Adsorption of Hydrogen Sulfide on Activated Carbon Materials Derived from the Solid Fibrous Digestate
Abstract
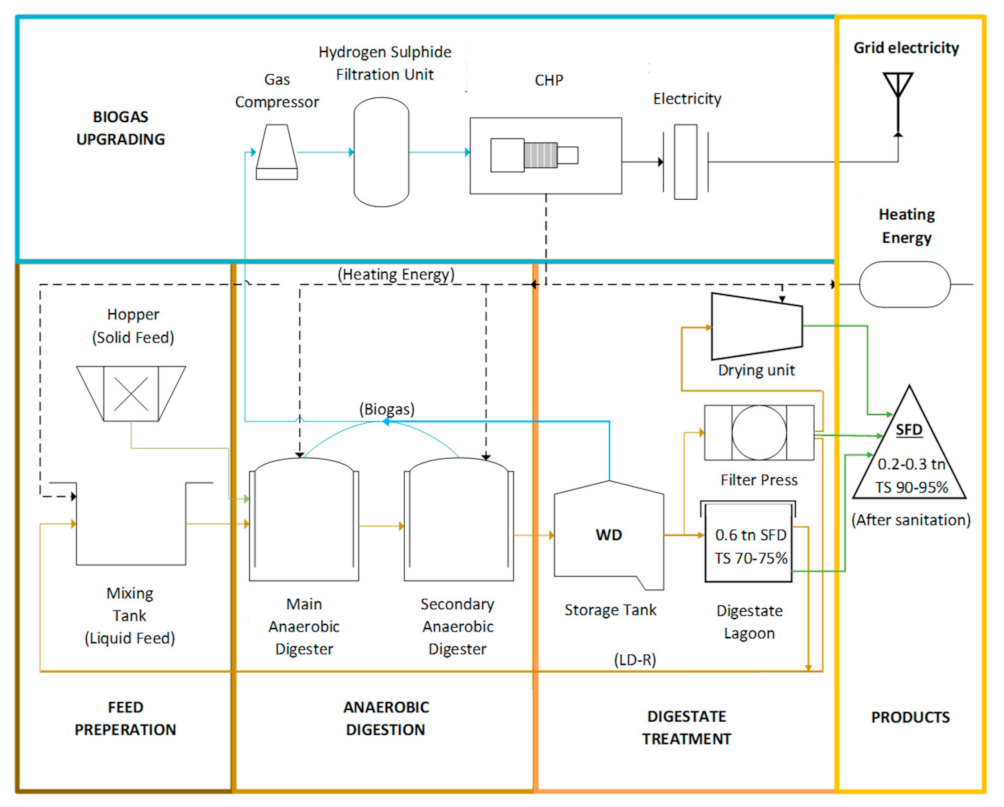
:1. Introduction
2. Experimental Procedures
2.1. Materials and Methods
2.2. Equipment and Procedure for SFD Pyrolysis and BC Activation
2.2.1. Pyrolysis of SFD and SFD-W
2.2.2. Activation of BC-SFD-W
2.3. Test Rig for Biogas Breakthrough Experiments
3. Results and Discussion
3.1. Biochar Yield
3.2. Elemental Analysis Results and Ash Content of BC
3.3. Pore Structural Characteristics of the Developed BCs and ACs
3.4. Surface Chemistry and Structural and Morphological Characteristics of ACs
3.5. Biogas Breakthrough Curves. Gas Separation Performance of the Developed ACs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pyrolysis Temperature | Pyrolysis Time | BC Yield (wt.%, DM * Feed) | |
|---|---|---|---|
| (°C) | (min) | BC-SFD | BC-SFD-Washed |
| 600 | 30 | 35.90 | 28.3 |
| 600 | 60 | 35.30 | 28.4 |
| 600 | 120 | 34.50 | 27.4 |
| 700 | 30 | 34.10 | 26.8 |
| 700 | 60 | 34.00 | 26.6 |
| 700 | 120 | 32.70 | 26.5 |
| 800 | 30 | 32.70 | 25.0 |
| 800 | 60 | 32.80 | 24.8 |
| 800 | 120 | 32.10 | 24.5 |
| Pyrolysis Temperature | Pyrolysis Time | Ash | C | H | N | O | H/C | O/C |
|---|---|---|---|---|---|---|---|---|
| (°C) | (min) | (wt.%, DM * Feed) | ||||||
| 600 | 30 | 35.30 | 58.80 | 1.60 | 0.50 | 3.70 | 0.33 | 0.05 |
| 600 | 60 | 33.90 | 58.60 | 1.60 | 0.80 | 5.10 | 0.32 | 0.07 |
| 600 | 120 | 33.60 | 61.40 | 1.30 | 0.90 | 2.80 | 0.25 | 0.03 |
| 700 | 30 | 38.20 | 58.70 | 1.10 | 0.50 | 1.40 | 0.23 | 0.02 |
| 700 | 60 | 38.00 | 58.90 | 1.30 | 0.70 | 1.20 | 0.27 | 0.02 |
| 700 | 120 | 37.60 | 60.90 | 0.90 | 0.70 | 0.00 | 0.18 | 0.00 |
| 800 | 30 | 39.10 | 61.10 | 0.70 | 0.30 | 0.00 | 0.14 | 0.00 |
| 800 | 60 | 37.00 | 61.20 | 0.60 | 0.50 | 0.70 | 0.12 | 0.01 |
| 800 | 120 | 38.30 | 60.30 | 0.40 | 0.90 | 0.10 | 0.07 | 0.00 |
| Pyrolysis Temperature | Pyrolysis Time | Ash | C | H | N | O | H/C | O/C |
