm, Rp0.2, HV, KCU);
- -
- The morphology of the microstructural constituents.
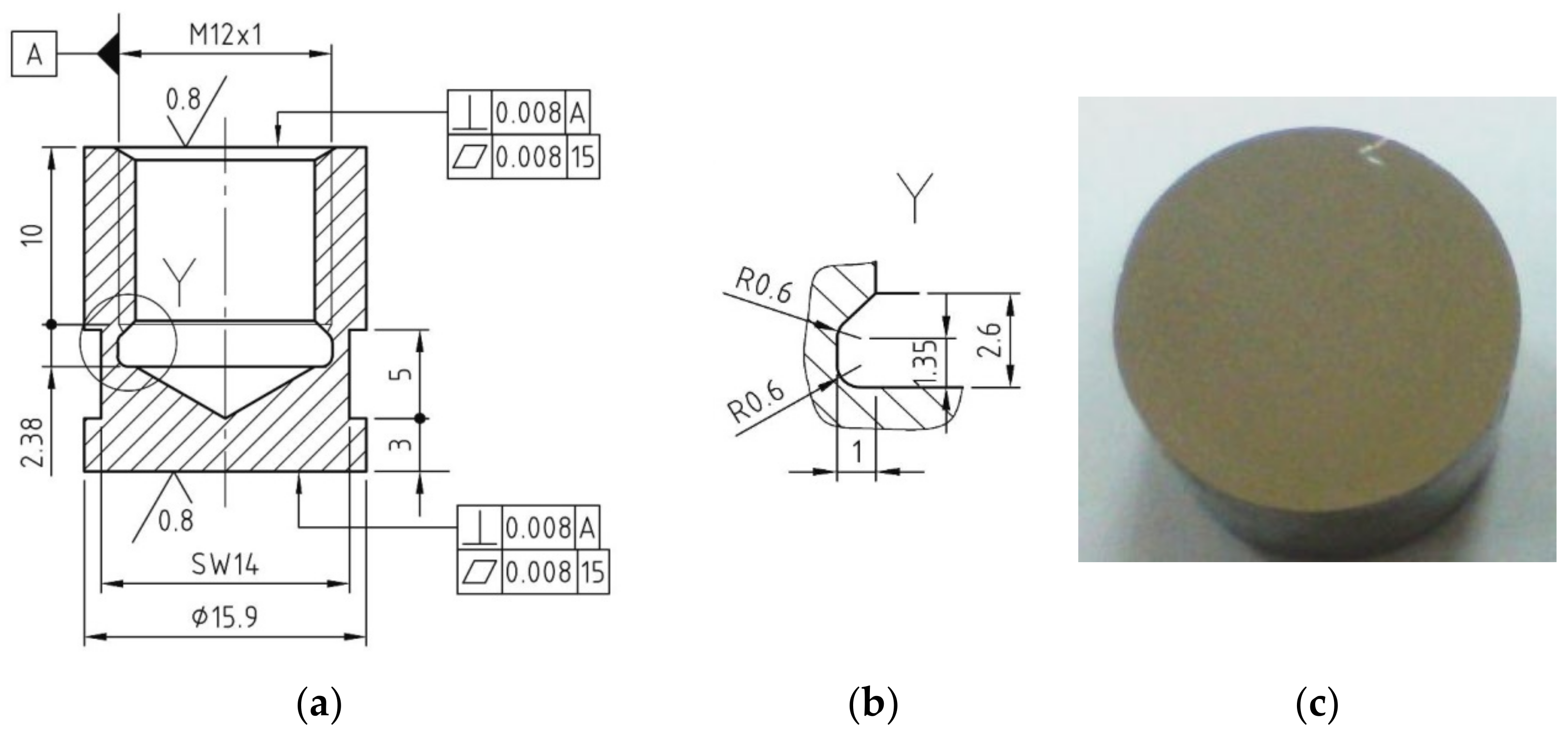
2. Researched Material
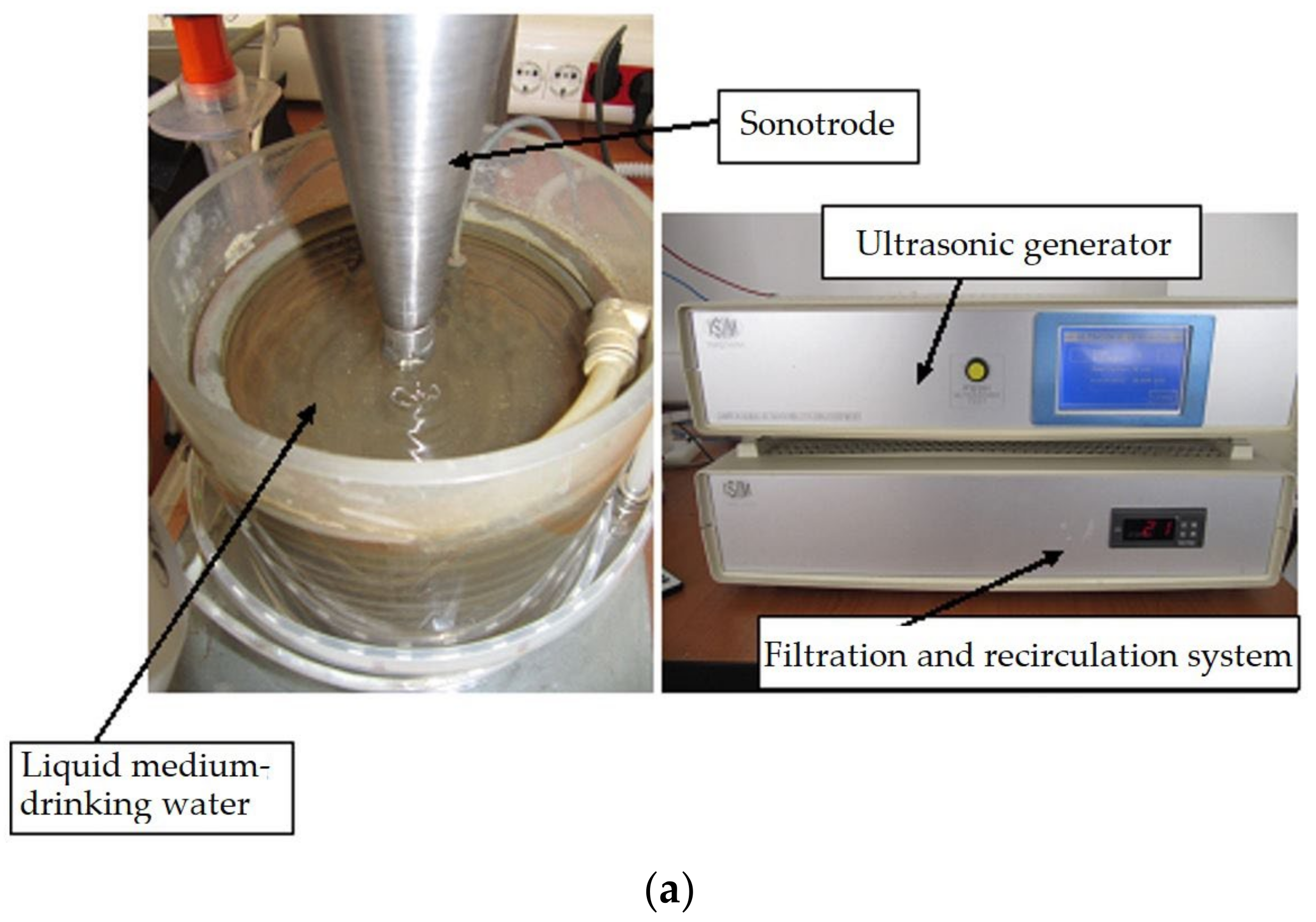
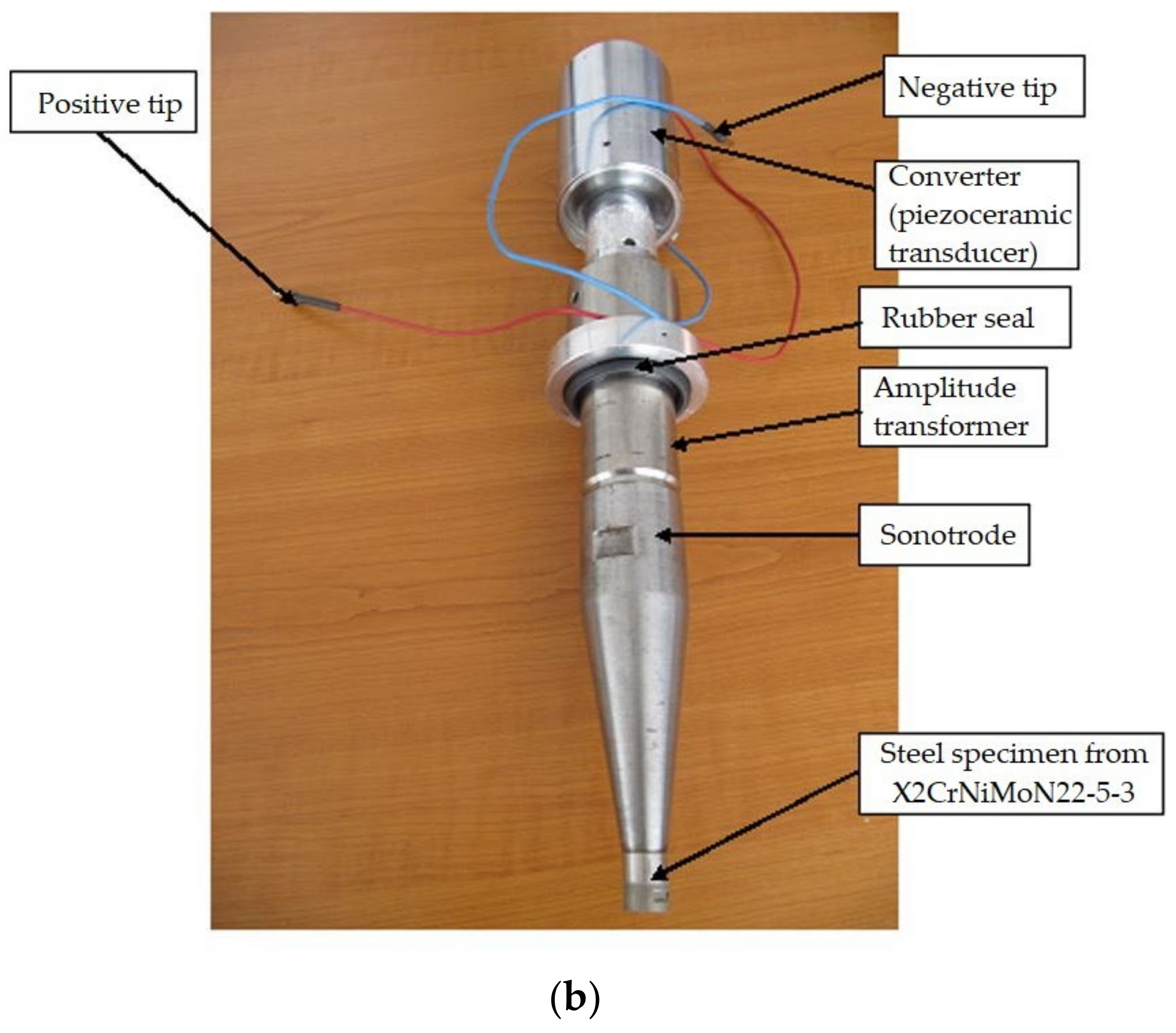
3. Experimental Stand and Work Procedure
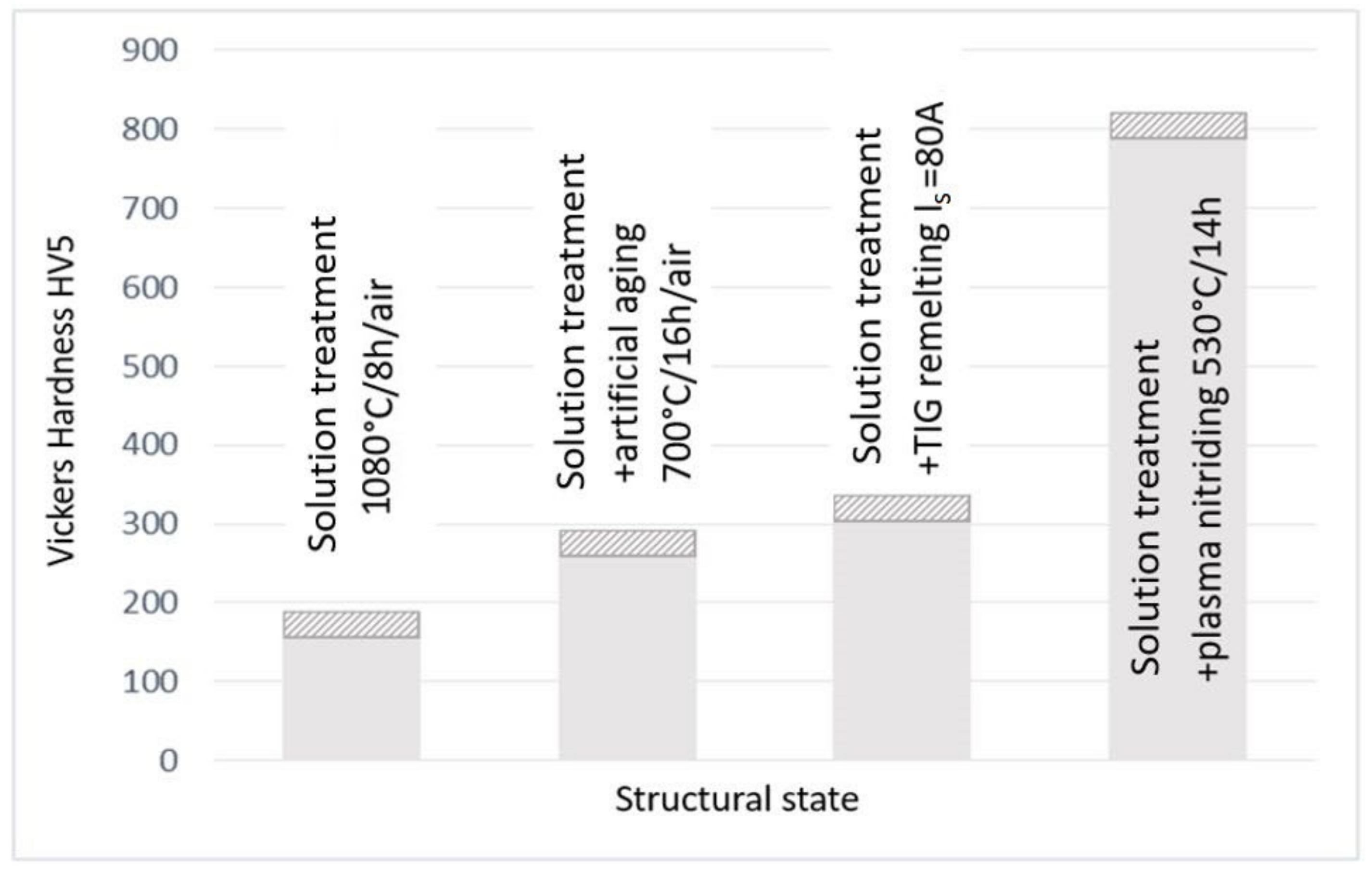
