Key Role of Corncob Based-Hydrochar (HC) in the Enhancement of Visible Light Photocatalytic Degradation of 2,4-Dichlorophenoxyacetic Acid Using a Derivative of ZnBi-Layered Double Hydroxides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photocatalysts (ZnBi-LDO and HC-ZnBi-LDO)
2.3. Characterization
2.4. Photocatalytic Experiment
3. Results and Discussion
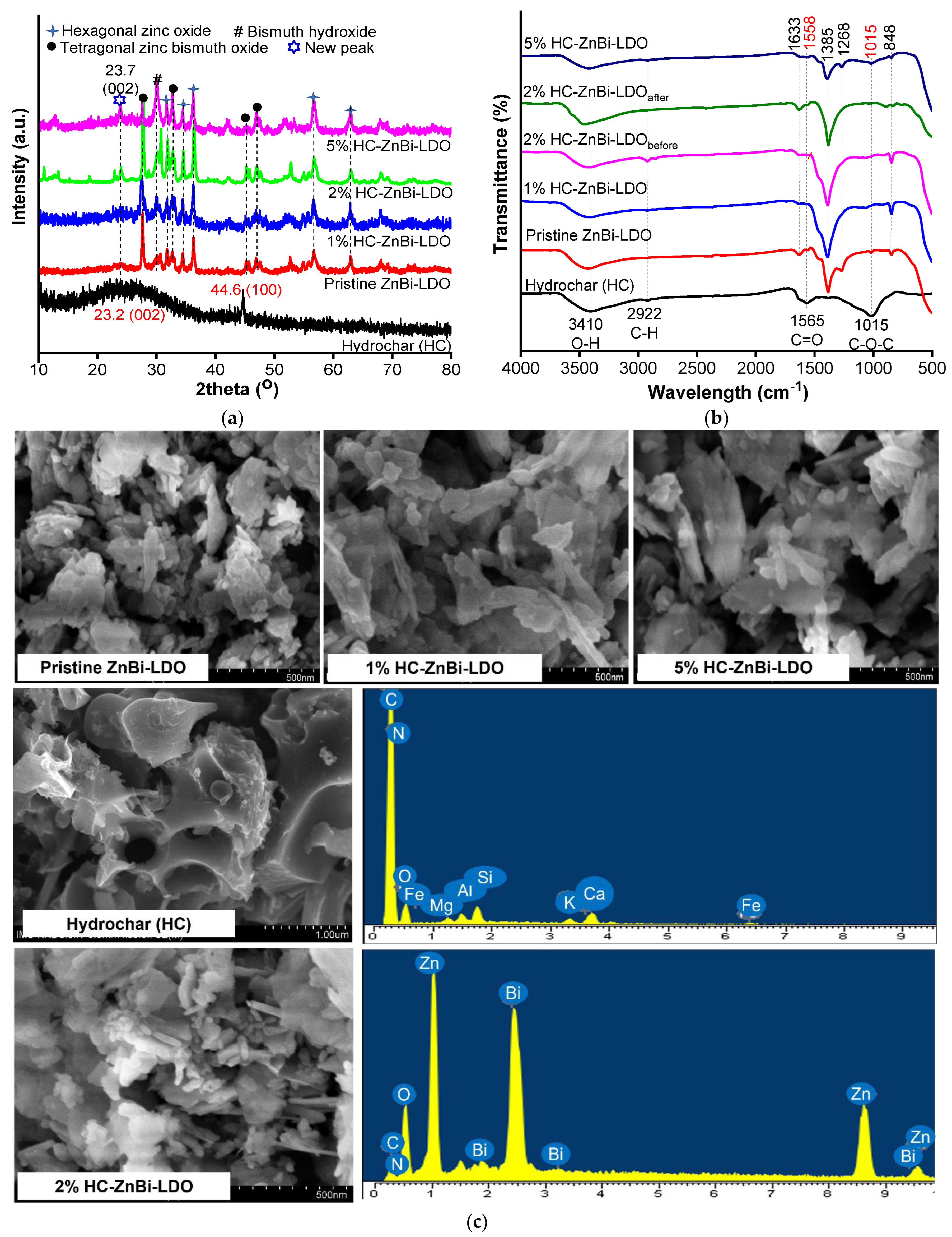
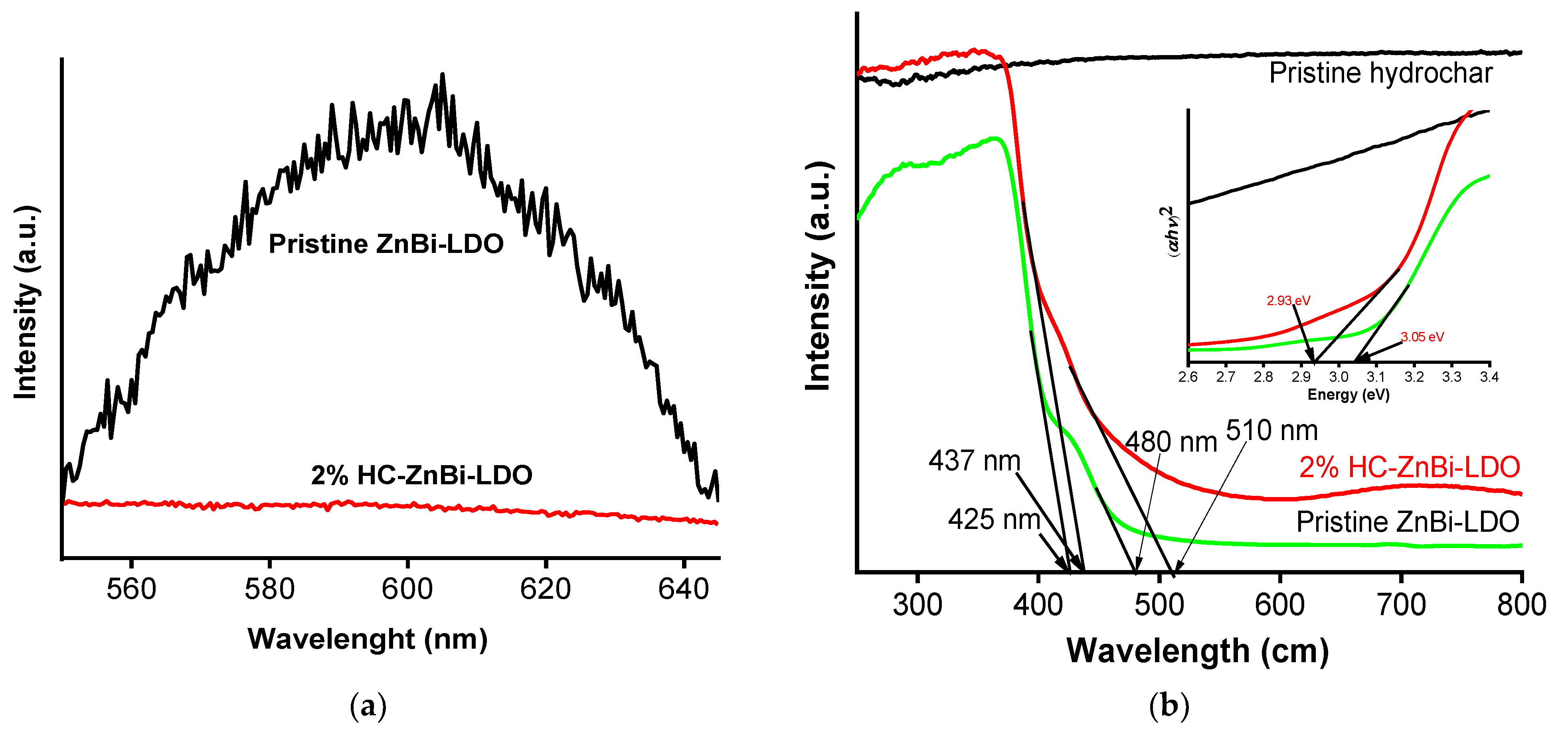
3.1. Material Characterization
3.2. Photodegradation Process
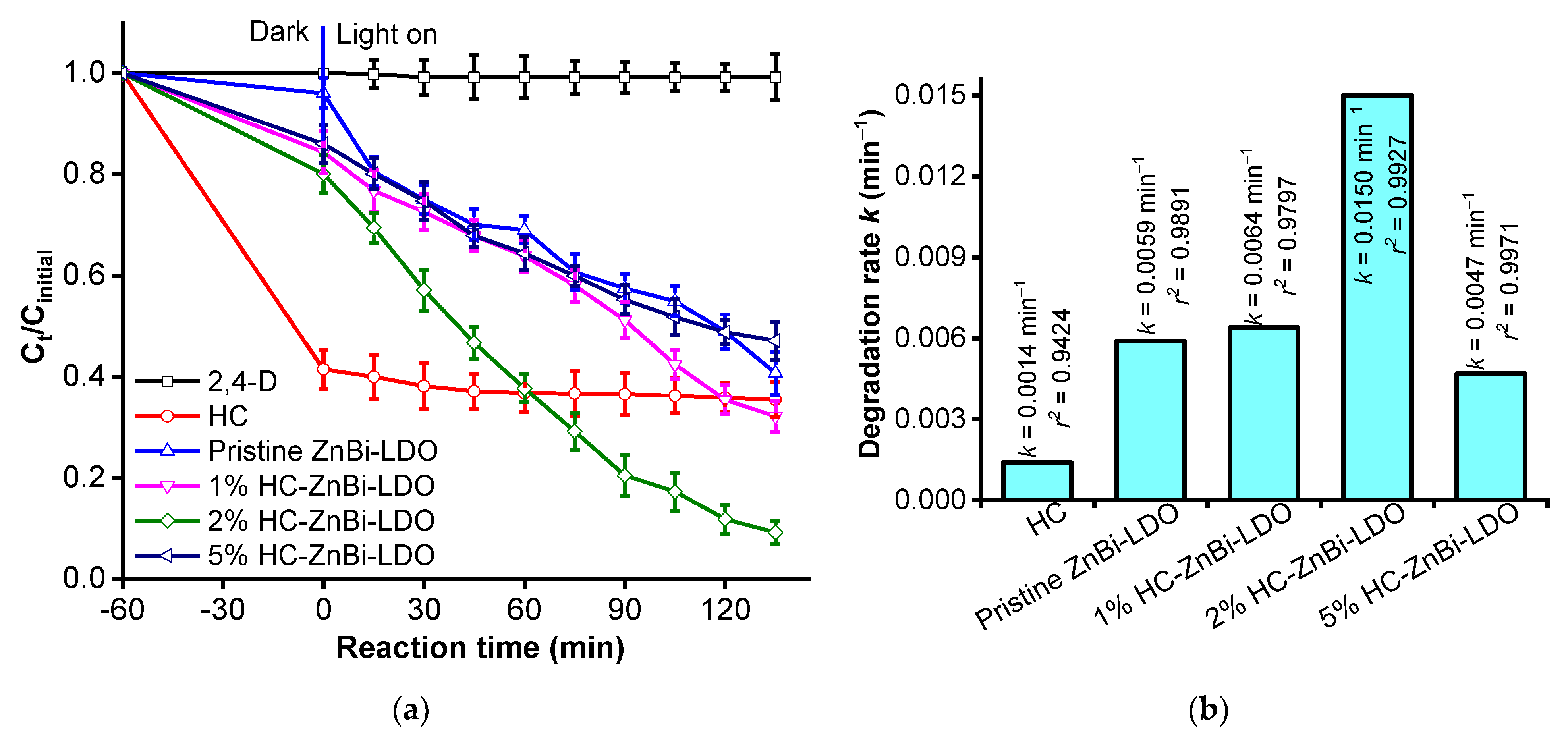
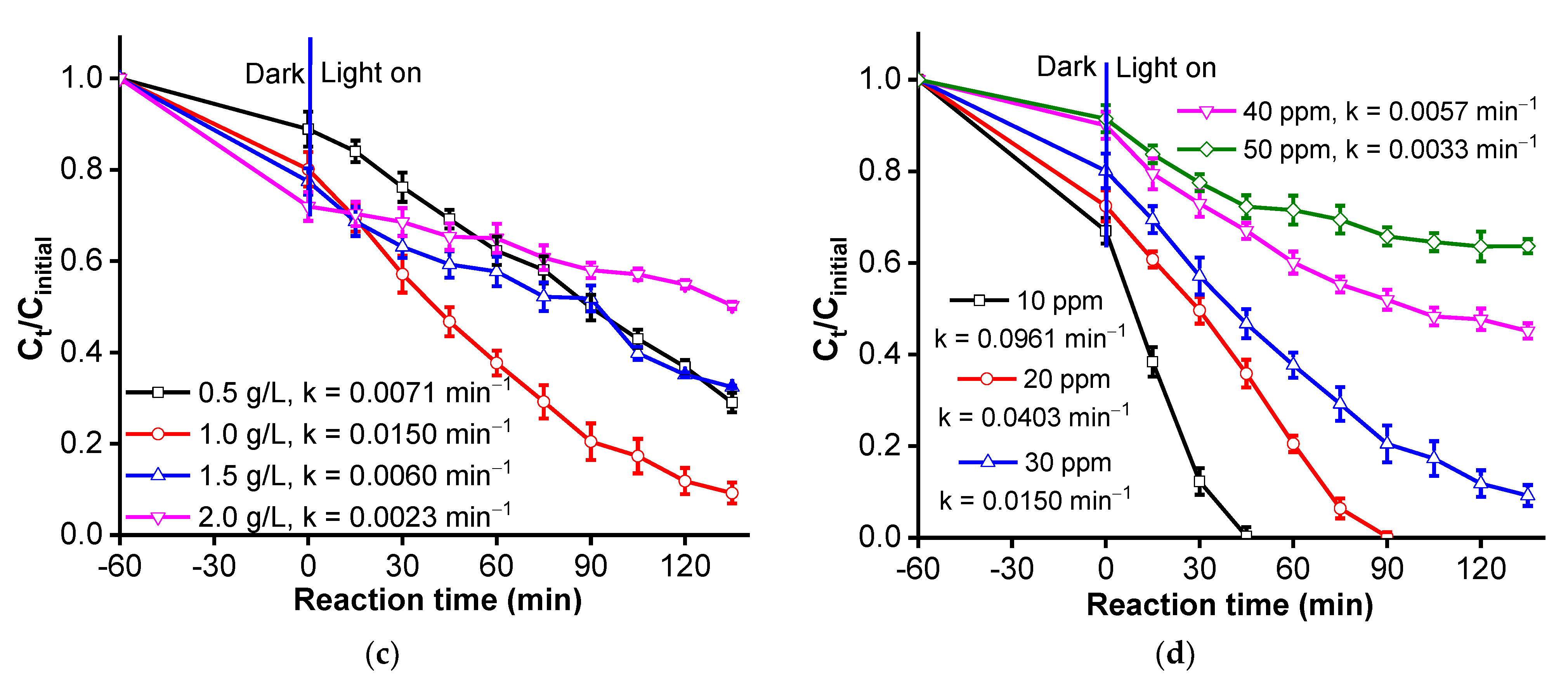
3.2.1. Effect of Hydrochar Source Loadings on Heterojunction HC-ZnBi-LDO
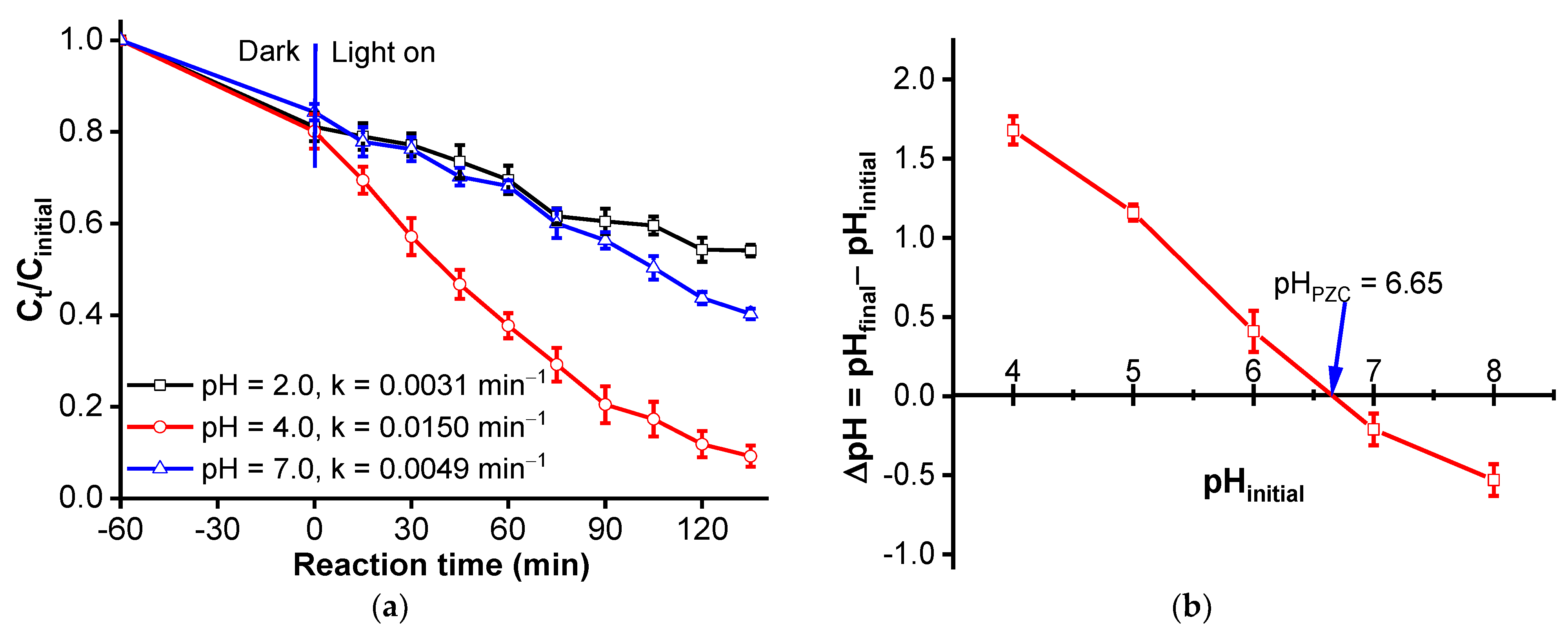
3.2.2. Effect of pH
3.2.3. Effect of 2% HC-ZnBi-LDO Dosage
3.2.4. Effect of Initial 2,4-D Concentration
3.2.5. Reusability of the 2% HC-ZnBi-LDO Photocatalyst
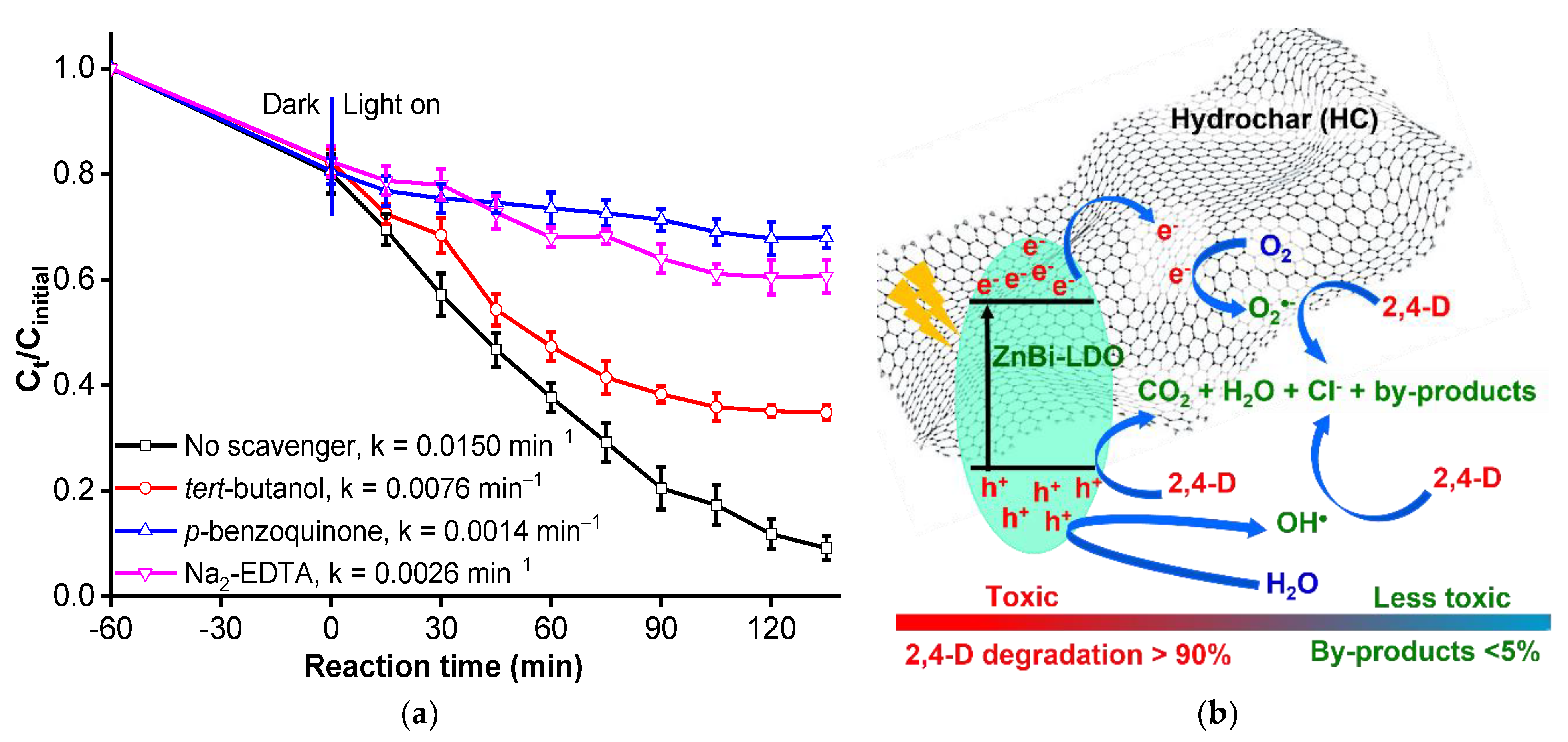
3.2.6. Effect of Scavengers on the Degradation and Proposed Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padhye, L.P.; Bandala, E.R.; Wijesiri, B.; Goonetilleke, A.; Bolan, N. Hydrochar: A Promising Step Towards Achieving a Circular Economy and Sustainable Development Goals. Front. Chem. Eng. 2022, 4, 867228. [Google Scholar] [CrossRef]
- Nadarajah, K.; Bandala, E.R.; Zhang, Z.; Mundree, S.; Goonetilleke, A. Removal of heavy metals from water using engineered hydrochar: Kinetics and mechanistic approach. J. Water Process Eng. 2021, 40, 101929. [Google Scholar] [CrossRef]
- Sumaraj; Xiong, Z.; Sarmah, A.K.; Padhye, L.P. Acidic surface functional groups control chemisorption of ammonium onto carbon materials in aqueous media. Sci. Total Environ. 2020, 698, 134193. [Google Scholar] [CrossRef]
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Galletti, A.M.R.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13. [Google Scholar] [CrossRef]
