Effect of MBF-20 Interlayer on the Microstructure and Corrosion Behaviour of Inconel 625 Super Alloy after Diffusion Brazing
Abstract
:1. Introduction
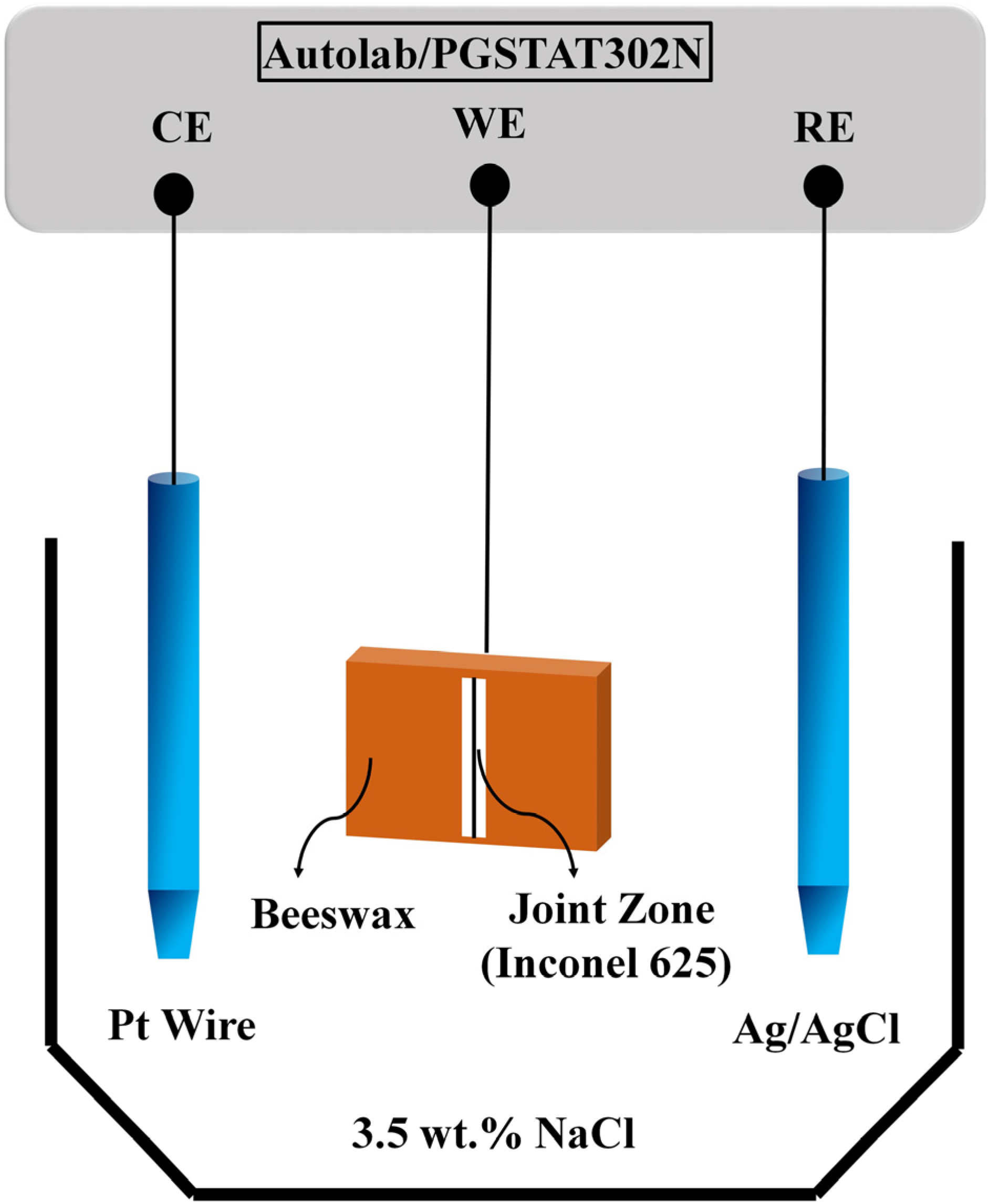
2. Materials and Methods
3. Results
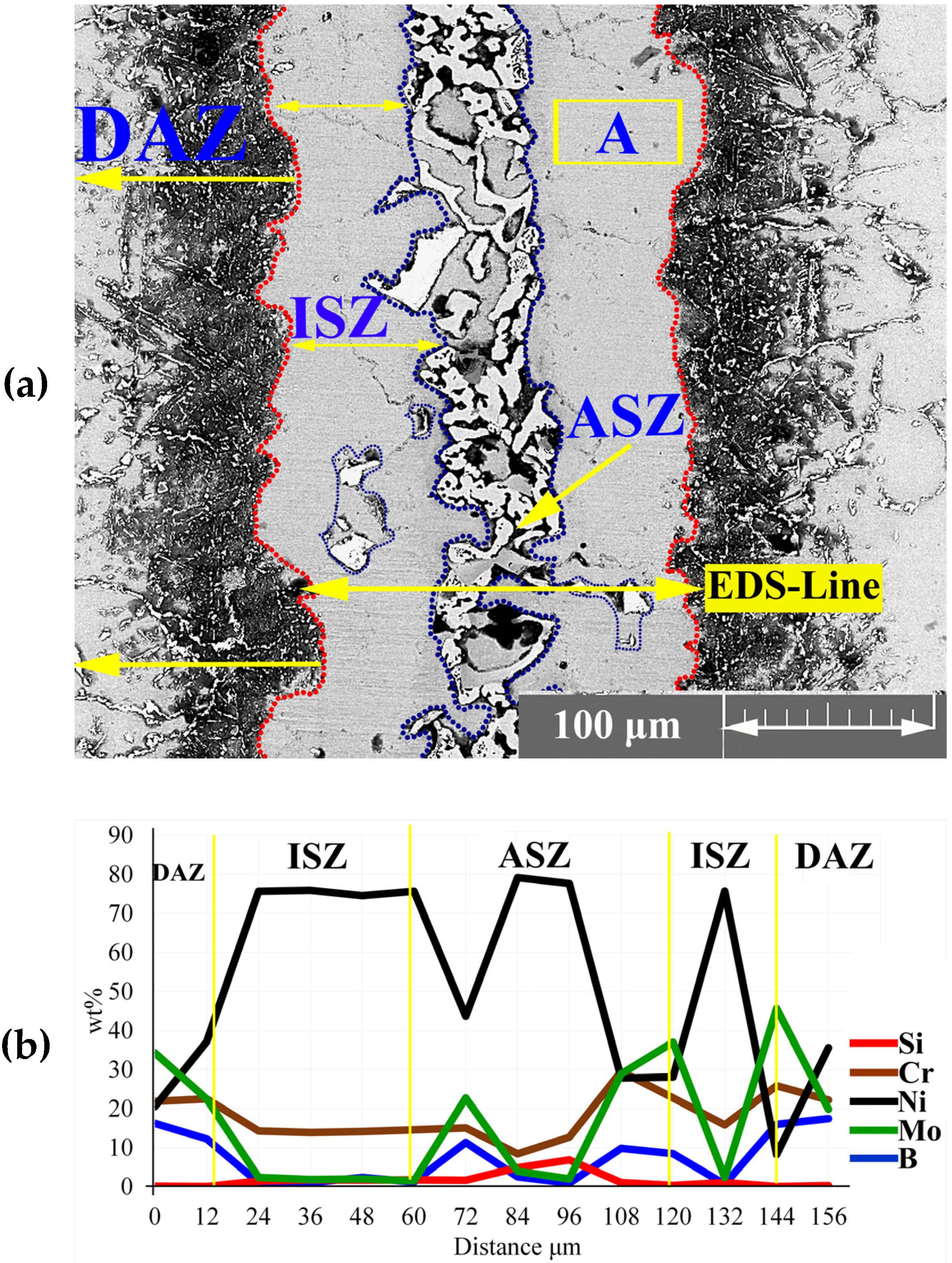
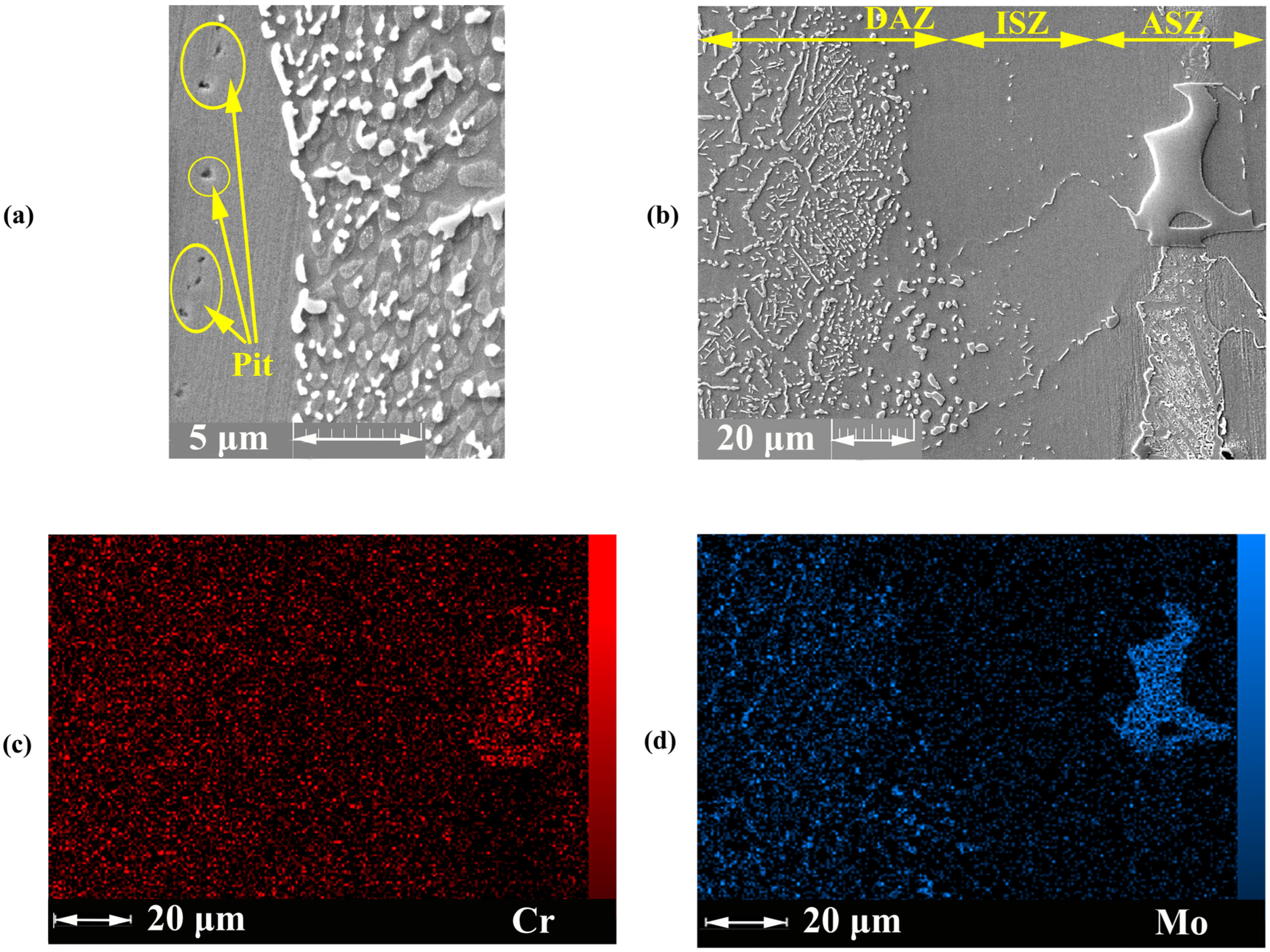
3.1. Microstructure Characterization
3.1.1. Isothermal Solidification Zone
3.1.2. Athermal Solidification Zone
- (1)
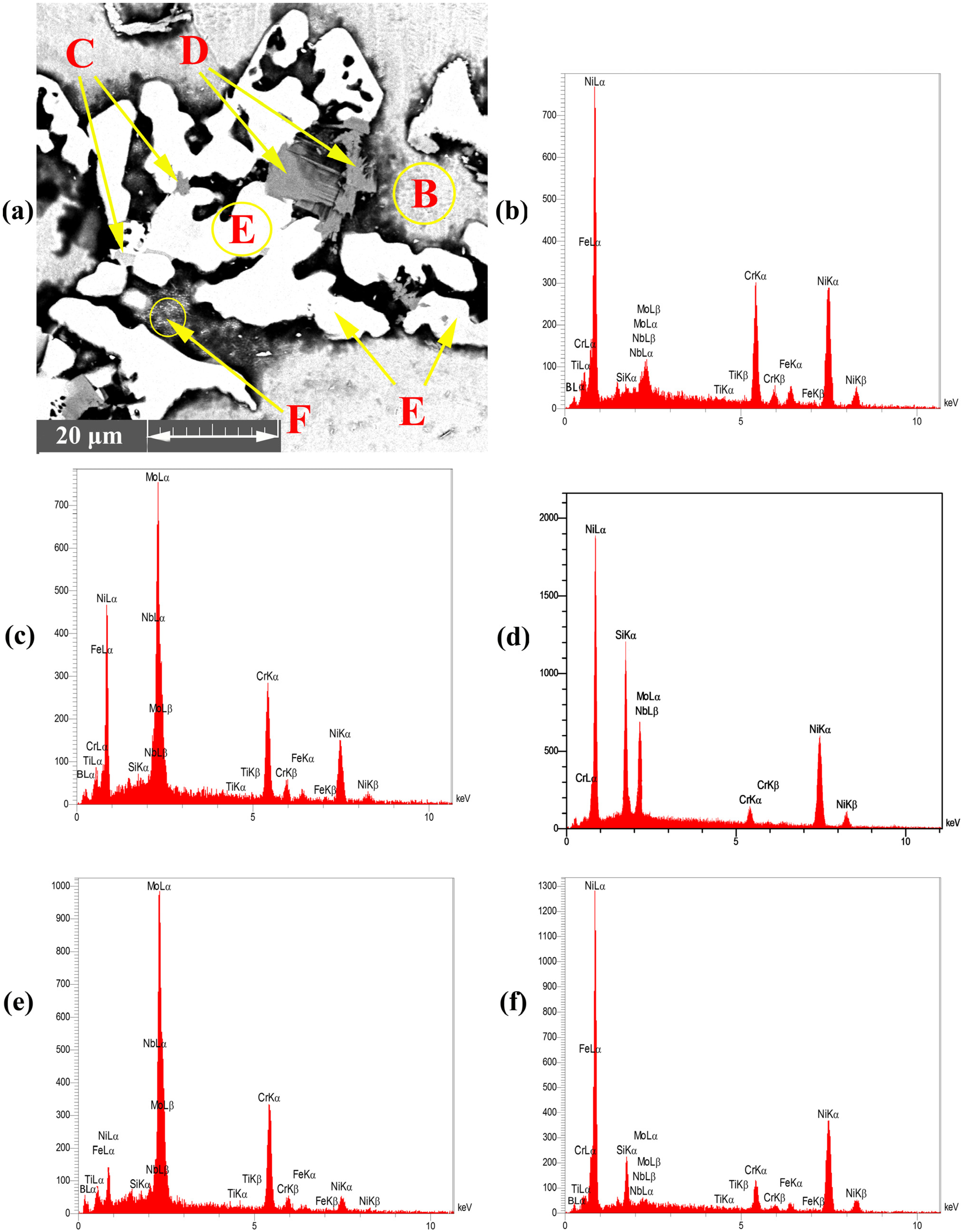
- The Ni-Cr-B phase might be formed in the bonding zone due to the dissolution of BM alloying elements, such as Cr. The EDS analysis of point B in Figure 4b probably showed the high concentrations of Ni and Cr because of the formation of Cr-Ni-rich boride