|---|---|---|---|---|---|---|---|---|
| (°C) | (min) | (wt.%, DM * Feed) | ||||||
| 600 | 30 | 14.20 | 77.70 | 2.20 | 0.60 | 5.10 | 0.35 | 0.05 |
| 600 | 60 | 16.80 | 76.20 | 1.90 | 1.30 | 3.80 | 0.31 | 0.04 |
| 600 | 120 | 14.60 | 80.60 | 1.70 | 0.00 | 3.10 | 0.25 | 0.03 |
| 700 | 30 | 16.80 | 77.20 | 1.80 | 1.20 | 3.00 | 0.28 | 0.03 |
| 700 | 60 | 17.80 | 77.70 | 1.50 | 1.10 | 1.80 | 0.24 | 0.02 |
| 700 | 120 | 15.70 | 78.30 | 1.60 | 1.00 | 3.50 | 0.24 | 0.03 |
| 800 | 30 | 16.30 | 86.80 | 0.70 | 1.40 | 0.00 | 0.09 | 0.00 |
| 800 | 60 | 17.70 | 78.80 | 0.90 | 0.00 | 2.50 | 0.14 | 0.02 |
| 800 | 120 | 18.10 | 82.30 | 0.60 | 0.90 | 0.00 | 0.09 | 0.00 |
| Pyrolysis Temperature | Pyrolysis Time | BET | Micropore Surface | External Surface | Micropore Volume | Total Pore Volume |
|---|---|---|---|---|---|---|
| (°C) | (min) | (m2/g) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) |
| 600 | 30 | 18 | 10 | 8 | 0.004 | 0.021 |
| 600 | 60 | 17 | 11 | 6 | 0.004 | 0.019 |
| 600 | 120 | 15 | 7 | 8 | 0.003 | 0.020 |
| 700 | 30 | 43 | 28 | 15 | 0.011 | 0.040 |
| 700 | 60 | 35 | 25 | 10 | 0.010 | 0.035 |
| 700 | 120 | 27 | 16 | 12 | 0.006 | 0.034 |
| 800 | 30 | 38 | 18 | 20 | 0.007 | 0.043 |
| 800 | 60 | 43 | 22 | 21 | 0.009 | 0.056 |
| 800 | 120 | 38 | 20 | 18 | 0.008 | 0.055 |
| Pyrolysis Temperature | Pyrolysis Time | BET | Micropore Surface | External Surface | Micropore Volume | Total Pore Volume |
|---|---|---|---|---|---|---|
| (°C) | (min) | (m2/g) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) |
| 600 | 30 | 279 | 263 | 16 | 0.103 | 0.125 |
| 600 | 60 | 276 | 265 | 13 | 0.103 | 0.129 |
| 600 | 120 | 287 | 268 | 20 | 0.104 | 0.135 |
| 700 | 30 | 291 | 279 | 12 | 0.108 | 0.127 |
| 700 | 60 | 275 | 263 | 12 | 0.102 | 0.122 |
| 700 | 120 | 288 | 278 | 10 | 0.107 | 0.125 |
| 800 | 30 | 363 | 339 | 24 | 0.132 | 0.165 |
| 800 | 60 | 313 | 298 | 15 | 0.115 | 0.138 |