- Sumaraj;Padhye, L.P. Influence of surface chemistry of carbon materials on their interactions with inorganic nitrogen contaminants in soil and water. Chemosphere 2017, 184, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yan, Z.; Cheng, X.; Li, Y.; Wang, A.; Chen, L. Quaternary ammonium-functionalized rice straw hydrochar as efficient adsorbents for methyl orange removal from aqueous solution. Clean Technol. Environ. Policy 2019, 21, 1269–1279. [Google Scholar] [CrossRef]
- Teng, F.; Zhang, Y.; Wang, D.; Shen, M.; Hu, D. Iron-modified rice husk hydrochar and its immobilization effect for Pb and Sb in contaminated soil. J. Hazard. Mater. 2020, 398, 122977. [Google Scholar] [CrossRef] [PubMed]
- Çatlıoğlu, F.N.; Akay, S.; Gözmen, B.; Turunc, E.; Anastopoulos, I.; Kayan, B.; Kalderis, D. Fe-modified hydrochar from orange peel as adsorbent of food colorant Brilliant Black: Process optimization and kinetic studies. Int. J. Environ. Sci. Technol. 2020, 17, 1975–1990. [Google Scholar] [CrossRef]
- Mittapalli, S.; Sharma, H.B.; Dubey, B.K. Hydrothermal carbonization of anaerobic granular sludge and co-pelletization of hydrochar with yard waste. Bioresour. Technol. Rep. 2021, 14, 100691. [Google Scholar] [CrossRef]
- Amarzadeh, M.; Azqandi, M.; Nateq, K.; Ramavandi, B.; Khan, N.A.; Nasseh, N. Heterogeneous Fenton-like Photocatalytic Process towards the Eradication of Tetracycline under UV Irradiation: Mechanism Elucidation and Environmental Risk Analysis. Water 2023, 15, 2336. [Google Scholar] [CrossRef]
- Nasab, E.A.; Nasseh, N.; Damavandi, S.; Amarzadeh, M.; Ghahrchi, M.; Hoseinkhani, A.; Alver, A.; Khan, N.A.; Farhadi, A.; Danaee, I. Efficient purification of aqueous solutions contaminated with sulfadiazine by coupling electro-Fenton/ultrasound process: Optimization, DFT calculation, and innovative study of human health risk assessment. