Degradation and Bone-Contact Biocompatibility of Two Drillable Magnesium Phosphate Bone Cements in an In Vivo Rabbit Bone Defect Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation and Characterization
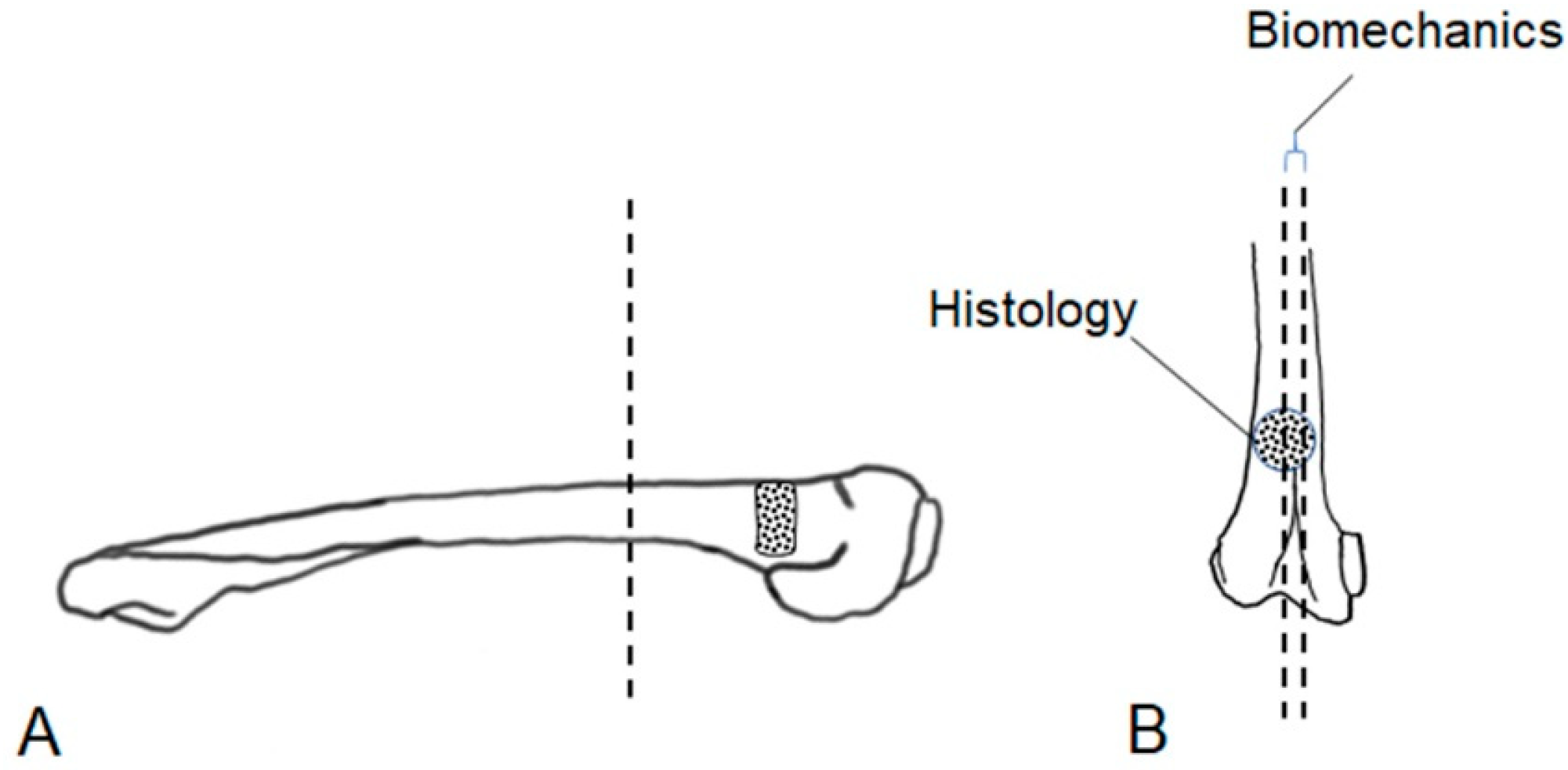
2.2. Animal Study Design and Surgery
2.3. Optical Assessment: Bone Mineral Density
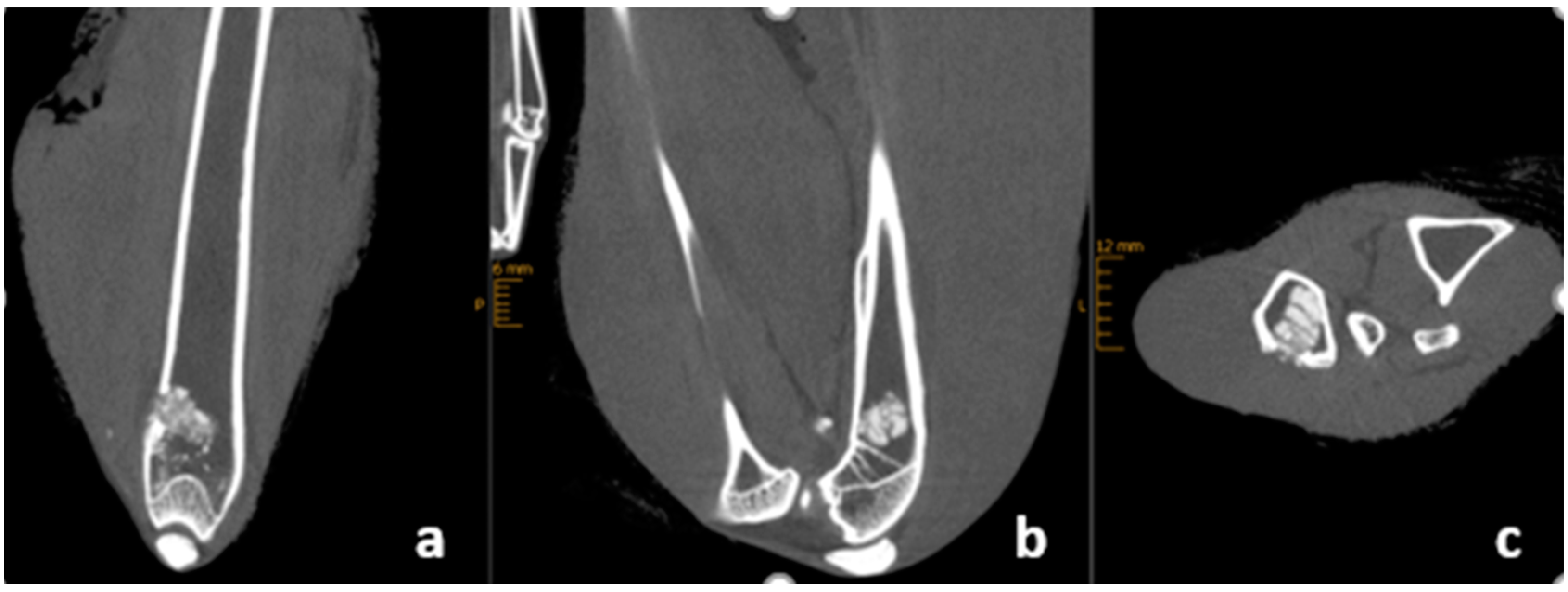
2.4. X-ray and CBCT Imaging
2.5. Biomechanical Evaluation and Histological Examination
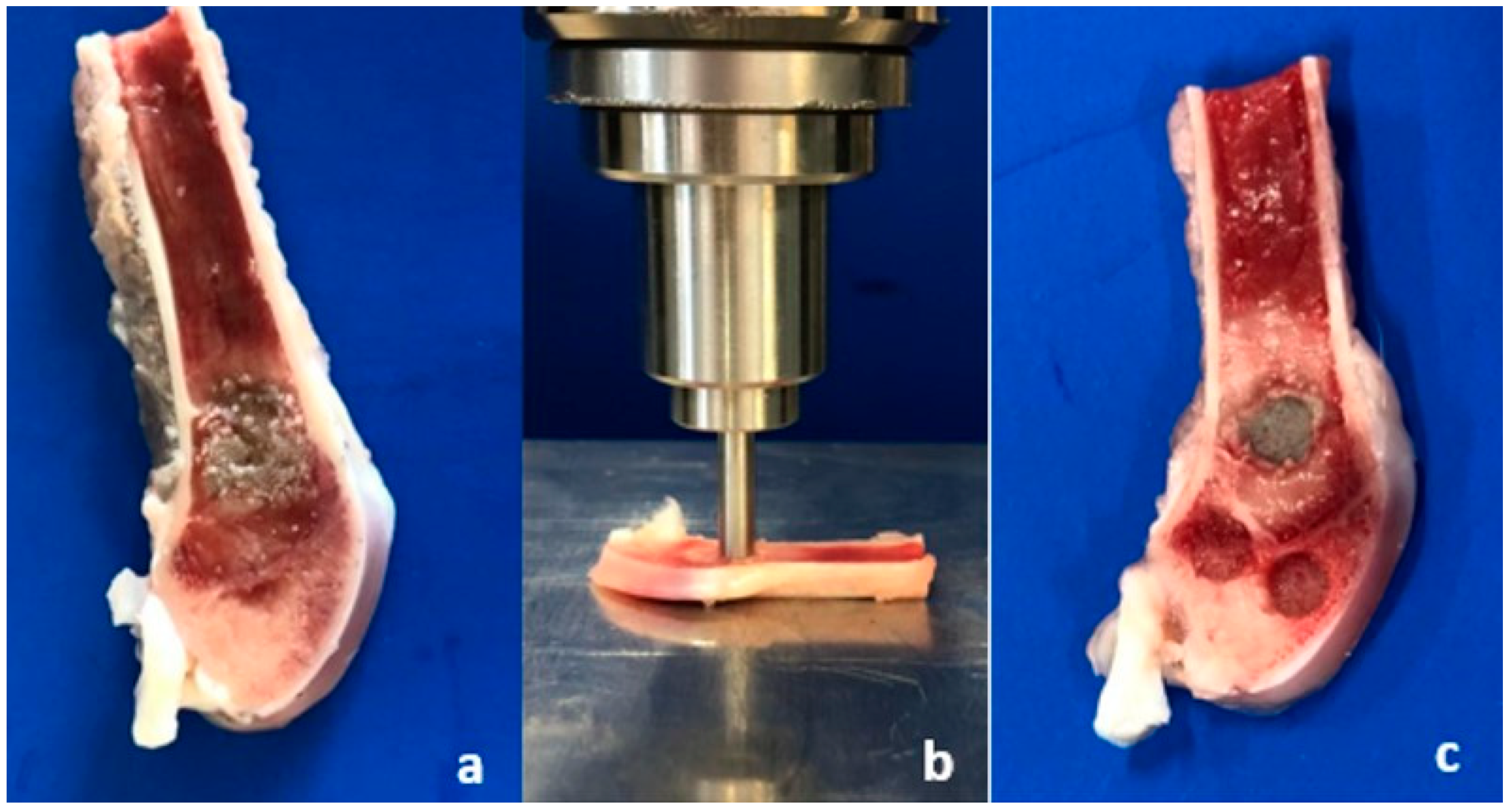
2.5.1. Sample Preparation
2.5.2. Biomechanical Evaluation
2.5.3. Histological Examination
2.6. Statistical Analysis
3. Results
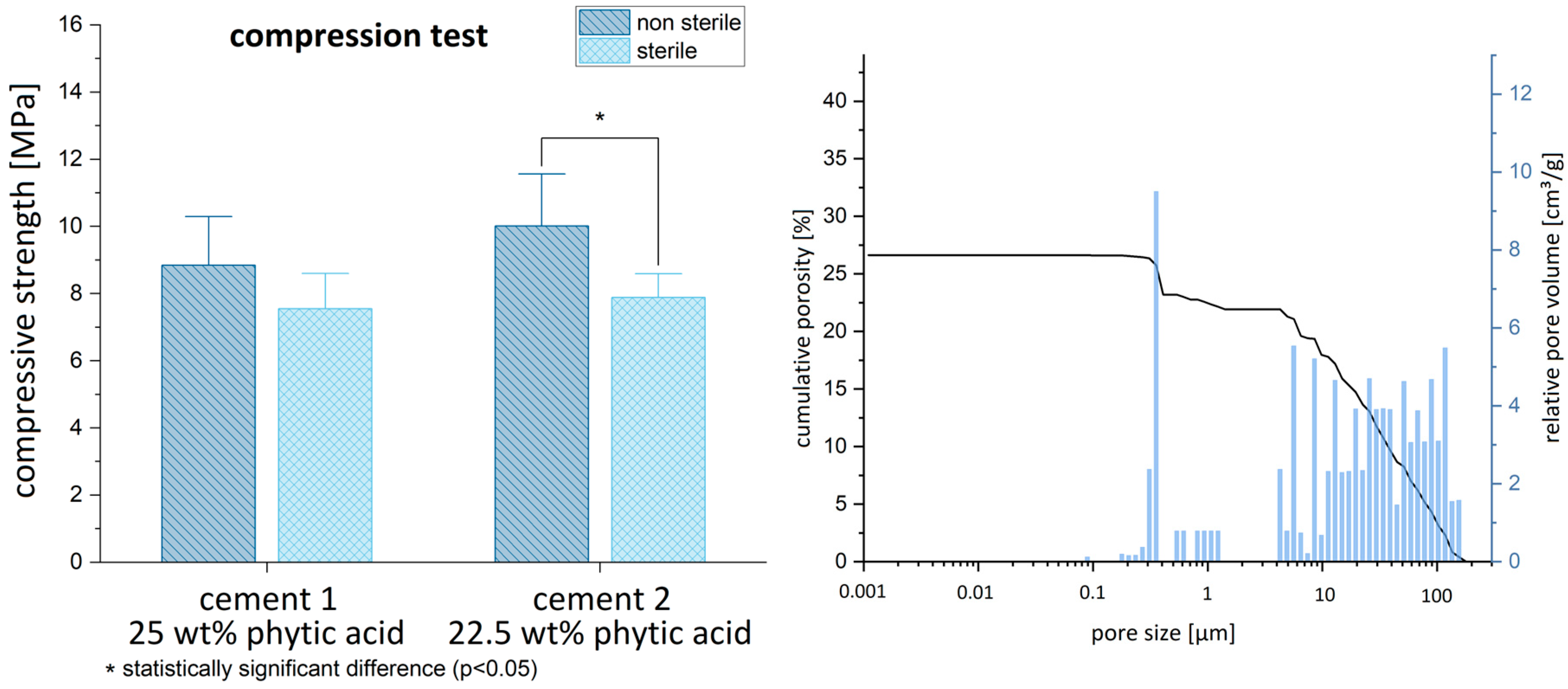
3.1. Material Characterization: Biomechanics and Porosimetry
3.2. Phase Composition before and after Implantation
3.3. Perioperative Animal Condition
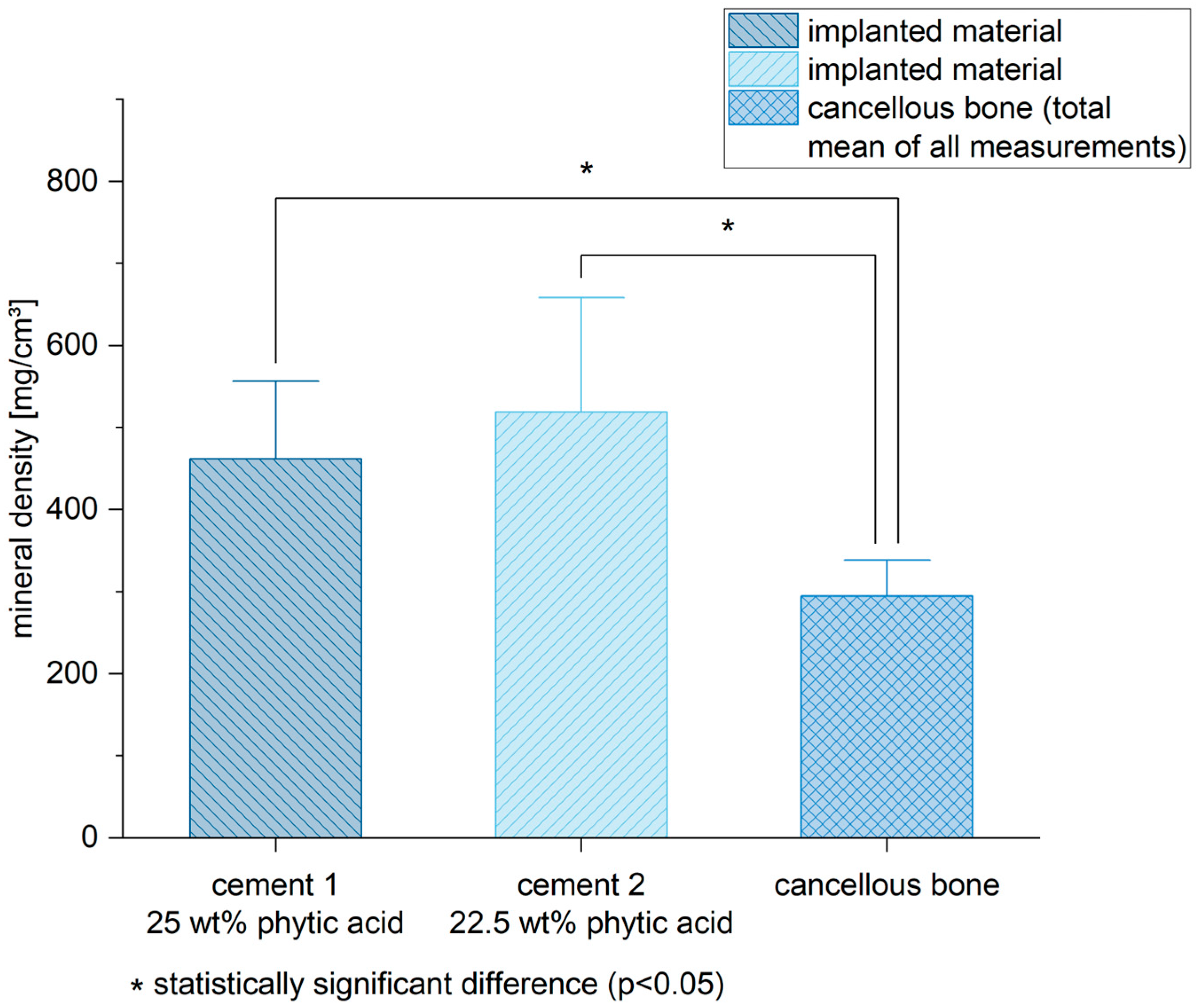
3.4. Mineral Density
3.5. Radiological Outcome
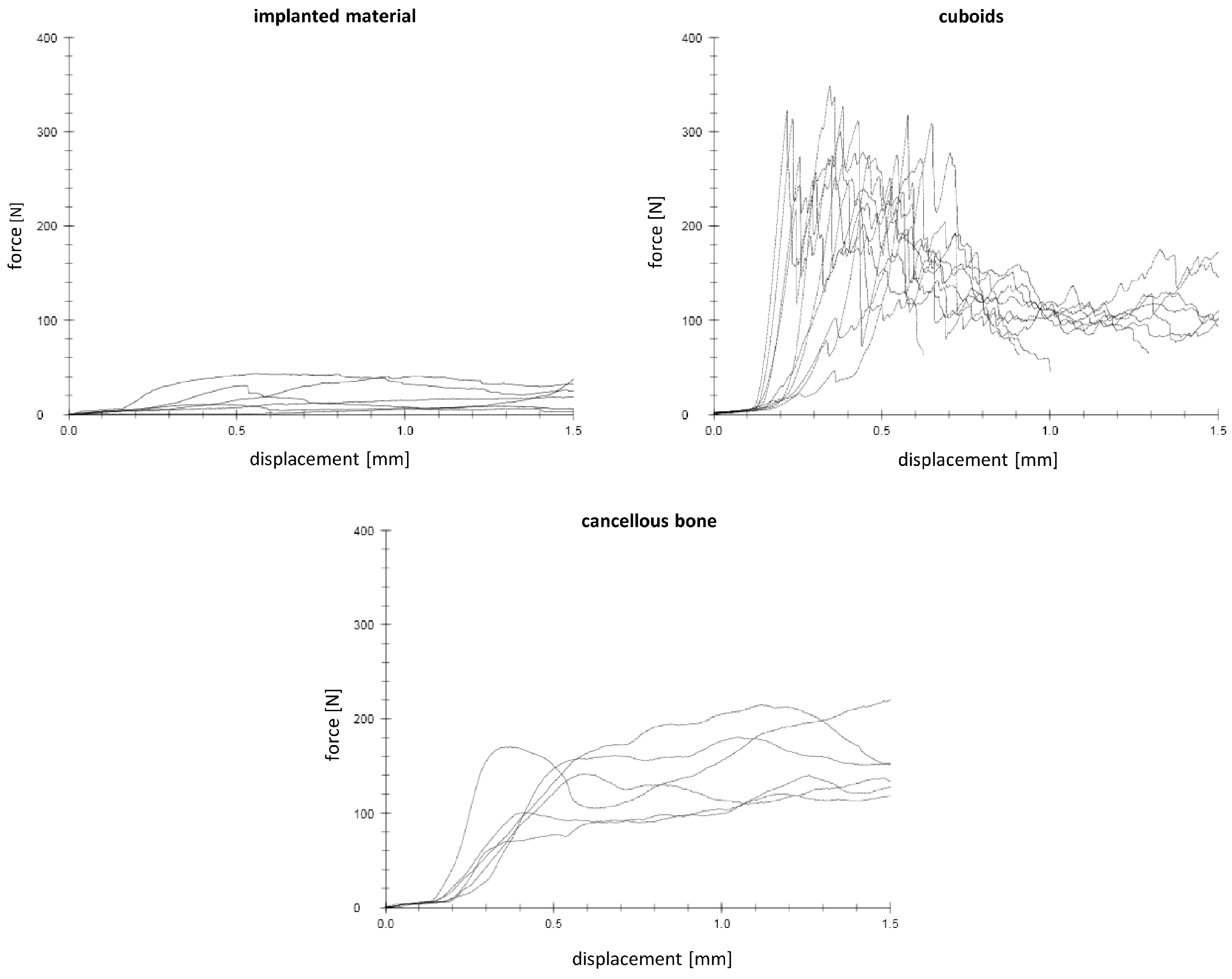
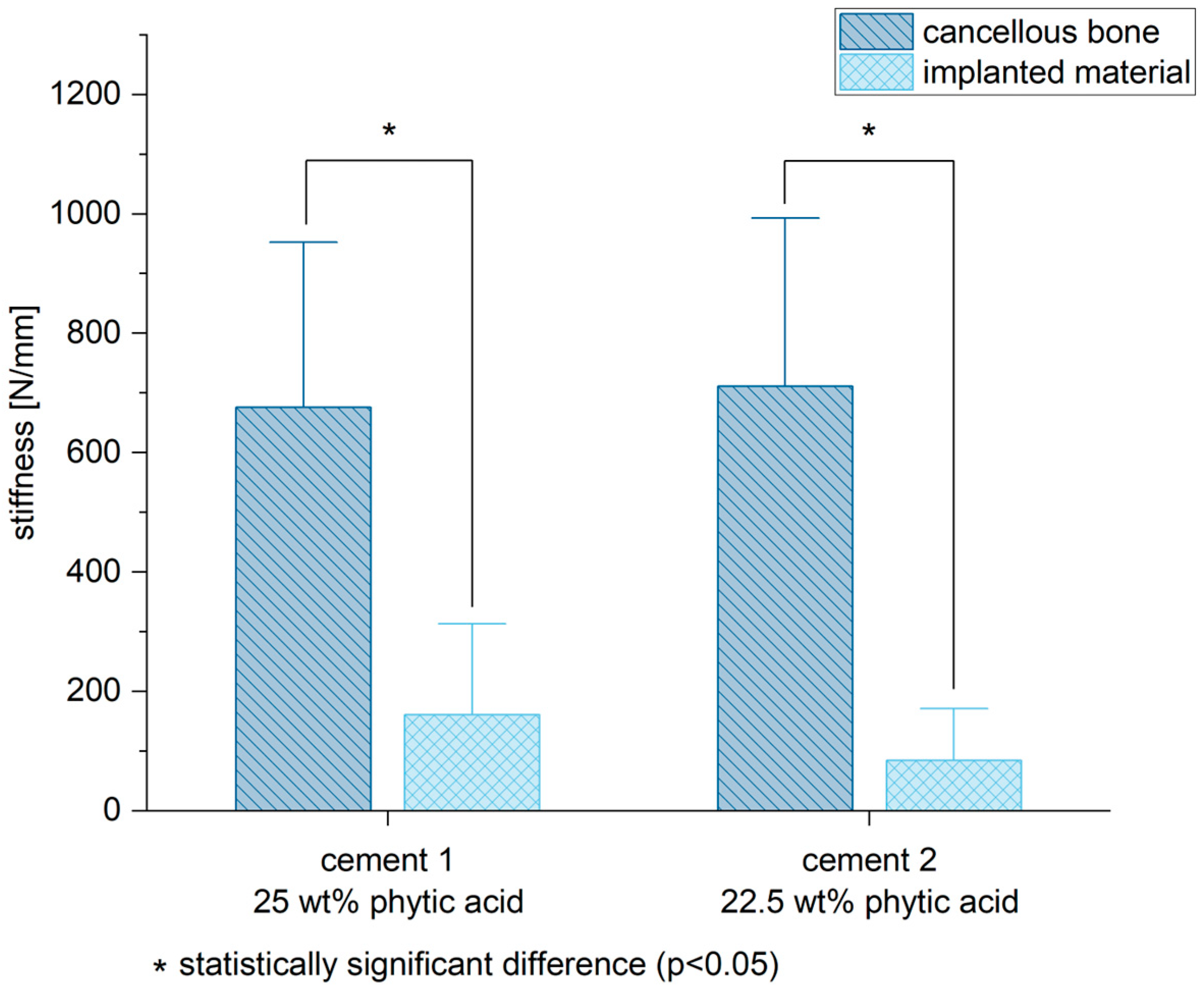
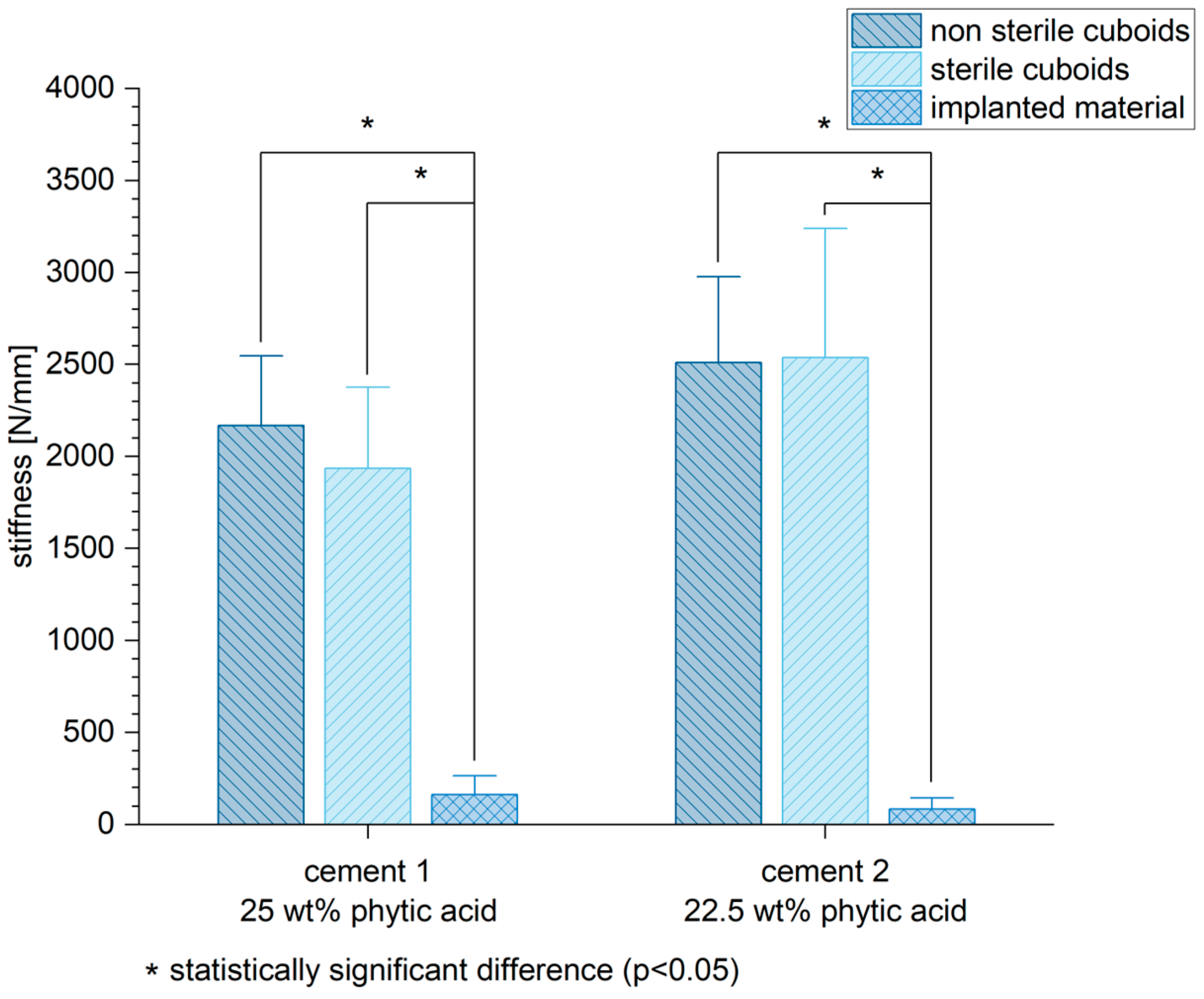
3.6. Biomechanical Stability
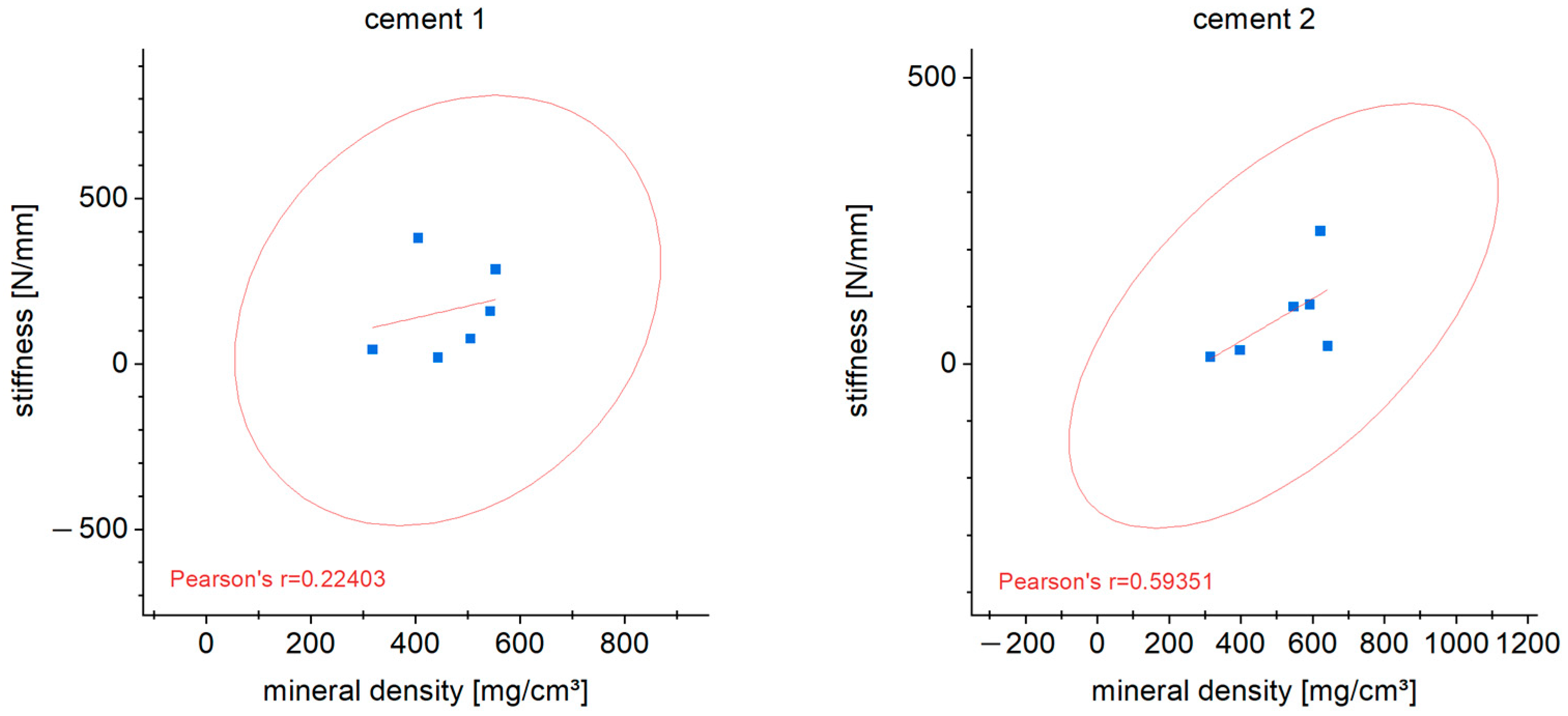
3.7. Correlation of Stiffness and Mineral Density
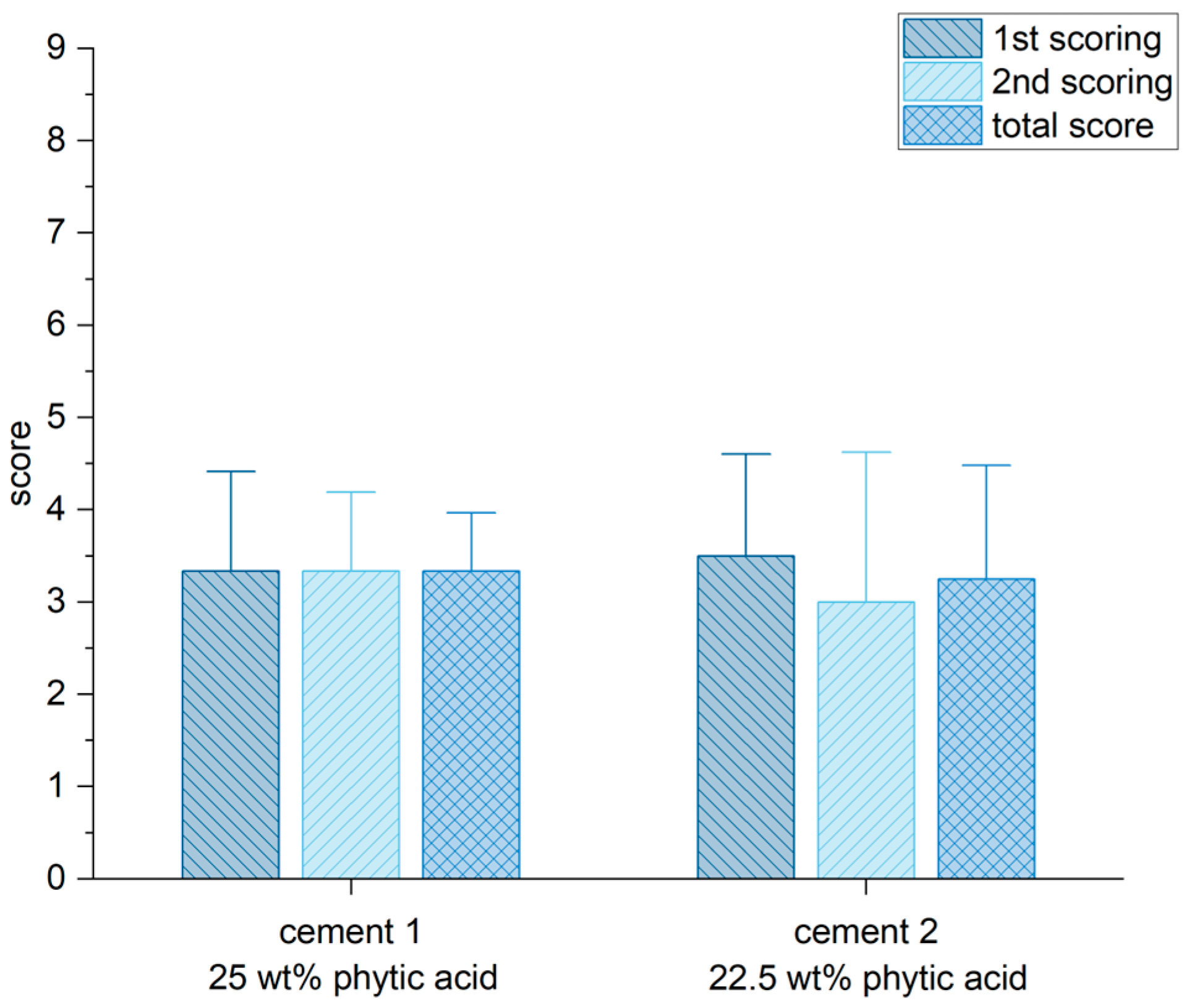
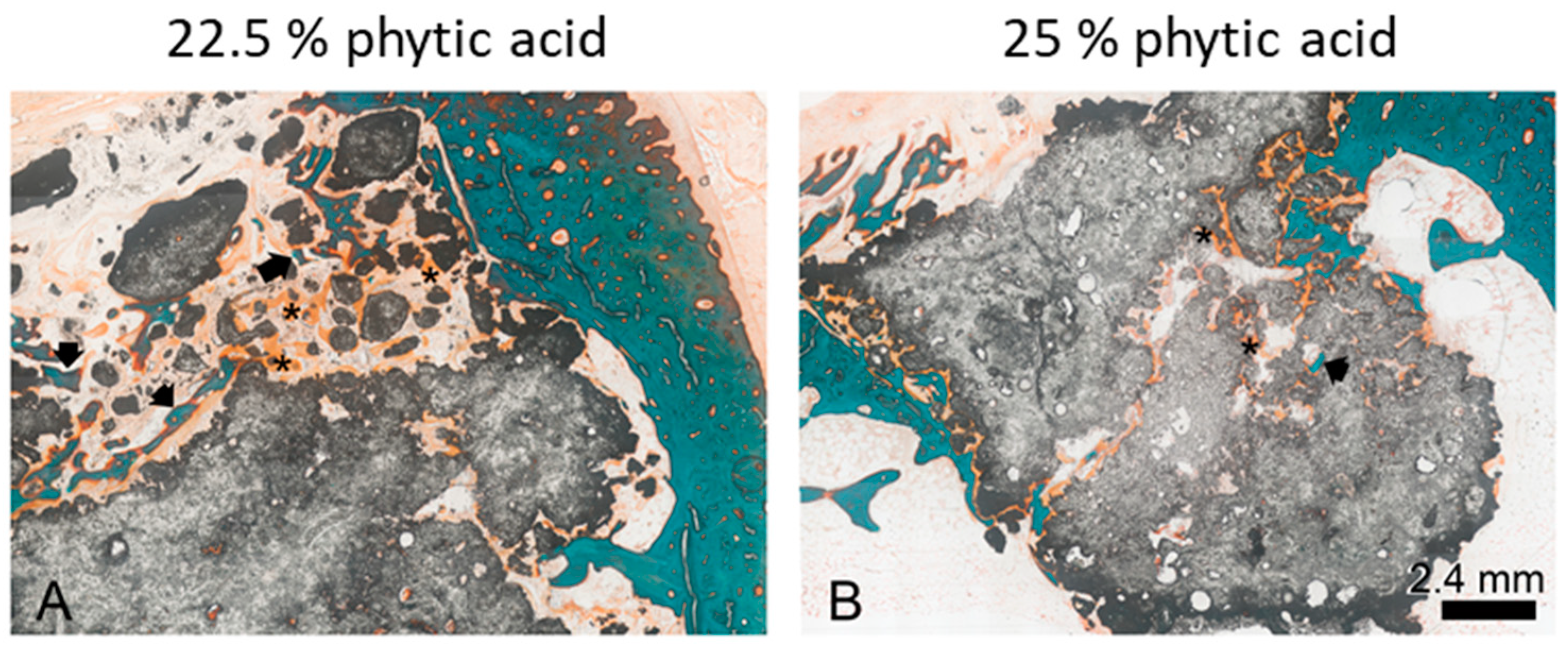
