Low Temperature Nitriding of Metal Alloys for Surface Mechanical Performance
Abstract
1. Introduction
2. Nitriding with Plasma, Implantation and Gas
2.1. Plasma Nitriding
2.2. Implantation Nitriding
2.3. Gas Nitriding
2.4. Plasma Based Ion Implantation (PBII) Nitriding
3. Surface Mechanical Benefits
3.1. General Features and Scales
3.2. Elastic-Plastic Response and Wear
3.3. Surface Mechanical Performance
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le Bourhis, E. Glass Mechanics and Technology, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Borgioli, F. The “Expanded” Phases in the Low-Temperature Treated Stainless Steels: A Review. Metals 2022, 12, 331. [Google Scholar] [CrossRef]
- Kamat, A.M.; Copley, S.M.; Segall, A.E.; Todd, J.A. Laser-Sustained Plasma (LSP) Nitriding of Titanium: A Review. Coatings 2019, 9, 283. [Google Scholar] [CrossRef]
- Czerviec, T.; Michel, H.; Bergmann, E. Low-pressure, high-density plasma nitriding: Mechanisms, technology and results. Surf. Coat. Technol. 1998, 182, 108–109. [Google Scholar]
- Yan, H.; Zhao, L.; Chen, Z.; Hu, X.; Yan, Z. Investigation of the Surface Properties and Wear Properties of AISI H11 Steel Treated by Auxiliary Heating Plasma Nitriding. Coatings 2020, 10, 528. [Google Scholar] [CrossRef]
- Dong, H. S-phase surface engineering of Fe-Cr, Co-Cr and Ni-Cr alloys. Int. Mater. Rev. 2010, 55, 65–98. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Akbari, A.; Drouet, M. Microstructure and wear properties of AISI M2 tool steel on RF plasma nitriding at different N 2 –H 2 gas compositions. Surf. Coat. Technol. 2014, 258, 566–573. [Google Scholar] [CrossRef]
- Gammeltoft-Hansen, N.; Munch, S.S.; Jellesen, M.S.; Somers, M.A.J.; Christiansen, T.L. Characterization of Thermochemically Surface-Hardened Titanium by Light Optical Microscopy. Mater. Perform. Charact. 2017, 6, 298–310. [Google Scholar] [CrossRef]
- Fouquet, V.; Pichon, L.; Straboni, A.; Drouet, M. Nitridation of Ti6Al4V by PBII: Study of the nitrogen diffusion and of the nitride growth mechanism. Surf. Coat. Technol. 2004, 186, 34–39. [Google Scholar] [CrossRef]
- Silva, M.; Pichon, L.; Drouet, M.; Otubo, J. Roughness studies of NiTi shape memory alloy treated by nitrogen plasma based ion implantation at high temperatures. Surf. Coat. Technol. 2012, 211, 209–212. [Google Scholar] [CrossRef]
- Miyamoto, J.; Inoue, T.; Tokuno, K.; Tsutamori, H.; Abraha, P. Surface Modification of Tool Steel by Atmospheric-Pressure Plasma Nitriding Using Dielectric Barrier Discharge. Tribol. Online 2016, 11, 460–465. [Google Scholar] [CrossRef]
