Abstract
The superheating process is a unique grain refining method found only in aluminum-containing magnesium alloys. It is a relatively simple method of controlling the temperature of the melt without adding a nucleating agent or refining agent for grain refinement. Although previous studies have been conducted on this process, the precise mechanism underlying this phenomenon has yet to be elucidated. In this study, a new approach was used to investigate the grain refinement mechanism of aluminum-containing magnesium alloys by the melting superheating process. AZ91 alloy, a representative Mg-Al alloy, was used in the study, and a rapid solidification process was designed to enable precise temperature control. Temperature control was successfully conducted in a unique way by measuring the temperature of the ceramic tube during the rapid solidification process. The presence of Al8Mn5 and Al10Mn3 particles in non-superheated and superheated AZ91 ribbon samples, respectively, manufactured by the rapid solidification process, was revealed. The role of these Al-Mn particles as nucleants in non-superheated and superheated samples was examined by employing STEM equipment. The crystallographic coherence between Al8Mn5 particles and magnesium was very poor, while Al10Mn3 particles showed better coherence than Al8Mn5. We speculated that Al10Mn3 particles generated by the superheating process may act as nucleants for α-Mg grains; this was the main cause of the superheating grain refinement of the AZ91 alloy.
1. Introduction
Magnesium alloys are among the lightest metals, featuring low density and high specific strength properties [1]. These characteristics are considered as important factors in various industrial fields, particularly in aerospace, automotive, and electronics industries where weight reduction is crucial for specific applications [2]. The utilization of magnesium alloys for creating lightweight and robust structures can reduce fuel consumption, enhance energy efficiency, and decrease environmental impact [3]. However, the strength and ductility of magnesium alloys may be lower compared to other lightweight materials, such as aluminum alloys, which highlights the need to develop methods to enhance the strength and ductility of magnesium alloys in order to make them more competitive in various applications [3].
Grain refinement in magnesium alloys has proven to be an efficient method for enhancing strength and ductility simultaneously [4]. Research on the grain refinement of magnesium alloys is primarily divided into two categories: aluminum-free and aluminum-bearing magnesium alloys [5]. Zirconium is recognized as the most potent grain refiner for aluminum-free magnesium alloys. Qian et al. asserted that the zirconium-rich core structures in Mg-Zr alloys serve as nucleation sites for α-Mg grains, emphasizing zirconium’s remarkable grain refining capabilities [6]. However, zirconium forms stable compounds with Al, Mn, Si, Fe, Sn, Ni, Co, and Sb, so it cannot serve as a nucleating agent in magnesium alloys containing aluminum, which are mostly used in commercial applications [7]. Various techniques have been reported for the grain refinement of aluminum-containing magnesium alloys, but, unlike the addition of zirconium in aluminum-free magnesium alloys, there are no commercially available and reliable grain-refining processes for aluminum-containing magnesium alloys. Additionally, the mechanisms of grain refinement in these alloys have not been fully elucidated [8]. Therefore, further research is required to understand the grain refinement mechanisms and develop effective grain refining processes for aluminum-containing magnesium alloys.
One of the grain refinement mechanisms for aluminum-containing magnesium (Mg-Al) alloys is the superheating process, which enables grain refinement through a relatively simple method of controlling only the temperature of the melt without the addition of nucleating or refining agents [9]. In this process, the molten alloy is heated to a temperature well above its liquidus temperature (150–260 °C or higher) for a brief period, after which it is rapidly cooled down to the pouring temperature and held for a short time before being cast [10]. The effectiveness of superheating as a grain refinement method depends on the alloy’s composition, particularly its aluminum content. Alloys with higher aluminum content tend to exhibit better grain refinement when subjected to superheating. Other elements, such as Fe, Mn, and Si, can also influence the grain refinement effect, but excessive amounts of these elements can be counterproductive [11]. The key to successful grain refinement through superheating lies in determining the optimal temperature range and holding time. Once these optimal conditions are achieved, further increasing the holding time or repeating the process does not result in additional grain refinement. Rapid cooling and immediate pouring after reaching the superheating temperature are essential to prevent grain coarsening [12].
Although grain refinement by superheating has been known for a long time, the exact mechanism has not yet been identified. Some hypotheses have been proposed to explain the effect, but they all have their limitations. The oxide nucleation theory [13] suggests that grain refinement occurs through the formation of magnesium oxide and other oxide particles produced in the molten metal during the superheating process. However, grain refinement is observed even in a vacuum atmosphere where oxide formation is difficult. Moreover, it cannot explain why grain refinement occurs with compounds composed of Al, C, Fe, and Mn. Temperature-solubility theory [14] posits that particles too large for good nucleants at normal pouring temperatures are dissolved during superheating and re-precipitated as finer particles that act as nucleation sites. However, the theory does not identify specific particle species. Al4C3 particle nucleation theory [15] suggests that grain refinement in Mg-Al alloys occurs due to the nucleation of α-Mg grains on Al4C3 particles formed during the superheating process. Superheating causes the diffusion of carbon atoms from the steel crucible into the Mg molten metal, leading to the formation of Al4C3 particles, which are believed to be nucleation sites for α-Mg grains. Motegi’s study [16] on commercial AZ91E alloys showed the presence of numerous Al4C3 particles after superheating, supporting this hypothesis. However, there is no direct experimental evidence to confirm the formation of Al4C3 particles during the superheating process. Al-Mn intermetallic compound nucleation theory [17] proposes α-Mg grain nucleation by Al-Mn intermetallic compounds precipitated during cooling after superheating. It has been proposed that aluminum contributes to reducing the solubility of manganese in Mg melts, leading to the formation of various Al-Mn intermetallic compounds during the superheating process. Byun et al. [18] suggested that Al8Mn5 compounds provided heterogeneous nucleation sites for primary α-Mg grains. However, the edge-to-edge matching model by Zhang et al. [19] indicated that Al8Mn5 had low nucleating efficiency as nucleation sites for α-Mg grains. Cao et al. [20] reported ε-AlMn as apotent nucleant for α-Mg grains due to its presence in the Al-60Mn master alloy, which showed high grain refining efficiency. Qin et al. [21] supported these findings, showing that single-phase ε-AlMn refined AZ31 grains, while single-phase Al8Mn5 did not. In contrast, Qiu et al. [22] suggested that metastable τ-AlMn, which could be generated during the melt superheating process, had better crystallographic matching with the Mg matrix than ε-AlMn and acted as a nucleant for α-Mg grains. However, no direct evidence currently exists to confirm the presence of τ-AlMn and ε-AlMn in Mg-Al alloys, and methods to control their phase and morphology are still unknown. Duplex nucleation theory [9,10] suggests that Al4C3 particles are responsible for grain refinement in Mg-Al alloys, while Mn and Fe may interfere with the nucleation potency of Al4C3 particles. During the superheating process, Mn and Fe are dissolved, allowing Al4C3 particles to act as nucleants for α-Mg grains. However, prolonged holding at the pouring temperature causes Mn and Fe to re-wrap around the Al4C3 particles, coarsening the grains. Han et al. [23] supported these findings, showing that Al4C3 particle clusters act as nucleants for α-Mg grains rather than individual particles. In Mn-containing AZ91 alloy, the attachment of Al8Mn5 particles to Al4C3 reduces nucleation efficiency. Nevertheless, Al4C3 particles not attached to Al8Mn5 within a nucleating cluster can still play an effective role in refining a-Mg grains. Thus, it is deduced that Al4C3 particle clusters are beneficial in overcoming the hindering effect of Mn. However, definitive evidence regarding the formation of duplex structures involving Al8Mn5 and Al4C3 has not yet been established.
Previous studies have proposed various theories to explain the mechanism of grain refinement by superheating in Mg-Al alloys. However, these studies have certain shortcomings, such as a lack of direct experimental evidence to validate the theories and incomplete understanding due to the inability of any one theory to fully explain all observed phenomena. In this study, the rapid solidification process was employed to identify the nucleants generated by the superheating process. This approach was specifically designed to identify and analyze potential nucleants that may exist as solid phases within the molten Mg-Al alloy during the superheating process. By harnessing the rapid solidification process, these solid particles could be effectively captured and scrutinized, allowing for a deeper and clearer understanding of their structure and composition. Therefore, this study presents new perspectives on the mechanism of superheating grain refinement that have not been reported in any previous studies.
2. Materials and Experiments
2.1. Materials and Casting Process
AZ91D alloy, which is most widely used in industrial fields, was used in this study. Approximately 1000 g of the alloys were melted in an alumina crucible using an electric furnace. Samples were prepared with and without the superheating process. For samples without superheating, the AZ91D alloy was melted at 670 °C under a protective gas of 1.0% SF6 and 99.0% N2 and then poured into a mild steel mold preheated to 250 °C. For samples subjected to the superheating process, the alloy was melted at 670 °C under a protective gas, then rapidly heated to 770 °C and held at this temperature for 15 min, followed by rapid cooling to 670 °C, and finally poured into a mild steel mold preheated to 250 °C. The chemical composition of the samples was analyzed by an optical emission spectrometer (Spectro MAXx, SPECTRO, Kleve, Germany).