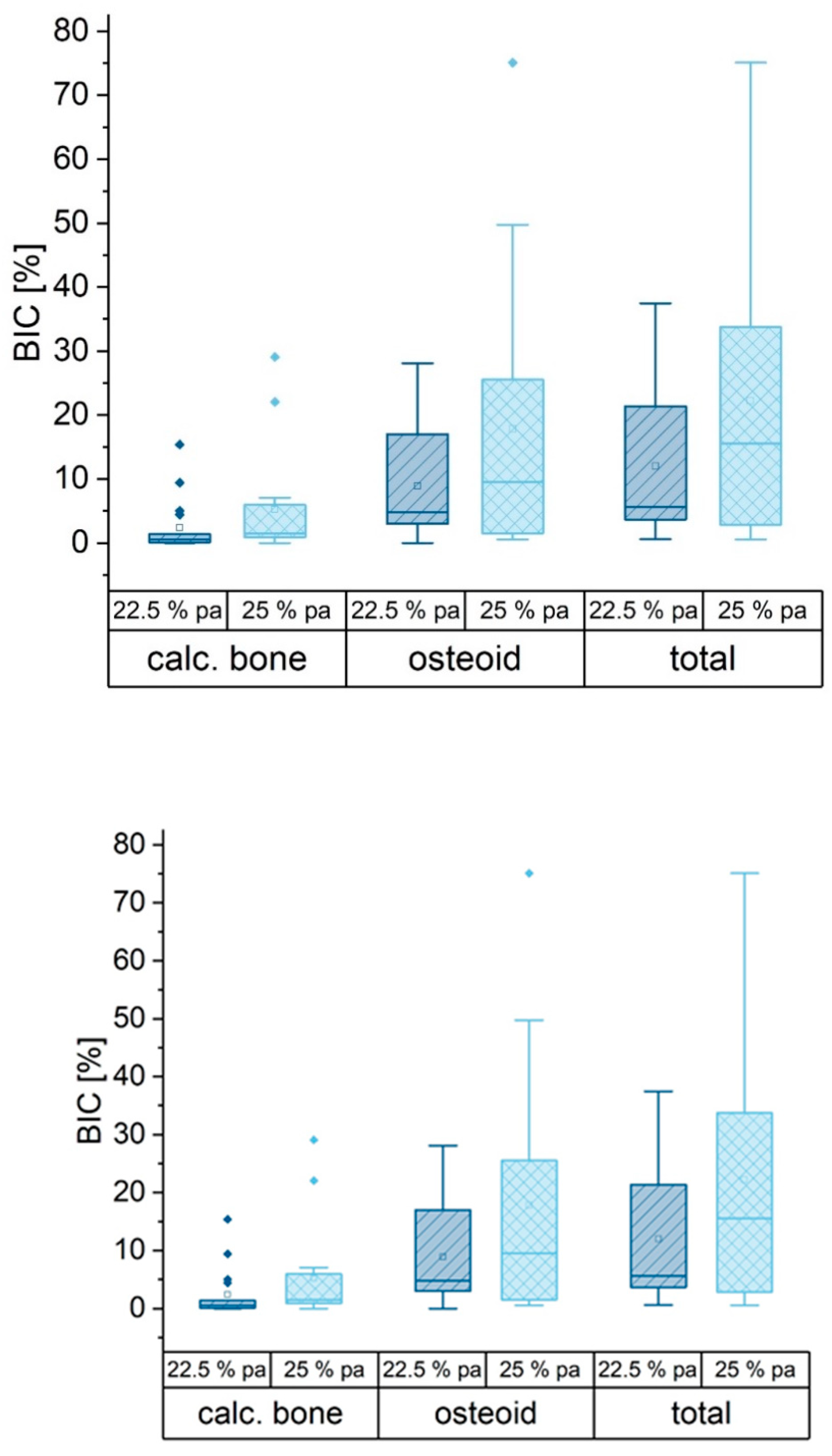
3.8. Histological Examination
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Bree, R.; Rinaldo, A.; Genden, E.M.; Suárez, C.; Rodrigo, J.P.; Fagan, J.J.; Kowalski, L.P.; Ferlito, A.; Leemans, C.R. Modern reconstruction techniques for oral and pharyngeal defects after tumor resection. Eur. Arch. Otorhinolaryngol. 2007, 265, 1–9. [Google Scholar] [CrossRef]
- Marecek, G.; Centomo, H. Augmented Fixation for Fractures of the Appendicular Skeleton. J. Am. Acad. Orthop. Surg. 2019, 27, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 82–91. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.P.; Hollinger, J.O.; Milam, S.B. Reconstruction of bone using calcium phosphate bone cements: A critical review. J. Oral Maxillofac. Surg. 1999, 57, 1122–1126. [Google Scholar] [CrossRef]
- Hoelscher-Doht, M.C.S.; Jordan, C.; Bonhoff, S.; Frey, T.; Blunk, R.H.M. Bone substitute first or screws first? A biome-chanical comparison of two operative techniques for tibial-head depression fractures. J. Orthop. Sci. 2014, 19, 978–983. [Google Scholar] [CrossRef]
