Highly Active Nickel (II) Oxide-Supported Cerium Oxide Catalysts for Valorization of Glycerol into Oxygenated Fuel Additives
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Reaction Study and Products Analysis
3. Results and Discussion
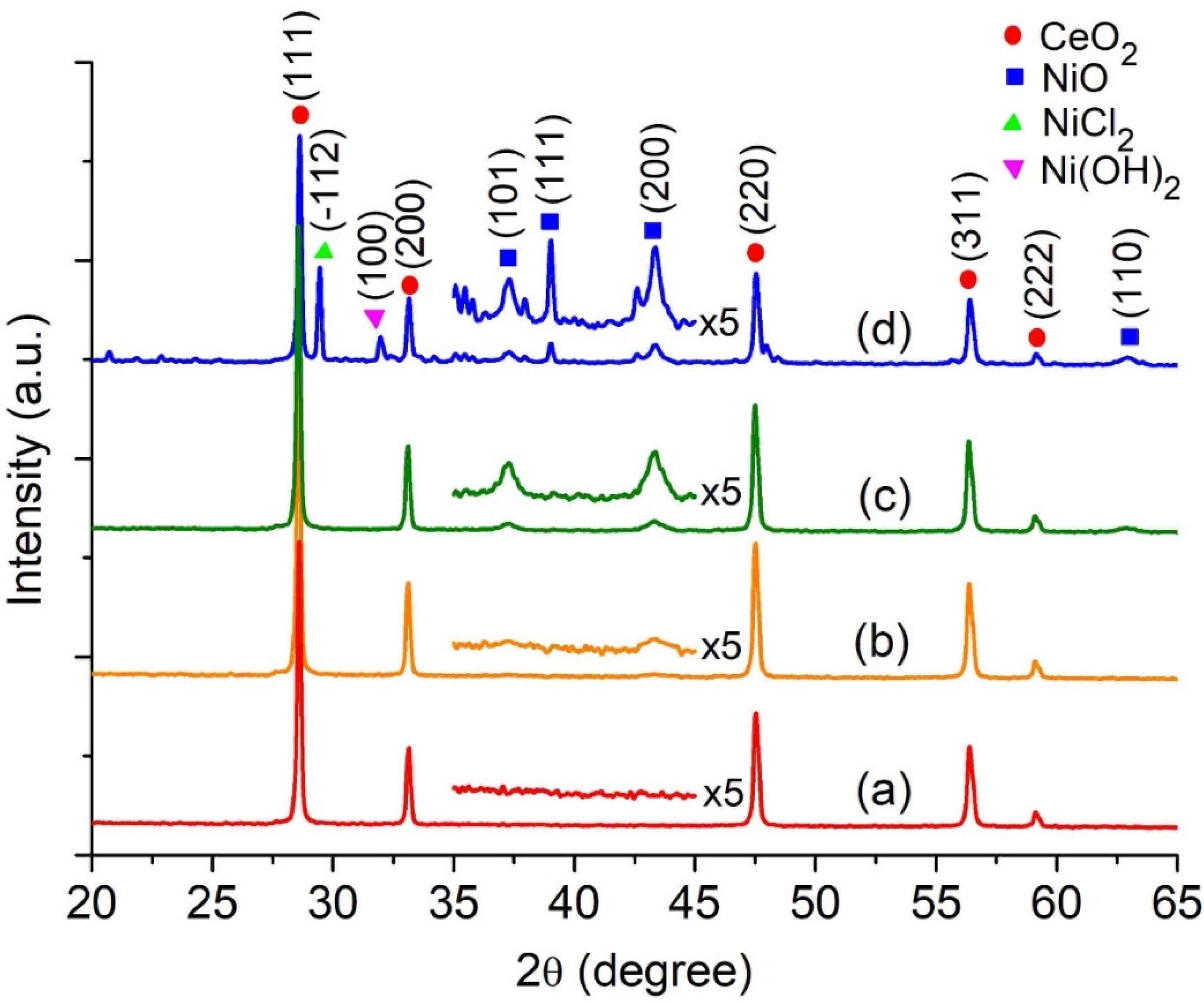
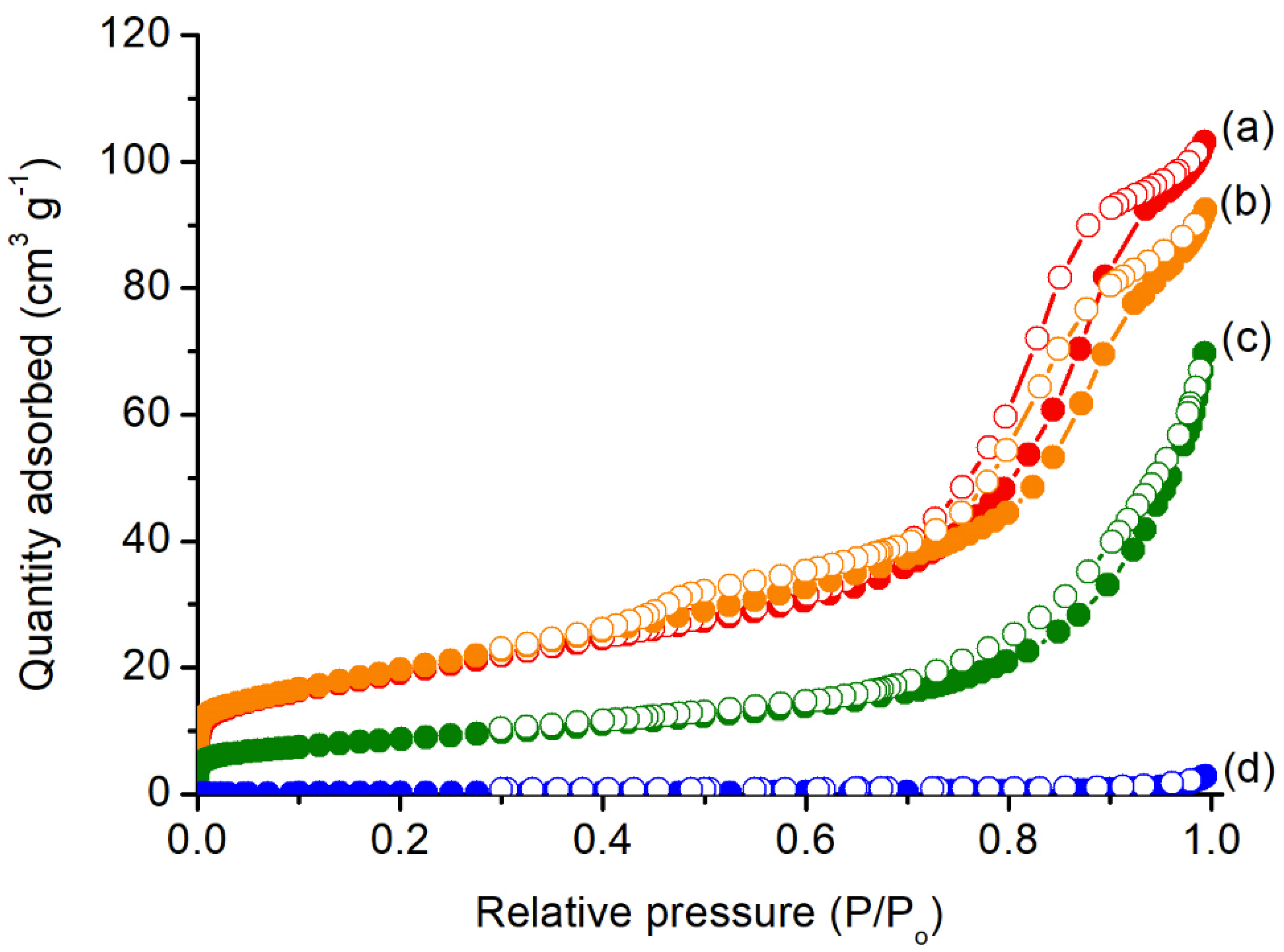
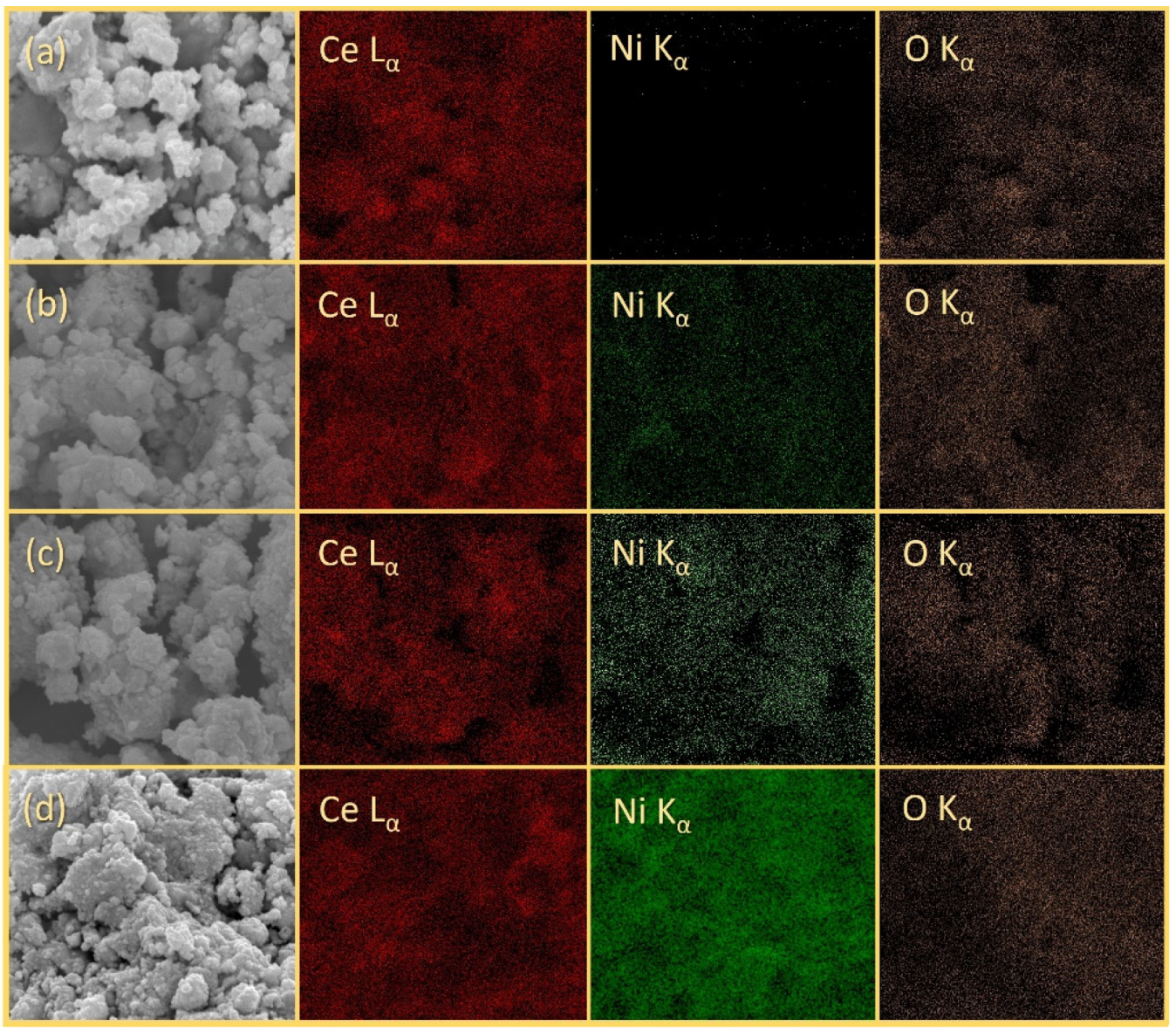
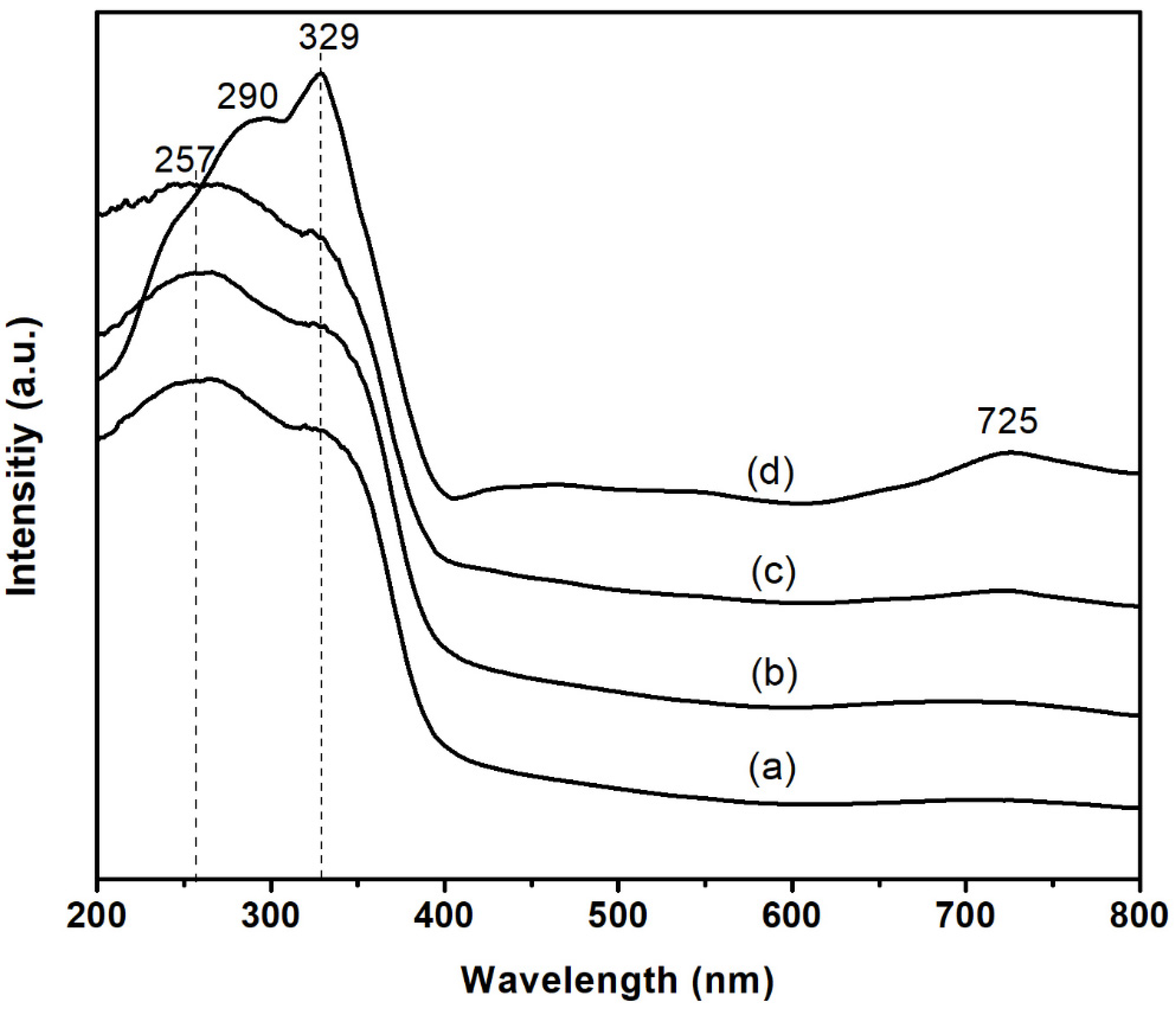
3.1. Characterizations of xNiO/CeO2 Catalysts
3.2. Catalytic Study
3.2.1. Catalytic Comparative Study of xNiO/CeO2 Catalysts
3.2.2. Effect of Reaction Temperature and Duration
3.2.3. Effect of the Amount of Catalyst
3.2.4. Effect of Glycerol:Ethanoic Acid Molar Ratio
3.2.5. Catalyst Recyclability Study
3.3. Thermodynamics and Kinetics Properties of Reaction
3.4. Possible Mechanism of the Acetylation Reaction of Glycerol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kale, S.S.; Armbruster, U.; Eckelt, R.; Bentrup, U.; Umbarkar, S.B.; Dongare, M.K.; Martin, A. Understanding the role of Keggin type heteropolyacid catalysts for glycerol acetylation using toluene as an entrainer. Appl. Catal. A Gen. 2016, 527, 9–18. [Google Scholar] [CrossRef]
- Arora, S.; Gosu, V.; Kumar, U.K.A.; Subbaramaiah, V. Valorization of glycerol into glycerol carbonate using the stable heterogeneous catalyst of Li/MCM-41. J. Clean. Prod. 2021, 295, 126437. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, A.; Quan, W.; Gong, W. Efficient synthesis of biodiesel from Hyoscyamus niger L. seed oil by base catalysis. Fuel Process. Technol. 2023, 241, 107630. [Google Scholar] [CrossRef]
- Kodgire, P.; Sharma, A.; Kachhwaha, S.S. Biodiesel production with enhanced fuel properties via appropriation of non-edible oil mixture using conjoint ultrasound and microwave reactor: Process optimization and kinetic studies. Fuel Process. Technol. 2022, 230, 107206. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Synthesis of oxygenated fuel additives via glycerol esterification with acetic acid over bio-derived carbon catalyst. Fuel 2017, 209, 538–544. [Google Scholar] [CrossRef]
- Mou, R.; Wang, X.; Wang, Z.; Zhang, D.; Yin, Z.; Lv, Y.; Wei, Z. Synthesis of fuel bioadditive by esterification of glycerol with acetic acid over hydrophobic polymer-based solid acid. Fuel 2021, 302, 121175. [Google Scholar] [CrossRef]
- Almas, Q.; Sievers, C.; Jones, C.W. Role of the mesopore generation method in structure, activity and stability of MFI catalysts in glycerol acetylation. Appl. Catal. A Gen. 2019, 571, 107–117. [Google Scholar] [CrossRef]
- Yang, X.; Wang, F.; Wei, R.; Li, S.; Wu, Y.; Shen, P.; Wang, H.; Gao, L.; Xiao, G. Synergy effect between hierarchical structured and Sn-modified H [Sn, Al] ZSM-5 zeolites on the catalysts for glycerol aromatization. Microporous Mesoporous Mater. 2018, 257, 154–161. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Dumeignil, F. Recent developments in the field of catalytic dehydration of glycerol to acrolein. ACS Catal. 2013, 3, 1819–1834. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Wu, Y.; Wang, C.; Yang, M.; Yan, P.; Welz-Biermann, U. Esterification of glycerol with acetic acid using double SO3H-functionalized ionic liquids as recoverable catalysts. Green Chem. 2011, 13, 697–701. [Google Scholar] [CrossRef]