2.2. Rapid Solidification Process
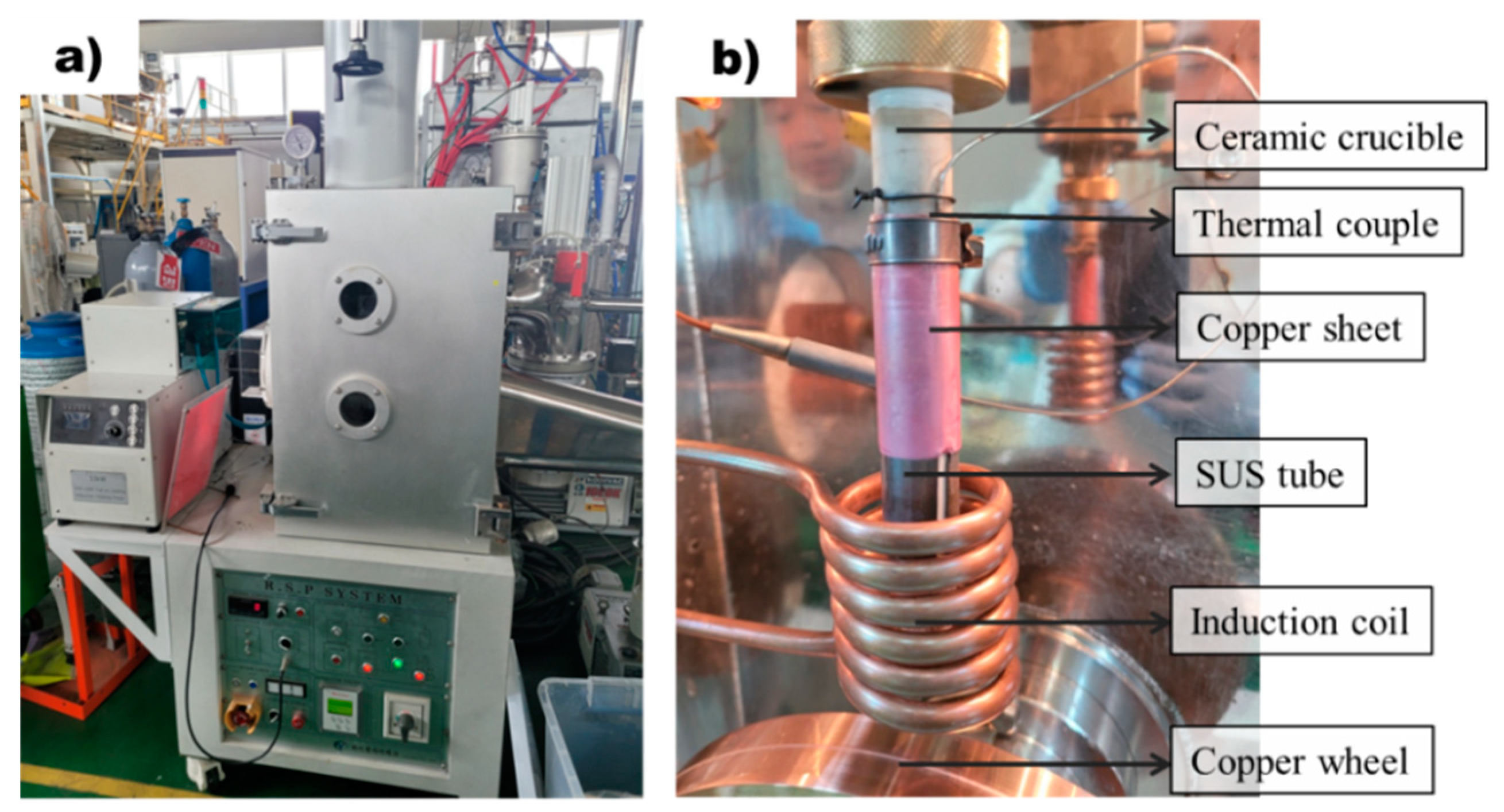
Rapidly solidified ribbon samples were prepared using the melt spinner equipment (MSE 170, Yein Tech, Seoul, Republic of Korea) shown in Figure 1a. Rapidly solidified ribbon samples were prepared by melting 2 g of AZ91D alloy within a ceramic tube and subsequently injecting the molten metal into a rapidly rotating copper wheel at 1500 rpm. As shown in Figure 1b, each sample was melted using a ceramic crucible that has little reactivity with Mg molten metal. Cracking of the ceramic crucibles due to thermal shock was prevented by wrapping them with SUS304 tube. The thermocouple was attached to a ceramic crucible and was set at a position not affected by the induction heating coil. To enhance the contact between the ceramic crucible and the thermocouple, a copper sheet was used to wrap around the thermocouple. The high thermal conductivity of copper enabled the temperatures of the molten metal and crucible to reach an equilibrium state over time, ensuring proper melting. The process temperature was recorded by a data logger (NI cDAQ-9174, National Instruments, Austin, TX, USA) at the frequency of 20 Hz. Conventional casting samples were produced by melting the molten metal at a temperature of 650 °C, followed by a spraying process onto the rotating wheel. In contrast, superheating samples were prepared by first heating the molten metal to a temperature of 800 °C, then rapidly cooling it down to 650 °C before proceeding with the spraying process.
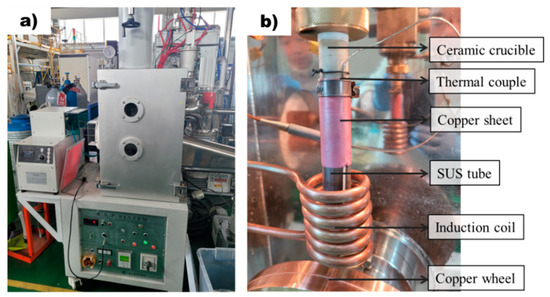
Figure 1.
Apparatus for the rapid solidification process: (a) Equipment for rapid solidification process. (b) Rapid solidification process system with precise temperature control.
2.3. Measurement of Grain Size
Metallographic specimens were polished and then etched using an acetic acid-picral etchant to obtain a clear color contrast that enabled detailed investigation of the microstructure via optical microscopy. Microstructural analysis was performed using a high-resolution optical microscope (Leica MC 170, Leica, Teaneck, NJ, USA) to capture images of the specimen surfaces. The average grain size of the specimens was measured by focusing on the center of the cross-section of each specimen.
2.4. Thermal Analysis
Thermal analysis experiments were carried out using cylindrical graphite crucibles to determine the undercooling during the solidification of the alloys. The graphite crucible was submerged in the melt until its temperature reached the temperature of the molten metal. The crucible, filled with molten metal, was then transferred to a ceramic board. A K-type thermocouple, calibrated using the equilibrium melting temperature of high-purity (99.99%) aluminum, was inserted into the center of the melt to monitor the temperature throughout the solidification process. Cooling curves were recorded using a data logger (NI cDAQ-9174, National Instruments, Austin, TX, USA) at a frequency of 20 Hz.
2.5. Analysis of Microstructure on Rapidly Solidified Sibbon Samples
The microstructure of samples was analyzed using a field emission scanning transmission electron microscope (Talos F200X, Thermo Fisher Scientific, Waltham, MA, USA) after preparing the samples with focused ion beam (Scios2, Thermo Fisher Scientific, Waltham, MA, USA) equipment. The sample was first protected with a thin layer of Pt, then FIB milling was performed to create thin samples. Energy-dispersive x-ray spectroscopy (EDS, Thermo Fisher Scientific, Waltham, MA, USA) was employed to obtain more detailed elemental information, and high-resolution images were acquired to study the microstructure of samples. Crystallographic analysis of the phases observed in each sample was carried out utilizing Gatan software (ver 3.43) and CrysTbox software (ver 1.10).
3. Results
3.1. Microstructure and Chemical Composition
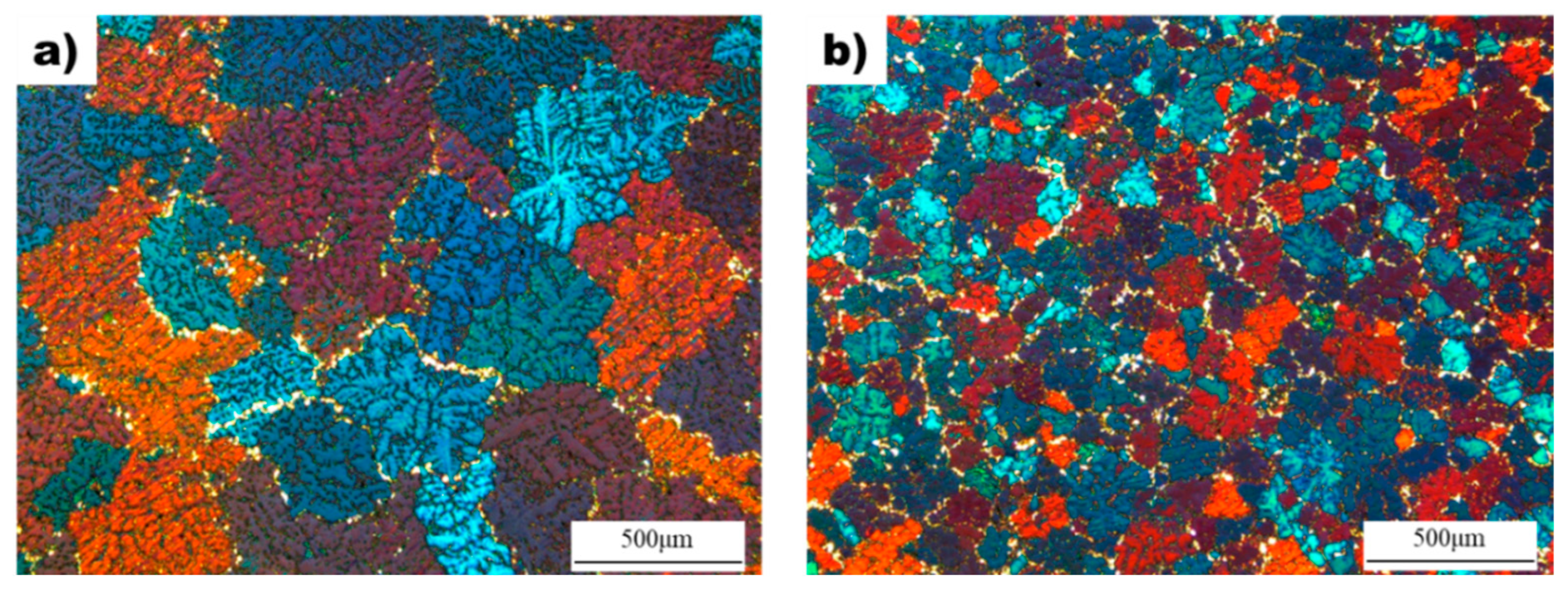
Figure 2 shows the effect of the superheating process on the microstructure and grain size of AZ91D alloy. The average grain size of the non-superheated sample was measured to be 310 µm, while the grain size decreased to 108 µm after the superheating process. Both samples exhibited dendritic microstructures with equiaxed grains, but the superheated sample had grains that were approximately three times smaller than the non-superheated sample. Additionally, the superheated sample showed enhanced uniformity in grain size and shape compared to the non-superheated sample, which had non-uniform grain shapes and sizes.
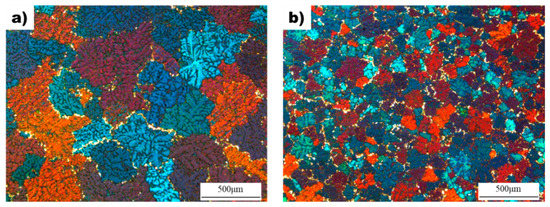
Figure 2.
The microstructure of the as-cast samples: (a) Non-superheated AZ91D alloy. (b) Superheated AZ91D alloy with superheating.
As shown in Table 1, the composition of Al, Zn, and Mn of both alloys is remarkably similar, thus indicating that the possibility of grain refinement effects resulting from constitutional undercooling can be excluded. Furthermore, because both specimens are manufactured under identical casting conditions, a more accurate comparison can be made, eliminating the potential for grain refinement effects due to differences in cooling rates. The consistency in casting conditions and composition ensures that the observed differences in microstructure can be ascribed to the superheating process, rather than variations in the manufacturing process.

Table 1.
Chemical compositions on non-superheated and superheated AZ91D alloy.