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Z.; Su, Y.; Wang, Y. Visible light-driven Bi2Sn2O7/reduced graphene oxide nanocomposite for efficient photocatalytic degradation of organic contaminants. Sep. Purif. Technol. 2015, 142, 25–32. [Google Scholar] [CrossRef]
- Xu, W.; Fang, J.; Chen, Y.; Lu, S.; Zhou, G.; Zhu, X.; Fang, Z. Novel heterostructured Bi2S3/Bi2Sn2O7 with highly visible light photocatalytic activity for the removal of rhodamine B. Mater. Chem. Phys. 2015, 154, 30–37. [Google Scholar] [CrossRef]
- Huy, B.T.; Jung, D.-S.; Kim Phuong, N.T.; Lee, Y.-I. Enhanced photodegradation of 2,4-dichlorophenoxyacetic acid using a novel TiO2@MgFe2O4 core@shell structure. Chemosphere 2017, 184, 849–856. [Google Scholar] [CrossRef]
- Thi Mai Tho, N.; The Huy, B.; Nha Khanh, D.N.; Quoc Thang, N.; Thi Phuong Dieu, N.; Dai Duong, B.; Thi Kim Phuong, N. Mechanism of Visible-Light Photocatalytic Mineralization of Indigo Carmine Using ZnBi2O4-Bi2S3 Composites. ChemistrySelect 2018, 3, 9986–9994. [Google Scholar] [CrossRef]
- Huy, B.T.; Paeng, D.S.; Thi Bich Thao, C.; Kim Phuong, N.T.; Lee, Y.-I. ZnO-Bi2O3/graphitic carbon nitride photocatalytic system with H2O2-assisted enhanced degradation of Indigo carmine under visible light. Arab. J. Chem. 2020, 13, 3790–3800. [Google Scholar] [CrossRef]
- Lee, S.-S.; Huy, B.T.; Phuong, N.T.K.; Tung, D.K.; Lee, Y.-I. Enhanced performance in the photocatalytic degradation of 2,4,5-Trichlorophenoxyacetic acid over Eu-doped Bi2WO6 under visible light irradiation. Korean J. Chem. Eng. 2019, 36, 1716–1723. [Google Scholar] [CrossRef]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A. g-C3N4/carbon dot-based nanocomposites serve as efficacious photocatalysts for environmental purification and energy generation: A review. J. Clean. Prod. 2020, 276, 124319. [Google Scholar] [CrossRef]