- Vasiliadou, E.; Lemonidou, A. Kinetic study of liquid-phase glycerol hydrogenolysis over Cu/SiO2 catalyst. Chem. Eng. J. 2013, 231, 103–112. [Google Scholar] [CrossRef]
- da Silva, M.J.; Chaves, D.M.; da Silva, R.C.; Gabriel Filho, J.B.; Bruziquesi, C.G.O.; Al-Rabiah, A.A. Impacts of Sn (II) doping on the Keggin heteropolyacid-catalyzed etherification of glycerol with tert-butyl alcohol. Chem. Eng. Sci. 2022, 247, 116913. [Google Scholar] [CrossRef]
- Güemez, M.B.; Requies, J.; Agirre, I.; Arias, P.L.; Barrio, V.L.; Cambra, J.F. Acetalization reaction between glycerol and n-butyraldehyde using an acidic ion exchange resin. Kinetic modelling. Chem. Eng. J. 2013, 228, 300–307. [Google Scholar] [CrossRef]
- Saikia, K.; Rajkumari, K.; Moyon, N.S.; Basumatary, S.; Halder, G.; Rashid, U.; Rokhum, S.L. Sulphonated biomass-based catalyst for solketal synthesis by acetalization of glycerol—A byproduct of biodiesel production. Fuel Process. Technol. 2022, 238, 107482. [Google Scholar] [CrossRef]
- Betiha, M.A.; Hassan, H.M.A.; El-Sharkawy, E.A.; Al-Sabagh, A.M.; Menoufy, M.F.; Abdelmoniem, H.E.M. A new approach to polymer-supported phosphotungstic acid: Application for glycerol acetylation using robust sustainable acidic heterogeneous–homogenous catalyst. Appl. Catal. B Environ. 2016, 182, 15–25. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Ramalingam, R.J.; Selvaraj, M.; Chia, S.; Tan, S.H.; Khoerunnisa, F.; Ling, T.C.; Ng, E.-P. Selective synthesis of triacetyl glyceride biofuel additive via acetylation of glycerol over NiO-supported TiO2 catalyst enhanced by non-microwave instant heating. Appl. Surf. Sci. 2021, 545, 149017. [Google Scholar] [CrossRef]
- Dizoğlu, G.; Sert, E. Fuel additive synthesis by acetylation of glycerol using activated carbon/UiO-66 composite materials. Fuel 2020, 281, 118584. [Google Scholar] [CrossRef]
- Balaraju, M.; Nikhitha, P.; Jagadeeswaraiah, K.; Srilatha, K.; Sai Prasad, P.S.; Lingaiah, N. Acetylation of glycerol to synthesize bioadditives over niobic acid supported tungstophosphoric acid catalysts. Fuel Process. Technol. 2010, 91, 249–253. [Google Scholar] [CrossRef]
- Mertsoy, E.Y.; Sert, E.; Atalay, S.; Atalay, F.S. Fabrication of chromium based metal organic framework (MIL-101)/activated carbon composites for acetylation of glycerol. J. Taiwan Inst. Chem. Eng. 2021, 120, 93–105. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Triwahyono, S.; Hameed, B.H.; Jalil, A.A. Improved production of fuel oxygenates via glycerol acetylation with acetic acid. Chem. Eng. J. 2014, 243, 473–484. [Google Scholar] [CrossRef]