- Heilig, P.; Sandner, P.; Jordan, M.C.; Jakubietz, R.G.; Meffert, R.H.; Gbureck, U.; Hoelscher-Doht, S. Experimental Drillable Magnesium Phosphate Cement Is a Promising Alternative to Conventional Bone Cements. Materials 2021, 14, 1925. [Google Scholar] [CrossRef] [PubMed]
- Demir-Oğuz, A.R.; Boccaccini, D.L. Injectable bone cements: What benefits the combination of calcium phosphates and bioactive glasses could bring? Bioact. Mater. 2023, 19, 217–236. [Google Scholar] [CrossRef]
- Wong, S.K.; Wong, Y.H.; Chin, K.-Y.; Ima-Nirwana, S. A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing. Polymers 2021, 13, 3075. [Google Scholar] [CrossRef]
- Apelt, D.; Theiss, F.; El-Warrak, A.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.; von Rechenberg, B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2003, 25, 1439–1451. [Google Scholar] [CrossRef]
- Ostrowski, N.; Roy, A.; Kumta, P.N. Magnesium Phosphate Cement Systems for Hard Tissue Applications: A Review. ACS Biomater. Sci. Eng. 2016, 2, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.B.; Lalzawmliana, V.; Saidivya, M.; Kumar, V.; Roy, M.; Nandi, S.K. Magnesium Phosphate Bioceramics for Bone Tissue Engineering. Chem. Rec. 2022, 22, e202200136. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Qi, C.; Cai, K. Biodegradable magnesium phosphates in biomedical applications. J. Mater. Chem. B 2022, 10, 2097–2112. [Google Scholar] [CrossRef]
