Study on the Influence of Surface Treatment Process on the Corrosion Resistance of Aluminum Alloy Profile Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Static Immersion Corrosion Experiment
2.3. Electrochemical Testing
3. Results and Discussion
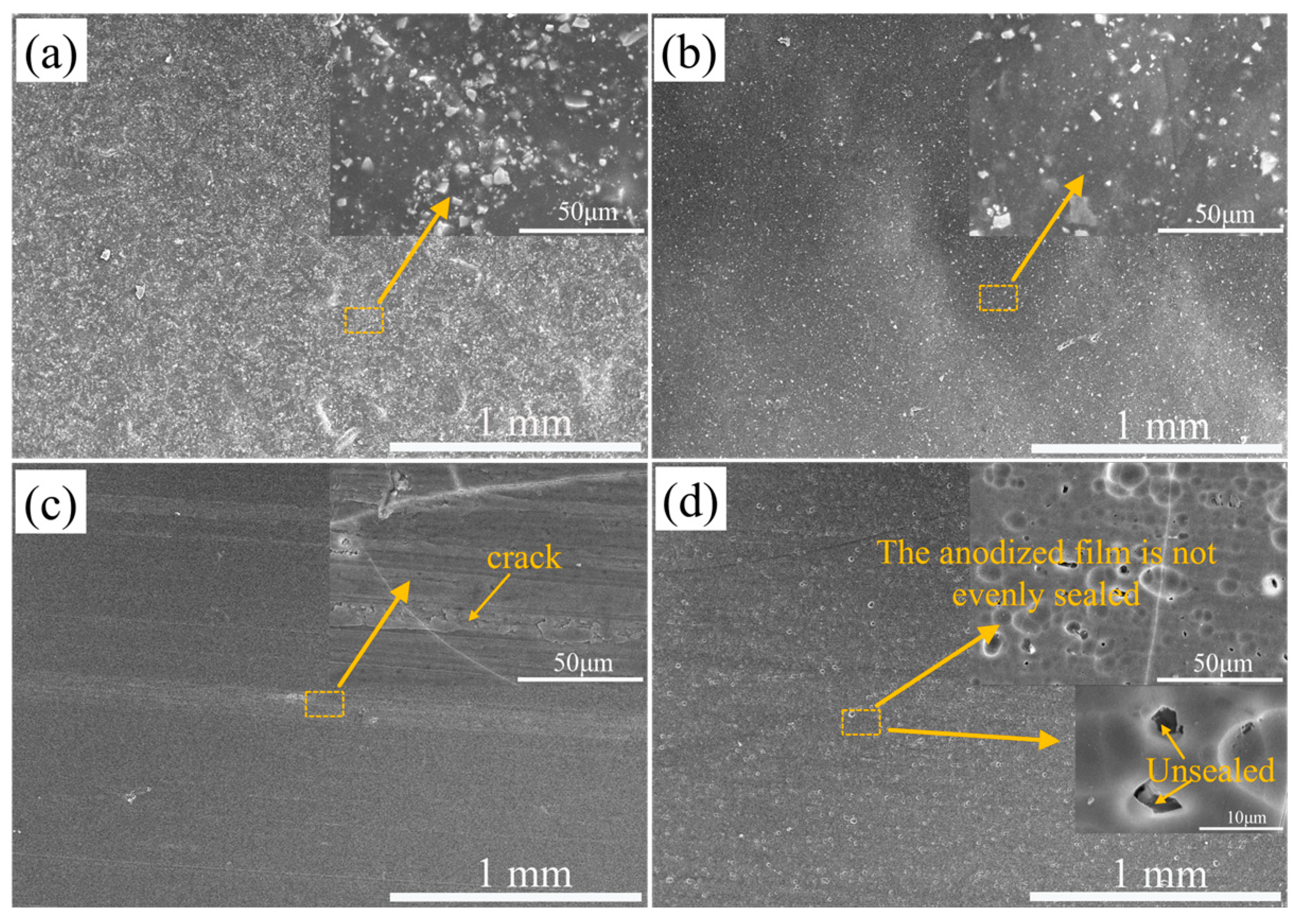
3.1. Micromorphology of the Coating after Corrosion
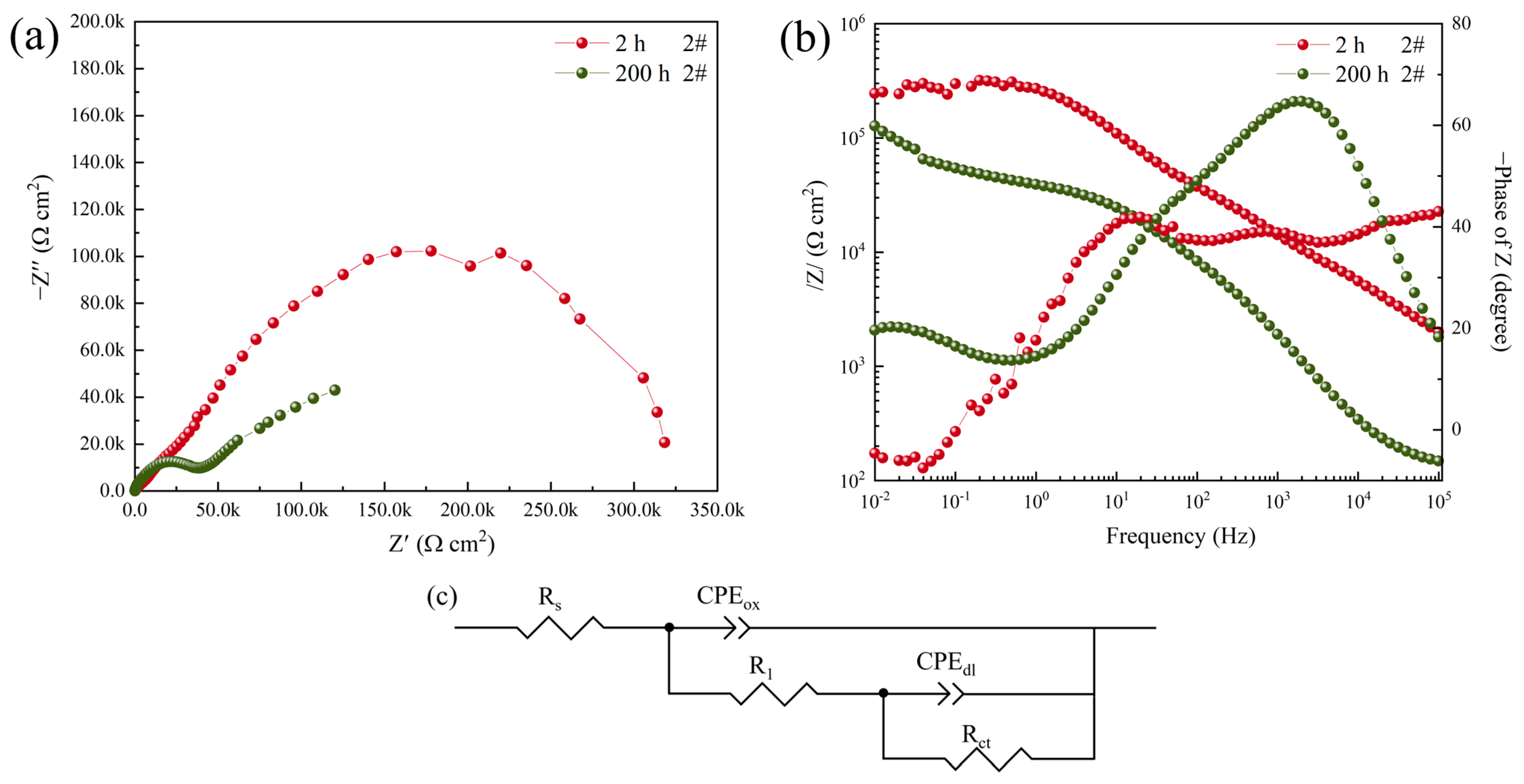
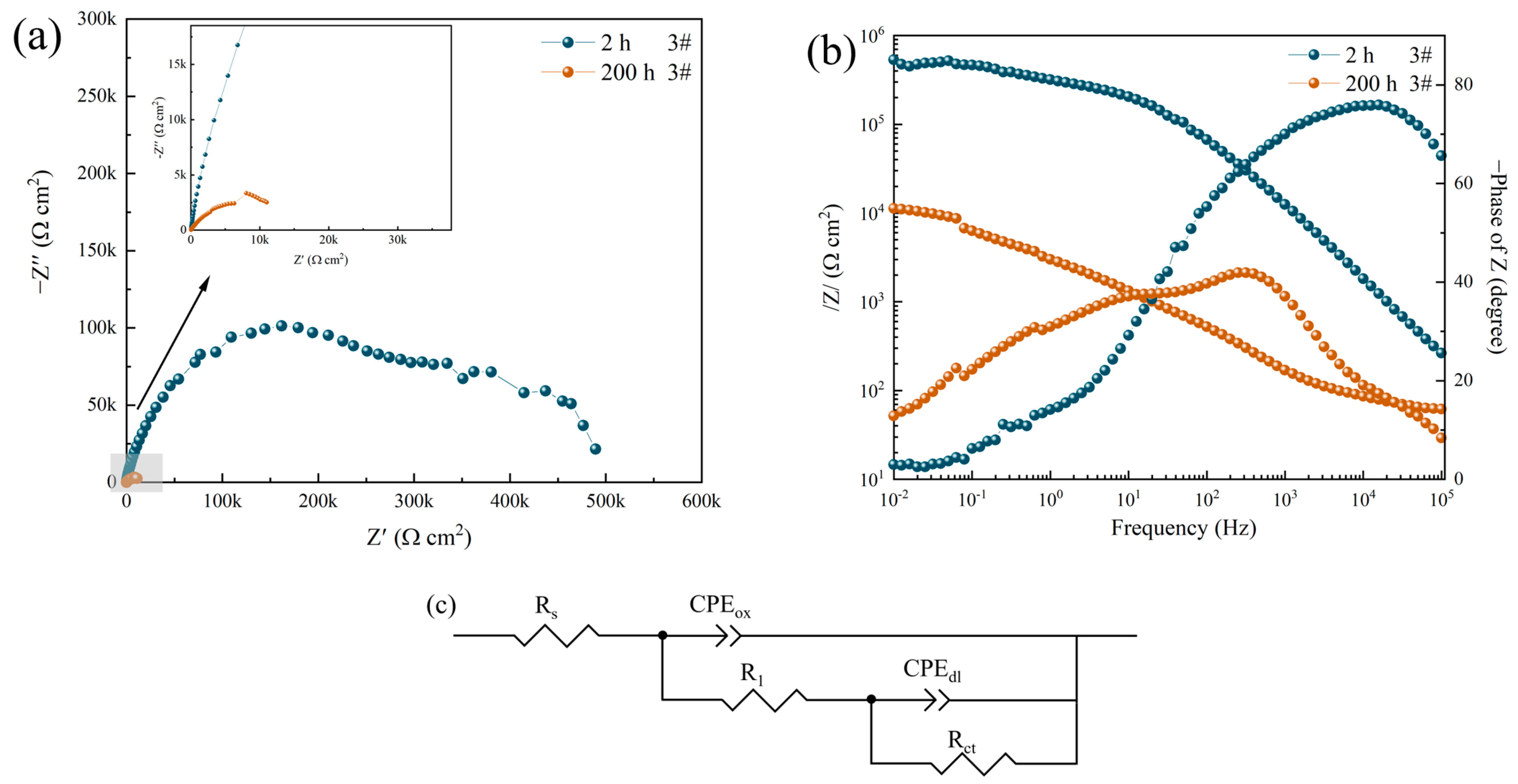
3.2. Electrochemical Impedance Spectroscopy
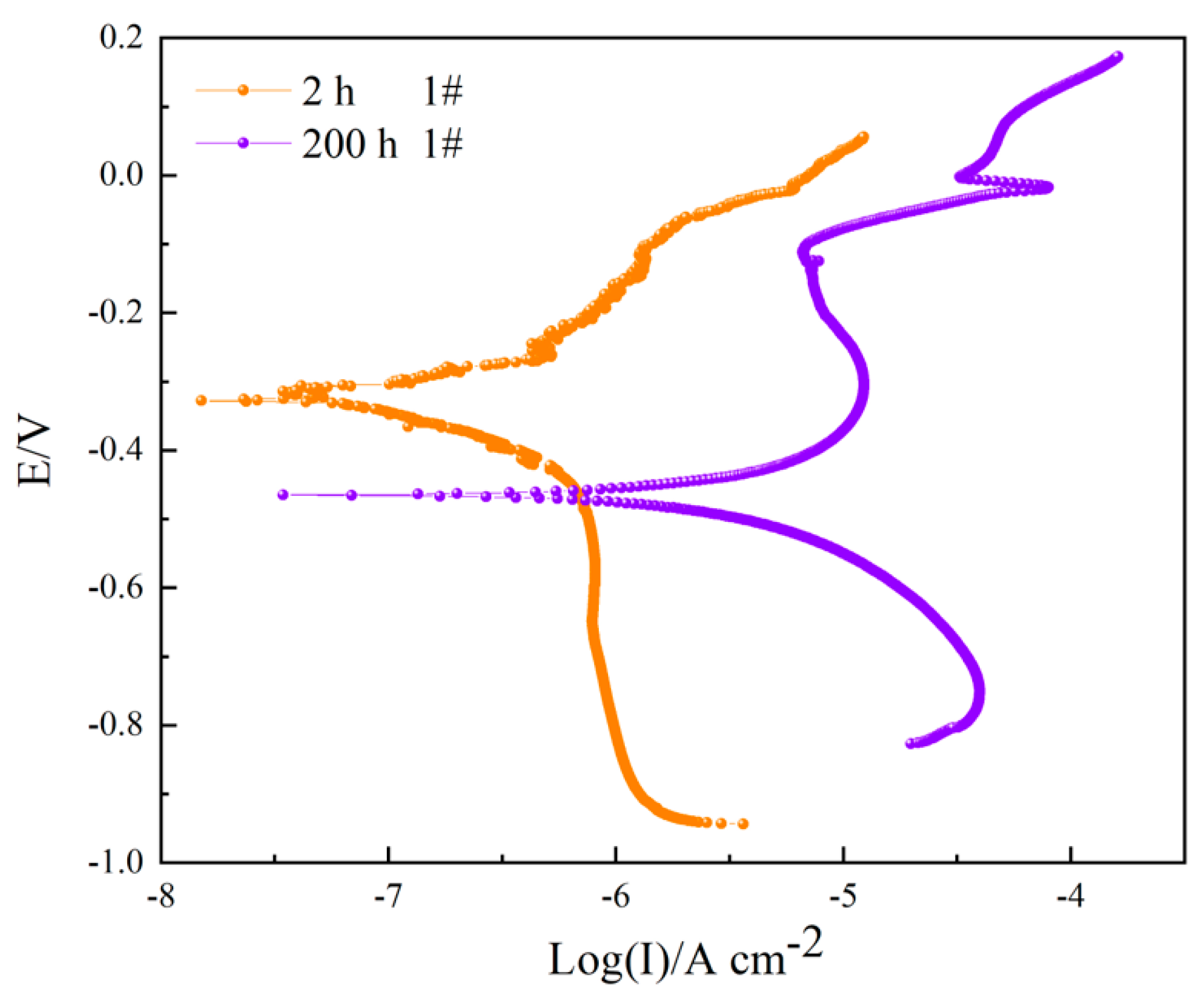
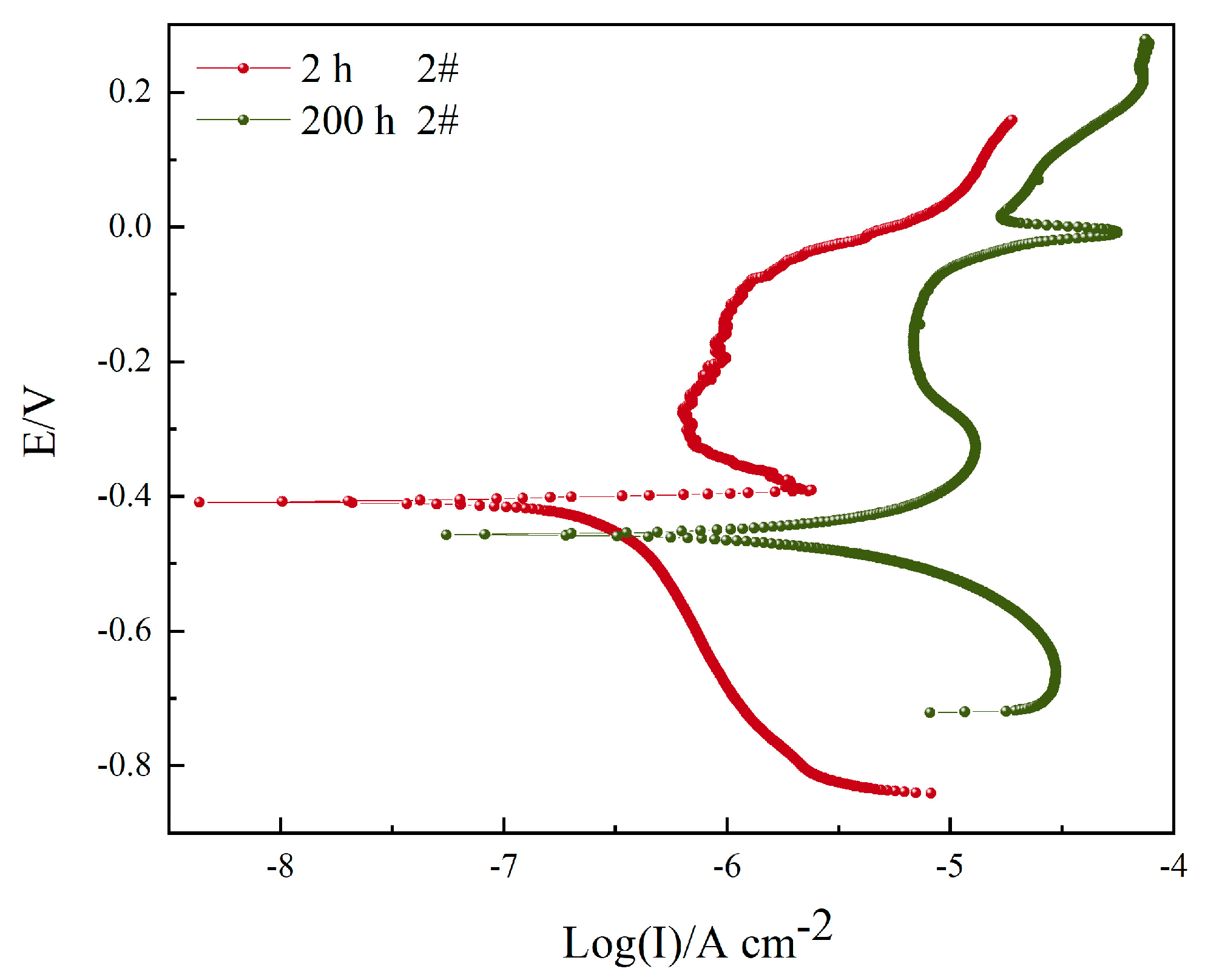
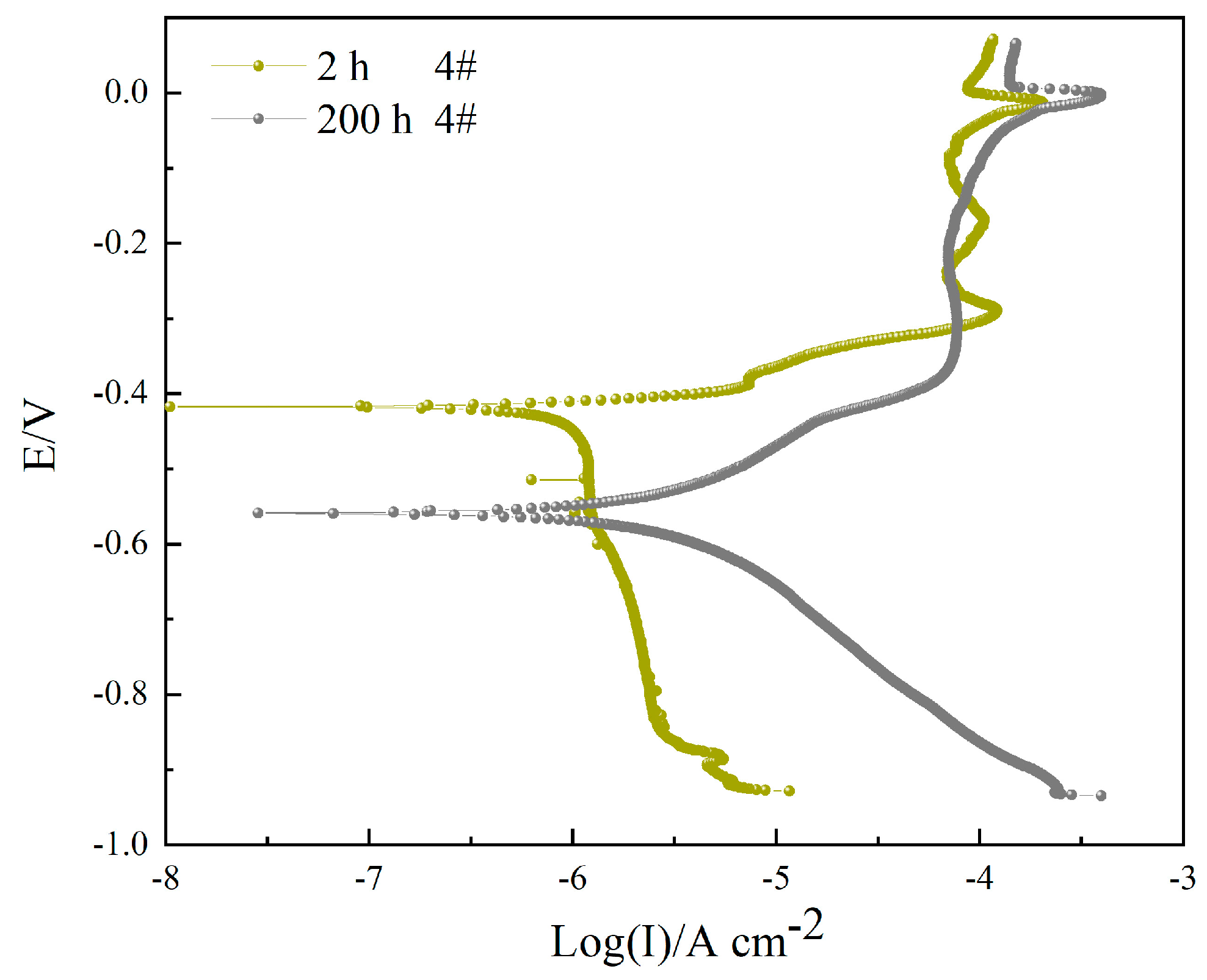
3.3. Polarization Curve
3.4. Summary
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sill, T.E.; Zaheer, W.; Valdes, C.G.; Balcorta, V.H.; Douglas, L.; Fletcher, T.; Steiger, S.; Spinner, N.S.; Verkhoturov, S.V.; Kalyanaraman, V.; et al. Mechanistic origins of corrosion protection of aluminum alloys by graphene/polyetherimide nanocomposite coatings. NPJ Mater. Degrad. 2023, 7, 35. [Google Scholar] [CrossRef]
- Rahmani, M.H.; Marhamati, F.; Haddadi, S.A.; Olivier, M.-G.; Mahdavian, M. N-doped carbon hollow sphere reservoirs containing Zn2+-mercaptobenzoxazole with pH-responsive function to fabricate a high-performance corrosion protective epoxy coating for aluminum alloy. Corros. Sci. 2023, 219, 111202. [Google Scholar] [CrossRef]
- Yang, M.; Lei, L.; Jiang, Y.; Xu, F.; Yin, C. Simultaneously improving tensile properties and stress corrosion cracking resistance of 7075-T6 aluminum alloy by USRP treatment. Corros. Sci. 2023, 218, 111211. [Google Scholar] [CrossRef]
