Corrosion Passivation in Simulated Body Fluid of Ti-Zr-Ta-xSn Alloys as Biomedical Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Electrochemical Cell and Electrochemical Techniques
3. Results and Discussion
3.1. CPP Measurements
3.2. CCt Measurements
3.3. SEM and EDX Analysis
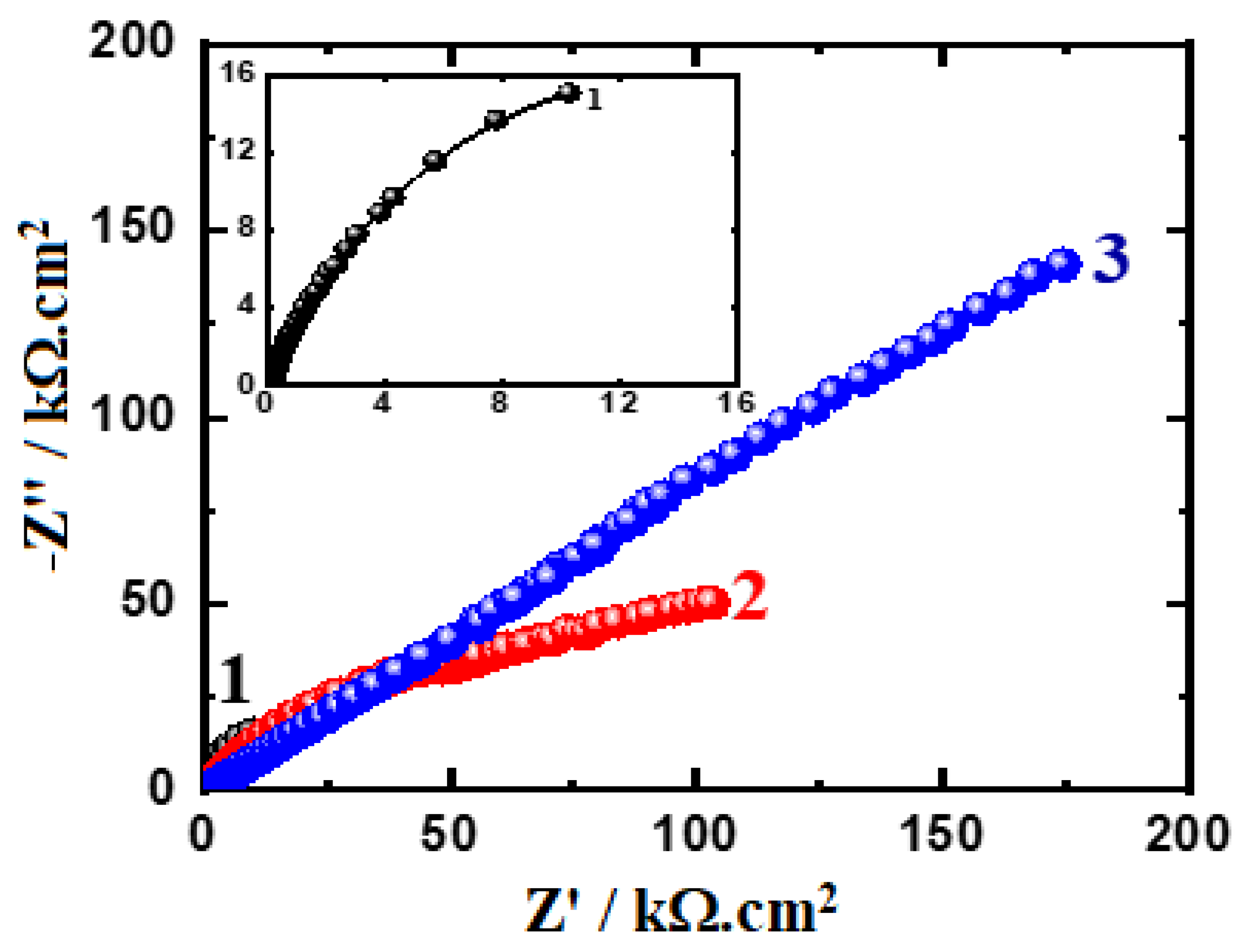
3.4. EIS Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Muraleedharan, K.; Gogia, A.K.; Asokamani, R. Effect of thermomechanical processing on microstructure of a Ti-13Nb-13Zr alloy. J. Alloys Compd. 2001, 329, 214–223. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Gilbert, J.L.; Urban, R.M. Current Concepts Review-Corrosion of Metal Orthopaedic Implants. J. Bone Jt. Surg. 1998, 80, 268–282. [Google Scholar] [CrossRef]
- Yu, Z. Titanium Alloys for Biomedical Development and Applications. In Design, Microstrcature, Properties, and Application; Elsevier Inc.: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-823927-8. [Google Scholar] [CrossRef]
- Chicardi, E.; García-Garrido, C.; Sayagués, M.; Torres, Y.; Amigó, V.; Aguilar, C. Development of a novel fcc structure for an amorphous-nanocrystalline Ti-33Nb-4Mn (at.%) ternary alloy. Mater. Charact. 2018, 135, 46–56. [Google Scholar] [CrossRef]
- Roubing, Z.; Lin, A. First principles study of Ti-Zr-Ta alloy phase stability and elastic properties. Mater. Res. Exp. 2023, 10, 026503. [Google Scholar] [CrossRef]
- Smith, S.M.; Gilbert, J.L. Interfacial compliance, energy dissipation, frequency effects, and long-term fretting corrosion performance of Ti-6Al-4V/CoCrMo interfaces. J. Biomed. Mater. Res. A 2022, 110, 409–423. [Google Scholar] [CrossRef]