- Reddy, P.S.; Sudarsanam, P.; Raju, G.; Reddy, B.M. Selective acetylation of glycerol over CeO2–M and SO42−/CeO2–M (M= ZrO2 and Al2O3) catalysts for synthesis of bioadditives. J. Ind. Eng. Chem. 2012, 18, 648–654. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.; Ramos, A.; Vital, J.; Castanheiro, J. Glycerol acetylation over dodecatungstophosphoric acid immobilized into a silica matrix as catalyst. Appl. Catal. B Environ. 2009, 91, 416–422. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.; Ramos, A.; Vital, J.; Castanheiro, J. Acetylation of glycerol over heteropolyacids supported on activated carbon. Catal. Commun. 2011, 12, 573–576. [Google Scholar] [CrossRef]
- Gonçalves, V.L.; Pinto, B.P.; Silva, J.C.; Mota, C.J. Acetylation of glycerol catalyzed by different solid acids. Catal. Today 2008, 133, 673–677. [Google Scholar] [CrossRef]
- Gonzalez-Arellano, C.; De, S.; Luque, R. Selective glycerol transformations to high value-added products catalysed by aluminosilicate-supported iron oxide nanoparticles. Catal. Sci. Technol. 2014, 4, 4242–4249. [Google Scholar] [CrossRef]
- Wang, B.; Shen, Y.; Sun, J.; Xu, F.; Sun, R. Conversion of platform chemical glycerol to cyclic acetals promoted by acidic ionic liquids. RSC Adv. 2014, 4, 18917–18923. [Google Scholar] [CrossRef]
- Testa, M.L.; La Parola, V.; Liotta, L.F.; Venezia, A.M. Screening of different solid acid catalysts for glycerol acetylation. J. Mol. Catal. A Chem. 2013, 367, 69–76. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Liu, T.; Yao, X.; Zhao, Z.; Pei, C.; Gong, J. Dynamic oxygen migration and reaction over ceria-supported nickel oxides in chemical looping partial oxidation of methane. Appl. Catal. B Environ. 2023, 328, 122478. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, C.; Xu, T.; Sun, M.; Zhang, J.; Wang, Y.; Wan, H. NiO–polyoxometalate nanocomposites as efficient catalysts for the oxidative dehydrogenation of propane and isobutane. Chem. Commun. 2009, 17, 2376–2378. [Google Scholar] [CrossRef]
- Gao, S.; Li, Y.; Guo, W.; Ding, X.; Zheng, L.; Wu, L.; Yan, H.; Wang, Y. Morphology effect of ceria support with hierarchical structure on the catalytic performance for nickel-based catalysts in dry reforming of methane. Mol. Catal. 2022, 533, 112766. [Google Scholar] [CrossRef]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene. Chem. Eng. J. 2016, 306, 1234–1246. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mierczynska, A.; Ciesielski, R.; Mosinska, M.; Nowosielska, M.; Czylkowska, A.; Maniukiewicz, W.; Szynkowska, M.I.; Vasilev, K. High active and selective Ni/CeO2–Al2O3 and Pd–Ni/CeO2–Al2O3 catalysts for oxy-steam reforming of methanol. Catalysts 2018, 8, 380. [Google Scholar] [CrossRef]
- Andas, J.; Adam, F. One-pot synthesis of nanoscale silver supported biomass-derived silica. Mater. Today: Proc. 2016, 3, 1345–1350. [Google Scholar] [CrossRef]
