Preparation, Modification, and Application of Biochar in the Printing Field: A Review
Abstract
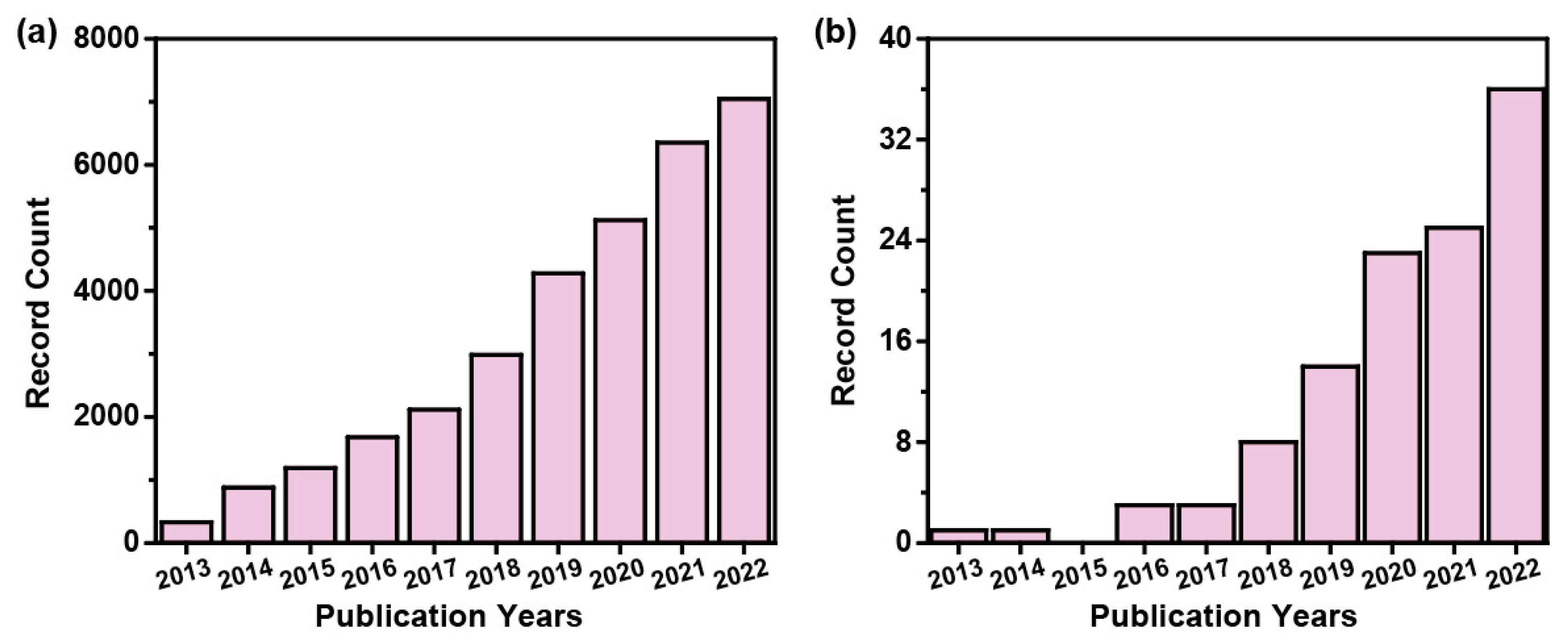
:1. Introduction
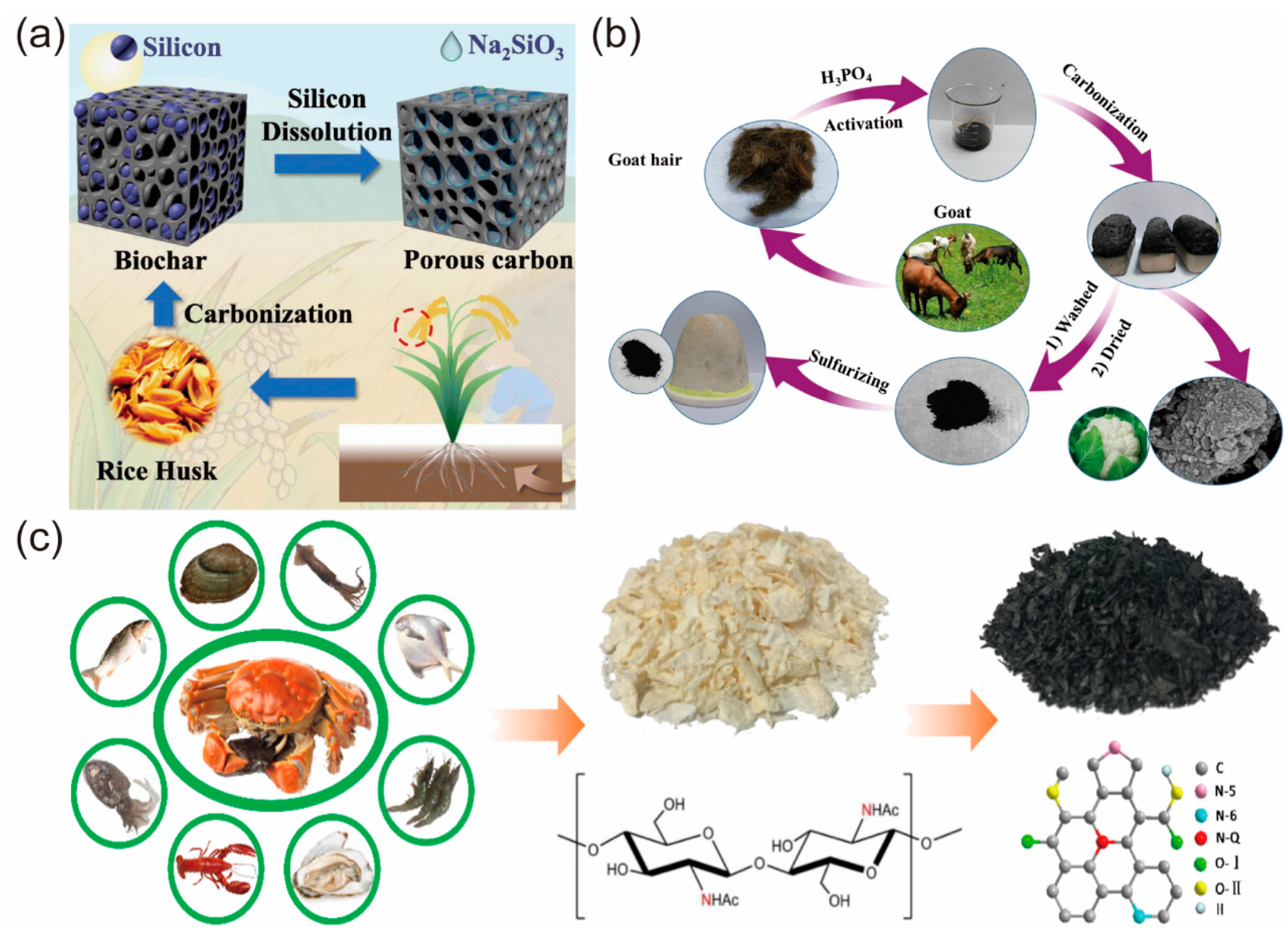
2. Raw Material and Preparation Method of Biochar
2.1. Pyrolysis Carbonization

2.2. Hydrothermal Carbonization
2.3. Laser-Induced Carbonization
2.4. Microwave-Assisted Carbonization
3. Modification of Biochar
3.1. Physical Modification
| Raw Materials | Modification Methods | Heating Temperature (°C) | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Application | Reference |
|---|---|---|---|---|---|---|
| Burcucumber | Steam | 300 and 700 | 7.10 | 0.038 | Remove sulfamethazine | [78] |
| Tea waste | Steam | 300 and 700 | 576.09 | 0.1091 | Remove sulfamethazine | [79] |
| Corncobs | Thermal air oxidation | 300–700 | ~350 | - | - | [80] |
| Softwood | Steam | 400 | 672 | - | Adsorb tetracycline | [81] |
| Wheat straw | Ball milling | 400–800 | 271.10 | 0.1445 | Adsorb volatile organic compounds | [82] |
| Danshen | Grind | 250–800 | 70.3 | 0.068 | Adsorb sulfamethoxazole | [83] |
3.2. Chemical Modification
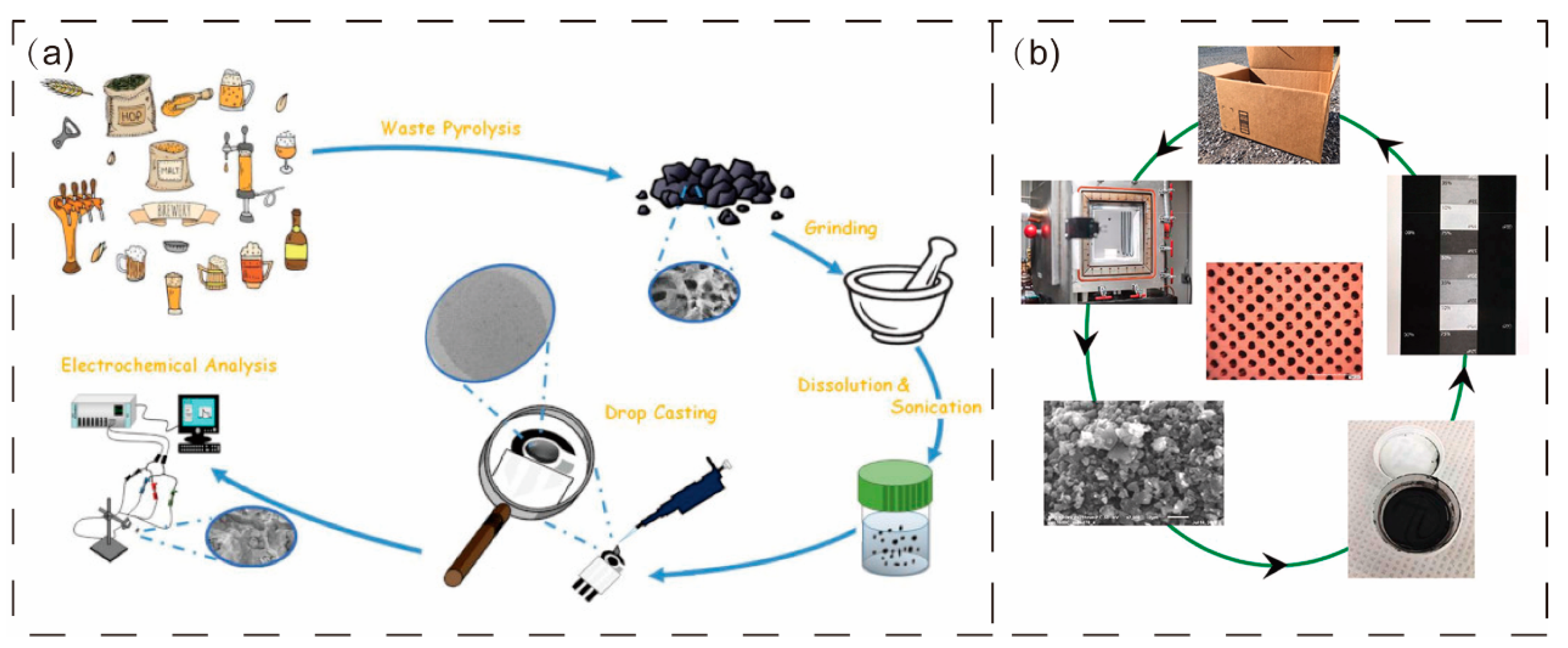
4. Application of Biochar in the Printing Field
4.1. Biochar as Printing Material
4.2. Application of Biochar in 3D Printing
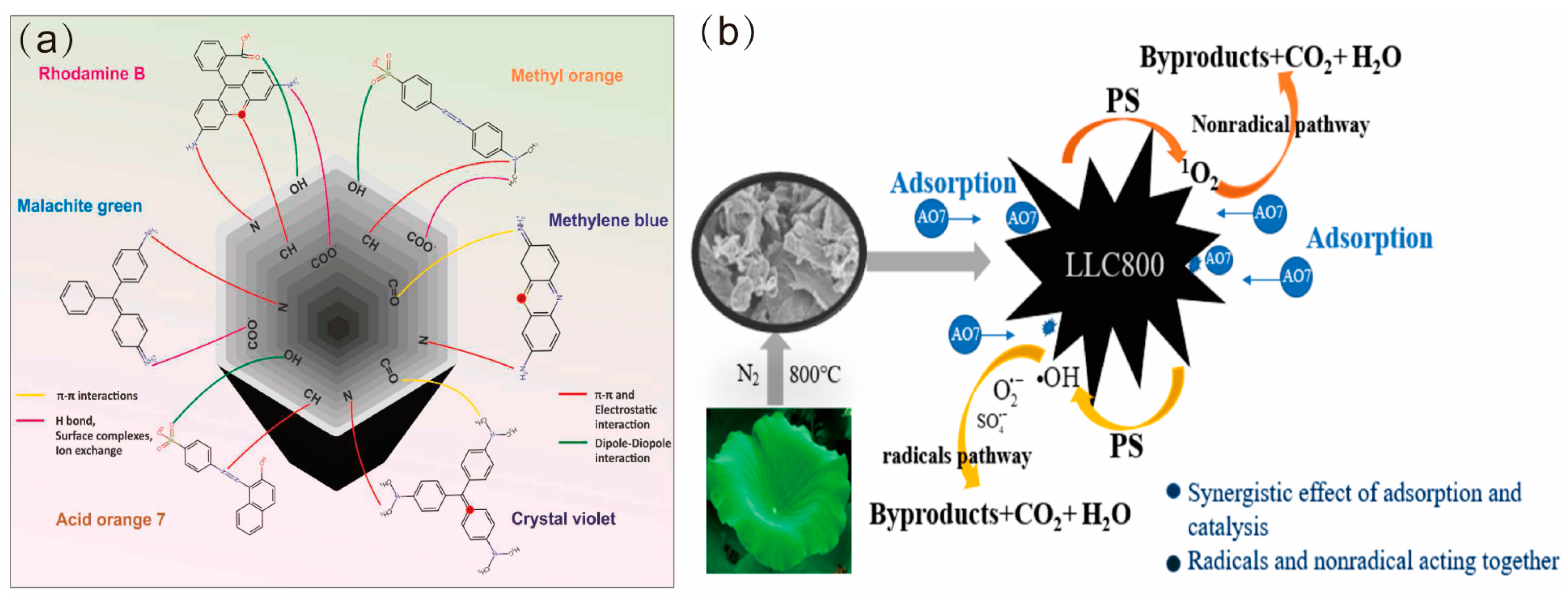
4.3. Biochar as Adsorbent
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent Advancement of Nanostructured Carbon for Energy Applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.K.; Thomas, B.; Kar, V.R. A Comprehensive Review on CNTs and CNT-Reinforced Composites: Syntheses, Characteristics and Applications. Mater. Today Commun. 2020, 25, 101546. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Jeon, M.S.; Gupta, R.K.; Jeon, Y.; Kalia, V.C.; Kim, S.C.; Cho, B.K.; Kim, D.R.; Lee, J.-K. Hierarchical Macroporous Particles for Efficient Whole-Cell Immobilization: Application in Bioconversion of Greenhouse Gases to Methanol. ACS Appl. Mater. Interfaces 2019, 11, 18968–18977. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Shen, X.; Wang, H.; Wang, H.; Xia, K.; Yin, Z.; Zhang, Y. Biomass-Derived Carbon Materials: Controllable Preparation and Versatile Applications. Small 2021, 17, 2008079. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, A.R.; Muthusamy, A.; Inho, C.; Kim, H.-J.; Senthil, K.; Prabakar, K. Ultrahigh surface area biomass derived 3D hierarchical porous carbon nanosheet electrodes for high energy density supercapacitors. Carbon 2021, 174, 463–474. [Google Scholar] [CrossRef]
- Sun, T.; Pei, P.; Sun, Y.; Xu, Y.; Jia, H. Performance and mechanism of As(III/V) removal from aqueous solution by novel positively charged animal-derived biochar. Sep. Purif. Technol. 2022, 290, 120836. [Google Scholar] [CrossRef]
- Ren, J.; Zhou, Y.; Wu, H.; Xie, F.; Xu, C.; Lin, D. Sulfur-encapsulated in heteroatom-doped hierarchical porous carbon derived from goat hair for high performance lithium–sulfur batteries. J. Energy Chem. 2019, 30, 121–131. [Google Scholar] [CrossRef]
- Gao, L.; Gan, W.; Qiu, Z.; Cao, G.; Zhan, X.; Qiang, T.; Li, J. Biomorphic Carbon-Doped TiO2 for Photocatalytic Gas Sensing with Continuous Detection of Persistent Volatile Organic Compounds. ACS Appl. Nano Mater. 2018, 1, 1766–1775. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, Y.; Dionysiou, D.D.; Sharma, V.K.; Ma, X. Biochar as a novel carbon-negative electron source and mediator: Electron exchange capacity (EEC) and environmentally persistent free radicals (EPFRs): A review. Chem. Eng. J. 2022, 429, 132313. [Google Scholar] [CrossRef]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.-H.; Antonietti, M.; Titirici, M.-M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gong, Y.; Wei, Z.; Wang, J.; Zhang, Z.; Li, H.; Dai, S.; Wang, Y. Updating Biomass into Functional Carbon Material in Ionothermal Manner. ACS Appl. Mater. Interfaces 2014, 6, 12515–12522. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Shen, L.; Xu, Y.; Hui, D.; Zhang, X. Lamellar-Structured Biomass-Derived Phosphorus- and Nitrogen-co-Doped Porous Carbon for High Performance Supercapacitors. New J. Chem. 2015, 39, 9497–9503. [Google Scholar] [CrossRef]