3.2. Cooling Curve and Undercooling
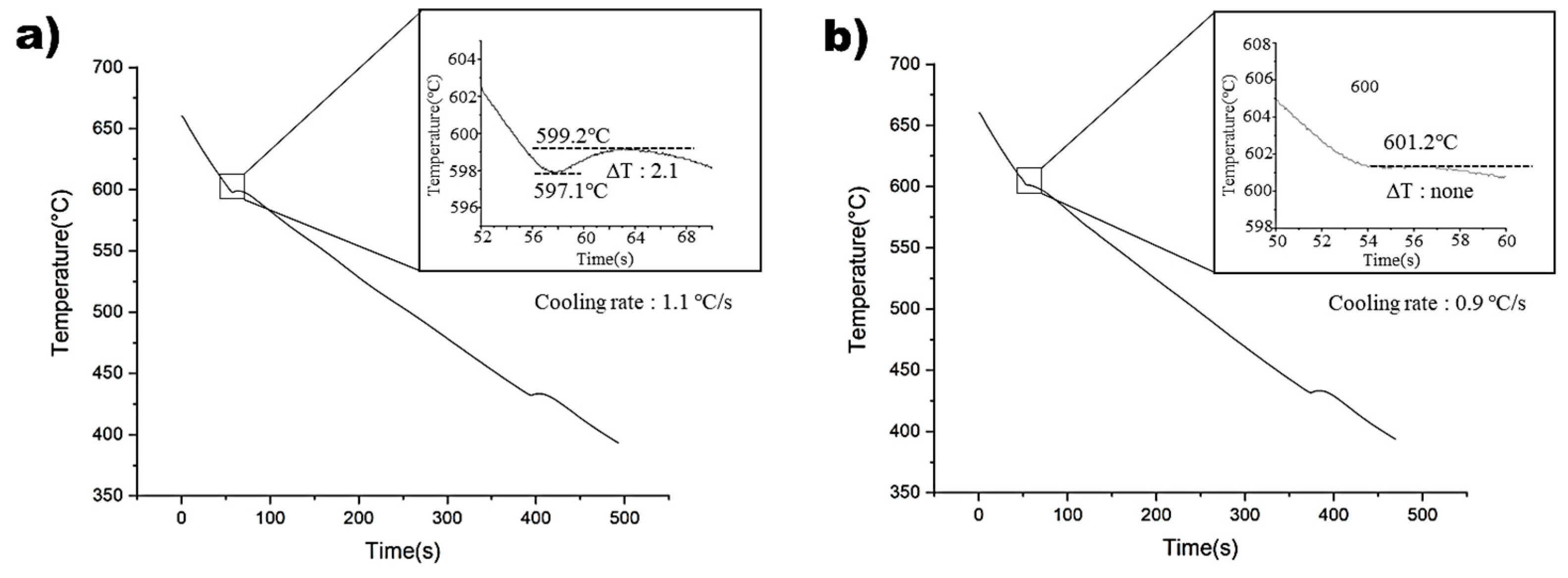
Figure 3 illustrates the cooling curves of the non-superheated and superheated AZ91 alloys. The non-superheated AZ91 alloy exhibited an undercooling of 2.1 °C, whereas the superheated AZ91 alloy showed negligible undercooling. This suggests that the superheated AZ91 alloy contained effective nucleants that could initiate nucleation with minimal activation energy [24]. Cooling rates were measured at 1.1 °C and 0.9 °C for the non-superheated and superheated samples, respectively, and it was found that the impact of cooling rates on the observed undercooling variations was negligible. Therefore, it can be concluded that the observed differences in microstructure between the two samples can be attributed to the superheating process, rather than variations in cooling rates [25].
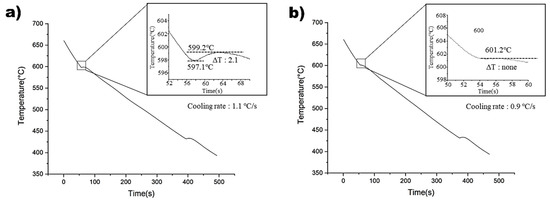
Figure 3.
Cooling curves of: (a) Non-superheated AZ91 alloy. (b) Superheated AZ91 alloy.
3.3. Design of the Precise Temperature Measurement System for the Rapid Solidification Process
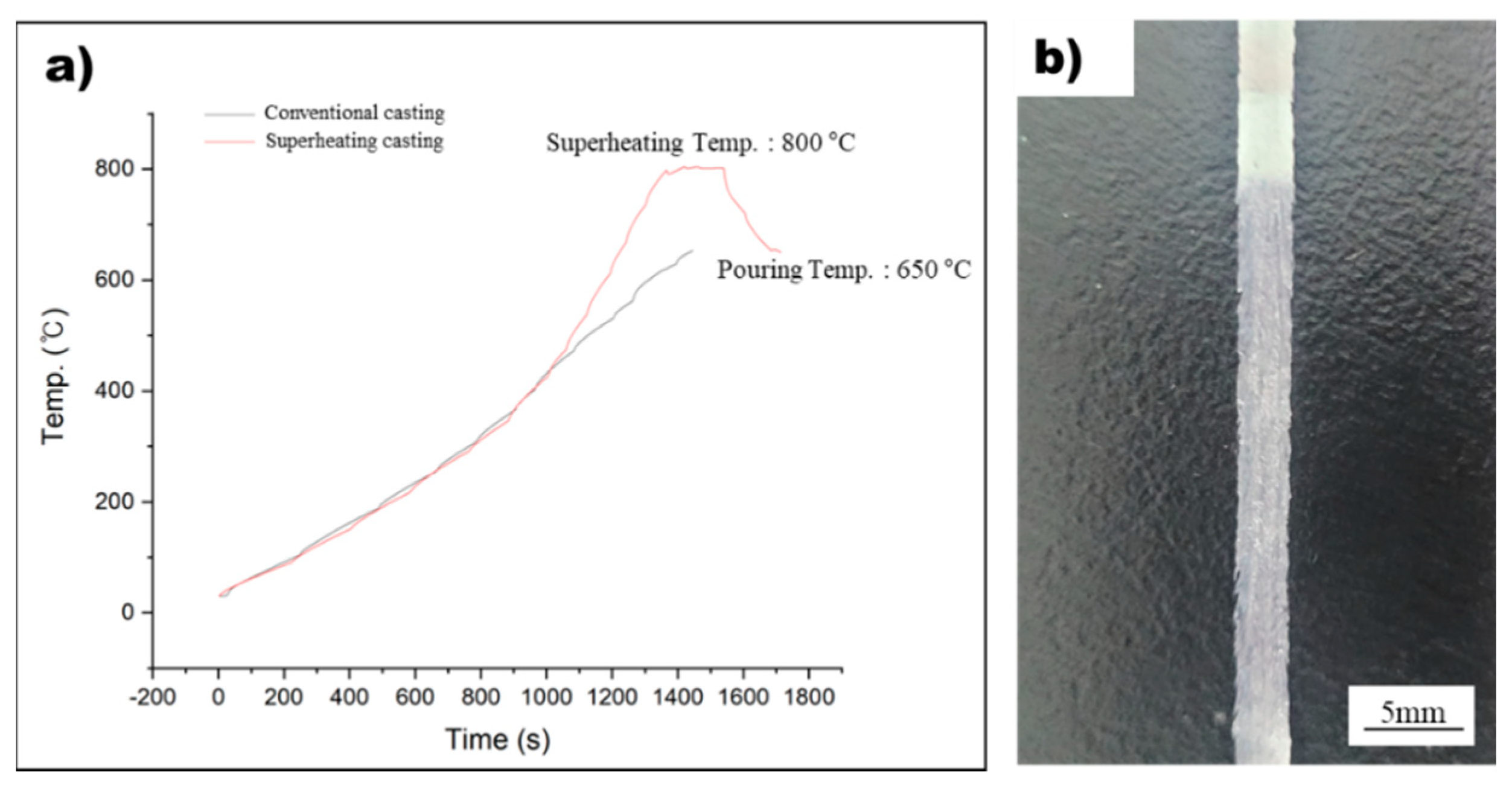
In this study, the rapid solidification process was used to detect nucleants within the molten metal that play a significant role in grain refinement. However, measuring the temperature of the molten metal during rapid solidification using melt spinner equipment can be challenging due to the absence of a dedicated temperature measurement system and thermocouple inaccuracies caused by induction coil influence. To overcome this challenge, a method was designed to precisely measure the temperature of the molten metal, and this method was used to manufacture rapidly solidified AZ91 ribbon samples, as shown in Figure 1. By integrating this precise temperature measurement system into conventional melt spinner equipment, it is possible to produce rapidly solidified AZ91 ribbon samples with a precisely controlled superheat process. Figure 4a displays the temperature profile of the non-superheated and superheated AZ91 ribbon samples during the manufacturing process, while Figure 4b illustrates the resulting rapidly solidified AZ91 ribbon samples. The non-superheated AZ91 ribbon sample was heated at a rate of 0.43 °C/s until it reached the target temperature of 650 °C. The molten metal was then immediately sprayed onto the rapidly rotating copper wheel, which led to the formation of rapidly solidified samples. For the superheated AZ91 ribbon sample, a two-stage heating process was employed. First, the sample was heated to 454 °C at a rate of 0.41 °C/s. Then, the heating rate was increased and the sample was rapidly heated to 800 °C at a rate of 1 °C/s. Once the desired superheating temperature was achieved, the molten metal was held at 800 °C for 180 s to ensure uniform temperature distribution. The molten metal was then cooled at a controlled rate of 0.9 °C/s until it reached the target temperature of 650 °C, at which point it was sprayed onto the copper wheel to produce rapidly solidified samples.
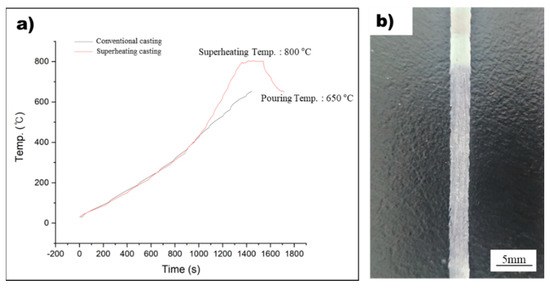
Figure 4.
(a) The temperature profile during the manufacturing of non-superheated and superheated AZ91 ribbon samples. (b) The rapidly solidified AZ91 ribbon samples.