- Brading, H.J.; Morton, P.H.; Bell, T.; Earwaker, L.G. The structure and composition of plasma nitrided coatings on titanium. Nucl. Instrum. Methods Phys. Res. B 1992, 66, 230–236. [Google Scholar] [CrossRef]
- Yang, S.; Kitchen, M.; Luo, Q.; Ievlev, D.; Cooke, K. Effect of Nitriding Time on the Structural Evolution and Properties of Austenitic Stainless Steel Nitrided Using High Power Pulsed DC Glow Discharge Ar/N2 Plasma. J. Coat. Sci. Technol. 2016, 3, 62–74. [Google Scholar] [CrossRef]
- Czerwiec, T.; Greer, F.; Graves, D.B. Nitrogen dissociation in a low pressure cylindrical ICP discharge studied by actinometry and mass spectrometry. J. Phys. D Appl. Phys. 2005, 38, 4278–4289. [Google Scholar] [CrossRef]
- Ricard, A.; Czerwiec, T.; Belmonte, T.; Bockel, S.; Michel, H. Detection by emission spectroscopy of active species in plasma–surface processes. Thin Solid Films 1999, 341, 1–8. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Nakao, S.; Ikeyama, M.; Saitoh, K.; Miyagawa, S. High fluence implantation of nitrogen into titanium: Fluence dependence of sputtering yield, retained fluence and nitrogen depth profile. Nucl. Instrum. Methods Phys. Res. B 1997, 12, 340–344. [Google Scholar] [CrossRef]
- Schneider, R.S.E.; Hiebler, H. Influence of increased nitriding temperatures on the hardness profile of low-alloy steels. J. Mater. Sci. 1998, 33, 1737–1744. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Somers, M.A.J. Low-temperature gaseous surface hardening of stainless steel: The current status. Int. J. Mater. Res. 2009, 100, 1361–1377. [Google Scholar] [CrossRef]
- Nam, N.D.; Xuan, N.A.; Van Bach, N.; Nhung, L.T.; Chieu, L.T. Control gas nitriding process: A review. J. Mech. Eng. Res. Dev. 2019, 42, 17–25. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A. On the crystallographic structure of S-phase. Scr. Mater. 2004, 50, 35–37. [Google Scholar] [CrossRef]
- Conrad, J.R.; Radtke, J.L.; Dodd, R.A.; Worzala, F.J.; Tran, N.C. Plasma source ion-implantation technique for surface modification of materials. J. Appl. Phys. 1987, 62, 4591–4596. [Google Scholar] [CrossRef]
- Collins, G.; Hutchings, R.; Short, K.; Tendys, J.; Van Der Valk, C. Development of a plasma immersion ion implanter for the surface treatment of metal components. Surf. Coat. Technol. 1996, 84, 537–543. [Google Scholar] [CrossRef]
- Marot, L.; Straboni, A.; Drouet, M. A new thermally assisted, plasma based, ionic implantation system of treatment and deposition. Surf. Coat. Technol. 2001, 142–144, 384–387. [Google Scholar] [CrossRef]
