Preheating Influence on the Precipitation Microstructure, Mechanical and Corrosive Properties of Additively Built Al–Cu–Li Alloy Contrasted with Conventional (T83) Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
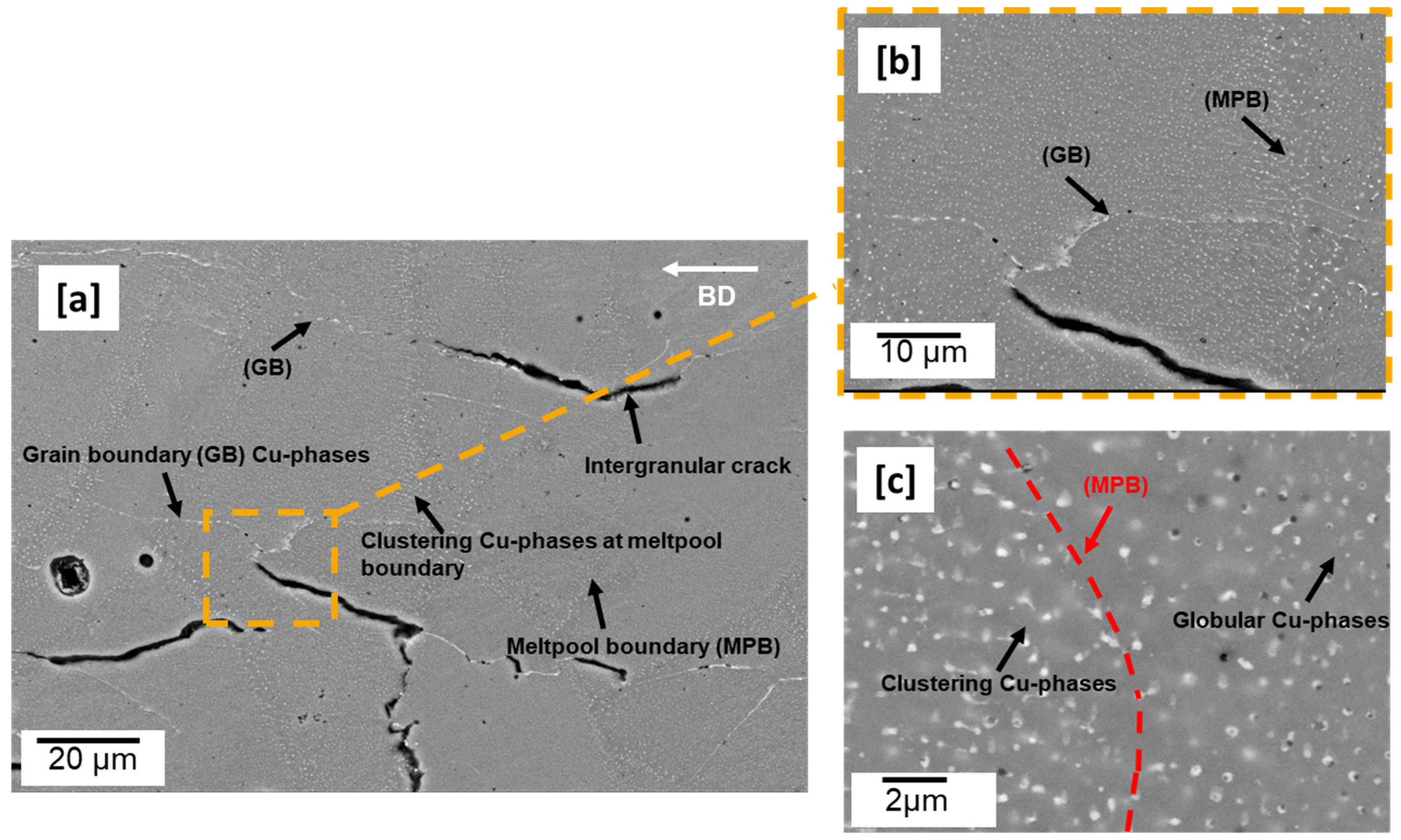
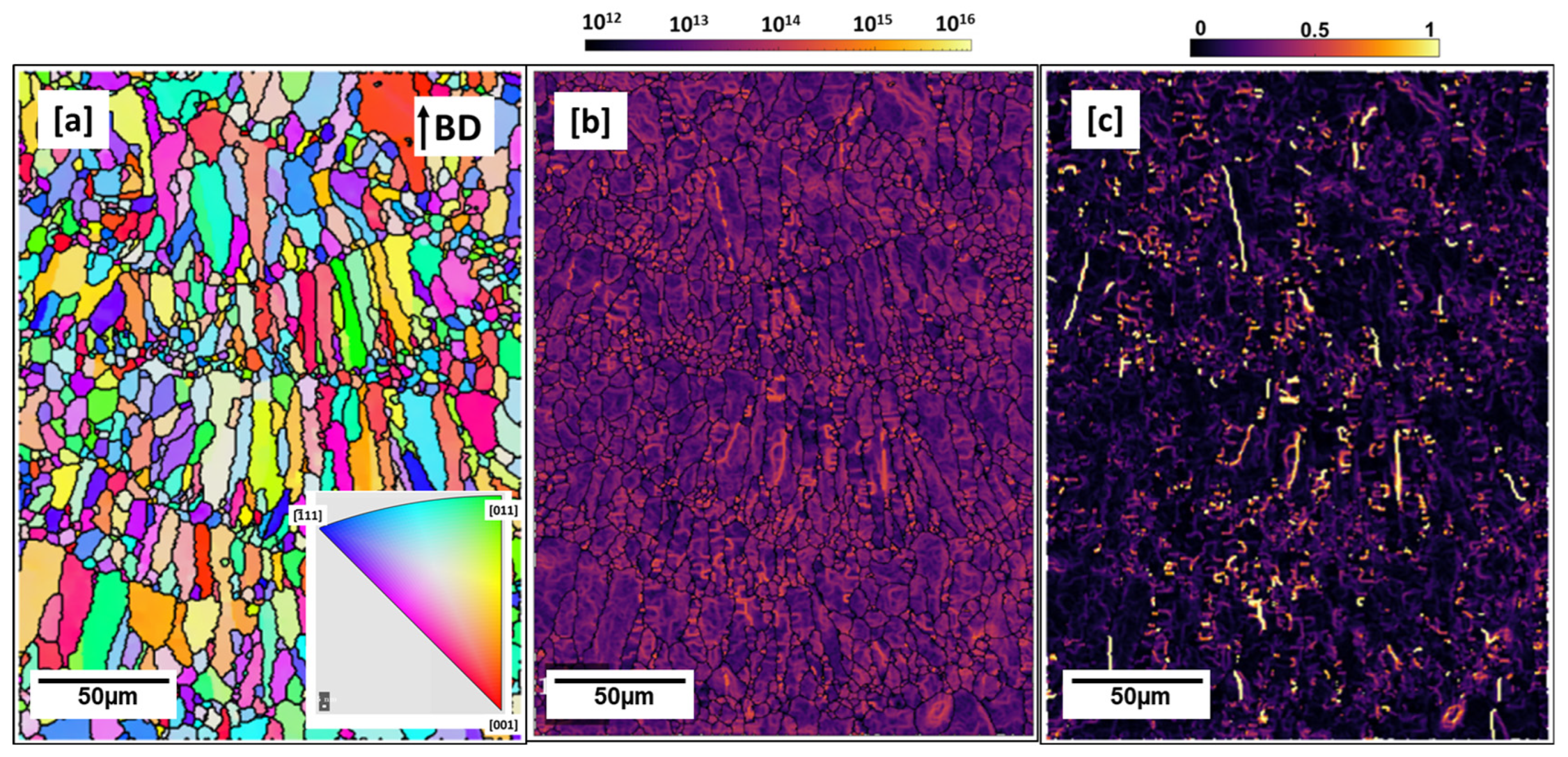
3.1. LPBF Microstructure—As-Built Cuboid
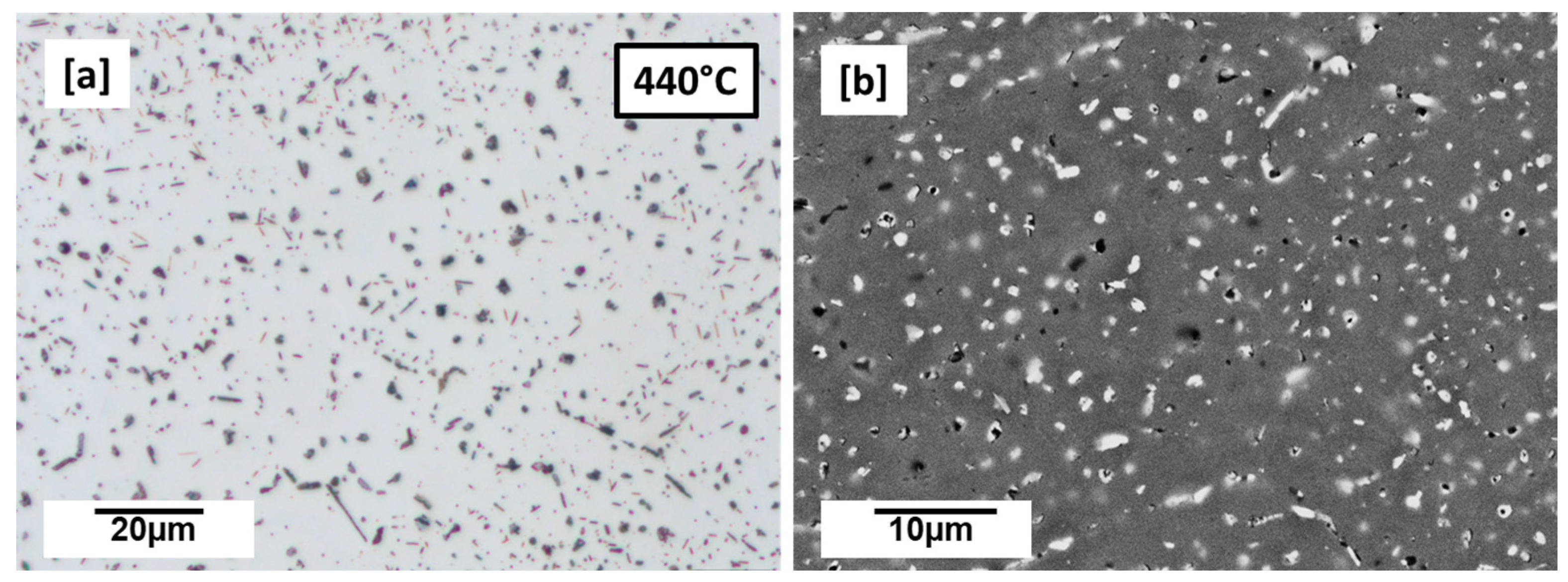
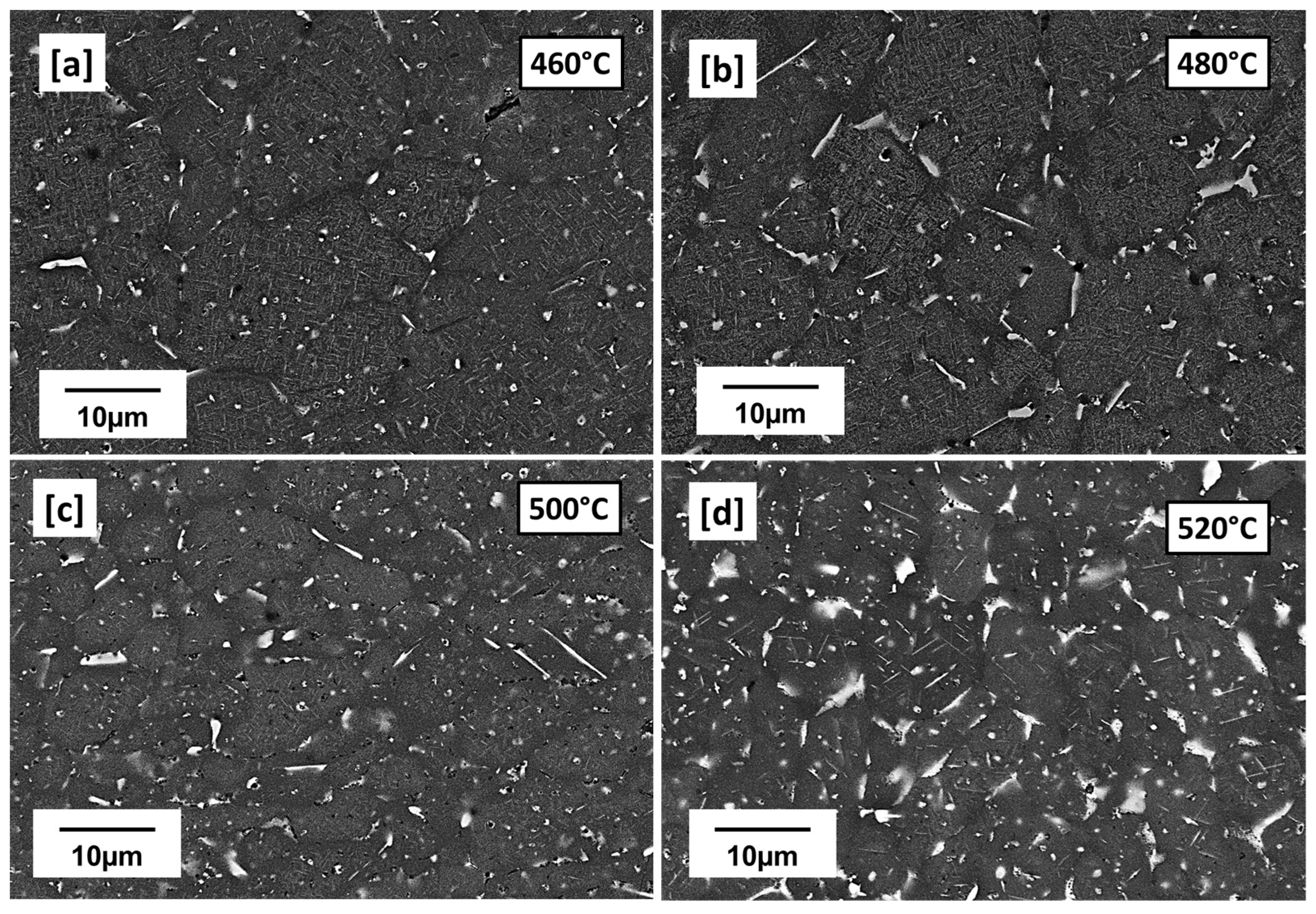
3.2. LPBF Microstructure—Preheated Cuboid (440 °C, 460 °C, 480 °C, 500 °C, and 520 °C)
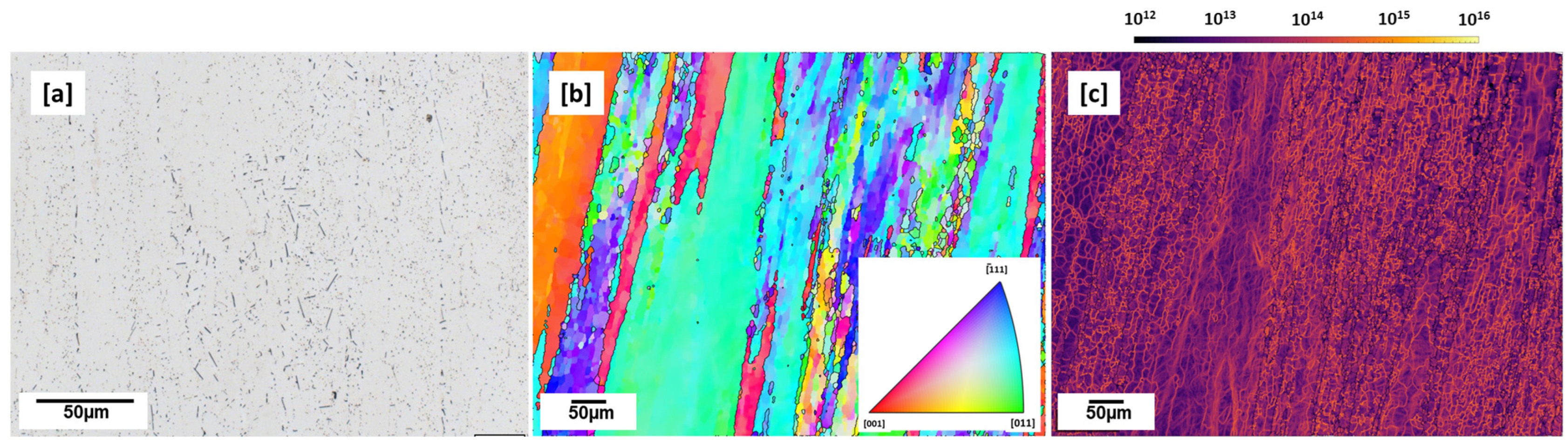
3.3. Conventional Microstructure—T83 Alloy