- Brueckner, T.; Heilig, P.; Jordan, M.C.; Paul, M.M.; Blunk, T.; Meffert, R.H.; Gbureck, U.; Hoelscher-Doht, S. Biomechanical Evaluation of Promising Different Bone Substitutes in a Clinically Relevant Test Set-Up. Materials 2019, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, J.; Liu, C.; Zhang, B.; Chen, H.; Guo, H.; Zhong, G.; Qu, W.; Jiang, S.; Huang, H. Evaluation of inherent toxicology and biocompatibility of magnesium phosphate bone cement. Colloids Surf. B Biointerfaces 2010, 76, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Kanter, B.; Vikman, A.; Brückner, T.; Schamel, M.; Gbureck, U.; Ignatius, A. Bone regeneration capacity of magnesium phosphate cements in a large animal model. Acta Biomater. 2018, 69, 352–361. [Google Scholar] [CrossRef]
- Kazakova, G.; Safronova, T.; Golubchikov, D.; Shevtsova, O.; Rau, J.V. Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair. Materials 2021, 14, 4857. [Google Scholar] [CrossRef]
- Mestres, G.; Ginebra, M.-P. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011, 7, 1853–1861. [Google Scholar] [CrossRef]
- Christel, T.; Christ, S.; Barralet, J.E.; Groll, J.; Gbureck, U. Chelate Bonding Mechanism in a Novel Magnesium Phosphate Bone Cement. J. Am. Ceram. Soc. 2015, 98, 694–697. [Google Scholar] [CrossRef]
- Medvecky, L.; Štulajterová, R.; Giretova, M.; Luptakova, L.; Sopčák, T. Injectable Enzymatically Hardened Calcium Phosphate Biocement. J. Funct. Biomater. 2020, 11, 74. [Google Scholar] [CrossRef]
- Hurle, K.; Maia, F.; Ribeiro, V.; Pina, S.; Oliveira, J.; Goetz-Neunhoeffer, F.; Reis, R. Osteogenic lithium-doped brushite cements for bone regeneration. Bioact. Mater. 2021, 16, 403–417. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Chalikias, S.; Papaioannou, N.; Koundis, G.; Pappa, E.; Galanos, A.; Anastassopoulos, G.; Sarris, I.N.; Panteliou, S., Sr.; Chronopoulos, E.; Dontas, I.A. Evaluation of Femoral Bone Fracture Healing in Rats by the Modal Damping Factor and Its Correlation with Peripheral Quantitative Computed Tomography. Cureus 2021, 13, 13342. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, K.; Huflage, H.; Kunz, A.; Ritschl, L.; Herbst, M.; Kappler, S.; Ergün, S.; Goertz, L.; Pennig, L.; Bley, T.; et al. The effect of tin prefiltration on extremity cone-beam CT imaging with a twin robotic X-ray system. Radiography 2021, 28, 433–439. [Google Scholar] [CrossRef]