- Zhang, P.; Xiong, Q.; Cai, Y.; Zhai, W.; Cai, M.; Cai, Z.; Zhu, J.; Gu, L. Dry fretting and sliding wear behavior of 7075-T651 aluminum alloy under linear reciprocating motion: A comparative study. Wear 2023, 526–527, 204942. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Jiang, L.; Lu, Y.; Gong, M.; Zhang, C.; Gao, M. Droplet detachment and motion behaviors in laser-MIG hybrid welding of aluminum alloy. Int. J. Heat Mass Transf. 2023, 210, 124165. [Google Scholar] [CrossRef]
- Van Truong, P.; Van Bo, N.; Van Minh, N.; Anh, N.V.; Kumar, G.S.; Shkir, M. Investigation of corrosion and wear resistance of PEO coated D16T aluminium alloys in the marine tropical climate conditions. Mater. Chem. Phys. 2022, 290, 126587. [Google Scholar] [CrossRef]
- Li, T.; Li, X.G.; Dong, C.F.; Cheng, Y.F. Characterization of Atmospheric Corrosion of 2A12 Aluminum Alloy in Tropical Marine Environment. J. Mater. Eng. Perform. 2010, 19, 591–598. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Li, X.G.; Man, C.; Xiao, K.; Dong, C.F.; Wang, X.; Liu, Z.Y. Corrosion Behavior of Field-Exposed 7A04 Aluminum Alloy in the Xisha Tropical Marine Atmosphere. J. Mater. Eng. Perform. 2015, 24, 2885–2897. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Jiang, H.; Li, X.; Mu, X. Atmospheric corrosion comparison of antirust aluminum exposed to industrial and coastal atmospheres. Mater. Corros. 2018, 69, 1516–1525. [Google Scholar] [CrossRef]
- Gu, T.; Liu, Y.; Peng, C.; Zhang, P.; Wang, Z. Initial atmospheric corrosion of zinc-aluminum-magnesium coated steel and galvanized steel in regions of extremely cold and industrial climate. Mater. Chem. Phys. 2022, 291, 126686. [Google Scholar] [CrossRef]