precipitates. The EDS analysis of point H showed the high value of Ni, and the EDS line that passed in Figure 5c at 0–2 µm indicated the high values of Ni and B; this area may be an Ni-B compound. In addition, the investigation of Ni-Cr-B ternary systems showed that eutectic phases and single-phase γ-solid solutions were formed from the remaining liquid phase at 1110 °C and 1096 °C based on the following phase transitions [36,37]:
- (2)
- The Mo-rich boride binary eutectic and Ni-rich γ-phase (Ni-Mo-B) were formed based on the EDS spectrum of point C in Figure 4c and the EDS analysis (Table 2). These phases were formed by a combination of Mo from the BM (K > 1) and the MPD elements (K < 1) that remained in the liquid phase. According to the study of the Mo-Ni-B ternary phase diagram, Ni-Mo boride precipitates were formed at 1080 °C through the following phases [38]:
- (3)
- phases were possibly formed at 1040 °C based on the Ni-Si phase diagram in Figure 6b and the concentration of Si and Ni (EDS analysis of point D, I, and K and Figure 4d in this area). Moreover, γ-eutectic phase including was formed in this area because the Si content was more than its solubility. Oikawa et al. [39] reported that during cooling, excessive amounts of Si atoms were repelled from the γ-solid solution, so small cubic-shape precipitates were formed. Figure 5b shows that Si and Ni are present in high amounts, in contrast to other elements.
