A Comprehensive Review of the Current Research Status of Biodegradable Zinc Alloys and Composites for Biomedical Applications
Abstract
1. Introduction
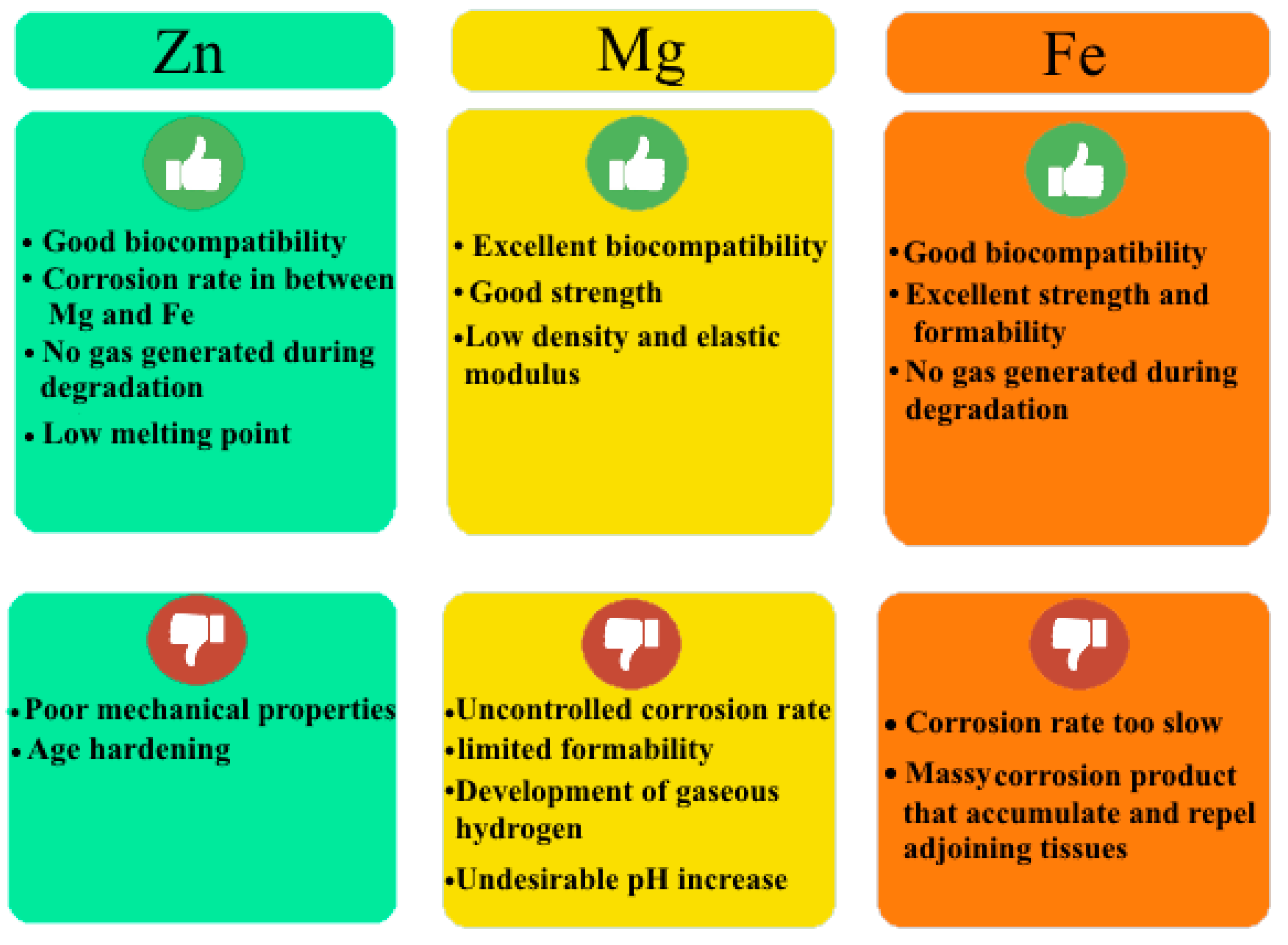
2. Zn Alloy Candidates for Biomedical Applications
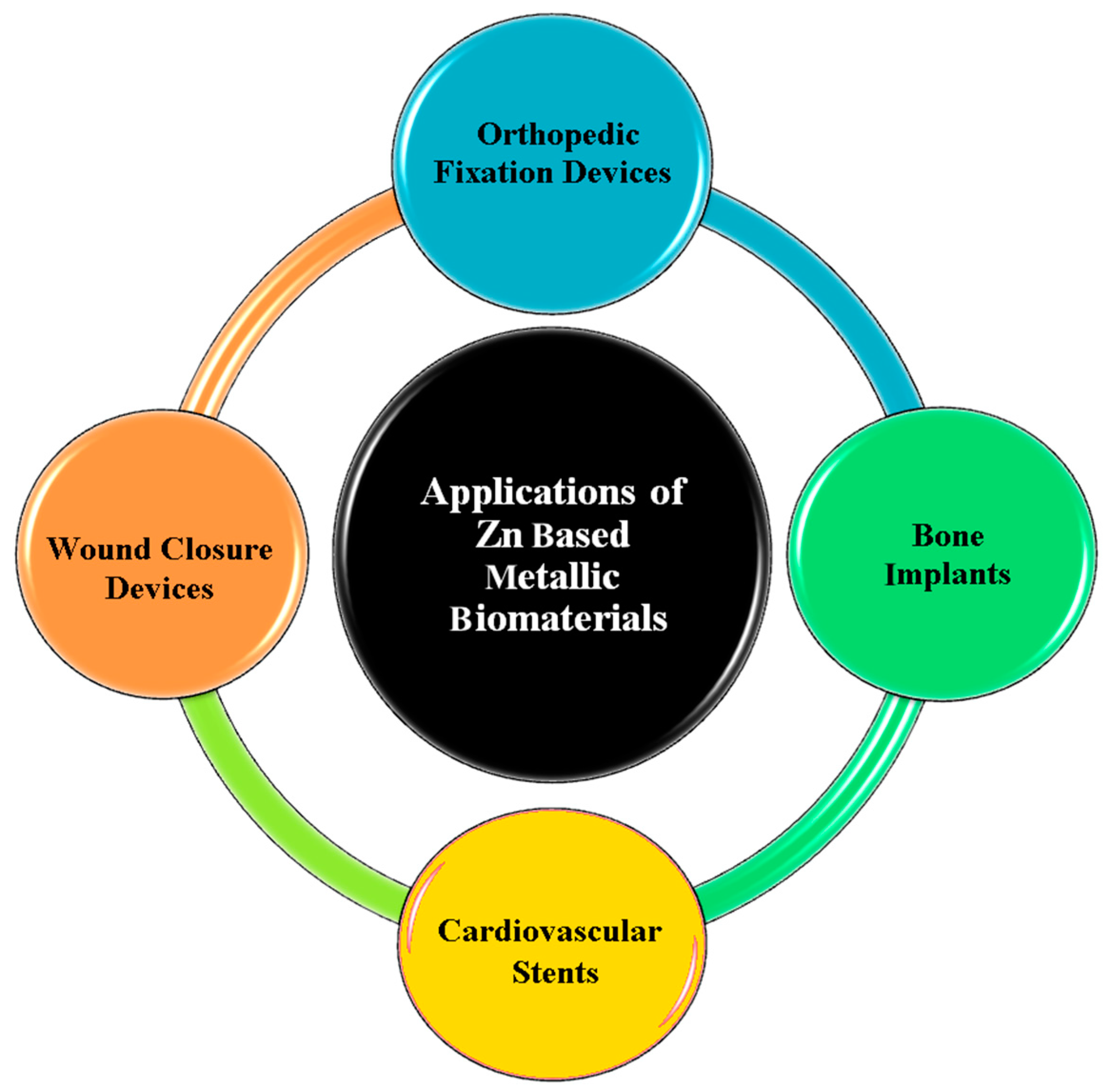
2.1. Pure Zn
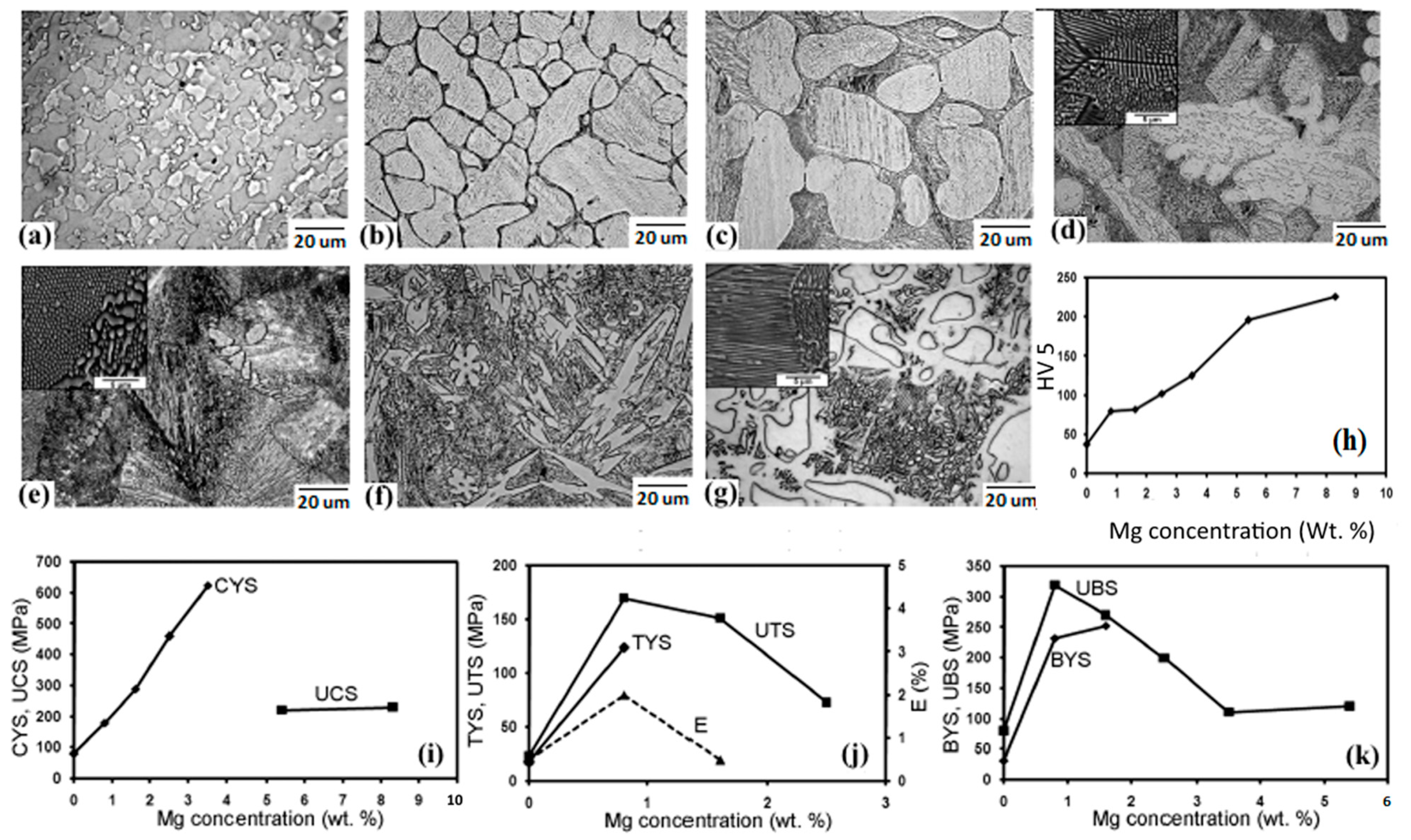
2.2. Zn-Mg Alloys
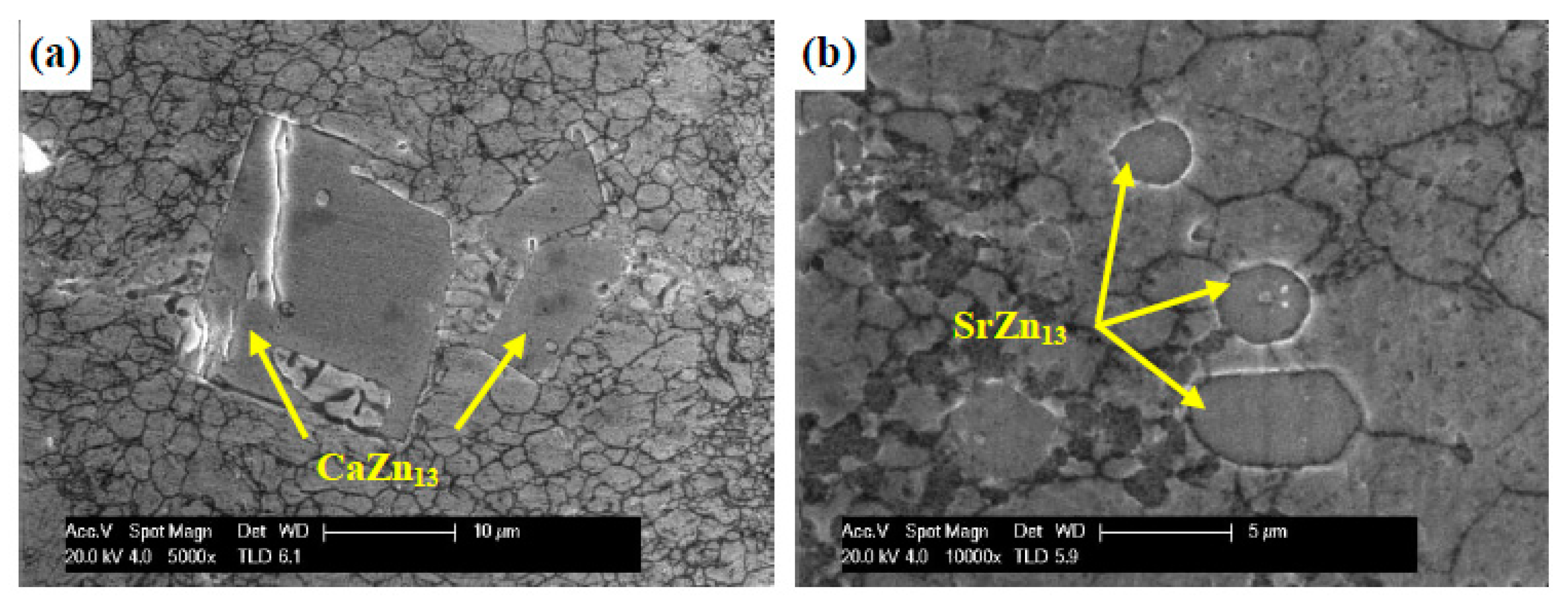
2.3. Zn-Ca\Sr Alloys
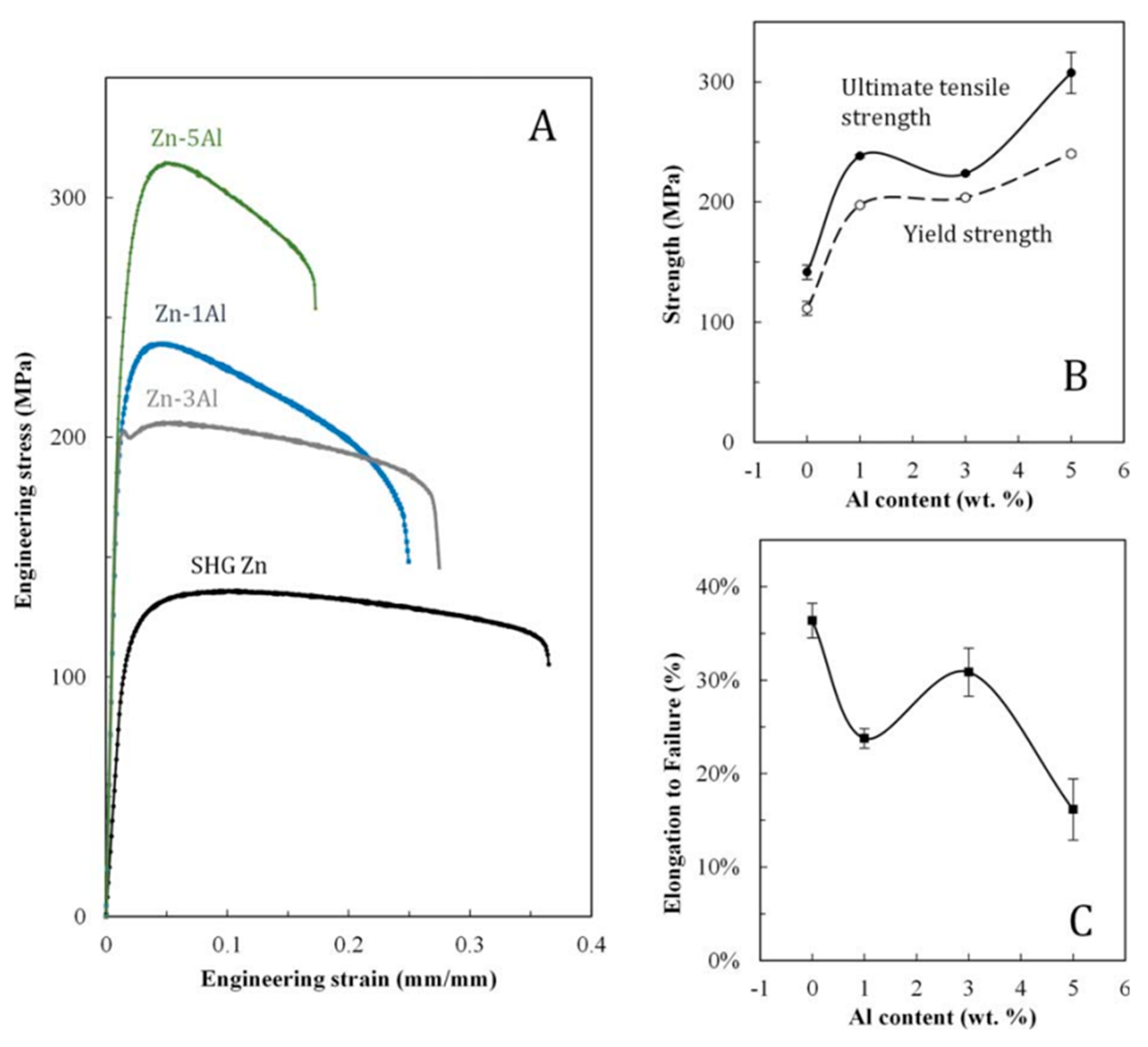
2.4. Zn-Al Alloys
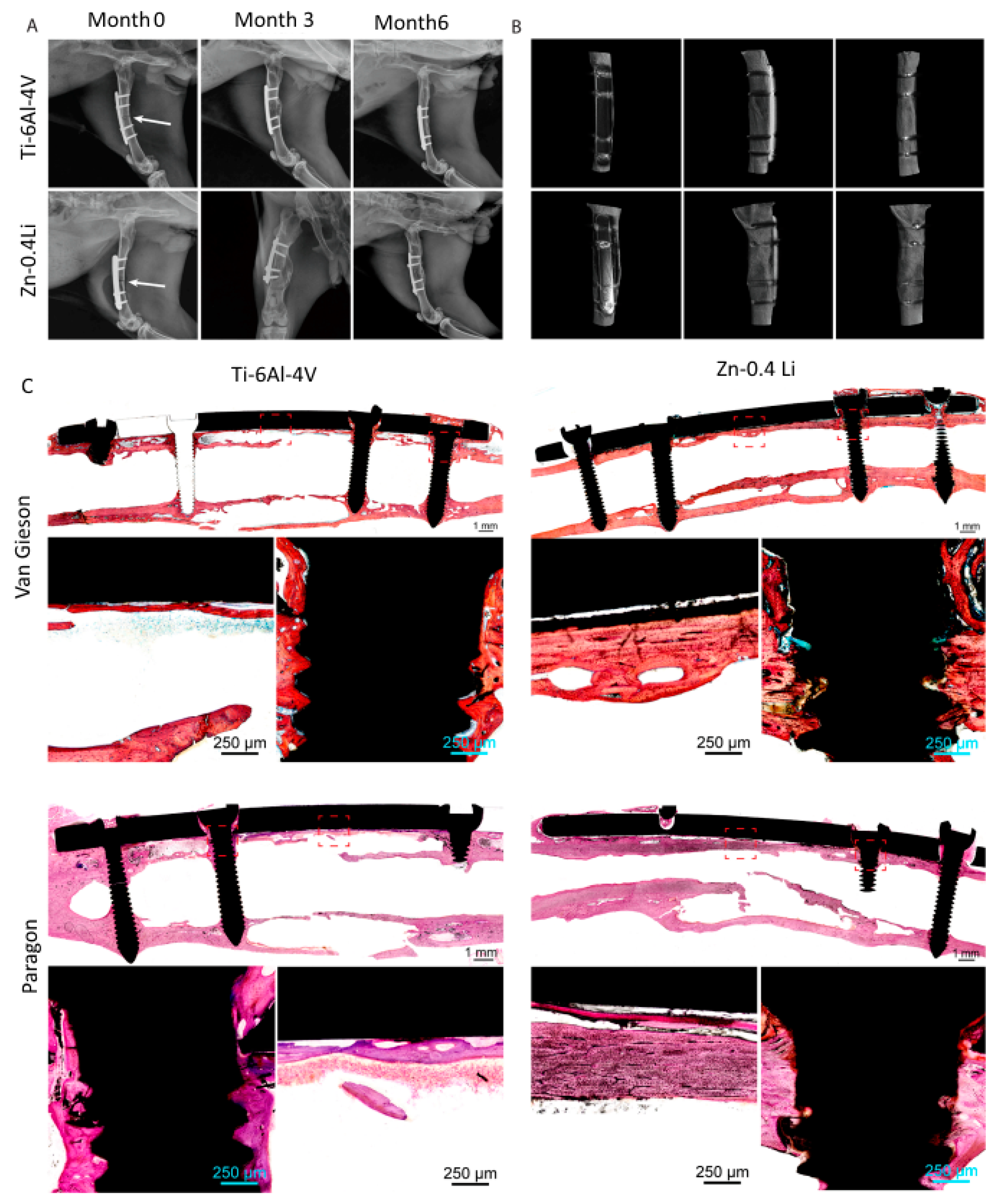
2.5. Zn-Li Alloys
2.6. Zn-Ag Alloys
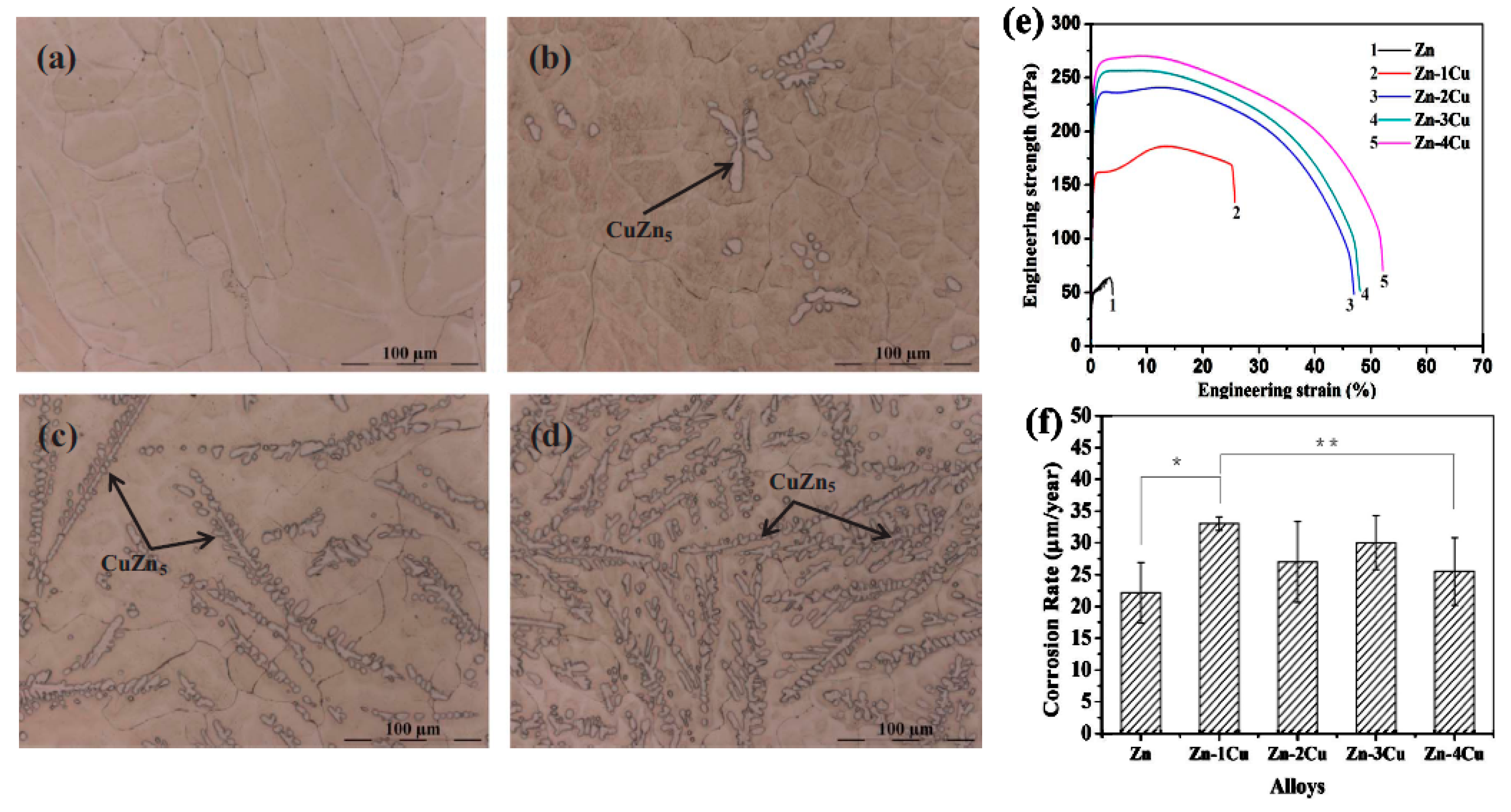
2.7. Zn-Cu Alloys
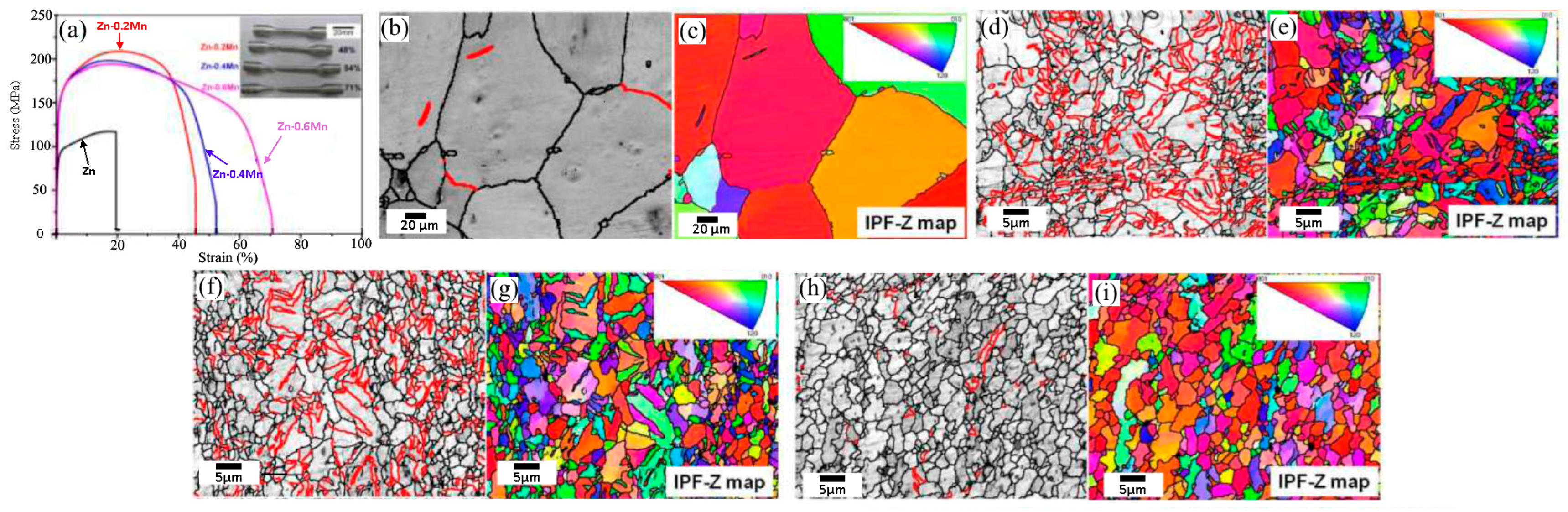
2.8. Zn-Mn Alloys
2.9. Zn-Mg-X (X = Sr, Ca, Mn, Fe, Cu, Al) Alloys
2.9.1. Zn-Mg-Sr\Ca Alloys
2.9.2. Zn-Mg-Mn Alloys
2.9.3. Zn-Mg-Fe Alloys
3. Zn-Based Composites
3.1. Carbon Elements Reinforcement
3.2. Bioceramic Reinforcements
4. Manufacture Methods for Biodegradable Zn
4.1. Casting
4.2. Conventional Wrought Procedures
4.3. Advanced Processing Methods
5. Conclusions and Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, J.; Tong, X.; Shi, Z.; Zhang, D.; Zhang, L.; Wang, K.; Wei, A.; Jin, L.; Lin, J.; Li, Y.; et al. A Biodegradable Zn-1Cu-0.1Ti Alloy with Antibacterial Properties for Orthopedic Applications. Acta Biomater. 2020, 106, 410–427. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Spataru, M.C.; Verestiuc, L.; Balan, V.; Solcan, C.; Sandu, A.V.; Geanta, V.; Voiculescu, I.; Vizureanu, P. Design, Synthesis, and Preliminary Evaluation for Ti–Mo–Zr–Ta–Si Alloys for Potential Implant Applications. Materials 2021, 14, 6806. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Sandu, A.V.; Nabialek, M.; Vizureanu, P.; Ciobanu, G. Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys. Micromachines 2021, 12, 1447. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The Influence of Alloying and Fabrication Techniques on the Mechanical Properties, Biodegradability and Biocompatibility of Zinc: A Comprehensive Review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Istrate, B.; Munteanu, C.; Geanta, V.; Baltatu, S.; Focsaneanu, S.; Earar, K. Microstructural Analysis of Biodegradable Mg-0.9Ca-1.2Zr Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2016, 147, 012033. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Karamian, E.; Kasiri-Asgarani, M.; Ghomi, H. Magnesium-Graphene Nano-Platelet Composites: Corrosion Behavior, Mechanical and Biological Properties. J. Alloys Compd. 2020, 821, 153379. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Karamian, E.; Kasiri-Asgarani, M.; Ghomi, H.; Omidi, M.; Abazari, S.; Ismail, A.F.; Sharif, S.; Berto, F. Synthesis and Characterization of Hot Extruded Magnesium-Zinc Nano-Composites Containing Low Content of Graphene Oxide for Implant Applications. Phys. Mesomech. 2021, 24, 486–502. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Abazari, S.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Daroonparvar, M.; Berto, F. A Comprehensive Review on Surface Modifications of Biodegradable Magnesium-Based Implant Alloy: Polymer Coatings Opportunities and Challenges. Coatings 2021, 11, 747. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Karamian, E.; Kasiri-Asgarani, M.; Ghomi, H. A Study on the Corrosion Behavior and Biological Properties of Polycaprolactone/Bredigite Composite Coating on Biodegradable Mg-Zn-Ca-GNP Nanocomposite. Prog. Org. Coatings 2020, 147, 105822. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. The Effect of Co-Encapsulated GO-Cu Nanofillers on Mechanical Properties, Cell Response, and Antibacterial Activities of Mg–Zn Composite. Metals 2022, 12, 207. [Google Scholar] [CrossRef]
- Gao, C.; Yao, M.; Li, S.; Feng, P.; Peng, S.; Shuai, C. Highly Biodegradable and Bioactive Fe–Pd-Bredigite Biocomposites Prepared by Selective Laser Melting. J. Adv. Res. 2019, 20, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Venezuela, J.; Dargusch, M.S. Addressing the Slow Corrosion Rate of Biodegradable Fe–Mn: Current Approaches and Future Trends. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100822. [Google Scholar] [CrossRef]
- Scarcello, E.; Lobysheva, I.; Bouzin, C.; Jacques, P.J.; Lison, D.; Dessy, C. Endothelial Dysfunction Induced by Hydroxyl Radicals—The Hidden Face of Biodegradable Fe-Based Materials for Coronary Stents. Mater. Sci. Eng. C 2020, 112, 110938. [Google Scholar] [CrossRef]
- Nayak, P.; Biswal, A.K.; Sahoo, S.K. Processing and Characterization of Fe-35Mn Biodegradable Metallic Materials. Mater. Today Proc. 2020, 33, 5284–5289. [Google Scholar] [CrossRef]
- Hofmann, G.O.; Wagner, F.D. New Implant Designs for Bioresorbable Devices in Orthopaedic Surgery. Clin. Mater. 1993, 14, 207–215. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable Metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Emily Walker, M.H. Magnesium, Iron and Zinc Alloys, the Trifecta of Bioresorbable Orthopaedic and Vascular Implantation—A Review. J. Biotechnol. Biomater. 2015, 5, 1. [Google Scholar] [CrossRef]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design Strategy for Biodegradable Fe-Based Alloys for Medical Applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef]
- Zhu, D.; Cockerill, I.; Su, Y.; Zhang, Z.; Fu, J.; Lee, K.-W.; Ma, J.; Okpokwasili, C.; Tang, L.; Zheng, Y.; et al. Mechanical Strength, Biodegradation, and in Vitro and in Vivo Biocompatibility of Zn Biomaterials. ACS Appl. Mater. Interfaces 2019, 11, 6809–6819. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Jia, C.; Xu, M.; Kaseem, M.; Tayebi, M. Microstructural Changes Caused by the Creep Test in ZK60 Alloy Reinforced by SiCp at Intermediate Temperature after KOBO Extrusion and Aging. Materials 2023, 16, 3885. [Google Scholar] [CrossRef]