- Tho, N.T.M.; Huy, B.T.; Khanh, D.N.N.; Vy, N.T.T.; Thang, N.Q.; Sy, D.T.; Hai, L.H.; Phuong, N.T.K. Visible-Light Degradation of Organic Dye Based on a Heterostructure Photocatalyst. Top. Catal. 2020, 63, 1157–1168. [Google Scholar] [CrossRef]
- Tho, N.T.M.; Khanh, D.N.N.; Thang, N.Q.; Lee, Y.-I.; Phuong, N.T.K. Novel reduced graphene oxide/ZnBi2O4 hybrid photocatalyst for visible light degradation of 2,4-dichlorophenoxyacetic acid. Environ. Sci. Pollut. Res. 2020, 27, 11127–11137. [Google Scholar] [CrossRef] [PubMed]
- Benjwal, P.; Sharma, R.; Kar, K.K. Effects of surface microstructure and chemical state of featherfiber-derived multidoped carbon fibers on the adsorption of organic water pollutants. Mater. Des. 2016, 110, 762–774. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, Z.; Qu, D.; Li, D.; Du, P.; Jing, X.; Sun, Z. On–Off–On Fluorescent Carbon Dot Nanosensor for Recognition of Chromium(VI) and Ascorbic Acid Based on the Inner Filter Effect. ACS Appl. Mater. Interfaces 2013, 5, 13242–13247. [Google Scholar] [CrossRef]
- Dong, H.; Kuzmanoski, A.; Gößl, D.M.; Popescu, R.; Gerthsen, D.; Feldmann, C. Polyol-mediated C-dot formation showing efficient Tb3+/Eu3+ emission. Chem. Commun. 2014, 50, 7503–7506. [Google Scholar] [CrossRef] [PubMed]
- Premalatha, N.; Rose Miranda, L. Surfactant modified ZnO–Bi2O3 nanocomposite for degradation of lambda- cyhalothrin pesticide in visible light: A study of reaction kinetics and intermediates. J. Environ. Manag. 2019, 246, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.; Rajasekaran, P.; Selvakumar, K.; Arunpandian, M.; Kaviyarasu, K.; Asath Bahadur, S.; Swaminathan, M. Visible active reduced graphene oxide-BiVO4-ZnO ternary photocatalyst for efficient removal of ciprofloxacin. Sep. Purif. Technol. 2020, 233, 115996. [Google Scholar] [CrossRef]