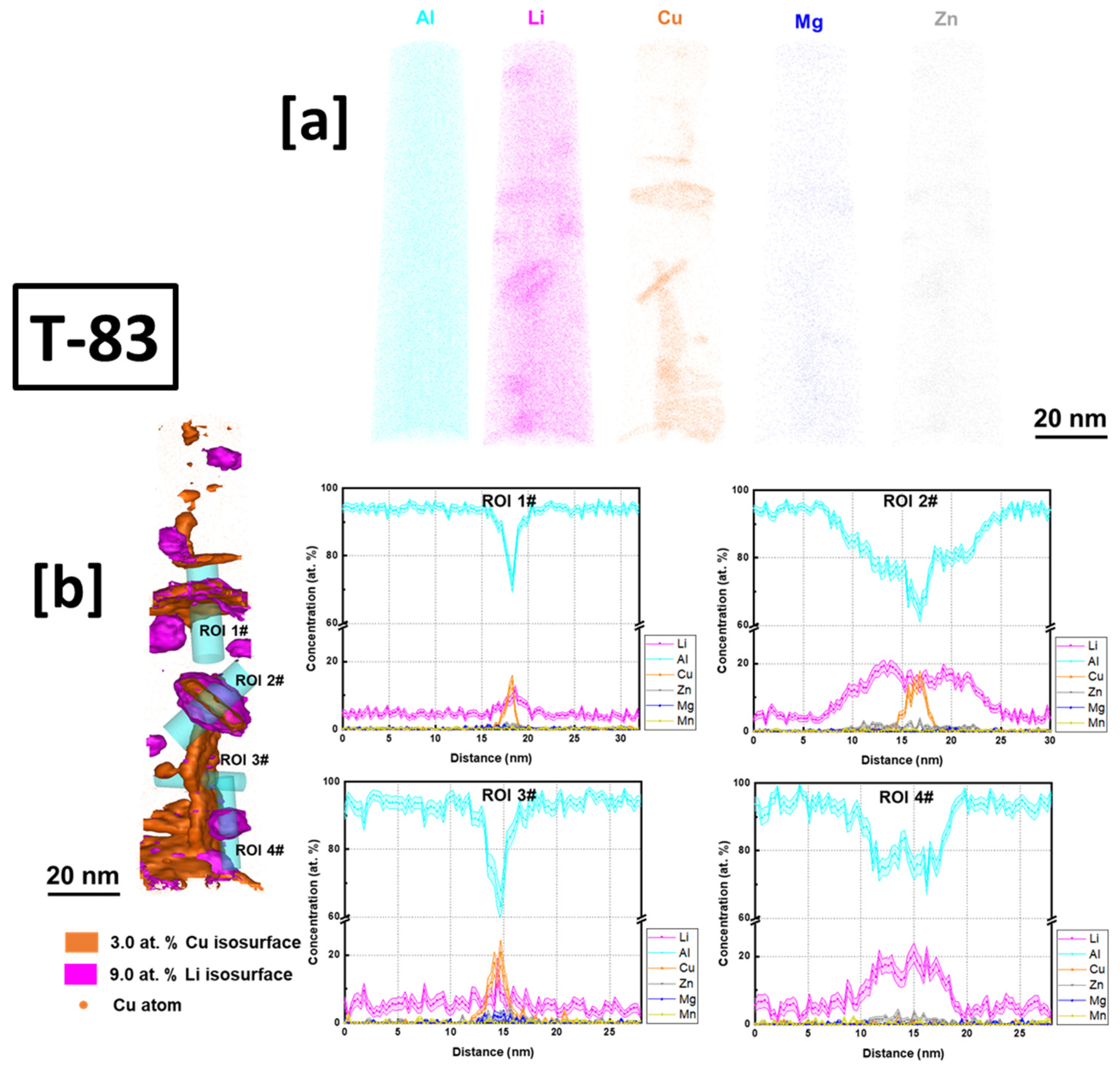
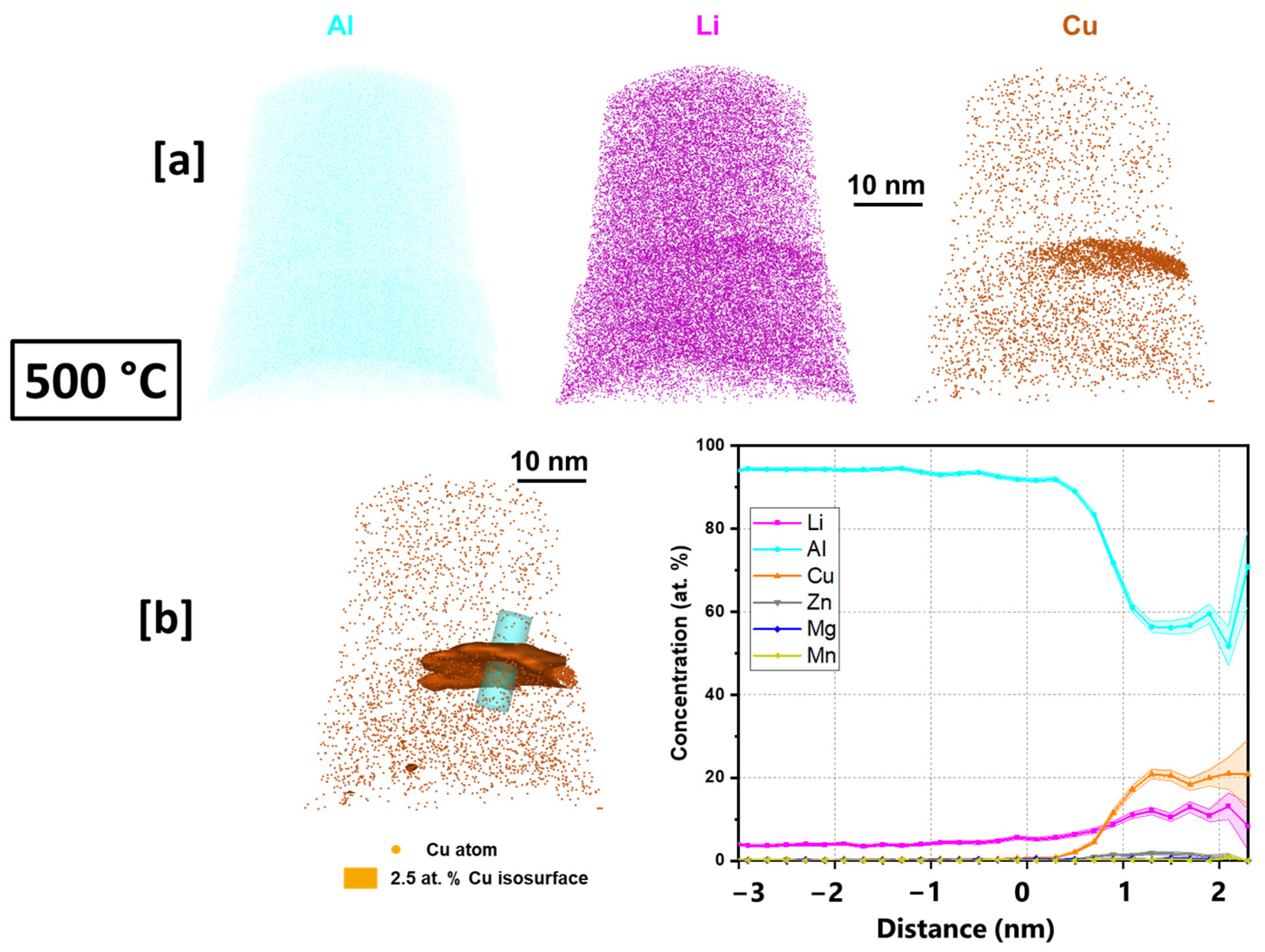
3.4. Atom Probe Tomography Characterization of T83, Preheated 500 °C
3.5. Mechanical Properties—Hardness, and Tensile properties
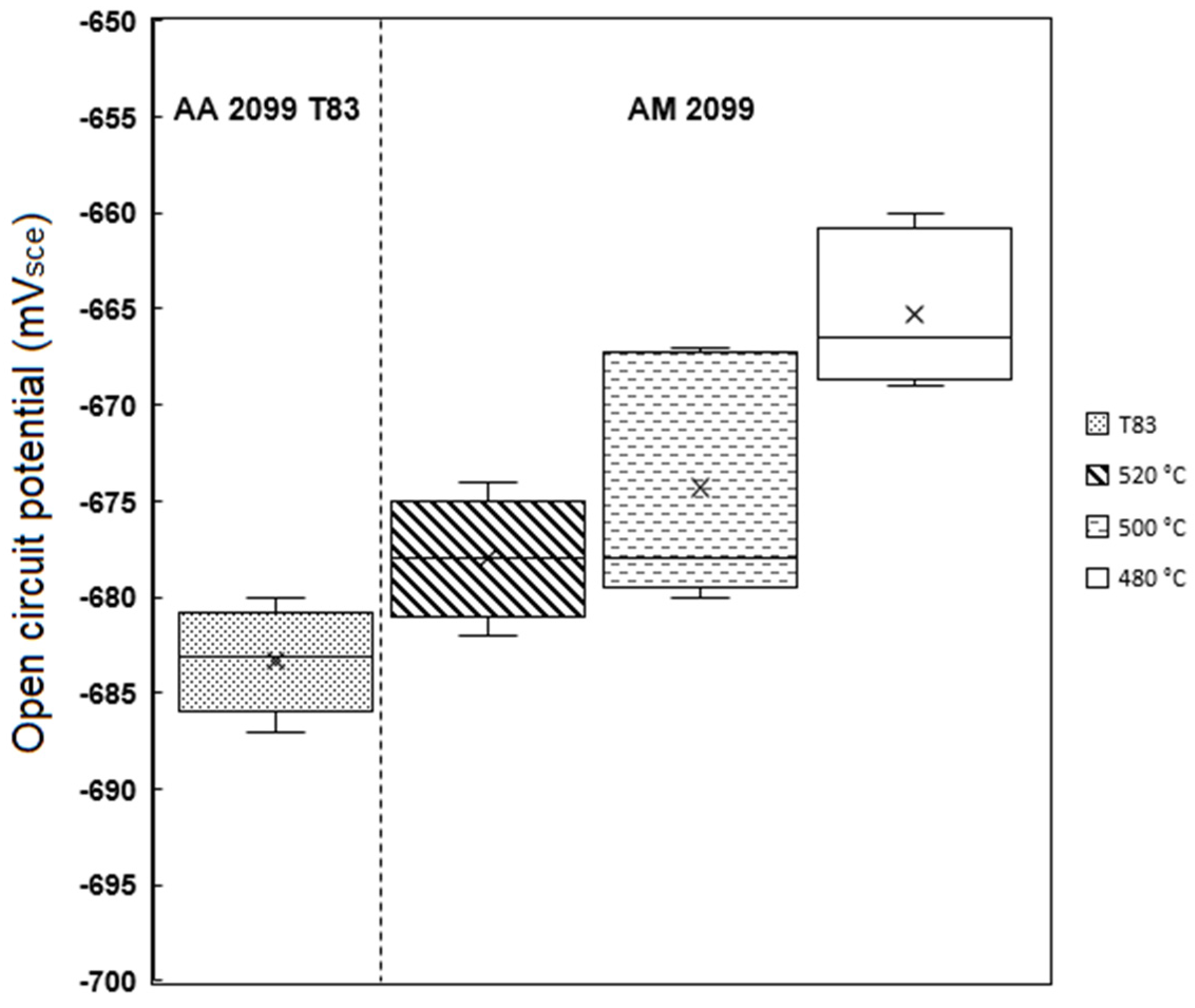
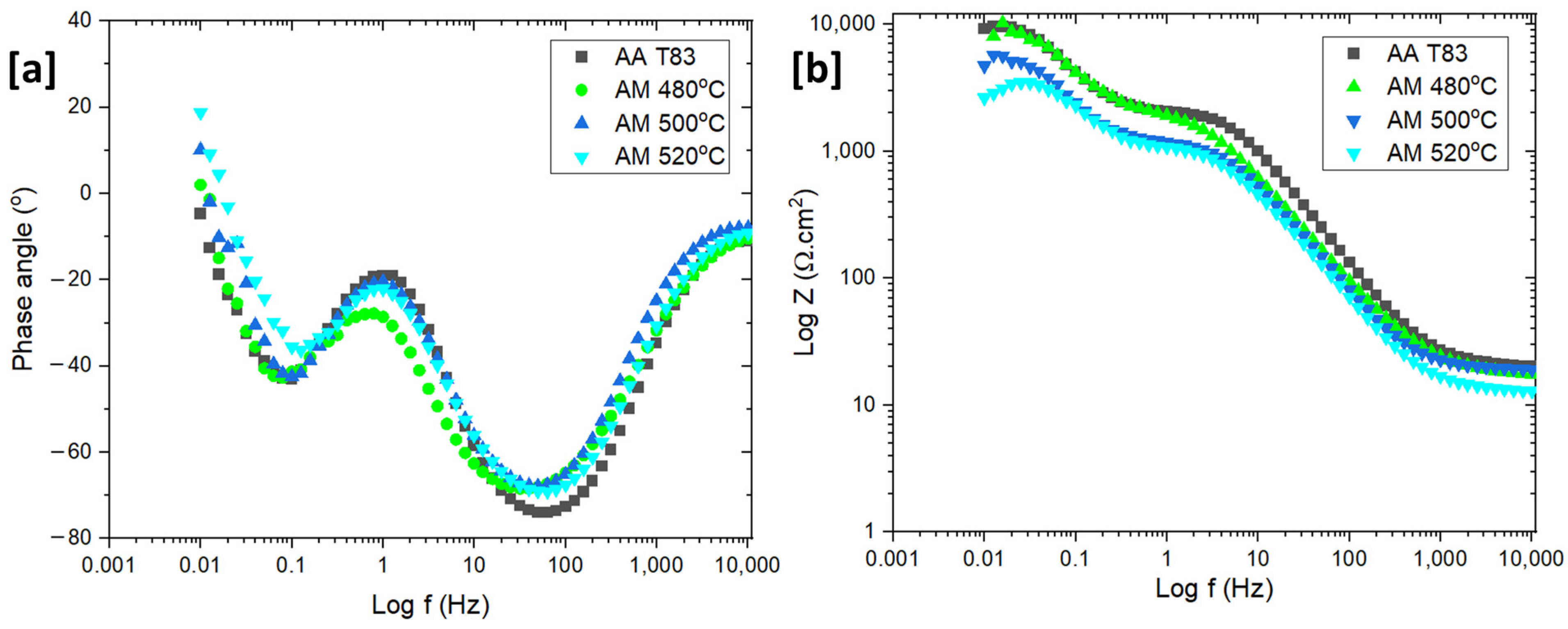
3.6. Corrosion Properties—T83 (Preheated: 480 °C, 500 °C, and 520 °C)
4. Conclusions
- As-built microstructure was dominated by coarse columnar grains along the building direction with many intergranular cracks. The as-built microstructure was also characterized