Effect of Cellulose-Based Bioplastics on Current LDPE Recycling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Recycled LPDE–CAT Blends and Films
2.3. Characterization of Recycled LDPE–CAT Blends
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Tensile Testing
2.3.6. Dynamic Rheological Measurement
3. Results
3.1. Morphological Analysis
3.2. FT-IR Spectroscopy
3.3. Thermal Characterization
3.3.1. Thermogravimetric Analysis
3.3.2. DSC Analysis
3.4. Rheological Properties
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Bioplastics Bioplastics Market Development Update. 2022. Available online: https://www.european-bioplastics.org/market/ (accessed on 17 May 2023).
- Wojnowska-Baryła, I.; Kulikowska, D.; Bernat, K. Effect of Bio-Based Products on Waste Management. Sustainability 2020, 12, 2088. [Google Scholar] [CrossRef] [Green Version]
- UNI EN 13432:2002; Requirements for Packaging Recoverable through Composting and Biodegradation. Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. UNI—Ente Nazionale Italiano di Unificazione: Milan, Italy, 2002.
- De Gisi, S.; Gadaleta, G.; Gorrasi, G.; La Mantia, F.P.; Notarnicola, M.; Sorrentino, A. The role of (bio)degradability on the management of petrochemical and bio-based plastic waste. J. Environ. Manag. 2022, 310, 114769. [Google Scholar] [CrossRef] [PubMed]
- Freeland, B.; McCarthy, E.; Balakrishnan, R.; Fahy, S.; Boland, A.; Rochfort, K.D.; Dabros, M.; Marti, R.; Kelleher, S.M.; Gaughran, J. A Review of Polylactic Acid as a Replacement Material for Single-Use Laboratory Components. Materials 2022, 15, 2989. [Google Scholar] [CrossRef] [PubMed]
- Dilkes-Hoffman, L.; Ashworth, P.; Laycock, B.; Pratt, S.; Lant, P. Public attitudes towards bioplastics–knowledge, perception and end-of-life management. Resour. Conserv. Recycl. 2019, 151, 104479. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Picuno, C.; Heerenklage, J.; Cafiero, L.; Oliviero, M.; Notarnicola, M.; Kuchta, K.; Sorrentino, A. The influence of bio-plastics for food packaging on combined anaerobic digestion and composting treatment of organic municipal waste. Waste Manag. 2022, 144, 87–97. [Google Scholar] [CrossRef]
- Alaerts, L.; Augustinus, M.; Van Acker, K. Impact of Bio-Based Plastics on Current Recycling of Plastics. Sustainability 2018, 10, 1487. [Google Scholar] [CrossRef] [Green Version]
- Gadaleta, G.; De Gisi, S.; Todaro, F.; Notarnicola, M. Carbon Footprint and Total Cost Evaluation of Different Bio-Plastics Waste Treatment Strategies. Clean Technol. 2022, 4, 570–583. [Google Scholar] [CrossRef]
- Gadaleta, G.; Ferrara, C.; De Gisi, S.; Notarnicola, M.; De Feo, G. Life cycle assessment of end-of-life options for cellulose-based bioplastics when introduced into a municipal solid waste management system. Sci. Total Environ. 2023, 871, 161958. [Google Scholar] [CrossRef]
- Cimpan, C.; Maul, A.; Jansen, M.; Pretz, T.; Wenzel, H. Central sorting and recovery of MSW recyclable materials: A review of technological state-of-the-art, cases, practice and implications for materials recycling. J. Environ. Manag. 2015, 156, 181–199. [Google Scholar] [CrossRef]