- Kadam, A.N.; Lee, J.; Nipane, S.V.; Lee, S.-W. Nanocomposites for visible light photocatalysis. In Nanostructured Materials for Visible Light Photocatalysis; Nayak, A.K., Sahu, N.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 295–317. [Google Scholar]
- Beranek, R. (Photo)electrochemical Methods for the Determination of the Band Edge Positions of TiO2-Based Nanomaterials. Adv. Phys. Chem. 2011, 2011, 786759. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Gao, W.; Hao, H. Synthesis and visible photocatalytic activity of new photocatalyst MBi2O4(M = Cu, Zn). J. Mater. Sci. Mater. Electron. 2015, 26, 1866–1873. [Google Scholar] [CrossRef]
- Guo, P.; Jiang, J.; Shen, S.; Guo, L. ZnS/ZnO heterojunction as photoelectrode: Type II band alignment towards enhanced photoelectrochemical performance. Int. J. Hydrogen Energy 2013, 38, 13097–13103. [Google Scholar] [CrossRef]
- Wu, L.Q.; Li, Y.C.; Li, S.Q.; Li, Z.Z.; Tang, G.D.; Qi, W.H.; Xue, L.C.; Ge, X.S.; Ding, L.L. Method for estimating ionicities of oxides using O1s photoelectron spectra. AIP Adv. 2015, 5, 097210. [Google Scholar] [CrossRef]
- Jia, X.; Li, J.; Wang, E. One-pot green synthesis of optically pH-sensitive carbon dots with upconversion luminescence. Nanoscale 2012, 4, 5572–5575. [Google Scholar] [CrossRef] [PubMed]
- Das, G.S.; Shim, J.P.; Bhatnagar, A.; Tripathi, K.M.; Kim, T. Biomass-derived Carbon Quantum Dots for Visible-Light-Induced Photocatalysis and Label-Free Detection of Fe(III) and Ascorbic acid. Sci. Rep. 2019, 9, 15084. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chen, M.; Chen, G.; Zou, W.; Zhao, Y.; Zhang, H.; Zhao, Q. Visible–Ultraviolet Upconversion Carbon Quantum Dots for Enhancement of the Photocatalytic Activity of Titanium Dioxide. ACS Omega 2021, 6, 4247–4254. [Google Scholar] [CrossRef]