3.4. Cooling Rate Calculation for Rapid Solidification Process
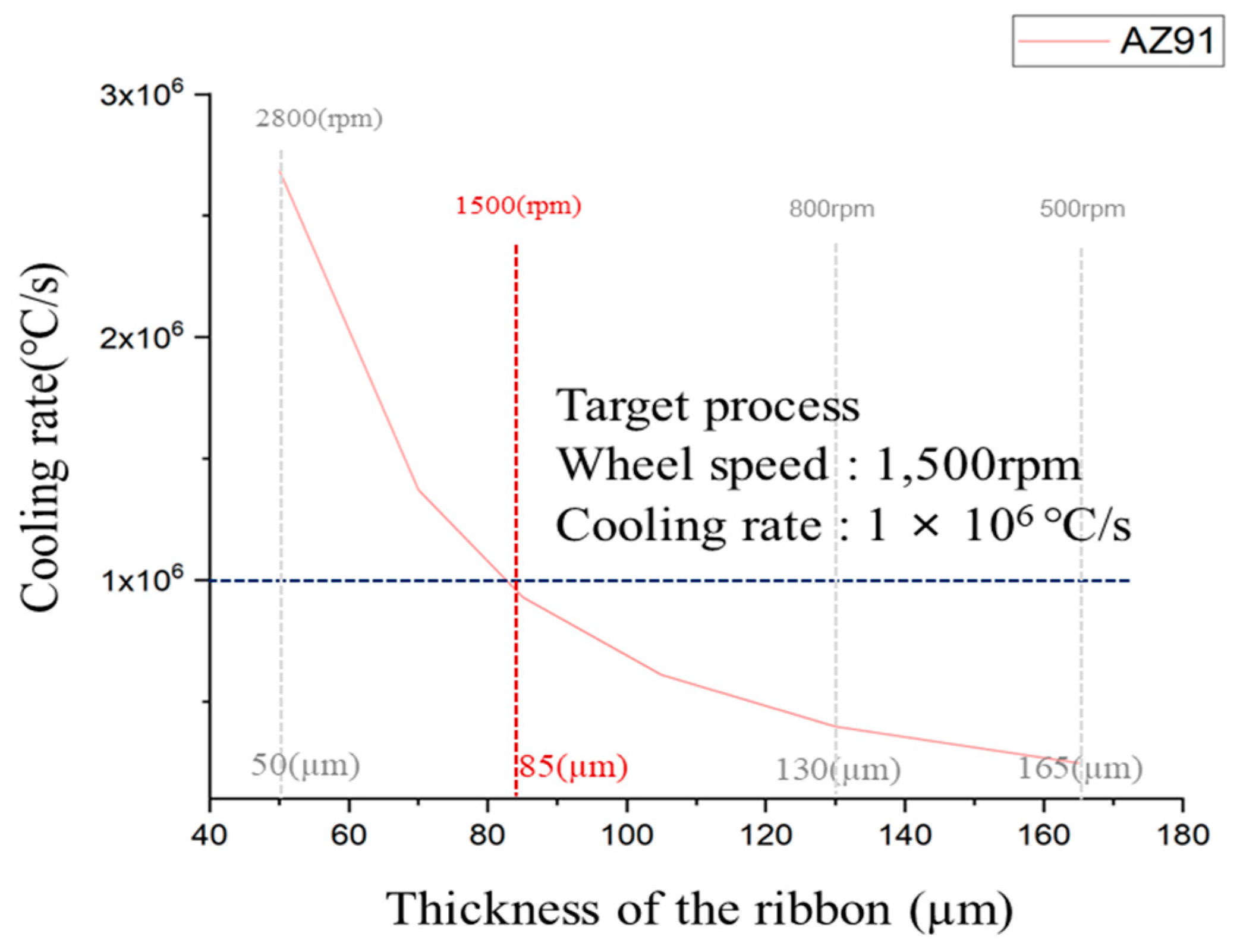
Determining the cooling rate of ribbons fabricated by rapid solidification is essential for the identification and analysis of solid-phase nucleants present in the molten AZ91 magnesium alloy. The cooling rate significantly influences the formation and growth of solid-phase nucleants within molten metals. By establishing and controlling the cooling rate, a systematic investigation of the formation of nucleants and their contribution to grain refinement can be conducted more effectively. Figure 5 shows the cooling rate of AZ91 ribbon samples at different copper wheel speeds. The cooling rate of the rapidly solidified AZ91 magnesium alloy ribbon was determined using an equation derived from a previous study [26]. The thickness of the ribbon samples was measured at different copper wheel speeds, and the data were used to calculate the cooling rate for each. For a specimen with a thickness of 50 μm, the cooling rate reached 2.69 × 106 °C/s, while for a specimen with a thickness of 165 μm, the cooling rate was notably lower at 2.47 × 105 °C/s. It was observed that the cooling rate demonstrated a tendency to increase as the thickness of the specimen decreased. For optimal results in rapid solidification, a higher cooling rate is preferable. However, when the copper wheel speed exceeded 2000 rpm, it became challenging to produce ribbon samples with consistent size and shape, making microstructure analysis difficult due to their small dimensions. Consequently, the rapid solidification process was conducted with a copper wheel speed of 1500 rpm, resulting in a ribbon sample with a thickness of 85 μm.
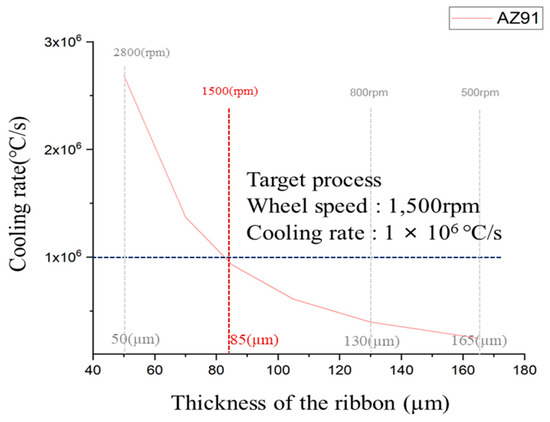
Figure 5.
Cooling of AZ91 ribbon samples according Cu wheel to rpm.
3.5. EDS Analysis of Rapidly Solidified AZ91D Ribbon Samples
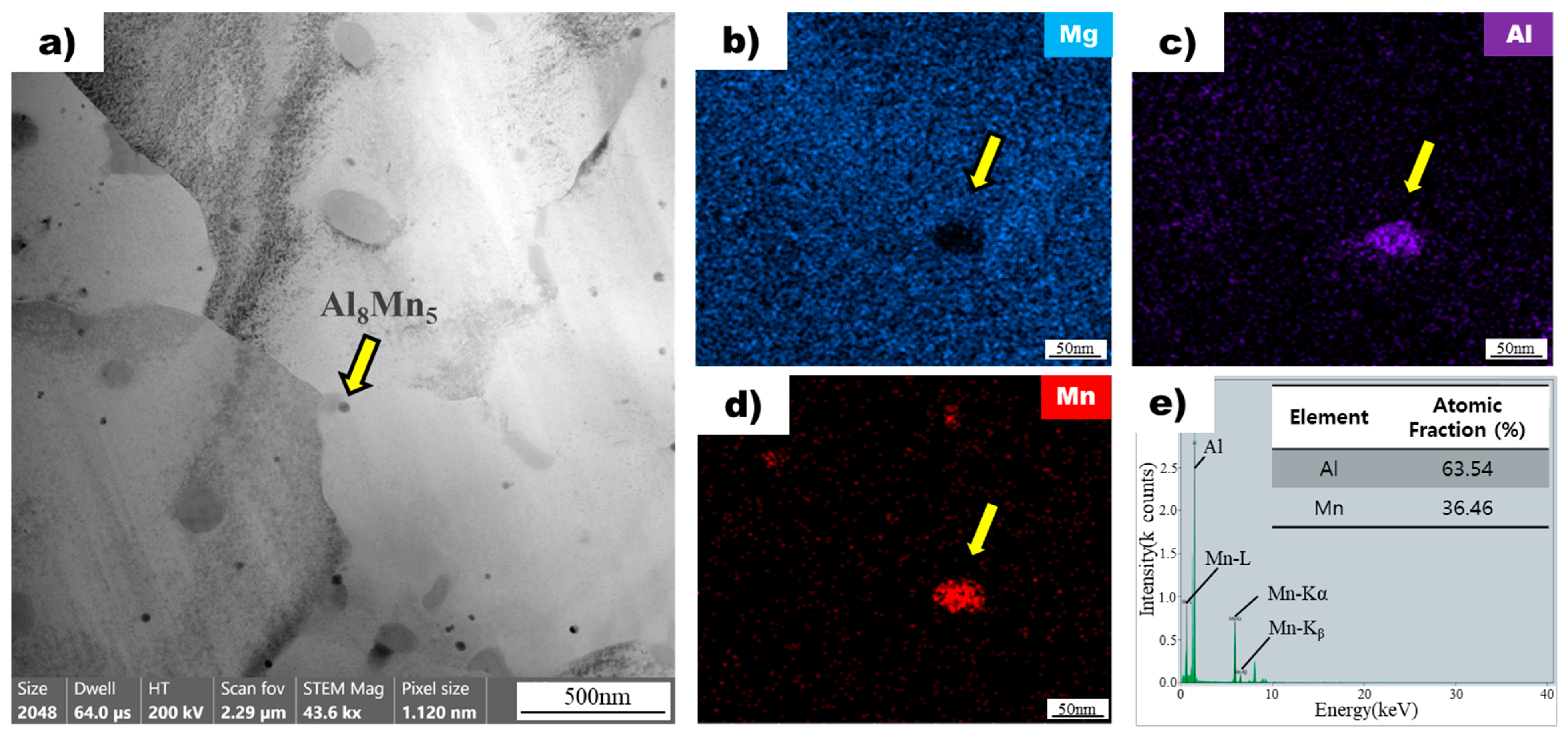
Figure 6a shows an image of a non-superheated AZ91 alloy ribbon sample obtained using bright-field transmission electron microscopy. The microstructure of the sample contains dispersed particles within the magnesium matrix. One of these particles, indicated by a yellow arrow, was selected for further analysis using energy-dispersive X-ray spectroscopy mapping (Figure 6b). The analysis revealed that the particle did not contain magnesium but had significant amounts of aluminum (Figure 6c) and manganese (Figure 6d). The atomic ratios of aluminum and manganese in the particle were found to be 63.54 and 36.46, respectively, based on EDS point analysis (Figure 6e). This composition ratio is consistent with the Al8Mn5 intermetallic compound, which was confirmed through further analysis of other particles in the sample. The results demonstrate that the particles observed in the non-superheated AZ91 alloy ribbon sample were Al8Mn5 intermetallic compounds [27]. Upon conducting further analysis of the other particles using the same method, results consistent with the previous observation were obtained.
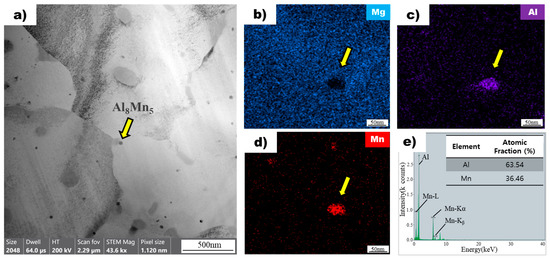
Figure 6.
(a) Bright field TEM image of non-superheated AZ91 ribbon samples. The yellow arrow points to the Al8Mn5 particle. EDS mapping profile pointed by the yellow arrow of Mg (b), Al (c), and Mn (d) for the particle observed in non-superheated AZ91 ribbon samples. (e) EDS point analysis of the particle observed in non-superheated AZ91 ribbon samples.