- Kovtun, M.J.A.; Goeckelmann, A.A.; Niclas, E.B.; Montufar, M.P.; Ginebra, J.A.; Planell, M.; Santin, A.I. In vivo performance of novel soybean/gelatin-based bioactive and injectable hydroxyapatite foams. Acta Biomater. 2015, 12, 242–249. [Google Scholar] [CrossRef]
- Donath, K. Die Trenn-Dünnschliff-Technik zur Herstellung histologischer Präparate von nicht schneidbaren Geweben und Materialien—Apparate und Methodenbeschreibung. In Kulzer Druckschrift; Electronic Arts GmbH, Ed.; Institut Universitaire de Pathologie: Hamburg, Germany, 1987. [Google Scholar]
- Goldner, J. A modification of the masson trichrome technique for routine laboratory purposes. Am. J. Pathol. 1938, 14, 237–243. [Google Scholar]
- Brückner, T.; Meininger, M.; Groll, J.; Kübler, A.C.; Gbureck, U. Magnesium Phosphate Cement as Mineral Bone Adhesive. Materials 2019, 12, 3819. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Kreczy, D.; Brückner, T.; Gbureck, U.; Stahlhut, P.; Bengel, M.; Hoess, A.; Nies, B.; Bator, J.; Klammert, U.; et al. Bone regeneration capacity of newly developed spherical magnesium phosphate cement granules. Clin. Oral Investig. 2021, 26, 2619–2633. [Google Scholar] [CrossRef] [PubMed]