- Gaustad, G.; Olivetti, E.; Kirchain, R. Improving aluminum recycling: A survey of sorting and impurity removal technologies. Resour. Conserv. Recycl. 2012, 58, 79–87. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Binetti, S.M.; Notarnicola, M. Outlining a comprehensive techno-economic approach to evaluate the performance of an advanced sorting plant for plastic waste recovery. Process. Saf. Environ. Prot. 2020, 143, 248–261. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Picuno, C.; Heerenklage, J.; Kuchta, K.; Sorrentino, A.; Notarnicola, M. Energy recovery options for the management of cellulose-based bio-plastics and mixed municipal solid waste. Biomass Bioenergy 2022, 166, 106628. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Todaro, F.; D’alessandro, G.; Binetti, S.; Notarnicola, M. Assessing the Sorting Efficiency of Plastic Packaging Waste in an Italian Material Recovery Facility: Current and Upgraded Configuration. Recycling 2023, 8, 25. [Google Scholar] [CrossRef]
- Yadav, N.; Hakkarainen, M. Degradable or not? Cellulose acetate as a model for complicated interplay between structure, environment and degradation. Chemosphere 2020, 265, 128731. [Google Scholar] [CrossRef] [PubMed]
- Phuong, V.T.; Verstiche, S.; Cinelli, P.; Anguillesi, I.; Coltelli, M.-B.; Lazzeri, A. Cellulose Acetate Blends—Effect of Plasticizers on Properties and Biodegradability. J. Renew. Mater. 2014, 2, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Dreux, X.; Majesté, J.-C.; Carrot, C.; Argoud, A.; Vergelati, C. Viscoelastic behaviour of cellulose acetate/triacetin blends by rheology in the melt state. Carbohydr. Polym. 2019, 222, 114973. [Google Scholar] [CrossRef] [PubMed]
- Ganster, J.; Fink, H.P. Cellulose and Cellulose Acetate. In Bio-Based Plastics: Materials and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 35–62. ISBN 9781118676646. [Google Scholar]
- Wang, D.; Sun, G.; Yu, L. Recyclability of cellulose acetate butyrate (CAB) matrix for controllable and productive fabrication of thermoplastic nanofibers. Carbohydr. Polym. 2011, 83, 1095–1100. [Google Scholar] [CrossRef]
- Tajeddin, B.; Abdul Rahman, R.; Chuah Abdulah, L. Mechanical and Morphological Properties of Kenaf Cellulose/LDPE Biocomposites. J. Agric. Environ. Sci. 2009, 5, 777–785. [Google Scholar]
- Kosaka, P.M.; Kawano, Y.; Fantini, M.C.A.; Petri, D.F.S. Structure and Properties of Maleated Linear Low-Density Polyethylene and Cellulose Acetate Butyrate Blends. Macromol. Mater. Eng. 2006, 291, 531–539. [Google Scholar] [CrossRef]
- Sailaja, R.R.N.; Seetharamu, S. Mechanical and thermal properties of LDPE-cellulose acetate phthalate blends-Effect of maleic anhydride-grafted LDPE compatibilizer. J. Appl. Polym. Sci. 2009, 112, 649–659. [Google Scholar] [CrossRef]
- Pedroso, A.; Rosa, D. Mechanical, thermal and morphological characterization of recycled LDPE/corn starch blends. Carbohydr. Polym. 2005, 59, 1–9. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM—American Society for Testing and Materials: West Conshohocken, PA, USA, 2018.