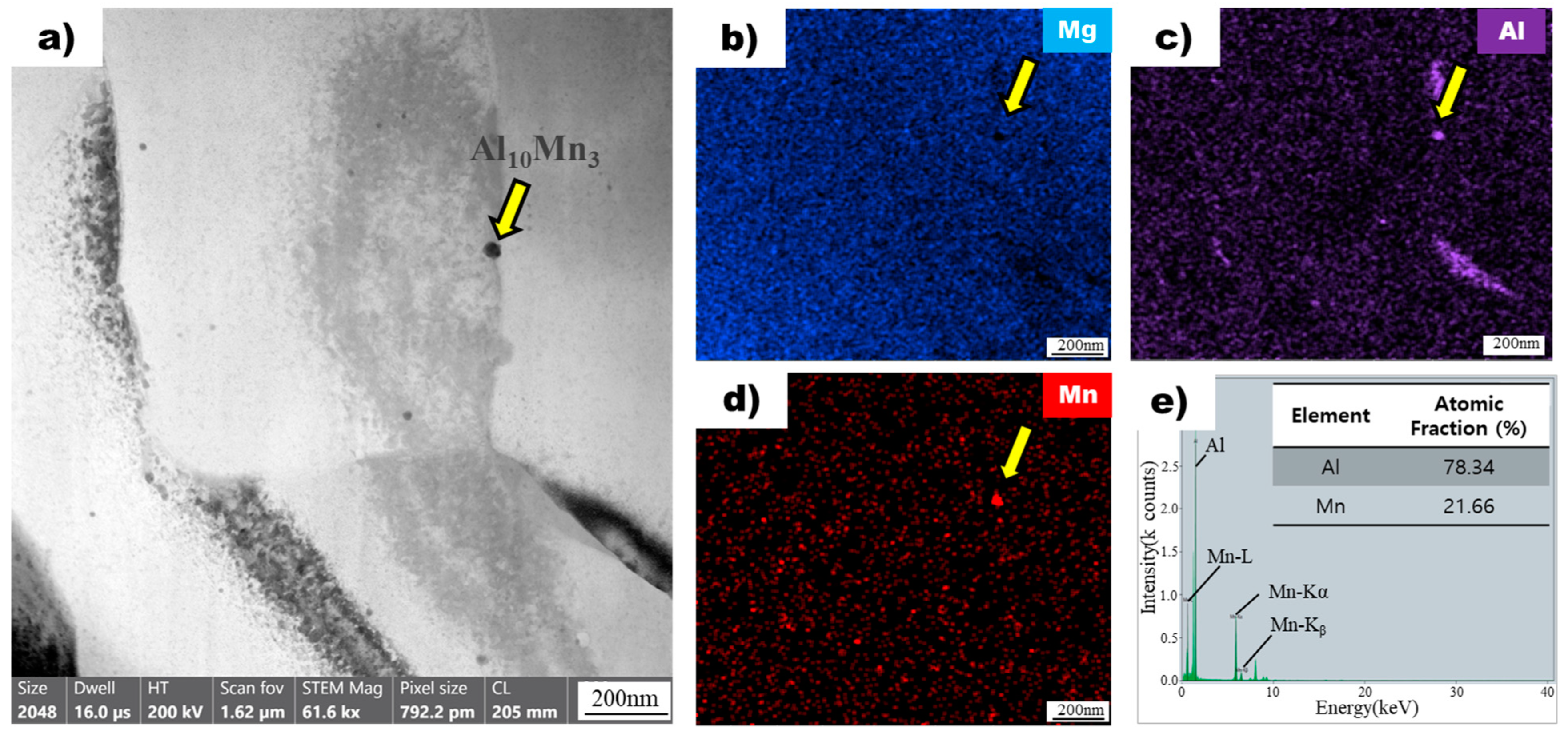
The image in Figure 7a shows a bright-field transmission electron microscopy image of a superheated AZ91 alloy ribbon sample, revealing the presence of particles dispersed throughout the magnesium matrix. One particle, indicated by a yellow arrow, was chosen for detailed analysis using energy-dispersive X-ray spectroscopy mapping. The analysis confirmed the absence of magnesium in the particle (Figure 7b) but the presence of aluminum (Figure 7c) and manganese (Figure 7d), consistent with the particles observed in the non-superheated sample. However, the atomic ratios of aluminum and manganese in the particle of the superheated sample (Figure 7e) were different from those in the non-superheated sample (Figure 6e). Specifically, the particle in the superheated sample exhibited an atomic ratio of 78.34 for aluminum and 21.66 for manganese, whereas the particle in the non-superheated sample had a ratio of 63.54 for aluminum and 36.46 for manganese. Based on these ratios, the particles were identified as Al10Mn3 in the superheated sample and Al8Mn5 intermetallic compounds in the non-superheated sample [27].
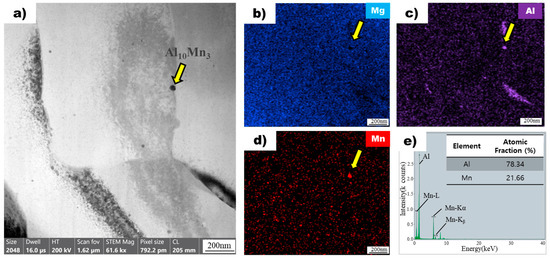
Figure 7.
(a) Bright field TEM image of superheated AZ91 ribbon samples. The yellow arrow points to the Al8Mn5 particle. EDS mapping profile pointed by the yellow arrow of (b) Mg, (c) Al, and (d) Mn for particle observed in superheated AZ91 ribbon samples. (e) EDS point analysis of particle observed in superheated AZ91 ribbon samples.
3.6. HR-TEM Images of Rapidly Solidified AZ91D Ribbon Samples
Table 2 shows the crystal structures of Mg, Al8Mn5, and Al10Mn3 [27]. It was observed that the crystal structure and lattice parameters of Al8Mn5 significantly diverge from those of Mg. Conversely, Al10Mn3 and Mg share the same crystal structure and even belong to the same space group; however, a distinction in their lattice parameters was observed. Considering the crystal structures of each phase, Al10Mn3 appears to present a higher propensity to function as a nucleat for α-Mg grains compared to Al8Mn5. However, to elucidate this with higher precision, it is essential to evaluate the interplanar spacings and investigate potential crystallographic mismatches between the planes interfacing with each phase.

Table 2.
The crystal structures for Mg, Al8Mn5, and Al10Mn3 [27].
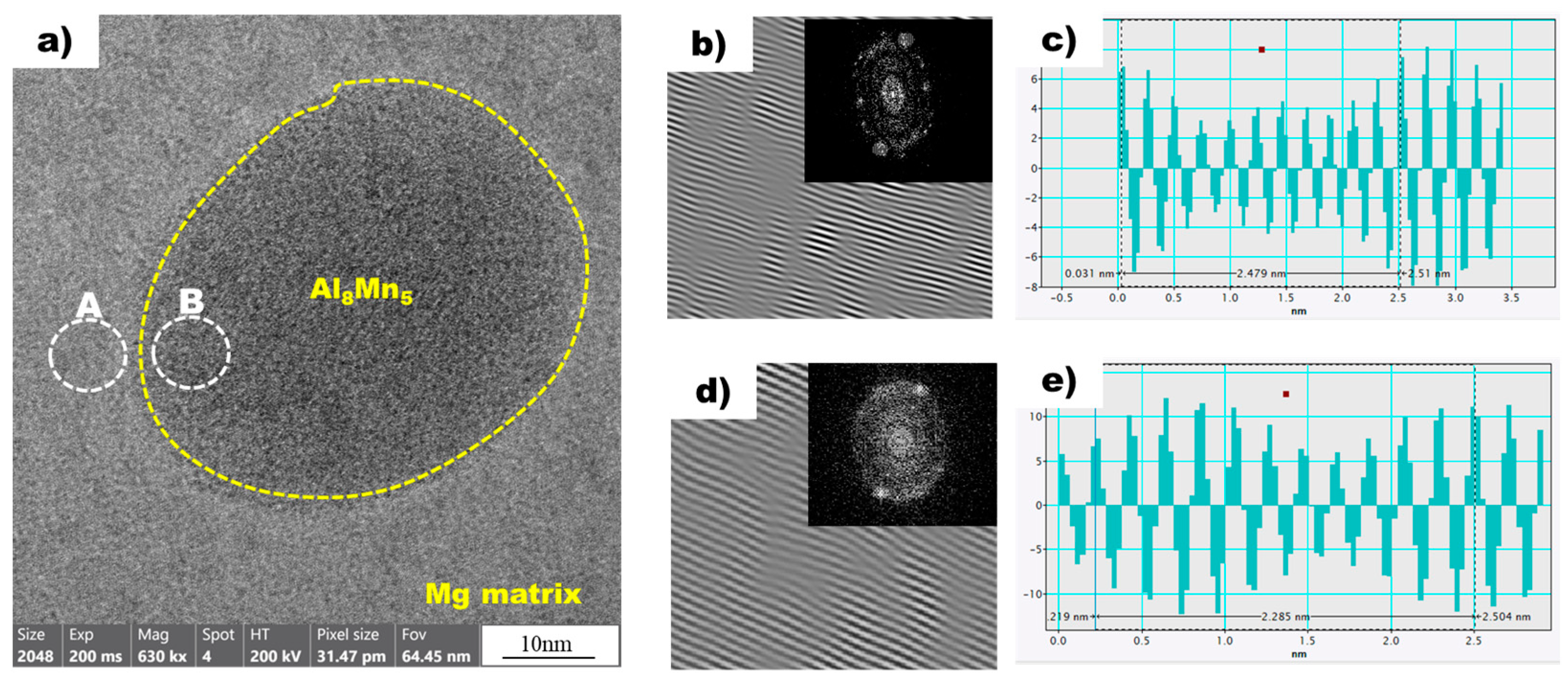
Figure 8a shows a high-resolution transmission electron microscopy (HR-TEM) image of the magnesium matrix and Al8Mn5 particles in the non-superheated AZ91 alloy ribbon sample. A local inverse fast Fourier transform (IFFT) image of white circle A (Mg) is shown in Figure 8b. The spacing of white line in the IFFT image denotes the interplanar spacing of the region being measured. Using the IFFT image as a reference, a profile image was derived, as shown in Figure 8c, enabling the measurement of the white line distance. As there is a variance between the peak intervals, an average value was acquired by measuring over an extended distance, which resulted in a value of 0.2479 nm. Table 3 presents the planes and corresponding d-spacing (in nm) for Mg, Al8Mn5, and Al10Mn3, derived using the CrysTBox software (ver 1.10). The plane of Mg, with a D-spacing of 0.2453 nm, displayed the closest proximity to the 0.2479 nm value obtained through the IFFT profile image. The IFFT image of the white circle B, representing Al8Mn5, is shown in Figure 8d, and Figure 8e displays the profile image derived from this IFFT image. The Al8Mn5 particles were identified on the (1 4 1) plane and the interplanar spacing was calculated to be 0.2275 nm, using the same method employed for the determination of the plane and interplanar spacing of Mg. Through the analysis of the HR-TEM image, IFFT images were acquired for the contact area between magnesium and Al8Mn5 particles, leading to the identification of the magnesium plane as and the Al8Mn5 plane as (1 4 1). Furthermore, the interplanar spacing was determined for both planes: the magnesium plane had an interplanar spacing of 0.2453 nm, while the Al8Mn5 particle (1 4 1) plane had a slightly smaller interplanar spacing of 0.2275 nm.