- Zhu, Y.-Q.; Li, J.-M.; Zhao, F.-C.; Dai, L.; Su, Y. The Characterization Research on Atmospheric Environmental Corrosion of Aluminum Alloy. In Proceedings of the 4th International Conference on System Reliability and Safety Engineering (SRSE), Guangzhou, China, 15–18 December 2022; pp. 399–406. [Google Scholar] [CrossRef]
- Miramontes, J.C.; Tiburcio, C.G.; Mata, E.G.; Alcála, M.E.; Maldonado-Bandala, E.; Lara-Banda, M.; Nieves-Mendoza, D.; Olguín-Coca, J.; Zambrano-Robledo, P.; López-León, L.D.; et al. Corrosion Resistance of Aluminum Alloy AA2024 with Hard Anodizing in Sulfuric Acid-Free Solution. Materials 2022, 15, 6401. [Google Scholar] [CrossRef]
- EL Mazyani, A.; Chafi, M.; Essahli, M. Assessment of AA5005 aluminum alloy corrosion resistance by Direct Blue 15 inhibitor in sodium chloride medium. Mater. Today Proc. 2021, 37, 3882–3888. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Zhao, S.; Niu, A.; Ma, Y.; Liu, B. Enhanced long-term corrosion protection of 2A14 aluminum alloy: Hybrid effect of micro-arc oxidation coating and cerium based conversion treatment. Surf. Coat. Technol. 2023, 464, 129579. [Google Scholar] [CrossRef]
- Minhas, B.; Dino, S.; Huang, L.; Wu, D. Active Corrosion Protection by Epoxy Coating on Li2CO3-Pretreated Anodized Aluminum Alloy 2024-T3. Front. Mater. 2022, 8, 804328. [Google Scholar] [CrossRef]
- Lin, X.H.; Huang, H.B.; Zhou, C.S.; Liu, J.C.; Saleh, M.; Wang, Z.Z. Research on surface modification of anodized aluminum alloy using piezoelectric machine hammer peening. Int. J. Adv. Manuf. Technol. 2019, 104, 1211–1219. [Google Scholar] [CrossRef]
- Badhotiya, G.K.; Saini, A.; Singh, D.; Chauhan, A.S. Oxime-modified aluminum (III) isopropoxide: A promising sol-gel precursor coating to improve mechanical strength of an aluminum alloy. Mater. Today Proc. 2021, 46, 10600–10603. [Google Scholar] [CrossRef]