- Ijaz, M.F.; Alharbi, H.F.; Bahri, Y.A.; Sherif, E.-S.M. Alloy Design and Fabrication of Duplex Titanium-Based Alloys by Spark Plasma Sintering for Biomedical Implant Applications. Materials 2022, 15, 8562. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. JOM J. Mater. Met. Mater. Soc. (TMS) 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Chong, Y.; Bhattacharjee, T.; Tsuji, N. Bi-lamellar microstructure in Ti–6Al–4V: Microstructure evolution and mechanical properties. Mater. Sci. Eng. A 2019, 762, 138077. [Google Scholar] [CrossRef]
- Héraud, L.; Castany, P.; Ijaz, M.F.; Gordin, D.-M.; Gloriant, T. Large-strain functional fatigue properties of superelastic metastable β titanium and NiTi alloys: A comparative study. J. Alloys Compd. 2023, 953, 170170. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-Z.; Zhang, J.; Chen, Q.; Wu, M. Corrosion behavior of selective laser melted Ti-6Al-4 V alloy in NaCl solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Loch, J.; Erazmus-Vignal, P. Influence of plastic deformation on the microstructure and corrosion behaviour of Ti–10Mo–4Zr and Ti–6Al–4V alloys in the Ringer’s solution at 37 °C. Corros. Sci. 2015, 96, 160–170. [Google Scholar] [CrossRef]
- Tkachenko, S.; Datskevich, O.; Kulak, L.; Jacobson, S.; Engqvist, H.; Persson, C. Wear and friction properties of experimental Ti–Si–Zr alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2014, 39, 61–72. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Li, R.; Liu, G.; Yang, L.; Qing, Y.A.; Tang, X.; Guo, D.; Zhang, K.; Qin, Y. Tantalum boride as a biocompatible coating to improve osteogenesis of the bionano interface. J. Biomed. Mater. Res. A 2020, 108, 1726–1735. [Google Scholar] [CrossRef]
- Kandimalla, R.; Vallamkondu, J.; Corigat, E.B.; Gill, K.D. Understanding Aspects of Aluminum Exposure in Alzheimer's Disease Development. Brain Pathol. 2016, 26, 139–154. [Google Scholar] [CrossRef]
- Kawahara, M. Kato-Negishi; M. Link between Aluminum and the Pathogenesis of Alzheimer’s Disease: The Integration of the Aluminum and Amyloid Cascade Hypotheses. Int. J. Alzheimer’s Dis. 2011, 2011, 276393. [Google Scholar]
- Lin, J.; Ozan, S.; Li, Y.; Ping, D.; Tong, X.; Li, G.; Wen, C. Novel Ti-Ta-Hf-Zr alloys with promising mechanical properties for prospective stent applications. Sci. Rep. 2016, 6, 37901. [Google Scholar] [CrossRef]
- Grandin, H.M.; Berner, S.; Dard, M. A review of titanium zirconium (TiZr) alloys for use in endosseous dental implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef]
- Hu, Q.-M.; Li, S.-J.; Hao, Y.-L.; Yang, R.; Johansson, B.; Vitos, L. Phase stability and elastic modulus of Ti alloys containing Nb, Zr, and/or Sn from first-principles calculations. Appl. Phys. Lett. 2008, 93, 121902. [Google Scholar] [CrossRef]
- Tahara, M.; Kim, H.Y.; Hosoda, H.; Miyazaki, S. Cyclic deformation behavior of a Ti-26 at.% Nb alloy. Acta Mater. 2009, 57, 2461–2469. [Google Scholar] [CrossRef]
