The Effect of Zirconia Nanoparticles on Thermal, Mechanical, and Corrosion Behavior of Nanocomposite Epoxy Coatings on Steel Substrates
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
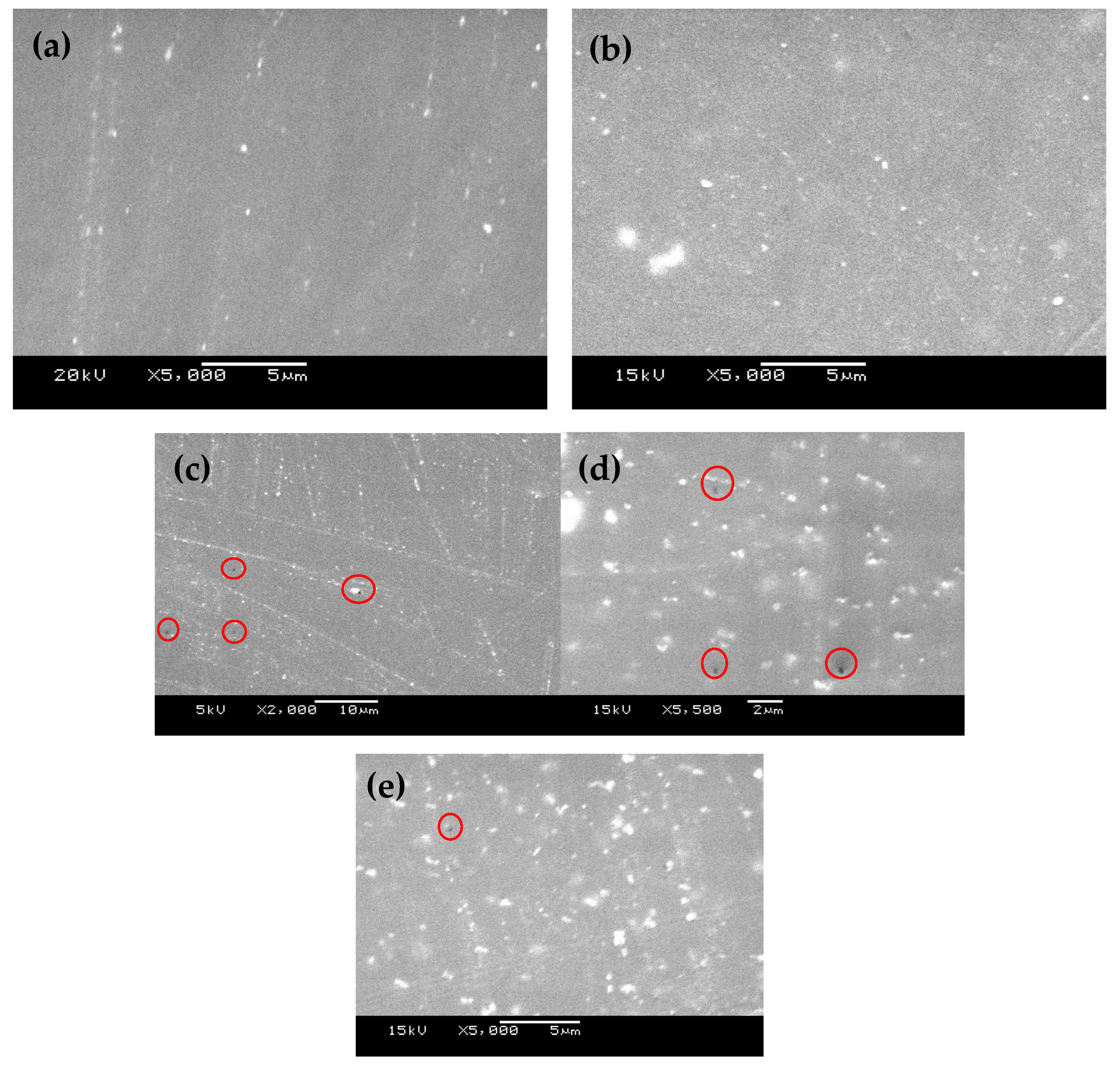
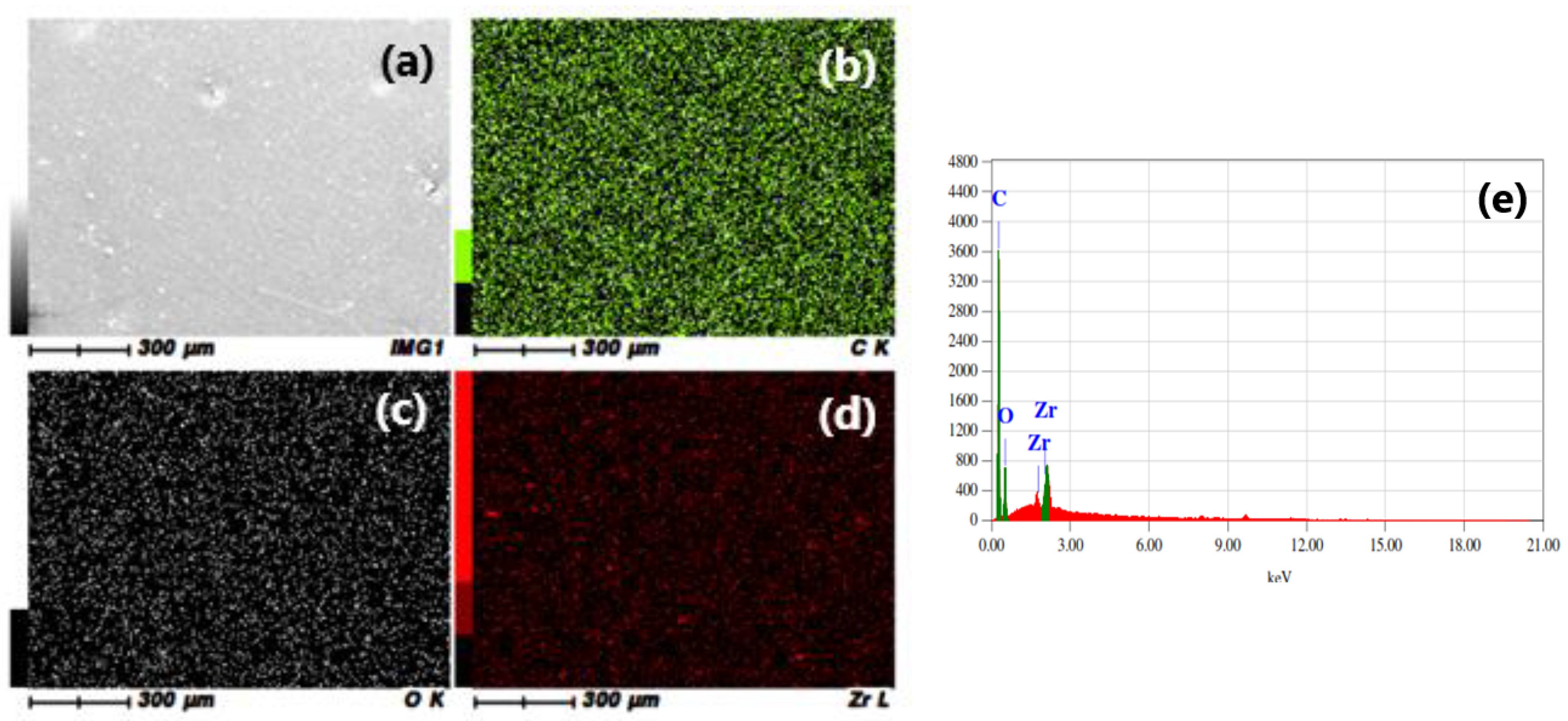
3.1. FE-SEM and EDS Investigations
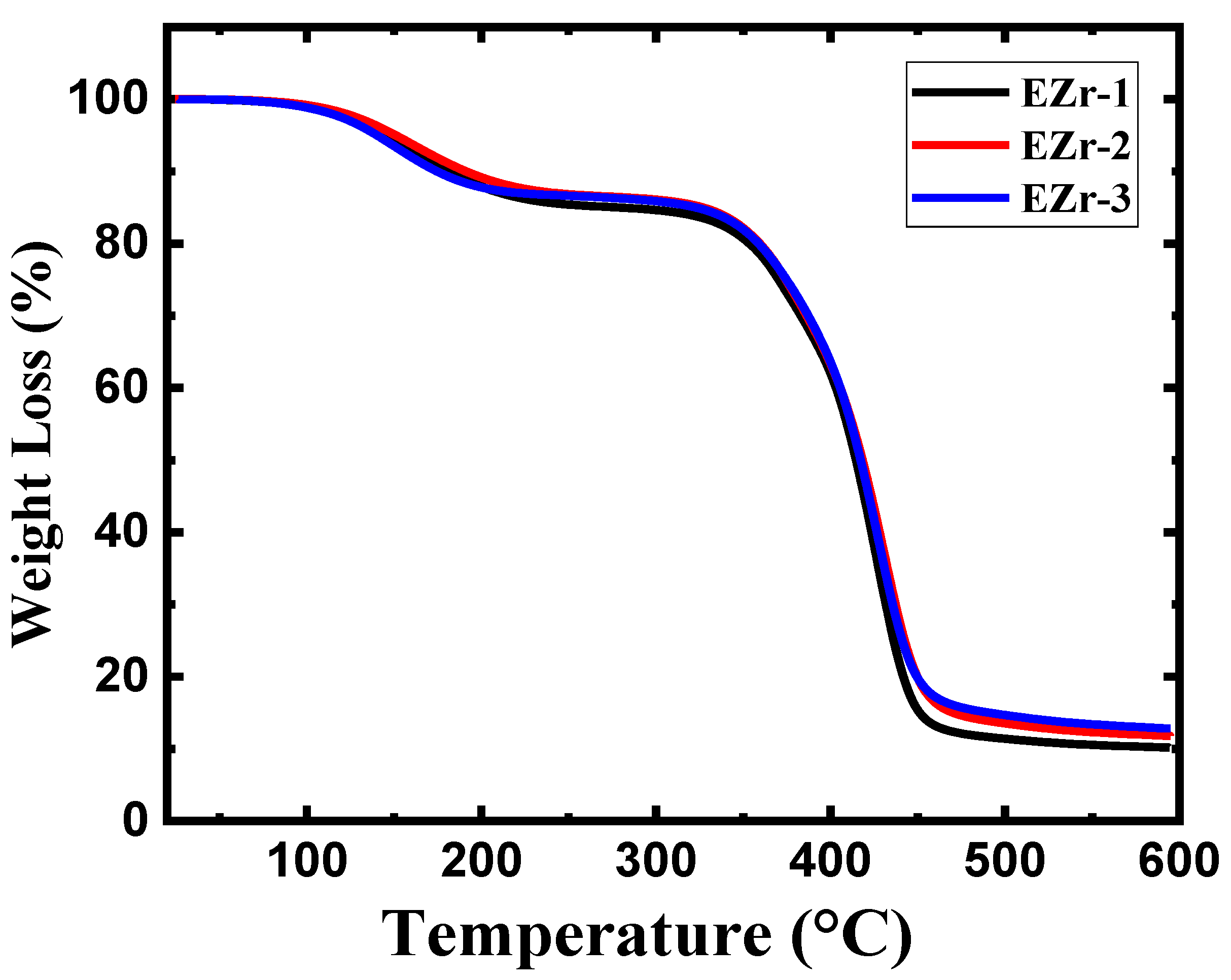
3.2. Thermogravimetric Analysis (TGA)
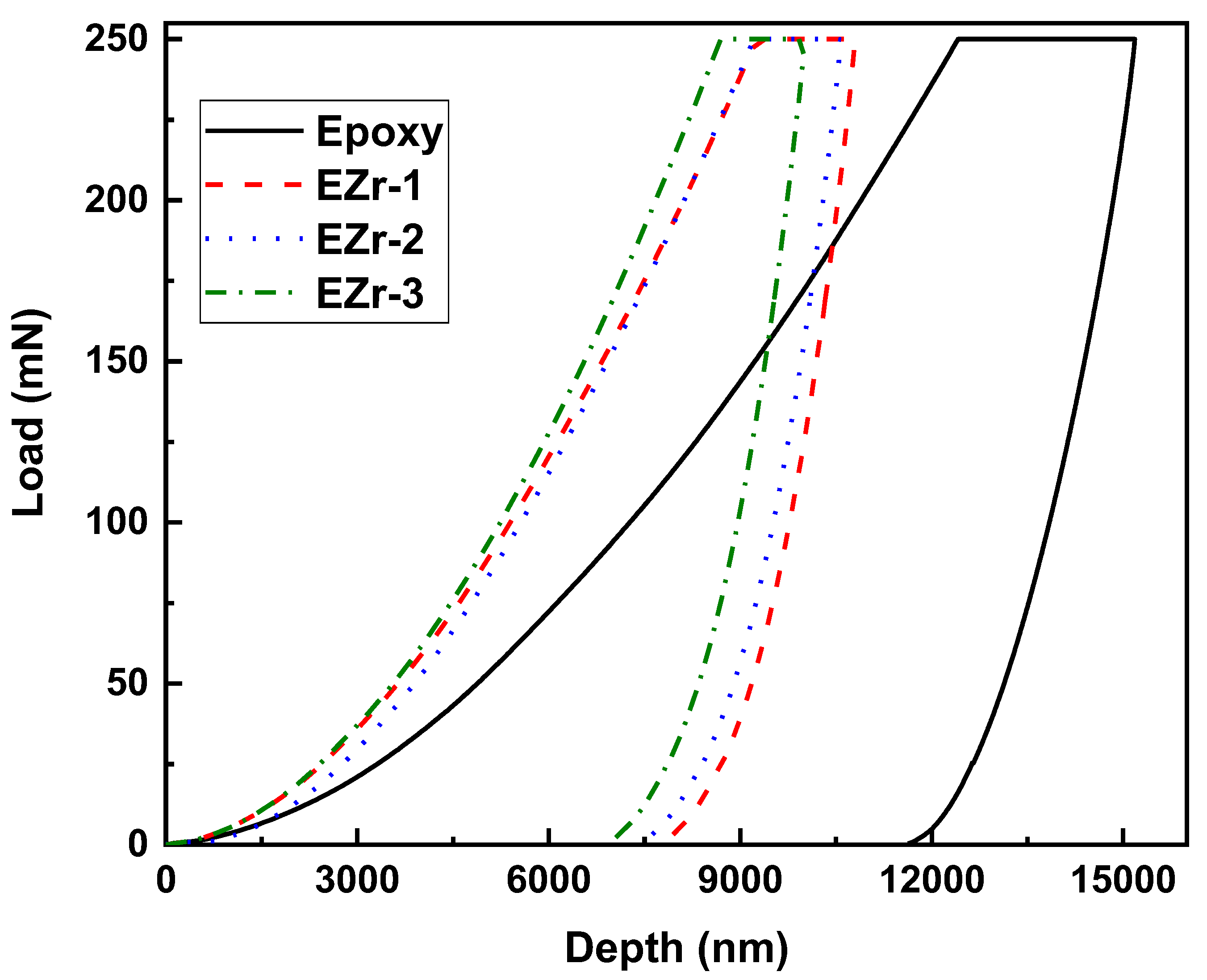
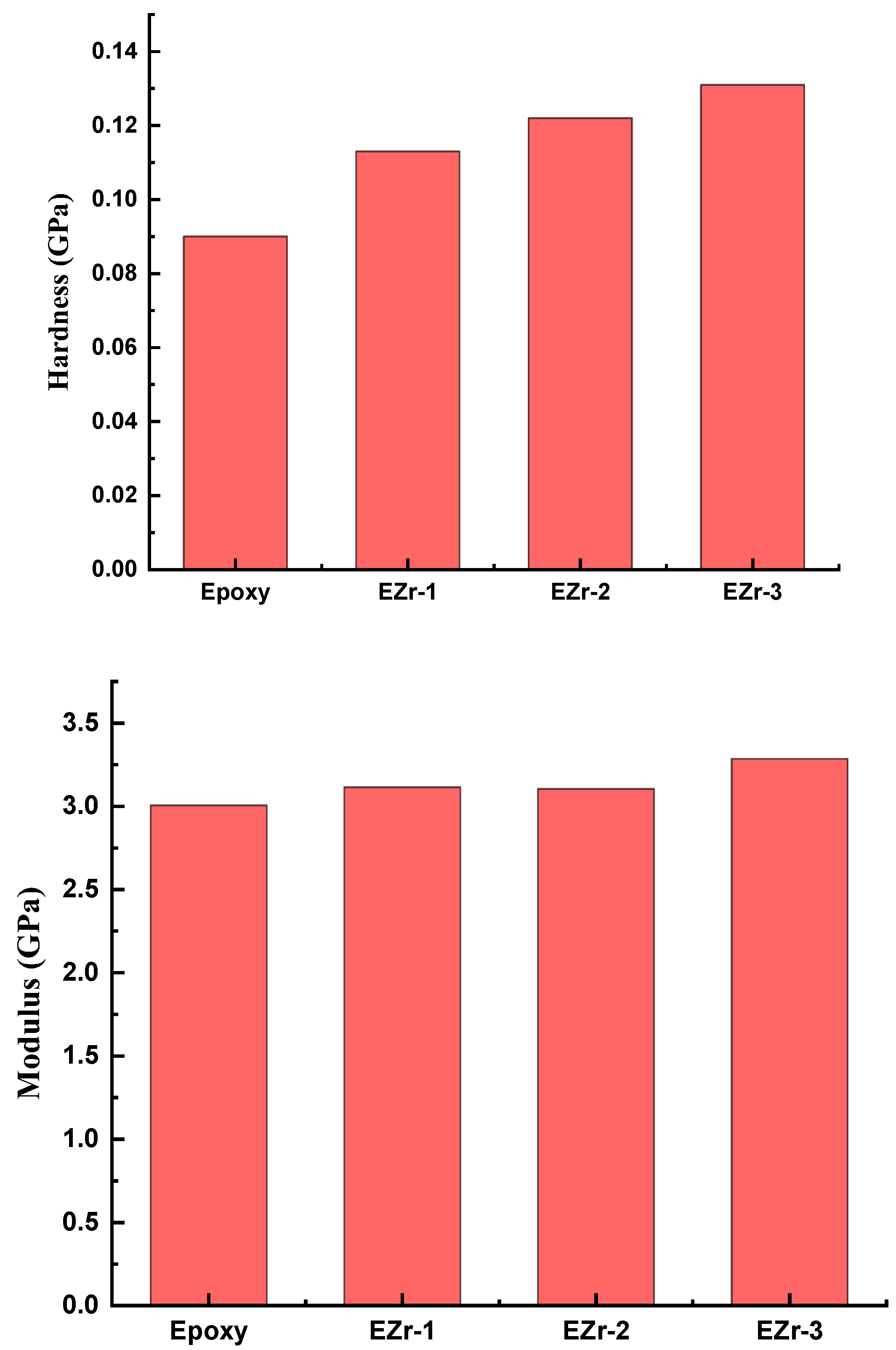
3.3. Mechanical Properties and Nanoindentation