- Solution treatment, 1080 °C/8 h/air;
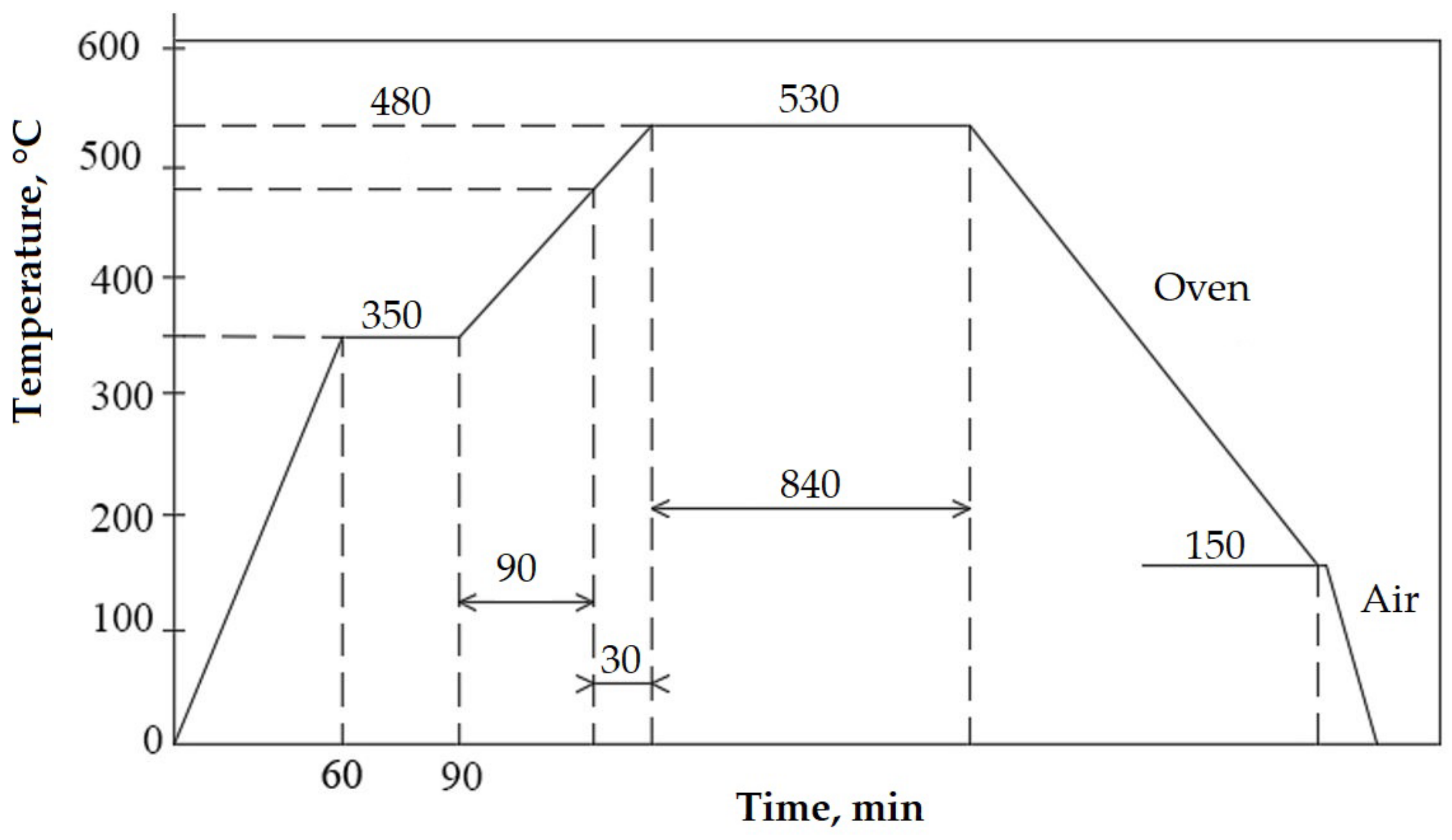
- Solution treatment followed by artificial ageing, 700 °C/16 h/air (the cyclogram shown in Figure 3);
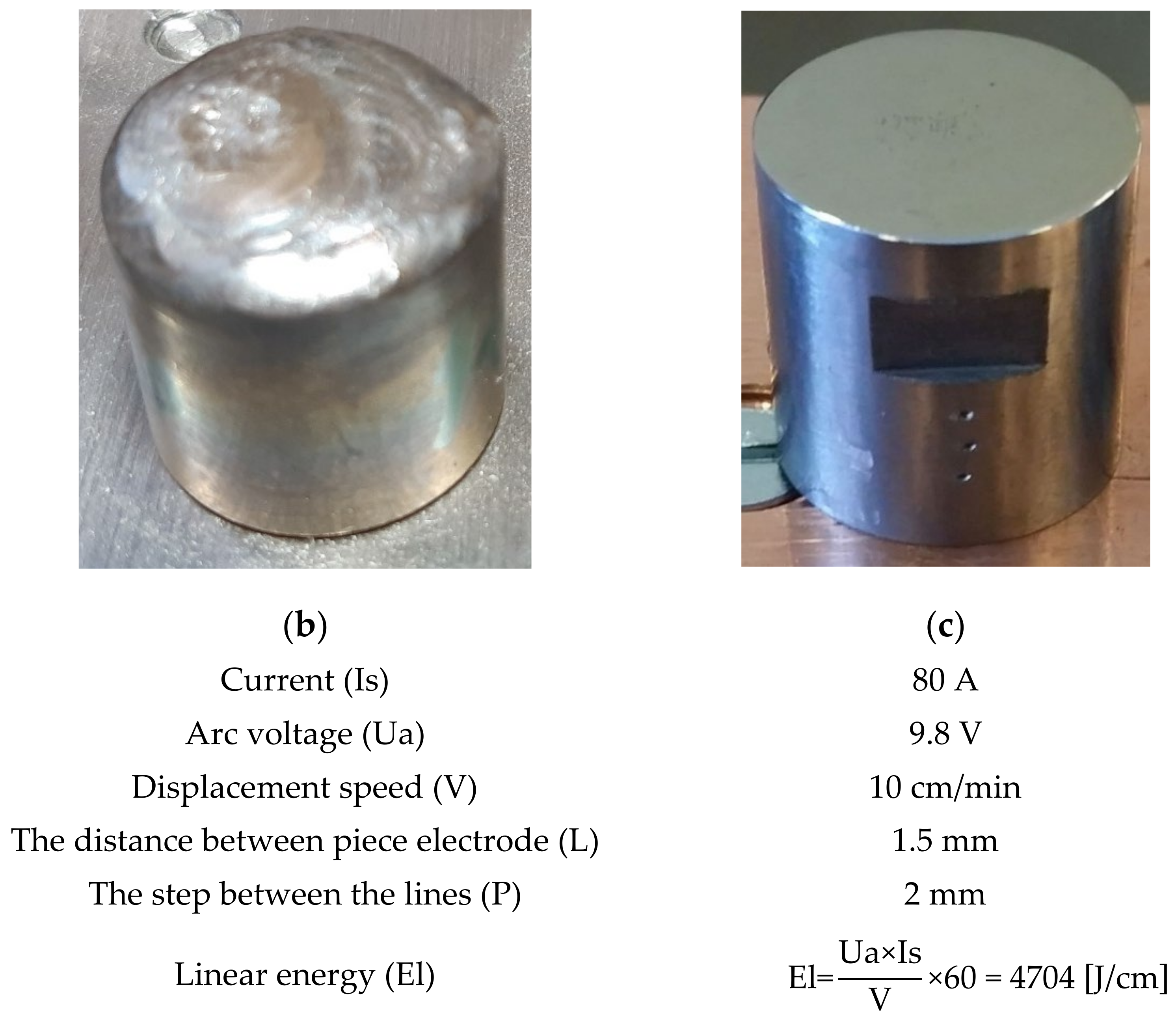
- Solution treatment followed by surface local remelting using the TIG technique;
- Solution treatment followed by plasma nitriding at 530 °C/14 h.
- Electronic ultrasonic generator power, 500 W;
- Vibration frequency, 20,000 ± 2% Hz;
- Vibration amplitude, 50 μm;
- Sample diameter: 15.8 ± 0.05 mm;
- Power supply: 220 V/50 Hz;
- Working media: drinking water from the industrial network, with a temperature of 22 ± 1 °C.