| 800 | 120 | 317 | 292 | 25 | 0.113 | 0.151 |
| Activation Temperature | Steam Flow Rate | Activation Time | BET | Micropore Surface | External Surface | Micropore Volume | Total Pore Volume |
|---|---|---|---|---|---|---|---|
| (°C) | (mL/min) | (min) | (m2/g) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) |
| 700 | 1 | 30 | 470 | 338 | 132 | 0.139 | 0.277 |
| 700 | 1 | 60 | 485 | 307 | 178 | 0.129 | 0.327 |
| 700 | 1 | 90 | 520 | 295 | 225 | 0.125 | 0.362 |
| 800 | 1 | 30 | 526 | 251 | 275 | 0.107 | 0.445 |
| 800 | 1 | 45 | 530 | 222 | 308 | 0.095 | 0.472 |
| 800 | 1 | 60 | 585 | 220 | 366 | 0.095 | 0.569 |
| 900 | 1 | 15 | 444 | 281 | 163 | 0.116 | 0.339 |
| 900 | 1 | 30 | 528 | 278 | 250 | 0.117 | 0.525 |
| 900 | 1 | 45 | 533 | 235 | 299 | 0.100 | 0.563 |
| Activation Temperature | KOH Molar Ratio | Activation Time | BET | Micropore Surface | External Surface | Micropore Volume | Total Pore Volume |
|---|---|---|---|---|---|---|---|
| (°C) | (min) | (m2/g) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) | |
| 600 | 4 | 30 | 658 | 511 | 47 | 0.246 | 0.307 |
| 700 | 4 | 30 | 1818 | 1746 | 73 | 0.688 | 0.767 |
| 800 | 1 | 30 | 1196 | 1143 | 52 | 0.446 | 0.502 |
| 800 | 2 | 30 | 367 | 335 | 32 | 0.134 | 0.178 |
| 800 | 4 | 30 | 2299 | 2174 | 125 | 0.887 | 1.010 |
| 800 | 4 | 60 | 1434 | 876 | 558 | 0.341 | 0.655 |
| 800 | 4 | 120 | 2032 | 1911 | 121 | 0.779 | 0.940 |
| Activated Carbon | Adsorption Temperature (°C) | H2S Capacity (g [H2S]/g) | ||
|---|---|---|---|---|
| Breakthrough Time (C/C0 = 0.05) 1 | Reference Time C/C0 = 0.5 1 | Exhaustion Time (C/C0 = 0.95) 2 | ||
| AC-H2O | 25 | 2.79 | 20.93 | 62.47 |
| 50 | 2.70 | 35.94 | 95.53 | |
| 70 | 1.20 | 15.02 | 45.13 | |
| AC-KOH | 25 | 1.46 | 5.66 | 25.95 |
| 50 | 1.40 | 9.03 | 41.75 | |
| 70 | 2.12 | 10.47 | 23.23 | |
| AC-CO2 | 25 | 22.14 | 53.62 | 137.83 |
| 50 | 10.62 | 53.44 | 135.65 | |
| 70 | 25.19 | 102.69 | 220.17 | |