- Kumar, J.J.; Satyanarayana, V.V.; Reddy, G.S.; Reddy, B.R.; Dharmavarapu, P. Effect of Al2TiO5 powder coating on the tribological, corrosion and mechanical properties of AISI 316L stainless steel. Mater. Res. Express 2023, 10, 066511. [Google Scholar] [CrossRef]
- Li, M.; Huang, H.-L.; Wu, J.-M.; Wu, Y.-R.; Shi, Z.-A.; Zhang, J.-X.; Shi, Y.-S. Preparation and properties of Si3N4 ceramics via digital light processing using Si3N4 powder coated with Al2O3-Y2O3 sintering additives. Addit. Manuf. 2022, 53, 102713. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Gordeeva, E.O.; Roslyakov, I.V.; Napolskii, K.S. Aluminium anodizing in selenic acid: Electrochemical behaviour, porous structure, and ordering regimes. Electrochim. Acta 2019, 307, 13–19. [Google Scholar] [CrossRef]
- Ali, I.; Quazi, M.M.; Zalnezhad, E.; Sarhan, A.A.D.; Sukiman, N.L.; Ishak, M. Hard Anodizing of Aerospace AA7075-T6 Aluminum Alloy for Improving Surface Properties. Trans. Indian Inst. Met. 2019, 72, 2773–2781. [Google Scholar] [CrossRef]
- Li, J.; Wei, H.; Zhao, K.; Wang, M.; Chen, D.; Chen, M. Effect of anodizing temperature and organic acid addition on the structure and corrosion resistance of anodic aluminum oxide films. Thin Solid Film. 2020, 713, 138359. [Google Scholar] [CrossRef]
- Atz-Dick, P.; Konrath, A.; Melo, Y.R.; Radtke, C.; Dick, L.F. Aluminum anodizing with simultaneous silanization for increased hydrophobicity and corrosion protection. Appl. Surf. Sci. 2022, 593, 153392. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Moradian, S.; Scantlebury, J.D.; Thompson, G.E. Characterization and corrosion performance of powder coated aluminium alloy. Iran Polym. J. 2003, 12, 261–269. [Google Scholar]