- Chen, Y.; Syed-Hassan, S.S.A.; Xiong, Z.; Li, Q.; Hu, X.; Xu, J.; Ren, Q.; Deng, Z.; Wang, X.; Su, S.; et al. Temporal and spatial evolution of biochar chemical structure during biomass pellet pyrolysis from the insights of micro-Raman spectroscopy. Fuel Process. Technol. 2021, 218, 106839. [Google Scholar] [CrossRef]
- Hidalgo, P.; Navia, R.; Hunter, R.; Coronado, G.; Gonzalez, M. Synthesis of carbon nanotubes using biochar as precursor material under microwave irradiation. J. Environ. Manag. 2019, 244, 83–91. [Google Scholar] [CrossRef]
- Chu, G.; Zhao, J.; Chen, F.; Dong, X.; Zhou, D.; Liang, N.; Wu, M.; Pan, B.; Steinberg, C.E.W. Physi-chemical and sorption properties of biochars prepared from peanut shell using thermal pyrolysis and microwave irradiation. Environ. Pollut. 2017, 227, 372–379. [Google Scholar] [CrossRef]
- Aubriet, F.; Ghislain, T.; Hertzog, J.; Sonnette, A.; Dufour, A.; Mauviel, G.; Carré, V. Characterization of biomass and biochar by LDI-FTICRMS—Effect of the laser wavelength and biomass material. J. Am. Soc. Mass Spectrom. 2018, 29, 1951–1962. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Lee, C.-W.; Lee, J.-U.; Ma, Y.-W.; Shin, B.-S. Laser-Induced Biochar Formation through 355 nm Pulsed Laser Irradiation of Wood, and Application to Eco-Friendly pH Sensors. Nanomaterials 2020, 10, 1904. [Google Scholar] [CrossRef]
- Kamali, M.; Appels, L.; Kwon, E.E.; Aminabhavi, T.M.; Dewil, R. Biochar in water and wastewater treatment—A sustainability assessment. Chem. Eng. J. 2021, 420, 129946. [Google Scholar] [CrossRef]
- Cuong, D.V.; Matsagar, B.M.; Lee, M.; Hossain, M.S.A.; Yamauchi, Y.; Vithanage, M.; Sarkar, B.; Ok, Y.S.; Wu, K.C.W.; Hou, C.-H. A critical review on biochar-based engineered hierarchical porous carbon for capacitive charge storage. Renew. Sustain. Energy Rev. 2021, 145, 111029. [Google Scholar] [CrossRef]
- Spanu, D.; Binda, G.; Dossi, C.; Monticelli, D. Biochar as an alternative sustainable platform for sensing applications: A review. Microchem. J. 2020, 159, 105506. [Google Scholar] [CrossRef]
- Sant’Anna, M.V.S.; Carvalho, S.W.M.M.; Gevaerd, A.; Silva, J.O.S.; Santos, E.; Carregosa, I.S.C.; Wisniewski, A.; Marcolino-Junior, L.H.; Bergamini, M.F.; Sussuchi, E.M. Electrochemical sensor based on biochar and reduced graphene oxide nanocomposite for carbendazim determination. Talanta 2020, 220, 121334. [Google Scholar] [CrossRef] [PubMed]
- Anae, J.; Ahmad, N.; Kumar, V.; Thakur, V.K.; Gutierrez, T.; Yang, X.J.; Cai, C.; Yang, Z.; Coulon, F. Recent advances in biochar engineering for soil contaminated with complex chemical mixtures: Remediation strategies and future perspectives. Sci. Total Environ. 2021, 767, 144351. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.; Lauro, S.; Barber, S.T.; Williams, S.A.; Trabold, T.A. Cleaner production of flexographic ink by substituting carbon black with biochar. J. Clean. Prod. 2021, 324, 129262. [Google Scholar] [CrossRef]
- Cancelliere, R.; Cianciaruso, M.; Carbone, K.; Micheli, L. Biochar: A Sustainable Alternative in the Development of Electrochemical Printed Platforms. Chemosensors 2022, 10, 344. [Google Scholar] [CrossRef]
- Pal, A.K.; Mohanty, A.K.; Misra, M. Additive manufacturing technology of polymeric materials for customized products: Recent developments and future prospective. RSC Adv. 2021, 11, 36398–36438. [Google Scholar] [CrossRef]