by many globular Cu-rich phases both within the grains and on the grain boundaries which tended to be clustered at melt pool boundaries. Calculated dislocation density (3.8949 × 1013 m−2) was highest in the as-built condition compared to all preheated conditions.
- With preheating at 440 °C, the precipitation microstructure was close to the as-built with the presence of many globular Cu-rich phases dominating the microstructure. However, from 460 °C to 520 °C preheating temperatures, the microstructures were dominated by plate-like and rod-like precipitations suspected to be T1 and TB, respectively. Increasing preheating temperature was found to influence particle density and size of the precipitates particularly T1 in terms of its length.
- As the preheating temperature proceeded further from 500 °C to 520 °C, precipitation-free zones (PFZ) began to widen as more low-melting eutectic Cu-rich phases formed as films around the grain. The remaining globular intragranular Cu-rich phases also coarsen, especially at 520 °C.
- The highest recorded hardness for the AM samples was achieved at 480 °C (93.6 HV0.1), marginally higher than the 460 °C (89.8 HV0.1) and 500 °C (89.9 HV0.1) hardness. The T83 alloy was far superior to the measured hardness of the AM samples. It likewise had superior tensile strength properties compared to the AM (500 °C), although the AM had a better elongation (14.1%).
- The presence of Mg atoms in the characterized T1 precipitates in both the AM and the T83 supports the T1 nucleation theory that Mg aids in its nucleation. T1 plate was found to be shelled by a δ precipitate, a phenomenon which has only previously been reported between δ and Al3TM; TM—transitional metals.