- Marot, L.; Drouet, M.; Berneau, F.; Straboni, A. High temperature plasma based ionic implantation of titanium alloys and silicon. Surf. Coat. Technol. 2002, 156, 155–158. [Google Scholar] [CrossRef]
- Fouquet, V.; Bourhis, L.; Pichon, L.; Drouet, M.; Straboni, A. Elastic–plastic resistance profile of PBII nitrided titanium. Scr. Mater. 2004, 51, 899–903. [Google Scholar] [CrossRef]
- Parry, V.; Le Bourhis, E.; Pichon, L.; Drouet, M. Relation between Mechanical Hardening and Nitrogen Profile of PBII Nitrided Titanium Alloy. Materials 2022, 15, 9028. [Google Scholar] [CrossRef]
- Peripolli, S.; Amaral, L.; Oliviero, E.; David, M.; Beaufort, M.; Barbot, J.; Pichon, L.; Drouet, M.; Fichtner, P.; Donnelly, S. Formation of neon induced cavities in silicon by plasma based ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2006, 249, 193–195. [Google Scholar] [CrossRef]
- David, M.-L.; Barbot, J.F.; Rousselet, S.; Pailloux, F.; Babonneau, D.; Beaufort, M.-F.; Pizzagalli, L.; Drouet, M.; Simoen, E.R.; Claeys, C. Extended Defects Created by Light Ion Implantation in Ge. ECS Trans. 2008, 16, 163–175. [Google Scholar] [CrossRef]
- Marot, L.; Pichon, L.; Drouet, M.; Straboni, A. Improved nitrogen transport in Fe–C alloys during NH3 plasma nitridation. Mater. Lett. 2000, 44, 35–38. [Google Scholar] [CrossRef]
- Zagonel, L.; Figueroa, C.; Droppa, R.; Alvarez, F. Influence of the process temperature on the steel microstructure and hardening in pulsed plasma nitriding. Surf. Coat. Technol. 2006, 201, 452–457. [Google Scholar] [CrossRef]
- Ruset, C.; Ciuca, S.; Grigore, E. The influence of the sputtering process on the constitution of the compound layers obtained by plasma nitriding. Surf. Coat. Technol. 2003, 174–175, 1201–1205. [Google Scholar] [CrossRef]
- Mandl, S.; Scholze, F.; Neumann, H.; Rauschenbach, B. Nitrogen diffusivity in expanded austenite. Surf. Coat. Technol. 2003, 174–175, 1191–1195. [Google Scholar] [CrossRef]
- El-Hossary, F.; Negm, N.; El-Rahman, A.A.; Hammad, M. Duplex treatment of 304 AISI stainless steel using rf plasma nitriding and carbonitriding. Mater. Sci. Eng. C 2009, 29, 1167–1173. [Google Scholar] [CrossRef]
- Martinavičius, A.; Abrasonis, G.; Möller, W.; Templier, C.; Rivière, J.P.; Declémy, A.; Chumlyakov, Y. Anisotropic ion-enhanced diffusion during ion nitriding of single crystalline austenitic stainless steel. J. Appl. Phys. 2009, 105, 093502. [Google Scholar] [CrossRef]
- Chollet, S.; Pichon, L.; Cormier, J.; Dubois, J.; Villechaise, P.; Drouet, M.; Declemy, A.; Templier, C. Plasma assisted nitriding of Ni-based superalloys with various microstructures. Surf. Coat. Technol. 2013, 235, 318–325. [Google Scholar] [CrossRef]