- Heilig, P.; Jordan, M.C.; Paul, M.M.; Kupczyk, E.; Meffert, R.H.; Gbureck, U.; Hoelscher-Doht, S. Augmentation of suture anchors with magnesium phosphate cement—Simple technique with striking effect. J. Mech. Behav. Biomed. Mater. 2022, 128, 105096. [Google Scholar] [CrossRef]
- Stübinger, S.; Dard, M. The Rabbit as Experimental Model for Research in Implant Dentistry and Related Tissue Regeneration. J. Investig. Surg. 2013, 26, 266–282. [Google Scholar] [CrossRef]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]



| Cement 1 | Cement 2 | |
|---|---|---|
| Powder | 7.929 g Mg3(PO4)2 0.643 g MgO | 7.989 g Mg3(PO4)2 0.583 g MgO |
| Liquid | 25% IP 6 | 22.5% IP 6 |
| Resorption of the Bone Substitute | |
| None | 0 |
| Resorption of 0–25% of the material | 1 |
| Resorption of 25–50% of the material | 2 |
| Resorption of 50–75% of the material | 3 |
| Resorption of 75–100% of the material | 4 |
| Bone regeneration | |
| None | 0 |
| Evidence of bone regeneration | 1 |
| Radiological unity | |
| None | 0 |
| Possible unity | 1 |
| Radiological unity | 2 |
| Structural reconstruction | |
| None | 0 |
| Reconstruction in progress | 1 |
| Completed reconstruction | 2 |