- Kurtz, M.A.; Wessinger, A.C.; Mace, A.; Moreno-Reyes, A.; Gilbert, J.L. Additively manufactured Ti-29Nb-21Zr shows improved oxide polarization resistance versus Ti-6Al-4V in inflammatory simulating solution. J. Biomed. Mater. Res. A. 2023, 1–16. [Google Scholar] [CrossRef]
- Stenlund, P.; Omar, O.; Brohede, U.; Norgren, S.; Norlindh, b.; Johansson, A.; Lausmaa, J.; Thomesn, P.; Palmquist, A. Bone response to a novel Ti-Ta-Nb-Zr alloy. Acta Biomater. 2015, 20, 165–175. [Google Scholar] [CrossRef]
- Fojt, J.; Joska, L.; Malek, J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros. Sci. 2013, 71, 78–83. [Google Scholar] [CrossRef]
- Mohan, L.; Anandan, C.; Rajendran, N. Electrochemical behavior and effect of heat treatment on morphology, crystalline structure of self-organized TiO2 nanotube arrays on Ti–6Al–7Nb for biomedical applications. Mater. Sci. Eng. C 2015, 50, 394–401. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.W. Influence of a large pulsed electron beam (LPEB) on the corrosion resistance of Ti6Al7Nb alloys. Corros. Sci. 2015, 96, 153–160. [Google Scholar] [CrossRef]
- Abdel-Hady, M.; Fuwa, H.; Hinoshita, K.; Kimura, H.; Shinzato, Y.; Morinaga, M. Phase stabilities change with Zr content in β-type Ti–Nb alloys. Scr. Mater. 2007, 57, 1000–1003. [Google Scholar] [CrossRef]
- Ijaz, M.F.; Vasilescu, C.; Drob, S.I.; Osiceanu, P.; Marcu, M.; Kim, H.Y.; Miyazaki, S.; Gordin, D.-M.; Gloriant, T. Electrochemical characterization of the superelastic (Ti-Zr)-Mo-Sn biomedical alloy displaying a large recovery strain. Mater. Corros. 2017, 68, 1220–1227. [Google Scholar] [CrossRef]