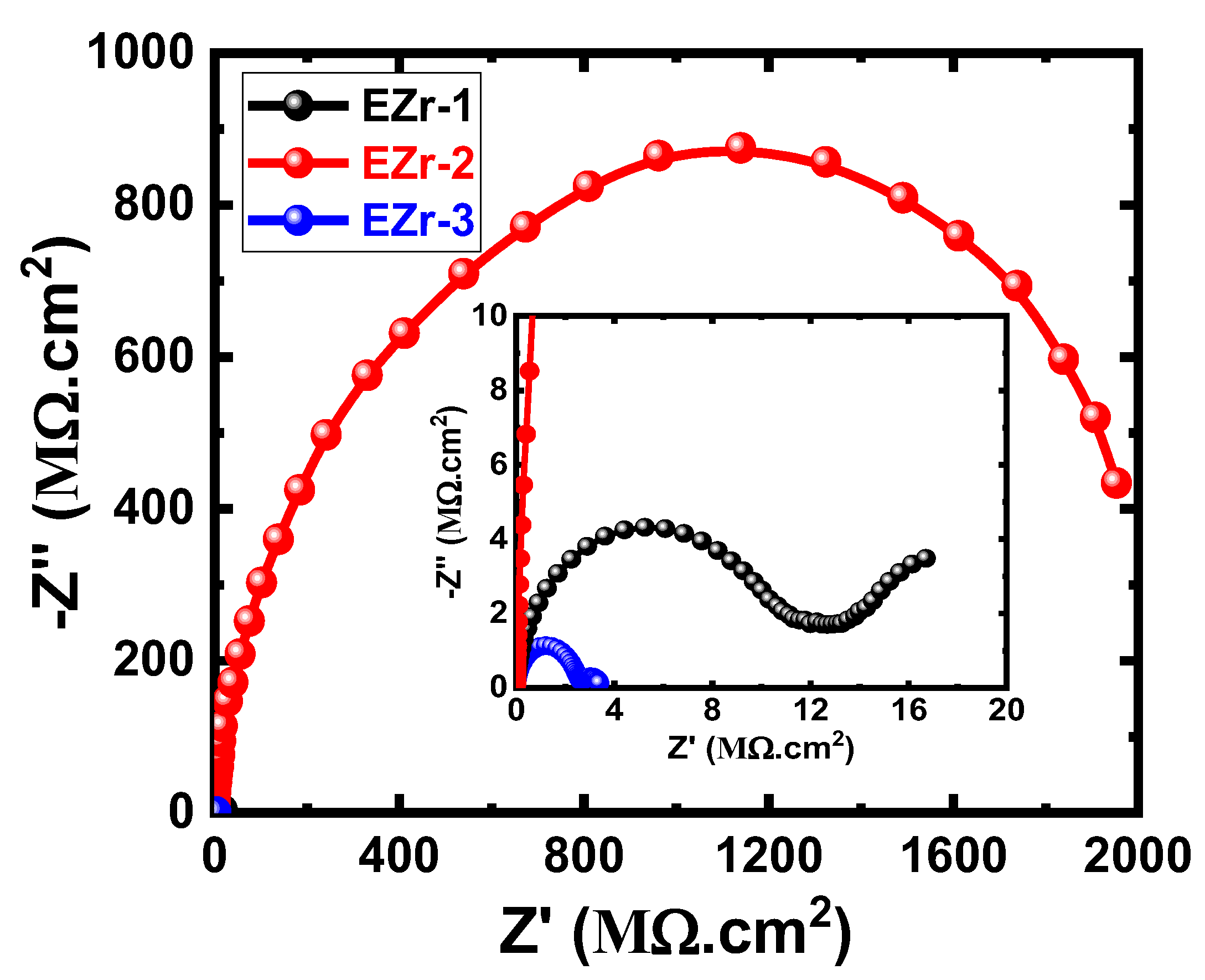
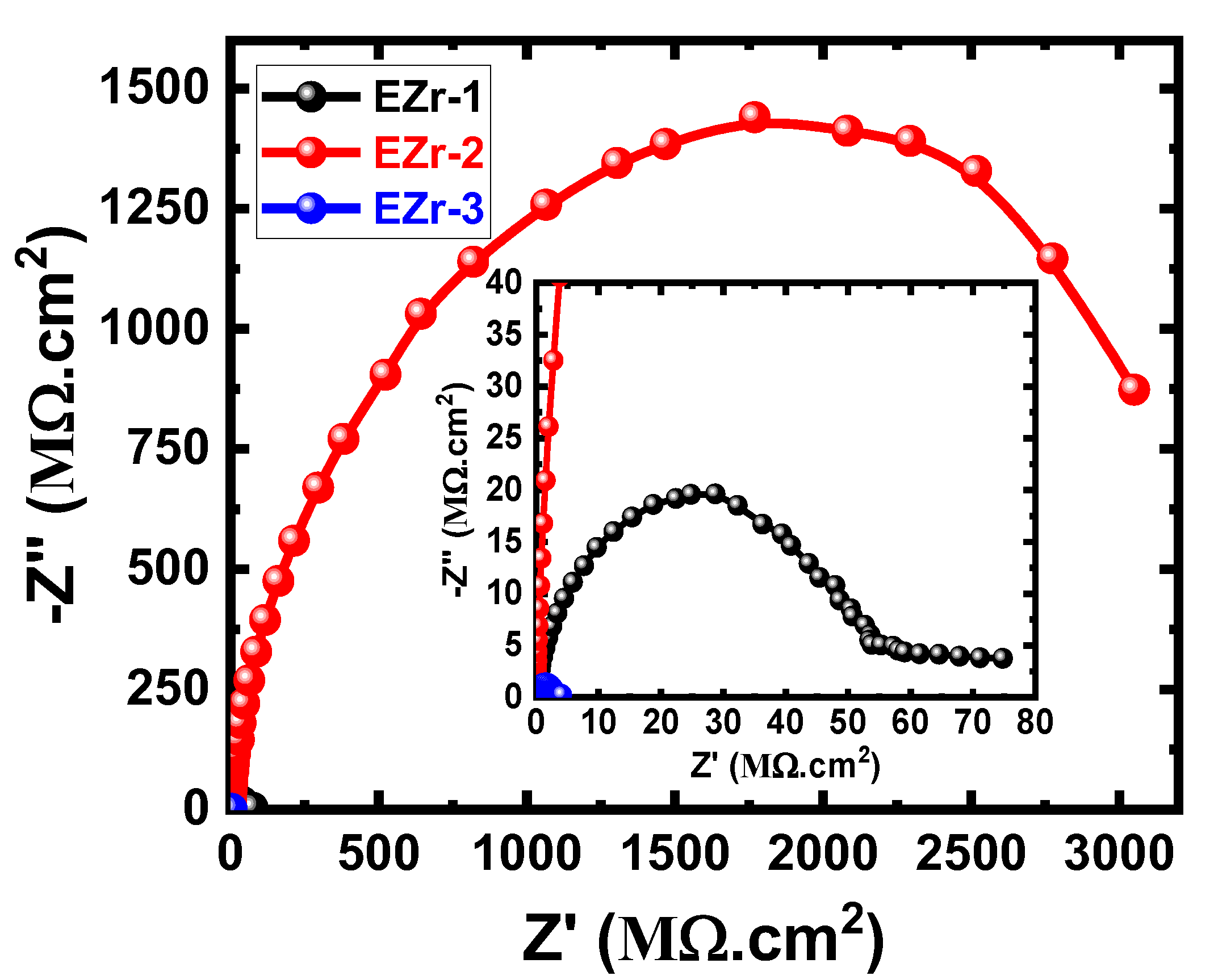
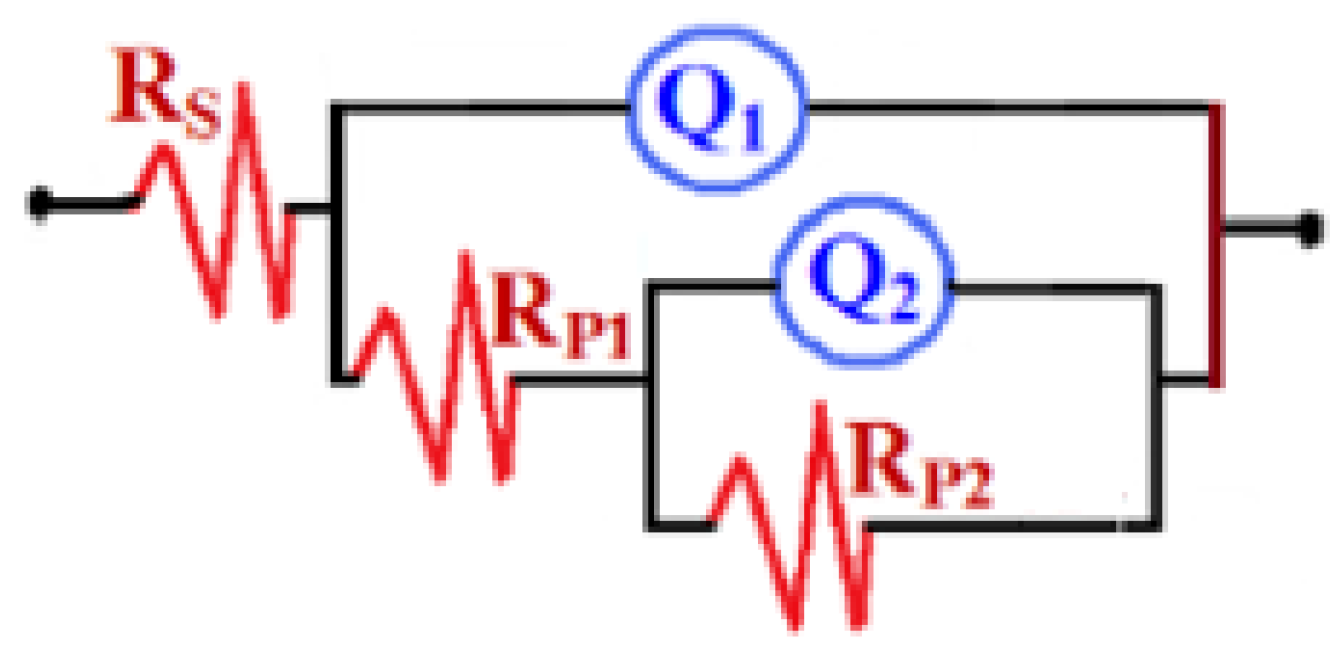
3.4. EIS Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, H.B.; Ma, C.; Gu, J.W.; Guo, J.; Yan, X.R.; Huang, J.N.; Zhang, Q.Y.; Guo, Z.H. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Singh, R.P.; Zhang, M.; Chan, D. Toughening of a brittle thermosetting polymer: Effects of reinforcement particle size and volume fraction. J. Mater. Sci. 2002, 37, 781–788. [Google Scholar] [CrossRef]