- Kheradmand, A.B.; Tayebi, M.; Lalegani, Z. Design of Fe–SiC–Cu–G Composite Alloy and Optimization of Graphite Contribution for High Sliding Speed Applications. Trans. Indian Inst. Met. 2022, 75, 2311–2322. [Google Scholar] [CrossRef]
- Mousavi, S.F.; Sharifi, H.; Tayebi, M.; Hamawandi, B.; Behnamian, Y. Thermal Cycles Behavior and Microstructure of AZ31/SiC Composite Prepared by Stir Casting. Sci. Rep. 2022, 12, 15191. [Google Scholar] [CrossRef] [PubMed]
- Istrate, B.; Munteanu, C.; Antoniac, I.V.; Lupescu, Ș.C. Current Research Studies of Mg–Ca–Zn Biodegradable Alloys Used as Orthopedic Implants—Review. Crystals 2022, 12, 1468. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Bakhsheshi-Rad, H.R.; Saberi, A.; Razzaghi, M.; Kasar, A.K.; Ramakrishna, S.; Menezes, P.L.; Misra, M.; Ismail, A.F.; Sharif, S.; et al. Surface Modification of Magnesium Alloys Using Thermal and Solid-State Cold Spray Processes: Challenges and Latest Progresses. J. Magnes. Alloy. 2022, 10, 2025–2061. [Google Scholar] [CrossRef]
- Tayebi, M.; Najafi, H.; Nategh, S.; Khodabandeh, A. Creep Behavior of ZK60 Alloy and ZK60/SiCw Composite After Extrusion and Precipitation Hardening. Met. Mater. Int. 2021, 27, 3905–3917. [Google Scholar] [CrossRef]
- Tayebi, M.; Nategh, S.; Najafi, H.; Khodabandeh, A. Tensile Properties and Microstructure of ZK60/SiCw Composite after Extrusion and Aging. J. Alloys Compd. 2020, 830, 154709. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Chi, P.; Bahonar, E.; Tayebi, M. Effects of the Microstructure and Precipitation Hardening on the Thermal Expansion Behavior of ZK60 Magnesium Alloy. J. Alloys Compd. 2022, 901, 163422. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Yarigarravesh, M.; Tayyebi, M.; Tayebi, M. Evaluation of Whisker Alignment and Anisotropic Mechanical Properties of ZK60 Alloy Reinforced with SiCw during KOBO Extrusion Method. J. Manuf. Process. 2022, 84, 344–356. [Google Scholar] [CrossRef]
- Wang, Y.; Tayyebi, M.; Tayebi, M.; Yarigarravesh, M.; Liu, S.; Zhang, H. Effect of Whisker Alignment on Microstructure, Mechanical and Thermal Properties of Mg-SiCw/Cu Composite Fabricated by a Combination of Casting and Severe Plastic Deformation (SPD). J. Magnes. Alloy. 2023, 11, 966–980. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Ebrahimi, S.; Yarmand, B. In-Vitro Corrosion and Bioactivity Behavior of Tailored Calcium Phosphate-Containing Zinc Oxide Coating Prepared by Plasma Electrolytic Oxidation. Corros. Sci. 2020, 173, 108781. [Google Scholar] [CrossRef]
- Li, P.; Zhang, W.; Spintzyk, S.; Schweizer, E.; Krajewski, S.; Alexander, D.; Dai, J.; Xu, S.; Wan, G.; Rupp, F. Impact of Sterilization Treatments on Biodegradability and Cytocompatibility of Zinc-Based Implant Materials. Mater. Sci. Eng. C 2021, 130, 112430. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Zhu, D.; Ruan, L.; Takashima, K. Challenges in the Use of Zinc and Its Alloys as Biodegradable Metals: Perspective from Biomechanical Compatibility. Acta Biomater. 2019, 97, 23–45. [Google Scholar] [CrossRef]
- Lynch, R.F. Zinc: Alloying, Thermomechanical Processing, Properties, and Applications. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 9869–9883. [Google Scholar]
- Pola, A.; Tocci, M.; Goodwin, F.E. Review of Microstructures and Properties of Zinc Alloys. Metals 2020, 10, 253. [Google Scholar] [CrossRef]
- Katarivas Levy, G.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants—A Review Paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent Research and Progress of Biodegradable Zinc Alloys and Composites for Biomedical Applications: Biomechanical and Biocorrosion Perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Zhang, L.N.; Hou, Z.T.; Ye, X.; Xu, Z.B.; Bai, X.L.; Shang, P. The Effect of Selected Alloying Element Additions on Properties of Mg-Based Alloy as Bioimplants: A Literature Review. Front. Mater. Sci. 2013, 7, 227–236. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Florido-Suarez, N.; Saceleanu, M.V.; Mirza-Rosca, J.C. New Titanium Alloys, Promising Materials for Medical Devices. Materials 2021, 14, 5934. [Google Scholar] [CrossRef]
- Li, J.W.; Du, C.F.; Yuchi, C.X.; Zhang, C.Q. Application of Biodegradable Materials in Orthopedics. J. Med. Biol. Eng. 2019, 39, 633–645. [Google Scholar] [CrossRef]
- Letzig, D.; Swiostek, J.; Bohlen, J.; Beaven, P.A.; Kainer, K.U. Wrought Magnesium Alloys for Structural Applications. Mater. Sci. Technol. 2008, 24, 991–996. [Google Scholar] [CrossRef]
- Liu, Y.; Du, T.; Qiao, A.; Mu, Y.; Yang, H. Zinc-Based Biodegradable Materials for Orthopaedic Internal Fixation. J. Funct. Biomater. 2022, 13, 164. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Zheng, Y.; Zhou, F.; Qiu, K.; Wang, X. Design and Characterizations of Novel Biodegradable Ternary Zn-Based Alloys with IIA Nutrient Alloying Elements Mg, Ca and Sr. Mater. Des. 2015, 83, 95–102. [Google Scholar] [CrossRef]
- Hagelstein, S.; Zankovic, S.; Kovacs, A.; Barkhoff, R.; Seidenstuecker, M. Mechanical Analysis and Corrosion Analysis of Zinc Alloys for Bioabsorbable Implants for Osteosynthesis. Materials 2022, 15, 421. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Y.; Zhou, J.; Li, H.; Chang, J.; Huan, Z. In Vitro Degradation and Surface Bioactivity of Iron-Matrix Composites Containing Silicate-Based Bioceramic. Bioact. Mater. 2017, 2, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, J.; Qiu, K.; Yang, Y.; Pu, Z.; Li, L.; Zheng, Y. Effects of Alloying Elements (Ca and Sr) on Microstructure, Mechanical Property and in Vitro Corrosion Behavior of Biodegradable Zn–1.5Mg Alloy. J. Alloys Compd. 2016, 664, 444–452. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Goldman, J.; Drelich, J.; Aghion, E. The Suitability of Zn–1.3%Fe Alloy as a Biodegradable Implant Material. Metals 2018, 8, 153. [Google Scholar] [CrossRef]