| Activated Carbon | Adsorption Temperature (°C) | CO2 Capacity (g [CO2]/g) | |||
|---|---|---|---|---|---|
| Breakthrough Time (C/C0 = 0.05) 1 | C/C0 = 0.5 1 | Exhaustion Time (C/C0 = 0.95) 1 | Reference Time C/C0 = 0.5 2 | ||
| AC-H2O | 25 | 1.82 | 4.32 | 6.82 | 6.82 |
| 50 | 0.00 | 3.41 | 8.18 | 8.18 | |
| 70 | 2.50 | 4.54 | 8.63 | 8.63 | |
| AC-KOH | 25 | 4.77 | 14.09 | 26.58 | 26.58 |
| 50 | 2.95 | 11.13 | 19.54 | 19.54 | |
| 70 | 0.91 | 8.41 | 12.27 | 12.27 | |
| AC-CO2 | 25 | 1.59 | 3.86 | 8.63 | 8.63 |
| 50 | 1.36 | 2.27 | 2.27 | 2.27 | |
| 70 | 1.14 | 2.50 | 2.73 | 2.73 | |
References
- McKinsey, S.Z. Removal of Hydrogen Sulfide from Biogas Using Cow-Manure Compost. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2003. [Google Scholar]
- Kulkarni, M.; Ghanegaonkar, P. Hydrogen sulfide removal from biogas using chemical absorption technique in packed column reactors. Glob. J. Environ. Sci. Manag. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Shareefdeen, Z.; Singh, A. Biotechnology for Odor and Air Pollution Control; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Weiland, P. Notwendigkeit der Biogasaufbereitung, Ansprüche einzelner Nutzungsrouten und Stand der Technik. Gülzower Fachgespr. 2003, 21, 7–23. [Google Scholar]
- Sitthikhankaew, R.; Chadwick, D.; Assabumrungrat, S.; Laosiripojana, N. Effects of humidity, O2, and CO2 on H2S adsorption onto upgraded and KOH impregnated activated carbons. Fuel Process. Technol. 2014, 124, 249–257. [Google Scholar] [CrossRef]
- Council Directive of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources (91/676/EEC). Available online: https://eur-lex.europa.eu/eli/dir/1991/676/2008-12-11 (accessed on 7 June 2023).
- Allegue, L.B.; Hinge, J. Biogas Upgrading Evaluation of Methods for H2S removal. Danish Technological Institute. 2014. Available online: https://www.teknologisk.dk/_/media/60599_Biogas%20upgrading.%20Evalution%20of%20metods%20for%20H2S%20removal.pdf (accessed on 22 May 2023).