- Lutz, A.; Halseid, M.C.; De Graeve, I. Corrosion performance of powder-coated aluminum profiles with increased trace element content. Mater. Corros. 2022, 73, 1575–1585. [Google Scholar] [CrossRef]
- Halseid, M.; Gundersen, J.T.B.; Bauger, Ø.; Hentschel, T. The effect of trace additions of copper and pretreatment conditions on the filiform corrosion of powder-coated aluminium alloy AA6060. Surf. Interface Anal. 2019, 51, 1225–1230. [Google Scholar] [CrossRef]
- Niedźwiedź, M.; Bara, M.; Barylski, A. Dependence of the Surface Morphology and Micromechanical and Sclerometric Properties of Al2O3 Layers on the Parameters of Anodizing Aluminum Alloy. Materials 2022, 15, 8482. [Google Scholar] [CrossRef]
- Conde, A.; de Damborenea, J. Electrochemical impedance spectroscopy for studying the degradation of enamel coatings. Corros. Sci. 2002, 44, 1555–1567. [Google Scholar] [CrossRef]
- Wong, K.P.; Alkire, R.C. Local Chemistry and Growth of Single Corrosion Pits in Aluminum. J. Electrochem. Soc. 1990, 137, 3010. [Google Scholar] [CrossRef]
- Ferreira, S.; Rocha, L.; Ariza, E.; Sequeira, P.; Watanabe, Y.; Fernandes, J. Corrosion behaviour of Al/Al3Ti and Al/Al3Zr functionally graded materials produced by centrifugal solid-particle method: Influence of the intermetallics volume fraction. Corros. Sci. 2011, 53, 2058–2065. [Google Scholar] [CrossRef]

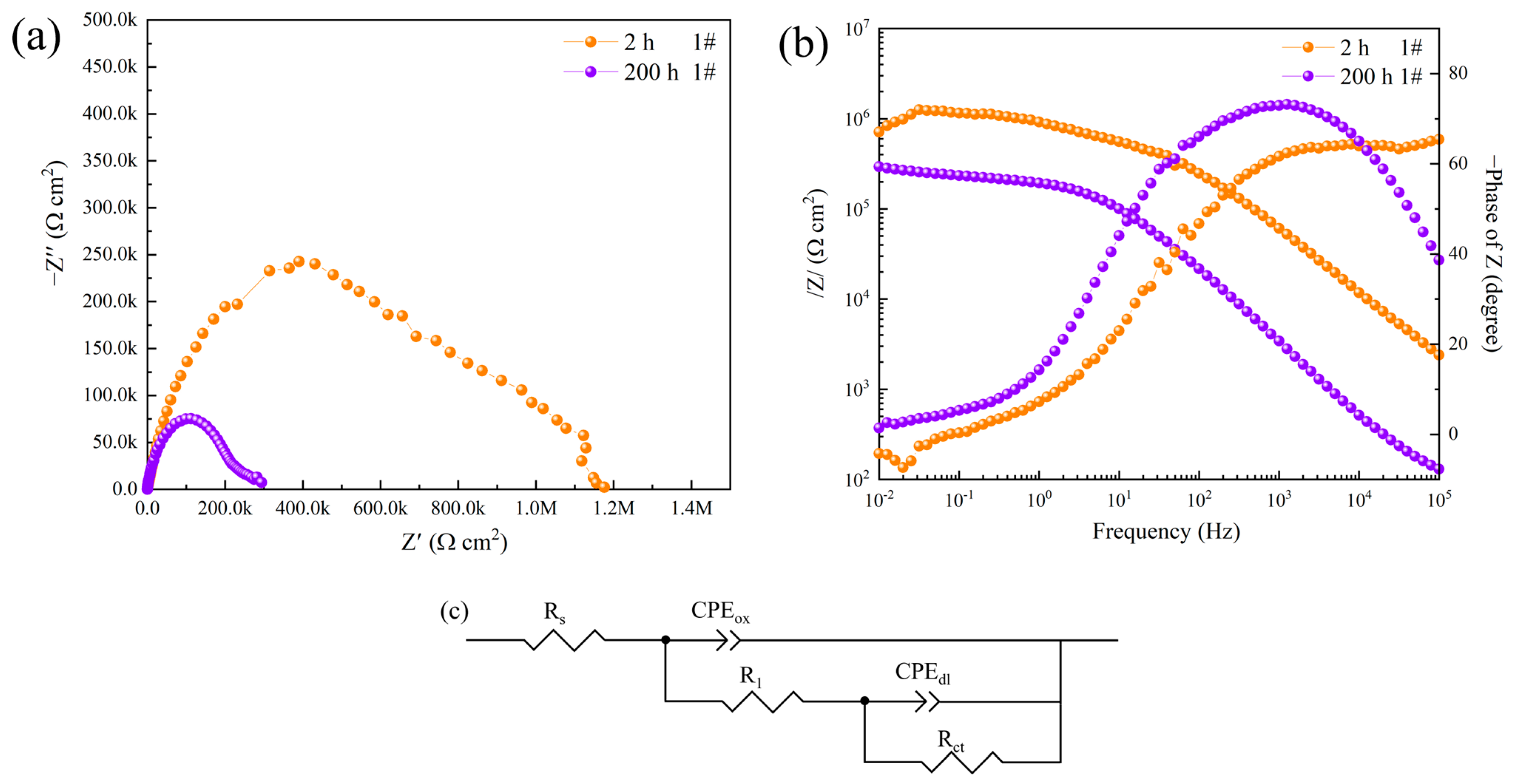
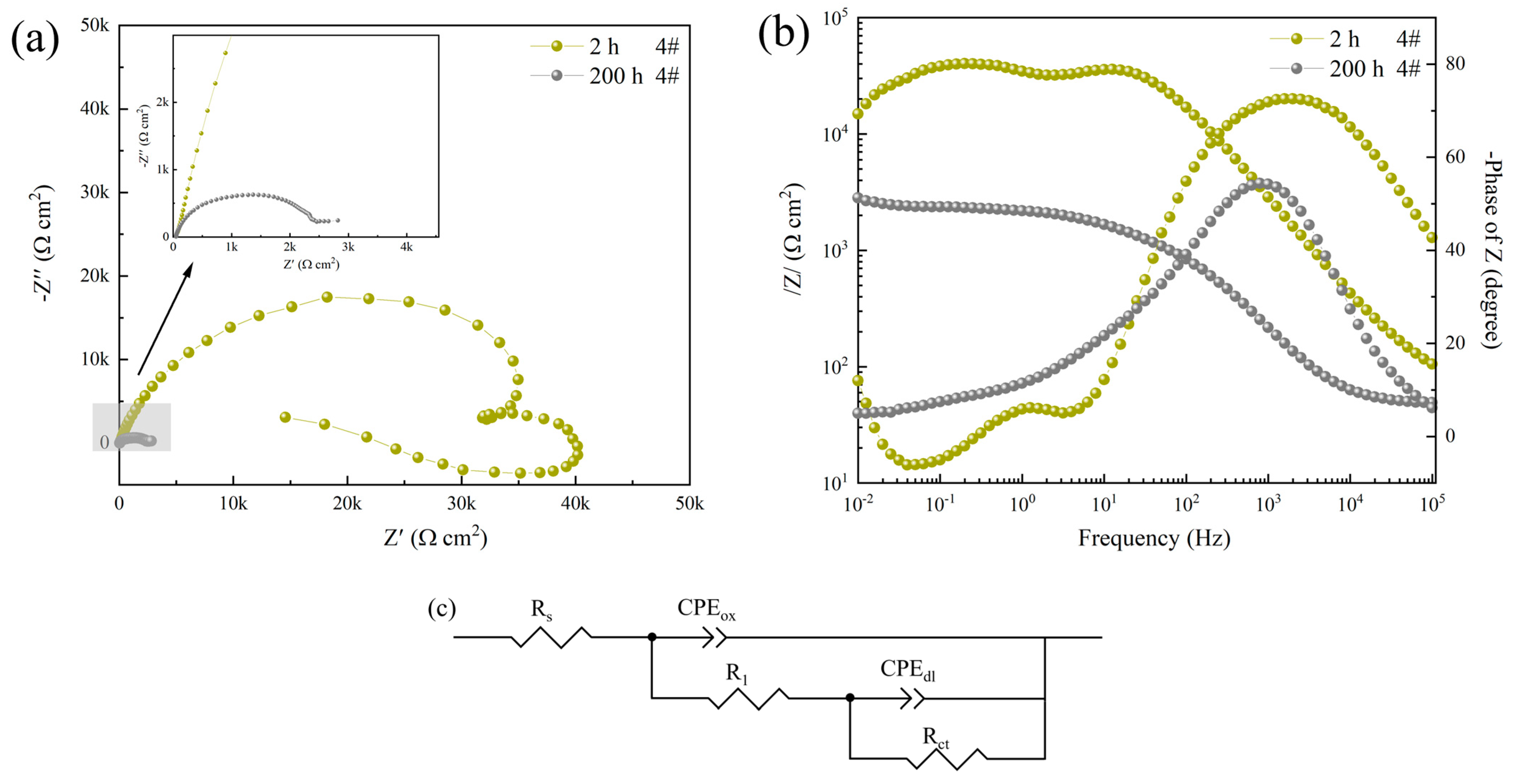

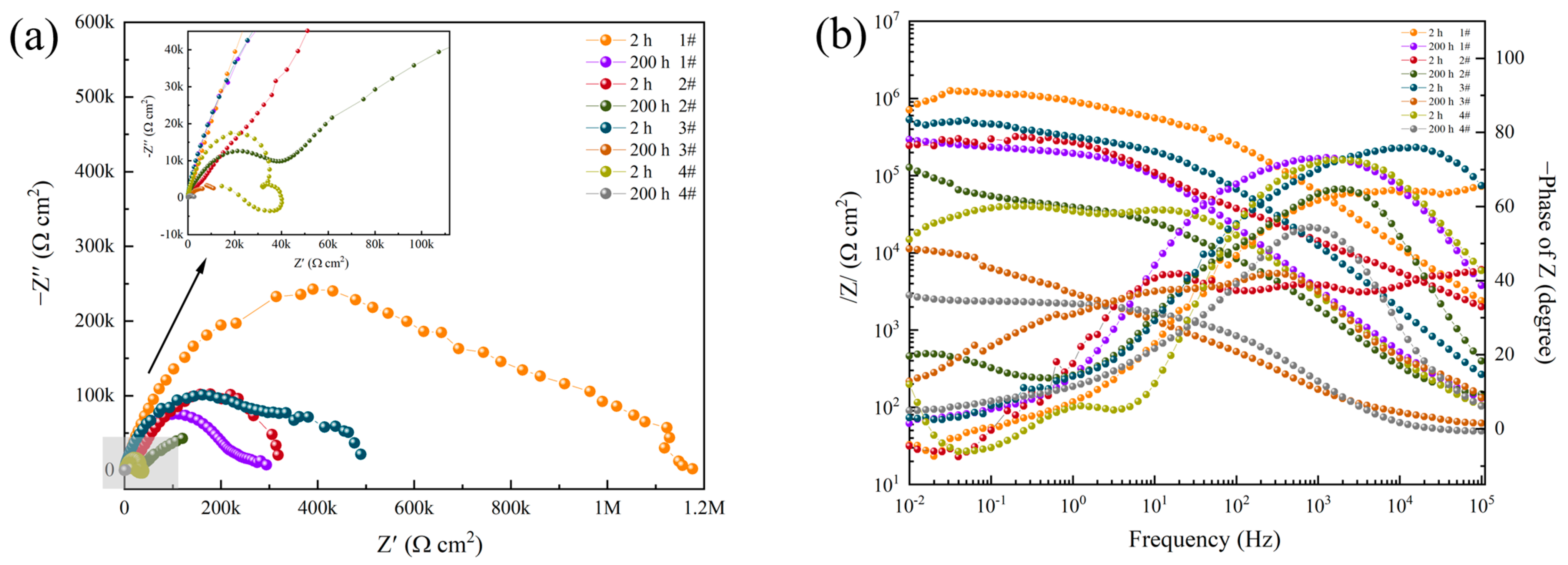
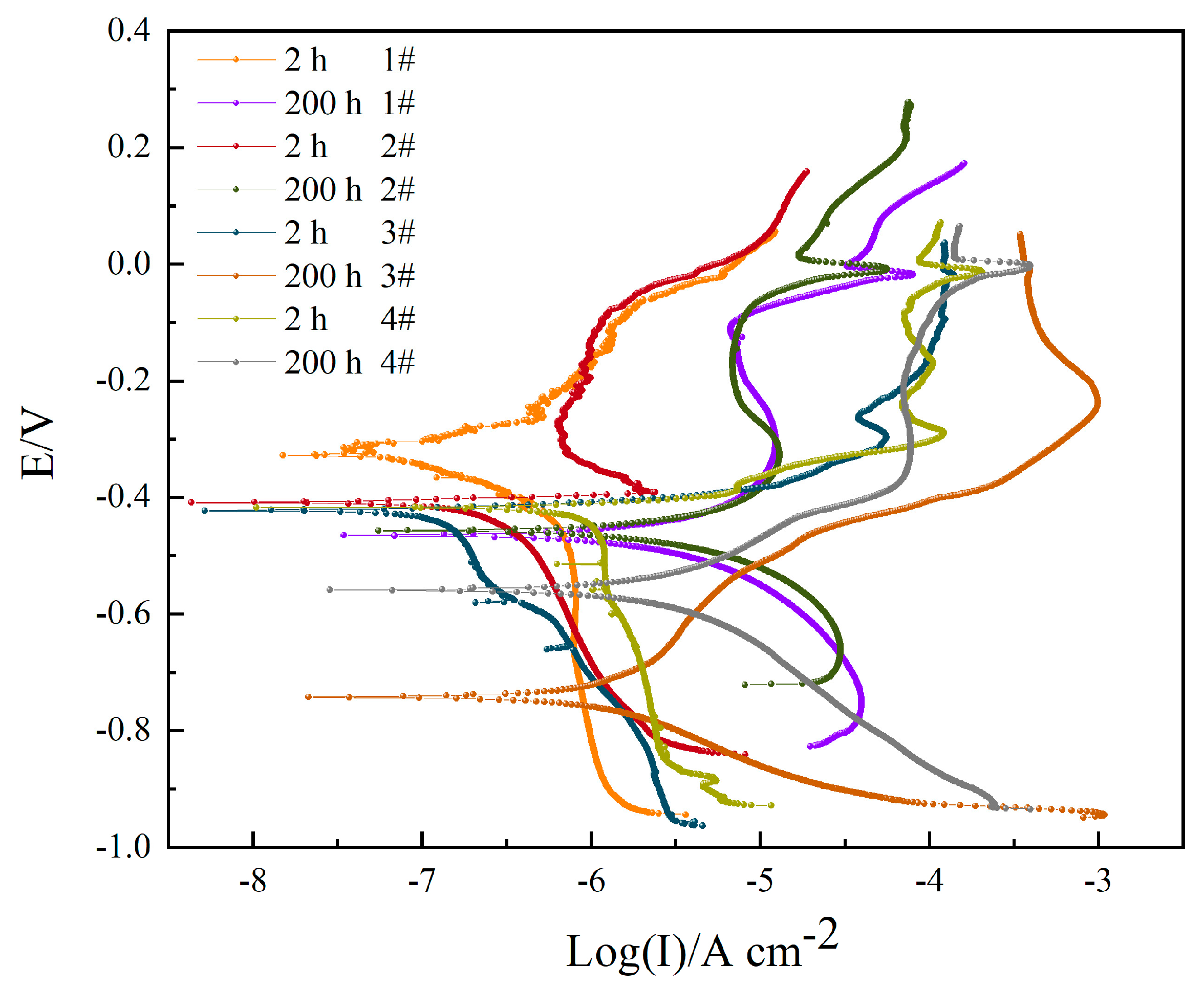