- The potentiodynamic polarization results also demonstrated that increasing preheating temperature increased with the corrosion current density (Icorr) which also indicated higher corrosion rate. The corrosion performance of the conventional T83 alloy was found to be better compared to the AM-preheated alloy.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gruško, O.E.; Ovsânnikov, B.D.; Ovčinnikov, V.V. Aluminum-Lithium Alloys: Process Metallurgy, Physical Metallurgy, and Welding; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lavernia, E.J.; Srivatsan, T.S.; Mohamed, F.A. Strength, deformation, fracture behaviour and ductility of aluminium-lithium alloys. J. Mater. Sci. 1990, 25, 1137–1158. [Google Scholar] [CrossRef]
- Sankaran, K.; Grant, N. The structure and properties of splat-quenched aluminum alloy 2024 containing lithium additions. Mater. Sci. Eng. 1980, 44, 213–227. [Google Scholar] [CrossRef]
- Dorin, T.; De Geuser, F.; Lefebvre, W.; Sigli, C.; Deschamps, A. Strengthening mechanisms of T1 precipitates and their influence on the plasticity of an Al–Cu–Li alloy. Mater. Sci. Eng. A 2014, 605, 119–126. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, L.; Wu, G.; Peng, Y.; Li, Y. Effect of Mn addition on microstructure and mechanical properties of cast Al–2Li–2Cu–0.8Mg–0.4Zn–0.2Zr alloy. J. Mater. Res. 2016, 31, 250–258. [Google Scholar] [CrossRef]
- Gong, X.; Luo, S.; Li, S.; Wu, C. Dislocation-Induced Precipitation and Its Strengthening of Al–Cu–Li–Mg Alloys with High Mg. Acta Metall. Sin. 2021, 34, 597–605. [Google Scholar] [CrossRef]
- Balducci, E.; Ceschini, L.; Messieri, S. High Temperature Tensile Tests of the Lightweight 2099 and 2055 Al-Cu-Li Alloy: A Comparison. JOM 2018, 70, 2716–2725. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, Z.; Li, S. Effect of solution treatment on microstructures and mechanical properties of 2099 Al–Li alloy. Arch. Civ. Mech. Eng. 2014, 14, 61–71. [Google Scholar] [CrossRef]
- Hengsbach, F.; Koppa, P.; Duschik, K.; Holzweissig, M.J.; Burns, M.; Nellesen, J.; Tillmann, W.; Tröster, T.; Hoyer, K.-P.; Schaper, M. Duplex stainless steel fabricated by selective laser melting Microstructural and mechanical properties. Mater. Des. 2017, 133, 136–142. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Nie, X.; Hu, Z.; Zhu, H.; Zeng, X. A high strength Al–Li alloy produced by laser powder bed fusion: Densification, microstructure, and mechanical properties. Addit. Manuf. 2020, 35, 101346. [Google Scholar] [CrossRef]
- Qi, Y.; Hu, Z.; Zhang, H.; Nie, X.; Zhang, C.; Zhu, H. High strength Al–Li alloy development for laser powder bed fusion. Addit. Manuf. 2021, 47, 102249. [Google Scholar] [CrossRef]
- Wu, S.; Lei, Z.; Li, B.; Liang, J.; Chen, Y. Hot cracking evolution and formation mechanism in 2195 Al-Li alloy printed by laser powder bed fusion. Addit. Manuf. 2022, 54, 102762. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Zhu, H.; Nie, X.; Zeng, X. An Aluminum-Lithium Allloy Produced by Laser Powder Bed Fusion; University of Texas at Austin: Austin, TX, USA, 2019. [Google Scholar]
- Raffeis, I.; Adjei-Kyeremeh, F.; Vroomen, U.; Suwanpinij, P.; Ewald, S.; Bührig-Polazcek, A. Investigation of the Lithi-um-Containing Aluminum Copper Alloy (AA2099) for the Laser Powder Bed Fusion Process [L-PBF]: Effects of Process Pa-rameters on Cracks, Porosity, and Microhardness. JOM 2019, 71, 1543–1553. [Google Scholar] [CrossRef]
- Raffeis, I.; Adjei-Kyeremeh, F.; Vroomen, U.; Richter, S.; Bührig-Polaczek, A. Characterising the Microstructure of an Additively Built Al-Cu-Li Alloy. Materials 2020, 13, 5188. [Google Scholar] [CrossRef] [PubMed]
- Köhnen, P.; Létang, M.; Voshage, M.; Schleifenbaum, J.H.; Haase, C. Understanding the process-microstructure correlations for tailoring the mechanical properties of L-PBF produced austenitic advanced high strength steel. Addit. Manuf. 2019, 30, 100914. [Google Scholar] [CrossRef]
- Carrick, D.M.; Hogg, S.C.; Wilcox, G.D. Influence of Li Additions on the Microstructure and Corrosion Response of 2XXX Series Aluminium Alloys. Mater. Sci. Forum 2014, 794–796, 193–198. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Zhou, X.-R.; Meng, X.-M.; Huang, W.-J.; Liao, Y.; Chen, X.-L.; Yi, Y.-N.; Zhang, X.-X.; Thompson, G. Influence of thermomechanical treatments on localized corrosion susceptibility and propagation mechanism of AA2099 Al–Li alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 1472–1481. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Huang, W.; Thompson, G.; Zhang, X.; Luo, C.; Sun, Z. Localized corrosion in AA2099-T83 aluminum–lithium alloy: The role of intermetallic particles. Mater. Chem. Phys. 2015, 161, 201–210. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; He, Q.; Jiang, J.; Li, D.; Shao, W.; Zhen, L. Intergranular corrosion of an Al-Cu-Li alloy: The influence from grain structure. Corros. Sci. 2023, 211, 110845. [Google Scholar] [CrossRef]
- Zhu, Y.; Poplawsky, J.D.; Li, S.; Unocic, R.R.; Bland, L.G.; Taylor, C.D.; Locke, J.S.; Marquis, E.; Frankel, G.S. Localized corrosion at nm-scale hardening precipitates in Al-Cu-Li alloys. Acta Mater. 2020, 189, 204–213. [Google Scholar] [CrossRef]
- Leon, A.; Shirizly, A.; Aghion, E. Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing (AM) vs. Its Counterpart Gravity Cast Alloy. Metals 2016, 6, 148. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Frankel, G.S.; Miller, L.G.; Garves, J.; Pope, J.; Locke, J. Electrochemical Characteristics of Intermetallic Phases in Al–Cu–Li Alloys. J. Electrochem. Soc. 2023, 170, 21502. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Liao, Y.; Yi, Y.; Wu, H.; Wang, Z.; Huang, W. Localised corrosion in AA 2099-T83 aluminium-lithium alloy: The role of grain orientation. Corros. Sci. 2016, 107, 41–48. [Google Scholar] [CrossRef]