- Avior, O.; Ben Ghedalia-Peled, N.; Ron, T.; Goldman, J.; Vago, R.; Aghion, E. Stress Corrosion Analysis and Direct Cell Viability of Biodegradable Zn-Fe-Ca Alloy in In-Vitro Conditions. Metals 2022, 12, 76. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Wang, L.; Zeng, X.; Jin, Z. Cluster Hardening Effects on Twinning in Mg-Zn-Ca Alloys. Metals 2022, 12, 693. [Google Scholar] [CrossRef]
- Yu, H.; Ren, J.; Kang, S.; Yu, W.; Wang, Z.; Feng, J.; Wang, Q.; Ji, P.; Zhang, X.; Yin, F. Effect of 1wt% Zn Addition on Microstructure and Mechanical Properties of Mg-6Er Alloys under High Strain Rates. Metals 2022, 12, 883. [Google Scholar] [CrossRef]
- Kumar, C.M.P.; Lakshmikanthan, A.; Chandrashekarappa, M.P.G.; Pimenov, D.Y.; Giasin, K. Electrodeposition Based Preparation of Zn–Ni Alloy and Zn–Ni–WC Nano-Composite Coatings for Corrosion-Resistant Applications. Coatings 2021, 11, 712. [Google Scholar] [CrossRef]
- Dong, H.; Virtanen, S. Anodic ZnO Microsheet Coating on Zn with Sub-Surface Microtrenched Zn Layer Reduces Risk of Localized Corrosion and Improves Bioactivity of Pure Zn. Coatings 2021, 11, 486. [Google Scholar] [CrossRef]
- Wu, R.; Wang, Q.; Yang, S.; Wu, L.; Gong, S.; Han, Q.; Wu, W. Enhanced Thermal Stability of Exciton Recombination in CsPbI3 Perovskite Nanocrystals via Zinc Alloying. J. Alloys Compd. 2021, 857, 157574. [Google Scholar] [CrossRef]
- Elrouby, M.; El –Shafy Shilkamy, H.A.; Elsayed, A. Development of the Electrochemical Performance of Zinc via Alloying with Indium as Anode for Alkaline Batteries Application. J. Alloys Compd. 2021, 854, 157285. [Google Scholar] [CrossRef]
- Pachla, W.; Przybysz, S.; Jarzębska, A.; Bieda, M.; Sztwiertnia, K.; Kulczyk, M.; Skiba, J. Structural and Mechanical Aspects of Hypoeutectic Zn–Mg Binary Alloys for Biodegradable Vascular Stent Applications. Bioact. Mater. 2021, 6, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H.; Huang, L.; Tian, L.; et al. Development of Biodegradable Zn-1X Binary Alloys with Nutrient Alloying Elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Mostaed, A.; Loffredo, S.; Demir, A.G.; Previtali, B.; Mantovani, D.; Beanland, R.; Vedani, M. Novel Zn-Based Alloys for Biodegradable Stent Applications: Design, Development and in Vitro Degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef]
- Sotoudeh Bagha, P.; Khaleghpanah, S.; Sheibani, S.; Khakbiz, M.; Zakeri, A. Characterization of Nanostructured Biodegradable Zn-Mn Alloy Synthesized by Mechanical Alloying. J. Alloys Compd. 2018, 735, 1319–1327. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Jablonská, E.; Pospíšilová, I.; Lipov, J.; Ruml, T. Structure, Mechanical Characteristics and in Vitro Degradation, Cytotoxicity, Genotoxicity and Mutagenicity of Novel Biodegradable Zn–Mg Alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ren, Y.; Wang, L.; Yang, B.; Li, H.; Qin, G. Abnormal Effect of Mn Addition on the Mechanical Properties of As-Extruded Zn Alloys. Mater. Sci. Eng. A 2017, 701, 129–133. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying Design of Biodegradable Zinc as Promising Bone Implants for Load-Bearing Applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Kubásek, J.; Vojtěch, D. Zn-Based Alloys as an Alternative Biodegradable Materials. Met. 2012 Conf. Proc. 21st Int. Conf. Metall. Mater. 2012, 5, 1355–1361. [Google Scholar]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and Corrosion Properties of Newly Developed Biodegradable Zn-Based Alloys for Bone Fixation. Acta Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Seitz, J.-M.; Eifler, R.; Maier, H.J.; Guillory, R.J.; Earley, E.J.; Drelich, A.; Goldman, J.; Drelich, J.W. Zn-Li Alloy after Extrusion and Drawing: Structural, Mechanical Characterization, and Biodegradation in Abdominal Aorta of Rat. Mater. Sci. Eng. C 2017, 76, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Schille, C.; Schweizer, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Mechanical Characteristics, In Vitro Degradation, Cytotoxicity, and Antibacterial Evaluation of Zn-4.0Ag Alloy as a Biodegradable Material. Int. J. Mol. Sci. 2018, 19, 755. [Google Scholar] [CrossRef] [PubMed]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, Mechanical Properties and in Vitro Degradation Behavior of Newly Developed Zn Ag Alloys for Degradable Implant Applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential Biodegradable Zn-Cu Binary Alloys Developed for Cardiovascular Implant Applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.; Han, Y.; Zhang, Z.; Qu, X.; Zhuang, Y.; Wu, Q.; Zheng, Y.; Dai, K. In Vitro and in Vivo Studies of Zn-Mn Biodegradable Metals Designed for Orthopedic Applications. Acta Biomater. 2020, 108, 358–372. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Chen, D.; Zhu, D.; Dai, K.; Zheng, Y. Enhanced Osseointegration of Zn-Mg Composites by Tuning the Release of Zn Ions with Sacrificial Mg-Rich Anode Design. ACS Biomater. Sci. Eng. 2019, 5, 453–467. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, L.; Wang, L.; Zhang, Q.; Zhu, X.; Sun, W.; Shen, J.; Lu, T.; Song, Z.; Liu, H. Corrosion and Biocompatibility of Pure Zn with a Micro-Arc-Oxidized Layer Coated with Calcium Phosphate. Coatings 2021, 11, 1425. [Google Scholar] [CrossRef]