- Cai, Q.; Lin, W.-Y.; Xiao, F.-S.; Pang, W.-Q.; Chen, X.-H.; Zou, B.-S. The preparation of highly ordered MCM-41 with extremely low surfactant concentration. Microporous Mesoporous Mater. 1999, 32, 1–15. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Li, X.; Wang, C.; Wang, R.; Wang, B.; Yan, H. MOF-derived NiO/CeO2 heterojunction: A photocatalyst for degrading pollutants and hydrogen evolution. J. Mater. Sci. 2020, 55, 15930–15944. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Bi, S.; Cao, L.; Sun, Y. Variable-temperature preparation and performance of NiCl2 as a cathode material for thermal batteries. Sci. China Mater. 2017, 60, 251–257. [Google Scholar] [CrossRef]
- Li, C.; Liu, S. Preparation and characterization of Ni(OH)2 and NiO mesoporous nanosheets. J. Nanomater. 2012, 2012, 1. [Google Scholar] [CrossRef]
- Melchiorre, M.; Amendola, R.; Benessere, V.; Cucciolito, M.E.; Ruffo, F.; Esposito, R. Solvent-free transesterification of methyl levulinate and esterification of levulinic acid catalyzed by a homogeneous iron (III) dimer complex. Mol. Catal. 2020, 483, 110777. [Google Scholar] [CrossRef]
- Rao, G.S.; Sudhakar, B.; Prasanna, H.; Devasahayam, V.; Rao, M.C.S. Spectroscopic studies of lead arsenate glasses doped with nickel oxide. J. Non-Cryst. Solids 2011, 357, 1130–1135. [Google Scholar] [CrossRef]
- Kaneko, H.; Taku, S.; Tamaura, Y. Reduction reactivity of CeO2–ZrO2 oxide under high O2 partial pressure in two-step water splitting process. Sol. Energy 2011, 85, 2321–2330. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.; Zhang, X.; Bairq, Z.A.S.; Huang, Y.; Tontiwachwuthikul, P.; Liang, Z. Catalytic performance and mechanism of SO42−/ZrO2/SBA-15 catalyst for CO2 desorption in CO2-loaded monoethanolamine solution. Appl. Energy 2020, 259, 114179. [Google Scholar] [CrossRef]
- Zou, W.; Ge, C.; Lu, M.; Wu, S.; Wang, Y.; Sun, J.; Pu, Y.; Tang, C.; Gao, F.; Dong, L. Engineering the NiO/CeO2 interface to enhance the catalytic performance for CO oxidation. RSC Adv. 2015, 5, 98335–98343. [Google Scholar] [CrossRef]
- Putri, G.E.; Arief, S.; Jamarun, N.; Gusti, F.R.; Zainul, R. Microstructural analysis and optical properties of nanocrystalline cerium oxides synthesized by precipitation method. Rasayan J. Chem. 2019, 12, 85–90. [Google Scholar] [CrossRef]
- Srinivas, D.; Satyanarayana, C.V.V.; Potdar, H.S.; Ratnasamy, P. Structural studies on NiO-CeO2-ZrO2 catalysts for steam reforming of ethanol. Appl. Catal. A Gen. 2003, 246, 323–334. [Google Scholar] [CrossRef]
- El-Kemary, M.; Nagy, N.; El-Mehasseb, I. Nickel oxide nanoparticles: Synthesis and spectral studies of interactions with glucose. Mater. Sci. Semicond. Process. 2013, 16, 1747–1752. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Mihaylov, M.; Klissurski, D.; Stefanov, P.; Abadjieva, N.; Vassileva, E.; Mintchev, L. Characterization of Ni/SiO2 catalysts prepared by successive deposition and reduction of Ni2+ ions. J. Catal. 1999, 185, 314–323. [Google Scholar] [CrossRef]