- Guo, M.; Chen, H.; Maia, J.M. Effects of structure and processing on the surface roughness of extruded co-continuous poly(ethylene) oxide/ethylene-vinyl acetate blends. J. Polym. Eng. 2019, 40, 763–770. [Google Scholar] [CrossRef]
- Arrigo, R.; Malucelli, G.; La Mantia, F.P. Effect of the Elongational Flow on the Morphology and Properties of Polymer Systems: A Brief Review. Polymers 2021, 13, 3529. [Google Scholar] [CrossRef] [PubMed]
- Samper, M.D.; Bertomeu, D.; Arrieta, M.P.; Ferri, J.M.; López-Martínez, J. Interference of Biodegradable Plastics in the Polypropylene Recycling Process. Materials 2018, 11, 1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, A.K.; Chaitanya, K.; Dalmia, R.; Vijayakumar, R.P. Synergistic effect of UV, thermal, and chemical treatment on biological degradation of low-density polyethylene (LDPE) by Thermomyces lanuginosus. Environ. Monit. Assess. 2021, 193, 1–11. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Chong, Z.K.; Heerenklage, J.; Notarnicola, M.; Kuchta, K.; Cafiero, L.; Oliviero, M.; Sorrentino, A.; Picuno, C. Degradation of thermoplastic