- Katarivas Levy, G.; Leon, A.; Kafri, A.; Ventura, Y.; Drelich, J.W.; Goldman, J.; Vago, R.; Aghion, E. Evaluation of Biodegradable Zn–1%Mg and Zn–1%Mg–0.5%Ca Alloys for Biomedical Applications. J. Mater. Sci. Mater. Med. 2017, 28, 174. [Google Scholar] [CrossRef] [PubMed]
- Fosmire, G.J. Zinc Toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium Matrix Nanocomposites for Orthopedic Applications: A Review from Mechanical, Corrosion, and Biological Perspectives. Acta Biomater. 2019, 96, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, M.; Bizari, D.; Hassanzade, Z. Investigation of Mechanical Properties and Biocorrosion Behavior of in Situ and Ex Situ Mg Composite for Orthopedic Implants. Mater. Sci. Eng. C 2020, 113, 110974. [Google Scholar] [CrossRef]
- Kubásek, J.; Pospíšilová, I.; Vojtěch, D.; Jablonská, E.; Ruml, T. Structural, Mechanical and Cytotoxicity Characterization of as-Cast Biodegradable Zn-XMg (x = 0.8–8.3%) Alloys. Mater. Tehnol. 2014, 48, 623–629. [Google Scholar]
- Kawahara, M.; Kato-Negishi, M. Link between Aluminum and the Pathogenesis of Alzheimer’s Disease: The Integration of the Aluminum and Amyloid Cascade Hypotheses. Int. J. Alzheimers Dis. 2011, 2011, 276393. [Google Scholar] [CrossRef]
- Kvakaj, T.; Bidulsk, J.; Koiko, R.; Bidulsk, R. Effect of Severe Plastic Deformation on the Properties and Structural Developments of High Purity Al and Al-Cu-Mg-Zr Aluminium Alloy. In Aluminium Alloys, Theory and Applications; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Longauerová, M.; Hodur, M.; Vojtko, M.; Zubko, P.; Glogovský, M.; Demčáková, M.; Matvija, M.; Kvačkaj, T. Structural Nature of Znal4cu1 Alloy Plasticity Affected by Various Technological Treatments. In Defect and Diffusion Forum; Trans Tech Publications: Stafa-Zurich, Switzerland, 2020; Volume 405 DDF. [Google Scholar]
- Bowen, P.K.; Seitz, J.M.; Guillory, R.J.; Braykovich, J.P.; Zhao, S.; Goldman, J.; Drelich, J.W. Evaluation of Wrought Zn–Al Alloys (1, 3, and 5 Wt % Al) through Mechanical and in Vivo Testing for Stent Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 245–258. [Google Scholar] [CrossRef]
- Bao, G.; Fan, Q.; Ge, D.; Wang, K.; Sun, M.; Zhang, Z.; Guo, H.; Yang, H.; He, B.; Zheng, Y. In Vitro and in Vivo Studies to Evaluate the Feasibility of Zn-0.1Li and Zn-0.8Mg Application in the Uterine Cavity Microenvironment Compared to Pure Zinc. Acta Biomater. 2021, 123, 393–406. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Wang, M.; Cheng, H.; Jia, B.; Nie, J.; Dai, K.; Zheng, Y. Zn-0.4Li Alloy Shows Great Potential for the Fixation and Healing of Bone Fractures at Load-Bearing Sites. Chem. Eng. J. 2021, 417, 129317. [Google Scholar] [CrossRef]
- Oliver, A.A.; Guillory, R.J.; Flom, K.L.; Morath, L.M.; Kolesar, T.M.; Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Goldman, J. Analysis of Vascular Inflammation against Bioresorbable Zn–Ag-Based Alloys. ACS Appl. Bio Mater. 2020, 3, 6779–6789. [Google Scholar] [CrossRef]
- Finney, L.; Vogt, S.; Fukai, T.; Glesne, D. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009, 36, 88–94. [Google Scholar] [CrossRef]
- Qu, X.; Yang, H.; Jia, B.; Yu, Z.; Zheng, Y.; Dai, K. Biodegradable Zn–Cu Alloys Show Antibacterial Activity against MRSA Bone Infection by Inhibiting Pathogen Adhesion and Biofilm Formation. Acta Biomater. 2020, 117, 400–417. [Google Scholar] [CrossRef]
- Yu, L.; Tian, Y.; Qiao, Y.; Liu, X. Mn-Containing Titanium Surface with Favorable Osteogenic and Antimicrobial Functions Synthesized by PIII&D. Colloids Surf. B Biointerfaces 2017, 152, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.M.C.; Vieira, S.I.; Cerqueira, A.R.; Pina, S.; da Cruz Silva, O.A.B.; Abrantes, J.C.C.; Ferreira, J.M.F. Effects of Mn-Doping on the Structure and Biological Properties of β-Tricalcium Phosphate. J. Inorg. Biochem. 2014, 136, 57–66. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, P.; Wang, Q.; Wu, H.; Liu, Y.; Deng, Y.; Zhou, Y.; Shuai, C. The Enhancement of Mg Corrosion Resistance by Alloying Mn and Laser-Melting. Materials 2016, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Shi, Z.-Z.; Gao, X.-X.; Liu, X.-F.; Wang, J.-Q.; Wang, L.-N. Adjusting Comprehensive Properties of Biodegradable Zn-Mn Alloy through Solution Heat-Treatment. Mater. Today Commun. 2020, 23, 101150. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Wang, L.; Yan, K.; Li, Y.; Jiang, J.; Ma, A.; Xue, F.; Bai, J. Revealing the Effect of Minor Ca and Sr Additions on Microstructure Evolution and Mechanical Properties of Zn-0.6 Mg Alloy during Multi-Pass Equal Channel Angular Pressing. J. Alloys Compd. 2020, 844, 155923. [Google Scholar] [CrossRef]
- Ghaziof, S.; Gao, W. The Effect of Pulse Electroplating on Zn–Ni Alloy and Zn–Ni–Al2O3 Composite Coatings. J. Alloys Compd. 2015, 622, 918–924. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhou, F.; Yang, Y.; Chang, R.; Qiu, K.; Pu, Z.; Li, L.; Zheng, Y. Micro-Alloying with Mn in Zn–Mg Alloy for Future Biodegradable Metals Application. Mater. Des. 2016, 94, 95–104. [Google Scholar] [CrossRef]
- Kubásek, J.; Pinc, J.; Hosová, K.; Straková, M.; Molnárová, O.; Duchoň, J.; Nečas, D.; Čavojský, M.; Knapek, M.; Godec, M.; et al. The Evolution of Microstructure and Mechanical Properties of Zn-0.8Mg-0.2Sr Alloy Prepared by Casting and Extrusion. J. Alloys Compd. 2022, 906, 164308. [Google Scholar] [CrossRef]
