Abstract
The goal of this work is to develop a sustainable value chain of carbonaceous adsorbents that can be produced from the solid fibrous digestate (SFD) of biogas plants and further applied in integrated desulfurization-upgrading (CO2/CH4 separation) processes of biogas to yield high-purity biomethane. For this purpose, physical and chemical activation of the SFD-derived BC was optimized to afford micro-mesoporous activated carbons (ACs) of high BET surface area (590–2300 m2g−1) and enhanced pore volume (0.57–1.0 cm3g−1). Gas breakthrough experiments from fixed bed columns of the obtained ACs, using real biogas mixture as feedstock, unveiled that the physical and chemical activation led to different types of ACs, which were sufficient for biogas upgrade and biogas desulfurization, respectively. Performing breakthrough experiments at three temperatures close to ambient, it was possible to define the optimum conditions for enhanced H2S/CO2 separation. It was also concluded that the H2S adsorption capacity was significantly affected by the restriction to gas diffusion. Hence, the best performance was obtained at 50 °C, and the maximum observed in the H2S adsorption capacity vs. the temperature was attributed to the counterbalance between adsorption and diffusion processes.
1. Introduction
Biogas use as a source of renewable energy has increased over the years and the demand for economically attractive methods for biogas upgrading is of growing interest. Hydrogen sulfide is one of the main contaminants that needs to be removed before the biogas stream enters the CHP due to its corrosive effect. One of the most effective, low cost and easy-to-maintain methods for H2S removal is the in situ biological reduction, implemented either by adding iron salts/oxides or by air dosing to the digester’s slurry where biological anaerobic oxidation of H2S to elemental sulfur and sulfates happens by Thiobacilus bacteria. Adding iron salts/oxides is a very effective practice in reducing high H2S levels down to 100–200 ppmv, but it fails to maintain a stable level of H2S. Most regularly practiced is the use of liquid FeCl2, while Fe(OH)3, Fe(OH)2 and ferrous chloride FeCl3 can also be involved in their solid form. The method of dosing 3–6% air to the biogas ratio can achieve 80–99% H2S reduction, down to 20–100 ppm H2S [1], while the oxygen content will be 0.5–1.8% per volume. The second most frequently applied process is adsorption, which entails trapping pollutants on a solid with a high surface area (activated carbon or crystalline material with high porosity, e.g., zeolites, silica gel, activated alumina), holding the pollutants through physical (weak) attraction forces or via chemical bonding. Adsorption is one of the most competitive technologies for precision desulfurization because it is simple and effective (>99%). The most competitive adsorbents for H2S biogas removal are impregnated activated carbons and iron oxides [1,2]. Adsorption systems are typically suitable for flow rates between 10 and 10,000 m3/h and pollutant concentrations between 0.1 and 8 g/m3 [3,4]. Impregnated activated carbons are preferably applied when it is necessary to significantly reduce or eliminate the concentration of H2S. This is because, in addition to the physical adsorption, activated carbons provide catalytic sites for oxidation to elemental sulfur and sulfate, thus enhancing the removal capacity of H2S. The activated carbons (ACs) must maintain a content of 20–30% moisture and the required volume of oxygen [5]. When the levels of H2S in the feed stream are high (>3000 ppmv), the adsorptive and catalytic sites are saturated, making periodic regeneration of the AC adsorbents necessary. Impregnated products usually exhibit enhanced H2S removal capacity, from a normal 10–20 kg H2S/(m3 C) for virgin carbon to 120–140 kg H2S/(m3 AC) for activated carbon. The cons are that the regeneration of those used in the process ACs is not sustainable and, consequently, the spent carbon must either be landfilled, applying to policy on nitrogen pollution (EU Nitrates Directive (1991) [6]), or re-impregnated, adding up to the logistic costs [7].
There is thereby a new interest field that emerged in using the biomass, even better the solid fibrous digestate (SFD), to produce the ACs on-site for the desulfurization of the biogas. In this way, a waste product (SFD) is transformed into an effective adsorbent that can be used either on-site, creating a closed loop value chain within the biogas plant, or implemented in other gas separation and wastewater treatment processes, achieving the effective integration between value chains [8,9].
To date, activated carbon adsorbents for biogas desulfurization have been prepared and studied using the common raw materials usually involved to yield ACs. Guo et al. [10] prepared chemically and physically activated carbons based on palm and coconut shells and investigated the different mechanisms of H2S adsorption, physisorption, chemisorption and H2S oxidation, depending on the activation agent, using H2O, CO2, ΚOH and H2SO4 and concluded that chemical activation has better dynamic adsorption performances. Longer breakthrough times and prolonged exhaustion times seem to increase the H2S adsorption capacities. Javier et al. [11] investigated the production of AC from barley straw via a physical activation method with CO2 and steam and concluded that the optimal conditions for the activation stage with CO2 were at 800 °C and a residence time of 1 h and at 700 °C and a hold time of 1 h when H2O is the activating agent [12,13,14]. The maximum BET surface area and micropore volume achieved by carbon dioxide activation were 789 m2/g and 0.3268 cm3/g, while for steam activation, they were 552 m2/g and 0.2304 cm3/g, representing an increase on both values of more than 42% for the case of activation with carbon dioxide. Those ACs functionalized with CO2 presented a well-developed micropore structure compared to the lower degree of microporosity endowed in the steam-activated carbons. Calderon et al. achieved 94% retention of H2S using AC as an adsorbent. This AC was derived from the sludge of a wastewater treatment plant while activation was performed with 1 M KOH for 20 min at 700 °C. Furthermore, simulation studies predicted that the retention of H2S could be enhanced to 96% [15], for an activation temperature of 635 °C and KOH molarity of 2.6 M. Cattle manure-based activated carbon materials prepared with a flow rate of 1000 mL/min CO2 has a total feasible manufacturing cost of €0.97 per kg [16]. In this study, AC activated with increased CO2 flow reached a maximum of 408.36 m2/g in surface area and 0.0528 cm3/g in micro-pore volume and possessed a superior H2S adsorption capacity of 868.45 mg/g. Akhtar Hussain [17] investigated 2, 3, 6 and 9-ring carbon structured adsorbent surfaces using parameters such as the planar and non-planar mode and the surface defects. The 6-ring with the vacancy centrally located in non-planar mode illustrated the highest steric heat of sorption (qst) for H2S, while adsorption was performed with significant strength both on a non-defected and defected 3-ring model in planar mode. As a general outcome, the increase in the size and structure decreases the qst and the most suitable configuration for the phenomenon to happen is central in a non-planar mode. Tangsathitkulchai et al. [18] investigated the equilibrium and kinetics of CO2 adsorption at 273 K by coconut shell activated carbon impregnated with sodium hydroxide (NaOH) and concluded that the maximum effective pore diffusivity occurred at an optimum NaOH loading of 180 mg/g, in line with the equilibrium adsorption results and achieved retention of CO2 up to 200 mg/g. Equilibrium isotherms of CO2 adsorption displayed an initial part of the Type I isotherm, with most adsorption taking place in micropores in the range of 0.7–0.9 nm by pore-filling mechanisms.
Many studies have shown that impregnated ACs are suitable adsorbents for many gases. Fomkin et al. [19] investigated the adsorption of hydrogen on KOH-activated ACs. Their study was conducted at the temperature range of 30 to 90 °C and pressures up to 200 bar, and the optimum conditions for enhanced adsorptivity of 11 mmol[H2]/g were found to be at 30 °C and 160 bar. Higher pressure favored the adsorption phenomenon, regardless. Nahum et al. [20] applied physical activation under CO2 atmosphere, at 850 °C for 2 h on carbon materials derived from cigarette butts and achieved a maximum adsorption capacity of 269 mg[C6H6O]/g of phenol at 10 °C, concluding that for phenol adsorption, the microporosity of the material is a crucial parameter. Moreover, ACs show good adsorbance behavior on the aqueous phase to remove toxic organic chemical compounds, such as benzene derivatives [21], and work well as heavy metal adsorbents [22]. Concerning the use of Solid Fibrous Digestate as a precursor material to produce activated carbons appropriate for the adsorption of on-site contaminants of the biogas production process, research oriented to phosphate adsorption on the liquid fraction of the digestate [23] concluded that the amount of solid digestate produced is sufficient for the removal of approximately 20% of the phosphate present. Biochar produced at 1000 °C pyrolysis temperature up to 100% recovery of phosphate solution at 50 mg/L or lower for higher phosphate concentration.
Further to the investigations on the development of highly efficient and selective for H2S, activated carbon adsorbents, there are also many studies focusing on the engineering part of the desulfurization process, elaborating either standalone processes or cascades that combine different processes such as adsorption on activated carbons and steel wool and absorption into aqueous solutions of amine, sodium hydroxide and calcium hydroxide [24,25]. The target of these studies is to instigate the overall process with the desired functionality, which can be either to enhance the CH4 content of biogas or to effectively remove H2S and CO2. Relevant reported results are presented in Table 1, concluding that the combination of calcium hydroxide (1 Molar) and steel wool (Fe and Zn elements) favors CO2 and H2S removal (max −44% and −97% respectively) while a combination of sodium hydroxide (1.5 Molar), activated carbon and steel wool favors CH4% content enhancement (up to +30% max) and CO2 removal (up to −41% max).

Table 1.
Results obtained from cascade processes that combine adsorption and absorption columns for biogas treatment. Absorbers are filled with 1 L of solvent, while adsorber columns are filled with 500 g of adsorbent [1].
For other parameters for optimization related to the process conditions, it was shown that the biogas inlet pressure had a varying effect on the performance depending on the composition of the solvent (absorbent) and the type of adsorbent, whereas the amplification of the biogas feed flow rate had a negative effect on the targets of high CH4 content and effective CO2 removal [26,27,28]. On the contrary, H2S was favored for flow rates up to 10 LPM. While cascades of absorption and adsorption processes offer the flexibility to select among a great variety of solvents and adsorbents and to fine-tune the conditions toward achieving the required performance, they also present major difficulties related to the need to design and integrate completely different absorber and adsorber columns and the great variety of processes required for the regeneration of solvents and adsorbents along with the different frequencies of regeneration. These difficulties are showcased in Table 2.