4. Results and Discussions
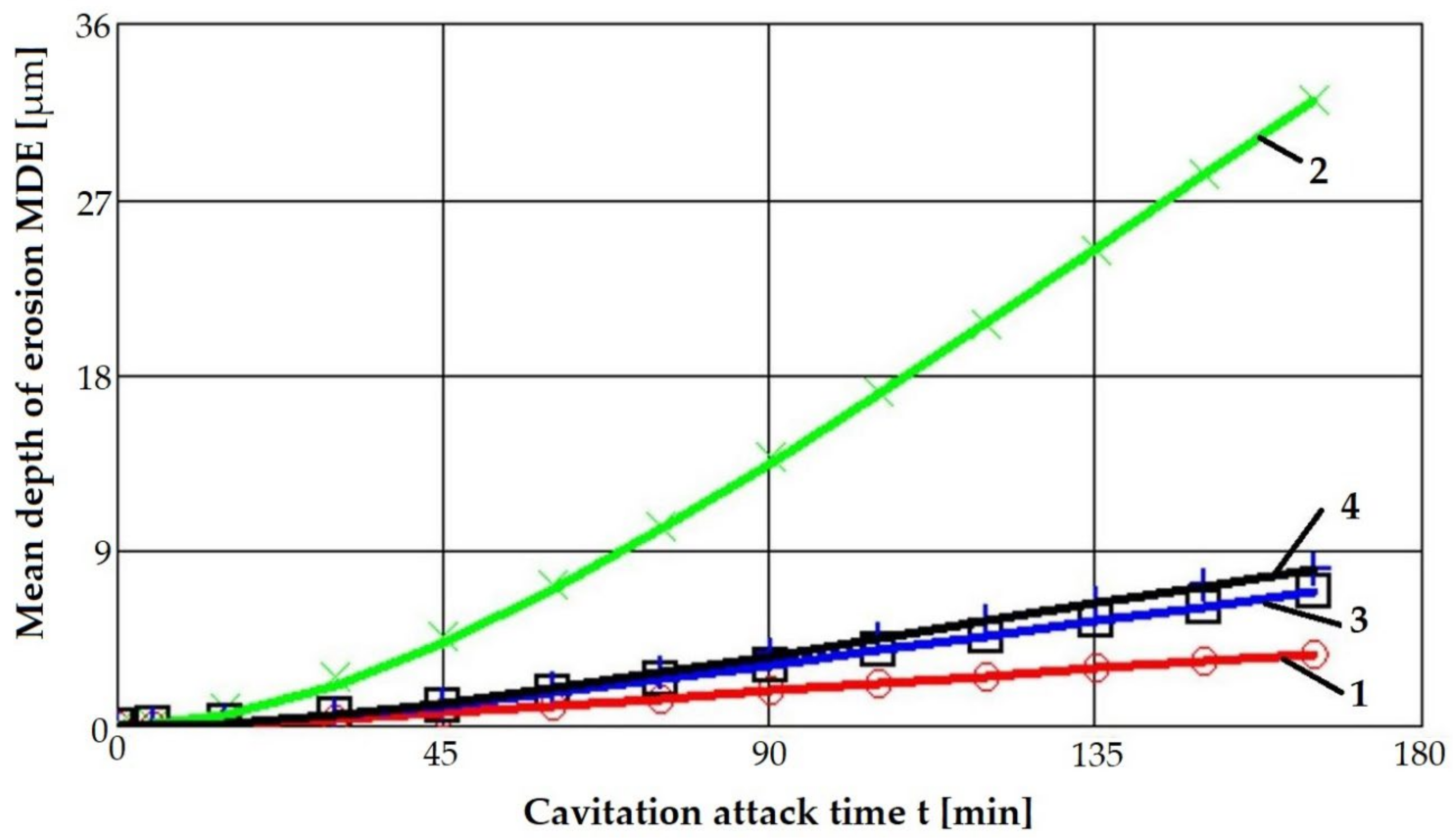
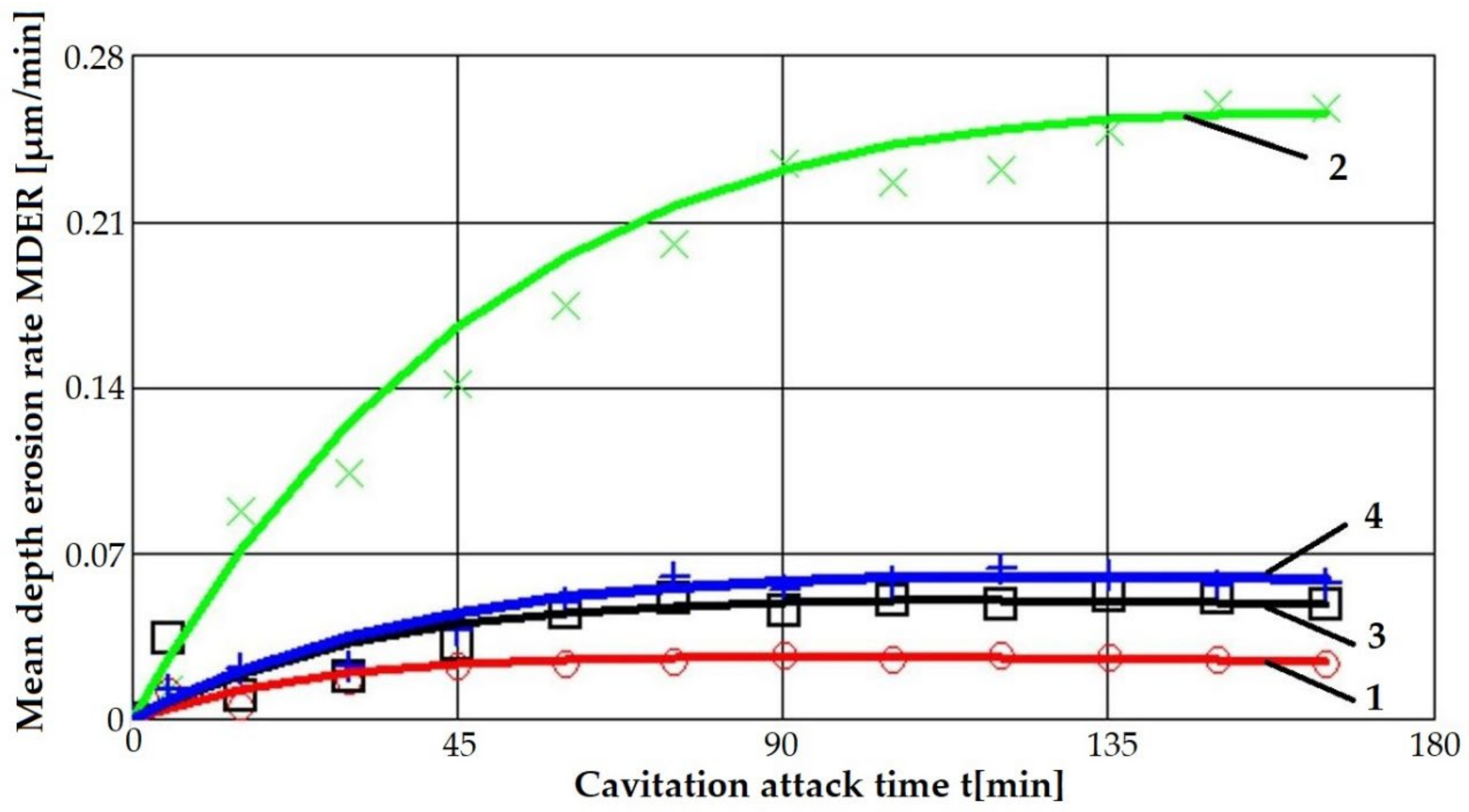
4.1. Cavitation Curves
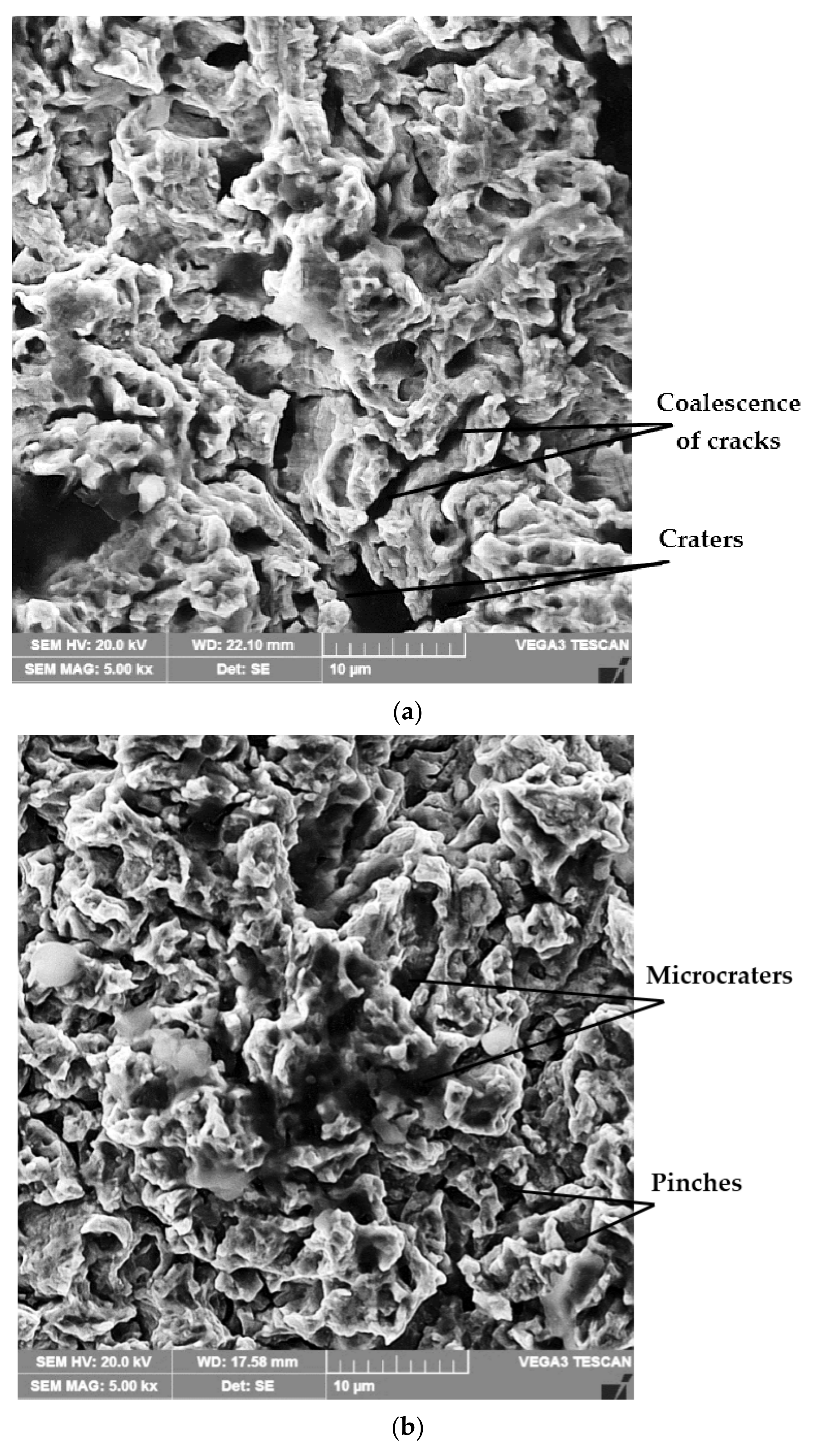
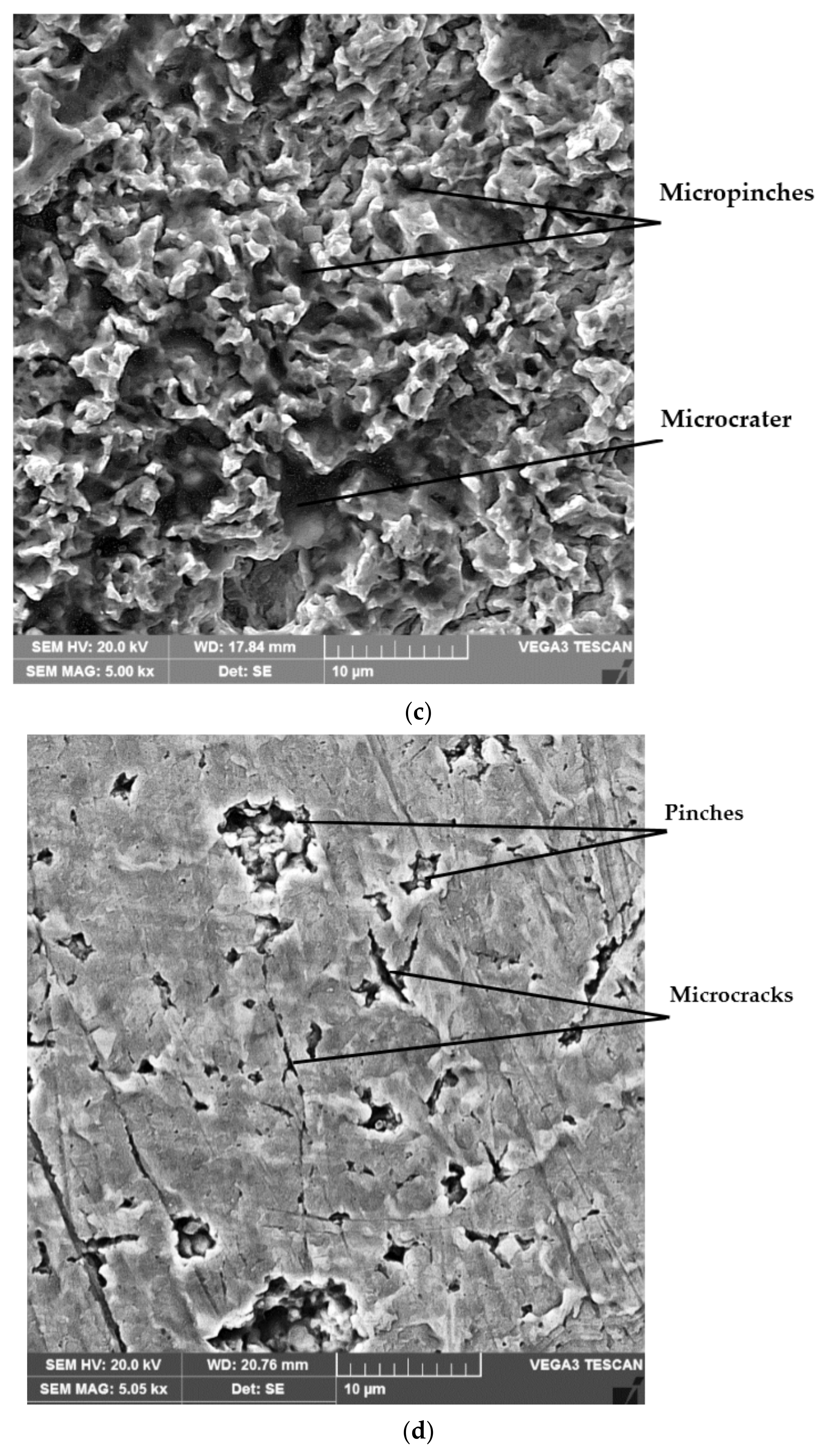
- The highest values of the mean penetration depth of erosion, MDE, are characteristic of the solution treatment;
- The lowest values of MDE were obtained after applying the thermochemical nitriding treatment;
- The local modification by TIG remelting (Is = 80 A) of the material surface led to lower cavitation losses compared to the heat treatment composed of a solution followed by artificial aging (MDE = 6865 µm).