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.-H.; Jiang, L.-H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [PubMed]
- Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. Removal of Hydrogen Sulfide From Various Industrial Gases: A Review of The Most Promising Adsorbing Materials. Catalysts 2020, 10, 521. [Google Scholar] [CrossRef]
- Guo, J.; Luo, Y.; Lua, A.C.; Chi, R.-A.; Chen, Y.-L.; Bao, X.-T.; Xiang, S.-X. Adsorption of hydrogen sulphide (H2S) by activated carbons derived from oil-palm shell. Carbon 2007, 45, 330–336. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Chiung-Fen, C.; Ching-Yuan, C.; Wen-Tien, T. Effects of Burn-off and Activation Temperature on Preparation of Activated Carbon from Corn Cob Agrowaste by CO2 and Steam. J. Colloid Interface Sci. 2000, 232, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Farma, R.; Deraman, M.; Awitdrus, A.; Talib, I.; Taer, E.; Basri, N.; Manjunatha, J.; Ishak, M.; Dollah, B.; Hashmi, S. Preparation of highly porous binderless activated carbon electrodes from fibres of oil palm empty fruit bunches for application in supercapacitors. Bioresour. Technol. 2013, 132, 254–261. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; Gonzalez, M.; Rodriguez-Reinoso, F.; Sepúlveda-Escribano, A. Effect of steam and carbon dioxide activation in the micropore size distribution of activated carbon. Carbon 1996, 34, 505–509. [Google Scholar] [CrossRef]
- Calderon, S.T.; Pampa-Quispe, N.B.; Huaranga, M.A.C. Adsorption of hydrogen sulfide by activated carbon produced by regeneration of sludge from upflow anaerobic sludge blanket. Eng. Rural. Dev. 2022, 5, 25–27. [Google Scholar] [CrossRef]
- Tuerhong, T.; Kuerban, Z. Preparation and characterization of cattle manure-based activated carbon for hydrogen sulfide removal at room temperature. J. Environ. Chem. Eng. 2022, 10, 107177. [Google Scholar] [CrossRef]
- Hussain, A. A computational study of adsorption of H2S and SO2 on the activated carbon surfaces. J. Mol. Graph. Model. 2023, 122, 108463. [Google Scholar] [CrossRef] [PubMed]
- Tangsathitkulchai, C.; Naksusuk, S.; Wongkoblap, A.; Phadungbut, P.; Borisut, P. Equilibrium and Kinetics of CO2 Adsorption by Coconut Shell Activated Carbon Impregnated with Sodium Hydroxide. Processes 2021, 9, 201. [Google Scholar] [CrossRef]
- Fomkin, A.; Pribylov, A.; Men’shchikov, I.; Shkolin, A.; Aksyutin, O.; Ishkov, A.; Romanov, K.; Khozina, E. Adsorption-Based Hydrogen Storage in Activated Carbons and Model Carbon Structures. Reactions 2021, 2, 209–226. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.A.; Ocampo-Pérez, R.; Forgionny, A.; Labrada-Delgado, G.J.; Zárate-Guzmán, A.I.; Cruz-Briano, S.A.; Flores-Ramírez, R. Insights into Equilibrium and Adsorption Rate of Phenol on Activated Carbon Pellets Derived from Cigarette Butts. Processes 2021, 9, 934. [Google Scholar] [CrossRef]