Table 2.
Details of the frequency of regeneration and related data for different reagents [1].
Conclusively, the design of cascade processes integrating different solid adsorbents with tailor-made gas adsorption and separation capacity seems to be a more feasible solution for applications in biogas desulfurization and upgrading.
In this context, the present study achieves the dual target of developing effective adsorbents from the waste effluent of a biogas plant and further endowing them with enhanced CO2 separating or H2S separating capacity. Hence, the developed, in this work, activated carbons can be applied in stand-alone or cascade processes with the targets of desulfurizing biogas and enhancing its CH4 content. Starting from the solid fraction of digestate, pre-treatment and pyrolysis techniques are first optimized to achieve high yields of biochar (BC) along with a high external surface [29,30,31,32]. Furthermore, biochar is converted to activated carbon by applying a variety of physical and chemical activation methods with CO2, H2O and KOH, optimizing the functionalization conditions to further enlarge the carbon’s surface and the porosity and aiming for higher adsorption yields. Breakthrough experiments in fixed bed columns at different temperatures using real biogas mixtures are performed, and the obtained gas uptake and separation performances of the various ACs are scrutinized against their pore structural and surface chemistry properties. Conclusively, the outcome of this work is an optimized workflow that starts with SFD and ends up with a tailor-made adsorbent for either enhanced CO2 or H2S separation.
2. Experimental Procedures
2.1. Materials and Methods
The precursor material, namely solid fibrous digestate (SFD), used for the AC preparation was collected from a biogas plant in Greece (Biogas Lagada, Kolchiko, Greece), which uses mostly agricultural waste to feed the anaerobic digester (AD). The SFD is obtained from the whole digestate (WD) of the AD after separation in a screw filter press separator, followed by drying and sanitation. The total solids (TS) content of the SFD is between 90% and 95%.
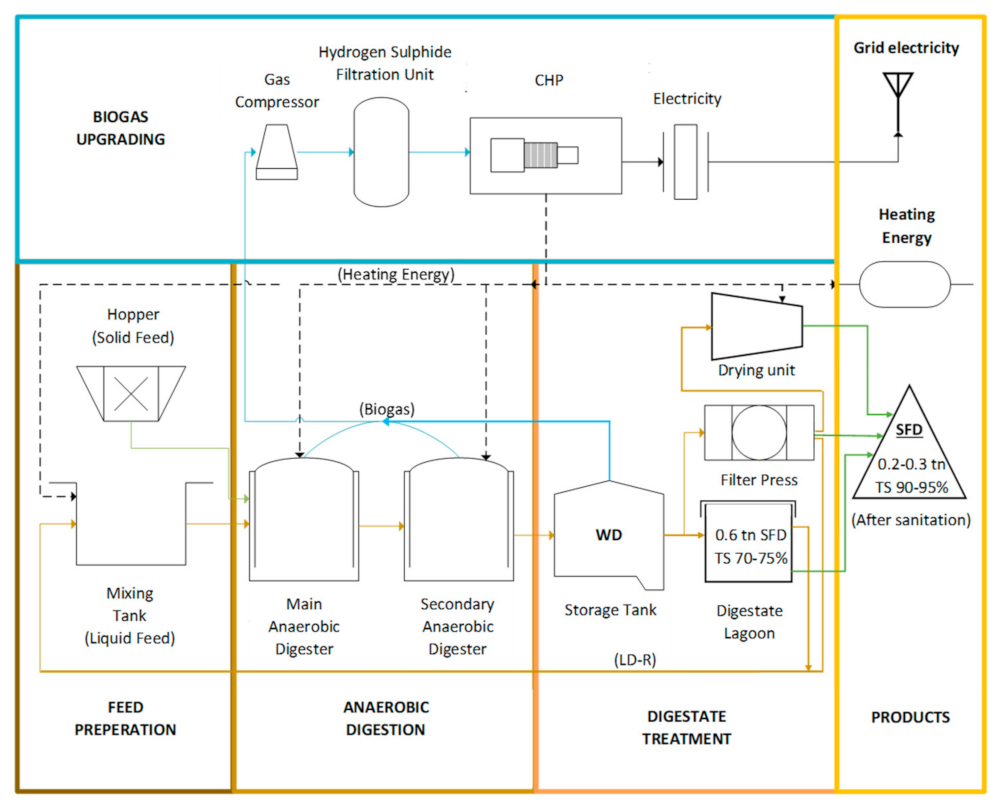
The process flowchart of the biogas plant is presented in Figure 1, starting from the feed preparation stage where Solid Feed is mixed and then driven into the anaerobic digester along with the liquid feed from a mixing tank. The feedstock of the digesters is planned ahead, taking into account the biogas methane content and the digester’s biological equilibrium. The solid feedstock is mostly silage and other agricultural waste, and the liquid most common feedstock is cow and chicken manure, olive mill waste, meat processing waste, etc. Fresh feedstock enters the primary anaerobic digester (AD-1) while part of the previously digested material is led to the secondary one (AD-2) to remain there for a longer time, assisting the process of anaerobic digestion and maintaining the digester’s health. Part of the AD-2’s content is removed, leading to a storage tank and the material is then called Whole Digestate (WD). Sedimentation of Solid Digestate happens due to gravity and the supernatant Liquid Digestate (LD) is used as a recirculation liquid feed (LD-R) to the AD-1. The sediment of the Digestate Lagoon (Solid Digestate with TS 70–75%) is transferred to the screw filter press separator, followed by a drying unit, and after sanitation at 70 °C for 1 h, the Solid Fibrous Digestate (SFD) is attained, with TS 90–95%. Biogas produced in AD-1, AD-2 and the storage tank of the WD is led to a gas compressor, followed by a hydrogen sulfide filtration unit before entering the CHP engine. Electrical energy is turned into electricity and enters the national grid, while the heating energy produced on site is sufficient for the heating demands of the biogas plant (preheating the feed, heating the reactors, drying and sanitating the solid digestate).
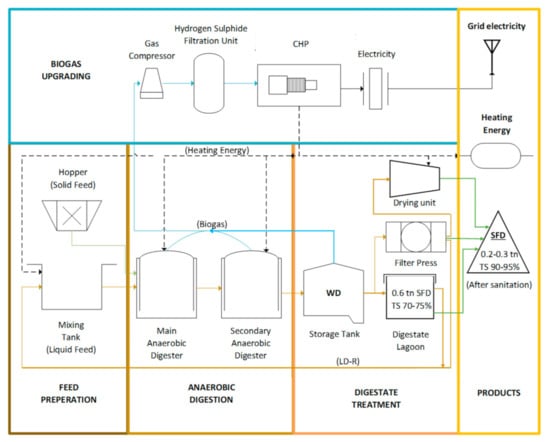
Figure 1.
Biogas Plant Process Flowchart (SFD: solid fibrous digestate, WD: whole digestate, LD-R: liquid digestate recirculation, TS: total solids, CHP: combined heat and power).
In order to free the SFD precursor materials from their inorganic content, wash steps with 1% HNO3 solution were included (50 g SFD in 500 mL 1% HNO3 solution, at room temperature for 24 h). Thus, the inorganic matter was reduced from 12.9 wt.% to 4.6 wt.%, as shown in Table 3, whereas the carbon content increased from 42.2% for the SFD to 48.6% for the SFD washed with 1% HNO3 solution (15% increase). The resulting washed materials are further abbreviated as SFD-W. After washing, the SFD-W was dried at 105 °C for 24 h.

Table 3.
Moisture, ash content and elemental analysis of SFD and SFD-W.
2.2. Equipment and Procedure for SFD Pyrolysis and BC Activation
2.2.1. Pyrolysis of SFD and SFD-W
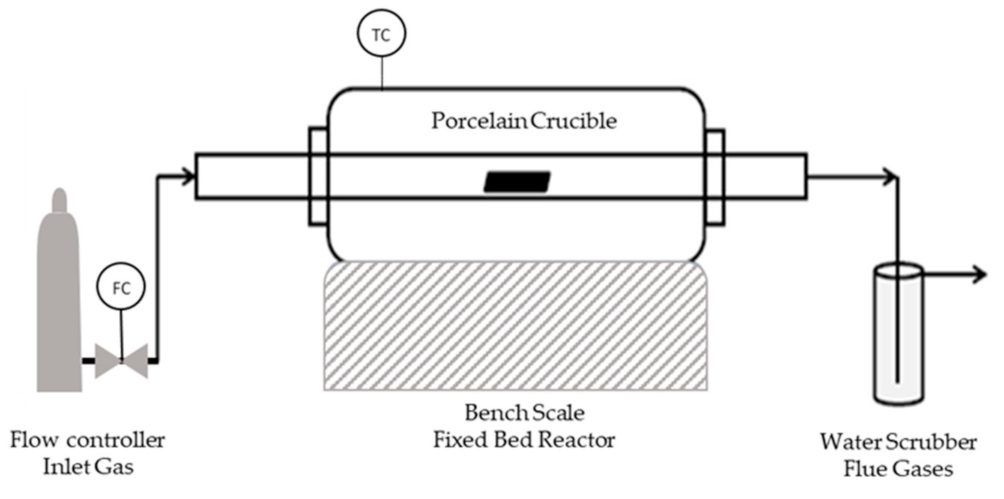
The carbonization of the SFD and SFD-W was carried out via slow pyrolysis in a bench-scale fixed bed reactor (Figure 2), investigating the temperature parameter along with the residence time of the pyrolysis. A sample size of 5 g was carbonized at different temperatures (600–800 °C) and duration (30–120 min) (Table A1).
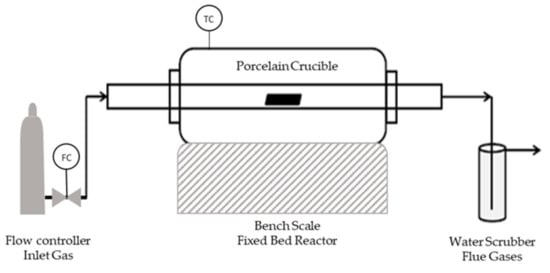
Figure 2.
Pyrolysis Reactor.
Achieving a higher pyrolysis yield was of high priority in this study since the overall yield of the whole process is relatively low. Thus, as long as the surface and pore characteristics are good, the pyrolysis parameters that favor the BC yield are preferred.
BC materials derived from the carbonization of the untreated SFD (BC-SFD) had a higher ash content (more than 33.60%), while the pretreated BC-SFD-W materials had less than half the ash content (up to 18.10%) (Table A2 and Table A3). This indicates that the removal of part of the inorganic compounds (~72%) from the precursor material with the HNO3 pretreatment is a highly important stage that must be common to any type of activated carbon development, as it allows the carbonization process to expand the carbon matrix.
2.2.2. Activation of BC-SFD-W
BC activation was carried out via slow pyrolysis in a bench-scale fixed-bed reactor. Physical activation was carried out (a) with H2O (1 mL/min) at different temperatures (700–900 °C) and activation duration (15–90 min) (Table 4), with the water vapor flow controlled via a Bronkhorst CEM-System (Controlled Evaporation and Mixing); (b) with CO2 (50 mL/min) at 850 °C and activation duration of 150 min. For chemical activation with KOH, BC was mixed with solutions of KOH of different concentrations to achieve KOH/BC ratios of 1:4 (Table 5). After evaporation of the H2O, the mixture was activated at 600–800 °C for 30–120 min.

Table 4.
Activated carbon yield (wt.%, dry matter feed), with physical activation (H2O) at various activation temperatures, steam flow and activation time.

Table 5.
Activated carbon yield (wt.%, dry matter feed), with chemical activation (KOH) at various activation temperatures, reagent KOH to BC ratio and activation time.
2.3. Test Rig for Biogas Breakthrough Experiments
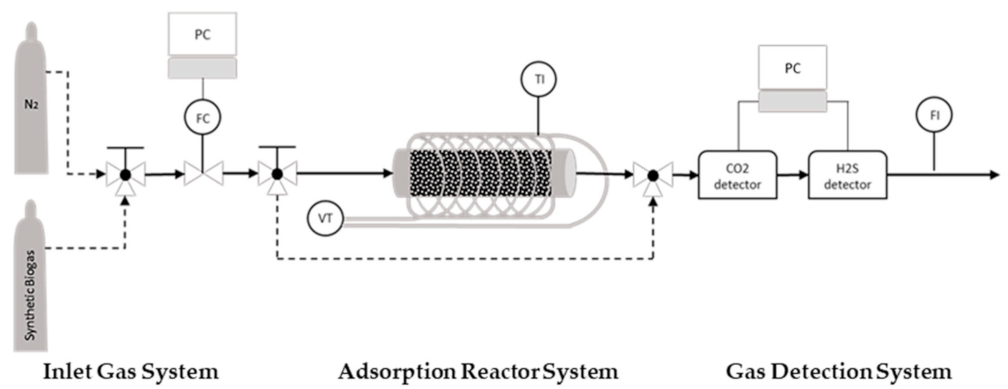
The experimental system for obtaining the breakthrough curves consists of 3 parts; the inlet gas manifold, the fixed bed adsorber and a gas detection system (Figure 3). There are 2 bottles with gases, one with nitrogen and one with synthetic biogas (57% CH4, 42% CO2, 0.5% O2 and 500 ppm H2S). The inlet gas flow rates are controlled by a mass flow controller (MFC) and a three-way valve, which is installed upstream of the MFC to allow the selection of either a nitrogen or biogas mixture. The adsorber is a horizontal stainless steel tubular fixed bed, 7.9 cm long and 4.8 mm in the inner diameter. To initiate the experimental procedure, the activated carbon sample is positioned inside the adsorber tube, which is wall-heated by a heating mantle powered by a Variac Variable AC transformer. A temperature sensor is used to observe the temperature. Before each breakthrough experiment, the samples were regenerated under a nitrogen stream and a temperature of 250 °C. After lowering the temperature to the experimental value, the inlet stream is switched to by-pass, and the nitrogen gas is then switched to the biogas stream. As soon as the concentration of H2S is stabilized to the expected level (500 ppm), the inlet biogas stream is switched and allowed to pass through the adsorber containing the activated carbon sample. Thus, the adsorption phenomena happen and the breakthrough curves of H2S and CO2 can be logged via the software of the respective gas sensors. The concentrations of H2S and CO2 in the biogas stream escaping the adsorber column are quantitatively monitored using the SGX ECVW EK3 Electrochemical and Pellistor Gas Sensor Evaluation Kit and Rapidox Logger 7100 multigas analyzer, respectively. The experiments are conducted at an adsorption temperature of 25–70 °C, and the inlet gas flow is set to 50 mL/min. Measuring the real flow of the outlet biogas mixture downstream of the adsorber and taking into account the dimensional characteristics of the tubular reactor and the consistency of the synthetic biogas mixture, the pressure inside the reactor is calculated at an average of 0.044 millibars and the overflow rate at 220 cm/min (3.66 cm/s) on average. The adsorption temperature affects the kinematic viscosities of the gases in the mixture; thus, the flow characteristics vary, although slightly, for every single experiment (Table 6).
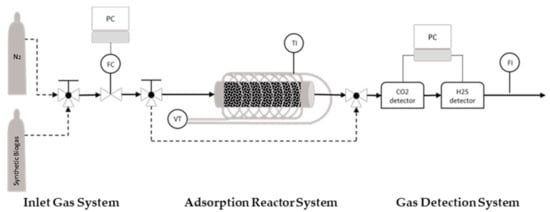
Figure 3.
Adsorption Reactor; FC: Flow Controller; VT: Variac Transformer; TI: Temperature Indicator; FI: Flow Indicator.