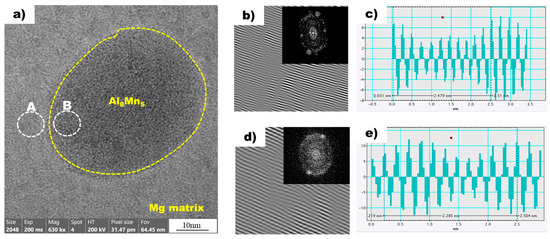
Figure 8.
(a) HRTEM image of Mg (background) and Al8Mn5 (yellow dot circle) in the non-superheated AZ91 ribbon sample. (b) The local IFFT image of the white circle A (Mg) in (a). (c) The profiles of the local IFFT image (b). (d) The local IFFT image of the white circle B (Al8Mn5) in (a). (e) The profiles of the local IFFT image (d).

Table 3.
List of Plane and D-Spacing (nm) for Mg, Al8Mn5, and Al10Mn3 (used CrysTbox ver1.10).
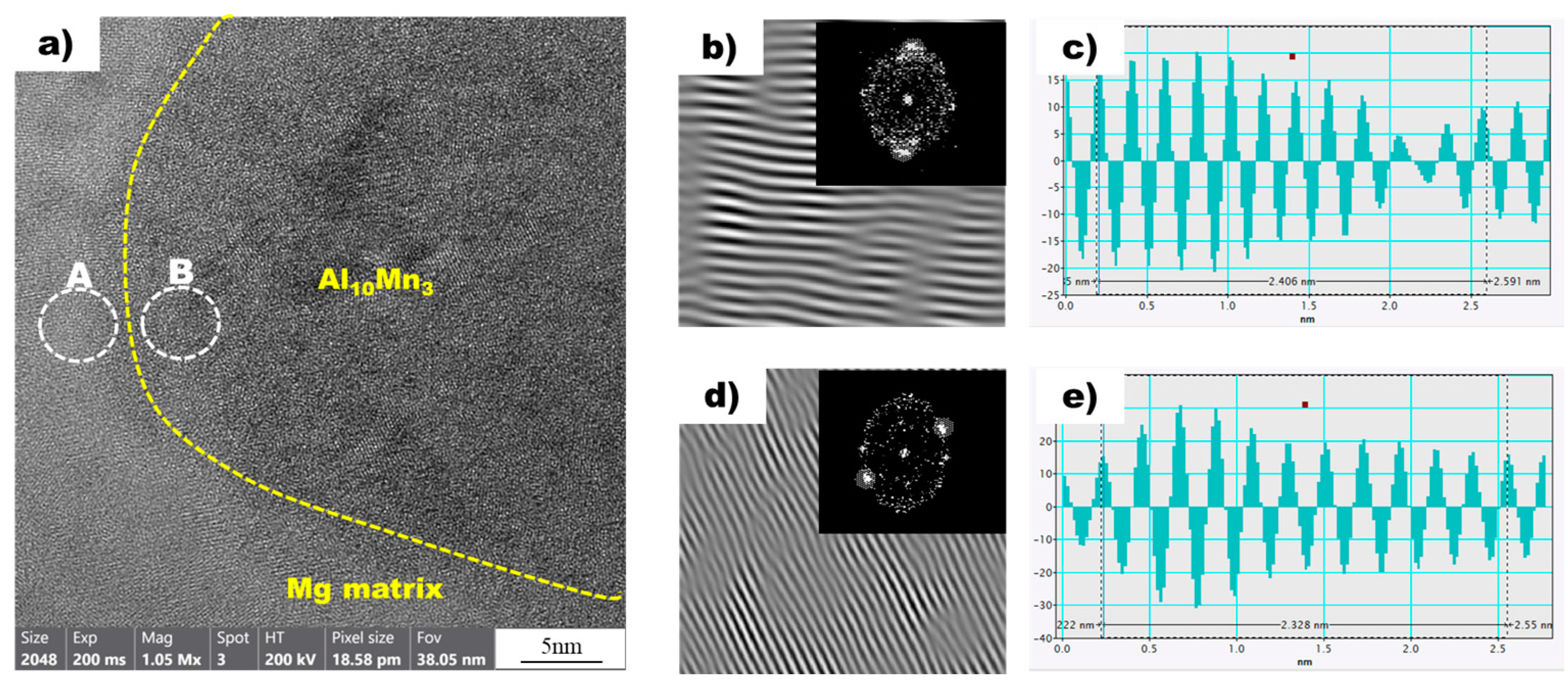
Figure 9a shows a high-resolution transmission electron microscopy (HRTEM) image of the magnesium matrix and Al10Mn3 particles in the superheated AZ91 alloy ribbon sample. Local inverse fast Fourier transform (IFFT) images of two white circles, A and B, are also shown in Figure 9b,d, respectively. In addition, Figure 9c,d are profile images derived through IFFT images of two white circles, A and B, respectively. In the profile images of Mg and Al10Mn3, the average distance between the peaks was calculated to be 0.2406 nm and 0.2328 nm, respectively. The values obtained from the profile images were compared with the D-spacing values listed in Table 3, allowing for the confirmation of the Mg and Al10Mn3 planes. In the superheated sample, the magnesium plane was identified as , which is consistent with the non-superheated sample. However, the Al10Mn3 plane was observed as (1 2 1), which is distinct from the Al8Mn5 plane found in the non-superheated sample. Furthermore, a detailed examination of the interplanar spacing was conducted, revealing an interplanar spacing of 0.2347 nm for the Al10Mn3 particle (1 2 1) plane in the superheated sample.
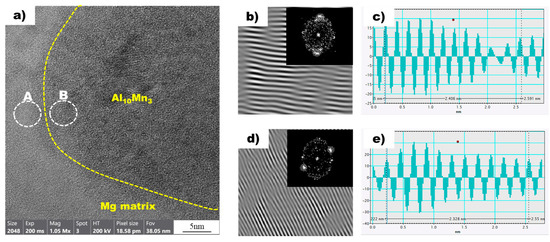
Figure 9.
(a) HRTEM image of Mg (background) and Al10Mn3 (yellow dot circle) in the non-superheated AZ91 ribbon sample. (b) The local IFFT image of the white circle A (Mg) in (a). (c) The profiles of the local IFFT image (b). (d) The local IFFT image of the white circle B (Al10Mn3) in (a). (e) The profiles of the local IFFT image (d).
4. Discussions
The microstructure of as-cast AZ91D samples was investigated to determine the effect of superheating, and the results are presented in Figure 2. The non-superheated alloy exhibited an average grain size of 310 µm, while superheating reduced it to 108 µm. This indicates that the superheating process has a significant impact on the grain size of the alloy. Furthermore, the superheated AZ91D alloy had a more uniform and finer grain size than the non-superheated sample. The average grain size of the superheated sample was approximately three times smaller than that of the non-superheated sample. The chemical composition of the cast AZ91D samples was analyzed and is shown in Table 1. The primary additives, Al, Zn, and Mn, were found to have similar compositions in both alloys, thereby eliminating constitutional undercooling as a possible cause of grain refinement. In addition, because the samples were manufactured under the same casting conditions, any grain refinement effect due to the difference in cooling rate can be excluded. Thus, the observed difference in grain size between the non-superheated and superheated samples can be attributed to the superheating process. The cooling curves of non-superheated and superheated AZ91 alloys are shown in Figure 3. The extent of undercooling during solidification can reveal the presence of nucleants in the melt. When potent nucleants are present in the melts, the thermodynamic driving force required for the transition from the liquid phase to the solid phase is decreased, facilitating the formation of solid-phase nuclei with minimal undercooling [24]. This suggests that the cooling curves of the non-superheated and superheated AZ91 alloys provide information about the extent of undercooling during solidification, which can reveal the presence of nucleants in the melt. When potent nucleants are present, the thermodynamic driving force required for the transition from liquid to solid phase is decreased, facilitating the formation of solid-phase nuclei with minimal undercooling. Although a quantitative analysis of the nucleants was not conducted in this study, the degree of undercooling measured during solidification provides a basis for qualitatively deducing that the superheated AZ91 alloy possessed more nucleants promoting grain refinement than the non-superheated AZ91 alloy.
The rapid solidification process was used in this study to investigate the effect of nucleants on grain refinement in molten metal, as it can detect nucleants present in the molten metal. Ribbon samples were fabricated using non-superheated and superheated AZ91 alloys to study the impact of the superheating process on grain refinement and the contribution of nucleants to the formation of α-Mg grains. Rapid solidification also allows for the preservation of metastable phases within the molten metal, which is important for understanding the underlying mechanisms of grain refinement during the superheating process. Accurately measuring the temperature of the molten metal during the production of rapidly solidified ribbon samples using conventional melt spinner equipment is a significant challenge in this study. This is because precise temperature measurement is crucial to understanding the mechanism of grain refinement induced by the superheating process. However, conventional melt spinner equipment lacks an integrated temperature measurement system, and obtaining precise temperature control using a thermocouple is difficult due to the interference of the induction coil responsible for heating the metal material. In this study, a system capable of precise temperature measurement was designed and used to produce these rapidly solidified ribbon samples by modifying the existing melt spinner equipment. The temperature profiles during the fabrication of non-superheated and superheated AZ91 ribbon samples are presented in Figure 4a. It is evident that both samples were manufactured using a rapid solidification process at a precisely controlled temperature. The cooling rate is an important factor in identifying and analyzing solid-phase nucleants in AZ91 magnesium alloy produced by rapid solidification. It significantly affects the formation and growth of solid-phase nucleants, and controlling it allows for a more systematic investigation of their formation and contribution to grain refinement. The study by Wang et al. [26] investigated the heat transfer during rapid solidification of a ribbon prepared by the melt spinning process. They used a one-dimensional heat conduction equation to model the heat transfer, which allowed them to determine the temperature distribution and cooling rate within the ribbon. The cooling rate was found to be inversely proportional to the square of the thickness of the ribbon [26], and the same principle was applied for estimating the cooling rate of those rapidly solidified AZ91 alloy ribbon samples in this study. Various factors such as melting temperature, thermal conductivity, specific heat capacity, density, and latent heat were adjusted for the AZ91 magnesium alloy. The molten metal was rapidly solidified by spraying it onto a copper wheel rotating at a speed of 1500 rpm, resulting in the manufacturing of a ribbon sample with a thickness of 85 μm. This approach builds upon the findings of previous research and allowed for a systematic investigation of the cooling rate and its effect on the formation of solid-phase nucleants and subsequent grain refinement in the AZ91 magnesium alloy.