| Sample | Rs /(Ω·cm2) | CPEox-Y0 /(sn·Ω−1·cm−1) | CPE-nox | R1 /(Ω·cm2) | CPEdl-Y0 /(sn·Ω−1·cm−1) | CPE-ndl | Rct /(kΩ·cm2) | v (%) |
|---|---|---|---|---|---|---|---|---|
| 1#, 2 h | 99.53 ± 6.23 | 2.1170 × 10−9 | 0.9283 | 8.0740 × 104 | 9.0023 × 10−8 | 0.5434 | 1091.20 ± 50.12 | - |
| 1#, 200 h | 83.20 ± 4.30 | 2.1803 × 10−7 | 0.8246 | 1.4101 × 106 | 5.0555 × 10−6 | 0.2777 | 179.15 ± 2.44 | 83.58 |
| Sample | Rs /(Ω·cm2) | CPEox-Y0 /(sn·Ω−1·cm−1) | CPE-nox | R1 /(Ω·cm2) | CPEdl-Y0 /(sn·Ω−1·cm−1) | CPE-ndl | Rct /(kΩ·cm2) | v (%) |
|---|---|---|---|---|---|---|---|---|
| 2#, 2 h | 133.2 ± 10.2 | 1.4130 × 10−9 | 0.9543 | 3.8050 × 103 | 7.976 × 10−7 | 0.5412 | 451.16 ± 23.87 | - |
| 2#, 200 h | 116.1 ± 7.3 | 6.7045 × 10−7 | 0.7699 | 2.5064 × 105 | 3.1047 × 10−5 | 0.3362 | 108.43 ± 2.17 | 75.97 |
| Sample | Rs /(Ω·cm2) | CPEox-Y0 /(sn·Ω−1·cm−1) | CPE-nox | R1 /(Ω·cm2) | CPEdl-Y0 /(sn·Ω−1·cm−1) | CPE-ndl | Rct /(kΩ·cm2) | v (%) |
|---|---|---|---|---|---|---|---|---|
| 3#, 2 h | 49.39 ± 3.4 | 3.6644 × 10−8 | 0.8685 | 1.410 × 103 | 8.3306 × 10−7 | 0.2883 | 635.42 ± 10.15 | - |
| 3#, 200 h | 55.91 ± 2.6 | 4.5205 × 10−5 | 0.5788 | 2.512 × 103 | 1.2077 × 10−4 | 0.5467 | 11.94 ± 0.95 | 98.12 |
| Sample | Rs /(Ω·cm2) | CPEox-Y0 /(sn·Ω−1·cm−1) | CPE-nox | R1 /(Ω·cm2) | CPEdl-Y0 /(sn·Ω−1·cm−1) | CPE-ndl | Rct /(kΩ·cm2) | v (%) |
|---|---|---|---|---|---|---|---|---|
| 4#, 2 h | 62.41 ± 7.48 | 2.3807 × 10−7 | 0.8342 | 4.2038 × 104 | 1.8558 × 10−5 | 1.543 | 225.5 ± 3.4 | - |
| 4#, 200 h | 47.17 ± 6.26 | 2.534 × 10−6 | 0.8523 | 1.044 × 103 | 7.6539 × 10−5 | 0.2938 | 2.87 ± 0.22 | 98.73 |
| Sample | Icorr/(μA cm−2) | E0 (mV) | −bC/(mV dec−1) | bA/(mV dec−1) | η (%) |
|---|---|---|---|---|---|