Table 6.
Experimental conditions of the biogas breakthrough tests.
3. Results and Discussion
3.1. Biochar Yield
The BC yield of both untreated solid fibrous digestate and that pretreated with 1% HNO3, (Table A1) implies that pyrolysis at 600 °C achieves the highest yield compared to higher temperatures, while residence pyrolysis time seems to not affect the BC yield. It should be noted, however, that a shorter duration of pyrolysis has given the optimal yield of BC, e.g., 30 and 60 min for the BC produced from the non-washed SFD and the one washed with 1% HNO3 solution prior to the pyrolysis, respectively (Table 3). Since a decreased pyrolysis temperature and shorter carbonization time favor BC yield, the final preferred product is named BC-600 °C-30 min-SFD-W, which stands for BC produced from Pyrolysis at 600 °C for 30 min using the Solid Fibrous Digestate from the Biogas Lagada SA plant as a precursor material, pretreated with 1% HNO3 solution.
3.2. Elemental Analysis Results and Ash Content of BC
Elemental analysis shows that the carbon content is increased in the BC compared to its precursor material (Table A2 and Table A3). By increasing the pyrolysis temperature, the carbon content of the BC is increased while decreasing its hydrogen content. Likewise, though, this happens when extending the residence time of the pyrolysis. At low temperatures up to 200 °C the amount of hydrogen drops due to a decrease in the moisture content in the BC, which is also indicated by the reduction of the oxygen content. Up to 600 °C hydrogen content drops rapidly in the step of framework formation, presumably due to the completion of alkyl fragmentation. At higher temperatures up to 800 °C the nitrogen content drops and the same holds for hydrogen content. The ongoing lowering of the amount of nitrogen shows that further densification occurs, which mainly involves the elimination of nitrogen-containing side products. Furthermore, the processes occurring at 800 °C can be described as an ongoing condensation of the aromatic systems upon further elimination of elemental hydrogen and nitrogen.
3.3. Pore Structural Characteristics of the Developed BCs and ACs
The surface and pore structural properties of the resulting BCs and activated carbons are determined by N2 sorption-desorption at −196 °C using the Autosorb-1 MP (Kr-upgrade) gas sorption analyzer of Quantachrome. Before each measurement, all samples are outgassed at a high vacuum and a temperature of 250 °C for 24 h. As indicated from the results included in Table A4 and Table A5, BCs produced from SFD previously being washed with HNO3 1% have a higher BET (in the range of 300–350 m2/g) than those produced from non-washed SFD (<50 m2/g). Washing the SFD with HNO3 1% can increase the BET by at least 7 times (and up to 19 times) and the Total Pore Volume by at least 3 times (and up to 7 times), respectively. Pyrolysis temperature and residence time affect the material’s surface and pore characteristics; a higher pyrolysis temperature causes a higher BET and total pore volume, while longer pyrolysis residence time seems to not significantly affect porosity and surface characteristics.
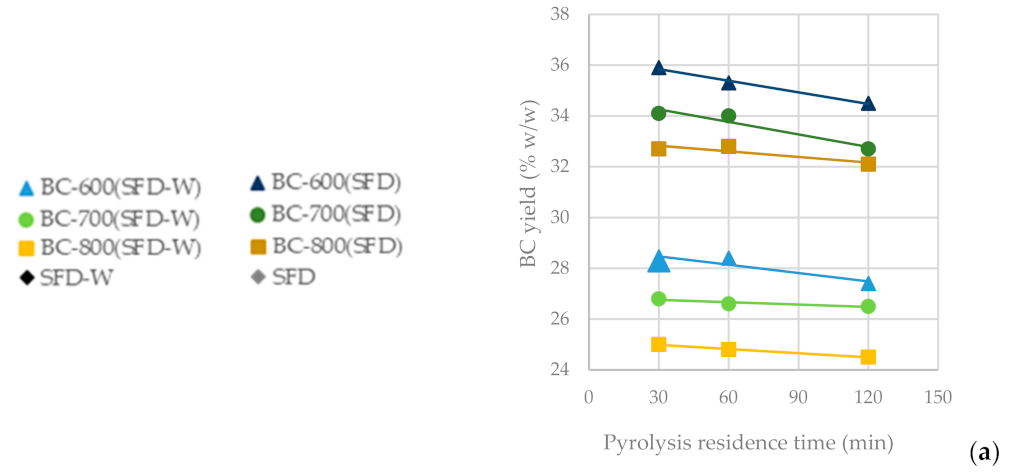
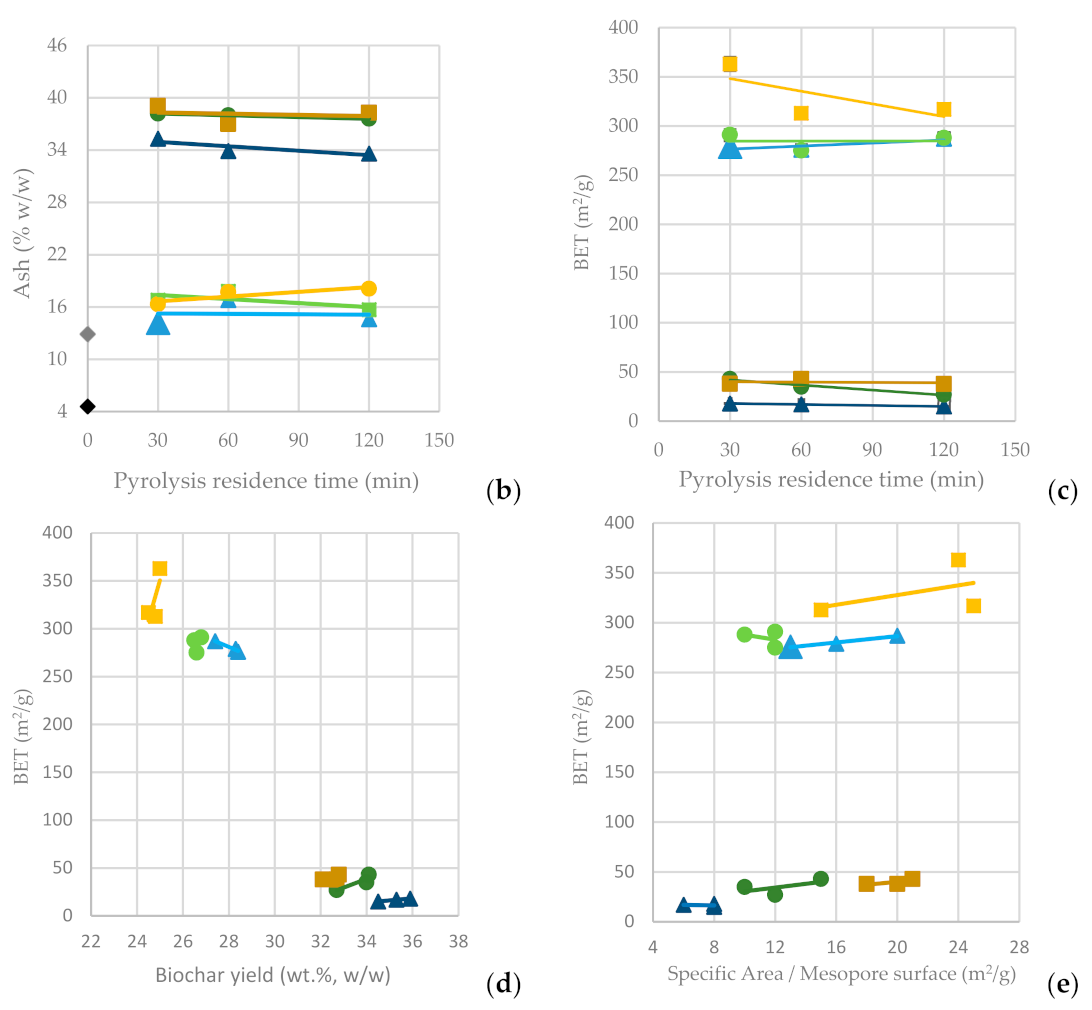
In Figure 4a, it is observed that as the pyrolysis temperature increases from 600 °C to 800 °C, the BC yield drops significantly (BC-SFD 32.10%; BC-SFD-W 24.50%), whereas the residence time seems not to significantly affect it. In Figure 4b, it can be observed that the ash content is affected inversely to the BC yield, meaning that the highest ash content (BC-SFD 39.10%; BC-SFD-W 18.10%) is achieved for the highest pyrolysis temperature (800 °C) and the lowest for the lowest pyrolysis temperature (600 °C), respectively, while the residence time does not significantly affect the ash content either. Aiming to obtain the most BC possible from the pyrolysis and considering (Figure 4c) that the BET is independent of whether the pyrolysis takes place at 600 or 700 °C, it becomes clear (Figure 4d,e) that a pyrolysis temperature of 600 °C serves both the purpose of achieving a high BC yield and a high BET, while the mesoporosity is also relatively high.
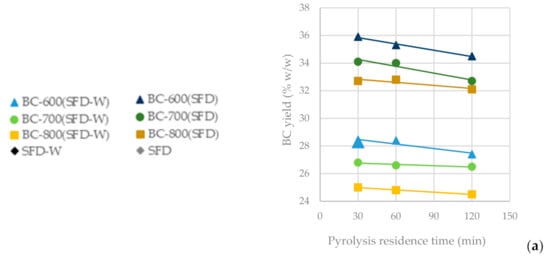
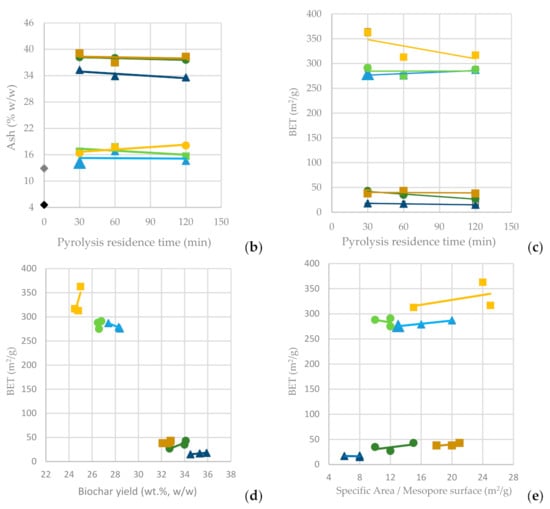
Figure 4.
(a) BC yield vs. pyrolysis residence time (b) Ash content vs. pyrolysis residence time (c) BET surface area vs. pyrolysis residence time (d) BET surface area vs. BC yield (e) BET surface area vs. specific area/mesopore surface area from the pyrolysis of SFD and SFD-W at different pyrolysis conditions.
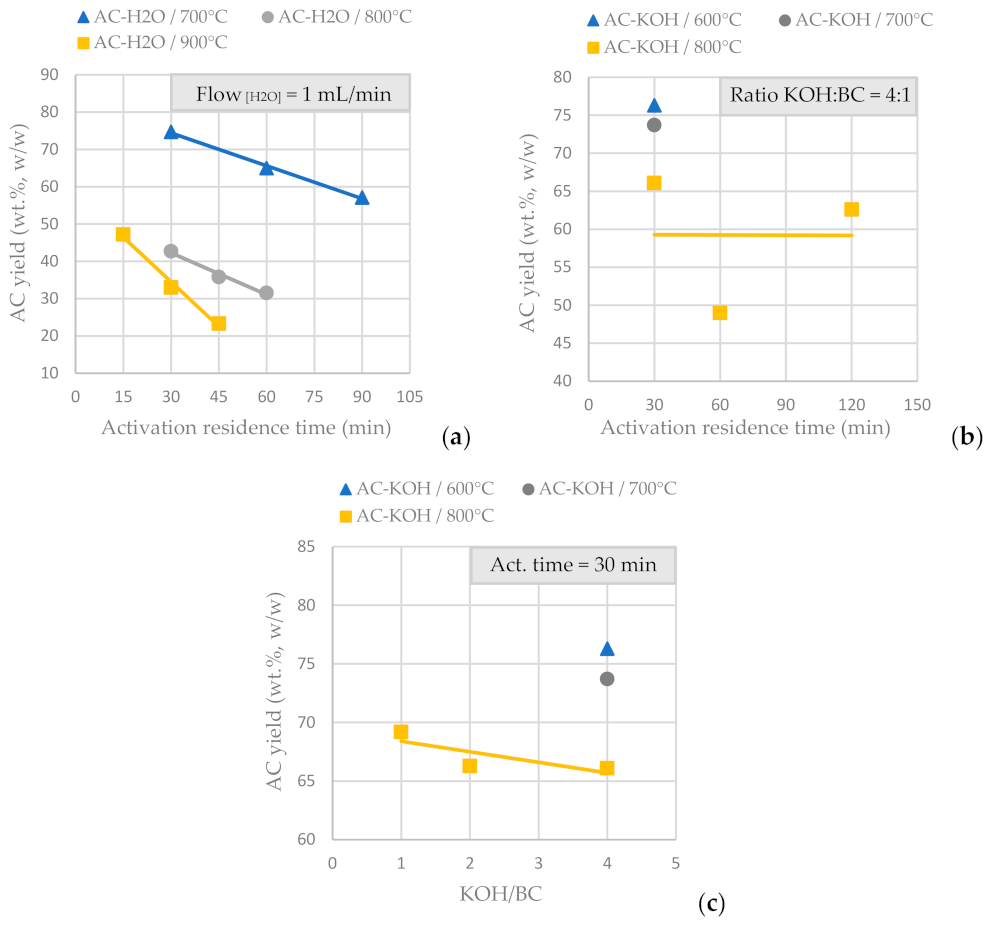
BCs obtained by SFD-W were transformed into activated carbon using physical activation with H2O (Table 4) and CO2 (50 mL/min at 850 °C and an activation duration of 150 min) and chemical activation with KOH (Table 5). Figure 5a presents the AC yield vs. the activation residence time for physical activation with fixed steam flow at 1mL/min and in Figure 5b presents the AC yield vs. the activation time for chemical activation with KOH at a fixed ratio of 4:1 over BC. It is concluded that for physical activation, prolonged residence time eliminates the AC yield (for a fixed steam flow), while for chemical activation, the AC yield is not affected significantly the longer the activation process lasts (for a fixed KOH/BC ratio). Figure 5c shows the AC yield vs. the KOH molar ratio for a fixed activation residence time; thus, it is concluded that the KOH molar ratio (from 1:1 to 4:1) doesn’t affect significantly the amount of AC produced from the functionalization process (for a fixed activation residence time). Temperature is the parameter that affects AC yield in both physical and chemical activation.
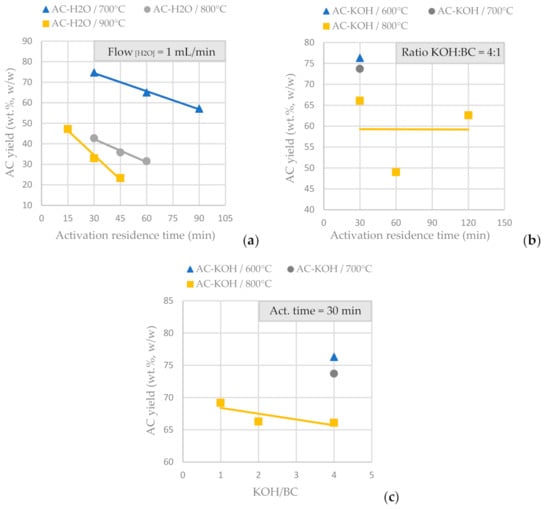
Figure 5.