Bright-field transmission electron microscopy images of ribbon samples from non-superheated and superheated AZ91 alloys are shown in Figure 6a and Figure 7a, respectively. In both samples, particles are dispersed throughout the magnesium matrix. The EDS results showed that the particle in the non-superheated sample was an intermetallic compound with a composition of Al8Mn5, while the particle in the superheated sample was identified as Al10Mn3. Although manganese is an element added to enhance the corrosion resistance of the AZ91 alloy [28], it reacts with aluminum to form Al-Mn intermetallic compounds. Among these, Al8Mn5 is known to form prior to the primary magnesium phase [29], and its role as a nucleant for α-Mg grains has been debated in numerous studies. However, no definitive mechanism has been established [17,30]. One widely accepted theory is the edge-to-edge matching model proposed by Zhang et al. [19], which suggests that, crystallographically, Al8Mn5 cannot act as a nucleant for α-Mg grains. There are various theories regarding the mechanism of the superheating process, and one of them is related to the Al-Mn intermetallic compound nucleation [17]. Cao et al. [20] found that ε-AlMn, which is present in Al-60Mn master alloy, can act as a potent nucleant for α-Mg grains, showing excellent grain refinement efficiency. This was further supported by Qin et al. [21], who reported that single-phase ε-AlMn could refine AZ31 grains, whereas single-phase Al8Mn5 could not. However, Qiu et al. [22] suggested that metastable τ-AlMn, potentially formed during the melt superheating process, could exhibit better crystallographic matching with the Mg matrix than ε-AlMn, making it a more effective nucleant for α-Mg grains. Nonetheless, there is currently no clear evidence to verify the presence of both τ-AlMn and ε-AlMn in Mg-Al alloys, and there are no established methods to regulate their phase and morphology. The finding of a new particle, Al10Mn3, during the superheating process of the AZ91 alloy is significant as it has not been previously reported in the literature. This new particle may provide novel insights into the mechanism of grain refinement during the superheating process. However, further research is needed to fully understand the thermodynamic calculations and physical and chemical processes involved in the formation of Al10Mn3 during the superheating process.
The coherent relationship between two phases occurs when their interatomic distances and atomic arrangements on their crystal faces are similar, enabling one phase to act as a highly efficient heterogeneous nucleation site for the other phase [31]. It is important to note that for a favorable coherent relationship to exist at the interface of the two phases, comparable interplanar distances are essential. Classical nucleation theory suggests that a substrate’s ability to promote nucleation depends on the interfacial energy between the substrate and the nuclei. This interfacial energy is influenced by the crystallographic structures of both the substrate and the nuclei [32]. Turnbull and Vonnegut [33] proposed a one-dimensional misfit model to describe the relationship between substrate particle effectiveness and crystallographic mismatch for nuclei. However, this model has limitations when the crystallographic structures of the nuclei and substrate particles differ significantly. Bramfitt [34,35] improved upon Turnbull’s model by creating a two-dimensional model that takes into account the angle between the crystal orientations of both phases. This two-dimensional model enables the calculation of two-dimensional lattice misfit for matching planes.
In this modified Turnbull’s model, the two-dimensional lattice misfit of two matching planes was calculated based on Equation (1),
where (h k l)s is a plane of the substrate, [uvw]s a direction in (h k l)s, (h k l)n a plane of the nucleated solid, [uvw]n a direction in (h k l)n, d[uvw]s the interatomic distance along [uvw]s, d[uvw]n the interatomic distance along [uvw]n, and θ is the angle between the [uvw]s and [uvw]n.
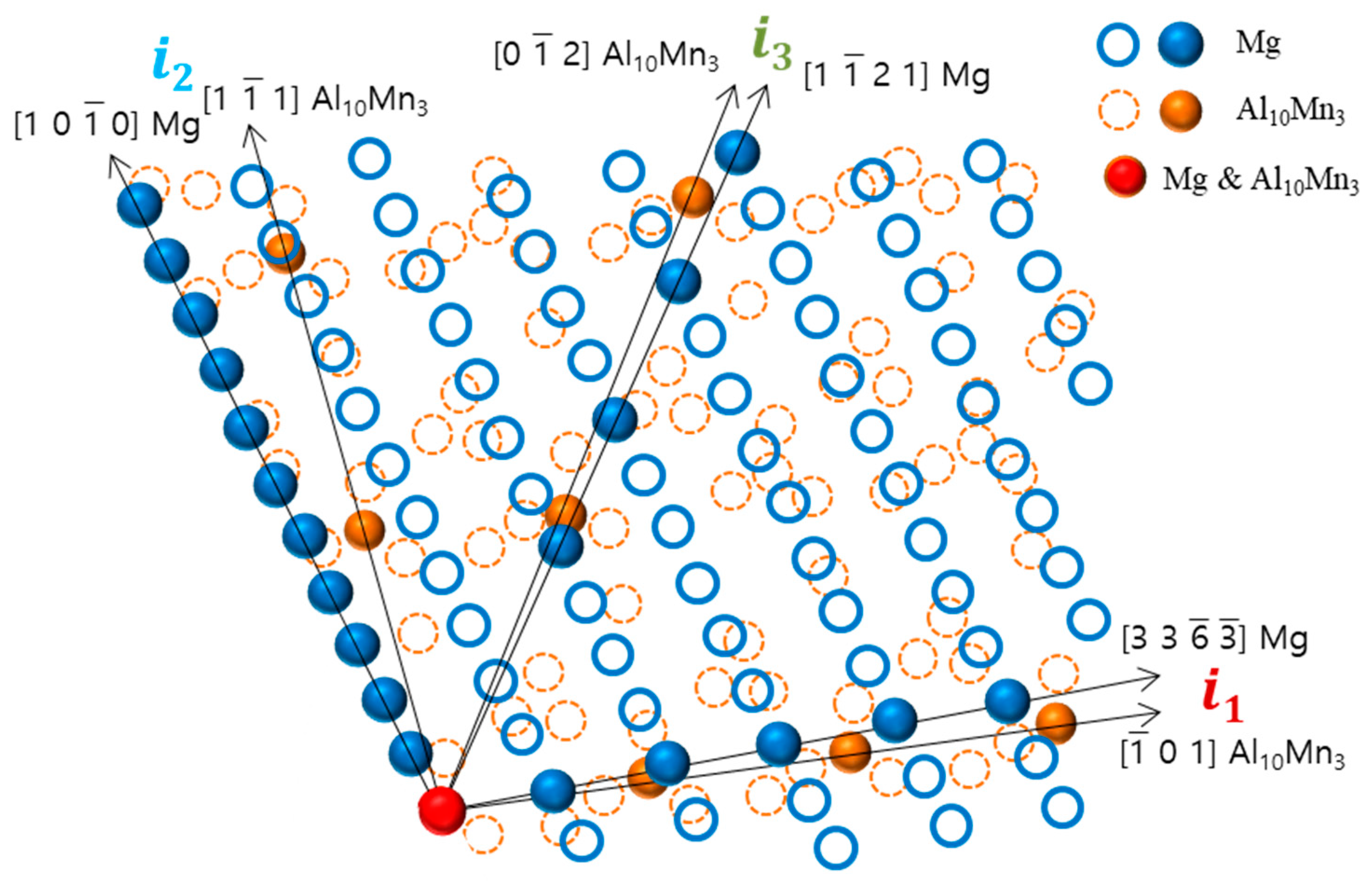
This study found that there is crystallographic coherence between the interfaces of Al8Mn5 and Al10Mn3 particles with the magnesium matrix. The high-resolution transmission electron microscopy (HR-TEM) and inverse fast Fourier transform (IFFT) images of the non-superheated AZ91 alloy ribbon samples are shown in Figure 8, which revealed that the contact planes for magnesium and Al8Mn5 particles were the magnesium plane and the Al8Mn5 plane (1 4 1). Similarly, the HR-TEM and IFFT images of the superheated AZ91 alloy ribbon samples are shown in Figure 9, which identified the contact planes for magnesium and Al10Mn3 particles as the magnesium plane and the Al10Mn3 plane (1 2 1). The modified Turnbull’s model is effective in analyzing the coherence of two planes when their crystallographic structures are not significantly different. However, in the case of the (1 4 1) plane of Al8Mn5 and the plane of Mg, their crystallographic coherence was poor, and thus the application of the modified Turnbull’s model was not feasible. On the other hand, the (1 2 1) plane of Al10Mn3 and the plane of Mg showed good crystallographic coherence, and the modified Turnbull’s model could be used to analyze their coherence. Figure 10 shows the typical planar atomic arrangements in the (1 2 1) plane of Al10Mn3 and in the plane of Mg. The brown dash circles represent the Al10Mn3 atoms in the (1 2 1) plane and the blue circles represent the Mg atoms in the plane. The Al10Mn3 and Mg atoms applied to the lattice misfit analysis are indicated by filling the center with the same color as the circle, and the place where the Al10Mn3 and Mg atoms exactly match is marked in red. The i1 indicate a line connecting the arrangement of Al10Mn3 atoms in the [1 0 1] direction with an interatomic distance of 1.224 nm and a line connecting the arrangement of Mg atoms in the [3 3 −3] direction with an interatomic distance of 1.084 nm; the angle (θ) between both lines is 4°. The i2 and the i3 are marked in the same way as the i1; the required parameters for Equation (1) are listed in Table 4. The disregistry, δ, for Mg ║Al10Mn3 (1 2 1) is as follows:
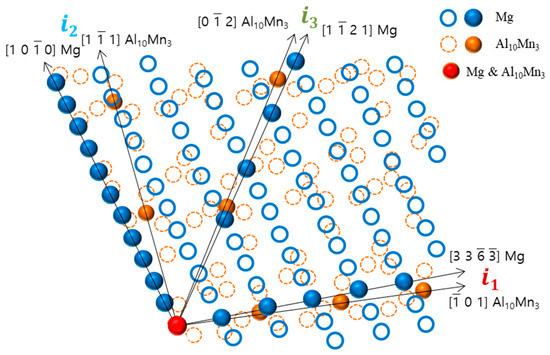
Figure 10.
The crystallographic relationship at the interface between the (1 2 1) plane of Al10Mn3 and the plane of Mg.

Table 4.
Parameters for Equation (1).