- Umegaki, T.; Yamamoto, Y.; Xu, Q.; Kojima, Y. Influence of the Water/Titanium Alkoxide Ratio on the Morphology and Catalytic Activity of Titania–Nickel Composite Particles for the Hydrolysis of Ammonia Borane. ChemistryOpen 2018, 7, 611–616. [Google Scholar] [CrossRef]
- Seo, J.; Moon, J.; Kim, J.H.; Lee, K.; Hwang, J.; Yoon, H.; Yi, D.K.; Paik, U. Role of the oxidation state of cerium on the ceria surfaces for silicate adsorption. Appl. Surf. Sci. 2016, 389, 311–315. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S.; Charojrochkul, S. Steam reforming of ethanol with co-fed oxygen and hydrogen over Ni on high surface area ceria support. Appl. Catal. A Gen. 2007, 327, 180–188. [Google Scholar] [CrossRef]
- Zhao, D.; Prinsen, P.; Wang, Y.; Ouyang, W.; Delbecq, F.; Len, C.; Luque, R. Continuous flow alcoholysis of furfuryl alcohol to alkyl levulinates using zeolites. ACS Sustain. Chem. Eng. 2018, 6, 6901–6909. [Google Scholar] [CrossRef]
- Zulkepli, S.; Juan, J.C.; Lee, H.V.; Rahman, N.S.A.; Show, P.L.; Ng, E.P. Modified mesoporous HMS supported Ni for deoxygenation of triolein into hydrocarbon-biofuel production. Energy Convers. Manag. 2018, 165, 495–508. [Google Scholar] [CrossRef]
- Sankar, E.S.; Reddy, K.S.; Jyothi, Y.; Raju, B.D.; Rao, K.S.R. Alcoholysis of furfuryl alcohol into n-butyl levulinate over SBA-16 supported heteropoly acid catalyst. Catal. Lett. 2017, 147, 2807–2816. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Zhang, Z.; Yu, W.-J.; Li, L.-D.; Zhang, M.-H.; Zhang, Z.-B. A swelling-changeful catalyst for glycerol acetylation with controlled acid concentration. Fuel Process. Technol. 2016, 142, 228–234. [Google Scholar] [CrossRef]
- Ramalingam, R.J.; Appaturi, J.N.; Pulingam, T.; Al-Lohedan, H.A.; Al-dhayan, D.M. In-situ incorporation of ruthenium/copper nanoparticles in mesoporous silica derived from rice husk ash for catalytic acetylation of glycerol. Renew. Energy 2020, 160, 564–574. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, K.; Zhao, Z.K. Efficient conversion of furfuryl alcohol into alkyl levulinates catalyzed by an organic–inorganic hybrid solid acid catalyst. ChemSusChem 2011, 4, 112–118. [Google Scholar] [CrossRef]
- Sun, J.; Tong, X.; Yu, L.; Wan, J. An efficient and sustainable production of triacetin from the acetylation of glycerol using magnetic solid acid catalysts under mild conditions. Catal. Today 2016, 264, 115–122. [Google Scholar] [CrossRef]
- Bhanja, P.; Modak, A.; Chatterjee, S.; Bhaumik, A. Bifunctionalized mesoporous SBA-15: A new heterogeneous catalyst for the facile synthesis of 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2017, 5, 2763–2773. [Google Scholar] [CrossRef]
- Ceondo GmbH. Chemeo—High Quality Chemical Properties. Available online: www.chemeo.com (accessed on 9 June 2023).