- Leroy, C.; Czerwiec, T.; Gabet, C.; Belmonte, T.; Michel, H. Plasma assisted nitriding of Inconel 690. Surf. Coat. Technol. 2001, 142–144, 241–247. [Google Scholar] [CrossRef]
- Möller, W.; Parascandola, S.; Telbizova, T.; Günzel, R.; Richter, E. Surface processes and diffusion mechanisms of ion nitriding of stainless steel and aluminium. Surf. Coat. Technol. 2001, 136, 73–79. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Dahl, K.; Somers, M.A. Simulation of Nitrogen Concentration Depth Profiles in Low Temperature Nitrided Stainless Steel. Defect Diffus. Forum 2006, 258–260, 378–383. [Google Scholar] [CrossRef]
- Zhecheva, A.; Sha, W.; Malinov, S.; Long, A. Enhancing the microstructure and properties of titanium alloys through nitriding and other surface engineering methods. Surf. Coat. Technol. 2005, 200, 2192–2207. [Google Scholar] [CrossRef]
- Gredelj, S.; Kumar, S.; Gerson, A.R.; Cavallaro, G.P. Radio frequency plasma nitriding of aluminium at higher power levels. Thin Solid Films 2006, 515, 1480–1485. [Google Scholar] [CrossRef]
- Berberich, F.; Matz, W.; Kreissig, U.; Richter, E.; Schell, N.; Möller, W. Structural characterisation of hardening of Ti–Al–V alloys after nitridation by plasma immersion ion implantation. Appl. Surf. Sci. 2001, 179, 13–19. [Google Scholar] [CrossRef]
- Drouet, M.; Pichon, L.; Dubois, J.; Le Bourhis, E.; Christiansen, T.L. Surface engineering of titanium by multi-interstitial diffusion using plasma processing. MATEC Web Conf. 2020, 321, 11010. [Google Scholar] [CrossRef]
- Drouet, M.; Pichon, L.; Vallet, Y.; Le Bourhis, E.; Christiansen, T.L. Surface engineering of titanium alloy TiAl6V4 by multi-interstitial diffusion using plasma processing. Eur. J. Mater. 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Geng, Y.; McCarthy, E.; Brabazon, D.; Harrison, N. Ti6Al4V functionally graded material via high power and high speed laser surface modification. Surf. Coat. Technol. 2020, 398, 126085. [Google Scholar] [CrossRef]
- Stinville, J.C.; Templier, C.; Villechaise, P.; Pichon, L. Swelling of 316L austenitic stainless steel induced by plasma nitriding. J. Mater. Sci. 2011, 46, 5503–5511. [Google Scholar] [CrossRef]
- Stinville, J.; Cormier, J.; Templier, C.; Villechaise, P. Modeling of the lattice rotations induced by plasma nitriding of 316L polycrystalline stainless steel. Acta Mater. 2015, 83, 10–16. [Google Scholar] [CrossRef]
- Tromas, C.; Stinville, J.; Templier, C.; Villechaise, P. Hardness and elastic modulus gradients in plasma-nitrided 316L polycrystalline stainless steel investigated by nanoindentation tomography. Acta Mater. 2012, 60, 1965–1973. [Google Scholar] [CrossRef]