- Shao, X.; Wang, X.; Xu, F.; Dai, T.; Zhou, J.G.; Liu, J.; Song, K.; Tian, L.; Liu, B.; Liu, Y. In Vivo Biocompatibility and Degradability of a Zn–Mg–Fe Alloy Osteosynthesis System. Bioact. Mater. 2022, 7, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Wątroba, M.; Bednarczyk, W.; Kawałko, J.; Mech, K.; Marciszko, M.; Boelter, G.; Banzhaf, M.; Bała, P. Design of Novel Zn–Ag–Zr Alloy with Enhanced Strength as a Potential Biodegradable Implant Material. Mater. Des. 2019, 183, 108154. [Google Scholar] [CrossRef]
- Dai, Q.; Peng, S.S.; Zhang, Z.; Liu, Y.; Fan, M.; Zhao, F. Microstructure and Mechanical Properties of Zinc Matrix Biodegradable Composites Reinforced by Graphene. Front. Bioeng. Biotechnol. 2021, 9, 635338. [Google Scholar] [CrossRef]
- Dai, Q.; Peng, S.S.; Zhang, Z.; Liu, Y.; Fan, M.; Zhao, F.; Liu, Y.; Yin, S.; Peng, S.S.; Zhang, Z.; et al. Biocompatibility of Zinc Matrix Biodegradable Composites Reinforced by Graphene Nanosheets. Materials 2022, 15, 6481. [Google Scholar] [CrossRef]
- Zhao, J.; Haowei, M.; Saberi, A.; Heydari, Z.; Baltatu, M.S. Carbon Nanotube (CNT) Encapsulated Magnesium-Based Nanocomposites to Improve Mechanical, Degradation and Antibacterial Performances for Biomedical Device Applications. Coatings 2022, 12, 1589. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Saberi, A.; Heydari, Z. Journal of the Mechanical Behavior of Biomedical Materials the Effect of Co-Encapsulated GNPs-CNTs Nanofillers on Mechanical Properties, Degradation and Antibacterial Behavior of Mg-Based Composite. J. Mech. Behav. Biomed. Mater. 2023, 138, 105601. [Google Scholar] [CrossRef] [PubMed]
- Amirzade-Iranaq, M.T.; Omidi, M.; Bakhsheshi-Rad, H.R.; Saberi, A.; Abazari, S.; Teymouri, N.; Naeimi, F.; Sergi, C.; Ismail, A.F.; Sharif, S.; et al. MWCNTs-TiO2 Incorporated-Mg Composites to Improve the Mechanical, Corrosion and Biological Characteristics for Use in Biomedical Fields. Materials 2023, 16, 1919. [Google Scholar] [CrossRef]
- Zhang, H.; Saberi, A.; Heydari, Z.; Baltatu, M.S. Bredigite-CNTs Reinforced Mg-Zn Bio-Composites to Enhance the Mechanical and Biological Properties for Biomedical Applications. Materials 2023, 16, 1681. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.; Peng, S.; Xu, L.; He, C.; Qi, F.; Zhao, M.; Shuai, C. Microstructure Evolution and Texture Tailoring of Reduced Graphene Oxide Reinforced Zn Scaffold. Bioact. Mater. 2021, 6, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhao, F.; Liu, Y.; Yin, S.; Peng, S.; Zhang, Z. Zinc Matrix Composites Reinforced with Partially Unzipped Carbon Nanotubes as Biodegradable Implant Materials. Crystals 2022, 12, 1110. [Google Scholar] [CrossRef]
- Owhal, A.; Choudhary, M.; Pingale, A.D.; Belgamwar, S.U.; Mukherjee, S.; Rathore, J.S. Non-Cytotoxic Zinc/f-Graphene Nanocomposite for Tunable Degradation and Superior Tribo-Mechanical Properties: Synthesized via Modified Electro Co-Deposition Route. Mater. Today Commun. 2023, 34, 105112. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In Vitro and in Vivo Studies on Zinc-Hydroxyapatite Composites as Novel Biodegradable Metal Matrix Composite for Orthopedic Applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef]
- Pinc, J.; Čapek, J.; Hybášek, V.; Průša, F.; Hosová, K.; Maňák, J.; Vojtěch, D. Characterization of Newly Developed Zinc Composite with the Content of 8 Wt.% of Hydroxyapatite Particles Processed by Extrusion. Materials 2020, 13, 1716. [Google Scholar] [CrossRef]
- Pinc, J.; Miklášová, E.; Průša, F.; Čapek, J.; Drahokoupil, J.; Vojtěch, D. Influence of Processing on the Microstructure and the Mechanical Properties of Zn/HA8 Wt.% Biodegradable Composite. Manuf. Technol. 2019, 19, 836–841. [Google Scholar] [CrossRef]
- Pan, C.; Sun, X.; Xu, G.; Su, Y.; Liu, D. The Effects of β-TCP on Mechanical Properties, Corrosion Behavior and Biocompatibility of β-TCP/Zn-Mg Composites. Mater. Sci. Eng. C 2020, 108, 110397. [Google Scholar] [CrossRef]
- Sun, X.; Yu, X.; Li, W.; Chen, M.; Liu, D. Fabrication and Characterization of Biodegradable Zinc Matrix Composites Reinforced by Uniformly Dispersed Beta-Tricalcium Phosphate via Graphene Oxide-Assisted Hetero-Agglomeration. Mater. Sci. Eng. C 2021, 130, 112431. [Google Scholar] [CrossRef]
- Mahesh, P.V.S.; Akhil, S.; Chiranjeevi, P.; Sivaji, Y.; Kondaiah, V.V.; Rao, M.A.; Dumpala, R.; Sunil, B.R. Developing Composites of Zinc and Hydroxyapatite for Degradable Orthopedic Implant Applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1116, 012002. [Google Scholar] [CrossRef]
- Narendra Kumar, Y.; Venkateswarlu, B.; Ratna Raju, L.; Dumpala, R.; Ratna Sunil, B. Developing Zn-MgO Composites for Degradable Implant Applications by Powder Metallurgy Route. Mater. Lett. 2021, 302, 130433. [Google Scholar] [CrossRef]
- Schlatter, R. Vacuum Induction Melting. Jom 1972, 24, 17–25. [Google Scholar] [CrossRef]
- ZHAO, J.; SHANGGUAN, J.; GU, C.; JIN, B.; SHI, Y. Microstructures and Mechanical Properties of TC4/AZ91D Bimetal Prepared by Solid–Liquid Compound Casting Combined with Zn/Al Composite Interlayer. Trans. Nonferr. Met. Soc. China 2022, 32, 1144–1158. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Koltygin, A.V.; Sung, M.C.; Park, S.H.; Tselovalnik, Y.V.; Stepashkin, A.A.; Rizhsky, A.A.; Belov, M.V.; Belov, V.D.; Malyutin, K.V. Development of Mg–Zn–Y–Zr Casting Magnesium Alloy with High Thermal Conductivity. J. Magnes. Alloy. 2021, 9, 1567–1577. [Google Scholar] [CrossRef]