- Ertane, E.G.; Dorner-Reisel, A.; Baran, O.; Welzel, T.; Matner, V.; Svoboda, S. Processing and Wear Behaviour of 3D Printed PLA Reinforced with Biogenic Carbon. Adv. Tribol. 2018, 2018, 1763182. [Google Scholar] [CrossRef] [Green Version]
- Idrees, M.; Jeelani, S.; Rangari, V. Three-Dimensional-Printed Sustainable Biochar-Recycled PET Composites. ACS Sustain. Chem. Eng. 2018, 6, 13940–13948. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Khan, M.U.; Lin, X.N.; Cai, H.Z.; Lei, H.W. Temperature varied biochar as a reinforcing filler for high-density polyethylene composites. Compos. Part B Eng. 2019, 175, 107151. [Google Scholar] [CrossRef]
- Sutar, S.; Patil, P.; Jadhav, J. Recent advances in biochar technology for textile dyes wastewater remediation: A review. Environ. Res. 2022, 209, 112841. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.X.; Pang, X.H.; Wei, X.Y.; Sun, X.; Liu, H.W.; Sheng, P.F.; Zhu, M.Q.; Yang, X.F. Efficient Degradation of Printing and Dyeing Wastewater by Lotus Leaf-Based Nitrogen Self-Doped Mesoporous Biochar Activated Persulfate: Synergistic Mechanism of Adsorption and Catalysis. Catalysts 2022, 12, 1004. [Google Scholar] [CrossRef]
- Khan, A.A.; Gul, J.; Naqvi, S.R.; Ali, I.; Farooq, W.; Liaqat, R.; AlMohamadi, H.; Stepanec, L.; Juchelkova, D. Recent progress in microalgae-derived biochar for the treatment of textile industry wastewater. Chemosphere 2022, 306, 135565. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Li, M.; Peng, H.; Wang, D.; Fu, S. Cost-effective resource utilization for waste biomass: A simple preparation method of photo-thermal biochar cakes (BCs) toward dye wastewater treatment with solar energy. Environ. Res. 2021, 194, 110720. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Thermochemical conversion of lignin to functional materials: A review and future directions. Green Chem. 2015, 17, 4888–4907. [Google Scholar] [CrossRef]
- Reffas, A.; Bernardet, V.; David, B.; Reinert, L.; Lehocine, M.B.; Dubois, M.; Batisse, N.; Duclaux, L. Carbons prepared from coffee grounds by H3PO4 activation: Characterization and adsorption of methylene blue and Nylosan Red N-2RBL. J. Hazard. Mater. 2010, 175, 779–788. [Google Scholar] [CrossRef]
- Ren, J.; Chen, N.; Wan, L.; Li, G.; Chen, T.; Yang, F.; Sun, S. Preparation of High-Performance Activated Carbon from Coffee Grounds after Extraction of Bio-Oil. Molecules 2021, 26, 257. [Google Scholar] [CrossRef]
- Gan, F.; Wang, B.; Jin, Z.; Xie, L.; Dai, Z.; Zhou, T.; Jiang, X. From typical silicon-rich biomass to porous carbon-zeolite composite: A sustainable approach for efficient adsorption of CO2. Sci. Total Environ. 2021, 768, 144529. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Xu, J.; Mei, Y.; Fan, S.; Xu, H. Effect of carbonization methods on the properties of tea waste biochars and their application in tetracycline removal from aqueous solutions. Chemosphere 2021, 267, 129283. [Google Scholar] [CrossRef]
- Katiyar, R.; Patel, A.K.; Nguyen, T.-B.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D. Adsorption of copper (II) in aqueous solution using biochars derived from Ascophyllum nodosum seaweed. Bioresour. Technol. 2021, 328, 124829. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J.; Ma, C.; Zhang, C.; Wu, D.; Zhu, H. Carbonized daikon for high efficient solar steam generation. Sol. Energy Mater. Sol. Cells 2019, 191, 83–90. [Google Scholar] [CrossRef]
- Hao, R.; Yang, Y.; Wang, H.; Jia, B.; Ma, G.; Yu, D.; Guo, L.; Yang, S. Direct chitin conversion to N-doped amorphous carbon nanofibers for high-performing full sodium-ion batteries. Nano Energy 2018, 45, 220–228. [Google Scholar] [CrossRef]