(a) AC yield vs. activation residence time for physical activation with steam, for a fixed H2O flow of 1mL/min at different activation temperatures, of the BC derived from the pyrolysis of SFD-W at 600 °C for 30 min. (b) AC yield vs. activation residence time for chemical activation with KOH, for a fixed KOH:BC ratio 4:1 at different activation temperatures, of the BC derived from the pyrolysis of SFD-W at 600 °C for 30 min (c) AC yield vs. KOH:BC ratio for chemical activation with KOH, for a fixed activation residence time of 30 min at different activation temperatures, of the BC derived from the pyrolysis of SFD-W at 600 °C for 30 min.
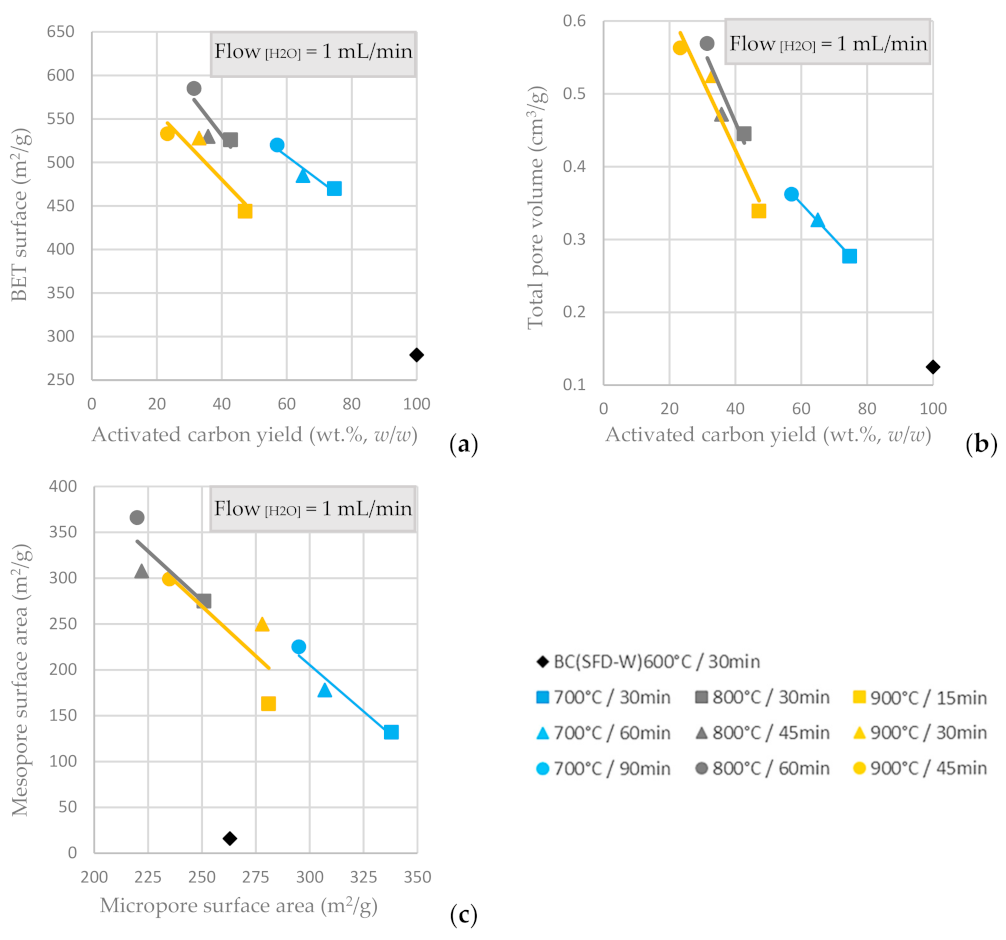
In Figure 6a,b, the pore structural characteristics (BET and total pore volume) of the ACs produced via physical activation with steam are presented relative to the AC yield and the respective residence time. It is indicated that the BET surface area is enhanced for prolonged activation time, but an activation temperature higher than 800 °C does not offer any benefit to the BET and total pore volume values. The highest BET surface was achieved for an activation temperature of 800 °C and residence time of 60 min. Likewise, the total pore volume is optimized for the same conditions, concerning the physical activation with steam flow of 1 mL/min. Mesopore surface area is inversely proportional to the micropore surface area, as shown in Figure 6c and the highest mesopore surface area is achieved at activation temperature 800 °C and residence time of 60 min, while the highest micropore surface area is achieved at activation temperature 700 °C and residence time of 30 min (the lowest activation temperature and residence time tested). The steam-activated ACs had both micropores and mesopores, the amount of which could be tuned by adjusting the temperature and the duration of activation; a higher temperature and/or longer duration resulted in a decrease of the microporosity and an increase of mesoporosity.
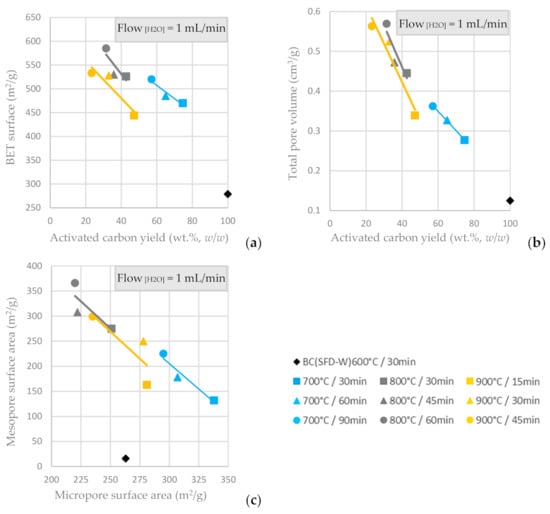
Figure 6.
(a) BET surface area vs. AC yield wt.% w/w (b) Total pore volume vs. AC yield wt.% w/w of input BC. (c) Mesopore surface area vs. micropore surface area from the physical activation of BC-600 °C/30 min derived from the pyrolysis of SFD-W at different activation conditions.
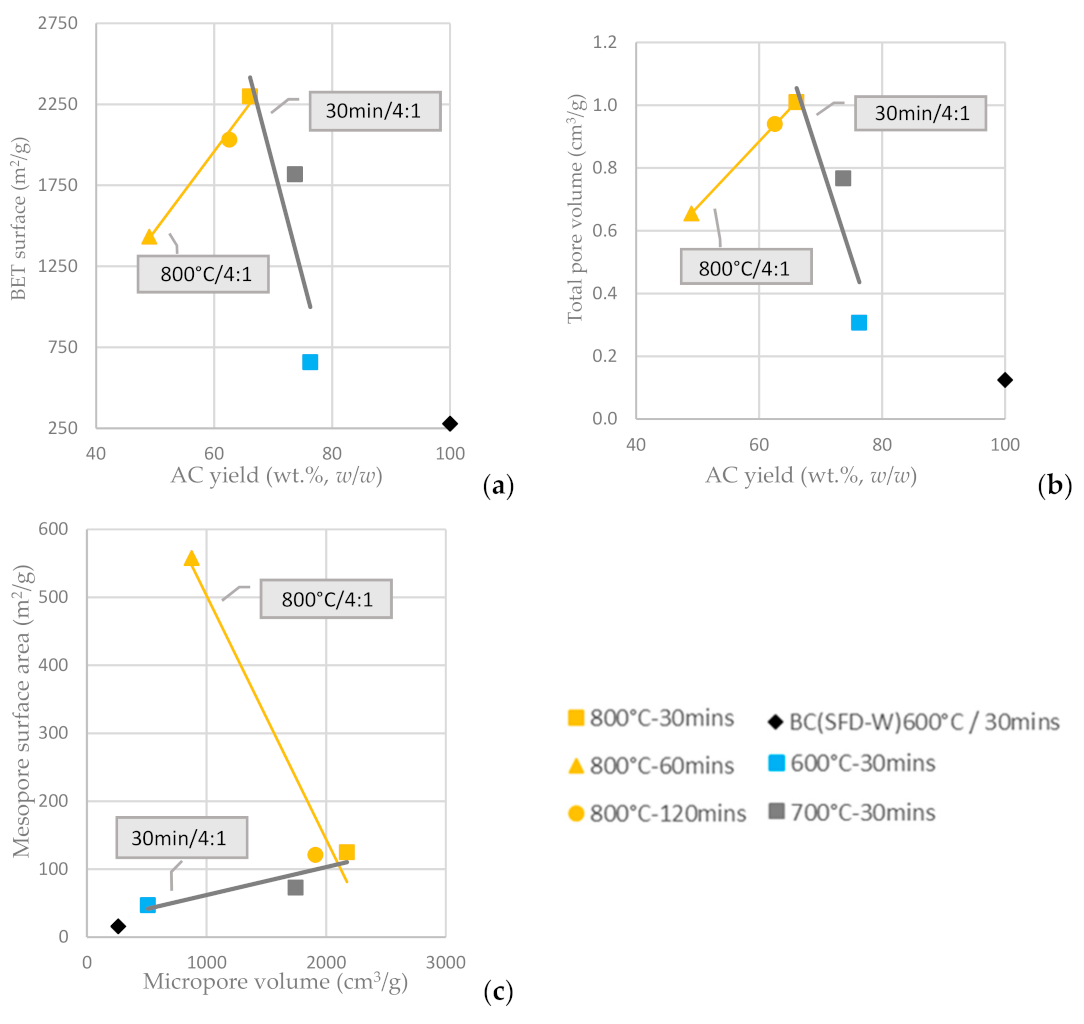
In Figure 7a, it is depicted that the BET surface area of the KOH-activated ACs (KOH:BC ratio of 4:1) is enhanced for longer residence time and is proportionally increased as long as AC yield is increased. On the other hand, for a fixed residence time and a fixed KOH:AC ratio, the BET surface area is increased as the activation temperature increases from 600 °C to 800 °C and the AC yield attenuates. The total pore volume of the KOH-activated ACs is affected in a similar way (Figure 7b). The mesopore surface area is, again, inversely proportional to the micropore surface area, as shown in Figure 7c and the highest mesopore surface area is achieved at activation temperature 800 °C and residence time of 60 min, while the highest micropore surface area is achieved at activation temperature 800 °C and residence time of 30 min (both at the highest activation temperature tested but different residence times). Furthermore, the chemically activated with KOH carbons had both micropores and mesopores, the amount of which could be also tuned by adjusting the temperature and the duration of activation; higher temperature resulted in an increase of both mesoporosity and microporosity, while the optimum residence time has been defined to be no more than 60 min.
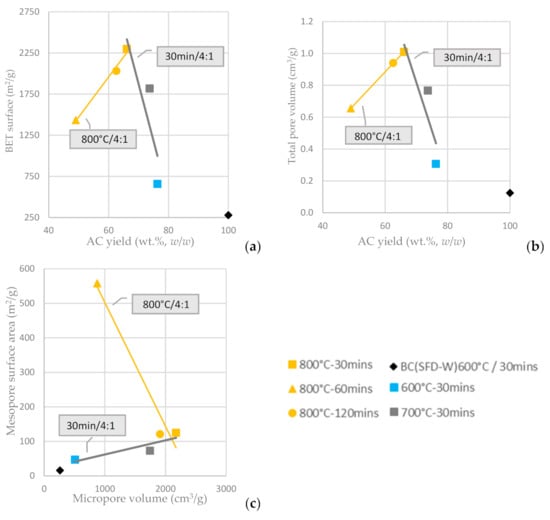
Figure 7.
(a) BET surface area vs. AC yield wt.% w/w (b) Total pore volume vs. AC yield wt.% w/w of input BC (c) Mesopore surface area vs. micropore surface area from the physical activation of BC-600 °C/30 min derived from the pyrolysis of SFD-W at different activation conditions.
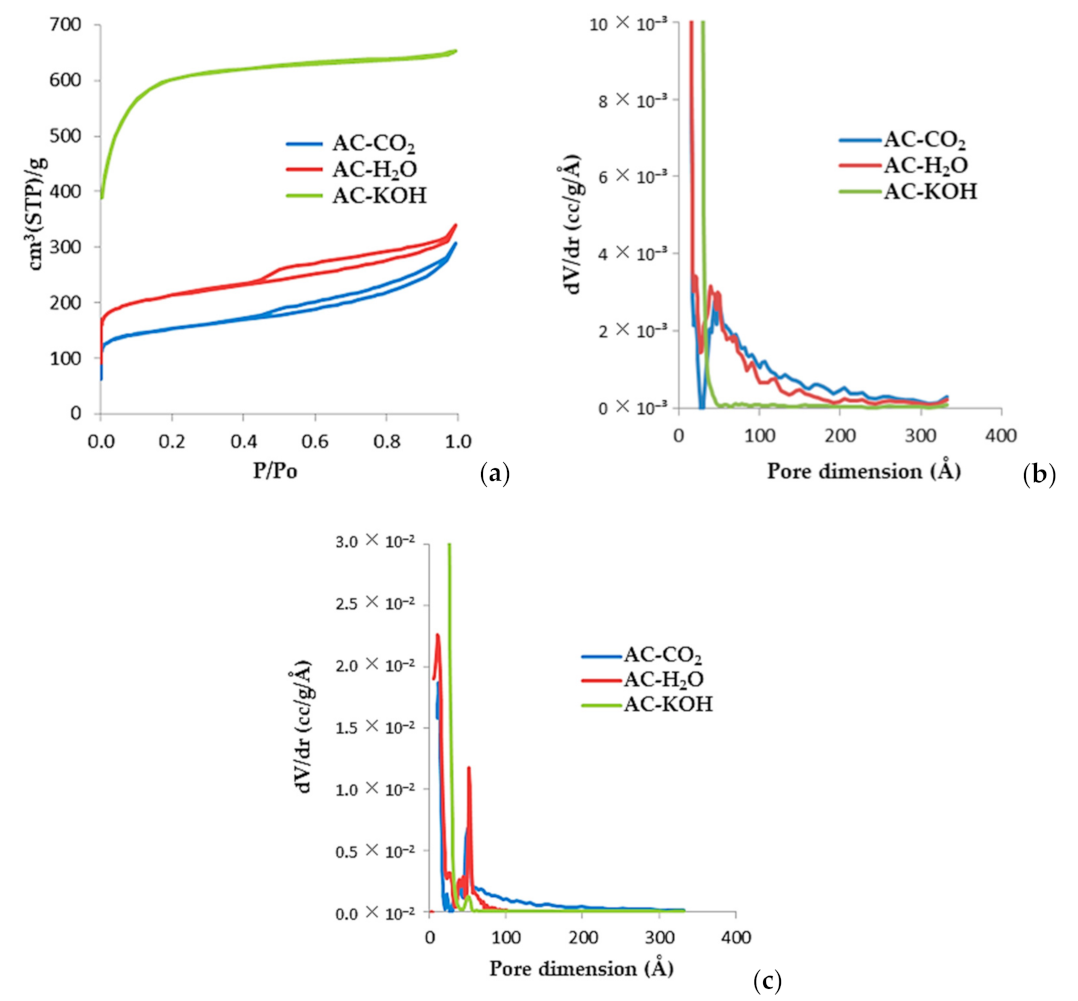
In Figure 8, we present the N2 (77K) adsorption/desorption isotherms and the respective pore size distribution of three selected samples, AC-H2O, AC-KOH, and AC-CO2). AC-H2O was produced with H2O activation for 60 min at 800 °C and a water vapor flow rate of 1 mL/min. Sample AC-KOH was produced via 30 min of chemical activation at a KOH molar ratio of 4:1 and temperature of 800 °C, while AC-CO2 was produced by physical activation at 850 °C for 150 min under a constant CO2 flow rate of 50 mL/min. The pore size distributions are derived with the QSDFT method for carbon and cylindrical pores, which is applied to both the adsorption and desorption branches of the isotherm. The micropore and external surfaces are presented in Table 7 and Table A4, Table A5, Table A6, Table A7 are derived from the analysis of the corresponding αs-plots using the N2 (77K) adsorption isotherm of a non-porous carbon as the reference. From the shape of the adsorption isotherms and the respective pore size distributions, it is concluded that physical activation of SFD-derived BC yields activated carbons with an extended mesopore structure. These carbons actually have a bimodal pore size distribution comprising micropores of the order of 12 Å and mesopores of the order of 50 Å. On the contrary, chemical activation with KOH leads to an almost purely microporous material with astonishing high surface area (2272 m2/g) and enhanced micropore volume of about 0.9 mL/g.
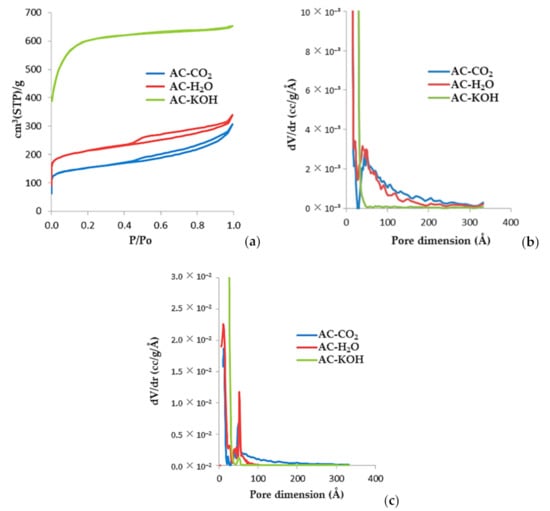
Figure 8.
(a) N2 (77K) adsorption isotherms of porous carbons activated using various methods of physical and chemical activation. (b) QSDFT-derived pore size distributions obtained from the adsorption branch of the isotherms. (c) QSDFT-derived pore size distributions obtained from the desorption branch of the isotherms.