- Sun, S.; Li, D.; Liu, D.; Yang, B.; Ren, Y.; Liu, J.; Xie, H. Effects of Bi and Ce Addition on Tensile Properties and Corrosion Resistance of Zn-15Al Alloys by Continuous Casting and Extrusion. Mater. Lett. 2020, 275, 128027. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Li, Z.-L.; Bai, W.-S.; Tuoliken, A.; Yu, J.; Liu, X.-F. (Fe, Mn)Zn13 Phase and Its Core-Shell Structure in Novel Biodegradable Zn-Mn-Fe Alloys. Mater. Des. 2019, 162, 235–245. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Li, H.-Y.; Xu, J.-Y.; Gao, X.-X.; Liu, X.-F. Microstructure Evolution of a High-Strength Low-Alloy Zn–Mn–Ca Alloy through Casting, Hot Extrusion and Warm Caliber Rolling. Mater. Sci. Eng. A 2020, 771, 138626. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Liu, D.; Zhao, Y.; Sun, X. Microstructure and Properties of β-TCP/Zn–1Mg Composites Processed by Hot Extrusion Combination of Multi-Pass ECAP. J. Alloys Compd. 2022, 903, 163988. [Google Scholar] [CrossRef]
- Svetlizky, D.; Eliaz, N. Sol–Gel Encapsulation of ZnAl Alloy Powder with Alumina Shell. Coatings 2021, 11, 1389. [Google Scholar] [CrossRef]
- Sun, X.; Su, Y.; Huang, Y.; Chen, M.; Liu, D. Microstructure Evolution and Properties of β-TCP/Mg-Zn-Ca Biocomposite Processed by Hot Extrusion Combined with Multi-Pass ECAP. Metals 2022, 12, 685. [Google Scholar] [CrossRef]
- Singh, A.; Osawa, Y.; Somekawa, H.; Mukai, T. Effect of Solidification Cooling Rate on Microstructure and Mechanical Properties of an Extruded Mg-Zn-Y Alloy. Metals 2018, 8, 337. [Google Scholar] [CrossRef]
- Nguyen, Y.N.; Dao, A.-T.; Le, M.-H.; Dang, K.Q.; Nanko, M. Fabrication of transparent MgAl2O4 spinel ceramics by PECS processing of combustion—Sythesized nanopowders. Acta Metall. Slovaca 2019, 25, 186–192. [Google Scholar] [CrossRef]
- Vida, T.A.; Conde, A.; Freitas, E.S.; Arenas, M.A.; Cheung, N.; Brito, C.; de Damborenea, J.; Garcia, A. Directionally Solidified Dilute Zn-Mg Alloys: Correlation between Microstructure and Corrosion Properties. J. Alloys Compd. 2017, 723, 536–547. [Google Scholar] [CrossRef]
- Jarzębska, A.; Bieda, M.; Kawałko, J.; Rogal, Ł.; Koprowski, P.; Sztwiertnia, K.; Pachla, W.; Kulczyk, M. A New Approach to Plastic Deformation of Biodegradable Zinc Alloy with Magnesium and Its Effect on Microstructure and Mechanical Properties. Mater. Lett. 2018, 211, 58–61. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Izman, S.; Kurniawan, D.; Farahany, S.; Yahaya, B.; Hermawan, H. Influence of Thermal Treatment on Microstructure, Mechanical and Degradation Properties of Zn–3Mg Alloy as Potential Biodegradable Implant Material. Mater. Des. 2015, 85, 431–437. [Google Scholar] [CrossRef]
- Krystýnová, M.; Doležal, P.; Fintová, S.; Březina, M.; Zapletal, J.; Wasserbauer, J. Preparation and Characterization of Zinc Materials Prepared by Powder Metallurgy. Metals 2017, 7, 396. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, P.; Voshage, M.; Chen, Y.; Georg, P.; Jauer, L.; Xia, D.; Guo, H.; Zheng, Y.; Henrich, J. Additive Manufacturing of Biodegradable Zn-XWE43 Porous Scaffolds: Formation Quality, Microstructure and Mechanical Properties. Mater. Des. 2019, 181, 107937. [Google Scholar] [CrossRef]
- Bidulský, R.; Bidulská, J.; Gobber, F.S.; Kvačkaj, T.; Petroušek, P.; Actis-Grande, M.; Weiss, K.-P.; Manfredi, D. Case Study of the Tensile Fracture Investigation of Additive Manufactured Austenitic Stainless Steels Treated at Cryogenic Conditions. Materials 2020, 13, 3328. [Google Scholar] [CrossRef]
- Gajdoš, I.; Spišák, E.; Kaščák, L.; Krasinskyi, V. Surface Finish Techniques for FDM Parts. Mater. Sci. Forum 2015, 818, 45–48. [Google Scholar] [CrossRef]
- Cao, Q.; Qi, B.; Zeng, C.; Zhang, R.; He, B.; Qi, Z.; Wang, F.; Wang, H.; Cong, B. Achieving Equiaxed Microstructure and Isotropic Mechanical Properties of Additively Manufactured AZ31 Magnesium Alloy via Ultrasonic Frequency Pulsed Arc. J. Alloys Compd. 2022, 909, 164742. [Google Scholar] [CrossRef]
- Parilak, L.; Dudrova, E.; Bidulsky, R.; Kabatova, M. Derivation, Testing and Application of a Practical Compaction Equation for Cold Die-Compacted Metal Powders. Powder Technol. 2017, 322, 447–460. [Google Scholar] [CrossRef]
- Gobber, F.S.; Bidulská, J.; Fais, A.; Bidulský, R.; Grande, M.A. Innovative Densification Process of a Fe-Cr-C Powder Metallurgy Steel. Metals 2021, 11, 665. [Google Scholar] [CrossRef]
- Lu, S.; Liu, S.; Hou, P.; Yang, B.; Liu, M.; Yin, L.; Zheng, W. Soft Tissue Feature Tracking Based on Deep Matching Network. Comput. Model. Eng. Sci. 2023, 136, 363–379. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, P.; Wang, N.; Peng, L.; Kang, B.; Zeng, H.; Yuan, G.; Ding, W. Challenges and Solutions for the Additive Manufacturing of Biodegradable Magnesium Implants. Engineering 2020, 6, 1267–1275. [Google Scholar] [CrossRef]
- Lima, D.D.; Campo, K.N.; Button, S.T.; Caram, R. 3D Thixo-Printing: A Novel Approach for Additive Manufacturing of Biodegradable Mg-Zn Alloys. Mater. Des. 2020, 196, 109161. [Google Scholar] [CrossRef]
- Dhandapani, R.; Krishnan, P.D.; Zennifer, A.; Kannan, V.; Manigandan, A.; Arul, M.R.; Jaiswal, D.; Subramanian, A.; Kumbar, S.G.; Sethuraman, S. Additive Manufacturing of Biodegradable Porous Orthopaedic Screw. Bioact. Mater. 2020, 5, 458–467. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Tomasina, C.; Calore, A.; Moroni, L. Additive Manufactured, Highly Resilient, Elastic, and Biodegradable Poly(Ester)Urethane Scaffolds with Chondroinductive Properties for Cartilage Tissue Engineering. Mater. Today Bio 2020, 6, 100051. [Google Scholar] [CrossRef]
- Shuai, C.; Cheng, Y.; Yang, Y.; Peng, S.; Yang, W.; Qi, F. Laser Additive Manufacturing of Zn-2Al Part for Bone Repair: Formability, Microstructure and Properties. J. Alloys Compd. 2019, 798, 606–615. [Google Scholar] [CrossRef]