cellulose acetate-based bioplastics by full-scale experimentation of industrial anaerobic digestion and composting. Chem. Eng. J. 2023, 462, 142301. [Google Scholar] [CrossRef]
- Babaghayou, M.I.; Mourad, A.-H.I.; Ochoa, A.; Beltrán, F.; Cherupurakal, N. Study on the thermal stability of stabilized and unstabilized low-density polyethylene films. Polym. Bull. 2020, 78, 5225–5241. [Google Scholar] [CrossRef]
- Sikora, J.; Majewski, Ł.; Puszka, A. Modern Biodegradable Plastics—Processing and Properties: Part I. Materials 2020, 13, 1986. [Google Scholar] [CrossRef] [Green Version]
- Sirin, H.; Tuna, B.; Ozkoc, G. The Effects of Thermomechanical Cycles on the Properties of PLA/TPS Blends. Adv. Polym. Technol. 2014, 33, 21458. [Google Scholar] [CrossRef]
- Gao, C.; Bao, X.; Yu, L.; Liu, H.; Simon, G.P.; Chen, L.; Liu, X. Thermal properties and miscibility of semi-crystalline and amorphous PLA blends. J. Appl. Polym. Sci. 2014, 131, 41205. [Google Scholar] [CrossRef]
- Aumnate, C.; Rudolph, N.; Sarmadi, M. Recycling of Polypropylene/Polyethylene Blends: Effect of Chain Structure on the Crystallization Behaviors. Polymers 2019, 11, 1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heeley, E.L.; Hughes, D.J.; Taylor, P.G.; Bassindale, A.R. Crystallization and morphology development in polyethylene–octakis(n-octadecyldimethylsiloxy)octasilsesquioxane nanocomposite blends. RSC Adv. 2015, 5, 34709–34719. [Google Scholar] [CrossRef] [Green Version]
- Heeley, E.L.; Hughes, D.J.; El Aziz, Y.; Williamson, I.; Taylor, P.G.; Bassindale, A.R. Properties and self-assembled packing morphology of long alkyl-chained substituted polyhedral oligomeric silsesquioxanes (POSS) cages. Phys. Chem. Chem. Phys. 2013, 15, 5518–5529. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Wang, J.H.; Bai, Y.; Li, Y. Effects and mechanism of cellulose acetate butyrate on the crystallization of polylactic acid. Eur. Polym. J. 2019, 121, 109286. [Google Scholar] [CrossRef]
- Zare, Y.; Garmabi, H.; Rhee, K.Y. Structural and phase separation characterization of poly(lactic acid)/poly(ethylene oxide)/carbon nanotube nanocomposites by rheological examinations. Compos. Part B Eng. 2018, 144, 1–10. [Google Scholar] [CrossRef]