- Atkins, P.; Paula, J. Physical Chemistry, 8th ed.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Lai, H.-W.; Chen, Y.; Jiang, C.-J. Thermodynamic analysis for reaction of glycerol and acetic acid. Chem. Eng. 2013, 41, 27–29,42. [Google Scholar]
- Sandesh, S.; Manjunathan, P.; Halgeri, A.B.; Shanbhag, G.V. Glycerol acetins: Fuel additive synthesis by acetylation and esterification of glycerol using cesium phosphotungstate catalyst. RSC Adv. 2015, 5, 104354–104362. [Google Scholar] [CrossRef]



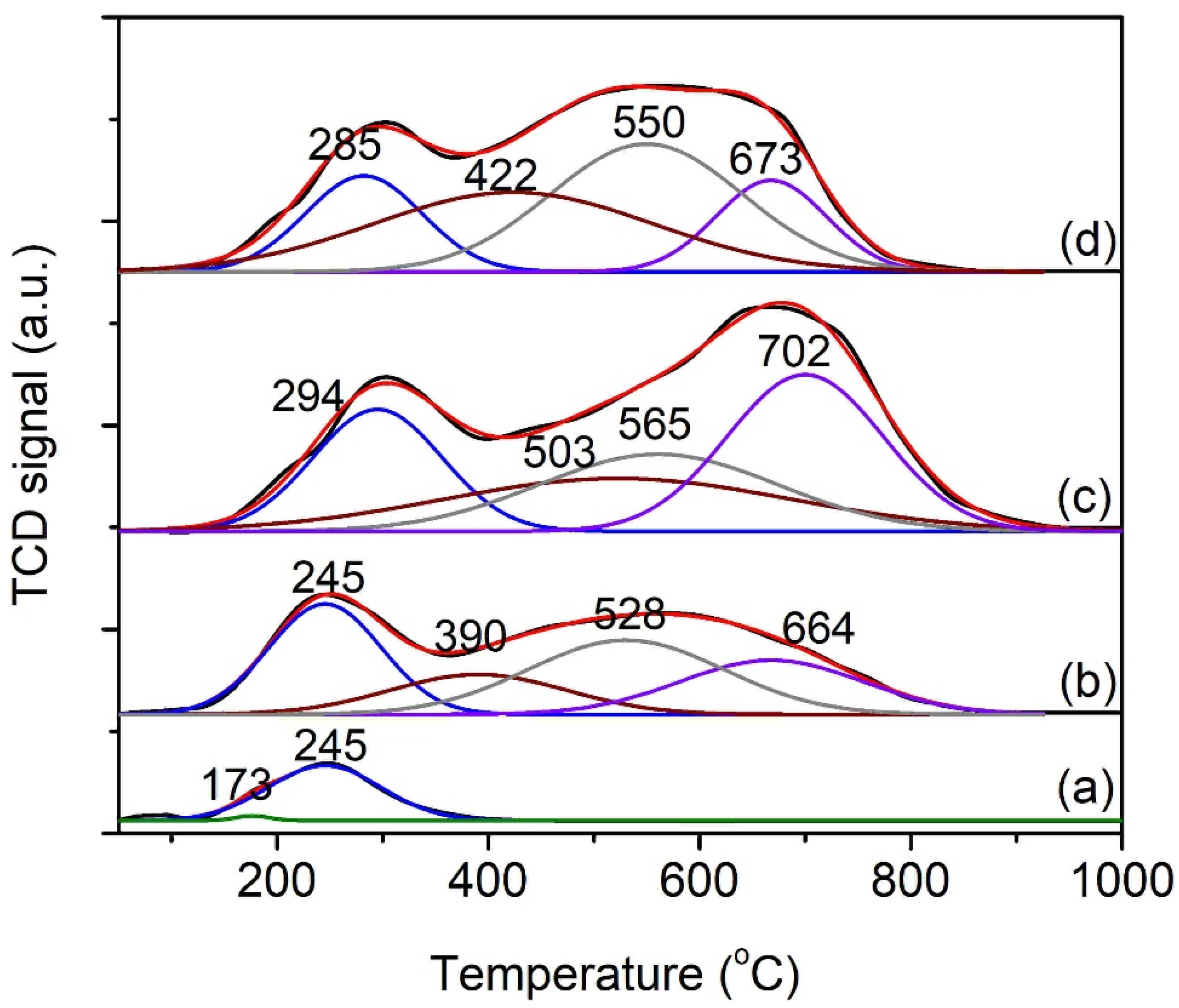
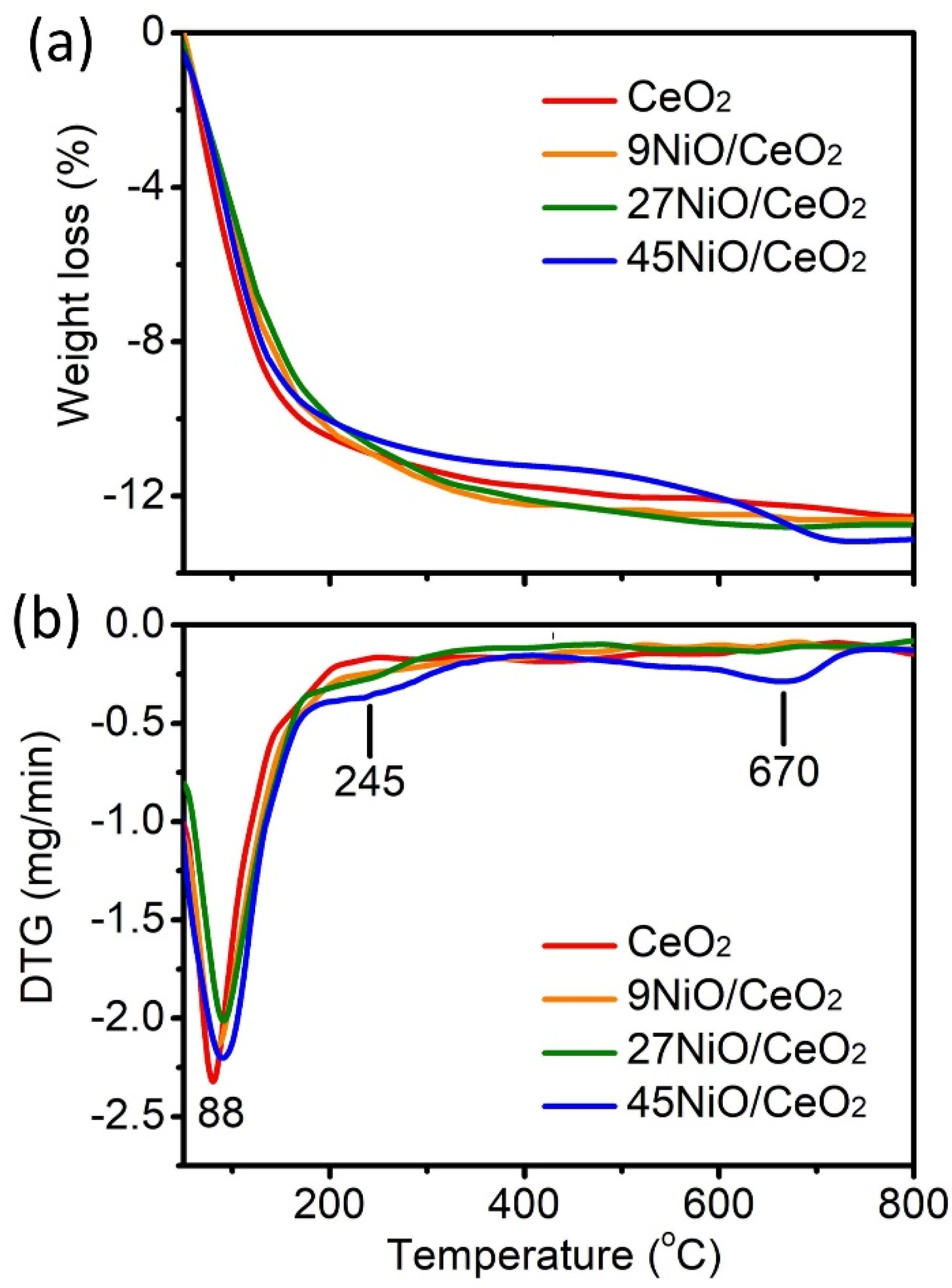
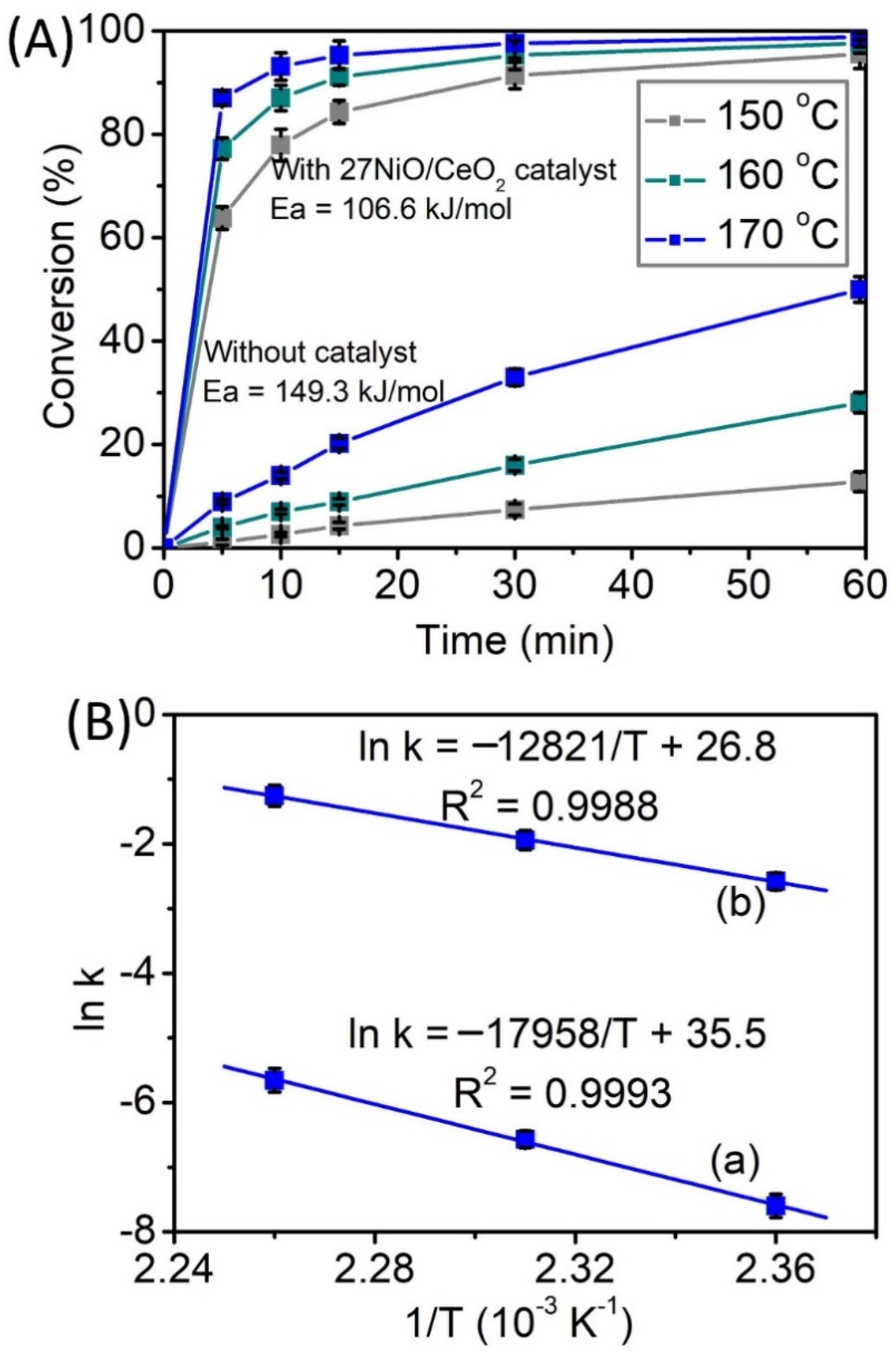




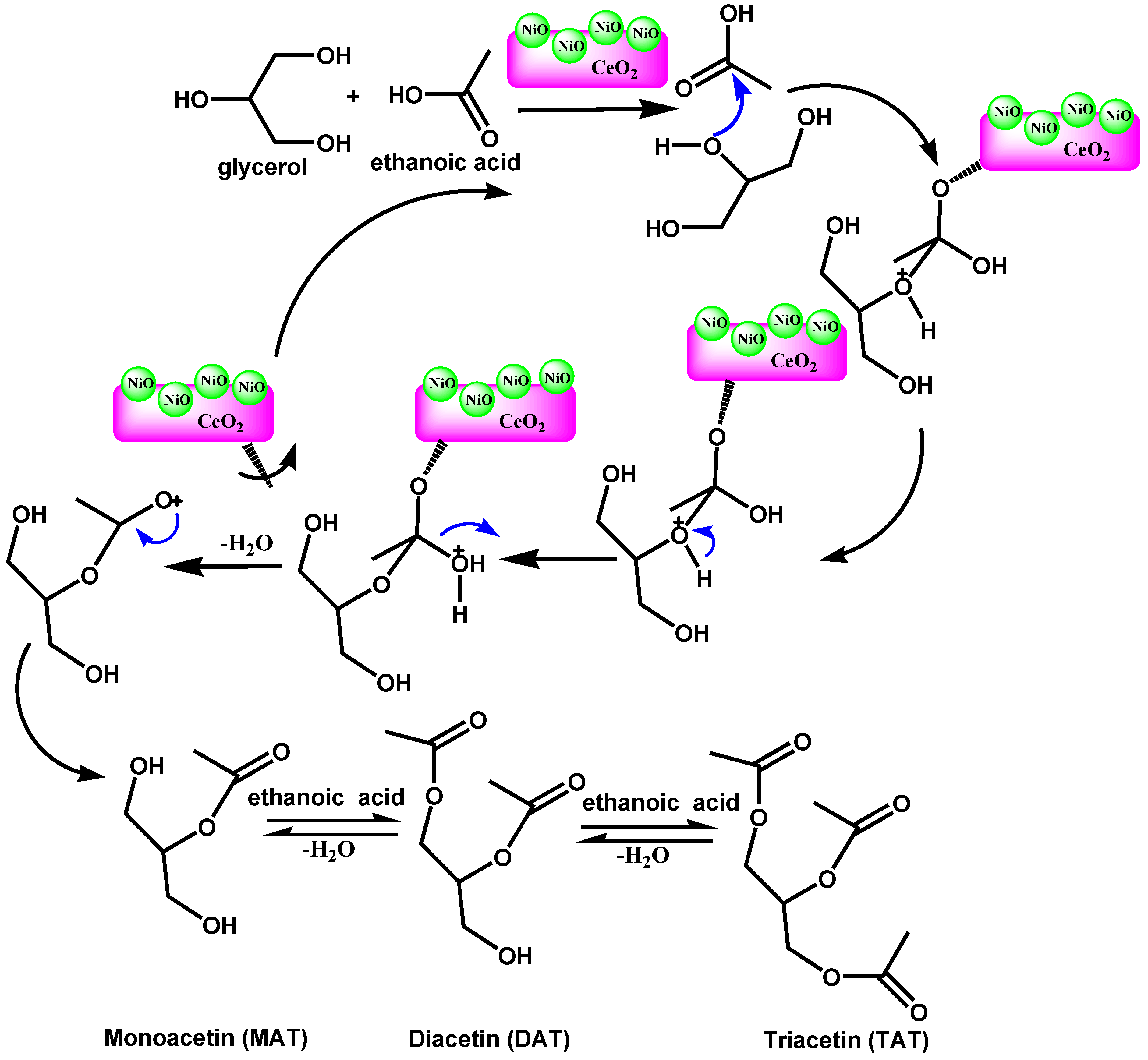
| Samples | NiO Content (%) | BET Surface Area (m2/g) | Micropore Surface Area (nm) | Average Pore Diameter (nm) | Total Pore Volume (cm3/g) | Particle Size (nm) | TPD-NH3 Acidity (μmol/g) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mild | Mild-to-Strong | Strong | Total | |||||||
| CeO2 | 0 | 69 | 12 | 9.86 | 0.15 | 125 ± 23 | 129 | 0 | 0 | 129 |