- Marot, L.; Le Bourhis, E.; Straboni, A. Improved nitridation efficiency and mechanical property of stainless steel surface after N2–H2 plasma nitridation at low temperature. Mater. Lett. 2002, 56, 76–79. [Google Scholar] [CrossRef]
- Stratton, P.; Graf, M. Wear of diffusion hardened Ti–6Al–4V sliding against tool steel. Wear 2010, 268, 612–616. [Google Scholar] [CrossRef]
- Gil, F.J.; Canedo, R.; Padrós, A.; Sada, E. Enhanced Wear Resistance of Ball-And-Socket Joints of Dental Implants by Means of Titanium Gaseous Nitriding. J. Biomater. Appl. 2002, 17, 31–43. [Google Scholar] [CrossRef]
- Basu, A.; Majumdar, J.D.; Alphonsa, J.; Mukherjee, S.; Manna, I. Corrosion resistance improvement of high carbon low alloy steel by plasma nitriding. Mater. Lett. 2008, 62, 3117–3120. [Google Scholar] [CrossRef]
- Stinville, J.; Tromas, C.; Villechaise, P.; Templier, C. Anisotropy changes in hardness and indentation modulus induced by plasma nitriding of 316L polycrystalline stainless steel. Scr. Mater. 2011, 64, 37–40. [Google Scholar] [CrossRef]
- de Ara, J.F.; Almandoz, E.; Palacio, J.; Fuentes, G. Simultaneous ageing and plasma nitriding of grade 300 maraging steel: How working pressure determines the effective nitrogen diffusion into narrow cavities. Surf. Coat. Technol. 2017, 317, 64–74. [Google Scholar] [CrossRef]
- Kücükyildiz, O.C.; Grumsen, F.B.; Christiansen, T.L.; Winther, G.; Somers, M.A. Anisotropy effects on gaseous nitriding of austenitic stainless steel single crystals. Acta Mater. 2020, 194, 168–177. [Google Scholar] [CrossRef]
- Sun, Y.; Bell, T.; Wood, G. Wear behaviour of plasma-nitrided martensitic stainless steel. Wear 1994, 178, 131–138. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, S.; Guagliano, M. Microstructural evolution during nitriding, finite element simulation and experimental assessment. Appl. Surf. Sci. 2013, 271, 156–163. [Google Scholar] [CrossRef]
- Frączek, T.; Prusak, R.; Ogórek, M.; Skuza, Z. Nitriding of 316L Steel in a Glow Discharge Plasma. Materials 2022, 15, 3081. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Surface Modification of a Nickel-Free Austenitic Stainless Steel by Low-Temperature Nitriding. Metals 2021, 11, 1845. [Google Scholar] [CrossRef]
- He, H.; Czerwiec, T.; Dong, C.; Michel, H. Effect of grain orientation on the nitriding rate of a nickel base alloy studied by electron backscatter diffraction. Surf. Coat. Technol. 2003, 163–164, 331–338. [Google Scholar] [CrossRef]