4.2. Surface Hardness Measurements
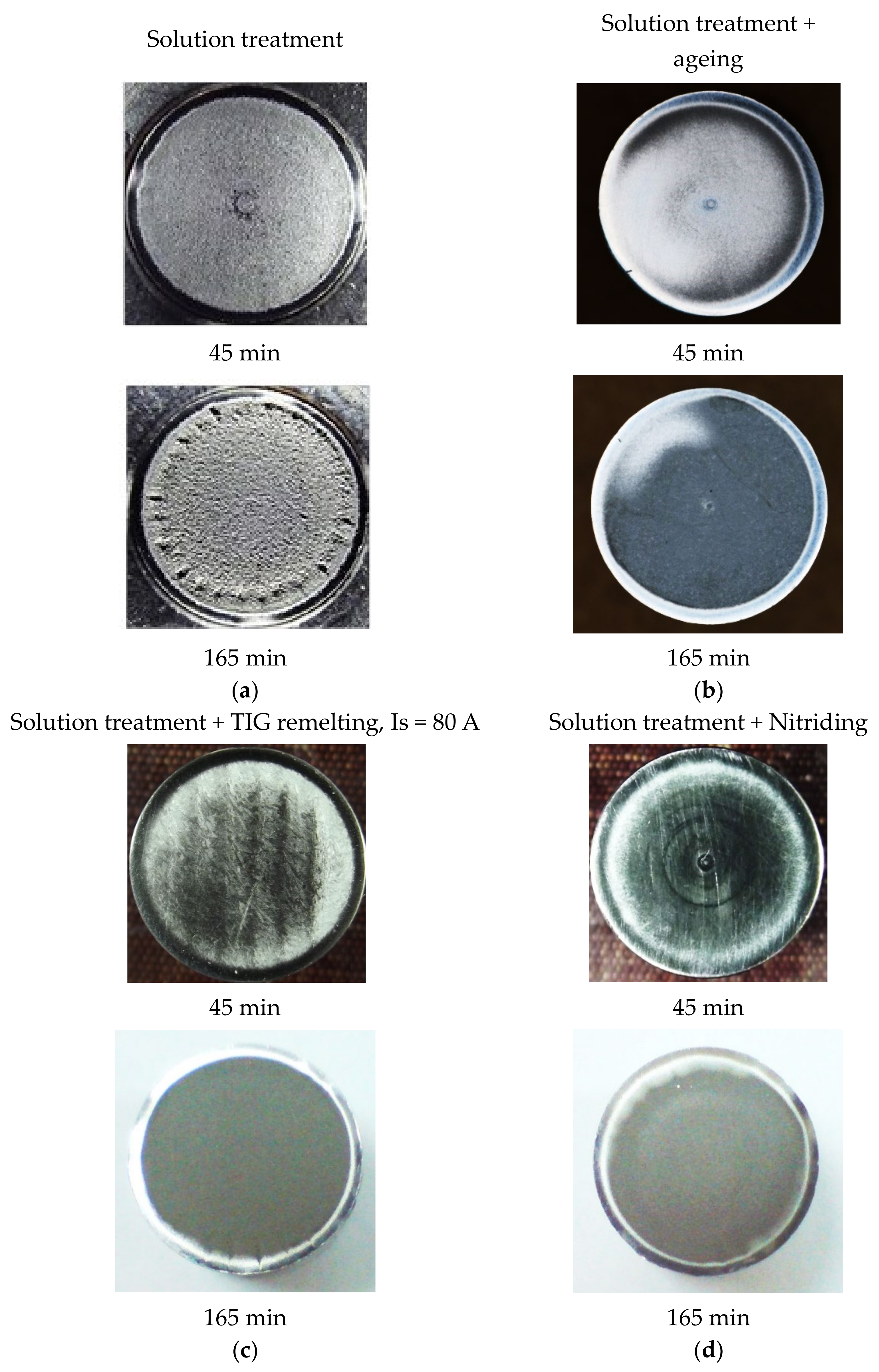
4.3. Roughness of the Cavitation Tested Surfaces
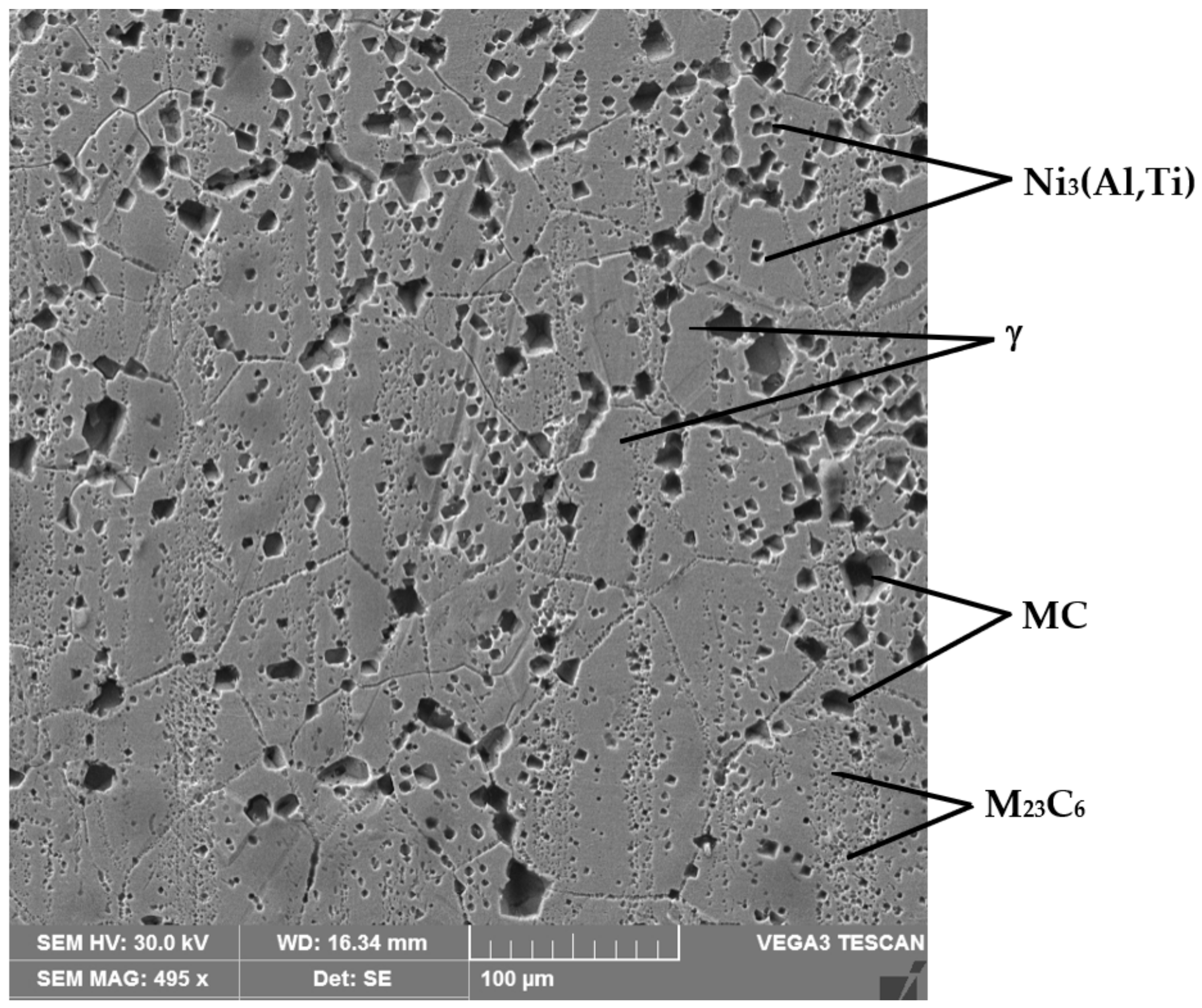
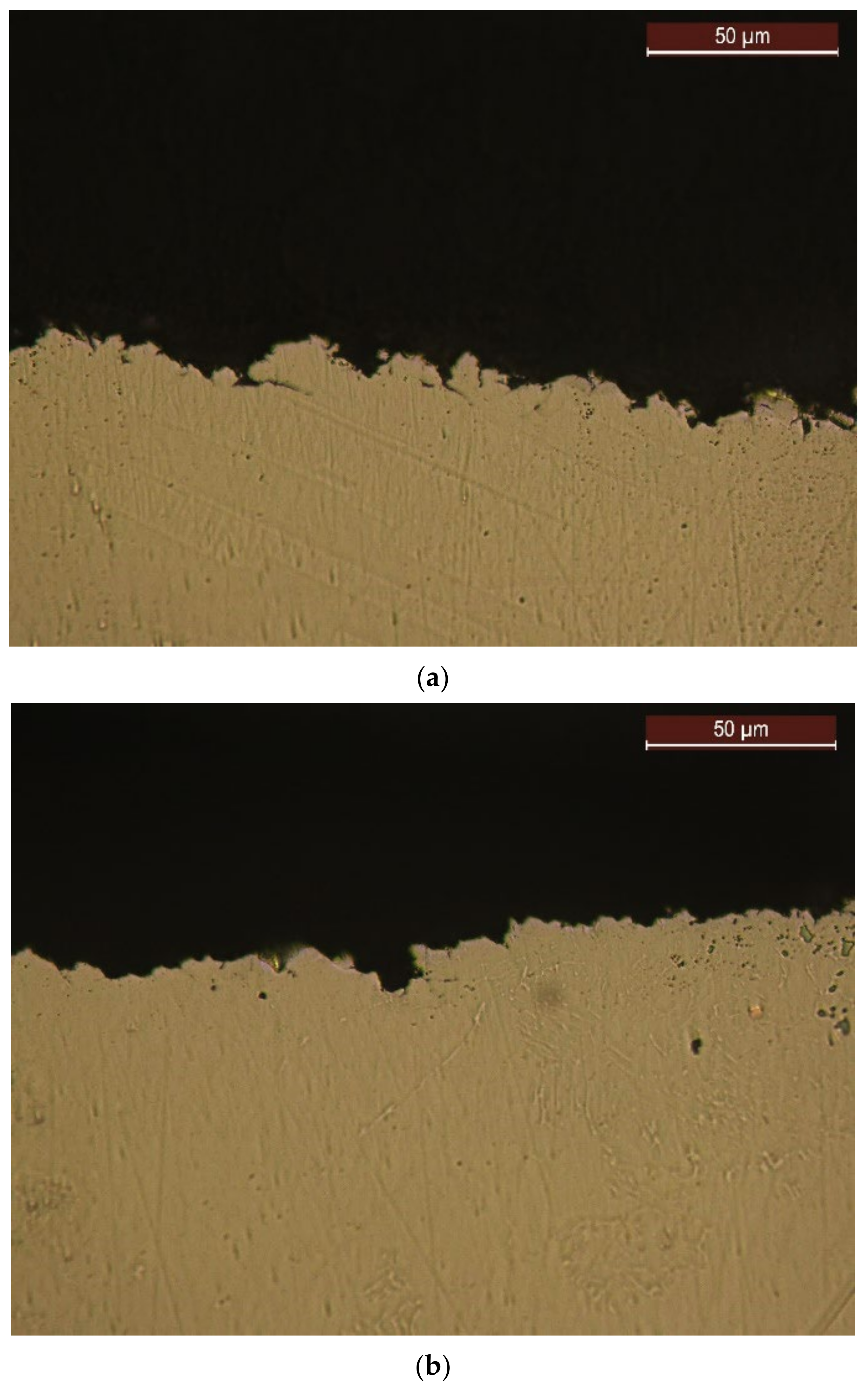
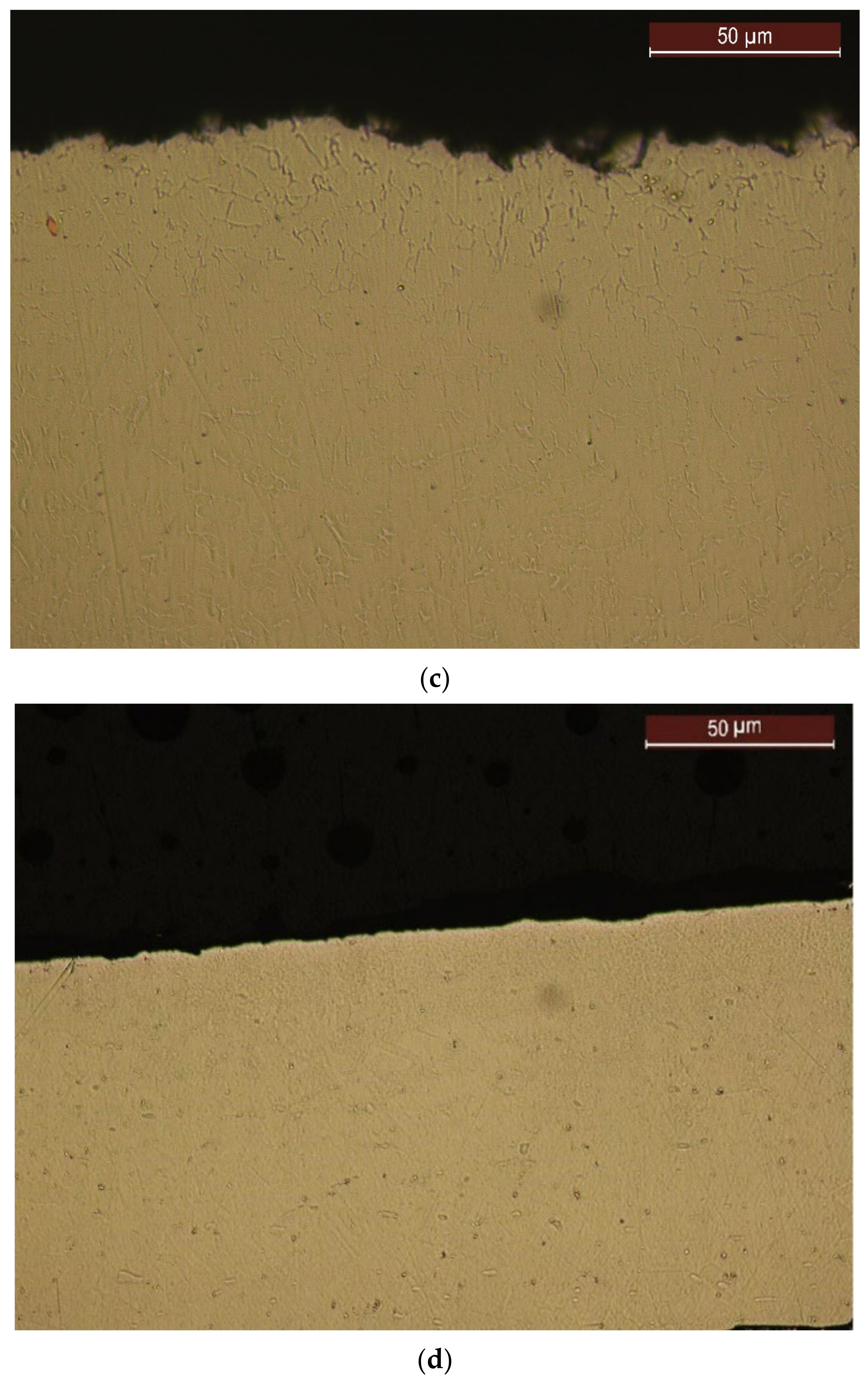
4.4. Structural Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franc, J.-P.; Michel, J.M. Fundamentals of Cavitation; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2004. [Google Scholar]
- Eliaseny, K.M.; Christiansen, T.; Somers, M. Low temperature gaseous nitriding of Ni based superalloys. Surf. Eng. 2010, 26, 248–255. [Google Scholar] [CrossRef]
- Chang, J.T.; Yeh, C.H.; He, J.J.; Chen, K.C. Cavitation erosion and corrosion behavior of Ni–Al intermetallic coatings. Wear 2003, 255, 162–169. [Google Scholar] [CrossRef]
- Krella, A.K.; Krupa, A. Effect of cavitation intensity on degradation of X6CrNiTi18-10 stainless steel. Wear 2018, 408–409, 180–189. [Google Scholar] [CrossRef]
- Chollet, S.; Pichont, L.; Cormier, J.; Dubois, J.B.; Villechaise, P.; Drouet, M.; Declemy, A.; Templier, C. Plasma assisted nitriding of Ni-based superalloys with various microstructures. Surf. Coat. Technol. 2013, 235, 318–325. [Google Scholar] [CrossRef]
- Han, S.; Lin, J.H.; Kuo, J.J.; He, J.L.; Shih, H.C. The cavitation-erosion phenomenon of chromium nitride coatings deposited using cathodic arc plasma deposition on steel. Surf. Coat. Technol. 2002, 161, 20–25. [Google Scholar] [CrossRef]
- Lo, K.H.; Kwok, C.T.; Wang, K.Y.; Ai, W. Implications of solution treatment on cavitation erosion and corrosion resistances and synergism of austenitic stainless steel. Wear 2017, 392–393, 159–166. [Google Scholar] [CrossRef]
- Mesa, D.H.; Pinedo, C.E.; Tschiptschin, A.P. Improvement of the cavitation erosion resistance of UNS S31803 stainless steel by duplex treatment. Surf. Coat. Technol. 2010, 205, 1552–1556. [Google Scholar] [CrossRef]
- Santa, J.F.; Blanco, J.A.; Giraldo, J.E.; Toro, A. Cavitation erosion of martensitic and austenitic stainless steel welded coatings. Wear 2011, 271, 1445–1453. [Google Scholar] [CrossRef]