- (4)
- Cr-Mo-rich compounds were observed in the joint centreline, considering the dissolution of BM alloying elements into the bonding area. The EDS analysis of point E showed the high concentrations of Cr and Mo. The EDS line of B (Figure 5) showed a high intensity in this area, indicating that it is super-saturated with B. According to the EDS analysis results and the clarification by Tojo et al. [40], the following phases were formed at 1000 °C during the cooling cycle:
- (5)
- The EDS line in Figure 5 showed that in the 15–20 µm, B, Si, and Ni have high values, which may be Ni-Si-B compounds. The EDS data for point F (Figure 4f) and point L (Figure 6a) in Table 2 and Table 3 indicated that Ni-B-Si may be formed in these areas. Tokunaga et al. [41,42] reported similar results after studying Ni–Si–B ternary systems. Ni2B, Ni3B and Ni6Si2B phases may be formed at 850–990 °C by eutectic transformation from the remaining liquid phase from the last stage and during cooling process to room temperature, as shown in the following reaction:
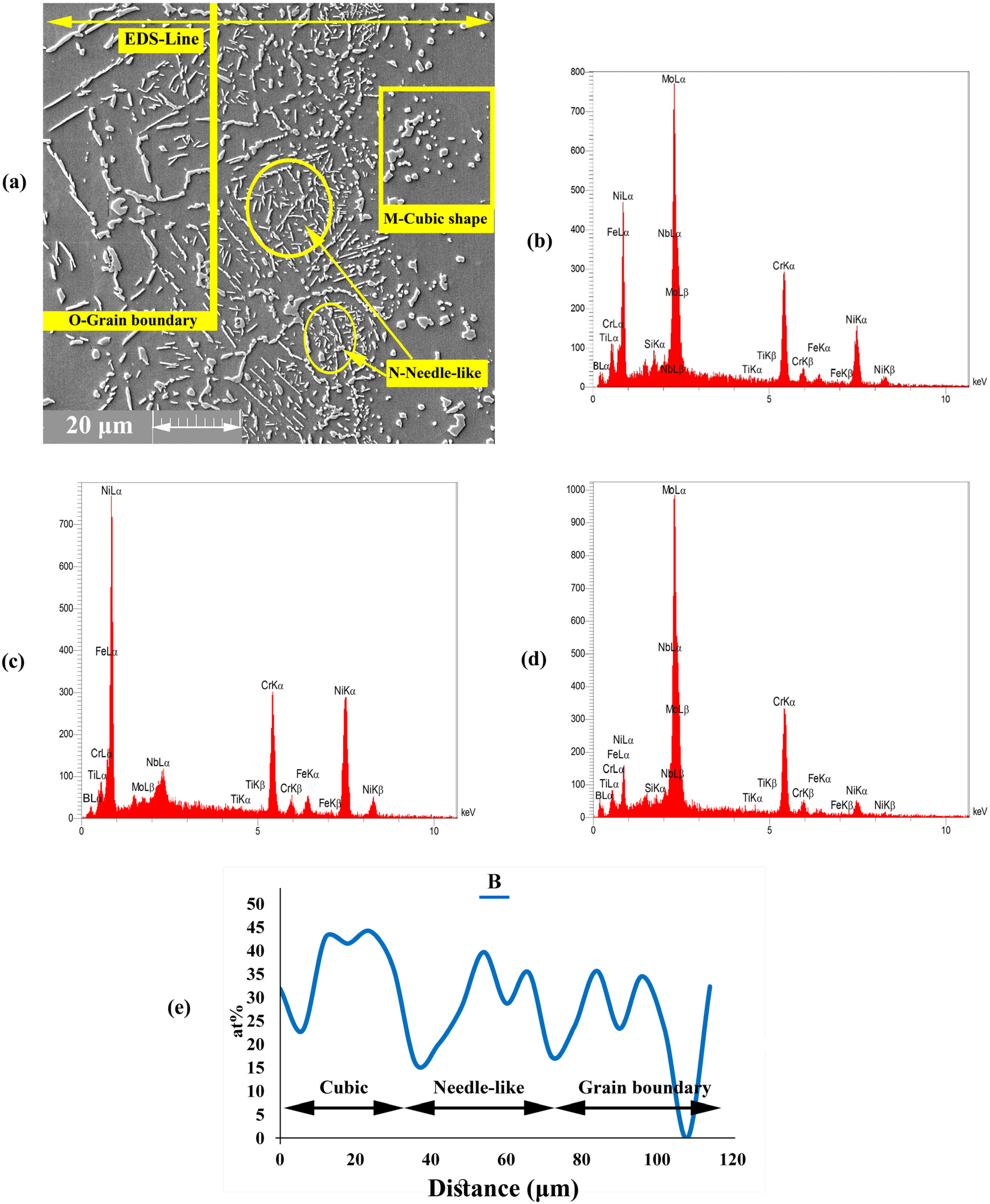
3.1.3. Diffusion Affected Zone
- (1)
- Cubic precipitates: based on EDS data for point M, the concentrations of Mo and Cr were very high in the precipitated phases. The formation of Cr- and Mo-rich borides was highly probable due to the high concentration of B in these areas. Additionally, the EDS analysis spectra illustrated in Figure 7b showed the high concentrations of Cr and Mo and the presence of B.
- (2)
- Needle-shaped precipitates: the analysis of point N (Figure 7c) showed that this area had higher concentrations of Cr and B inside the grains and higher solubility than that for the matrix. As a result, this phenomenon led to the formation of Cr-rich boride precipitates.
- (3)
- Grain boundary precipitates: these precipitates were formed in the grain boundary and based on the EDS analysis of point O (Figure 7d) and EDS line of the B element. These areas are Mo-rich borides. The grain boundaries are favourable paths for the diffusion of atoms, such as MPD elements (i.e., B element), due to low atomic density.