- Gharbi, O.; Jiang, D.; Feenstra, D.; Kairy, S.; Wu, Y.; Hutchinson, C.; Birbilis, N. On the corrosion of additively manufactured aluminium alloy AA2024 prepared by selective laser melting. Corros. Sci. 2018, 143, 93–106. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, H.; Zhu, H.; Hu, Z.; Ke, L.; Zeng, X. Analysis of processing parameters and characteristics of selective laser melted high strength Al-Cu-Mg alloys: From single tracks to cubic samples. J. Mater. Process. Technol. 2018, 256, 69–77. [Google Scholar] [CrossRef]
- Wang, P.; Deng, L.; Prashanth, K.; Pauly, S.; Eckert, J.; Scudino, S. Microstructure and mechanical properties of Al-Cu alloys fabricated by selective laser melting of powder mixtures. J. Alloys Compd. 2018, 735, 2263–2266. [Google Scholar] [CrossRef]
- Mair, P.; Braun, J.; Kaserer, L.; March, L.; Schimbäck, D.; Letofsky-Papst, I.; Leichtfried, G. Unique microstructure evolution of a novel Ti-modified Al-Cu alloy processed using laser powder bed fusion. Mater. Today Commun. 2022, 31, 103353. [Google Scholar] [CrossRef]
- Muránsky, O.; Balogh, L.; Tran, M.; Hamelin, C.; Park, J.-S.; Daymond, M. On the measurement of dislocations and dislocation substructures using EBSD and HRSD techniques. Acta Mater. 2019, 175, 297–313. [Google Scholar] [CrossRef]
- Pantleon, W. Resolving the geometrically necessary dislocation content by conventional electron backscattering diffraction. Scr. Mater. 2008, 58, 994–997. [Google Scholar] [CrossRef]
- Nye, J.F. Some geometrical relations in dislocated crystals. Acta Metall. 1953, 1, 153–162. [Google Scholar] [CrossRef]
- Rui, S.-S.; Han, Q.-N.; Wang, X.; Li, S.; Ma, X.; Su, Y.; Cai, Z.; Du, D.; Shi, H.J. Correlations between two EBSD-based metrics Kernel Average Misorientation and Image Quality on indicating dislocations of near-failure low alloy steels induced by tensile and cyclic de-formations. Mater. Today Commun. 2021, 27, 102445. [Google Scholar] [CrossRef]
- Saraf, L. Kernel Average Misorientation Confidence Index Correlation from FIB Sliced Ni-Fe-Cr alloy Surface. Microsc. Microanal. 2011, 17, 424–425. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Liu, J.; Chen, J.; Duan, S.; Liu, Z.; Ming, W.; Wu, C. Formation mechanism of precipitate T1 in AlCuLi alloys. J. Alloys Compd. 2015, 624, 22–26. [Google Scholar] [CrossRef]
- Gable, B.; Zhu, A.; Csontos, A.; Starke, E. The role of plastic deformation on the competitive microstructural evolution and mechanical properties of a novel Al–Li–Cu–X alloy. J. Light Met. 2001, 1, 1–14. [Google Scholar] [CrossRef]
- Dwyer, C.; Weyland, M.; Chang, L.Y.; Muddle, B.C. Combined electron beam imaging and ab initio modeling of T1 precipitates in Al–Li–Cu alloys. Appl. Phys. Lett. 2011, 98, 201909. [Google Scholar] [CrossRef]
- Wang, G.; Ouyang, H.; Fan, C.; Guo, Q.; Li, Z.; Yan, W.; Li, Z. The origin of high-density dislocations in additively manufactured metals. Mater. Res. Lett. 2020, 8, 283–290. [Google Scholar] [CrossRef]
- Yan, F.; Xiong, W.; Faierson, E.J. Grain Structure Control of Additively Manufactured Metallic Materials. Materials 2017, 10, 1260. [Google Scholar] [CrossRef] [Green Version]
- Gumbmann, E.; De Geuser, F.; Sigli, C.; Deschamps, A. Influence of Mg, Ag and Zn minor solute additions on the precipitation kinetics and strengthening of an Al-Cu-Li alloy. Acta Mater. 2017, 133, 172–185. [Google Scholar] [CrossRef] [Green Version]
- Gumbmann, E.; Lefebvre, W.; De Geuser, F.; Sigli, C.; Deschamps, A. The effect of minor solute additions on the precipitation path of an Al Cu Li alloy. Acta Mater. 2016, 115, 104–114. [Google Scholar] [CrossRef]
- Phyu, M.P.; Adjei-Kyeremeh, F.; Suwanpinij, P.; Raffeis, I.; Apel, M.; Bührig-Polaczek, A. Phase-Field Simulation of Microstructure Formation in Gas-Atomized Al-Cu-Li-Mg Powders. Materials 2023, 16, 1677. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Role of Ag and Mg on precipitation of T1 phase in an Al-Cu-Li-Mg-Ag alloy. Scr. Mater. 2001, 44, 701–706. [Google Scholar] [CrossRef]
- Araullo-Peters, V.; Gault, B.; de Geuser, F.; Deschamps, A.; Cairney, J.M. Microstructural evolution during ageing of Al–Cu–Li–x alloys. Acta Mater. 2014, 66, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Gault, B.; de Geuser, F.; Bourgeois, L.; Gabble, B.M.; Ringer, S.P.; Muddle, B.C. Atom probe tomography and transmission electron microscopy characterisation of precipitation in an Al-Cu-Li-Mg-Ag alloy. Ultramicroscopy 2011, 111, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Khushaim, M.; Boll, T.; Seibert, J.; Haider, F.; Al-Kassab, T. Characterization of Precipitation in Al-Li Alloy AA2195 by means of Atom Probe Tomography and Transmission Electron Microscopy. Adv. Condens. Matter Phys. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ovri, H. Micromechanisms Governing Plastic Instability in Al–Li Based Alloys. Ph.D. Thesis, Technische Universität Hamburg, Hamburg, Germany, 2015. [Google Scholar] [CrossRef]
- Belov, N.A.; Eskin, D.G.; Aksenov, A.A. Multicomponent Phase Diagrams; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Sun, S.; Brandt, M.; Easton, M. Powder bed fusion processes. In Laser Additive Manufacturing: Powder Bed Fusion Processes: An Overview; Sun, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 55–77. [Google Scholar]
- Jabra, J.; Romios, M.; Lai, J.; Setiawan, M.; Lee, E.W.; Witters, J.; Abourialy, N.; Ogren, J.R.; Clark, R.; Oppenheim, T.; et al. The Effect of Thermal Exposure on the Mechanical Properties of 2099-T6 Die Forgings, 2099-T83 Extrusions, 7075-T7651 Plate, 7085-T7452 Die Forgings, 7085-T7651 Plate, and 2397-T87 Plate Aluminum Alloys. J. Mater. Eng. Perform. 2006, 15, 601–607. [Google Scholar] [CrossRef]