- Luo, J.; Bo, S.; Qin, Y.; An, Q.; Xiao, Z.; Zhai, S. Transforming goat manure into surface-loaded cobalt/biochar as PMS activator for highly efficient ciprofloxacin degradation. Chem. Eng. J. 2020, 395, 125063. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.-F.; Chen, S. Amphiphilic Egg-Derived Carbon Dots: Rapid Plasma Fabrication, Pyrolysis Process, and Multicolor Printing Patterns. Angew. Chem. Int. Ed. 2012, 51, 9297–9301. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Lazaridis, P.A.; Fotopoulos, A.P.; Karakoulia, S.A.; Triantafyllidis, K.S. Catalytic Fast Pyrolysis of Kraft Lignin With Conventional, Mesoporous and Nanosized ZSM-5 Zeolite for the Production of Alkyl-Phenols and Aromatics. Front. Chem. 2018, 6, 295. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Luo, Z.; Guo, W.; Cai, W.; Zhu, X. Reutilization of biomass pyrolysis waste: Tailoring dual-doped biochar from refining residue of bio-oil through one-step self-assembly. J. Clean. Prod. 2022, 343, 131046. [Google Scholar] [CrossRef]
- Lei, L.; Pan, F.; Lindbrthen, A.; Zhang, X.; Guiver, M.D. Carbon hollow fiber membranes for a molecular sieve with precise-cutoff ultramicropores for superior hydrogen separation. Nat. Commun. 2021, 12, 268. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kirtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Vijayaraghavan, K. Recent advancements in biochar preparation, feedstocks, modification, characterization and future applications. Environ. Technol. Rev. 2019, 8, 47–64. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis —A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Aguado, R.; Bilbao, J.; Olazar, M. Biomass Oxidative Flash Pyrolysis: Autothermal Operation, Yields and Product Properties. Energy Fuels 2012, 26, 1353–1362. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulkornain, M.F.; Shamsuddin, A.H.; Normanbhay, S.; Md Saad, J.; Zhang, Y.S.; Samsuri, S.; Wan Ab Karim Ghani, W.A. Microwave-assisted Hydrothermal Carbonization for Solid Biofuel Application: A Brief Review. Carbon Capture Sci. Technol. 2021, 1, 100014. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Jin, C.; E, S.; Sheng, K.; Zhang, X. Effect of Swelling Pretreatment on Properties of Cellulose-Based Hydrochar. ACS Sustain. Chem. Eng. 2019, 7, 10821–10829. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal Carbon Spheres and Their Core/Shell Structures with Noble-Metal Nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef]
- Chen, C.; Liu, G.; An, Q.; Lin, L.; Shang, Y.; Wan, C. From wasted sludge to valuable biochar by low temperature hydrothermal carbonization treatment: Insight into the surface characteristics. J. Clean. Prod. 2020, 263, 121600. [Google Scholar] [CrossRef]
- Gascó, G.; Paz-Ferreiro, J.; Álvarez, M.L.; Saa, A.; Méndez, A. Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag. 2018, 79, 395–403. [Google Scholar] [CrossRef]
- González-Arias, J.; Sánchez, M.E.; Cara-Jiménez, J.; Baena-Moreno, F.M.; Zhang, Z. Hydrothermal carbonization of biomass and waste: A review. Environ. Chem. Lett. 2022, 20, 211–221. [Google Scholar] [CrossRef]
- Akbari, M.; Oyedun, A.O.; Kumar, A. Comparative energy and techno-economic analyses of two different configurations for hydrothermal carbonization of yard waste. Bioresour. Technol. Rep. 2019, 7, 100210. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A Comprehensive Review on Hydrothermal Carbonization of Biomass and its Applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Le, T.-S.D.; Phan, H.-P.; Kwon, S.; Park, S.; Jung, Y.; Min, J.; Chun, B.J.; Yoon, H.; Ko, S.H.; Kim, S.-W.; et al. Recent Advances in Laser-Induced Graphene: Mechanism, Fabrication, Properties, and Applications in Flexible Electronics. Adv. Funct. Mater. 2022, 32, 2205158. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, Y.; Su, X.; Wang, C.; Zhang, M.; Jian, M.; Xia, K.; Liang, X.; Lu, H.; et al. Laser Writing of Janus Graphene/Kevlar Textile for Intelligent Protective Clothing. ACS Nano 2020, 14, 3219–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.-S.D.; Park, S.; An, J.; Lee, P.S.; Kim, Y.-J. Ultrafast Laser Pulses Enable One-Step Graphene Patterning on Woods and Leaves for Green Electronics. Adv. Funct. Mater. 2019, 29, 1902771. [Google Scholar] [CrossRef]