Table 7.
Surface characteristics and porosity of activated carbons AC-H2O, AC-KOH and AC-CO2 produced by activation of BC, which has been derived from the pretreated SFD.
3.4. Surface Chemistry and Structural and Morphological Characteristics of ACs
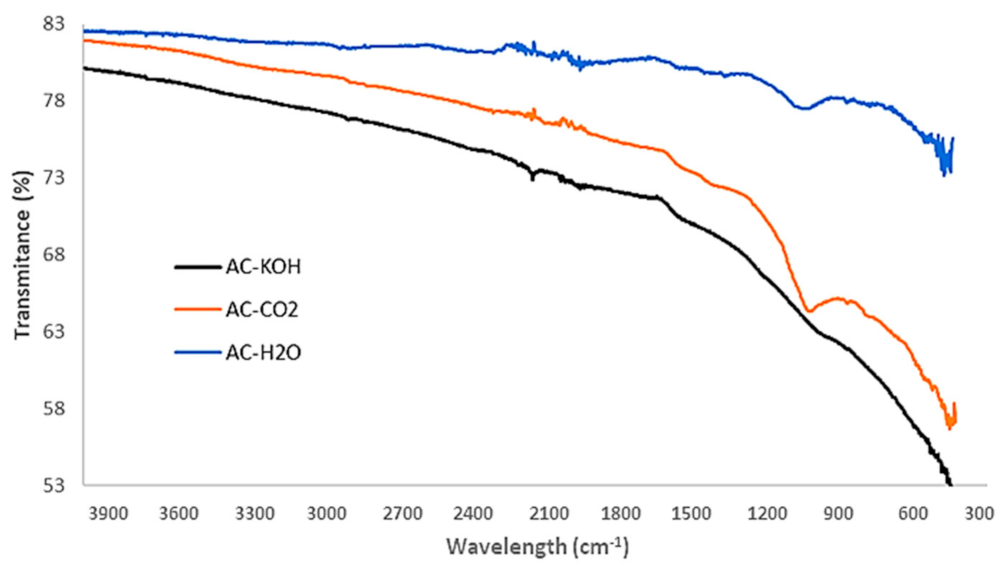
Apart from pore structural characteristics, the surface chemistry of the activated carbons may have a significant effect on their gas adsorption capacity. In particular, the selectivity of CO2 over CH4 and of H2S over CO2 can be defined by the specific interactions of the gases with the functional groups sprawled on the pore surface of the AC samples. Hence, ATR-FTIR analysis is conducted with an Attenuated Total Reflectance (Brucker FTTR Spectrometer Alpha II, which features a monolithic diamond crystal) to detect and determine the several oxygenated functional groups that usually exist on the surface of ACs. The results presented in Figure 9 show that the physical activation methods, apart from being technically easy and sustainable, do not gravely affect the chemical composition of the formed carbons.
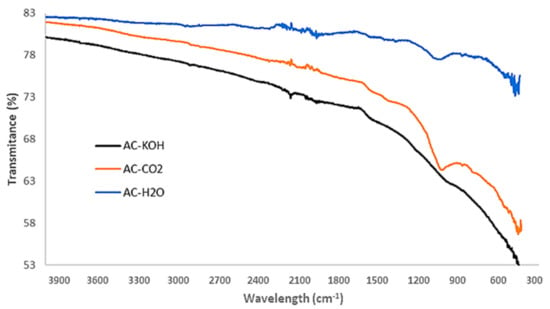
Figure 9.
FTIR spectra of chemically and physically activated carbons.
The peaks corresponding to C-O and C=O stretching vibrations are clearly distinguished in the AC-H2O and AC-CO2 samples whereas they are eliminated in the AC-KOH sample. This is an important result that will be discussed in the following section, in conjunction with the pore structural features of the AC samples and their capacity to selectively adsorb CO2 or H2S.
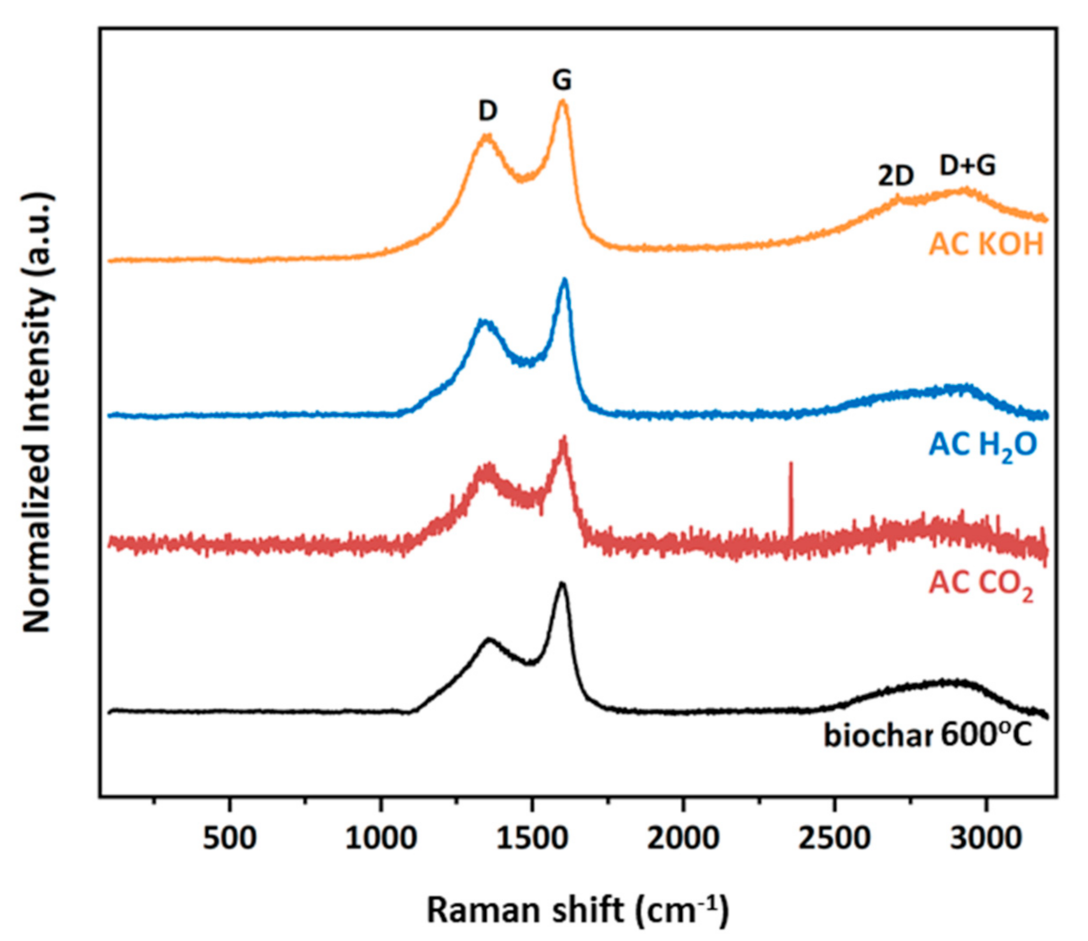
The Raman spectral analysis ranged from 100 to 3200 cm−1 in order to investigate the first- and second-orders of BC and the three derived activated carbons (ACs) samples. As depicted in Figure 10, the characteristic graphitic D and G peaks located at approximately 1375 and 1600 cm−1, respectively, are clearly observed for all studied materials. The D band is associated with a disordered graphitic structure, such as sp3 sites, and is denoted as a breathing mode with A1g symmetry. On the other hand, the G band has E2g symmetry corresponding to the in-plane stretching of sp2-bonded carbon atoms in a hexagonal graphitic ring. Furthermore, at this point, it could be mentioned that the intensity ratio of the D and G bands is a measure of the degree of disorder in a carbon material. For the precursor BC sample, the calculated ID/IG value is 0.616, whereas for the three activated carbons, it is 0.830, 0.727 and 0.827 under CO2, H2O and KOH treatments, respectively. The similar trend of ratio increase for the activated carbons reveals the higher number of structural defects and disorder present compared to the precursor, but in general, for all studied samples, the ID/IG ratio indicates similar obtained graphitic structure with formation of graphitized carbon and defects in lattice. The relatively broad D + G combination mode, due to the amorphous nature of samples in the range of 2800–2920 cm−1 is also presented for all samples. Finally, the 2D band, an overtone of the D band, is only observed in the AC KOH sample located at ~2700 cm−1. It is derived from a second-order Raman scattering process, and it is well-known that it is very sensitive to the stacking order of the graphene sheets. From its asymmetry and large Full Width at Half Maximum (FWHM), it is presumed that this sample has obtained a multi-layer graphitic structure.
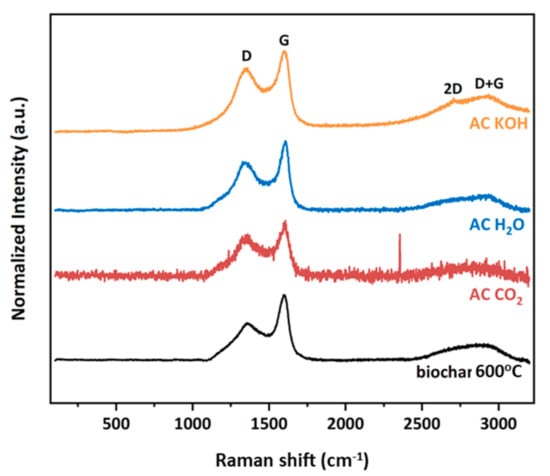
Figure 10.
Raman spectra of all samples.
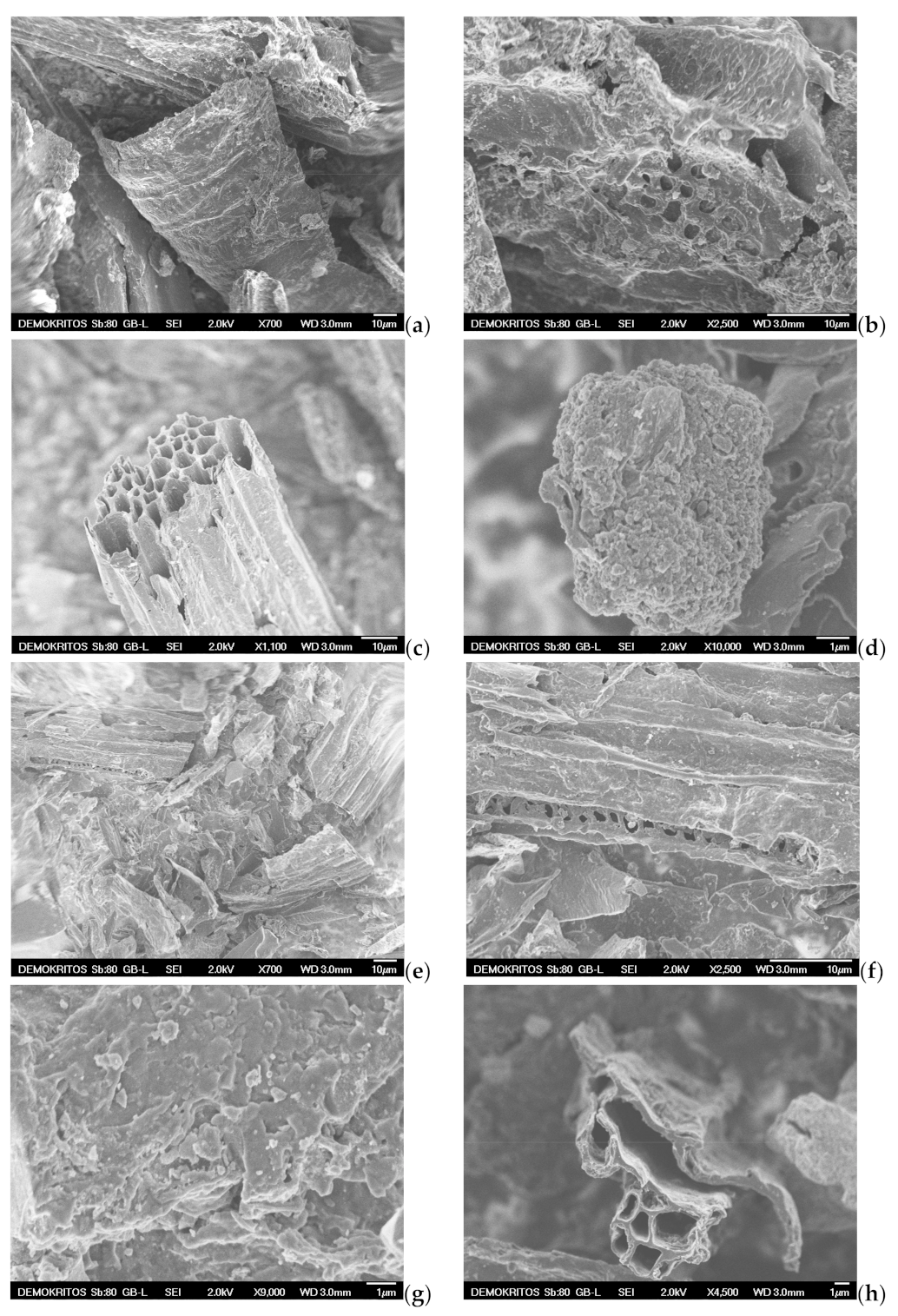
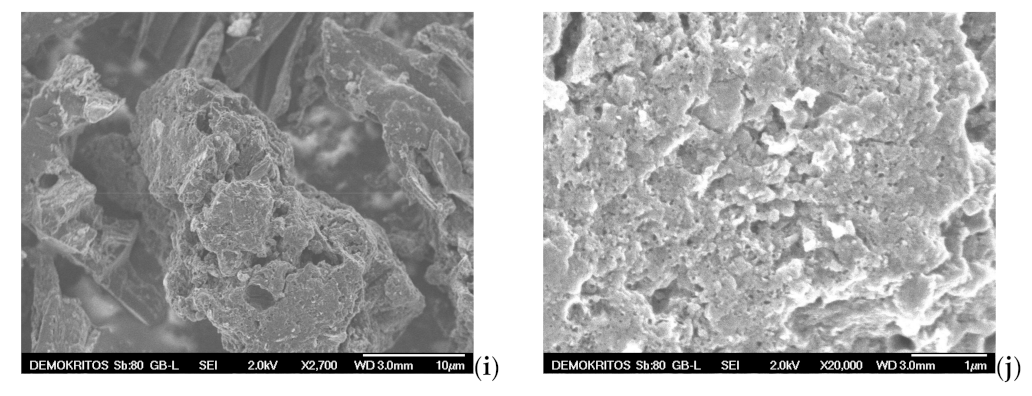
SEM analysis is also applied to examine the surface morphology and possibly define the pore size and shape of both the precursor BC (Figure 11a,b) and the prepared ACs, namely the AC-H2O-800 °C/60 min/1 mL·min−1, the AC-KOH-800 °C/30 min/4:1 and the AC-CO2-850 °C/150 min/50 mL·min−1 as shown in Figure 11c–j. SEM images magnified by 700 to 1100 times visualize the presence of many honeycomb-shaped large pores (Figure 11c,h) for the ACs that are physically activated with steam and CO2, respectively. Regarding the chemically activated AC, a plate-form surface can be observed (Figure 11e), which is very similar to that of the BC precursor (Figure 11a). At higher magnifications (×2500–2700), well distributed macropores (pore widths of approximately 1.5–2 μm) can be distinguished on the sponge-like surface and plate-form surface of the ACs (Figure 11b,f,i). When the magnification goes beyond ×10,000, mesopores start to become distinguishable. Hence, in the CO2 activated AC, these mesopores are in the range of 10–50 nm (Figure 11j), while in the steam-activated AC (Figure 11d), the mesopores appearing on the sponge-like surface are a bit larger (40–80 nm), and some of them can be classified as small macropores. It is also clear that the population of mesopores on the surface of chemically activated AC is very low (Figure 11g). This indicates that activation with steam and CO2 results in more and well distributed mesopores of various sizes, while the chemical activation with KOH does not show such an intense population of mesopores or a homogeneous size distribution. Given the fact that the BET surface area and the micropore surface of AC-KOH are the highest measured, it is concluded that the microporosity of AC-KOH is readily enhanced (Table 7). However, micropores cannot be distinguished by SEM analysis (Figure 11j). Additionally, it can be seen that activation with CO2 has destroyed the honeycomb-shaped surface of the AC (Figure 11h), which must be caused by the prolonged residence time of activation (150 min).
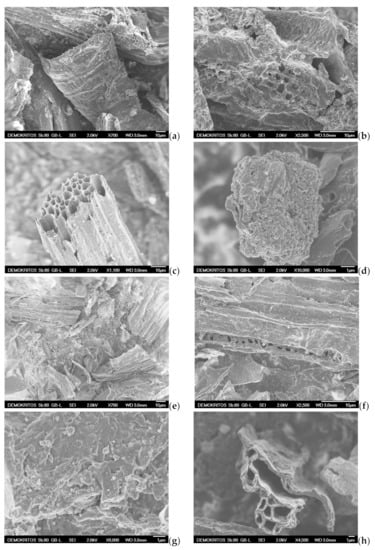
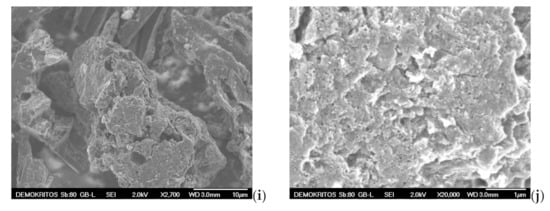
Figure 11.
Scanning electron micrographs (SEM) of (a,b) BC-600 °C/30 min-SFD-W, (c,d) AC-H2O-800 °C/60 min/1 mL·min−1, (e–g) AC-KOH-800 °C/30 min/4:1, (h–j) AC-CO2–850 °C/150 min/50 mL·min−1.
3.5. Biogas Breakthrough Curves. Gas Separation Performance of the Developed ACs
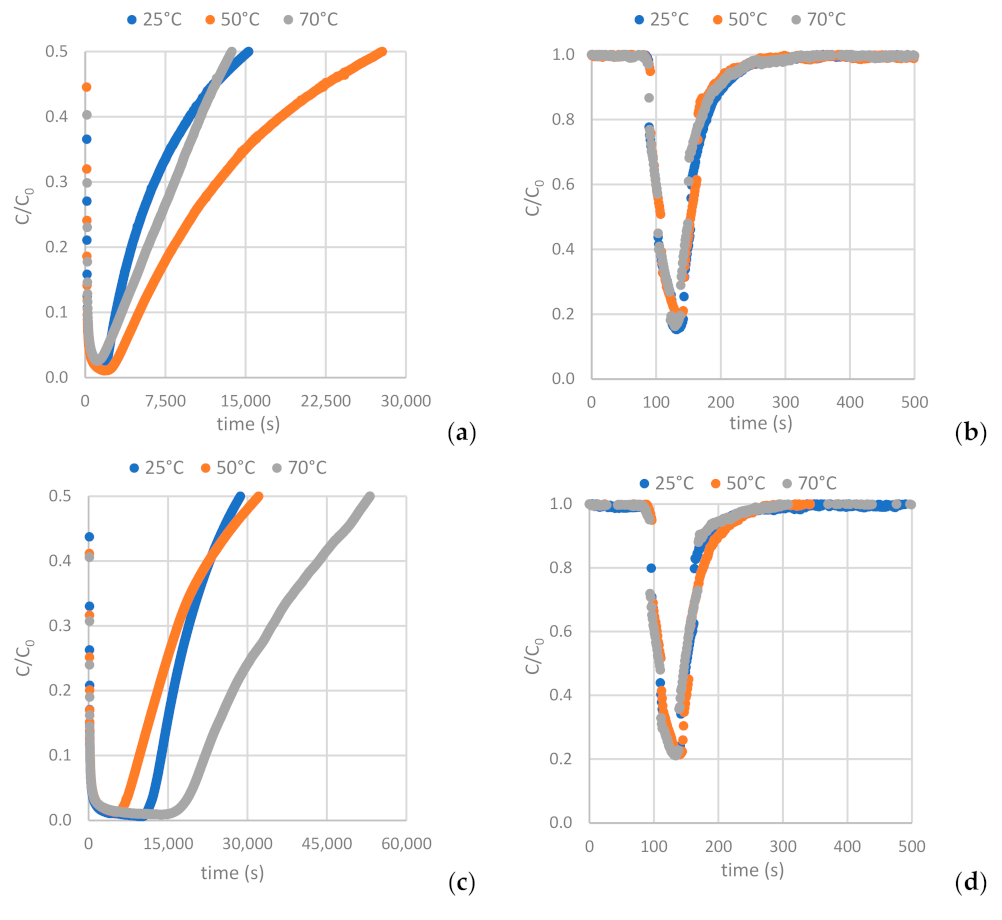
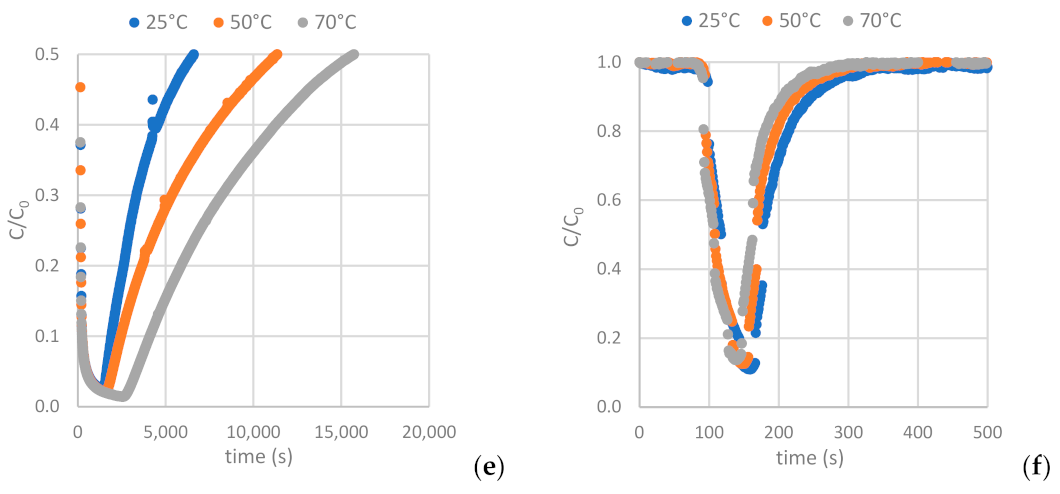
Figure 12 depicts the H2S and CO2 breakthrough curves obtained from samples AC-H2O, AC-CO2 and AC-KOH under the conditions described in Section 2.2.2 (Table 4 and Table 5). Having interpreted the breakthrough curves, the complete set of results is presented in Table 8 and Table 9 (see also Table A8 and Table A9).
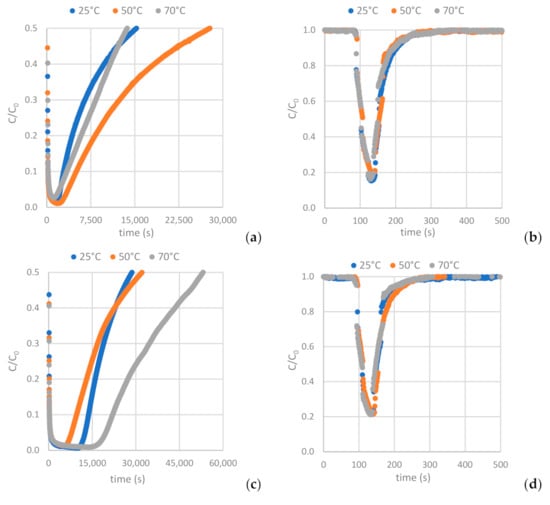
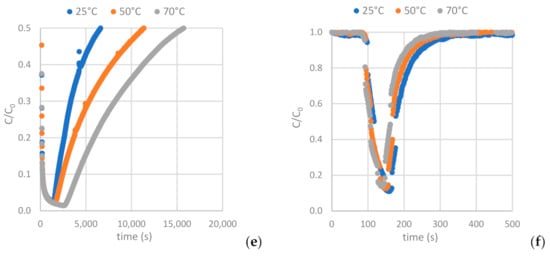
Figure 12.
Breakthrough curves of: (a,b) H2S and CO2 on AC-H2O. (c,d) H2S and CO2 on AC-KOH. (e,f) H2S and CO2 on AC-CO2.