- Sanchez, A.G.; Schreiner, W.; Duffó, G.U.S.T.A.V.O.; Ceré, S.I.L.V.I.A. Surface characterization of anodized zirconium for biomedical applications. Appl. Surf. Sci. 2011, 257, 6397–6405. [Google Scholar] [CrossRef]
- Festas, A.J.; Ramos, A.; Davim, J.P. Medical devices biomaterials–A review. Proc. Inst. Mech. Eng. Part L J. Mater. Design Appl. 2020, 234, 218–228. [Google Scholar] [CrossRef]
- Jeong, S. Basic knowledge about metal stent development. Clin. Endosc. 2016, 49, 108–112. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Kuroda, P.A.B.; Lourenço, M.L.; Buzalaf, M.A.R.; Grandini, C.R. Adjustment of the microstructure and selected mechanical properties of biomedical Ti-15Zr-Mo alloys through oxygen doping. J. Alloys Compd. 2019, 775, 158–167. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Pan, Y.; Xu, W.; Singh, H.-P.; Liu, B.; Lu, D.; Wang, H.; Zhang, J.; Lu, X. Effect of Sn addition on the mechanical properties and high-temperature oxidation resistance of intermetallic TiAl alloys by first principles study and experimental investigation. J. Mater. Res. Technol. 2022, 21, 3666–3677. [Google Scholar] [CrossRef]
- Keshtta, A.; Gepreel, M.A.-H. Effect of Sn-addition on the properties of the biomedical Ti17Nb-6Ta alloy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 553, 012032. [Google Scholar] [CrossRef]
- Torres-Sánchez, C.; Wang, J.; Norrito, M.; Zani, L.; Conway, P.P. Addition of Sn to TiNb alloys to improve mechanical performance and surface properties conducive to enhanced cell activity. Mater. Sci. Eng. C 2020, 115, 110839. [Google Scholar] [CrossRef]