3.2. Corrosion Study
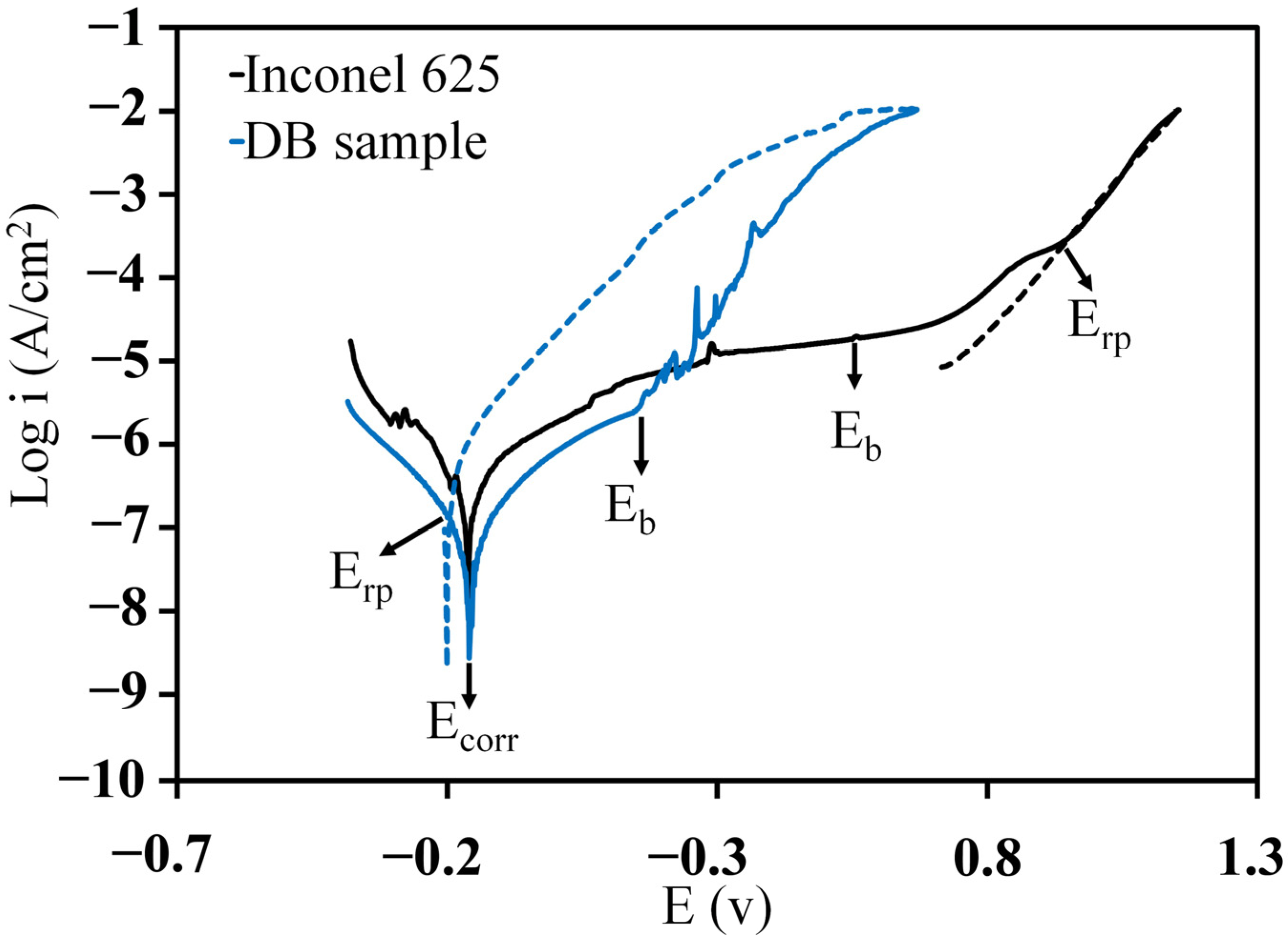
3.2.1. Polarisation Test
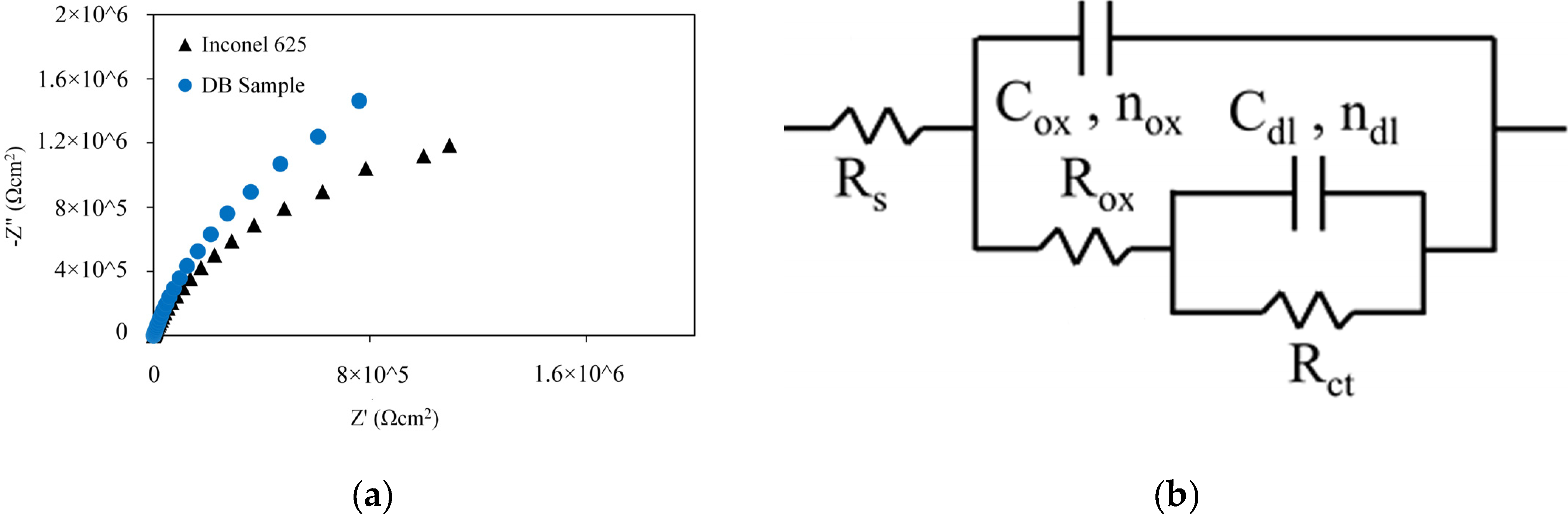
3.2.2. EIS test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sims, C.T.; Stoloff, N.S.; Hagel, W.C. Superalloys II; Wiley: Hoboken, NJ, USA, 1987; p. 615. ISBN 0471011479. [Google Scholar]
- Ramkumar, K.D.; Abraham, W.S.; Viyash, V.; Arivazhagan, N.; Rabel, A.M. Investigations on the Microstructure, Tensile Strength and High Temperature Corrosion Behaviour of Inconel 625 and Inconel 718 Dissimilar Joints. J. Manuf. Process. 2017, 25, 306–322. [Google Scholar] [CrossRef]
- Shamsabadi, A.Y.; Bakhtiari, R.; Eisaabadi, B.G. TLP Bonding of IN738/MBF20/IN718 System. J. Alloys Compd. 2016, 685, 896–904. [Google Scholar] [CrossRef]
- DABWF, G. Transient Liquid Phase Bonding-Overview. Sci. Technol. Weld. Join. 2004, 9, 1–18. [Google Scholar]
- Hadibeyk, S.; Beidokhti, B.; Sajjadi, S.A. Effect of Bonding Time and Homogenization Heat Treatment on the Microstructure and Mechanical Properties of the Transient Liquid Phase Bonded Dissimilar GTD-111/FSX-414 TLP Superalloys. J. Alloys Compd. 2018, 731, 929–935. [Google Scholar] [CrossRef]
- Doroudi, A.; Dastgheib, A.; Omidvar, H. The Bonding Temperature Effect of the Diffusion Brazing Inconel 625 Superalloy on the Microstructure Changes, Corrosion Resistance, and Mechanical Properties. J. Manuf. Process. 2020, 53, 213–222. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Transient Liquid Phase Bonding of Wrought IN718 Nickel Based Superalloy Using Standard Heat Treatment Cycles: Microstructure and Mechanical Properties. Mater. Des. 2013, 50, 694–701. [Google Scholar] [CrossRef]
- Samani, M.S.; Bahrami, A.; Karimzadeh, F.; Samani, M.S.; Bahrami, A.; Karimzadeh, F. Microstructure and Mechanical Properties of Transient Liquid Phase (TLP)-Bonded Ni3Al Intermetallic Compounds. Mater. Today Commun. 2019, 21, 100619. [Google Scholar] [CrossRef]
- Pilehrood, A.E.; Omidvar, H.; Shamsipur, A.; Sajuri, Z. Influence of Transient Liquid Phase Bonding Followed by Homogenization on the Fatigue Lifetimes of Inconel 738 at Elevated Temperature. J. Manuf. Process. 2020, 55, 348–358. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Peng, Z.; Wu, Z.; Cui, Z. Microstructure and Formation Mechanism of Ultrasound-Assisted Transient Liquid Phase Bonded Magnesium Alloys with Ni Interlayer. Materials 2019, 12, 3732. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Du, D.; Zhang, L.; Hao, Q.; Long, W. Vacuum Brazing of C/C Composite and TiAl Intermetallic Alloy Using BNi-2 Brazing Filler Metal. Materials 2021, 14, 1844. [Google Scholar] [CrossRef]
- Arafin, M.A.; Medraj, M.; Turner, D.P.; Bocher, P. Transient Liquid Phase Bonding of Inconel 718 and Inconel 625 with BNi-2: Modeling and Experimental Investigations. Mater. Sci. Eng. A 2007, 447, 125–133. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Solidification and Solid State Phenomena during TLP Bonding of IN718 Superalloy Using Ni-Si-B Ternary Filler Alloy. J. Alloys Compd. 2013, 563, 143–149. [Google Scholar] [CrossRef]
- Binesh, B.; Gharehbagh, A.J. Transient Liquid Phase Bonding of IN738LC/MBF-15/IN738LC: Solidification Behavior and Mechanical Properties. J. Mater. Sci. Technol. 2016, 32, 1137–1151. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Role of Base-Metal Composition in Isothermal Solidification during Diffusion Brazing of Nickel-Based Superalloys. Sci. Technol. Weld. Join. 2018, 23, 13–18. [Google Scholar] [CrossRef]
- Venkateswaran, T.; Ravi, K.R.; Sivakumar, D.; Pant, B.; Ram, G.D.J. Transient Liquid Phase Bonding of Cu-Cr-Zr-Ti Alloy Using Ni and Mn Coatings: Microstructural Evolution and Mechanical Properties. J. Mater. Eng. Perform. 2017, 26, 4064–4071. [Google Scholar] [CrossRef]