- Hara, A.; Świątek, Z.; Ozga, P. The Role of Surfactants in Induced Electrodeposition of Zn–Mo Layer from Citrate Solutions. J. Alloys Compd. 2020, 827, 154195. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Chen, Z.; He, X.; Li, X.; Sun, X. Fabrication, in Vitro and in Vivo Properties of β-TCP/Zn Composites. J. Alloys Compd. 2022, 913, 165223. [Google Scholar] [CrossRef]
- Safavi, M.S.; Bordbar-Khiabani, A.; Khalil-Allafi, J.; Mozafari, M.; Visai, L. Additive Manufacturing: An Opportunity for the Fabrication of Near-Net-Shape NiTi Implants. J. Manuf. Mater. Process. 2022, 6, 65. [Google Scholar] [CrossRef]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, W.; Wang, Y.; Zhao, Z. Mechanical Performance and Damage Monitoring of CFRP Thermoplastic Laminates with an Open Hole Repaired by 3D Printed Patches. Compos. Struct. 2023, 303, 116308. [Google Scholar] [CrossRef]
- Wang, L.; Wu, T.; Wang, D.; Liang, Z.; Yang, X.; Peng, Z.; Liu, Y.; Liang, Y.; Zeng, Z.; Oliveira, J.P. A Novel Heterogeneous Multi-Wire Indirect Arc Directed Energy Deposition for in-Situ Synthesis Al-Zn-Mg-Cu Alloy: Process, Microstructure and Mechanical Properties. Addit. Manuf. 2023, 72, 103639. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, S.; Deng, Y.; Zhang, Y.; Yin, L.; Zheng, W. An Improved Method for Soft Tissue Modeling. Biomed. Signal Process. Control 2021, 65, 102367. [Google Scholar] [CrossRef]

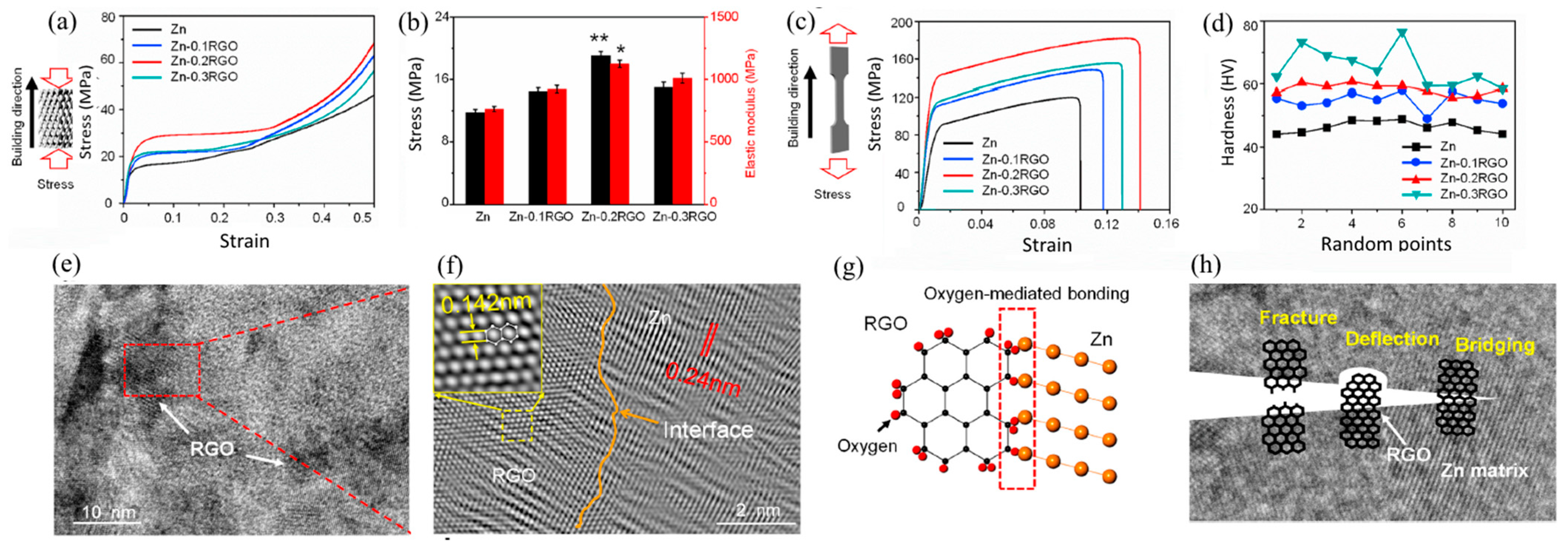
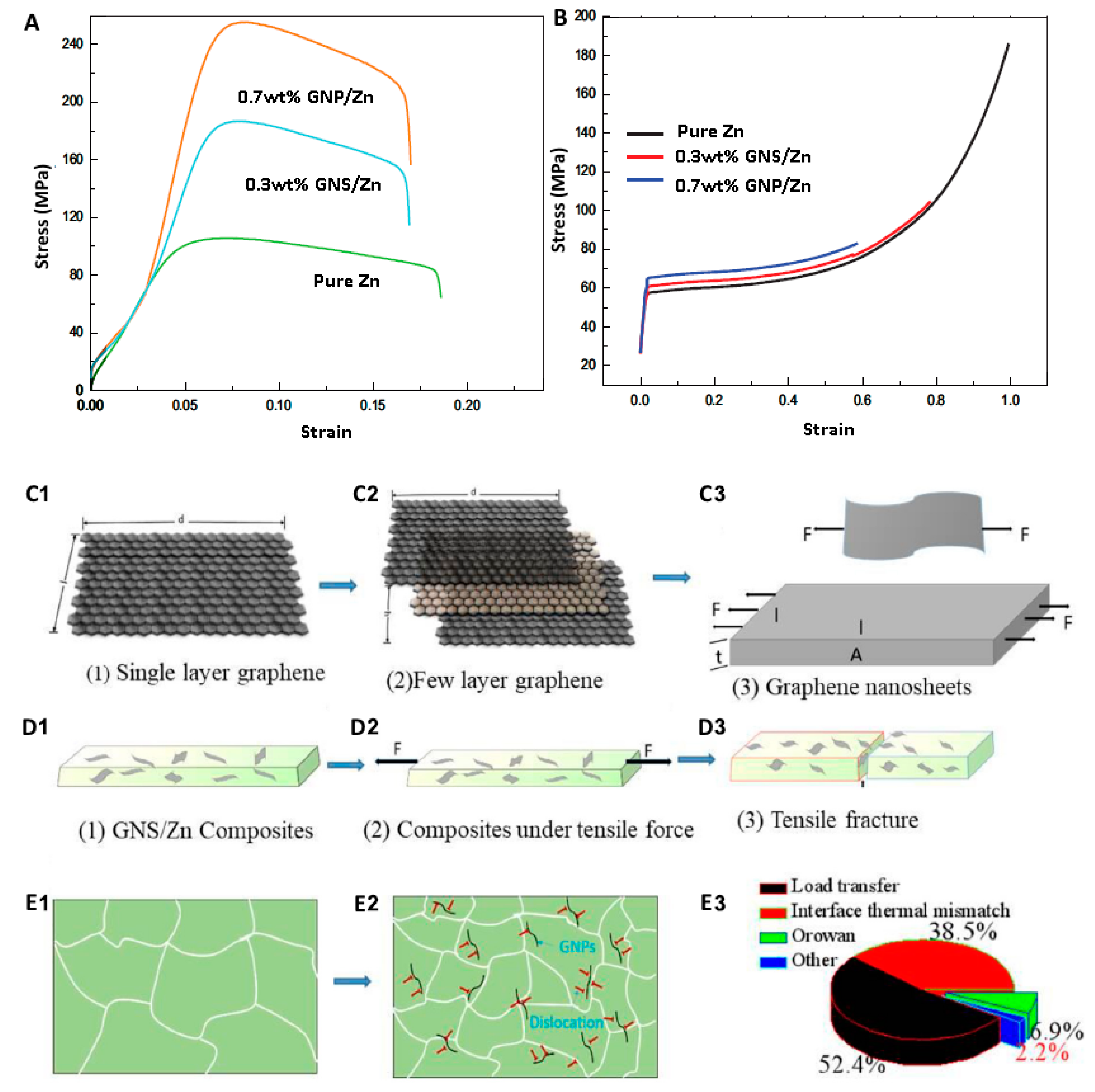
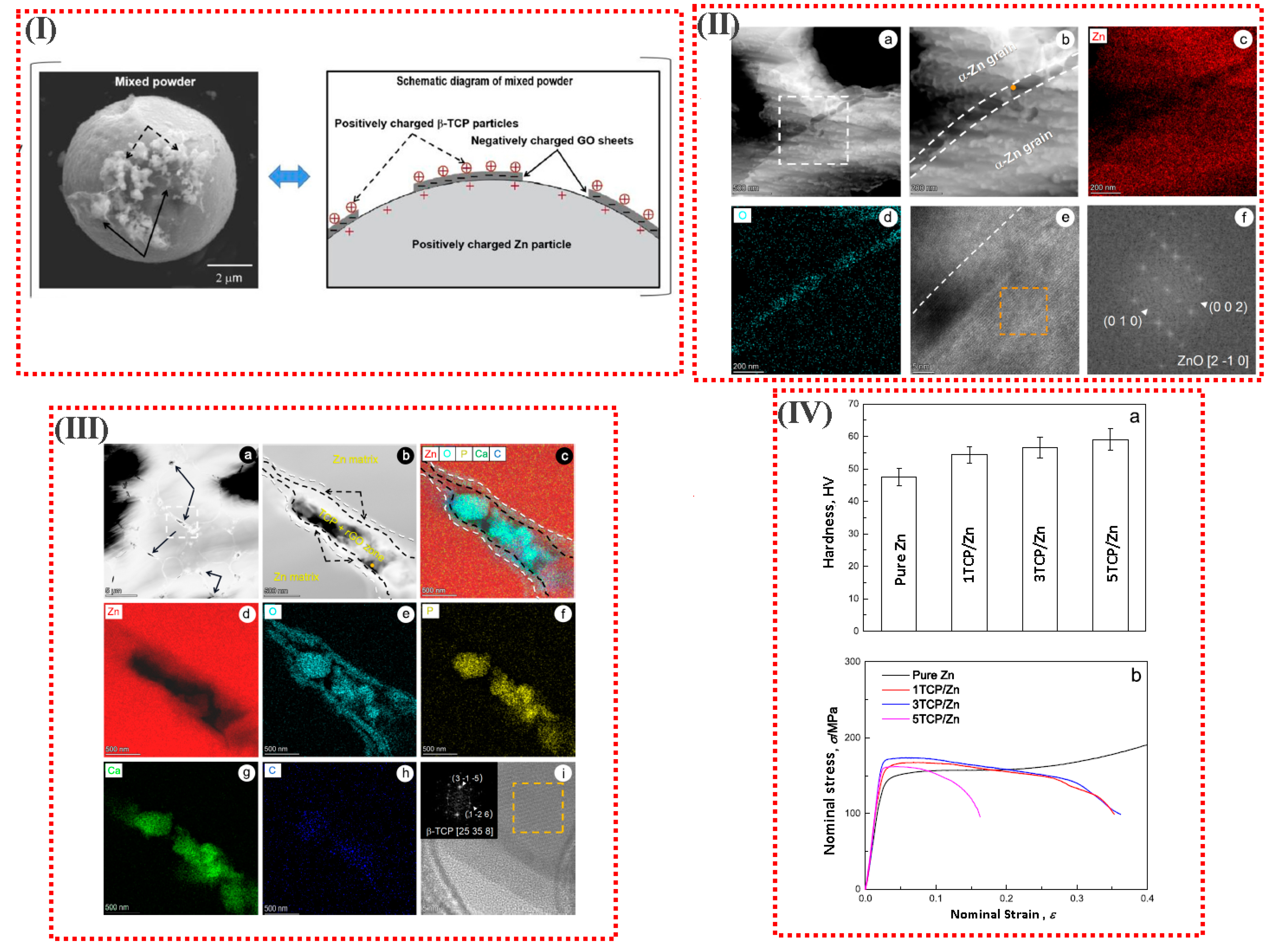


| Material Type | Density (g/cm3) | UCS (MPa) | UTS (MPa) | E (GPa) | ε (%) | Degradation Rate (mm y−1) | Refs. |
|---|---|---|---|---|---|---|---|
| Natural bone | 1.8–2.1 | 164–200 | 35–283 | 3–20 | 3–4 | NBR | [40,41] |
| Ti alloy | 4.4–4.5 | 900 | 900–1000 | 110–127 | 10–15 | No | [40,42] |
| Stainless Steel | 7.9–8.1 | 500–1000 | 460–1700 | 189–205 | 10–40 | No | [43] |
| Co-Cr alloy | 7.9–8.1 | 450–1000 | 860 | 230 | 20 | No | [39] |
| Mg pure | 1.74–2 | 65–100 | 135–285 | 41–45 | 7–40 | 0.8–2.7 | [40,44] |
| Zn pure | 7.1 | 30–100 | 100–150 | 78–121 | 0.3–2 | 0.1–0.3 | [45,46,47] |
| Fe pure | 7.8 | 560 | 300 | 213 | 37.5 | 0.1 | [11,48] |
| Mechanical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tissue/Alloy | Processing Technique | Tensile Yield Strength (TYS) (MPa) | Ultimate Tensile Strength (UTS) (MPa) | Compressive Yield Strength (CYS) | Ultimate Compressive Strength (UCS) | Elongation (%) | Hardness (HV) | Ref. |
| Pure Zn | ||||||||
| Zn | Cast + HE | 68 | 135 | - | - | 61.2 | 32 | [58] |
| Zn | Cast | 9.3 | 18.1 | - | - | 0.34 | 37.8 | [46] |
| Zn | Rolled | 30 | 50 | - | - | 5.62 | 40 | [46] |
| Zn | Extruded | 34.6 | 63.9 | 103.1 | - | 3.57 | - | [46] |
| Zn | Cast | 10 ± 2 | 18 ± 3 | 102.92 ± 6.73 | - | 0.3 ± 0.1 | 38.2 ± 1.0 | [59] |
| Zn | HE | 51 ± 3.7 | 111 ± 4.5 | - | - | 60 ± 5.9 | 34 ± 1.7 | [60] |
| Zn | PM | - | - | - | 33 | 16 | 18 | [61] |
| Zn | HE | 55 ± 8 | 97 ± 10 | 94 ± 13 | - | 77 ± 2.7 | 44 ± 6 | [62] |
| Zn | 60 | 117 | - | - | 14 | - | [63] | |
| Zn | HE | 124 | 164 | 94 | 276 | 39.3 | 44 | [64] |
| Zn-Mg | ||||||||
| Zn-1.5 Mg | Cast + HE | 356 | 463 | - | - | 38.6 | 120 | [58] |
| Zn-0.5 Mg | Cast + HE | 373 | 514 | - | - | 10.5 | 107 | |
| Zn-1Mg | Cast + HE | 367 | 478 | - | - | 24.9 | 111 | |
| Zn-1Mg | Cast | 130 ± 10 | 180 ± 21 | 284.50 ± 16.90 | - | 2 ± 0.2 | 78.26 ± 2.84 | [59] |
| Zn-0.15Mg | HE | 114 ± 7.7 | 250 ± 9.2 | - | - | 22 ± 4 | 52 ± 4.9 | [60] |
| Zn-0.5Mg | HE | 159 ± 8.9 | 297 ± 6.9 | - | - | 13 ± 0.9 | 65 ± 3.9 | [60] |
| Zn-1Mg | HE | 180 ± 7.3 | 340 ± 15.6 | - | - | 6 ± 1.1 | 75 ± 3.9 | [60] |
| Zn-3Mg | HE | 291 ± 9.3 | 399 ± 14.4 | - | - | 1 ± 0.1 | 117 ± 7.1 | [60] |
| Zn-0.8Mg | HE | 203 ± 7 | 301 ± 8 | 186 ± 10 | - | 13 ± 2 | 83 ± 5 | [62] |
| Zn-1.6Mg | HE | 232 ± 8 | 368 ± 8 | 257 ± 13 | - | 4 ± 0.3 | 97 ± 4 | [62] |
| Zn-1 Mg | Cast | - | 153 | - | - | 1.5 | 65 | [65] |
| Zn-0.1 Mg | HE | 214 | 274 | 201 | 534 | 10.2 | 70 | [64] |
| Zn-0.4Mg | HE | 284 | 353 | 281 | 604 | 15.2 | 82 | [64] |
| Zn-0.8 Mg | HE | 297 | 386 | 304 | 631 | 9.3 | 96 | [64] |
| Zn-Ca | ||||||||
| Zn-1Ca | Cast | 119 ± 7 | 165 ± 14 | 280.7 ± 20.7 | - | 2.1 ± 0.2 | 73 ± 7.43 | [59] |
| Zn-0.1 | HE | 127 | 169 | 122 | 289 | 37.9 | 45 | [64] |
| Zn-0.4 | HE | 116 | 166 | 111 | 269 | 26.7 | 44 | [64] |
| Zn-0.8 | HE | 127 | 173 | 111 | 303 | 27.9 | 44 | [64] |
| Zn-Sr | ||||||||
| Zn-1Sr | Cast | 120 ± 6 | 171 ± 14 | 340.9 ± 17.7 | - | 2 ± 0.2 | 61.8 ± 6.7 | [59] |
| Zn-0.1Sr | HE | 89 | 139 | 88 | 250 | 34.5 | 44 | [64] |
| Zn-0.4Sr | HE | 106 | 153 | 94 | 293 | 20.2 | 44 | [64] |
| Zn-0.8Sr | HE | 104 | 151 | 105 | 306 | 30.0 | 48 | [64] |
| Zn-Al | ||||||||
| ZnAl4Cu1 | Cast | 171 | 210 | - | - | 1 | 80 | [66] |
| Zn-0.5Al | As cast-Extruded | 119 ± 2.3 | 2.3 ± 9.6 | - | - | 33 ± 1.2 | 59 ± 5.8 | [60] |
| Zn-1Al | As cast-Extruded | 134 ± 5.8 | 223 ± 4.3 | - | - | 24 ± 4.2 | 73 ± 4.6 | [60] |
| Zn-Mg-Ca | ||||||||
| Zn-1Mg-1Ca | Cast | 79.8 | 129.6 | - | - | 1.02 | 91.5 | [46] |
| Zn-1Mg-1Ca | Rolled | 127.1 | 197.1 | - | - | 8.37 | 106.7 | [46] |
| Zn-1Mg-1Ca | Extruded | 205.9 | 257.4 | 299.4 | 3272.4 | 5.35 | - | [46] |
| Zn-Mg-Sr | ||||||||
| Zn-1Mg-1Sr | Cast | 85.6 | 136.1 | - | - | 1.20 | 85.2 | [46] |
| Zn-1Mg-1Sr | Rolled | 139.2 | 202.1 | - | - | 9.63 | 91.8 | [46] |
| Zn-1Mg-1Sr | Extruded | 202.4 | 253.8 | 383.5 | 3848.3 | 7.44 | - | [46] |
| Zn-Ca-Sr | ||||||||
| Zn-1Ca-1Sr | Cast | 84.4 | 139.6 | - | - | 1.13 | 90.1 | [46] |
| Zn-1Ca-1Sr | Rolled | 142.8 | 202.8 | - | - | 8.73 | 86 | [46] |
| Zn-1Ca-1Sr | Extruded | 213.0 | 260.9 | 340.9 | 3244.3 | 6.76 | - | [46] |