- Dal Bó, M.R.; Salvador, C.A.F.; Mello, M.G.; Lima, D.D.; Faria, G.A.; Ramirez, A.J.; Caram, R. The effect of Zr and Sn additions on the microstructure of Ti-Nb-Fe gum metals with high elastic admissible strain. Mater. Des. 2018, 160, 1186–1195. [Google Scholar] [CrossRef]
- Drob, S.I.; Drob, S.I.; Ijaz, M.F.; Vasilescu, C.; Osiceanu, P.; Gordin, D.-M.; Cimpean, A.; Gloriant, T. Surface Characterization, Corrosion Resistance and in Vitro Biocompatibility of a New Ti-Hf-Mo-Sn Alloy. Materials 2016, 9, 818. [Google Scholar] [CrossRef]
- Khalil, K.A.; Sherif, E.-S.M.; Almajid, A.A. Corrosion passivation in Simulated Body Fluid of Magnesium/Hydroxyapatite Nanocomposites Sintered by High Frequency Induction Heating. Int. J. Electrochem. Sci. 2011, 6, 6184–6199. [Google Scholar]
- Alharbi, H.F.; Bahri, Y.A.; Sherif, E.-S.M. Influence of Zirconium on the Corrosion Passivation of Titanium in Simulated Body Fluid. Crystals 2021, 11, 1391. [Google Scholar] [CrossRef]
- Calvo, E.J.; Mozhzhukhina, N. A rotating ring disk electrode study of the oxygen reduction reaction in lithium containing non aqueous electrolyte. Electrochem. Commun. 2013, 31, 56. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Bahri, Y.A.; Alharbi, H.F.; Ijaz, M.F.; Alnaser, I.A. Influence of Tantalum Addition on the Corrosion Passivation of Titanium-Zirconium Alloy in Simulated Body Fluid. Materials 2022, 15, 8812. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Li, Y.; Li, S.; Wang, L. A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 2009, 51, 291–300. [Google Scholar] [CrossRef]