- Yuan, X.J.; Kim, M.B.; Kang, C.Y. Effects of Boron and Silicon on Microstructure and Isothermal Solidification during TLP Bonding of a Duplex Stainless Steel Using Two Ni–Si–B Insert Alloys. Mater. Sci. Technol. 2011, 27, 1191–1197. [Google Scholar] [CrossRef]
- Murray, D.C.; Corbin, S.F. Determining the Kinetics of Transient Liquid Phase Bonding (TLPB) of Inconel 625/BNi-2 Couples Using Differential Scanning Calorimetry. J. Mater. Process. Technol. 2017, 248, 92–102. [Google Scholar] [CrossRef]
- Bansal, A.; Sharma, A.K.; Kumar, P. Galvanic Corrosion Behavior of Microwave Welded and Post-Weld Heat-Treated Inconel-718 Joints. J. Mater. Eng. Perform. 2017, 26, 2322–2330. [Google Scholar] [CrossRef]
- Crum, J.R. Metals Handbook; Corrosion, ASM Metals Handbook; ASM International Materials Park: Novelty, OH, USA, 1987; Volume 13. [Google Scholar]
- Kangazian, J.; Shamanian, M.; Ashrafi, A. Dissimilar Welding between SAF 2507 Stainless Steel and Incoloy 825 Ni-Based Alloy: The Role of Microstructure on Corrosion Behavior of the Weld Metals. J. Manuf. Process. 2017, 29, 376–388. [Google Scholar] [CrossRef]
- Cao, S.; Brooks, C.R.; Whittaker, G. The Structure of the Heat-Affected Zone in Welds of a Ni-29 Wt.% Mo Commercial Alloy (Hastelloy B2). Mater. Charact. 1994, 33, 21–32. [Google Scholar] [CrossRef]
- Henderson, J.D.; Ebrahimi, N.; Dehnavi, V.; Guo, M.; Shoesmith, D.W.; Noël, J.J. The Role of Internal Cathodic Support during the Crevice Corrosion of Ni-Cr-Mo Alloys. Electrochim. Acta 2018, 283, 1600–1608. [Google Scholar] [CrossRef]
- Arhami, F.; Mirsalehi, S.E.; Sadeghian, A.; Johar, M.H. The Joint Properties of a High-Chromium Ni-Based Superalloy Made by Diffusion Brazing: Microstructural Evolution, Corrosion Resistance and Mechanical Behavior. J. Manuf. Process. 2019, 37, 203–211. [Google Scholar] [CrossRef]
- Mirzaei, S.; Binesh, B. Microstructure Evolution Mechanism and Corrosion Behavior of Transient Liquid Phase Bonded 304L Stainless Steel. Met. Mater. Int. 2021, 27, 3417–3431. [Google Scholar] [CrossRef]
- Mosallaee, M.; Babanejhad, A. Correlation Between Microstructure and Pitting Corrosion Behavior of TLP Bonded DSS-2205/Ni–P/DSS-2205 Specimens. Met. Mater. Int. 2020, 27, 1–11. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Doroudi, A.; Pilehrood, A.E.; Mohebinia, M.; Dastgheib, A.; Rajabi, A.; Omidvar, H. Effect of the Isothermal Solidification Completion on the Mechanical Properties of Inconel 625 Transient Liquid Phase Bond by Changing Bonding Temperature. J. Mater. Res. Technol. 2020, 9, 10355–10365. [Google Scholar] [CrossRef]
- Doroudi, A.; Shamsipur, A.; Omidvar, H.; Vatanara, M. Effect of Transient Liquid Phase Bonding Time on the Microstructure, Isothermal Solidification Completion and the Mechanical Properties during Bonding of Inconel 625 Superalloy Using Cr-Si-B-Ni Filler Metal. J. Manuf. Process. 2019, 38, 235–243. [Google Scholar] [CrossRef]
- Avazkonandeh-Gharavol, M.H.; Haddad-Sabzevar, M.; Fredriksson, H. Effect of Partition Coefficient on Microsegregation during Solidification of Aluminium Alloys. Int. J. Miner. Metall. Mater. 2014, 21, 980–989. [Google Scholar] [CrossRef]
- Ghasemi, A.; Pouranvari, M. Thermal Processing Strategies Enabling Boride Dissolution and Gamma Prime Precipitation in Dissimilar Nickel-Based Superalloys Transient Liquid Phase Bond. Mater. Des. 2019, 182, 108008. [Google Scholar] [CrossRef]
- Bakhtiari, R.; Ekrami, A.; Khan, T.I. Microstructure-Mechanical Properties Relation of TLP-Bonded FSX-414 Superalloy: Effect of Homogenization Design. J. Mater. Eng. Perform. 2015, 24, 1687–1696. [Google Scholar] [CrossRef]
- Muhamed, M.N.; Omar, M.Z.; Abdullah, S.; Sajuri, Z.; Wan Zamri, W.F.H. Investigation of Low-Velocity Impact and Flexural Loading on AR500 Steel/AA7075 Aluminum Alloy Brazed Joint. Songklanakarin J. Sci. Technol. 2018, 40, 1034–1038. [Google Scholar]