- Scheuer, C.R.J.; Cardoso, R.P.; Brunatto, S.F. Sequential low-temperature plasma-assisted thermochemical treatments of the AISI 420 martensitic stainless steel. Surf. Coat. Technol. 2021, 421, 127459. [Google Scholar] [CrossRef]
- Mahboubi, F.; Samandi, M.; Dunne, D.; Bloyce, A.; Bell, T. Plasma nitriding of microalloyed steel. Surf. Coat. Technol. 1995, 71, 135–141. [Google Scholar] [CrossRef]
- Kværndrup, F.B.; Kücükyildiz, Ö.C.; Winther, G.; Somers, M.A.; Christiansen, T.L. Extreme hardening of titanium with colossal interstitial contents of nitrogen and oxygen. Mater. Sci. Eng. A 2021, 813, 141033. [Google Scholar] [CrossRef]
- Li, L.; Liu, R.; Liu, Q.; Wu, Z.; Meng, X.; Fang, Y. Effects of Initial Microstructure on the Low-Temperature Plasma Nitriding of Ferritic Stainless Steel. Coatings 2022, 12, 1404. [Google Scholar] [CrossRef]
- Sun, Y.; Bailey, R. Comparison of Wear Performance of Low Temperature Nitrided and Carburized 316L Stainless Steel under Dry Sliding and Corrosive-Wear Conditions. J. Mater. Eng. Perform. 2022, 32, 1238–1247. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Hu, Y.; Luo, L.; Jiang, C.; Liu, F.; Jin, W.; Zhu, K.; Long, Z.; Liu, K. Effect of hydrogen on microstructure and mechanical properties of plasma-nitrided pure titanium by cathodic cage plasma nitriding. Surf. Coat. Technol. 2023, 456, 129231. [Google Scholar] [CrossRef]
- Manova, D.; Mändl, S. Initial phase formation during nitriding of austenitic stainless steel. Surf. Coat. Technol. 2023, 456, 129258. [Google Scholar] [CrossRef]
- Silva, L.G.L.; Naeem, M.; Costa, T.H.C.; Libório, M.S.; Bandeira, R.M.; Ferreira, N.S.; Rossino, L.S.; Júnior, C.A.A.; Queiroz, J.C.A.; Neto, J.F.M.; et al. Wear and Corrosion of UNS S32750 Steel Subjected to Nitriding and Cathodic Cage Deposition. J. Mater. Eng. Perform. 2023. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, M.; Zhang, Z.; Yi, X.; Yan, J.; Zhou, Z.; Fang, D.; He, Y.; Li, Y. Corrosion Behavior of Nitrided Layer of Ti6Al4V Titanium Alloy by Hollow Cathodic Plasma Source Nitriding. Materials 2023, 16, 2961. [Google Scholar] [CrossRef]
- Ozturk, M.; Husem, F.; Karademir, I.; Maleki, E.; Amanov, A.; Unal, O. Fatigue crack growth rate of AISI 4140 low alloy steel treated via shot peening and plasma nitriding. Vacuum 2023, 207, 111552. [Google Scholar] [CrossRef]
- Niu, J.; Qureshi, M.W.; Ding, Z.; Ma, X.; He, Y. Effect of nitriding on the transformation of alloy carbides (VC and Mo2C) in 8Cr4Mo4V steel. Appl. Surf. Sci. 2023, 610, 155561. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L.; Sun, J. Characteristics and properties of Cr N compound layer produced by plasma nitriding of Cr-electroplated of AISI 304 stainless steel. Surf. Coat. Technol. 2020, 385, 125450. [Google Scholar] [CrossRef]