- Mitelea, I.; Bordeasu, I.; Mutascu, D.; Buzdugan, D.; Craciuneescu, C.M. Cavitation resistance of Stellite 21 coatings tungsten inert gas (TIG) deposited onto duplex stainless steel X2CrNiMoN22-5-3. Mater. Test. 2022, 64, 967–976. [Google Scholar] [CrossRef]
- Tong, Z.; Jiao, J.; Zhou, W.; Yang, Y.; Chen, L.; Liu, H.; Sun, Y.; Ren, X. Improvement in cavitation erosion resistance of AA5083 aluminium alloy by laser shock processing. Surf. Coat. Technol. 2019, 377, 124799. [Google Scholar] [CrossRef]
- Alabeedi, K.F.; Abboud, J.H.; Benyounis, K.Y. Microstructure and erosion resistance enhancement of nodular cast iron by laser melting. Wear 2009, 266, 925–933. [Google Scholar] [CrossRef]
- Mitelea, I.; Bordeasu, I.; Riemschneider, E.; Utu, I.D.; Craciunescu, C.M. Cavitation erosion improvement following TIG surface-remelting of gray cast iron. Wear 2022, 496–497, 204282. [Google Scholar] [CrossRef]
- Godoy, C.; Mancosu, R.D.; Lima, M.M.; Brandão, D.; Housden, J.; Avelar-Batista, J.C. Influence of plasma nitriding and PAPVD Cr1−xNx coating on the cavitation erosion resistance of an AISI 1045 steel. Surf. Coat. Technol. 2006, 200, 5370–5378. [Google Scholar] [CrossRef]
- Mitelea, I.; Dimian, E.; Bordeasu, I.; Craciunescu, C. Ultrasonic cavitation erosion of gas nitrided Ti-6Al-4V alloys. Ultrason. Sonochem. 2014, 21, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Manova, D.; Hirsch, D.; Gerlach, J.W.; Mändl, S.; Neumann, H.; Rauschenbach, B. In situ investigation of phase formation during low energy ion nitriding of Ni80Cr20 alloy. Surf. Coat. Technol. 2014, 259, 434–441. [Google Scholar] [CrossRef]
- Belin, C.; Mitelea, I.; Bordeasu, I.; Craciunescu, C.M. Ultrasonic cavitation erosion mechanism in TIG remelted surfaces of Nimonic 80 A alloy. Mater. Today Proc. 2021, 45, 4207–4210. [Google Scholar] [CrossRef]
- Mitelea, I.; Bordeasu, I.; Belin, C.; Utu, I.D.; Craciunescu, C.M. Cavitation Resistance, Microstructure, and Surface Topography of Plasma Nitrided Nimonic 80 A Alloy. Materials 2022, 15, 6654. [Google Scholar] [CrossRef] [PubMed]
- ASTM G 32 Standard; Standard Test Method for Cavitation Erosion Using Vibratory Apparatus. ASTM International: West Conshohocken, PA, USA, 2016.