- Riande, E.; Diaz-Calleja, R.; Prolongo, M.; Masegosa, R.; Salom, C. Polymer Viscoelasticity Stress and Strain in Practice 2000; Marcel Dekker, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Djellali, S.; Sadoun, T.; Haddaoui, N.; Bergeret, A. Viscosity and viscoelasticity measurements of low density polyethylene/poly(lactic acid) blends. Polym. Bull. 2015, 72, 1177–1195. [Google Scholar] [CrossRef]
- Baghaei, B.; Jafari, S.H.; Khonakdar, H.A.; Rezaeian, I.; As’habi, L.; Ahmadian, S. Interfacially compatibilized LDPE/POE blends reinforced with nanoclay: Investigation of morphology, rheology and dynamic mechanical properties. Polym. Bull. 2009, 62, 255–270. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, M.; Adhikari, B. Thermal stability and degradation of the post-use reclaim milk pouches during multiple extrusion cycles. Thermochim. Acta 2005, 430, 87–94. [Google Scholar] [CrossRef]
- Aniśko, J.; Barczewski, M. Uniaxial Rotational Molding of Bio-Based Low-Density Polyethylene Filled with Black Tea Waste. Materials 2023, 16, 3641. [Google Scholar] [CrossRef]
- Bekhta, P.; Pizzi, A.; Kusniak, I.; Bekhta, N.; Chernetskyi, O.; Nuryawan, A. A Comparative Study of Several Properties of Plywood Bonded with Virgin and Recycled LDPE Films. Materials 2022, 15, 4942. [Google Scholar] [CrossRef]


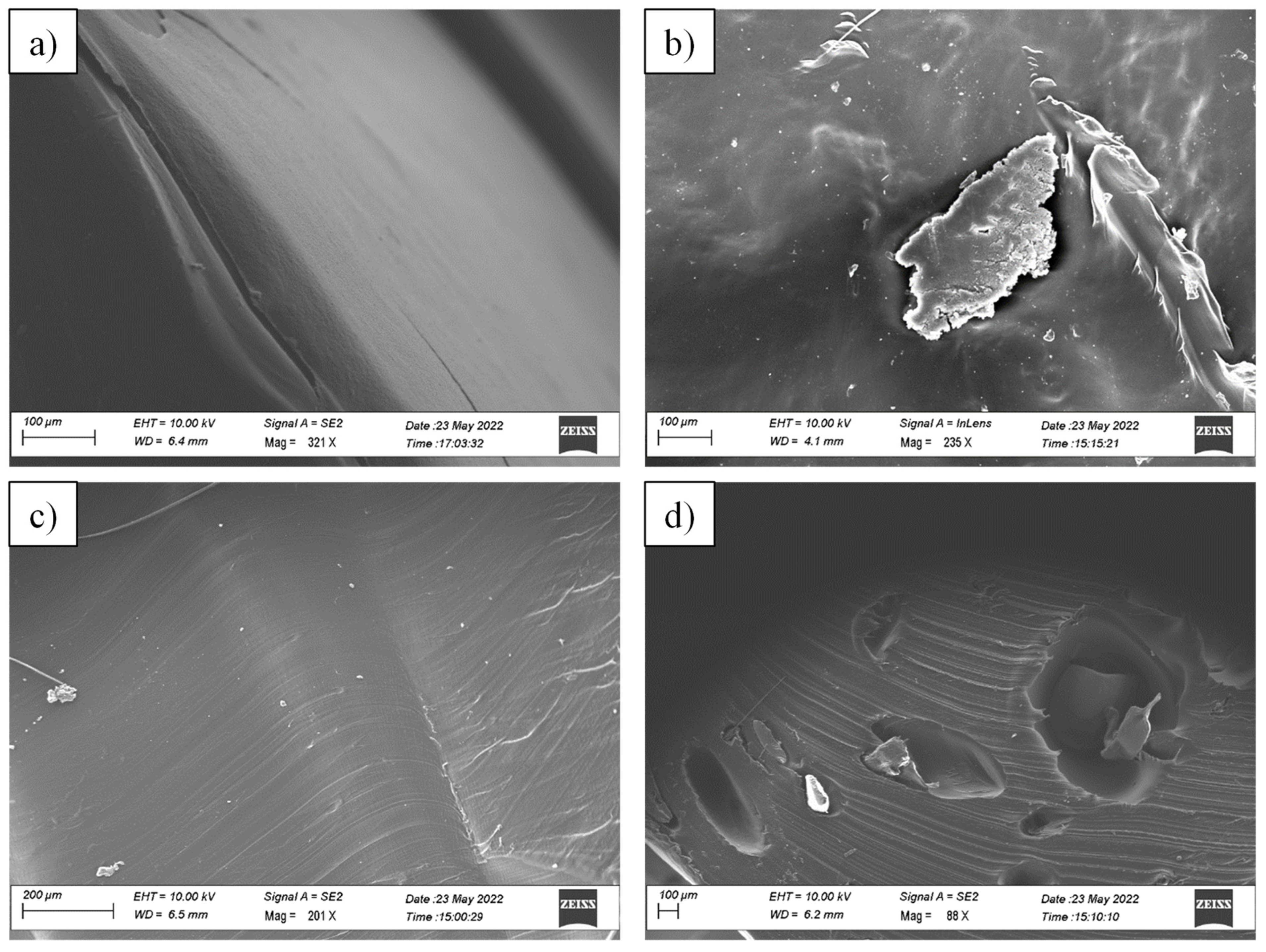
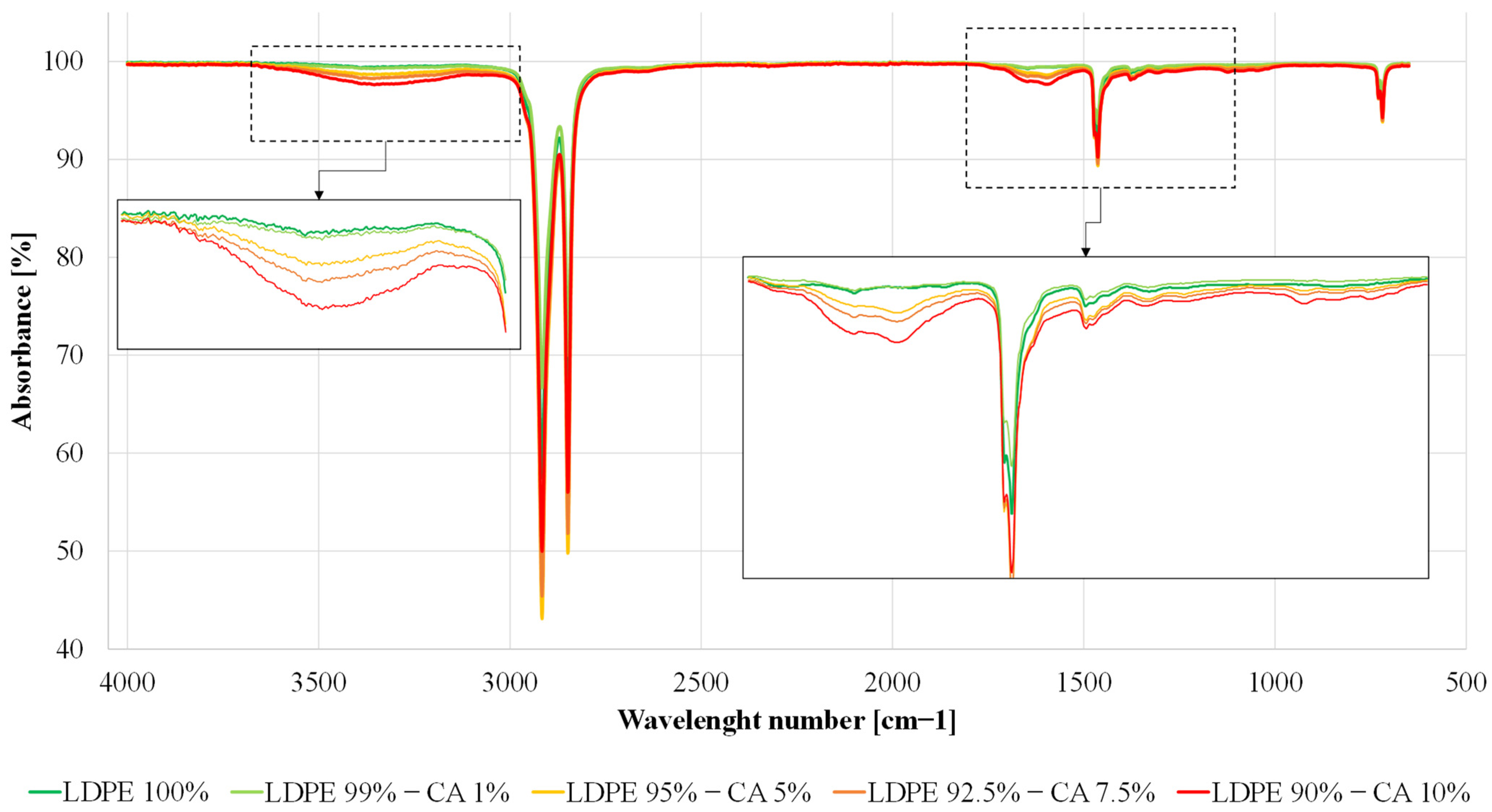

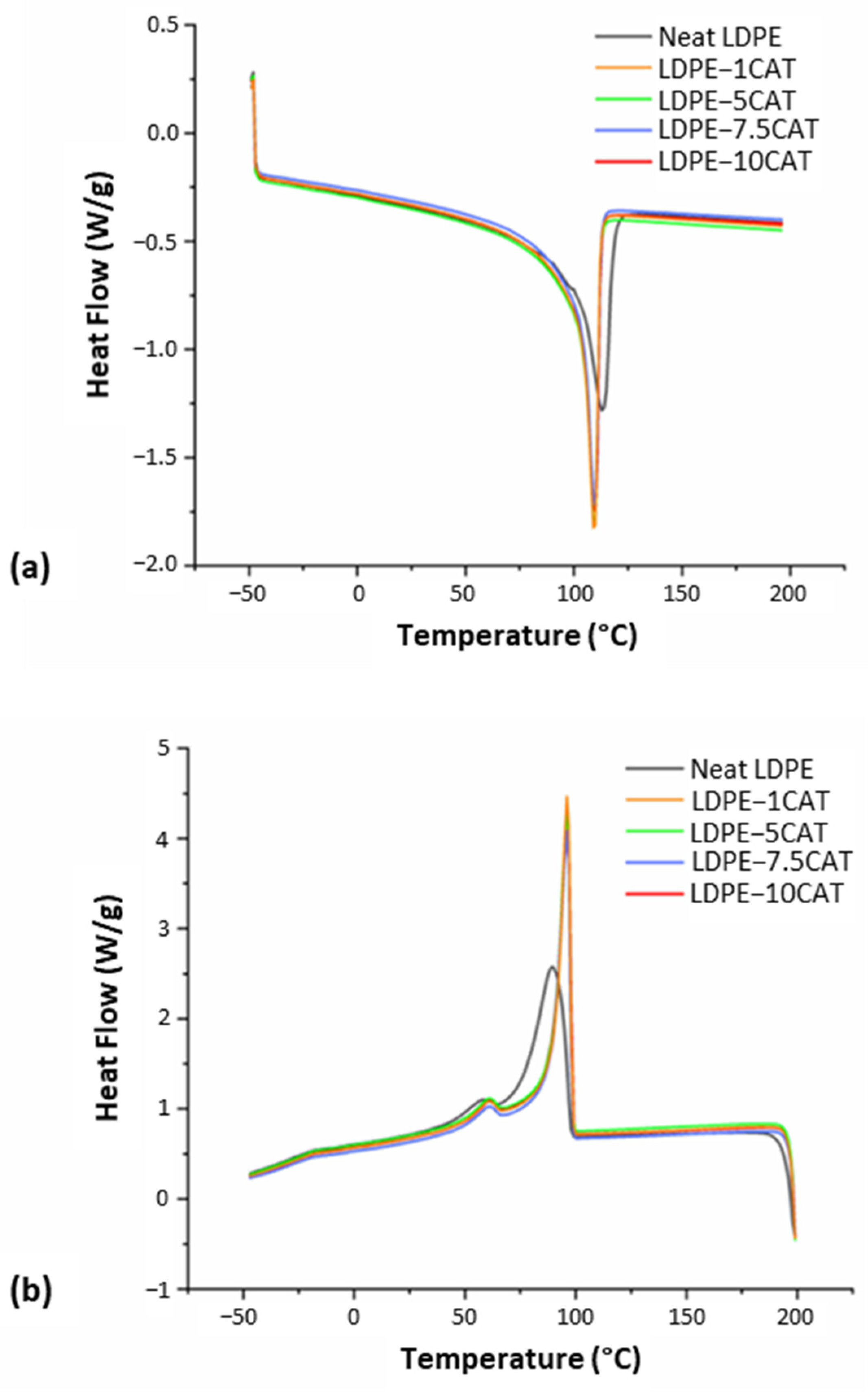

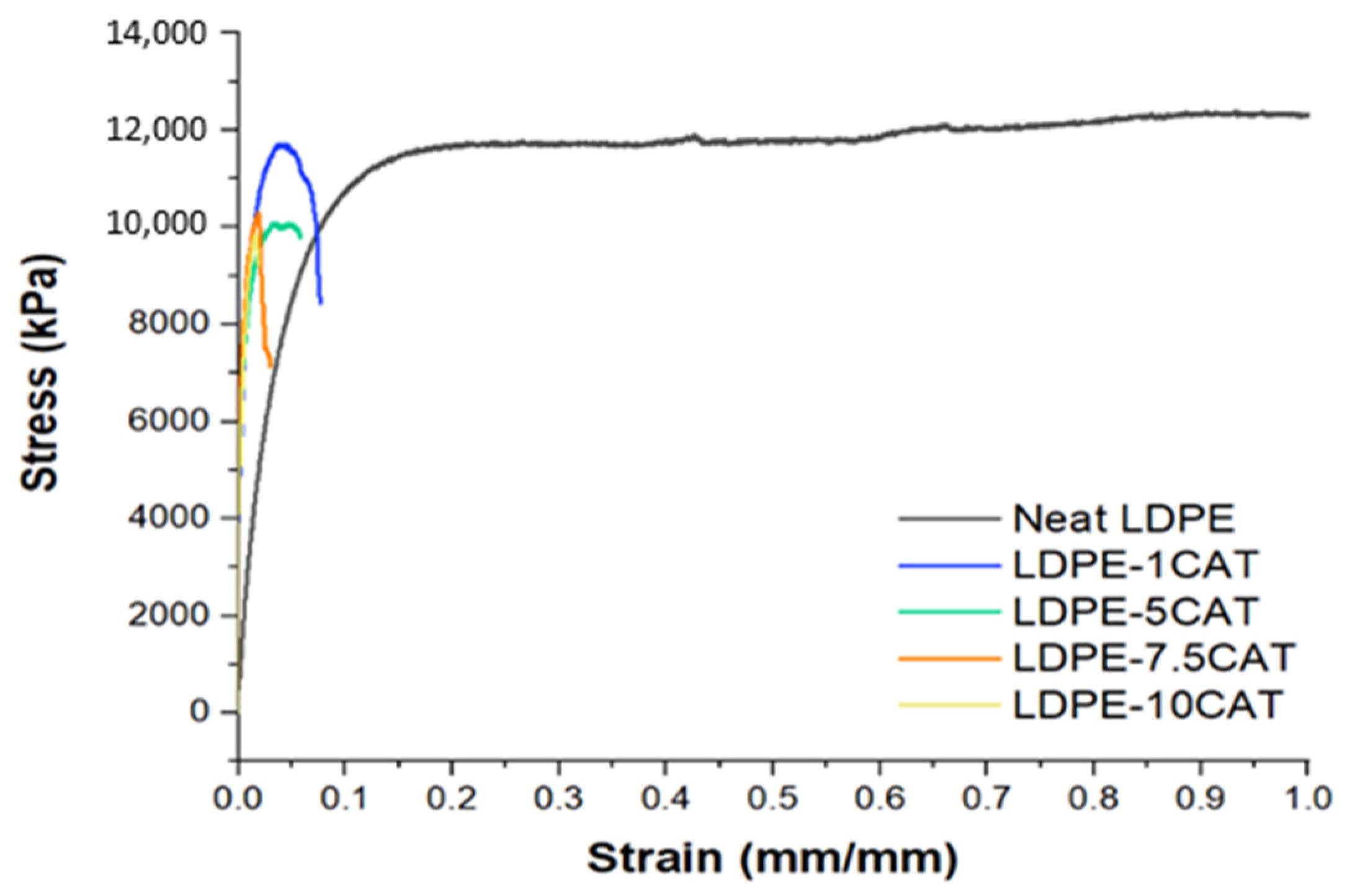
| Sample | Tdmax,1 [°C] | Tdmax,2 [°C] |
|---|---|---|
| CAT | 369 | - |
| Neat LDPE | - | 440 |
| LDPE-10CAT | 336 | 443 |
| Sample | Tm [°C] | Tc1 [°C] | Tc2 [°C] | ΔHc1 [J/g] | ΔHc2 [J/g] | Xc [%] |
|---|---|---|---|---|---|---|
| Neat LDPE | 116 | 90 | 58 | 77 | 5.1 | 27.8 |
| LDPE-1CAT | 110 | 96 | 60 | 74 | 4.2 | 27.4 |
| LDPE-5CAT | 110 | 96 | 60 | 75 | 4.3 | 27.1 |
| LDPE-7.5CAT | 110 | 96 | 60 | 74 | 4.1 | 26.7 |
| LDPE-10CAT | 110 | 96 | 60 | 75 | 4.2 | 26.7 |
| Sample | Tensile Modulus [MPa] | Yield Stress [MPa] | Yield Strain [mm/mm] | Elongation at Break [mm/mm] |
|---|---|---|---|---|
| Neat LDPE | 248.7 ± 6.9 | 11.9 ± 0.1 | 0.24 ± 0.00 | 1.1 ± 0.04 |