- Akbari, A.; Riviere, J.P.; Templier, C.; Le Bourhis, E. Structural and mechanical properties of IBAD deposited nanocomposite Ti–Ni–N coatings. Surf. Coat. Technol. 2006, 200, 6298–6302. [Google Scholar] [CrossRef]
- Özkan, D.; Yilmaz, M.A.; Karakurt, D.; Szala, M.; Walczak, M.; Bakdemir, S.A.; Türküz, C.; Sulukan, E. Effect of AISI H13 Steel Substrate Nitriding on AlCrN, ZrN, TiSiN, and TiCrN Multilayer PVD Coatings Wear and Friction Behaviors at a Different Temperature Level. Materials 2023, 16, 1594. [Google Scholar] [CrossRef]
- Wu, J.; Mao, C.; Wei, K.; Hu, J. Titanium-modified plasma nitriding layer and enhanced properties for 42CrMo steel. J. Mater. Res. Technol. 2022, 18, 3819–3825. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Jellesen, M.S.; Somers, M.A.J. Future trends in gaseous surface hardening of titanium and titanium alloys. La Metall. Ital. 2018, 9, 13–22. [Google Scholar]
- Mahboubi, F.; Fa, M. Duplex treatment of plasma nitriding and plasma oxidation of plain carbon steel. Vacuum 2005, 79, 1–6. [Google Scholar] [CrossRef]
- Le Bourhis, E.; Patriarche, G. TEM-nanoindentation studies of semiconducting structures. Micron 2007, 38, 377–389. [Google Scholar] [CrossRef]
- Czyrska-Filemonowicz, A.; Buffat, P.; Łucki, M.; Moskalewicz, T.; Rakowski, W.; Wierzchoń, T. Transmission electron microscopy and atomic force microscopy characterisation of titanium-base alloys nitrided under glow discharge. Acta Mater. 2005, 53, 4367–4377. [Google Scholar] [CrossRef]
- Jung, K.; Schacherl, R.; Bischoff, E.; Mittemeijer, E. Nitriding of ferritic Fe–Cr–Al alloys. Surf. Coat. Technol. 2010, 204, 1942–1946. [Google Scholar] [CrossRef]
- Bianco, M.; Poitel, S.; Hong, J.-E.; Yang, S.; Wang, Z.-J.; Willinger, M.; Steinberger-Wilckens, R.; Van Herle, J. Corrosion behaviour of nitrided ferritic stainless steels for use in solid oxide fuel cell devices. Corros. Sci. 2020, 165, 108414. [Google Scholar] [CrossRef]
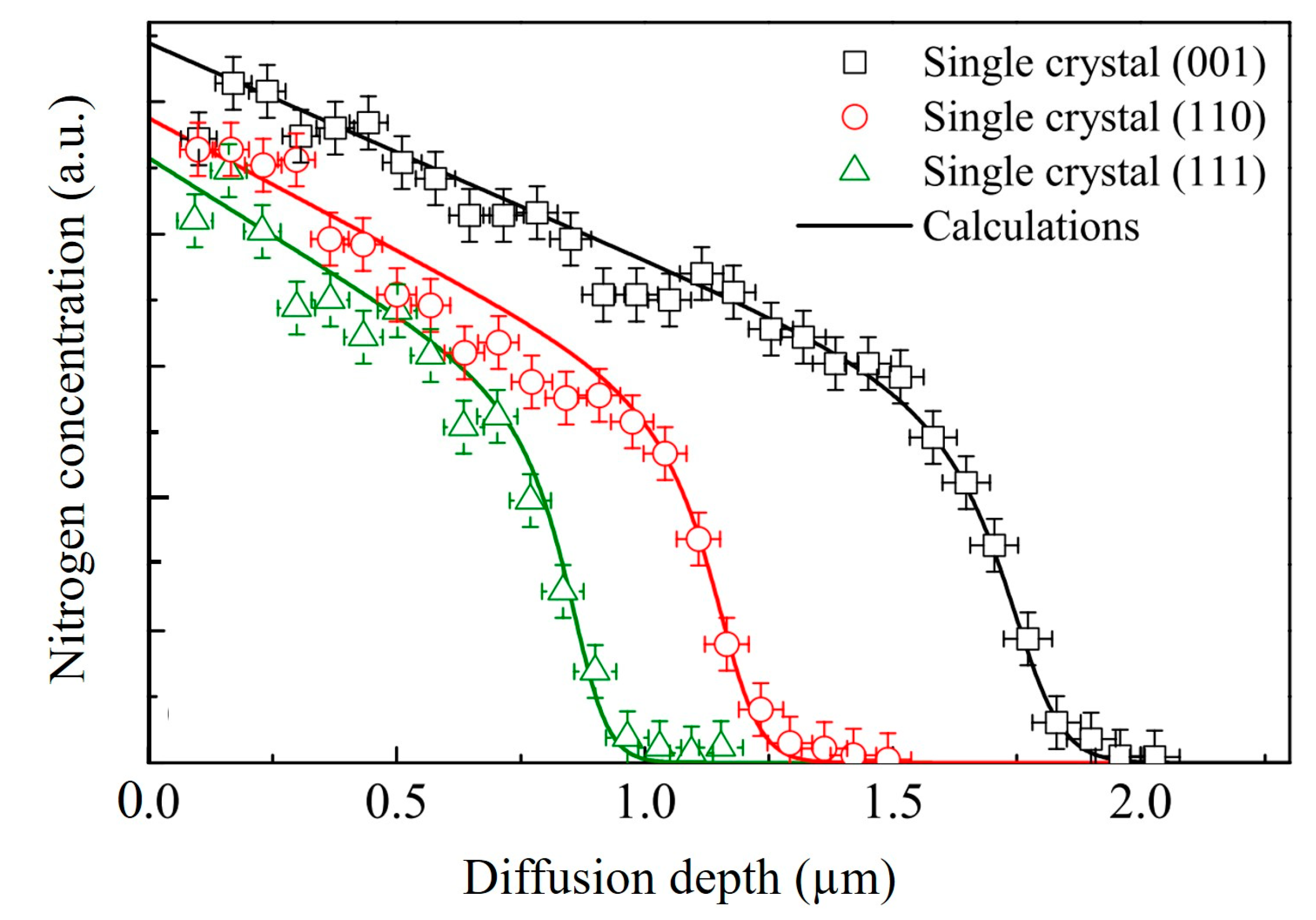
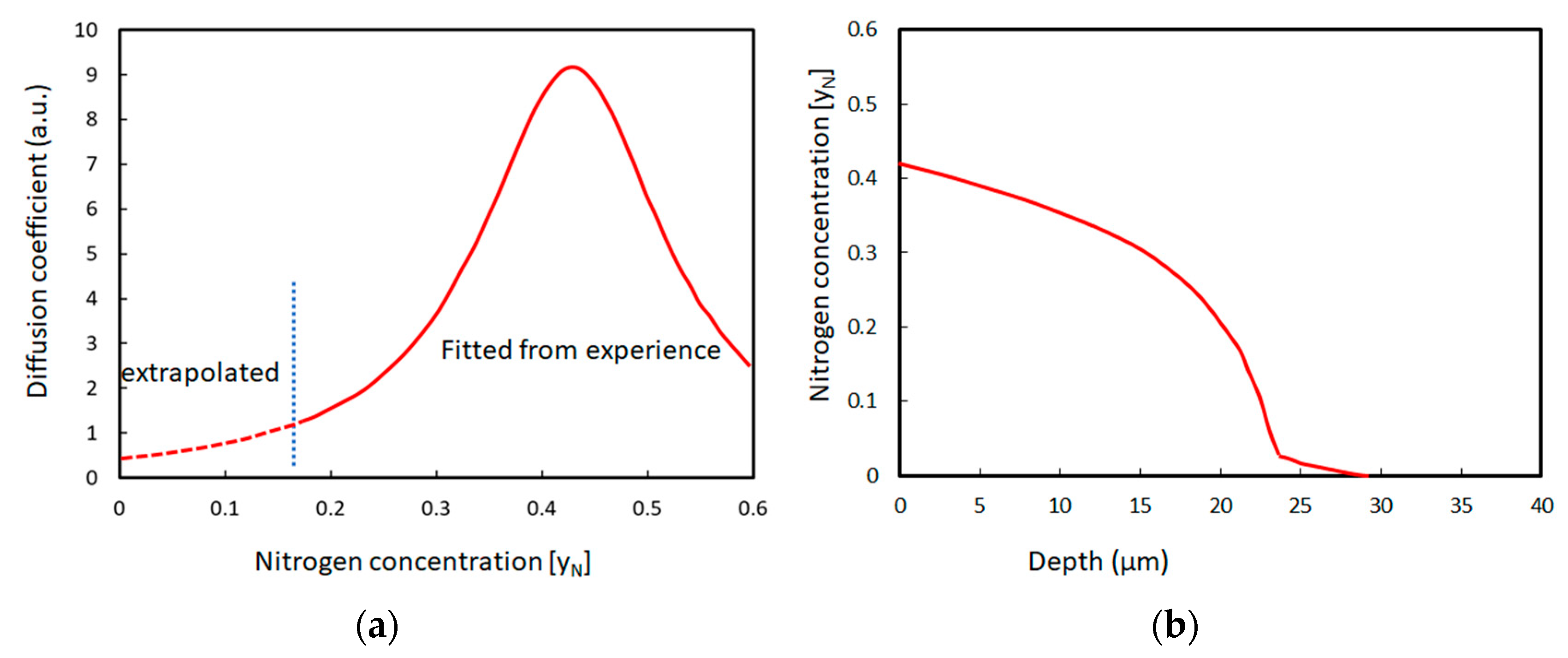
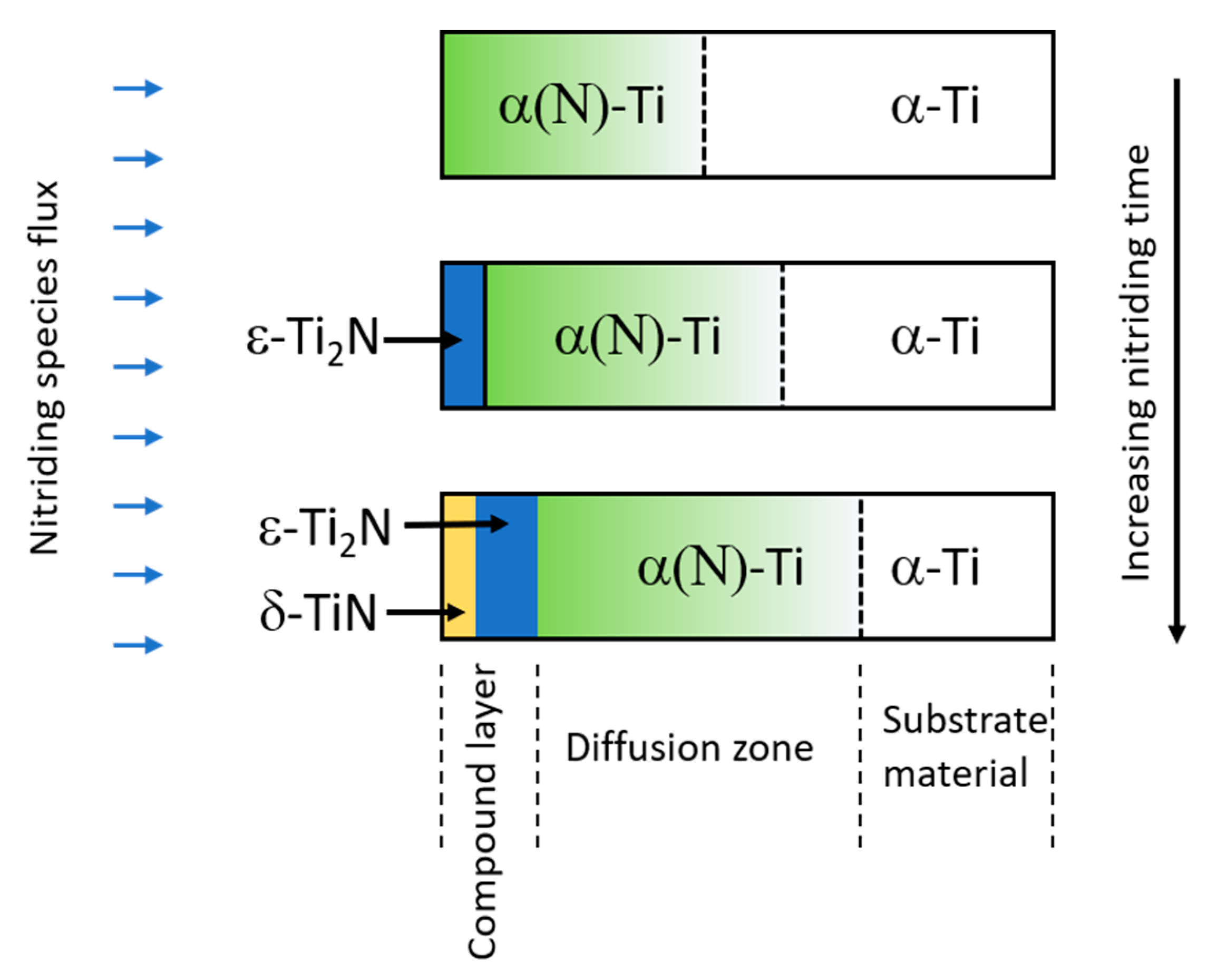
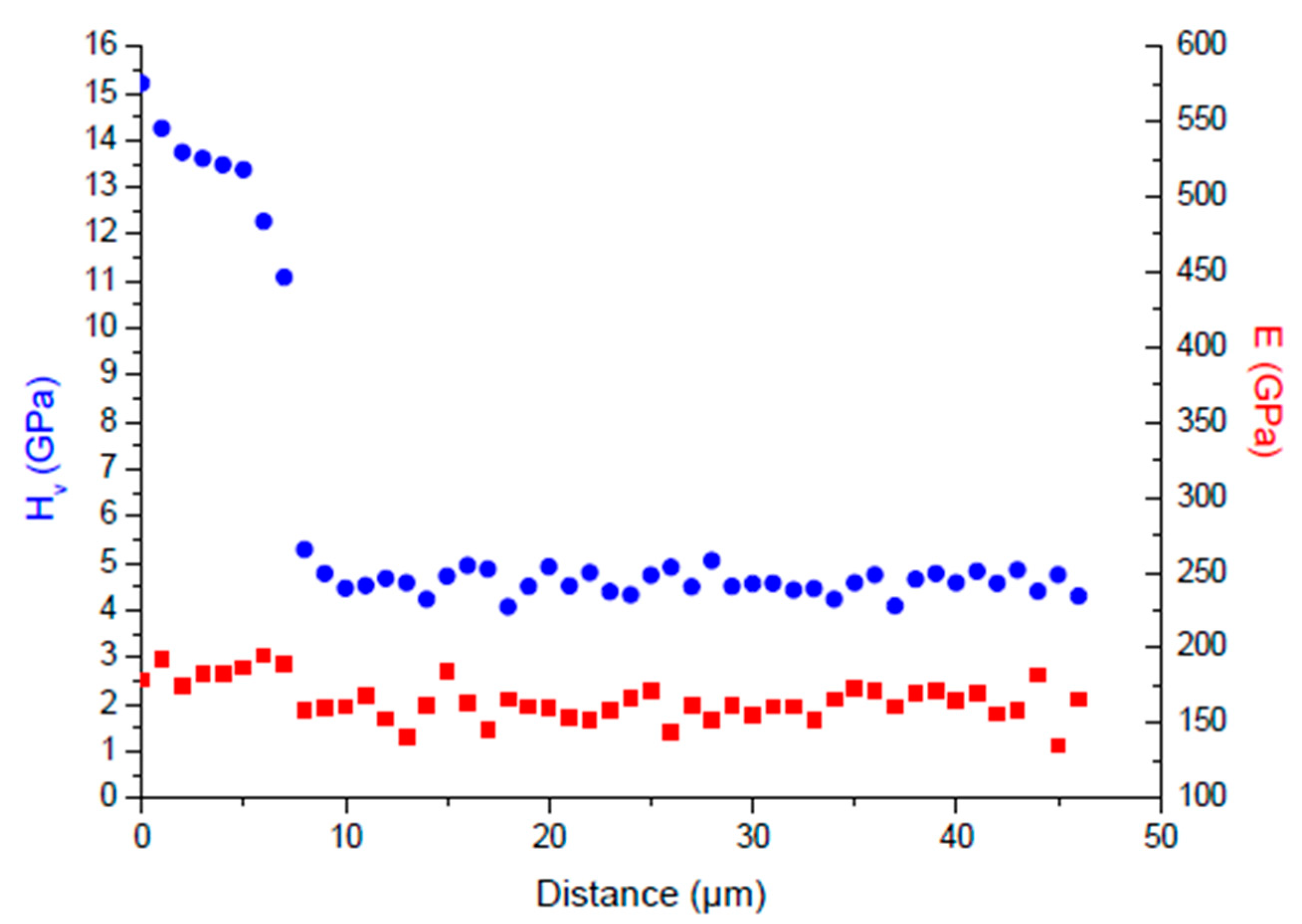
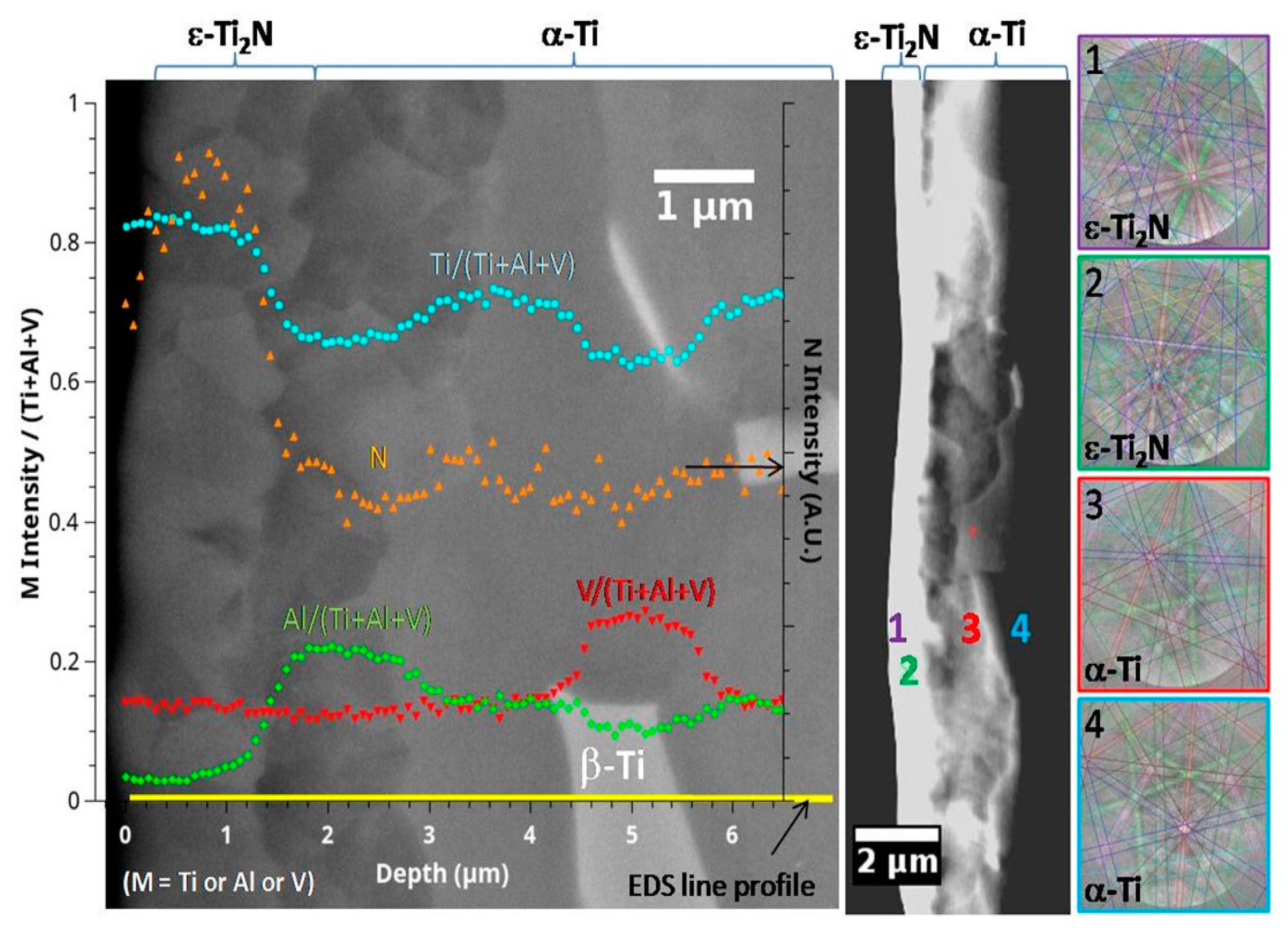
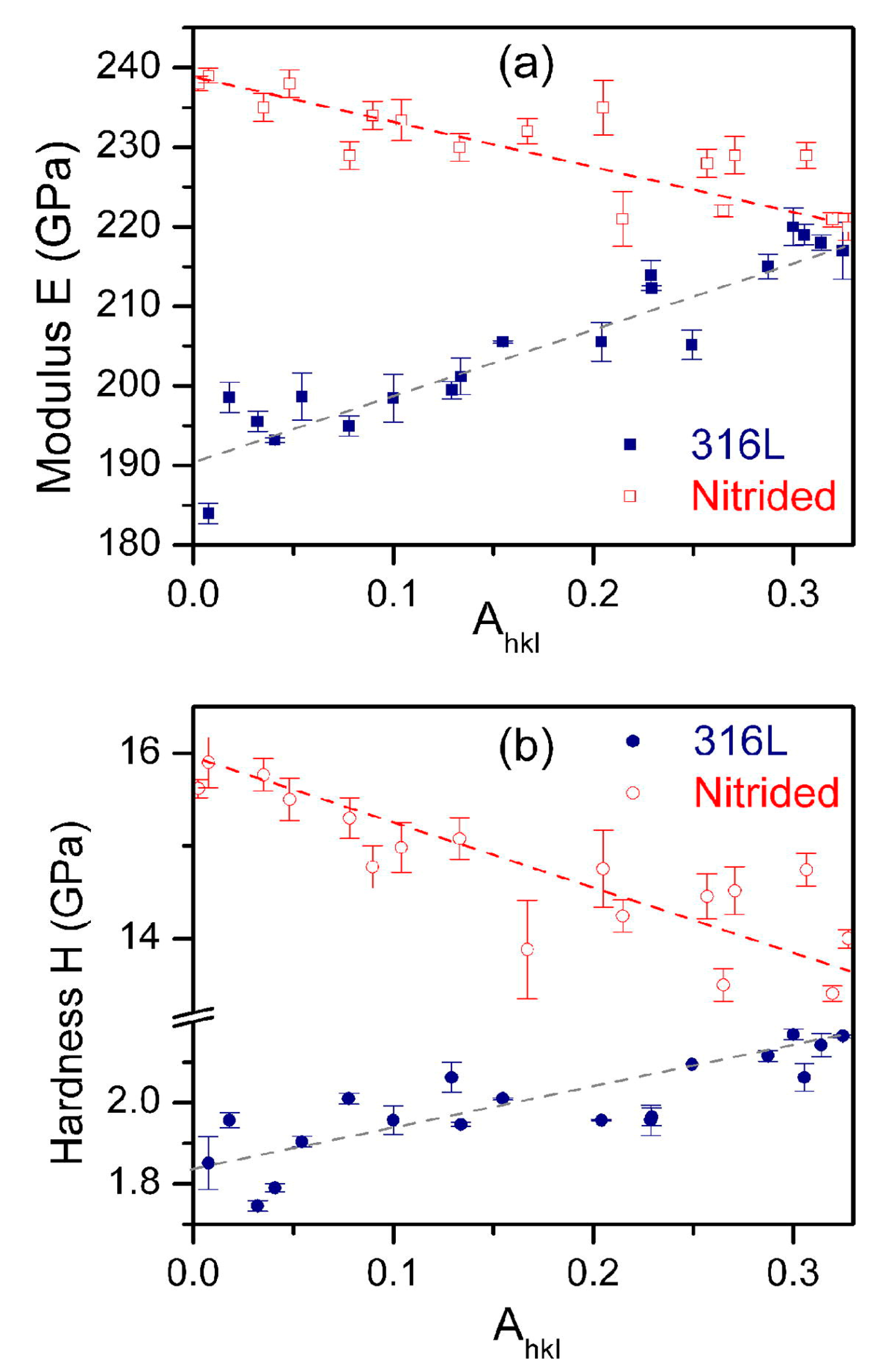
| Technique | Advantage | Drawback | Notes |
|---|---|---|---|
| Plasma | Moderate temperature, some complexity of the shape acceptable: lower gas consumption and less waste | High l/d ratio holes not well treated, sputtering, possible edge effects | |