| 1#, 2 h | 0.1709 ± 0.0422 | −350.97 ± 7.56 | 160.20 | 226.68 | - |
| 1#, 200 h | 4.0059 ± 0.1130 | −486.49 ± 4.50 | 163.94 | 298.19 | 95.73 |
| Sample | Icorr/(μA cm−2) | E0 (mV) | −bC/(mV dec−1) | bA/(mV dec−1) | η (%) |
|---|---|---|---|---|---|
| 2#, 2 h | 0.2418 ± 0.0330 | −401.15 ± 5.24 | 337.00 | 9.08 | - |
| 2#, 200 h | 5.4439 ± 0.2503 | −471.05 ± 4.16 | 176.98 | 331.61 | 95.56 |
| Sample | Icorr/(μA cm−2) | E0 (mV) | −bC/(mV dec−1) | bA/(mV dec−1) | η (%) |
|---|---|---|---|---|---|
| 3#, 2 h | 0.1371 ± 0.0214 | −416.36 ± 4.88 | 532.73 | 11.25 | - |
| 3#, 200 h | 1.4051 ± 0.0832 | −754.58 ± 9.00 | 125.20 | 323.83 | 90.24 |
| Sample | Icorr/(μA cm−2) | E0 (mV) | −bC/(mV dec−1) | bA/(mV dec−1) | η (%) |
|---|---|---|---|---|---|
| 4#, 2 h | 0.3069 ± 0.0412 | −412.16 ± 3.10 | 69.22 | 10.02 | - |
| 4#, 200 h | 2.8404 ± 0.1004 | −559.81 ± 5.24 | 173.79 | 165.02 | 89.19 |
| Sample | Rs /(Ω·cm2) | CPEox-Y0 /(sn·Ω−1·cm−1) | CPE-nox | R1 /(Ω·cm2) | CPEdl-Y0 /(sn·Ω−1·cm−1) | CPE-ndl | Rct /(kΩ·cm2) | v (%) |
|---|---|---|---|---|---|---|---|---|
| 2 h 1# | 995.03 | 2.117 × 10−9 | 0.9283 | 8074 | 9.0023 × 10−8 | 0.5434 | 1091.2 | - |
| 2 h 2# | 1343.2 | 1.413 × 10−9 | 0.9543 | 3805 | 7.976 × 10−7 | 0.5412 | 451.16 | 58.65 |
| 2 h 3# | 49.39 | 3.6644 × 10−8 | 0.8685 | 1410 | 8.3306 × 10−7 | 0.2883 | 635.42 | 41.77 |
| 2 h 4# | 62.41 | 2.3807 × 10−7 | 0.8342 | 4.2038 × 104 | 1.8558 × 10−5 | 1.543 | 225.5 | 79.33 |
| 200 h 1# | 83.2 | 2.1803 × 10−7 | 0.8246 | 141,010 | 5.0555 × 10−6 | 0.2777 | 179.15 | - |
| 200 h 2# | 116.1 | 6.7045 × 10−7 | 0.7699 | 25,064 | 3.1047 × 10−5 | 0.3362 | 108.43 | 39.48 |
| 200 h 3# | 55.91 | 4.5205 × 10−5 | 0.5788 | 2512 | 1.2077 × 10−4 | 0.5467 | 11.94 | 93.33 |