- Jurkiewicz, M.; Pełech, R. Adsorption of 1,2-Dichlorobenzene from the Aqueous Phase onto Activated Carbons and Modified Carbon Nanotubes. Int. J. Mol. Sci. 2021, 22, 13152. [Google Scholar] [CrossRef]
- Wang, R.-S.; Li, Y.; Shuai, X.-X.; Liang, R.-H.; Chen, J.; Liu, C.-M. Pectin/Activated Carbon-Based Porous Microsphere for Pb2+ Adsorption: Characterization and Adsorption Behaviour. Polymers 2021, 13, 2453. [Google Scholar] [CrossRef]
- Alberto, D.R.; Tyler, A.C.; Trabold, T.A. Phosphate adsorption using biochar derived from solid digestate. Bioresour. Technol. Rep. 2021, 16, 100864. [Google Scholar] [CrossRef]
- Plaza, M.; González, A.; Pis, J.; Rubiera, F.; Pevida, C. Production of microporous biochars by single-step oxidation: Effect of activation conditions on CO2 capture. Appl. Energy 2014, 114, 551–562. [Google Scholar] [CrossRef] [Green Version]
- Aworn, A.; Thiravetyan, P.; Nakbanpote, W. Preparation and characteristics of agricultural waste activated carbon by physical activation having micro- and mesopores. J. Anal. Appl. Pyrolysis 2008, 82, 279–285. [Google Scholar] [CrossRef]
- Kainourgiakis, M.; Steriotis, T.; Kikkinides, E.; Romanos, G.; Stubos, A. Adsorption and diffusion in nanoporous materials from stochastic and process-based reconstruction techniques. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 206, 321–334. [Google Scholar] [CrossRef]
- Romanos, G.; Vangeli, O.; Stefanopoulos, K.; Kouvelos, E.; Papageorgiou, S.; Favvas, E.; Kanellopoulos, N. Methods of evaluating pore morphology in hybrid organic–inorganic porous materials. Microporous Mesoporous Mater. 2008, 120, 53–61. [Google Scholar] [CrossRef]
- Pokhrel, J.; Bhoria, N.; Anastasiou, S.; Tsoufis, T.; Gournis, D.; Romanos, G.; Karanikolos, G.N. CO2 adsorption behavior of amine-functionalized ZIF-8, graphene oxide, and ZIF-8/graphene oxide composites under dry and wet conditions. Microporous Mesoporous Mater. 2018, 267, 53–67. [Google Scholar] [CrossRef]
- Aik Chong, L.; Fong Yow, L.; Jia, G. Influence of pyrolysis conditions on pore development of oil-palm-shell activated carbons. J. Anal. Appl. Pyrolysis 2006, 76, 96–102. [Google Scholar]
- Bouchelta, C.; Medjram, M.S.; Zoubida, M.; Chekkat, F.A.; Ramdane, N.; Bellat, J.-P. Effects of pyrolysis conditions on the porous structure development of date pits activated carbon. J. Anal. Appl. Pyrolysis 2012, 94, 215–222. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocel-lulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Antonakou, E.; Kalogiannis, K.; Stefanidis, S.; Karakoulia, S.; Triantafyllidis, K.; Lappas, A.; Achilias, D. Catalytic and thermal pyrolysis of polycarbonate in a fixed-bed reactor: The effect of catalysts on products yields and composition. Polym. Degrad. Stab. 2014, 110, 482–491. [Google Scholar] [CrossRef]
















| Gas | NaOH (1.5 Molar)+AC (mass 500 g) +Steel Wool (mass 500 g) | Ca(OH)2 (1 Molar) +Steel Wool (mass 500 g) | ||
|---|---|---|---|---|