| 9NiO/CeO2 | 8.10 | 71 | 7 | 8.95 | 0.13 | 124 ± 33 | 271 | 473 | 217 | 961 |
| 27NiO/CeO2 | 28.10 | 31 | 4 | 7.51 | 0.07 | 120 ± 33 | 320 | 871 | 373 | 1564 |
| 45NiO/CeO2 | 44.02 | 1 | 0 | 5.93 | 0.002 | 149 ± 30 | 235 | 1123 * | 154 * | 1512 * |
| Entry | Sample | Conversion (%) at 150 °C | Products Selectivity (%) at 150 °C | Conversion (%) at 170 °C | Products Selectivity (%) at 170 °C | ||||
|---|---|---|---|---|---|---|---|---|---|
| MAT | DAT | TAT | MAT | DAT | TAT | ||||
| 1 | CeO2 | 20.0 | 19.3 | 72.7 | 0.0 | 31.1 | 17.2 | 72.6 | 0.0 |
| 2 | 9NiO/CeO2 | 77.5 | 22.0 | 66.3 | 2.2 | 88.0 | 19.6 | 55.3 | 13.6 |
| 3 | 27NiO/CeO2 | 84.3 | 18.2 | 66.0 | 4.3 | 95.3 | 15.8 | 60.4 | 14.9 |
| 4 | 45NiO/CeO2 | 76.0 | 20.3 | 66.7 | 2.9 | 87.2 | 17.6 | 57.0 | 14.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appaturi, J.N.; Maireles-Torres, P.; Alomar, T.S.; AlMasoud, N.; El-Bahy, Z.M.; Ling, T.C.; Ng, E.-P. Highly Active Nickel (II) Oxide-Supported Cerium Oxide Catalysts for Valorization of Glycerol into Oxygenated Fuel Additives. Materials 2023, 16, 4713. https://doi.org/10.3390/ma16134713