- Medina, R.; Haupert, F.; Schlarb, A.K. Improvement of tensile properties and toughness of an epoxy resin by nanozirconium-dioxide reinforcement. J. Mater. Sci. 2008, 43, 3245–3252. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.M.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Behzadnasab, M.; Kabiri, K. Effect of various combinations of zirconia and organoclay nanoparticles on mechanical and thermal properties of an epoxy nanocomposite coating. Compos. Part A Appl. Sci. Manuf. 2012, 43, 2095–2106. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.M.; Esfandeh, M. Corrosion protection of steel by epoxy nanocomposite coatings containing various combinations of clay and nanoparticulate zirconia. Corros. Sci. 2013, 75, 134–141. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A.; Bondioli, F.; Messori, M. Improving Epoxy Adhesives with Zirconia Nanoparticles. Compos. Interface 2010, 17, 873–892. [Google Scholar] [CrossRef] [Green Version]
- Rao, T.N.; Hussain, I.; Lee, J.E.; Kumar, A.; Koo, B.H. Enhanced Thermal Properties of Zirconia Nanoparticles and Chitosan-Based Intumescent Flame Retardant Coatings. Appl. Sci. 2019, 9, 3464. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Kannan, K.; Parangusan, H.; Eldeib, S.; Shehata, O.; Ismail, M.; Zarandah, R.; Sadasivuni, K.K. Enhanced Corrosion Protection of Epoxy/ZnO-NiO Nanocomposite Coatings on Steel. Coatings 2020, 10, 783. [Google Scholar] [CrossRef]
- ASTM D-4366; Standard Test Methods for Hardness of Organic Coatings by Pendulum Damping Tests. ASTM: West Conshohocken, PA, USA, 2021.
- ASTM D-7027; Standard Test Method for Evaluation of Scratch Resistance of Polymeric Coatings and Plastics Using an Instrumented Scratch Machine. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM D-2794; Standard Test Method for Resistance of Organic Coatings to the Effects of Rapid Deformation (Impact). ASTM: West Conshohocken, PA, USA, 2019.