| 200 h 4# | 47.17 | 2.534 × 10−6 | 0.8523 | 10.44 | 7.6539 × 10−5 | 0.2938 | 2.87 | 98.39 |
| Sample | Icorr/(μA cm−2) | E0 (mV) | −bC/(mV dec−1) | bA/(mV dec−1) | η (%) |
|---|---|---|---|---|---|
| 2 h 1# | 0.1709 | −350.97 | 160.20 | 226.68 | - |
| 2 h 2# | 0.2418 | −401.15 | 337.00 | 9.08 | 41.49 |
| 2 h 3# | 0.1371 | −416.36 | 532.73 | 11.25 | −19.78 |
| 2 h 4# | 0.3069 | −412.16 | 69.22 | 10.02 | 79.58 |
| 200 h 1# | 4.0059 | −486.49 | 163.94 | 298.19 | - |
| 200 h 2# | 5.4439 | −471.05 | 176.98 | 331.61 | 35.90 |
| 200 h 3# | 1.4051 | −754.58 | 125.20 | 323.83 | −64.92 |
| 200 h 4# | 2.8404 | −559.81 | 173.79 | 165.02 | −29.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Wang, F.; Wang, Z.; Hao, X.; Yang, N.; Ran, D. Study on the Influence of Surface Treatment Process on the Corrosion Resistance of Aluminum Alloy Profile Coating. Materials 2023, 16, 6027. https://doi.org/10.3390/ma16176027
Fan L, Wang F, Wang Z, Hao X, Yang N, Ran D. Study on the Influence of Surface Treatment Process on the Corrosion Resistance of Aluminum Alloy Profile Coating. Materials. 2023; 16(17):6027. https://doi.org/10.3390/ma16176027
Chicago/Turabian StyleFan, Lei, Fatao Wang, Zhouhui Wang, Xuelong Hao, Neng Yang, and Denglin Ran. 2023. "Study on the Influence of Surface Treatment Process on the Corrosion Resistance of Aluminum Alloy Profile Coating" Materials 16, no. 17: 6027. https://doi.org/10.3390/ma16176027
APA StyleFan, L., Wang, F., Wang, Z., Hao, X., Yang, N., & Ran, D. (2023). Study on the Influence of Surface Treatment Process on the Corrosion Resistance of Aluminum Alloy Profile Coating. Materials, 16(17), 6027. https://doi.org/10.3390/ma16176027