Appaturi JN, Maireles-Torres P, Alomar TS, AlMasoud N, El-Bahy ZM, Ling TC, Ng E-P. Highly Active Nickel (II) Oxide-Supported Cerium Oxide Catalysts for Valorization of Glycerol into Oxygenated Fuel Additives. Materials. 2023; 16(13):4713. https://doi.org/10.3390/ma16134713
Chicago/Turabian StyleAppaturi, Jimmy Nelson, Pedro Maireles-Torres, Taghrid S. Alomar, Najla AlMasoud, Zeinhom M. El-Bahy, Tau Chuan Ling, and Eng-Poh Ng. 2023. "Highly Active Nickel (II) Oxide-Supported Cerium Oxide Catalysts for Valorization of Glycerol into Oxygenated Fuel Additives" Materials 16, no. 13: 4713. https://doi.org/10.3390/ma16134713
APA StyleAppaturi, J. N., Maireles-Torres, P., Alomar, T. S., AlMasoud, N., El-Bahy, Z. M., Ling, T. C., & Ng, E.-P. (2023). Highly Active Nickel (II) Oxide-Supported Cerium Oxide Catalysts for Valorization of Glycerol into Oxygenated Fuel Additives. Materials, 16(13), 4713. https://doi.org/10.3390/ma16134713