| Maximum points | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ewald, A.; Fuchs, A.; Boegelein, L.; Grunz, J.-P.; Kneist, K.; Gbureck, U.; Hoelscher-Doht, S. Degradation and Bone-Contact Biocompatibility of Two Drillable Magnesium Phosphate Bone Cements in an In Vivo Rabbit Bone Defect Model. Materials 2023, 16, 4650. https://doi.org/10.3390/ma16134650
Ewald A, Fuchs A, Boegelein L, Grunz J-P, Kneist K, Gbureck U, Hoelscher-Doht S. Degradation and Bone-Contact Biocompatibility of Two Drillable Magnesium Phosphate Bone Cements in an In Vivo Rabbit Bone Defect Model. Materials. 2023; 16(13):4650. https://doi.org/10.3390/ma16134650
Chicago/Turabian StyleEwald, Andrea, Andreas Fuchs, Lasse Boegelein, Jan-Peter Grunz, Karl Kneist, Uwe Gbureck, and Stefanie Hoelscher-Doht. 2023. "Degradation and Bone-Contact Biocompatibility of Two Drillable Magnesium Phosphate Bone Cements in an In Vivo Rabbit Bone Defect Model" Materials 16, no. 13: 4650. https://doi.org/10.3390/ma16134650
APA StyleEwald, A., Fuchs, A., Boegelein, L., Grunz, J.-P., Kneist, K., Gbureck, U., & Hoelscher-Doht, S. (2023). Degradation and Bone-Contact Biocompatibility of Two Drillable Magnesium Phosphate Bone Cements in an In Vivo Rabbit Bone Defect Model. Materials, 16(13), 4650. https://doi.org/10.3390/ma16134650