- Shi, J.; Ni, G.; Tu, J.; Jin, X.; Peng, J. Green synthesis of fluorescent carbon dots for sensitive detection of Fe2+ and hydrogen peroxide. J. Nanoparticle Res. 2017, 19, 209. [Google Scholar] [CrossRef]
- Chakinala, N.; Ranjan, P.; Chakinala, A.G.; Gogate, P.R. Performance comparison of photocatalysts for degradation of organic pollutants using experimental studies supported with DFT and fundamental characterization. Catal. Commun. 2023, 174, 106589. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Y.; Tang, H. Destruction of phenol aqueous solution by photocatalysis or direct photolysis. Chemosphere 2000, 41, 1205–1209. [Google Scholar]
- Singh, H.K.; Muneer, M. Photodegradation of a herbicide derivative, 2,4-dichlorophenoxy acetic acid in aqueous suspensions of titanium dioxide. Res. Chem. Intermed. 2004, 30, 317–329. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Mishra, K.P.; Gogate, P.R. Intensification of sonophotocatalytic degradation of p-nitrophenol at pilot scale capacity. Ultrason. Sonochemistry 2011, 18, 739–744. [Google Scholar] [CrossRef]
- Yang, L.; Luo, S.; Li, Y.; Xiao, Y.; Kang, Q.; Cai, Q. High efficient photocatalytic degradation of p-nitrophenol on a unique Cu2O/TiO2 p-n heterojunction network catalyst. Environ. Sci. Technol. 2010, 44, 7641–7646. [Google Scholar] [CrossRef]
- Zhu, C.-P.; Huang, B.; Han, Q.-B.; Ni, C.-H.; Zhu, G.-J.; Liu, M.-H.; Shan, M.-L. Frequency effect on p-nitrophenol degradation under conditions of strict acoustic and electric control. Water Sci. Eng. 2011, 4, 74–82. [Google Scholar]
- Amiri, F.; Dehghani, M.; Amiri, Z.; Yousefinejad, S.; Azhdarpoor, A. Photocatalytic degradation of 2,4-dichlorophenoxyacetic acid from aqueous solutions by Ag3PO4/TiO2 nanoparticles under visible light: Kinetic and thermodynamic studies. Water Sci. Technol. 2021, 83, 3110–3122. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, S.; Teng, Y.; Liu, C.; Xu, X.; Zhang, X.; Chen, L. Efficient removal of herbicide 2,4-dichlorophenoxyacetic acid from water using Ag/reduced graphene oxide co-decorated TiO2 nanotube arrays. J. Hazard. Mater. 2012, 241–242, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Shankar, M.V.; Anandan, S.; Venkatachalam, N.; Arabindoo, B.; Murugesan, V. Fine route for an efficient removal of 2,4-dichlorophenoxyacetic acid (2,4-D) by zeolite-supported TiO2. Chemosphere 2006, 63, 1014–1021. [Google Scholar] [CrossRef] [PubMed]

| Recycling | TOC in HC-ZnBi-LDO (mg/g) | TOC in Solution before Visible Light-On | TOC in Solution after Visible Light-Off | %TOC Removal | Dechlorination (Inorganic Cl−) | %Degradation | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Light-On | After Light-Off | mg/L | % | mg/L | % | mg/L | % | |||
| 1st | 2.60 ± 0.14 | ND | 10.41 ± 0.22 | 79.7 ± 1.7 | 1.77 ± 0.15 | 13.6 ± 1.2 | 86.4 ± 1.7 | 8.65 ± 0.23 | 89.5 ± 2.3 | 90.8 ± 2.1 |
| 2nd | 2.38 ± 0.19 | ND | 10.73 ± 0.29 | 82.1 ± 2.2 | 2.18 ± 0.13 | 16.7 ± 1.0 | 83.3 ± 1.0 | 8.56 ± 0.16 | 88.6 ± 1.7 | 89.8 ± 1.3 |
| 3rd | 1.87 ± 0.18 | ND | 11.26 ± 0.23 | 86.1 ± 1.7 | 2.44 ± 0.18 | 18.7 ± 1.4 | 81.3 ± 1.4 | 8.50 ± 0.28 | 87.9 ± 2.9 | 88.2 ± 1.7 |
| 4th | 1.72 ± 0.11 | ND | 11.42 ± 0.17 | 87.4 ± 1.3 | 2.75 ± 0.22 | 21.0 ± 1.7 | 79.0 ± 1.7 | 8.34 ± 0.19 | 86.3 ± 1.9 | 86.4 ± 2.5 |
| Catalyst | Light Source | 2,4-D (mg/L) | Amount of Catalyst (g/L) | Time (min) | pH | Degradation (%) | Mineralization (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Current study | Visible | 30 | 1.0 | 135 | 4.0 | 90.8 | 86.4 | |
| TiO2@MgFe2O4/H2O2 | Visible | 100 | 0.5 | 240 | 2.0 | >83.0 | - | [15] |
| rGO/ZnBi2O4 | Visible | 30 | 1.0 | 120 | 2.45 | >90.0 | 83.7 | [21] |
| Ag3PO4/TiO2 | Visible | 10 | 1.0 | 60 | 3.0 | 98.4 | - | [44] |
| Ag/RGO-TiO2 | Solar | 10 | - | 160 | - | 100 | - | [45] |