- Morosawa, F.; Hayashi, S.; Terakawa, M. Femtosecond Laser-Induced Graphitization of Transparent Cellulose Nanofiber Films. ACS Sustain. Chem. Eng. 2021, 9, 2955–2961. [Google Scholar] [CrossRef]
- Schwenke, A.M.; Hoeppener, S.; Schubert, U.S. Synthesis and Modification of Carbon Nanomaterials utilizing Microwave Heating. Adv. Mater. 2015, 27, 4113–4141. [Google Scholar] [CrossRef]
- Morgan, H.M., Jr.; Bu, Q.; Liang, J.; Liu, Y.; Mao, H.; Shi, A.; Lei, H.; Ruan, R. A review of catalytic microwave pyrolysis of lignocellulosic biomass for value-added fuel and chemicals. Bioresour. Technol. 2017, 230, 112–121. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Chen, P.; Liu, S.Y.; Peng, P.; Min, M.; Cheng, Y.L.; Anderson, E.; Zhou, N.; Fan, L.L.; Liu, C.H.; et al. Effects of feedstock characteristics on microwave-assisted pyrolysis—A review. Bioresour. Technol. 2017, 230, 143–151. [Google Scholar] [CrossRef]
- Selvam, S.M.; Paramasivan, B. Microwave assisted carbonization and activation of biochar for energy-environment nexus: A review. Chemosphere 2022, 286, 131631. [Google Scholar] [CrossRef]
- Haeldermans, T.; Claesen, J.; Maggen, J.; Carleer, R.; Yperman, J.; Adriaensens, P.; Samyn, P.; Vandamme, D.; Cuypers, A.; Vanreppelen, K.; et al. Microwave assisted and conventional pyrolysis of MDF—Characterization of the produced biochars. J. Anal. Appl. Pyrolysis 2019, 138, 218–230. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Ok, Y.S.; Nizami, A.-S.; Khoshnevisan, B.; Mussatto, S.I.; Aghbashlo, M.; Tabatabaei, M.; Lam, S.S. A comprehensive review of engineered biochar: Production, characteristics, and environmental applications. J. Clean. Prod. 2020, 270, 122462. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Fang, J. Recent advances in engineered biochar productions and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Luo, L.; Lan, Y.; Zhang, Q.; Deng, J.; Luo, L.; Zeng, Q.; Gao, H.; Zhao, W. A review on biomass-derived activated carbon as electrode materials for energy storage supercapacitors. J. Energy Storage 2022, 55, 105839. [Google Scholar] [CrossRef]
- Bardestani, R.; Kaliaguine, S. Steam activation and mild air oxidation of vacuum pyrolysis biochar. Biomass Bioenergy 2018, 108, 101–112. [Google Scholar] [CrossRef]
- Rutherford, D.W.; Wershaw, R.L.; Rostad, C.E.; Kelly, C.N. Effect of formation conditions on biochars: Compositional and structural properties of cellulose, lignin, and pine biochars. Biomass Bioenergy 2012, 46, 693–701. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.W.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Ahmad, M.; Seo, D.-C.; Cho, J.-S.; Lee, S.-E.; Lee, S.S.; Ok, Y.S. Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J. Hazard. Mater. 2015, 290, 43–50. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Ahmad, M.; Mohan, D.; Chang, S.X.; Ok, Y.S. Pyrolysis condition affected sulfamethazine sorption by tea waste biochars. Bioresour. Technol. 2014, 166, 303–308. [Google Scholar] [CrossRef]
- Xiao, F.; Bedane, A.H.; Zhao, J.X.; Mann, M.D.; Pignatello, J.J. Thermal air oxidation changes surface and adsorptive properties of black carbon (char/biochar). Sci. Total Environ. 2018, 618, 276–283. [Google Scholar] [CrossRef]