- Bordeaşu, I.; Patrascoiu, C.; Badarau, R.; Sucitu, L.; Popoviciu, M.; Balasoiu, V. New Contributions in Cavitation Erosion Curves Modeling; FME Transactions Faculty of Mechanical Engineering, New Series, vol. 34, Nr.1/2006, YU ISSN 1451-2092; University of Belgrade: Belgrade, Serbia, 2006; pp. 39–44. ISSN 1451-2092. [Google Scholar]
- Bordeasu, I. Eroziunea Cavitationala a Materialelor; Editura Politehnica Timisoara: Timișoara, Romania, 2006. [Google Scholar]




| Chromium | 20.5 |
| Titanium | 1.98 |
| Aluminum | 1.54 |
| Iron | 2.14 |
| Cobalt | 1.61 |
| Manganese | 0.63 |
| Silicon | 0.45 |
| Copper | 0.12 |
| Carbon | 0.08 |
| Sulfur | 0.014 |
| Zirconium | 0.11 |
| Nickel | Balance |
| Structural State | Cavitation Erosion Resistance Parameter | Variation Compared with Solution Treated State | |
|---|---|---|---|
| MDERs (µm/min) | Rcav. (min/µm) | ||
| Solution treatment | 0.255 | 3.92 | - |
| Solution treatment followed by artificial aging | 0.059 | 16.94 | Increase of 4.3 times |
| Solution treatment followed by local TIG remelting, Is = 80 A | 0.049 | 20.4 | Increase of 5.2 times |
| Solution treatment + nitiding | 0.024 | 41.67 | Increase of 10.6 times |
| Structural State | MDE (165 min.) | Ra, µm | Rz, µm | Rt, µm |
|---|---|---|---|---|
| Solution treatment | 32.11 | 5.539 | 31.598 | 48.026 |
| Solution treatment + artificial aging | 8.068 | 1.975 | 12.504 | 17.306 |
| TIG remelting, Is = 80 A | 6.86 | 1.659 | 10.507 | 14.543 |
| Nitriding | 3.71 | 0.465 | 3.172 | 4.663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belin, C.; Mitelea, I.; Bordeașu, I.; Crăciunescu, C.M.; Uțu, I.-D. Technological Processes for Increasing the Cavitation Erosion Resistance of Nimonic 80A Superalloys. Materials 2023, 16, 3206. https://doi.org/10.3390/ma16083206
Belin C, Mitelea I, Bordeașu I, Crăciunescu CM, Uțu I-D. Technological Processes for Increasing the Cavitation Erosion Resistance of Nimonic 80A Superalloys. Materials. 2023; 16(8):3206. https://doi.org/10.3390/ma16083206
Chicago/Turabian StyleBelin, Cosmin, Ion Mitelea, Ilare Bordeașu, Corneliu Marius Crăciunescu, and Ion-Dragoș Uțu. 2023. "Technological Processes for Increasing the Cavitation Erosion Resistance of Nimonic 80A Superalloys" Materials 16, no. 8: 3206. https://doi.org/10.3390/ma16083206