Table 8.
H2S adsorption capacity, molar mass of H2S adsorbed per AC mass, on the activated carbon materials at various temperatures.

Table 9.
CO2 adsorption capacity, molar mass of CO2 adsorbed per AC mass, on the activated carbon materials at various temperatures.
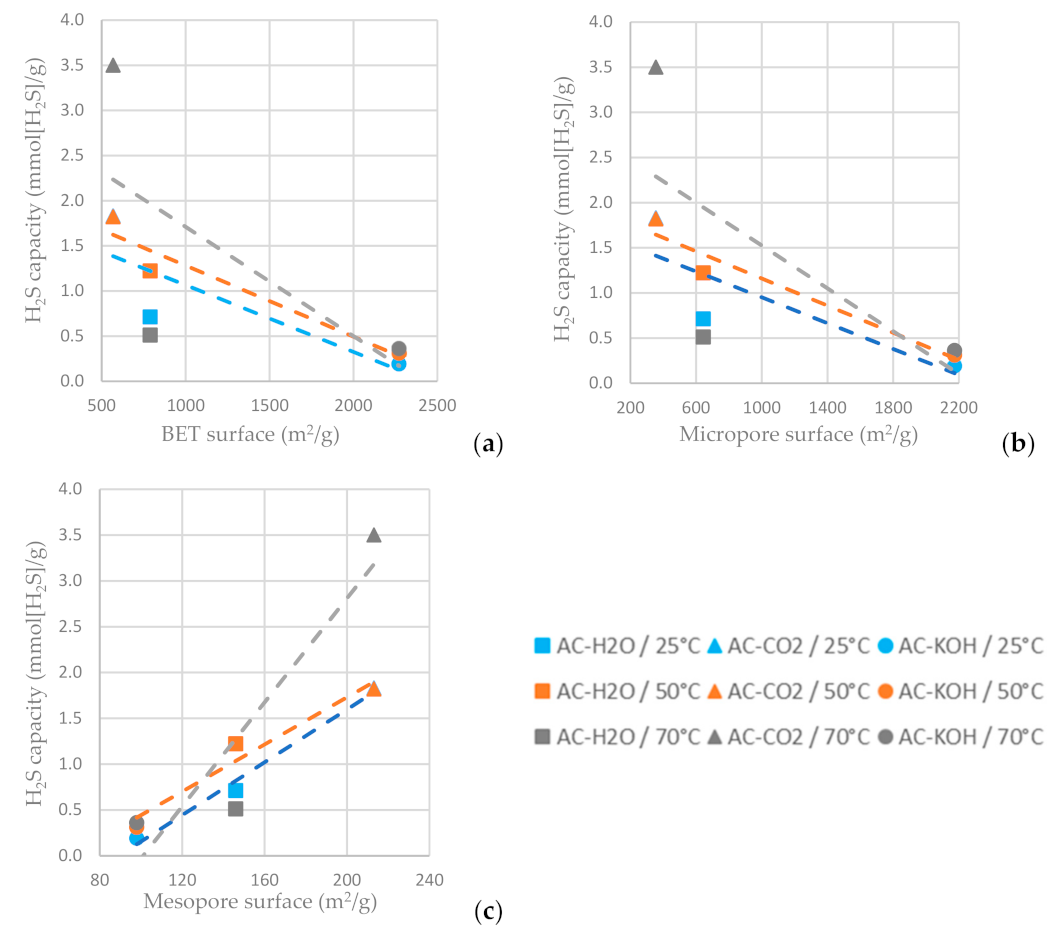
As shown in Figure 13c, the higher mesopore area of the AC favors hydrogen sulfide adsorption, while the increased BET surface area and micropore area decrease the H2S sulfide capacity of the AC (Figure 13a,b). For the ACs tested, the one physically functionalized with CO2 has the highest hydrogen sulfide sorption capacity when the sorption temperature is settled at 70 °C. It is indicated in Figure 13 that a higher adsorption temperature favors H2S adsorption on the ACs.
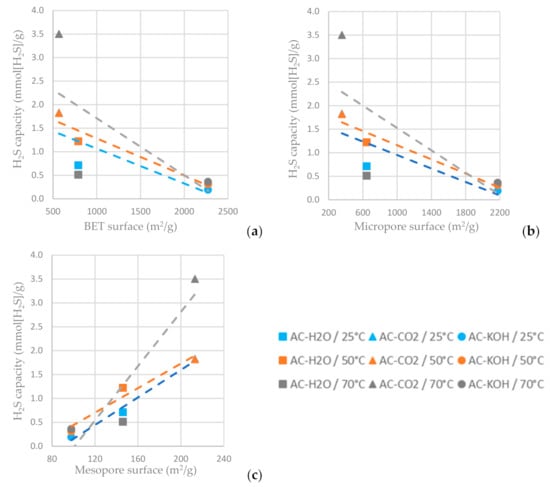
Figure 13.
H2S capacity at time reference when C/C0 = 0.5 vs. (a) BET surface area, (b) micropore surface area and (c) mesopore surface area of the ACs functionalized with H2O, CO2 and CO2 at different temperatures.
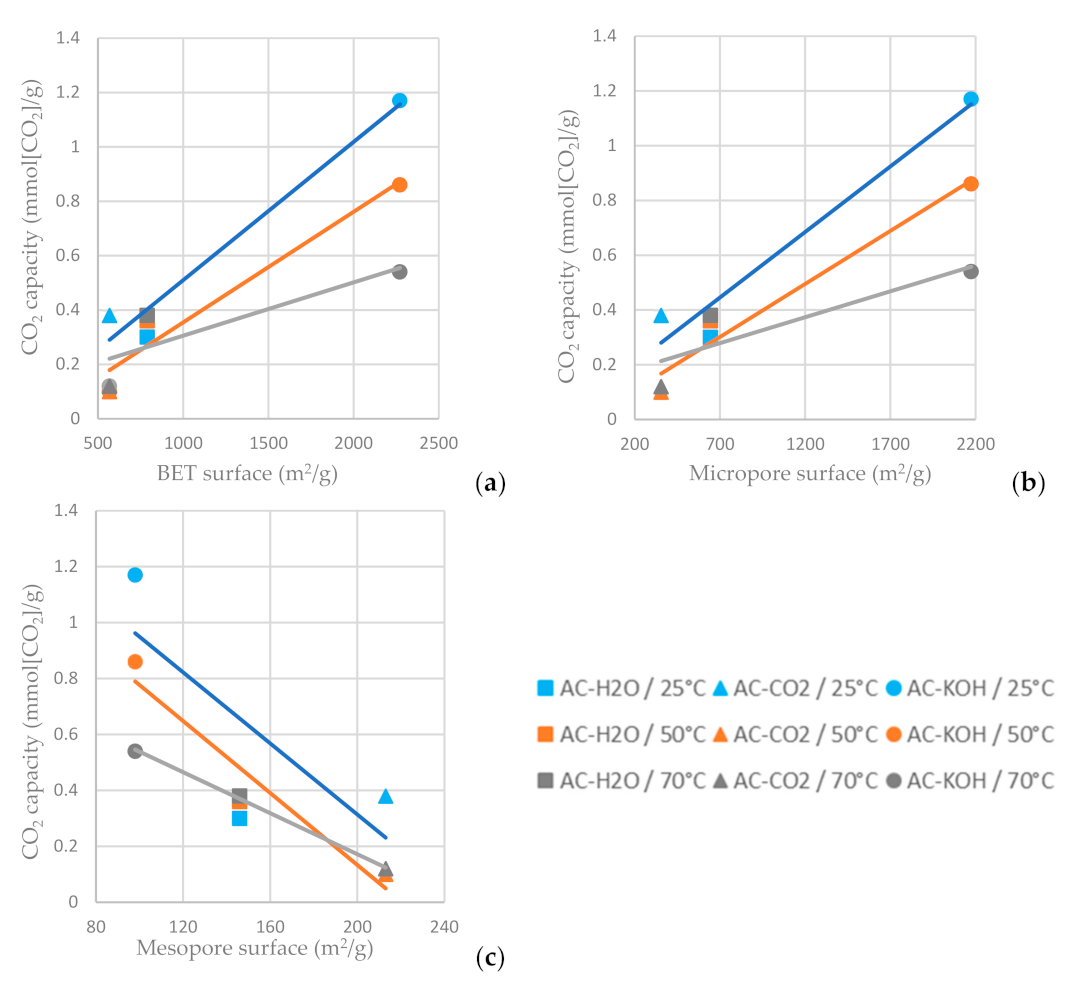
As shown in Figure 14a,b, the higher BET and micropore area of the AC favors carbon dioxide adsorption, while the increased mesopore area decreases the CO2 capacity of the AC (Figure 14c). For the ACs tested, the one chemically functionalized with KOH has the highest carbon dioxide sorption capacity when the sorption temperature is settled at 25 °C. It is indicated in Figure 14 that a lower adsorption temperature favors CO2 adsorption on the ACs.
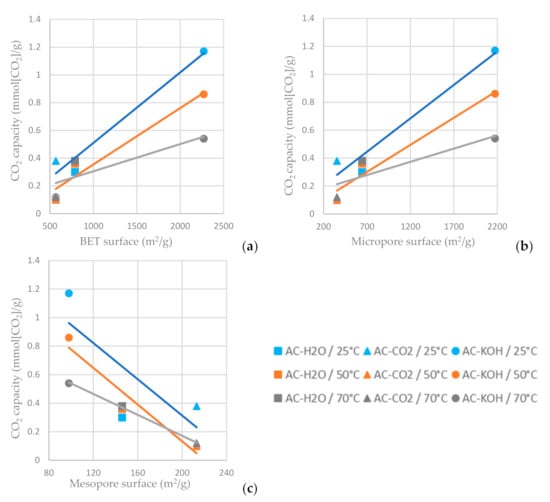
Figure 14.
CO2 capacity at time reference when C/C0 = 0.95 vs. (a) BET surface area, (b) micropore surface area and (c) mesopore surface area of the ACs functionalized with H2O, CO2 and CO2 at different temperatures.
The selectivity of hydrogen sulfide over carbon dioxide on the activated carbons tested has been calculated (Equation (1)) after obtaining the adsorbed amount results for both H2S and CO2 (Table 8 and Table 9). The results are displayed in Table 10, showing that the AC-CO2 is superior to the remaining ACs, resulting in a selectivity of at least 2 times more than the steam-activated ACs and more than 7 times higher than those chemically treated with KOH AC.
where : adsorbed amount of H2S and CO2 and : Partial pressure of H2S and CO2, respectively.