- Zhu, X.; Li, C.; Li, J.; Xie, B.; Lü, J.; Li, Y. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2018, 263, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Pan, Z.; Zhang, X.; Miao, X.; Xiang, W.; Gao, B. Effect of ball milling with hydrogen peroxide or ammonia hydroxide on sorption performance of volatile organic compounds by biochar from different pyrolysis temperatures. Chem. Eng. J. 2022, 450, 138027. [Google Scholar] [CrossRef]
- Lian, F.; Sun, B.; Song, Z.; Zhu, L.; Qi, X.; Xing, B. Physicochemical properties of herb-residue biochar and its sorption to ionizable antibiotic sulfamethoxazole. Chem. Eng. J. 2014, 248, 128–134. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.; Liu, S.; Tan, X.; Zeng, G.; Zeng, W.; Ding, Y.; Cao, W.; Zheng, B. Enhanced adsorption of methylene blue by citric acid modification of biochar derived from water hyacinth (Eichornia crassipes). Environ. Sci. Pollut. Res. 2016, 23, 23606–23618. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, D.; Wan, S.; Yu, Z. Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour. Technol. 2015, 198, 300–308. [Google Scholar] [CrossRef]
- Tomczyk, A.; Kondracki, B.; Szewczuk-Karpisz, K. Chemical modification of biochars as a method to improve its surface properties and efficiency in removing xenobiotics from aqueous media. Chemosphere 2023, 312, 137238. [Google Scholar] [CrossRef]
- Khoshnood Motlagh, E.; Asasian-Kolur, N.; Sharifian, S.; Ebrahimian Pirbazari, A. Sustainable rice straw conversion into activated carbon and nano-silica using carbonization-extraction process. Biomass Bioenergy 2021, 144, 105917. [Google Scholar] [CrossRef]
- Murge, P.; Dinda, S.; Roy, S. Adsorbent from Rice Husk for CO2 Capture: Synthesis, Characterization, and Optimization of Parameters. Energy Fuels 2018, 32, 10786–10795. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of nitrate and phosphate adsorption on Al-modified biochar: Influence of Al content. Sci. Total Environ. 2018, 631–632, 895–903. [Google Scholar] [CrossRef]
- Jung, K.-W.; Hwang, M.-J.; Jeong, T.-U.; Ahn, K.-H. A novel approach for preparation of modified-biochar derived from marine macroalgae: Dual purpose electro-modification for improvement of surface area and metal impregnation. Bioresour. Technol. 2015, 191, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhao, C.; Tsang, D.C.W.; Liu, K.; Zhu, L.; Zhang, W.; Zhang, J.; Tang, Y.; Qiu, R. Microscopic mechanism about the selective adsorption of Cr(VI) from salt solution on O-rich and N-rich biochars. J. Hazard. Mater. 2021, 404, 124162. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Shi, Y.; Gu, J.; Bi, J.; Yuan, H.; Luo, B.; Chen, Y. Aqueous Cr(VI) removal by biochar derived from waste mangosteen shells: Role of pyrolysis and modification on its absorption process. J. Environ. Chem. Eng. 2020, 8, 103885. [Google Scholar] [CrossRef]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Van Vinh, N.; Zafar, M.; Behera, S.K.; Park, H.S. Arsenic(III) removal from aqueous solution by raw and zinc-loaded pine cone biochar: Equilibrium, kinetics, and thermodynamics studies. Int. J. Environ. Sci. Technol. 2015, 12, 1283–1294. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Liu, W.-J.; Jiang, H.; Chen, J.-J.; Li, W.-W.; Yu, H.-Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, W.; Huang, W. Effect of removing silica in rice husk for the preparation of activated carbon for supercapacitor applications. Chin. Chem. Lett. 2019, 30, 1315–1319. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, S.; Zhou, N.; Fan, L.; Zhang, Y.; Peng, P.; Anderson, E.; Ding, K.; Wang, Y.; Liu, Y.; et al. Development and application of a continuous fast microwave pyrolysis system for sewage sludge utilization. Bioresour. Technol. 2018, 256, 295–301. [Google Scholar] [CrossRef]