- Ghosh, K.S.; Mukhopadhyay, S.; Konar, B.; Mishra, B. Study of aging and electrochemical behaviour of Al-Li-Cu-Mg alloys. Mater. Corros. 2013, 64, 890–901. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Liu, D.; Zhang, R.; Chen, Y.; Zhang, X.; Ma, P.; Gupta, R.K.; Birbilis, N. Correlation of intergranular corrosion behaviour with mi-crostructure in Al-Cu-Li alloy. Corros. Sci. 2018, 139, 215–226. [Google Scholar] [CrossRef]
- Liang, W.; Pan, Q.; He, Y.; Li, Y.; Zhou, Y.; Lu, C. Effect of aging on the mechanical properties and corrosion susceptibility of an Al-Cu-Li-Zr alloy containing Sc. Rare Met. 2008, 27, 146–152. [Google Scholar] [CrossRef]
- Krakhmalev, P.; Fredriksson, G.; Svensson, K.; Yadroitsev, I.; Yadroitsava, I.; Thuvander, M.; Peng, R. Microstructure, Solidification Texture, and Thermal Stability of 316 L Stainless Steel Manufactured by Laser Powder Bed Fusion. Metals 2018, 8, 643. [Google Scholar] [CrossRef] [Green Version]
- Risse, J. Additive Fertigung der Nickelbasis-Superlegierung IN738LC Mittels Selektivem Laserstrahlschmelzen; RWTH Aachen University: Aachen, Germany, 2019. [Google Scholar]
- Kürnsteiner, P. Precipitation Reactions during the Intrinsic Heat Treatment of Laser Additive Manufacturing. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2019. [Google Scholar] [CrossRef]
- Lei, X.; Nuam, V.L.; Yuan, Y.; Bai, Y.; Yao, W.; Wang, N. Investigation on laser beam remelted Al-Cu-Li alloy part II: Corrosion behavior and mechanical properties. J. Alloys Compd. 2021, 873, 159765. [Google Scholar] [CrossRef]
- Xu, J.; Deng, Y.; Chen, J. Enhancing the Corrosion Resistance of Al–Cu–Li Alloys through Regulating Precipitation. Materials 2020, 13, 2628. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.S.; Chen, K.H.; Fang, H.C.; Chao, H.; Chen, S.Y. EIS study on pitting corrosion of 7150 aluminum alloy in sodium chloride and hydrochloric acid solution. Mater. Corros. 2010, 61, 783–789. [Google Scholar] [CrossRef]
- Jingling, M.A.; Jiuba, W.; Gengxin, L.I.; Chunhua, X.V. The corrosion behaviour of Al–Zn–In–Mg–Ti alloy in NaCl solution. Corros. Sci. 2010, 52, 534–539. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Zhao, G.; Wang, Y.; Xu, X. Effects of re-solution and re-aging treatment on mechanical property, corrosion resistance and electrochemical behavior of 2196 Al-Cu-Li alloy. Mater. Des. 2021, 204, 109662. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Niya, S.M.R.; Hoorfar, M. On a possible physical origin of the constant phase element. Electrochim. Acta 2016, 188, 98–102. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Ca-pacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Ralston, K.; Birbilis, N.; Weyland, M.; Hutchinson, C. The effect of precipitate size on the yield strength-pitting corrosion correlation in Al–Cu–Mg alloys. Acta Mater. 2010, 58, 5941–5948. [Google Scholar] [CrossRef]
- Youngseok, K.; Buchheit, R.G. A characterization of the inhibiting effect of Cu on metastable pitting in dilute Al–Cu solid solution alloys. Electrochim. Acta 2007, 52, 2437–2446. [Google Scholar]






| Chemical Composition (wt.%) | |||||||
|---|---|---|---|---|---|---|---|
| Al | Cu | Li | Zn | Mg | Mn | Zr | |
| Gas atomized | Bal. | 2.63 | 1.56 | 0.67 | 0.28 | 0.17 | 0.09 |
| AW 2099-T83 | Bal. | 2.40 | 1.60 | 0.40 | 0.10 | 0.10 | 0.05 |
| Variables | T83 | 480 °C | 500 °C | 520 °C |
|---|---|---|---|---|
| Beta A (V/dec) | 0.03 (±0.004) | 0.038 (±0.006) | 0.055 (±0.007) | 0.060 (±0.01) |
| Ecorr (mV) | −662 (±5) | −643 (±11) | −648 (±8) | −646 (±3) |
| Icorr (µA/cm2) | 0.97 (±0.3) | 1.31 (±0.6) | 5.11 (±1.3) | 10.3 (±3) |
| Materials | Rs | Rox | Yox | n | Rct | Cdl | n |
|---|---|---|---|---|---|---|---|
| (KΩ.cm) | (KΩ.cm2) | (μS·cm2·s) | (KΩ.cm2) | (µF.cm) | |||
| T83 | 0.022 | 2.91 (±0.5) | 32 (±0.06) | 0.94 | 7.71 (±0.7) | 0.36 | 0.89 |
| 480 °C | 0.016 | 1.89 (±0.3) | 57 (±0.08) | 0.95 | 6.6 (±0.9) | 0.6 | 0.85 |
| 500 °C | 0.015 | 1.5 (±0.2) | 44 (±0.09) | 0.97 | 4.06 (±0.7) | 0.9 | 0.89 |
| 520 °C | 0.014 | 1.25 (±0.1) | 34 (±0.09) | 0.99 | 2.78 (±0.02) | 0.98 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adjei-Kyeremeh, F.; Pratesa, Y.; Shen, X.; Song, W.; Raffeis, I.; Vroomen, U.; Zander, D.; Bührig-Polaczek, A. Preheating Influence on the Precipitation Microstructure, Mechanical and Corrosive Properties of Additively Built Al–Cu–Li Alloy Contrasted with Conventional (T83) Alloy. Materials 2023, 16, 4916. https://doi.org/10.3390/ma16144916
Adjei-Kyeremeh F, Pratesa Y, Shen X, Song W, Raffeis I, Vroomen U, Zander D, Bührig-Polaczek A. Preheating Influence on the Precipitation Microstructure, Mechanical and Corrosive Properties of Additively Built Al–Cu–Li Alloy Contrasted with Conventional (T83) Alloy. Materials. 2023; 16(14):4916. https://doi.org/10.3390/ma16144916
Chicago/Turabian StyleAdjei-Kyeremeh, Frank, Yudha Pratesa, Xiao Shen, Wenwen Song, Iris Raffeis, Uwe Vroomen, Daniela Zander, and Andreas Bührig-Polaczek. 2023. "Preheating Influence on the Precipitation Microstructure, Mechanical and Corrosive Properties of Additively Built Al–Cu–Li Alloy Contrasted with Conventional (T83) Alloy" Materials 16, no. 14: 4916. https://doi.org/10.3390/ma16144916
APA StyleAdjei-Kyeremeh, F., Pratesa, Y., Shen, X., Song, W., Raffeis, I., Vroomen, U., Zander, D., & Bührig-Polaczek, A. (2023). Preheating Influence on the Precipitation Microstructure, Mechanical and Corrosive Properties of Additively Built Al–Cu–Li Alloy Contrasted with Conventional (T83) Alloy. Materials, 16(14), 4916. https://doi.org/10.3390/ma16144916