| Implantation | Low temperature, overcomes surface barriers: extremely low gas consumption and waste | Mainly Planar treatment | Convex shapes possible but with complex experimental sets |
| Gas | Easy complex shape treatments | High temperature | High l/d ratio holes easy |
| Metallic System | Nitriding Treatment | Structural Change | Surface Mechanical Performance |
|---|---|---|---|
| Ferritic steel | Gas (550–650 °C) | Compound layer with cracks γ′-Fe4N ε-Fe3N/Fe2N nitrides and diffusion layer | 2–4 fold increase in surface hardness [17] |
| Ferritic steel | Plasma (450–560 °C) | γ′-Fe4N and ε-Fe3N/Fe2N nitrides and diffusion layer γ′-Fe4N micro precipitates | 2–3 fold increase in surface hardness [51] and refs therein [29] |
| Austenite stainless steel | Gas (430–450 °C) | Expanded austenite or γ𝑁 phase | 3 fold increase in surface hardness, limited elastic change [18] |
| Austenite stainless steel | Plasma (<430 °C) | Expanded austenite or or γ𝑁 phase | 3 fold increase in surface hardness, limited elastic change [48,52] |
| Austenite stainless steel | PBII (430 °C) | Expanded austenite or or γ𝑁 phase | 3 fold increase in surface hardness, limited elastic change [23] |
| Titanium alloys | Gas (700–950 °C) | Formation of δ-TiN and ε-Ti2N High temperature enhanced in-depth diffusion | 2–3 fold increase in surface hardness up to 17 GPa [50] In-depth hardness gradient [53] |
| Titanium alloys | Plasma (650–850 °C) | Formation of δ-TiN/ε-Ti2N, in the depth α-Ti(N) solid solution | 2 fold increase in surface hardness, limited elastic change [39] |
| Titanium alloys | PBII (500–800 °C) | Formation of δ-TiN/ε-Ti2N, in the depth α-Ti(N) solid solution nitrides formed at low temperature (500 °C) | 2 fold increase in surface hardness, limited elastic change [9,26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drouet, M.; Le Bourhis, E. Low Temperature Nitriding of Metal Alloys for Surface Mechanical Performance. Materials 2023, 16, 4704. https://doi.org/10.3390/ma16134704
Drouet M, Le Bourhis E. Low Temperature Nitriding of Metal Alloys for Surface Mechanical Performance. Materials. 2023; 16(13):4704. https://doi.org/10.3390/ma16134704
Chicago/Turabian StyleDrouet, Michel, and Eric Le Bourhis. 2023. "Low Temperature Nitriding of Metal Alloys for Surface Mechanical Performance" Materials 16, no. 13: 4704. https://doi.org/10.3390/ma16134704
APA StyleDrouet, M., & Le Bourhis, E. (2023). Low Temperature Nitriding of Metal Alloys for Surface Mechanical Performance. Materials, 16(13), 4704. https://doi.org/10.3390/ma16134704