- Muhamed, M.N.; Omar, M.Z.; Abdullah, S.; Sajuri, Z.; Wan Zamri, W.F.H.; Abdullah, M.F. Brazed Joint Interface Bonding Strength of AR500 Steel and AA7075 Aluminium Alloy. Metals 2018, 8, 668. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Omar, M.Z.; Abdullah, S.; Sajuri, Z.; Zamri, W.F.H.; Muhamed, M.N.; Abdullah, M.F. Effects Of Wetting Time On Properties Of Steel-Aluminium Brazed Joint. J. Teknol. 2016, 78, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Lugscheider, E.; Knotek, O.; Reimann, H. The Ternary System Nickel-Chromium-Boron. Mon. Fuer Chem. 1974, 105, 80–90. [Google Scholar] [CrossRef]
- Materials Science International Team MSIT®. B-Cr-Ni (Boron-Chromium-Nickel). In Non-Ferrous Metal Systems; Part 3; Springer: Berlin/Heidelberg, Germany, 2007; pp. 153–167. [Google Scholar]
- Kubliy, V.Z.; Bondar, A.A.; Utkin, S.V.; Petyukh, V.M.; Lysenko, S.I.; Velikanova, T.Y. Phase Equilibria in the Nickel Corner of the Mo-Ni-B System at Temperatures Close to Melting. Powder Metall. Met. Ceram. 2008, 47, 211–222. [Google Scholar] [CrossRef]
- Oikawa, K.; Saito, R.; Kobayashi, K.; Yaokawa, J.; Anzai, K. Phase Equilibria in Ni-Rich Portion of Ni-Si System. Mater. Trans. 2007, 48, 2259–2262. [Google Scholar] [CrossRef] [Green Version]
- Tojo, M.; Tokunaga, T.; Ohtani, H.; Hasebe, M. Thermodynamic Analysis of Phase Equilibria in the Cr–Mo–B Ternary System. Calphad 2010, 34, 263–270. [Google Scholar] [CrossRef]
- Tokunaga, T.; Nishio, K.; Ohtani, H.; Hasebe, M. Phase Equilibria in the Ni-Si-B System. Mater. Trans. 2003, 44, 1651–1654. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, T.; Nishio, K.; Hasebe, M. Thermodynamic Study of Phase Equilibria in the Ni-Si-B System. J. Phase Equilibria 2001, 22, 291–299. [Google Scholar] [CrossRef]
- Yue, S.; Gamage, G.; Mohebinia, M.; Mayerich, D.; Talari, V.; Deng, Y.; Tian, F.; Dai, S.-Y.; Sun, H.; Hadjiev, V.; et al. Photoluminescence Mapping and Time-Domain Thermo-Photoluminescence for Rapid Imaging and Measurement of Thermal Conductivity of Boron Arsenide. Mater. Today Phys. 2020, 13, 100194. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.J.; Emadoddin, E.; Omidvar, H. The Joint Properties of A564-630 Stainless Steel Made by Transient Liquid Phase Bonding: Microstructural and Mechanical Strength Evaluation. J. Mater. Eng. Perform. 2020, 29, 4745–4753. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, H.; Xu, L.; Han, Y.; Zhao, L.; Zhang, J. Influence of Microstructure and Elemental Partitioning on Pitting Corrosion Resistance of Duplex Stainless Steel Welding Joints. Appl. Surf. Sci. 2017, 394, 297–314. [Google Scholar] [CrossRef]
- Muñoz, A.I.; Antón, J.G.; Nuévalos, S.L.; Guiñón, J.L.; Herranz, V.P. Corrosion Studies of Austenitic and Duplex Stainless Steels in Aqueous Lithium Bromide Solution at Different Temperatures. Corros. Sci. 2004, 46, 2955–2974. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Matykina, E. Pitting Corrosion Behaviour of Austenitic Stainless Steels —Combining Effects of Mn and Mo Additions. Corros. Sci. 2008, 50, 1796–1806. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Xue, P.; Wu, L.H.; Ni, D.R.; Ma, Z.Y. Microstructural Evolution and Pitting Corrosion Behavior of Friction Stir Welded Joint of High Nitrogen Stainless Steel. Mater. Des. 2016, 110, 802–810. [Google Scholar] [CrossRef]
- Ahmed, M.H.O.; Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.H.; Mohamad, A.B.; Gaaz, T.S. Synthesis and Characterization of a Novel Organic Corrosion Inhibitor for Mild Steel in 1 M Hydrochloric Acid. Results Phys. 2018, 8, 728–733. [Google Scholar] [CrossRef]
- Popova, A.; Christov, M. Evaluation of Impedance Measurements on Mild Steel Corrosion in Acid Media in the Presence of Heterocyclic Compounds. Corros. Sci. 2006, 48, 3208–3221. [Google Scholar] [CrossRef]
- Kangazian, J.; Shamanian, M.; Ashrafi, A. Surface Characterization of Incoloy 825 Ni-Based Alloy/2507 Super Duplex Stainless Steel Dissimilar Friction Stir Welds. Corrosion 2018, 74, 1259–1271. [Google Scholar] [CrossRef]