- Badawy, W.A.; Al-Kharafi, F.M.; El-Azab, A.S. Electrochemical behaviour and corrosion inhibition of Al, Al-6061 and Al-Cu in neutral aqueous solutions. Corros. Sci. 1999, 41, 709–727. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Bolzoni, F.; Ormellese, M.; Pérez-Rosales, E.A.; Pedeferri, M.P. Characterization of titanium oxide films by potentiodynamic polarisation and electrochemical impedance spectroscopy. Corros. Eng. Sci. Technol. 2010, 45, 428–434. [Google Scholar] [CrossRef]
- Afzali, P.; Ghomashchi, R.; Oskouei, R.H. On the corrosion Behaviour of low modulus titanium alloys for medical implant applications: A review. Metals 2019, 9, 878. [Google Scholar] [CrossRef]
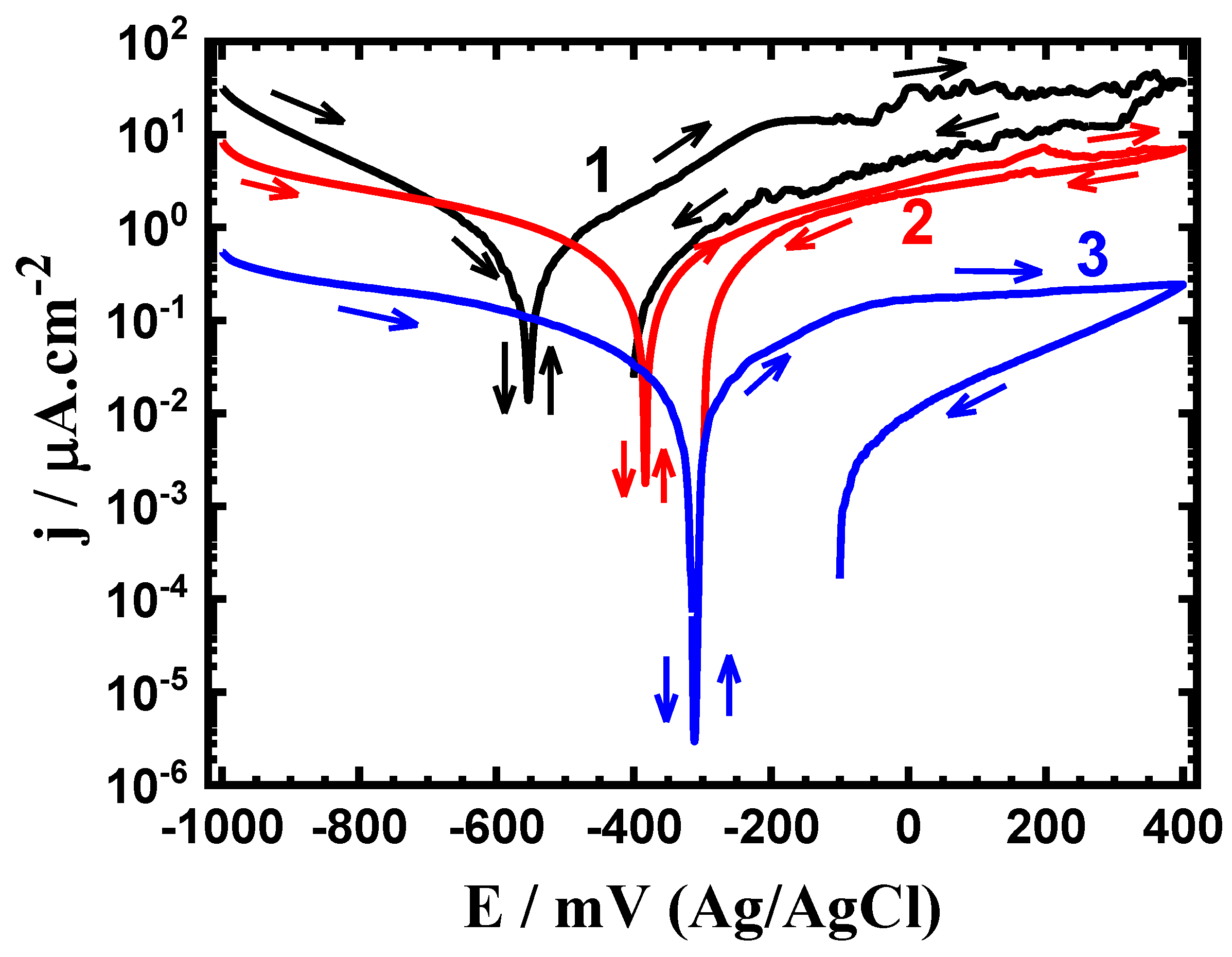
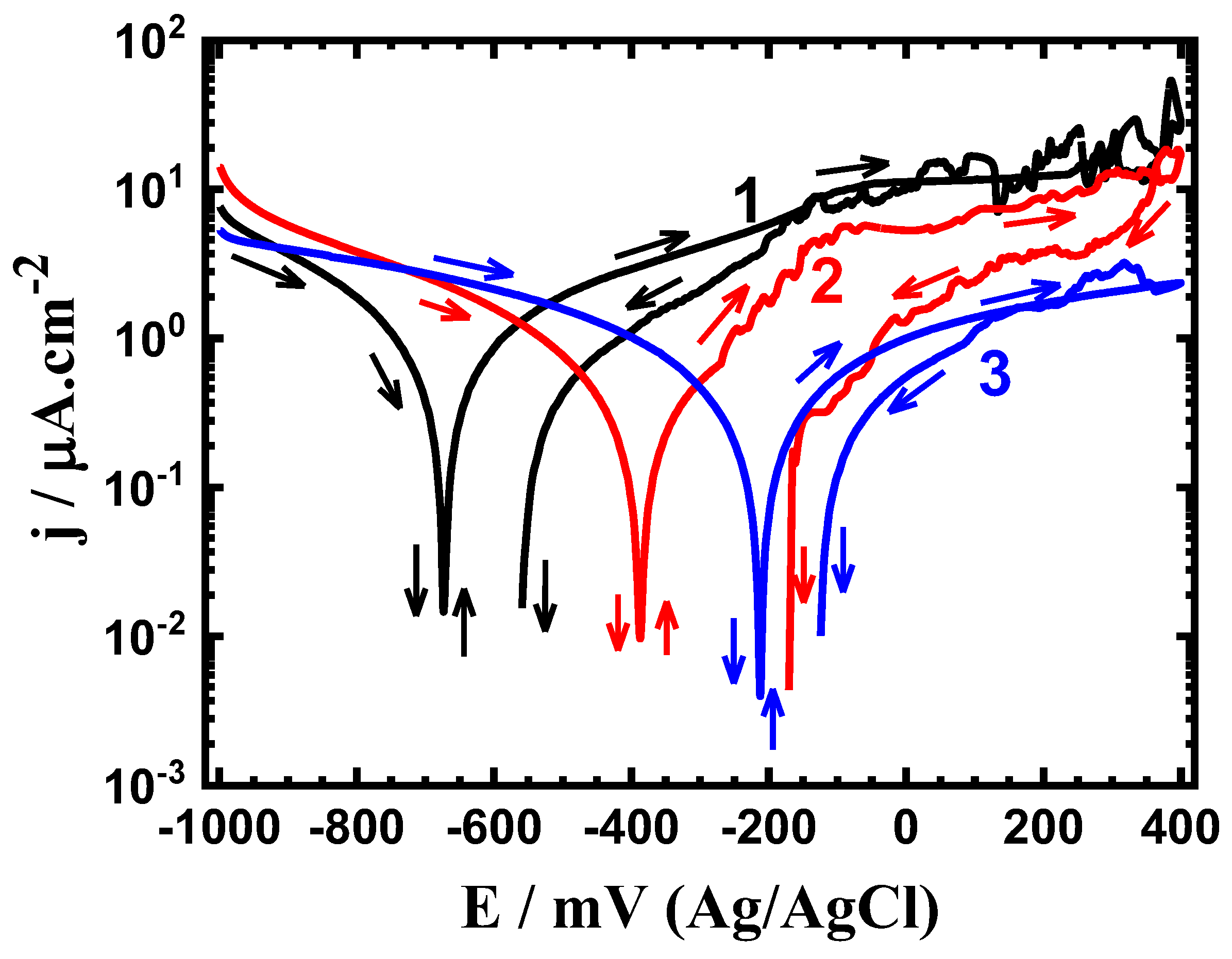
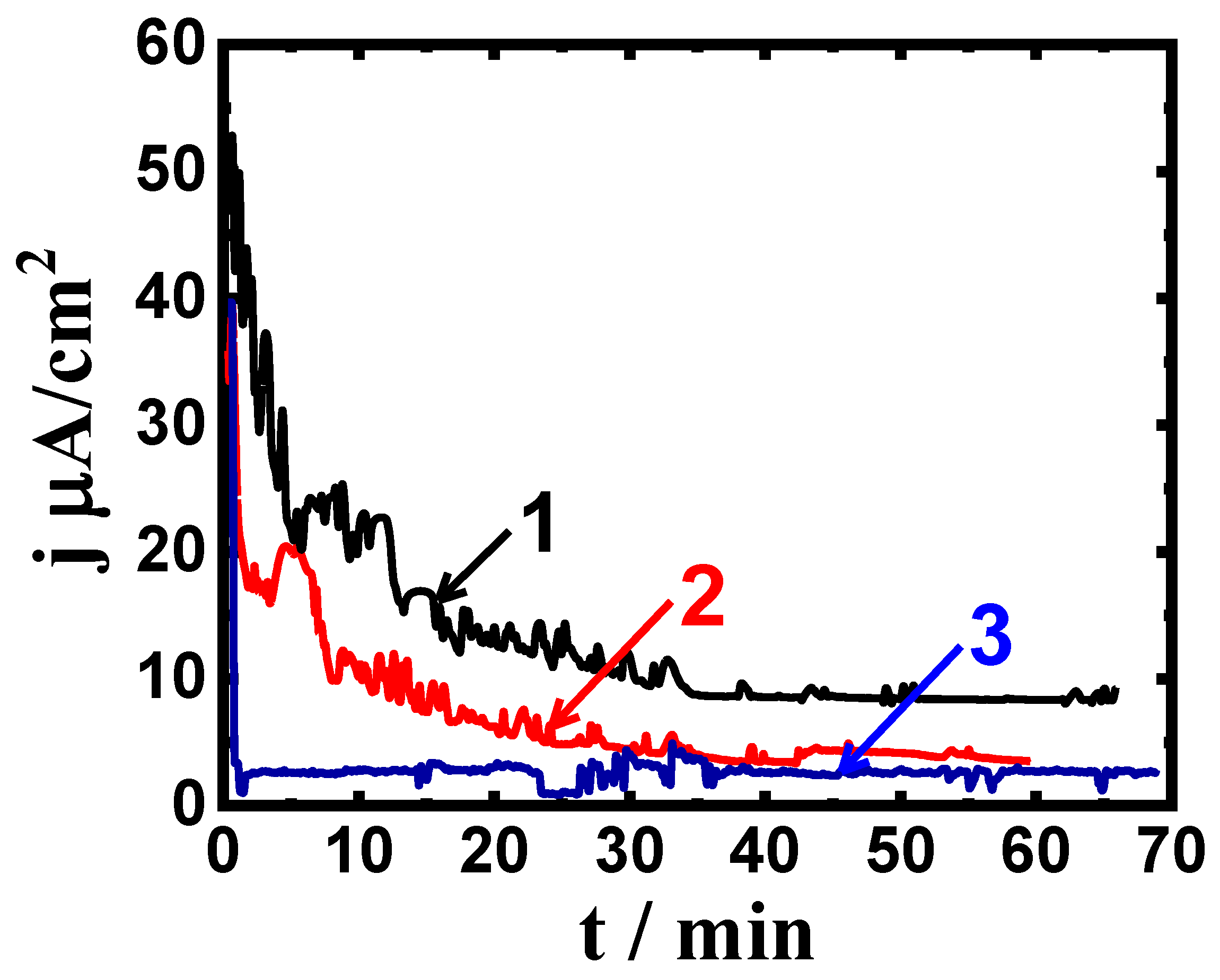



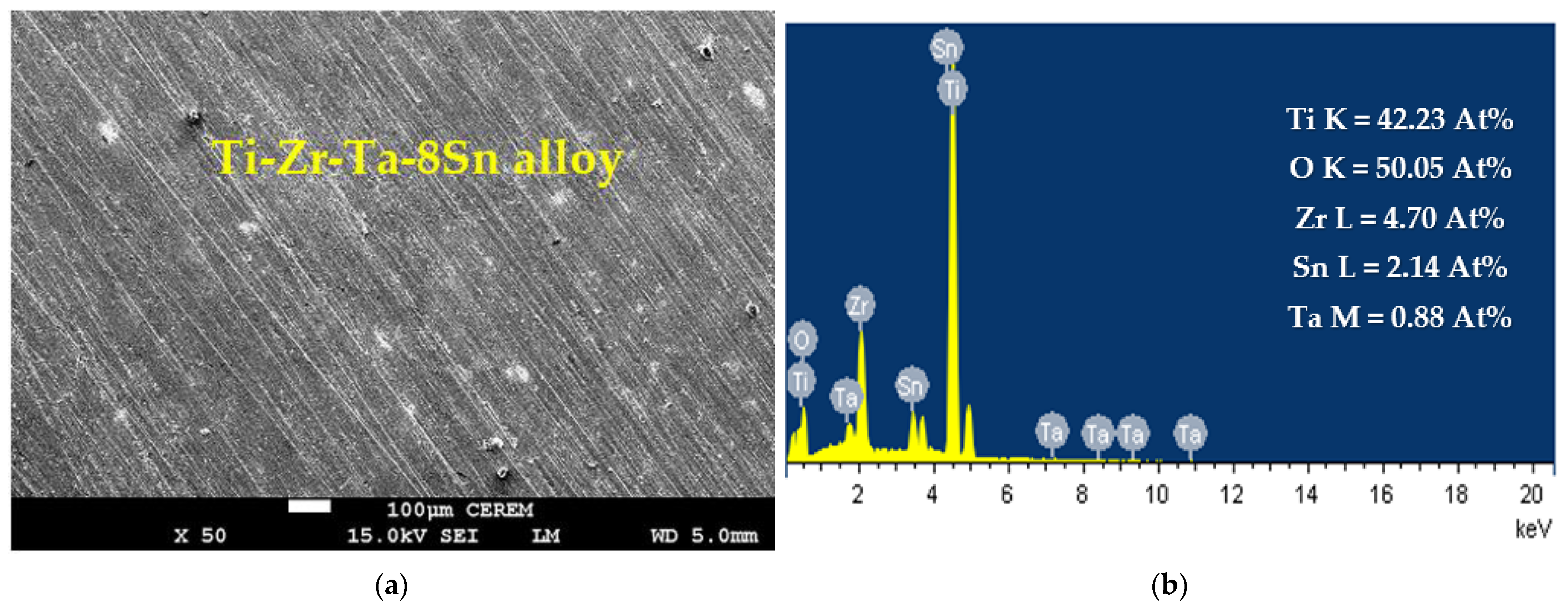


| Alloy/Exposure Time | βc (mV/dec) | βa (mV/dec) | ECorr (mV) | jCorr (μA/cm2) | RP (KΩ.cm2) | RCorr (mm/yr) |
|---|---|---|---|---|---|---|
| Ti-Zr-Ta-4Sn/one hour | 130 | 140 | −540 | 0.28 | 112.44 | 0.00244 |
| Ti-Zr-Ta-6Sn/one hour | 120 | 130 | −380 | 0.18 | 150.72 | 0.00157 |
| Ti-Zr-Ta-8Sn/one hour | 120 | 130 | −315 | 0.05 | 542.61 | 0.00044 |
| Ti-Zr-Ta-4Sn/72 h | 140 | 160 | −680 | 0.35 | 92.75 | 0.00305 |
| Ti-Zr-Ta-6Sn/72 h | 130 | 150 | −380 | 0.25 | 121.12 | 0.00218 |
| Ti-Zr-Ta-8Sn/72 h | 120 | 130 | −210 | 0.14 | 193.79 | 0.00122 |
| Sample | Impedance Data | ||||||
|---|---|---|---|---|---|---|---|