- Thomas, P.; Lai, C.W.; Bin Johan, M.R. Recent developments in biomass-derived carbon as a potential sustainable material for super-capacitor-based energy storage and environmental applications. J. Anal. Appl. Pyrolysis 2019, 140, 54–85. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, H.; Xu, W.; Tian, L.; Huang, J.; Liang, C.; Zhang, Y. Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors. Biosensors 2022, 12, 377. [Google Scholar] [CrossRef]
- Saletnik, B.; Zaguła, G.; Bajcar, M.; Tarapatskyy, M.; Bobula, G.; Puchalski, C. Biochar as a Multifunctional Component of the Environment—A Review. Appl. Sci. 2019, 9, 1139. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.M.; da Silva, A.D.; Camargo, J.R.; de Castro, B.S.; Meireles, L.M.; Silva, P.S.; Janegitz, B.C.; Silva, T.A. Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors 2023, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, R.; Di Tinno, A.; Di Lellis, A.M.; Tedeschi, Y.; Bellucci, S.; Carbone, K.; Signori, E.; Contini, G.; Micheli, L. An inverse-designed electrochemical platform for analytical applications. Electrochem. Commun. 2020, 121, 106862. [Google Scholar] [CrossRef]
- Arduini, F.; Di Nardo, F.; Amine, A.; Micheli, L.; Palleschi, G.; Moscone, D. Carbon Black-Modified Screen-Printed Electrodes as Electroanalytical Tools. Electroanalysis 2012, 24, 743–751. [Google Scholar] [CrossRef]
- Gecol, H.; Scamehorn, J.F.; Christian, S.D.; Grady, B.P.; Riddell, F. Use of surfactants to remove water based inks from plastic films. Colloids Surf. A 2001, 189, 55–64. [Google Scholar] [CrossRef]
- Cancelliere, R.; Carbone, K.; Pagano, M.; Cacciotti, I.; Micheli, L. Biochar from Brewers’ Spent Grain: A Green and Low-Cost Smart Material to Modify Screen-Printed Electrodes. Biosensors 2019, 9, 139. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Wang, Y.; He, S.; Liu, Z.; Fu, Y.; Sun, Z.; Li, M.; Wu, Z.-S.; Yu, H. Solid-state integrated micro-supercapacitor array construction with low-cost porous biochar. Mater. Chem. Front. 2021, 5, 4772–4779. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, Z.; Yang, Z.; Ke, L.; Kitipornchai, S.; Yang, J. Functionally graded graphene reinforced composite structures: A review. Eng. Struct. 2020, 210, 110339. [Google Scholar] [CrossRef]
- Compton, B.G.; Hmeidat, N.S.; Pack, R.C.; Heres, M.F.; Sangoro, J.R. Electrical and Mechanical Properties of 3D-Printed Graphene-Reinforced Epoxy. JOM 2018, 70, 292–297. [Google Scholar] [CrossRef]
- Vergara, L.A.; Perez, J.F.; Colorado, H.A. 3D printing of ordinary Portland cement with waste wood derived biochar obtained from gasification. Case Stud. Constr. Mater. 2023, 18, e02117. [Google Scholar] [CrossRef]
- Alhelal, A.; Mohammed, Z.; Jeelani, S.; Rangari, V.K. 3D printing of spent coffee ground derived biochar reinforced epoxy composites. J. Compos. Mater. 2021, 55, 3651–3660. [Google Scholar] [CrossRef]
- George, J.; Jung, D.; Bhattacharyya, D. Improvement of Electrical and Mechanical Properties of PLA/PBAT Composites Using Coconut Shell Biochar for Antistatic Applications. Appl. Sci. 2023, 13, 902. [Google Scholar] [CrossRef]
- Wei, M.; Li, Q.; Jiang, T.; Ding, H.; Wu, X.; Zhang, Y.; Wang, X. Improvement on the mechanical properties of maleic anhydride/polylactic acid composites with Pinus sylvestris-char. Mater. Today Commun. 2023, 34, 105278. [Google Scholar] [CrossRef]
- Mayakrishnan, V.; Mohamed, J.K.; Selvaraj, N.; SenthilKumar, D.; Annadurai, S. Effect of nano-biochar on mechanical, barrier and mulching properties of 3D printed thermoplastic polyurethane film. Polym. Bull. 2023, 80, 6725–6747. [Google Scholar] [CrossRef]
- Umerah, C.O.; Kodali, D.; Head, S.; Jeelani, S.; Rangari, V.K. Synthesis of carbon from waste coconutshell and their application as filler in bioplast polymer filaments for 3D printing. Compos. Part B 2020, 202, 108428. [Google Scholar] [CrossRef]