| LDPE-1CAT | 265.8 ± 15.6 | 12.0 ± 0.5 | 0.16 ± 0.01 | 0.43 ± 0.06 |
| LDPE-5CAT | 280.3 ± 11.1 | 10.3 ± 0.2 | 0.17 ± 0.01 | 0.37 ± 0.05 |
| LDPE-7.5CAT | 308.3 ± 23.5 | 10.0 ± 0.6 | 0.12 ± 0.02 | 0.28 ± 0.05 |
| LDPE-10CAT | 341.5 ± 49.0 | 9.0 ± 0.9 | 0.11 ± 0.03 | 0.16 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadaleta, G.; De Gisi, S.; Sorrentino, A.; Sorrentino, L.; Notarnicola, M.; Kuchta, K.; Picuno, C.; Oliviero, M. Effect of Cellulose-Based Bioplastics on Current LDPE Recycling. Materials 2023, 16, 4869. https://doi.org/10.3390/ma16134869
Gadaleta G, De Gisi S, Sorrentino A, Sorrentino L, Notarnicola M, Kuchta K, Picuno C, Oliviero M. Effect of Cellulose-Based Bioplastics on Current LDPE Recycling. Materials. 2023; 16(13):4869. https://doi.org/10.3390/ma16134869
Chicago/Turabian StyleGadaleta, Giovanni, Sabino De Gisi, Andrea Sorrentino, Luigi Sorrentino, Michele Notarnicola, Kerstin Kuchta, Caterina Picuno, and Maria Oliviero. 2023. "Effect of Cellulose-Based Bioplastics on Current LDPE Recycling" Materials 16, no. 13: 4869. https://doi.org/10.3390/ma16134869