| Zn-Li | ||||||||
| Zn-Li | Cast | 238 ± 60 | 274 ± 61 | 17 ± 7 | 97 ± 2 | [67] | ||
| Zn-0.1Li | HE | 341 | 431 | 306 | 784 | 28.1 | 108 | [64] |
| Zn-0.4Li | HE | 387 | 520 | 434 | 794 | 5 | 164 | [64] |
| Zn-0.8Li | HE | - | - | 454 | 1022 | - | 216 | [64] |
| Zn-Ag | ||||||||
| Zn-4Ag | TT | 157 | 261 | - | - | 37 | 73 | [68] |
| Zn-4Ag | APH | 149 | 215 | - | - | 24 | 82 | [68] |
| Zn-2.5Ag | 160 | 200 | - | - | 35 | - | [69] | |
| Zn-5.0Ag | 210 | 260 | - | - | 39 | - | [69] | |
| Zn-7.0Ag | 230 | 280 | - | - | 32 | - | [69] | |
| Zn-0.4Ag | HE | 127 | 167 | 88 | 162 | 38.1 | 50 | [64] |
| Zn-0.8Ag | HE | 134 | 184 | 82 | 177 | 58.3 | 58 | [64] |
| Zn-2.5Ag | HE | 186 | 231 | 145 | 221 | 36.7 | 55 | [64] |
| Zn-Cu | ||||||||
| Zn-1Cu | As cast- Extruded | 148.7 ± 0.5 | 186.3 ± 0.5 | - | - | 21.0 ± 4.4 | - | [70] |
| Zn-2Cu | As cast- Extruded | 199.7 ± 4.2 | 240.0 ± 1.4 | - | - | 46.8 ± 1.4 | - | [70] |
| Zn-3Cu | As cast- Extruded | 213.7 ± 1 | 257.0 ± 0.81 | - | - | 47.2 ± 1 | - | [70] |
| Zn-4Cu | As cast- Extruded | 227 ± 5 | 270.7 ± 0.5 | - | - | 50.6 ± 2.8 | - | [70] |
| Zn-0.4Cu | HE | 150 | 197 | 139 | 451 | 40.2 | 59 | [64] |
| Zn-0.8Cu | HE | 184 | 234 | 165 | 495 | 33.1 | 69 | [64] |
| Zn-2Cu | HE | 223 | 270 | 233 | 527 | 40.7 | 75 | [64] |
| Zn-Mn | ||||||||
| Zn-0.1 Mn | Extruded | 125 | 175 | - | - | 40 | - | [71] |
| Zn-0.4 Mn | Extruded | 165 | 220 | - | - | 45 | - | [71] |
| Zn-0.8 Mn | Extruded | 165 | 175 | - | - | 85 | - | [71] |
| Zn-4 Mn | PM | - | - | - | 290.8 | 14.9 | 102 | [61] |
| Zn-24Mn | PM | - | - | - | 132.4 | 6.7 | 71 | [61] |
| Zn-0.2Mn | As cast- Extruded | 132 | 220 | - | - | 48 | - | [63] |
| Zn-0.4Mn | As cast- Extruded | 123 | 198 | - | - | 54 | - | [63] |
| Zn-0.6Mn | As cast- Extruded | 118 | 182 | - | - | 71 | - | [63] |
| Zn-0.1Mn | HE | 131 | 177 | 125 | 383 | 39.8 | 54 | [64] |
| Zn-0.4Mn | HE | 160 | 214 | 136 | 439 | 43.4 | 57 | [64] |
| Zn-0.8Mn | HE | 156 | 190 | 145 | 383 | 83.8 | 50 | [64] |
| Mechanical Properties of Zn Based Composites | ||||||||
|---|---|---|---|---|---|---|---|---|
| Composite/Zn Based | Processing Technique | Tensile Yield Strength (TYS) (MPa) | Ultimate Tensile Strength (UTS) (MPa) | Compressive Yield Strength (CYS) | Ultimate Compressive Strength (UCS) | Elongation (%) | Hardness (HV) | Ref. |
| Carbon elements reinforcements | ||||||||
| Zn | LPBF | 91.6 ± 7.3 | 119.9 ± 8.5 | - | - | 9.5 ± 10 | - | [104] |
| Zn-0.1RGO | LPBF | 111.3 ± 9.1 | 148.5 ± 10.6 | - | - | 11.7 ± 1.2 | - | [104] |
| Zn-0.2RGO | LPBF | 142.9 ± 13.4 | 182.1 ± 15.4 | - | - | 14.1 ± 1.8 | - | [104] |
| Zn-0.3RGO | LPBF | 115.7 ± 17.5 | 155.2 ± 18.1 | - | - | 12.9 ± 2.3 | - | [104] |
| 0.1PUCNTs/Zn | SPS | 157 | 185 | - | - | 113 | [105] | |
| 0.2PUCNTs/Zn | SPS | 169 | 210 | - | - | 67 | - | [105] |
| 0.3PUCNTs/Zn | SPS | 118 | 131 | - | 58 | - | [105] | |
| 0.5PUCNTs/Zn | SPS | 125 | 133 | - | - | 52 | - | [105] |
| Pure Zn | SPS | - | 112 | - | 77 | - | 54 (AF) | [98] |
| 0.3GNS/Zn | SPS | - | 187 | - | 100 | - | 58 (AF) | [98] |
| 0.7GNS/Zn | SPS | - | 254 | - | 185 | - | 65 (AF) | [98] |
| Pure Zn | M-ECD + PM | - | - | 97 | - | - | 57 | [106] |
| Zn/f-GNP | M-ECD-PM | - | - | 182–284 | - | - | 68.8–108.5 | [106] |
| Bioceramic reinforcements | ||||||||
| Zn | SPS | - | - | ≈ 51 | ≈172 | - | ≈44 | [107] |
| Zn-1HA | SPS | - | - | ≈66 | ≈157 | - | ≈46 | [107] |
| Zn-5HA | SPS | - | - | ≈42 | ≈108 | - | ≈44 | [107] |
| Zn-10HA | SPS | - | - | ≈44 | ≈71 | - | ≈45 | [107] |
| Zn | Ex | - | - | 153.6 ± 11.1 | 243.8 ± 1.5 | - | 45.6 ± 2 | [108] |
| Zn/8HA | Ex | - | - | 112.8 ± 5.1 | 168.9 ± 3.7 | - | 44.7 ± 4.5 | [108] |
| Zn | SPS | - | - | 92.1 ± 1.2 | 128.7 ± 1.7 | - | 36.8 ± 1.4 | [109] |
| Zn/HA8 | SPS | - | - | 67.9 ± 7.4 | 88.9 ± 7.2 | - | 34.3 ± 4.5 | [109] |
| Zn-1Mg | UCHET | 235.7 ± 5 | 315.2 ± 8 | - | - | 6.7 ± 2 | - | [110] |
| Zn-1Mg-1TCP | UCHET | 250.8 ± 7 | 330.5 ± 9 | - | - | 11.7 ± 3 | - | [110] |
| Zn-1Mg-3TCP | UCHET | 220.3 ± 8 | 308.1 ± 7 | - | - | 4.5 ± 2 | - | [110] |
| Zn-1Mg-5TCP | UCHET | 194.7 ± 8 | 299.4 ± 9 | - | - | 3.9 ± 3 | - | [110] |
| Pure Zn | GHA-SPS | - | - | 121.3 ± 6.8 | 156.9 ± 4.8 | - | 47.3 ± 2.7 | [111] |
| 1TCP/Zn | GHA-SPS | - | - | 132.3 ± 5.3 | 167.5 ± 3.9 | - | 54.4 ± 2.5 | [111] |
| 3TCP/Zn | GHA-SPS | - | - | 141.8 ± 7.7 | 173.9 ± 4.9 | - | 56.5 ± 3.2 | [111] |
| 5TCP/Zn | GHA-SPS | - | - | 142.4 ± 6.4 | 161.8 ± 4.4 | - | 59.0 ± 3.3 | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Heydari, Z.; Lami, G.H.; Saberi, A.; Baltatu, M.S.; Vizureanu, P. A Comprehensive Review of the Current Research Status of Biodegradable Zinc Alloys and Composites for Biomedical Applications. Materials 2023, 16, 4797. https://doi.org/10.3390/ma16134797
Kong L, Heydari Z, Lami GH, Saberi A, Baltatu MS, Vizureanu P. A Comprehensive Review of the Current Research Status of Biodegradable Zinc Alloys and Composites for Biomedical Applications. Materials. 2023; 16(13):4797. https://doi.org/10.3390/ma16134797
Chicago/Turabian StyleKong, Lingyun, Zahra Heydari, Ghadeer Hazim Lami, Abbas Saberi, Madalina Simona Baltatu, and Petrica Vizureanu. 2023. "A Comprehensive Review of the Current Research Status of Biodegradable Zinc Alloys and Composites for Biomedical Applications" Materials 16, no. 13: 4797. https://doi.org/10.3390/ma16134797
APA StyleKong, L., Heydari, Z., Lami, G. H., Saberi, A., Baltatu, M. S., & Vizureanu, P. (2023). A Comprehensive Review of the Current Research Status of Biodegradable Zinc Alloys and Composites for Biomedical Applications. Materials, 16(13), 4797. https://doi.org/10.3390/ma16134797