This indicates that the Al10Mn3 particle is a better nucleating site for α-Mg grains than the Al8Mn5 particle, and α-Mg can nucleate on the Al10Mn3 nucleation substrates. Previous research has shown that a substrate can effectively act as a nucleant for a solid if the disregistry between the two is less than 5% [36]. However, this is not always the case. Despite an 11% disregistry between Al10Mn3 particles and Mg, it is not conclusive that Al10Mn3 particles cannot act as nucleants for α-Mg grains. This study found that Al8Mn5 particles, which have low crystallographic coherence with Mg, can transform into Al10Mn3 through superheating, allowing them to act as nucleants for α-Mg grains. This finding provides a new perspective on investigating the mechanism of superheating grain refinement in the AZ91 alloy.
This study investigated the mechanism of superheated grain refinement in the AZ91 magnesium alloy through rapid solidification with precise temperature control. The results showed that Al8Mn5 may transform into Al10Mn3 during the superheating process. At a higher temperature, the solubility of Mn in the Mg melt will be increased and that would also affect the phase transformation of Mn intermetallics. From the HR-TEM and IFFT images of the samples, the Al10Mn3 can effectively act as a nucleant for grain refinement in AZ91. The formation of Al10Mn3 particles was found to play a crucial role in the superheating grain refinement process of the alloy. However, the study did not provide objective thermodynamic evidence to support the phase transformation of Al8Mn5 to Al10Mn3 or the generation of Al10Mn3, which will be explored in future research. Overall, this study offers a new perspective on the superheating grain refinement mechanism in AZ91 and provides insights for future research.
5. Conclusions
In this work, the mechanism on superheating grain refinement of the AZ91 alloy was investigated using a rapid solidification process. The main conclusions can be summarized as follows:
- (1)
- The average grain size of the non-superheated sample manufactured by the mold casting methods was measured to be 310 µm, while the grain size decreased to 108 µm after the superheating process.
- (2)
- The non-superheated AZ91 alloy exhibited an undercooling of 2.1 °C, whereas the superheated AZ91 alloy showed negligible undercooling. This suggests that nucleants, which influence the refinement of α-Mg grains, were generated by the superheating process.
- (3)
- Through the utilization of a rapid solidification process with precise temperature control, Al8Mn5 particles were observed in the non-superheated AZ91 ribbon samples; the (1 4 1) plane of these particles and the plane of magnesium was found to be in contact. However, it was confirmed that the crystallographic coherence between the two planes was so inconsistent that the modified Turnbull–Vonnegut equation, which is used for quantitative crystallographic coherence analysis, could not be applied.
- (4)
- Al10Mn3 particles were observed in the superheated AZ91 ribbon samples; the (1 2 1) plane of these particles and the plane of magnesium was found to be in contact. The 11% mismatch between the two planes was calculated using the modified Turnbull–Vonnegut equation.
- (5)
- It is thought that the superheating process contributes to grain refinement of AZ91 alloy by generating Al10Mn3, which exhibits more good crystallographic matching with magnesium compared to Al8Mn5. However, the study did not provide objective thermodynamic evidence to support the phase transformation of Al8Mn5 to Al10Mn3 or the generation of Al10Mn3, and additional thermodynamic studies are planned to clarify our results.
Author Contributions
Planning and designing of experiments were performed by Y.L., and experiments were carried out by S.J., with the supervision of Y.L. Writing was carried out by S.J. and reviewed by Y.L.; Writing—review and editing, Y.P. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Korea Institute of Industrial Technology (“Development of Root Technology for multi-product flexible production (Grant number: KITECH EO–23–0008)”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
We would like to thank all ASTL (Advanced Solidification Technology Lab) members and very special thanks to Gwang-seok Son in Donga University for his valuable help in TEM analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaya, A.A. A Review on Developments in Magnesium Alloys. Front. Mater. 2020, 7, 1–26. [Google Scholar] [CrossRef]
- Mordike, B.L.; Ebert, T. Magnesium Properties-applications-potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy. 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Lee, Y.C.; Dahle, A.K.; Stjohn, D.H. The role of solute in grain refinement of magnesium. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2000, 31, 2895–2906. [Google Scholar] [CrossRef]
- Song, J.; Chen, J.; Xiong, X.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2021. J. Magnes. Alloy. 2022, 10, 863–898. [Google Scholar] [CrossRef]
- Qian, M.; Stjohn, D.H.; Frost, M.T. Characteristic zirconium-rich coring structures in Mg-Zr alloys. Scr. Mater. 2002, 46, 649–654. [Google Scholar] [CrossRef]
- Stjohn, D.H.; Qian, M.; Easton, M.A.; Cao, P. Grain Refinement of Magnesium Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2005, 36, 1669–1679. [Google Scholar] [CrossRef]
- Ali, Y.; Qiu, D.; Jiang, B.; Pan, F.; Zhang, M.X. Current research progress in grain refinement of cast magnesium alloys: A review article. J. Alloys Compd. 2015, 619, 639–651. [Google Scholar] [CrossRef]
- Cao, P.; Qian, M.; StJohn, D.H. Mechanism for grain refinement of magnesium alloys by superheating. Scr. Mater. 2007, 56, 633–636. [Google Scholar] [CrossRef]
- Cao, P.; Qian, M.; StJohn, D. Grain refinement of commercial purity Mg-9%Al alloys by superheating. Magnes. Technol. 2005, 6, 297–302. [Google Scholar]
- Jung, S.S.; Son, Y.G.; Park, Y.H.; Lee, Y.C. A Study on the Grain Refining Mechanisms and Melt Superheat Treatment of Aluminum-Bearing Mg Alloys. Metals 2022, 12, 464. [Google Scholar] [CrossRef]
- Zhao, P.; Geng, H.; Wang, Q. Effect of melting technique on the microstructure and mechanical properties of AZ91 commercial magnesium alloys. Mater. Sci. Eng. A 2006, 429, 320–323. [Google Scholar] [CrossRef]
- Karakulak, E. A review: Past, present and future of grain refining of magnesium castings. J. Magnes. Alloy. 2019, 7, 355–369. [Google Scholar] [CrossRef]
- Vinotha, D.; Raghukandan, K.; Pillai, U.T.S.; Pai, B.C. Grain refining mechanisms in magnesium alloys—An overview. Trans. Indian Inst. Met. 2009, 62, 521–532. [Google Scholar] [CrossRef]
- Stjohn, D.H.; Easton, M.A.; Qian, M.; Taylor, J.A. Grain refinement of magnesium alloys: A review of recent research, theoretical developments, and their application. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 2935–2949. [Google Scholar] [CrossRef]
- Motegi, T. Grain-refining mechanisms of superheat-treatment of and carbon addition to Mg-Al-Zn alloys. Mater. Sci. Eng. A 2005, 413–414, 408–411. [Google Scholar] [CrossRef]
- Han, G.; Liu, X. Phase control and formation mechanism of Al–Mn(–Fe) intermetallic particles in Mg–Al-based alloys with FeCl3 addition or melt superheating. Acta Mater. 2016, 114, 54–66. [Google Scholar] [CrossRef]
- Byun, J.; Kwon, S.I.; Ha, H.P.; Yoon, J. A Manufacturing Technology of AZ91-Alloy Slurry for Semi Solid Forming. Magnesium 2003, 27, 713–718. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Qian, M.; Taylor, J.A. Crystallography of grain refinement in Mg-Al based alloys. Acta Mater. 2005, 53, 3261–3270. [Google Scholar] [CrossRef]
- Cao, P.; Qian, M.; Stjohn, D.H. Effect of manganese on grain refinement of Mg-Al based alloys. Scr. Mater. 2006, 54, 1853–1858. [Google Scholar] [CrossRef]
- Qin, G.W.; Ren, Y.; Huang, W.; Li, S.; Pei, W. Grain refining mechanism of Al-containing Mg alloys with the addition of Mn-Al alloys. J. Alloys Compd. 2010, 507, 410–413. [Google Scholar] [CrossRef]
- Qiu, D.; Zhang, M.X.; Taylor, J.A.; Fu, H.M.; Kelly, P.M. A novel approach to the mechanism for the grain refining effect of melt superheating of Mg-Al alloys. Acta Mater. 2007, 55, 1863–1871. [Google Scholar] [CrossRef]
- Han, M.; Zhu, X.; Gao, T.; Liu, X. Revealing the roles of Al4C3and Al8Mn5during α-Mg nucleation in Mg-Al based alloys. J. Alloys Compd. 2017, 705, 14–21. [Google Scholar] [CrossRef]
- Men, H.; Jiang, B.; Fan, Z. Mechanisms of grain refinement by intensive shearing of AZ91 alloy melt. Acta Mater. 2010, 58, 6526–6534. [Google Scholar] [CrossRef]
- Xu, J.; Yang, T.; Li, Z.; Wang, X.; Xiao, Y.; Jian, Z. The recalescence rate of cooling curve for undercooled solidification. Sci. Rep. 2020, 10, 1380. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Chen, X.D.; Xia, T.D.; Yu, W.Y.; Wang, X.L. Influencing factors and estimation of the cooling rate within an amorphous ribbon. Intermetallics 2004, 12, 1233–1237. [Google Scholar] [CrossRef]
- Smith, J.F. (Ed.) Phase Diagrams of Binary Vanadium Alloys; Series: Monograph series on alloy phase diagrams; ASM International: Metals Park, OH, USA, 1989. [Google Scholar]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Zeng, G.; Xian, J.W.; Gourlay, C.M. Nucleation and growth crystallography of Al8Mn5 on B2-Al(Mn, Fe) in AZ91 magnesium alloys. Acta Mater. 2018, 153, 364–376. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, M.; Fan, Z.; Zhou, X.; Thompson, G.E. The effect of Al8Mn5 intermetallic particles on grain size of as-cast Mg-Al-Zn AZ91D alloy. Intermetallics 2010, 18, 1683–1689. [Google Scholar] [CrossRef]
- Rigsbee, J.M.; Aaronson, H.I. A computer modeling study of partially coherent f.c.c.:b.c.c. boundaries. Acta Metall. 1979, 27, 351–363. [Google Scholar] [CrossRef]
- Glicksman, M.E.; Childs, W.J. Nucleation catalysis in supercooled liquid tin. Acta Metall. 1962, 10, 925–933. [Google Scholar] [CrossRef]
- Turnbull, D.; Vonnegut, B. Nucleation Catalysis. Ind. Eng. Chem. 1952, 44, 1292–1298. [Google Scholar] [CrossRef]
- Bruce, L. BRAMFITT The Effect of Carbide and Nitride Additions on the Heterogeneous Nucleation Behavior of Liquid Iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar]
- Wang, D.; Chang, W.; Shen, Y.; Sun, J.; Sheng, C.; Zhang, Y.; Zhai, Q. The role of lattice mismatch in heterogeneous nucleation of pure Al on Al2O3 single-crystal substrates with different termination planes. J. Therm. Anal. Calorim. 2019, 137, 791–797. [Google Scholar] [CrossRef]
- Kim, B.; Hwang, J.; Park, Y.; Lee, Y. Microstructural improvement of eutectic al + mg2si phases on al–zn–si–mg cast alloy with tib2 particles additions. Materials 2021, 14, 2902. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).