- Aradhana, R.; Mohanty, S.; Nayak, S.K. Synergistic effect of polypyrrole and reduced graphene oxide on mechanical, electrical and thermal properties of epoxy adhesives. Polymer 2019, 166, 215–228. [Google Scholar] [CrossRef]
- Kunju, R.A.M.; Gopalakrishnan, J. Polyaniline nanorod adsorbed on reduced graphene oxide nanosheet for enhanced dielectric, viscoelastic and thermal properties of epoxy nanocomposites. Polym. Eng. Sci. 2021, 61, 1755–1773. [Google Scholar] [CrossRef]
- Tikhani, F.; Hadavand, B.S.; Bafghi, H.F.; Jouyandeh, M.; Vahabi, H.; Formela, K.; Hosseini, H.; Paran, S.M.R.; Esmaeili, A.; Mohaddespour, A.; et al. Polyurethane/Silane-Functionalized ZrO2 Nanocomposite Powder Coatings: Thermal Degradation Kinetics. Coatings 2020, 10, 413. [Google Scholar] [CrossRef] [Green Version]
- Atta, A.M.; Ahmed, M.A.; El-Saeed, A.M.; Abo-Elenien, O.M.; El-Sockary, M.A. Hybrid ZrO2/Cr2O3 Epoxy Nanocomposites as Organic Coatings for Steel. Coatings 2020, 10, 997. [Google Scholar] [CrossRef]
- Halder, S.; Goyat, M.S.; Ghosh, P.K. Morphological, structural, and thermophysical properties of zirconium dioxide–epoxy nanocomposites. High Perform. Polym. 2016, 28, 697–708. [Google Scholar] [CrossRef]
- Alam, M.A.; Sherif, E.-S.M.; Al-Zahrani, S.M. Fabrication of Various Epoxy Coatings for Offshore Applications and Evaluating Their Mechanical Properties and Corrosion Behavior. Int. J. Electrochem. Sci. 2013, 8, 3121–3131. [Google Scholar] [CrossRef]
- Chudoba, T.; Richter, F. Investigation of creep behaviour under load during indentation experiments and its influence on hardness and modulus results. Surf. Coat. Technol. 2001, 148, 191–198. [Google Scholar] [CrossRef]
- Alam, M.A.; Samad, U.A.; Sherif, E.-S.M.; Alothman, O.; Seikh, A.H.; Al-Zahrani, S.M. Effects of Minor Additions of Polypyrrole on the Thermal, Mechanical and Electrochemical Properties of Epoxy-2Pack Coatings. Int. J. Electrochem. Sci. 2017, 12, 74–89. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic-Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Arora, G.; Pathak, H. Nanoindentation characterization of polymer nanocomposites for elastic and viscoelastic properties: Experimental and mathematical approach. Compos. Part C Open Access 2021, 4, 100103. [Google Scholar] [CrossRef]
- Samad, U.A.; Alam, M.A.; Chafidz, A.; Al-Zahrani, S.M.; Alharthi, N.H. Enhancing mechanical properties of epoxy/polyaniline coating with addition of ZnO nanoparticles: Nanoindentation characterization. Prog. Org. Coat. 2018, 119, 109–115. [Google Scholar] [CrossRef]
- Abdus Samad, U.; Alam, M.A.; Sherif, E.-S.M.; Alam, M.; Shaikh, H.; Alharthi, N.H.; Al-Zahrani, S.M.J.C. Synergistic effect of Ag and ZnO nanoparticles on polypyrrole-incorporated epoxy/2pack coatings and their corrosion performances in chloride solutions. Coatings 2019, 9, 287. [Google Scholar] [CrossRef] [Green Version]
- Bansal, S.A.; Singh, A.P.; Kumar, S. Reinforcing Graphene Oxide Nanoparticles to Enhance Viscoelastic Performance of Epoxy Nanocomposites. J. Nanosci. Nanotechnol. 2019, 19, 4000–4006. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Samad, U.A.; Sherif, E.-S.M.; Poulose, A.M.; Mohammed, J.A.; Alharthi, N.; Al-Zahrani, S.M. Influence of SiO2 Content and Exposure Periods on the Anticorrosion Behavior of Epoxy Nanocomposite Coatings. Coatings 2020, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.A.; Samad, U.A.; Alam, M.; Anis, A.; Al-Zahrani, S.M. Enhancement in Nanomechanical, Thermal, and Abrasion Properties of SiO2 Nanoparticle-Modified Epoxy Coatings. Coatings 2020, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Davis, G.D.; Krebs, L.A.; Dacres, C.M. Coating evaluation and validation of accelerated test conditions using an in-situ corrosion sensor. J. Coat. Technol. 2002, 74, 69–74. [Google Scholar] [CrossRef]