| P: 2.5 cm of Hg | P: 5 cm of Hg | P: 2.5 cm of Hg | P: 7.5 cm of Hg | |
| Q: 10 LPM | Q: 2 LPM | Q: 10 LPM | Q: 2 LPM | |
| CH4 | +12% | +30% | +24% | +28% |
| CO2 | −55% | −41% | −22% | −44% |
| H2S | −97% | −96% | −97% | −97% |
| Reagent Chemical Formula | Cost (€) | Concentration of Aq. Solution or Adsorbent Mass | Volume of Biogas Purified before Saturation (m3) | Cost of Chemical for Purification (€/m3) |
|---|---|---|---|---|
| MEA, C2H7NO | 6.21/L | 10% v/v | 165 | Regeneration by heating |
| NaOH | 1.24/kg | 1.5 Molar | 178 | 0.42 |
| Granular AC | 0.18/kg (limestone) | mass 100 g | 117 | 0.12 |
| Steel wool, Fe2O3 | 4.97/kg | mass 500 g | 207 | 2.40 |
| Ca(OH)2 | 0.2/kg | 1 Molar | Regeneration up to 5 times | Regeneration by oxidization |
| TS | Ash | C | H | N | O | |
|---|---|---|---|---|---|---|
| (wt.%, DM * feed) | ||||||
| SFD | 94.3 | 12.9 | 42.2 | 5.6 | 1.7 | 37.3 |
| SFD-W | 93 | 4.6 | 48.6 | 5.9 | 1.4 | 39.6 |
| Activation Temperature | Steam Flow | Activation Time | AC Yield (wt.%, DM * Feed) |
|---|---|---|---|
| (°C) | (mL/min) | (min) | AC-H2O |
| 700 | 1 | 30 | 74.7 |
| 700 | 1 | 60 | 65.0 |
| 700 | 1 | 90 | 57.1 |
| 800 | 1 | 30 | 42.7 |
| 800 | 1 | 45 | 35.8 |
| 800 | 1 | 60 | 31.5 |
| 900 | 1 | 15 | 47.2 |
| 900 | 1 | 30 | 33.0 |
| 900 | 1 | 45 | 23.3 |
| Activation Temperature | Ratio KOH/BC | Activation Time | AC Yield (wt.%, DM * Feed) |
|---|---|---|---|
| (°C) | (min) | AC-KOH | |
| 600 | 4 | 30 | 76.3 |
| 700 | 4 | 30 | 73.7 |
| 800 | 1 | 30 | 69.2 |
| 800 | 2 | 30 | 66.3 |
| 800 | 4 | 30 | 66.1 |
| 800 | 4 | 60 | 49.0 |
| 800 | 4 | 120 | 62.6 |
| Activated Carbon | Reactor Temperature (°C) | Flow Characteristics | |||||
|---|---|---|---|---|---|---|---|
| Qbiogas (mL/min) | ΔΡbiogas (millibar) | Density (g/cm3) | Vaverage (cm/min) | ηbiogas (Poise) | H (mm) | ||
| AC-H2O | 25 | 39.75 | 0.0401 | 0.0423 | 219.78 | 9.97 × 10−6 | 1034.54 |
| 50 | 40.03 | 0.0447 | 0.0445 | 221.33 | 1.10 × 10−5 | 975.78 | |
| 70 | 39.10 | 0.0472 | 0.0449 | 216.18 | 1.19 × 10−5 | 934.02 | |
| AC-KOH | 25 | 39.77 | 0.0401 | 0.0423 | 219.89 | 9.97 × 10−6 | 1034.54 |
| 50 | 39.85 | 0.0445 | 0.0443 | 220.33 | 1.10 × 10−5 | 975.78 | |
| 70 | 39.73 | 0.0480 | 0.0457 | 219.67 | 1.19 × 10−5 | 934.02 | |
| AC-CO2 | 25 | 40.12 | 0.0404 | 0.0426 | 221.82 | 9.97 × 10−6 | 1034.54 |
| 50 | 40.05 | 0.0447 | 0.0445 | 221.44 | 1.10 × 10−5 | 975.78 | |
| 70 | 39.02 | 0.0471 | 0.0448 | 215.74 | 1.19 × 10−5 | 934.02 | |
| Sample | Activation Agent | BET | Micropore Surface | External Surface | Micropore Volume | Total Pore Volume |
|---|---|---|---|---|---|---|
| (m2/g) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) | ||
| BC-SFD-W | - | 279 | 263 | 16 | 0.103 | 0.125 |
| AC-H2O | H2O | 790 | 644 | 146 | 0.31 | 0.53 |
| AC-CO2 | CO2 | 568 | 355 | 213 | 0.224 | 0.48 |
| AC-KOH | KOH | 2272 | 2174 | 98 | 0.89 | 1.011 |
| Activated Carbon | Adsorption Temperature (°C) | H2S Capacity (mmol[H2S]/g) | ||