| Mn-ZnO/graphene | LED | 25 | 2.0 | 120 | 5.0 | 66.2 | - | [28] |
| TiO2/zeolite HY | UV | 200 | 2.0 | 300 | 3.0 | 100.0 | >80.0 | [46] |
| TiO2 | UVA | 25 | 1.5 | 180 | 5.0 | 97.5 | 39.9 | [32] |
| TiO2 + H2O2 | UVA | 25 | 1.5 | 180 | 5.0 | 99.7 | 56.0 | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuong Vy, N.T.; Nha Khanh, D.N.; Nghia, N.N.; Khoa, L.H.; Nhi, P.T.; Hung, L.X.; Minh Phuong, D.T.; Kim Phuong, N.T. Key Role of Corncob Based-Hydrochar (HC) in the Enhancement of Visible Light Photocatalytic Degradation of 2,4-Dichlorophenoxyacetic Acid Using a Derivative of ZnBi-Layered Double Hydroxides. Materials 2023, 16, 5027. https://doi.org/10.3390/ma16145027
Tuong Vy NT, Nha Khanh DN, Nghia NN, Khoa LH, Nhi PT, Hung LX, Minh Phuong DT, Kim Phuong NT. Key Role of Corncob Based-Hydrochar (HC) in the Enhancement of Visible Light Photocatalytic Degradation of 2,4-Dichlorophenoxyacetic Acid Using a Derivative of ZnBi-Layered Double Hydroxides. Materials. 2023; 16(14):5027. https://doi.org/10.3390/ma16145027
Chicago/Turabian StyleTuong Vy, Ngo Thi, Dang Nguyen Nha Khanh, Nguyen Ngoc Nghia, Le Hai Khoa, Pham Tuan Nhi, Le Xuan Hung, Doan Thi Minh Phuong, and Nguyen Thi Kim Phuong. 2023. "Key Role of Corncob Based-Hydrochar (HC) in the Enhancement of Visible Light Photocatalytic Degradation of 2,4-Dichlorophenoxyacetic Acid Using a Derivative of ZnBi-Layered Double Hydroxides" Materials 16, no. 14: 5027. https://doi.org/10.3390/ma16145027
APA StyleTuong Vy, N. T., Nha Khanh, D. N., Nghia, N. N., Khoa, L. H., Nhi, P. T., Hung, L. X., Minh Phuong, D. T., & Kim Phuong, N. T. (2023). Key Role of Corncob Based-Hydrochar (HC) in the Enhancement of Visible Light Photocatalytic Degradation of 2,4-Dichlorophenoxyacetic Acid Using a Derivative of ZnBi-Layered Double Hydroxides. Materials, 16(14), 5027. https://doi.org/10.3390/ma16145027