Table 10.
Selectivity of H2S over CO2 on activated carbon materials at various temperatures.
The results clearly show that the carbons derived from the physical activation of BC exhibit higher adsorption capacity for H2S and lower CO2 adsorptivity compared to the sample produced by the chemical activation method with KOH. A distinguishing feature of these samples is that, despite their moderate BET surface and micropore volume, they hold a quite extended mesopore structure with a PSD centered around 50 Å. In addition, contrary to what happens with the AC-KOH sample, the mesopores of the physically activated carbons preserve a high population of surface oxygenated functional groups. Hence, it becomes evident that H2S is strongly hindered from entering the micropore structure of ACs and is mostly adsorbed in the mesopores, also benefiting from its strong interaction with the functional groups. On the other hand, micropores are fully accessible for CO2, and this explains the much higher CO2 adsorptivity of AC-KOH as compared to AC-H2O and AC-CO2. Supporting these statements is that between the two physically activated samples, AC-CO2, despite its lower BET surface, is a more effective adsorbent for H2S because of its more extended mesopore structure (see Table 7) and possibly due to the higher population of oxygenated functional groups (Figure 4). Regarding the effect of temperature, in the case of H2S a maximum adsorptivity is observed systematically for all samples at 50 °C or a continuous increase up to 70 °C, something that does not happen with CO2, which in most of the experiments follows the normal trend of the adsorption exotherm. The maximum in H2S adsorptivity with temperature comes as a result of the counterbalance between adsorption and diffusion. This unveils that due to the strongly acidic character of H2S, its adsorptivity is controlled by diffusion, meaning that when the H2S molecules enter the pore, they reside for a long period on an adsorption site before hopping to the next unsaturated one. Diffusion is an activated process and is fortified as the temperature increases, whereas adsorption is attenuated. This counterbalance generates the maximum observed in our experiments.
4. Conclusions
This work concludes that in order to achieve the production of effective activated carbon adsorbents from the solid fibrous digestate (SFD) of biogas plants, washing with HNO3 to remove the inorganic content and expand the carbonaceous yield is a mandatory pre-treatment process. Moreover, BC intermediate can be produced by pyrolysis at moderate temperatures up to 600 °C with no effect on the quality of the subsequently derived ACs, which is of high importance for the sustainability of the proposed methodology. Notably, simple chemical and physical activation processes of the produced BCs conclude to very effective CO2 and H2S adsorbents respectively, paving the way for the achievement of a closed loop value chain where the waste effluent of a biogas plant is transformed to effective adsorbents that can be used in series to desulfurize and upgrade biogas.
Author Contributions
Conceptualization, G.E.R., E.K. and T.S.; methodology, G.E.R., A.L., S.S. and E.K.; software, G.E.R.; validation, G.E.R., E.K., A.L. and T.S.; formal analysis, G.P. and G.D.; investigation, A.M. and G.D.; resources, A.M.; data curation, E.C., S.S. and G.P.; writing—original draft preparation, E.C. and G.E.R.; writing—review and editing, G.E.R., T.S., K.B. and E.C.; visualization, E.C.; supervision, G.E.R. and K.B.; project administration, T.S.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by the European Regional Development Fund of the Eu- European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH-CREATE-INNOVATE (project code: T2EDK-00455 PYRO-D).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors acknowledge all staff members of Qlab P.C. and NCSR “Democritus” for their individual roles that contributed to the implementation of this study.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
BC yield (wt.%, dry matter feed) at various pyrolysis temperatures and times.
Table A1.
BC yield (wt.%, dry matter feed) at various pyrolysis temperatures and times.
| Pyrolysis Temperature | Pyrolysis Time | BC Yield (wt.%, DM * Feed) | |
|---|---|---|---|
| (°C) | (min) | BC-SFD | BC-SFD-Washed |
| 600 | 30 | 35.90 | 28.3 |
| 600 | 60 | 35.30 | 28.4 |
| 600 | 120 | 34.50 | 27.4 |
| 700 | 30 | 34.10 | 26.8 |
| 700 | 60 | 34.00 | 26.6 |
| 700 | 120 | 32.70 | 26.5 |
| 800 | 30 | 32.70 | 25.0 |
| 800 | 60 | 32.80 | 24.8 |
| 800 | 120 | 32.10 | 24.5 |
* on dry matter basis.