- El Rayes, M.M.; Sherif, E.-S.M.; Abdo, H.S. Comparative Study into Microstructural and Mechanical Characterization of HVOF-WC-Based Coatings. Crystals 2022, 12, 969. [Google Scholar] [CrossRef]
- Samad, U.A.; Alam, M.A.; Anis, A.; Abdo, H.S.; Shaikh, H.; Al-Zahrani, S.M. Nanomechanical and Electrochemical Properties of ZnO-Nanoparticle-Filled Epoxy Coatings. Coatings 2022, 12, 282. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; El Danaf, E.A.; Abdo, H.S.; El Abedin, S.Z.; Al-Khazraji, H. Effect of Annealing Temperature on the Corrosion Protection of Hot Swaged Ti-54M Alloy in 2 M HCl Pickling Solutions. Metals 2017, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Bacon, R.C.; Smith, J.J.; Rugg, F.M. Electrolytic Resistance in Evaluating Protective Merit of Coatings on Metals. Ind. Eng. Chem. 1948, 40, 161–167. [Google Scholar] [CrossRef]



| Sample | * Epoxy (gm) | MIBK (mL) | Xylene (mL) | ZrO2 (wt%) | Silane (gm) | * D-450 (gm) |
|---|---|---|---|---|---|---|
| EZr-1 | 83.34 | 10 | 10 | 1 | 2 | 16.66 |
| EZr-2 | 83.34 | 10 | 10 | 2 | 2 | 16.66 |
| EZr-3 | 83.34 | 10 | 10 | 3 | 2 | 16.66 |
| Element/Coating | EZr-1 | EZr-2 | EZr-3 |
|---|---|---|---|
| Carbon (C) | 69.64% | 69.40% | 69.25% |
| Oxygen (O) | 29.47% | 29.15% | 27.78% |
| Zirconium (Zr) | 0.89% | 1.45% | 2.97% |
| Total | 100 | 100 | 100 |
| Sample | 15% Loss Temp. (°C) | 25% Loss Temp. (°C) | 50% Loss Temp. (°C) | 75% Loss Temp. (°C) | Residue (%) |
|---|---|---|---|---|---|
| EZr-1 | 278.88 | 370.43 | 414.71 | 436.24 | 10.01 |
| EZr-2 | 326.93 | 374.48 | 418.33 | 442.47 | 11.98 |
| EZr-3 | 323.34 | 374.76 | 418.24 | 440.84 | 12.89 |
| Sample | DFT (µm) | Pendulum Hardness * | Scratch (Kg) | Impact (lb/in2) |
|---|---|---|---|---|
| EZr-1 | 100 ± 10 | 149 | 7.0 | 80 |
| EZr-2 | 100 ± 10 | 145 | 7.5 | 88 |
| EZr-3 | 100 ± 10 | 149 | 8.5 | 80 |
| Samples | RS/Ω cm2 | Q1 | RP1/MΩcm2 | Q2 | RP2/MΩcm2 | ||
|---|---|---|---|---|---|---|---|
| YQ1/MΩ cm2 | n1 | YQ2/MΩ cm2 | n2 | ||||
| EZr1 (1d) | 33.7 | 267 | 0.981 | 135 | 353 | 0.765 | 321 |
| EZr2 (1d) | 42.0 | 369 | 0.959 | 227 | 185 | 0.751 | 865 |
| EZr3 (1d) | 30.6 | 333 | 0.975 | 196 | 348 | 0.685 | 293 |
| EZr1 (7d) | 45.2 | 336 | 0.974 | 169 | 338 | 0.675 | 355 |
| EZr2 (7d) | 47.8 | 545 | 0.976 | 228 | 296 | 0.607 | 910 |
| EZr3 (7d) | 42.0 | 421 | 0.971 | 202 | 330 | 0.699 | 327 |
| EZr1 (14d) | 37.8 | 394 | 0.860 | 197 | 310 | 0.638 | 403 |
| EZr2 (14d) | 49.5 | 601 | 0.981 | 256 | 278 | 0.634 | 2400 |
| EZr3 (14d) | 43.5 | 432 | 0.961 | 217 | 324 | 0.626 | 351 |
| EZr1 (21d) | 41.2 | 479 | 0.995 | 239 | 322 | 0.609 | 384 |
| EZr2 (21d) | 51.5 | 813 | 0.980 | 299 | 263 | 0.649 | 2240 |
| EZr3 (21d) | 43.3 | 619 | 0.972 | 234 | 316 | 0.602 | 426 |
| EZr1 (30d) | 42.0 | 511 | 0.895 | 241 | 299 | 0.655 | 420 |
| EZr2 (30d) | 56.0 | 785 | 0.869 | 273 | 238 | 0.689 | 4010 |
| EZr3 (30d) | 45.8 | 608 | 0.982 | 255 | 292 | 0.620 | 443 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.A.; Samad, U.A.; Anis, A.; Sherif, E.-S.M.; Abdo, H.S.; Al-Zahrani, S.M. The Effect of Zirconia Nanoparticles on Thermal, Mechanical, and Corrosion Behavior of Nanocomposite Epoxy Coatings on Steel Substrates. Materials 2023, 16, 4813. https://doi.org/10.3390/ma16134813
Alam MA, Samad UA, Anis A, Sherif E-SM, Abdo HS, Al-Zahrani SM. The Effect of Zirconia Nanoparticles on Thermal, Mechanical, and Corrosion Behavior of Nanocomposite Epoxy Coatings on Steel Substrates. Materials. 2023; 16(13):4813. https://doi.org/10.3390/ma16134813
Chicago/Turabian StyleAlam, Mohammad Asif, Ubair Abdus Samad, Arfat Anis, El-Sayed M. Sherif, Hany S. Abdo, and Saeed M. Al-Zahrani. 2023. "The Effect of Zirconia Nanoparticles on Thermal, Mechanical, and Corrosion Behavior of Nanocomposite Epoxy Coatings on Steel Substrates" Materials 16, no. 13: 4813. https://doi.org/10.3390/ma16134813