- Kangazian, J.; Shamanian, M. Effect of Pulsed Current on the Microstructure, Mechanical Properties and Corrosion Behavior of Ni-Based Alloy/Super Duplex Stainless Steel Dissimilar Welds. Trans. Indian Inst. Met. 2019, 72, 2403–2416. [Google Scholar] [CrossRef]
- Ivušić, F.; Lahodny-Šarc, O.; Ćurković, H.O.; Alar, V. Synergistic Inhibition of Carbon Steel Corrosion in Seawater by Cerium Chloride and Sodium Gluconate. Corros. Sci. 2015, 98, 88–97. [Google Scholar] [CrossRef]
- Khaled, K.F. Application of Electrochemical Frequency Modulation for Monitoring Corrosion and Corrosion Inhibition of Iron by Some Indole Derivatives in Molar Hydrochloric Acid. Mater. Chem. Phys. 2008, 112, 290–300. [Google Scholar] [CrossRef]



| At.% | Ni | Cr | Fe | Mo | Nb | C | Mn | Al | Ti | Co | B | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inconel 625 | 58.95 | 25.46 | 5.08 | 5.84 | 2.38 | 0.99 | 0.43 | 0.87 | 0.49 | 0.87 | - | - |
| MBF-20 | 67.55 | 6.96 | 2.34 | - | - | 0.25 | - | - | - | - | 14.8 | 8.1 |
| Point | Ni | Mo | Cr | Nb | Si | Fe | Phase Predicted |
|---|---|---|---|---|---|---|---|
| Point A | 74.6 | 5.43 | 11.21 | 2.69 | 3.67 | 2.25 | γ-solid solution |
| Point B | 60.10 | 3.28 | 32.43 | 2.11 | Ni, Cr-rich boride | ||
| Point C | 61.15 | 34.73 | 1.87 | Ni, Mo-rich boride | |||
| Point D | 75.23 | 21.81 | 2.96 | Ni-rich silicide | |||
| Point E | 11.62 | 36.12 | 48.63 | 1.63 | Mo, Cr-rich boride | ||
| Point F | 76.35 | - | 7.44 | 10.65 | 2.58 | γ-eutectic Ni-Si-B |
| Point | Ni | Cr | Mo | Nb | Si | phases |
|---|---|---|---|---|---|---|
| Point G | 76.49 | 8.95 | 3.92 | 2.48 | 8.16 | Matrix |
| Point H | 73.08 | 14.09 | 2.45 | 4.65 | 5.62 | Ni-rich Boride |
| Point I | 64.18 | 3.87 | 3.52 | 5.31 | 23.18 | Ni-rich silicide |
| Point J | 81.79 | 7.37 | 5.47 | 1.13 | 4.24 | Matrix |
| Point K | 65.78 | 3.71 | 4.17 | 1.75 | 24.19 | Ni-rich silicide |
| Point L | 72.96 | 7.12 | 2.01 | 1.05 | 17.76 | Ni-Si-rich boride |
| Concentration | Ni | Cr | Mo | Fe | Nb |
|---|---|---|---|---|---|
| 58.95 | 24.07 | 5.84 | 5.08 | 2.38 | |
| 67.55 | 6.99 | 0 | 2.34 | 0 | |
| 63.24 | 15.53 | 2.92 | 3.71 | 1.19 | |
| 11.04 | −3.25 | −0.91 | 0.04 | 0.23 | |
| 73.94 | 12.28 | 2.01 | 3.75 | 1.42 |
| Point | Ni | Mo | Cr | Nb | Si | Fe | Phase Predicted |
|---|---|---|---|---|---|---|---|
| Point M | 20.43 | 32.23 | 42.28 | - | - | 2.31 | Cr, Mo-rich boride |
| Point N | 30.50 | 8.93 | 58.31 | - | - | 1.84 | Cr-rich boride |
| Pont O | 10.41 | 56.23 | 23.34 | - | - | 1.38 | Mo-rich boride |
| Sample | (V) | (V) | (V) | (V) | ||
|---|---|---|---|---|---|---|
| Inconel 625 | 0.330 | −0.16 | 0.56 | 0.94 | 0.72 | −0.38 |
| DB sample | 0.132 | −0.15 | 0.15 | −0.19 | 0.30 | 0.34 |
| Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inconel 625 | 29.8 | 62.82 | 3.53 | 0.84 | 3.10 | 0.003 | 0.76 | 3.16 | 1.4 |
| DB sample | 28.75 | 78.25 | 4.95 | 0.88 | 5.30 | 1.10 | 0.82 | 5.38 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroudi, A.; Omidvar, H.; Dastgheib, A.; Khorram, M.; Rajabi, A.; Baghdadi, A.H.; Ghazali, M.J. Effect of MBF-20 Interlayer on the Microstructure and Corrosion Behaviour of Inconel 625 Super Alloy after Diffusion Brazing. Materials 2023, 16, 5072. https://doi.org/10.3390/ma16145072
Doroudi A, Omidvar H, Dastgheib A, Khorram M, Rajabi A, Baghdadi AH, Ghazali MJ. Effect of MBF-20 Interlayer on the Microstructure and Corrosion Behaviour of Inconel 625 Super Alloy after Diffusion Brazing. Materials. 2023; 16(14):5072. https://doi.org/10.3390/ma16145072
Chicago/Turabian StyleDoroudi, Alireza, Hamid Omidvar, Ali Dastgheib, Mohammad Khorram, Armin Rajabi, Amir Hossein Baghdadi, and Mariyam Jameelah Ghazali. 2023. "Effect of MBF-20 Interlayer on the Microstructure and Corrosion Behaviour of Inconel 625 Super Alloy after Diffusion Brazing" Materials 16, no. 14: 5072. https://doi.org/10.3390/ma16145072