| RS/ Ω cm2 | Q1 | RP1/ Ω cm2 | Q2 | RP2/ Ω cm2 | |||
| YQ1/F cm−2 | n | YQ2/F cm−2 | n | ||||
| Ti-Zr-Ta-4Sn/one hour | 78.6 | 0.0042 | 0.83 | 9306 | 0.0047 | 0.89 | 9590 |
| Ti-Zr-Ta-6Sn/one hour | 110.1 | 0.0025 | 0.81 | 34,470 | 0.0038 | 0.87 | 36,542 |
| Ti-Zr-Ta-8Sn/one hour | 155.1 | 0.0019 | 0.75 | 128,978 | 0.0024 | 0.84 | 62,587 |
| Ti-Zr-Ta-4Sn/72 h | 135.9 | 0.0088 | 0.86 | 9829 | 0.0064 | 0.87 | 8982 |
| Ti-Zr-Ta-6Sn/72 h | 151.8 | 0.0075 | 0.79 | 19,523 | 0.0047 | 0.85 | 24,581 |
| Ti-Zr-Ta-8Sn/72 h | 164.1 | 0.0049 | 0.68 | 31,741 | 0.0036 | 0.92 | 48,395 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherif, E.-S.M.; Bahri, Y.A.; Alharbi, H.F.; Ijaz, M.F. Corrosion Passivation in Simulated Body Fluid of Ti-Zr-Ta-xSn Alloys as Biomedical Materials. Materials 2023, 16, 4603. https://doi.org/10.3390/ma16134603
Sherif E-SM, Bahri YA, Alharbi HF, Ijaz MF. Corrosion Passivation in Simulated Body Fluid of Ti-Zr-Ta-xSn Alloys as Biomedical Materials. Materials. 2023; 16(13):4603. https://doi.org/10.3390/ma16134603
Chicago/Turabian StyleSherif, El-Sayed M., Yassir A. Bahri, Hamad F. Alharbi, and Muhammad Farzik Ijaz. 2023. "Corrosion Passivation in Simulated Body Fluid of Ti-Zr-Ta-xSn Alloys as Biomedical Materials" Materials 16, no. 13: 4603. https://doi.org/10.3390/ma16134603
APA StyleSherif, E.-S. M., Bahri, Y. A., Alharbi, H. F., & Ijaz, M. F. (2023). Corrosion Passivation in Simulated Body Fluid of Ti-Zr-Ta-xSn Alloys as Biomedical Materials. Materials, 16(13), 4603. https://doi.org/10.3390/ma16134603