|---|---|---|---|---|
| Breakthrough Time (C/C0 = 0.05) 1 | Reference Time C/C0 = 0.5 1 | Exhaustion Time (C/C0 = 0.95) 2 | ||
| AC-H2O | 25 | 0.10 | 0.71 | 2.13 |
| 50 | 0.09 | 1.22 | 3.26 | |
| 70 | 0.04 | 0.51 | 1.54 | |
| AC-KOH | 25 | 0.05 | 0.19 | 0.88 |
| 50 | 0.05 | 0.31 | 1.42 | |
| 70 | 0.07 | 0.36 | 0.79 | |
| AC-CO2 | 25 | 0.75 | 1.83 | 4.70 |
| 50 | 0.36 | 1.82 | 4.63 | |
| 70 | 0.86 | 3.50 | 7.51 | |
| Activated Carbon | Adsorption Temperature (°C) | CO2 Capacity (mmol[CO2]/g) | |||
|---|---|---|---|---|---|
| Breakthrough Time (C/C0 = 0.05) 1 | C/C0 = 0.5 1 | Exhaustion Time (C/C0 = 0.95) 1 | Reference Time C/C0 = 0.5 2 | ||
| AC-H2O | 25 | 0.08 | 0.19 | 0.30 | 0.30 |
| 50 | 0.00 | 0.15 | 0.36 | 0.36 | |
| 70 | 0.11 | 0.20 | 0.38 | 0.38 | |
| AC-KOH | 25 | 0.21 | 0.62 | 1.17 | 1.17 |
| 50 | 0.13 | 0.49 | 0.86 | 0.86 | |
| 70 | 0.04 | 0.37 | 0.54 | 0.54 | |
| AC-CO2 | 25 | 0.07 | 0.17 | 0.38 | 0.38 |
| 50 | 0.06 | 0.10 | 0.10 | 0.10 | |
| 70 | 0.05 | 0.11 | 0.12 | 0.12 | |
| Activated Carbon | Reactor Temperature (°C) | Selectivity of H2S | ||
|---|---|---|---|---|
| Breakthrough Time (C/C0 = 0.05) 1 | Reference Time C/C0 = 0.5 1 | Exhaustion Time (C/C0 = 0.95) 2 | ||
| AC-H2O | 25 | 254 | 1903 | 5680 |
| 50 | 204 | 2719 | 7227 | |
| 70 | 86 | 1081 | 3248 | |
| AC-KOH | 25 | 34 | 132 | 606 |
| 50 | 45 | 288 | 1331 | |
| 70 | 106 | 525 | 1166 | |
| AC-CO2 | 25 | 1573 | 3810 | 9795 |
| 50 | 2902 | 14,606 | 37,075 | |
| 70 | 5607 | 22,859 | 49,010 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choleva, E.; Mitsopoulos, A.; Dimitropoulou, G.; Romanos, G.E.; Kouvelos, E.; Pilatos, G.; Beltsios, K.; Stefanidis, S.; Lappas, A.; Sfetsas, T. Adsorption of Hydrogen Sulfide on Activated Carbon Materials Derived from the Solid Fibrous Digestate. Materials 2023, 16, 5119. https://doi.org/10.3390/ma16145119
Choleva E, Mitsopoulos A, Dimitropoulou G, Romanos GE, Kouvelos E, Pilatos G, Beltsios K, Stefanidis S, Lappas A, Sfetsas T. Adsorption of Hydrogen Sulfide on Activated Carbon Materials Derived from the Solid Fibrous Digestate. Materials. 2023; 16(14):5119. https://doi.org/10.3390/ma16145119
Chicago/Turabian StyleCholeva, Evangelia, Anastasios Mitsopoulos, Georgia Dimitropoulou, George Em. Romanos, Evangelos Kouvelos, George Pilatos, Konstantinos Beltsios, Stylianos Stefanidis, Angelos Lappas, and Themistoklis Sfetsas. 2023. "Adsorption of Hydrogen Sulfide on Activated Carbon Materials Derived from the Solid Fibrous Digestate" Materials 16, no. 14: 5119. https://doi.org/10.3390/ma16145119
APA StyleCholeva, E., Mitsopoulos, A., Dimitropoulou, G., Romanos, G. E., Kouvelos, E., Pilatos, G., Beltsios, K., Stefanidis, S., Lappas, A., & Sfetsas, T. (2023). Adsorption of Hydrogen Sulfide on Activated Carbon Materials Derived from the Solid Fibrous Digestate. Materials, 16(14), 5119. https://doi.org/10.3390/ma16145119