- Mohammed, Z.; Jeelani, S.; Rangari, V. Effective reinforcement of engineered sustainable biochar carbon for 3D printed polypropylene biocomposites. Compos. Part C Open Access 2022, 7, 100221. [Google Scholar] [CrossRef]
- Li, Y.; Cao, P.; Wang, S.; Xu, X. Research on the treatment mechanism of anthraquinone dye wastewater by algal-bacterial symbiotic system. Bioresour. Technol. 2022, 347, 126691. [Google Scholar] [CrossRef]
- Gvoic, V.; Prica, M.; Turk Sekulic, M.; Pap, S.; Paunovic, O.; Kulic Mandic, A.; Becelic-Tomin, M.; Vukelic, D.; Kerkez, D. Synergistic effect of Fenton oxidation and adsorption process in treatment of azo printing dye: DSD optimization and reaction mechanism interpretation. Environ. Technol. 2022, 12, 1–20. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Dewage, N.B.; Karunanayake, A.G.; Farmer, E.L.; Perez, F.; Hassan, E.B.; Mlsna, T.E.; Pittman, C.U. Rhodamine B Adsorptive Removal and Photocatalytic Degradation on MIL-53-Fe MOF/Magnetic Magnetite/Biochar Composites. J. Inorg. Organomet. Polym. Mater. 2020, 30, 214–229. [Google Scholar] [CrossRef]
- Wang, K.; Peng, N.; Zhang, D.; Zhou, H.; Gu, J.; Huang, J.; Liu, C.; Chen, Y.; Liu, Y.; Sun, J. Efficient removal of methylene blue using Ca(OH)2 modified biochar derived from rice straw. Environ. Technol. Innov. 2023, 31, 103145. [Google Scholar] [CrossRef]
- Liu, S.; Shen, C.; Wang, Y.; Huang, Y.; Hu, X.; Li, B.; Karnowo; Zhou, J.; Zhang, S.; Zhang, H. Development of CO2/H2O activated biochar derived from pine pyrolysis: Application in methylene blue adsorption. J. Chem. Technol. Biotechnol. 2022, 97, 885–893. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Yang, Y.; Wang, Q.; He, Y.; Dong, N. Synergetic effect on methylene blue adsorption to biochar with gentian violet in dyeing and printing wastewater under competitive adsorption mechanism. Case Stud. Therm. Eng. 2021, 26, 101099. [Google Scholar] [CrossRef]

| Preparation Methods | Advantages | Disadvantages |
|---|---|---|
| Pyrolysis carbonization |
|
|
| Hydrothermal carbonization |
|
|
| Laser-induced carbonization |
|
|
| Microwave-assisted carbonization |
|
|
| Raw Materials | Modification Methods | Heating Temperature (°C) | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Application | Reference |
|---|---|---|---|---|---|---|
| Rice straw | HCl | 700–1000 | 2356 | 1.61 | - | [88] |
| Rice Husk | KOH | 700 | 403.0 | 0.35 | CO2 Capture | [89] |
| Poplar chips | AlCl3 | 550 | 418.14 | 0.413 | Adsorb NO3− and PO43− | [90] |
| Macroalgae | H2SO4 and NaOH | 450 | 45.463 | 0.0318 | Adsorb phosphate | [91] |
| Waste poplar leaves | Urea | 650 | - | - | Adsorb Cr(VI) | [92] |
| Mangosteen shells | HCl, KOH and ZnCl2 | 350, 700 | 1836.46 | 1.058 | Adsorb Cr(VI) | [93] |
| Swine manure | H3PO4 | 700 | 372.21 | 0.23 | Adsorb tetracycline | [94] |
| Pinecone | Zn(NO3)2·6H2O | 500 | 11.54 | 0.028 | Remove Arsenic(III) | [95] |
| Biochar | H2SO4/KOH | 60 °C stirrer | 117.8 | 0.073 | Remove tetracycline | [96] |
| Rice husk | KOH | 500 | 3263 | - | Supercapacitor | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zeng, J.; Zuo, S.; Lin, S.; Chen, G. Preparation, Modification, and Application of Biochar in the Printing Field: A Review. Materials 2023, 16, 5081. https://doi.org/10.3390/ma16145081
Li X, Zeng J, Zuo S, Lin S, Chen G. Preparation, Modification, and Application of Biochar in the Printing Field: A Review. Materials. 2023; 16(14):5081. https://doi.org/10.3390/ma16145081
Chicago/Turabian StyleLi, Xin, Jinyu Zeng, Shuai Zuo, Saiting Lin, and Guangxue Chen. 2023. "Preparation, Modification, and Application of Biochar in the Printing Field: A Review" Materials 16, no. 14: 5081. https://doi.org/10.3390/ma16145081