Table A2.
Ash content and elemental analysis of BC materials derived from the untreated SFD.
Table A2.
Ash content and elemental analysis of BC materials derived from the untreated SFD.
| Pyrolysis Temperature | Pyrolysis Time | Ash | C | H | N | O | H/C | O/C |
|---|---|---|---|---|---|---|---|---|
| (°C) | (min) | (wt.%, DM * Feed) | ||||||
| 600 | 30 | 35.30 | 58.80 | 1.60 | 0.50 | 3.70 | 0.33 | 0.05 |
| 600 | 60 | 33.90 | 58.60 | 1.60 | 0.80 | 5.10 | 0.32 | 0.07 |
| 600 | 120 | 33.60 | 61.40 | 1.30 | 0.90 | 2.80 | 0.25 | 0.03 |
| 700 | 30 | 38.20 | 58.70 | 1.10 | 0.50 | 1.40 | 0.23 | 0.02 |
| 700 | 60 | 38.00 | 58.90 | 1.30 | 0.70 | 1.20 | 0.27 | 0.02 |
| 700 | 120 | 37.60 | 60.90 | 0.90 | 0.70 | 0.00 | 0.18 | 0.00 |
| 800 | 30 | 39.10 | 61.10 | 0.70 | 0.30 | 0.00 | 0.14 | 0.00 |
| 800 | 60 | 37.00 | 61.20 | 0.60 | 0.50 | 0.70 | 0.12 | 0.01 |
| 800 | 120 | 38.30 | 60.30 | 0.40 | 0.90 | 0.10 | 0.07 | 0.00 |
* on dry matter basis.

Table A3.
Ash content and elemental analysis of BC materials derived from the pre-treated SFD.
Table A3.
Ash content and elemental analysis of BC materials derived from the pre-treated SFD.
| Pyrolysis Temperature | Pyrolysis Time | Ash | C | H | N | O | H/C | O/C |
|---|---|---|---|---|---|---|---|---|
| (°C) | (min) | (wt.%, DM * Feed) | ||||||
| 600 | 30 | 14.20 | 77.70 | 2.20 | 0.60 | 5.10 | 0.35 | 0.05 |
| 600 | 60 | 16.80 | 76.20 | 1.90 | 1.30 | 3.80 | 0.31 | 0.04 |
| 600 | 120 | 14.60 | 80.60 | 1.70 | 0.00 | 3.10 | 0.25 | 0.03 |
| 700 | 30 | 16.80 | 77.20 | 1.80 | 1.20 | 3.00 | 0.28 | 0.03 |
| 700 | 60 | 17.80 | 77.70 | 1.50 | 1.10 | 1.80 | 0.24 | 0.02 |
| 700 | 120 | 15.70 | 78.30 | 1.60 | 1.00 | 3.50 | 0.24 | 0.03 |
| 800 | 30 | 16.30 | 86.80 | 0.70 | 1.40 | 0.00 | 0.09 | 0.00 |
| 800 | 60 | 17.70 | 78.80 | 0.90 | 0.00 | 2.50 | 0.14 | 0.02 |
| 800 | 120 | 18.10 | 82.30 | 0.60 | 0.90 | 0.00 | 0.09 | 0.00 |
* on dry matter basis.

Table A4.
Surface characteristics and porosity of BC materials derived from the untreated SFD.
Table A4.
Surface characteristics and porosity of BC materials derived from the untreated SFD.
| Pyrolysis Temperature | Pyrolysis Time | BET | Micropore Surface | External Surface | Micropore Volume | Total Pore Volume |
|---|---|---|---|---|---|---|
| (°C) | (min) | (m2/g) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) |
| 600 | 30 | 18 | 10 | 8 | 0.004 | 0.021 |
| 600 | 60 | 17 | 11 | 6 | 0.004 | 0.019 |
| 600 | 120 | 15 | 7 | 8 | 0.003 | 0.020 |
| 700 | 30 | 43 | 28 | 15 | 0.011 | 0.040 |
| 700 | 60 | 35 | 25 | 10 | 0.010 | 0.035 |
| 700 | 120 | 27 | 16 | 12 | 0.006 | 0.034 |
| 800 | 30 | 38 | 18 | 20 | 0.007 | 0.043 |
| 800 | 60 | 43 | 22 | 21 | 0.009 | 0.056 |
| 800 | 120 | 38 | 20 | 18 | 0.008 | 0.055 |

Table A5.
Surface characteristics and porosity of BC materials derived from the pretreated SFD.
Table A5.
Surface characteristics and porosity of BC materials derived from the pretreated SFD.
| Pyrolysis Temperature | Pyrolysis Time | BET | Micropore Surface | External Surface | Micropore Volume | Total Pore Volume |
|---|---|---|---|---|---|---|
| (°C) | (min) | (m2/g) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) |
| 600 | 30 | 279 | 263 | 16 | 0.103 | 0.125 |
| 600 | 60 | 276 | 265 | 13 | 0.103 | 0.129 |
| 600 | 120 | 287 | 268 | 20 | 0.104 | 0.135 |
| 700 | 30 | 291 | 279 | 12 | 0.108 | 0.127 |
| 700 | 60 | 275 | 263 | 12 | 0.102 | 0.122 |
| 700 | 120 | 288 | 278 | 10 | 0.107 | 0.125 |
| 800 | 30 | 363 | 339 | 24 | 0.132 | 0.165 |
| 800 | 60 | 313 | 298 | 15 | 0.115 | 0.138 |
| 800 | 120 | 317 | 292 | 25 | 0.113 | 0.151 |

Table A6.
Surface characteristics and porosity of activated carbons AC-H2O produced by activation of BC derived from the pretreated SFD.
Table A6.
Surface characteristics and porosity of activated carbons AC-H2O produced by activation of BC derived from the pretreated SFD.
| Activation Temperature | Steam Flow Rate | Activation Time | BET | Micropore Surface | External Surface | Micropore Volume | Total Pore Volume |
|---|---|---|---|---|---|---|---|
| (°C) | (mL/min) | (min) | (m2/g) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) |
| 700 | 1 | 30 | 470 | 338 | 132 | 0.139 | 0.277 |
| 700 | 1 | 60 | 485 | 307 | 178 | 0.129 | 0.327 |
| 700 | 1 | 90 | 520 | 295 | 225 | 0.125 | 0.362 |
| 800 | 1 | 30 | 526 | 251 | 275 | 0.107 | 0.445 |
| 800 | 1 | 45 | 530 | 222 | 308 | 0.095 | 0.472 |
| 800 | 1 | 60 | 585 | 220 | 366 | 0.095 | 0.569 |
| 900 | 1 | 15 | 444 | 281 | 163 | 0.116 | 0.339 |
| 900 | 1 | 30 | 528 | 278 | 250 | 0.117 | 0.525 |
| 900 | 1 | 45 | 533 | 235 | 299 | 0.100 | 0.563 |

Table A7.
Surface characteristics and porosity of activated carbons AC-KOH produced by activation of BC derived from the pretreated SFD.
Table A7.
Surface characteristics and porosity of activated carbons AC-KOH produced by activation of BC derived from the pretreated SFD.
| Activation Temperature | KOH Molar Ratio | Activation Time | BET | Micropore Surface | External Surface | Micropore Volume | Total Pore Volume |
|---|---|---|---|---|---|---|---|
| (°C) | (min) | (m2/g) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) | |
| 600 | 4 | 30 | 658 | 511 | 47 | 0.246 | 0.307 |
| 700 | 4 | 30 | 1818 | 1746 | 73 | 0.688 | 0.767 |
| 800 | 1 | 30 | 1196 | 1143 | 52 | 0.446 | 0.502 |
| 800 | 2 | 30 | 367 | 335 | 32 | 0.134 | 0.178 |
| 800 | 4 | 30 | 2299 | 2174 | 125 | 0.887 | 1.010 |
| 800 | 4 | 60 | 1434 | 876 | 558 | 0.341 | 0.655 |
| 800 | 4 | 120 | 2032 | 1911 | 121 | 0.779 | 0.940 |

Table A8.
H2S adsorption capacity, mass of H2S adsorbed per AC mass, on the activated carbon materials at various temperatures.
Table A8.
H2S adsorption capacity, mass of H2S adsorbed per AC mass, on the activated carbon materials at various temperatures.
| Activated Carbon | Adsorption Temperature (°C) | H2S Capacity (g [H2S]/g) | ||
|---|---|---|---|---|
| Breakthrough Time (C/C0 = 0.05) 1 | Reference Time C/C0 = 0.5 1 | Exhaustion Time (C/C0 = 0.95) 2 | ||
| AC-H2O | 25 | 2.79 | 20.93 | 62.47 |
| 50 | 2.70 | 35.94 | 95.53 | |
| 70 | 1.20 | 15.02 | 45.13 | |
| AC-KOH | 25 | 1.46 | 5.66 | 25.95 |
| 50 | 1.40 | 9.03 | 41.75 | |
| 70 | 2.12 | 10.47 | 23.23 | |
| AC-CO2 | 25 | 22.14 | 53.62 | 137.83 |
| 50 | 10.62 | 53.44 | 135.65 | |
| 70 | 25.19 | 102.69 | 220.17 | |
1 Experimental. 2 Calculated.

Table A9.
CO2 adsorption capacity, mass of CO2 adsorbed per AC mass, on the activated carbon materials at various temperatures.
Table A9.
CO2 adsorption capacity, mass of CO2 adsorbed per AC mass, on the activated carbon materials at various temperatures.
| Activated Carbon | Adsorption Temperature (°C) | CO2 Capacity (g [CO2]/g) | |||
|---|---|---|---|---|---|
| Breakthrough Time (C/C0 = 0.05) 1 | C/C0 = 0.5 1 | Exhaustion Time (C/C0 = 0.95) 1 | Reference Time C/C0 = 0.5 2 | ||
| AC-H2O | 25 | 1.82 | 4.32 | 6.82 | 6.82 |
| 50 | 0.00 | 3.41 | 8.18 | 8.18 | |
| 70 | 2.50 | 4.54 | 8.63 | 8.63 | |
| AC-KOH | 25 | 4.77 | 14.09 | 26.58 | 26.58 |
| 50 | 2.95 | 11.13 | 19.54 | 19.54 | |
| 70 | 0.91 | 8.41 | 12.27 | 12.27 | |
| AC-CO2 | 25 | 1.59 | 3.86 | 8.63 | 8.63 |
| 50 | 1.36 | 2.27 | 2.27 | 2.27 | |
| 70 | 1.14 | 2.50 | 2.73 | 2.73 | |
1 CO2 breakthrough time, time when C/C0 = 0.5 and exhaustion time. 2 Reference time; when C/C0 = 0.5 at the adsorption curve of H2S.
References
- McKinsey, S.Z. Removal of Hydrogen Sulfide from Biogas Using Cow-Manure Compost. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2003. [Google Scholar]
- Kulkarni, M.; Ghanegaonkar, P. Hydrogen sulfide removal from biogas using chemical absorption technique in packed column reactors. Glob. J. Environ. Sci. Manag. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Shareefdeen, Z.; Singh, A. Biotechnology for Odor and Air Pollution Control; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Weiland, P. Notwendigkeit der Biogasaufbereitung, Ansprüche einzelner Nutzungsrouten und Stand der Technik. Gülzower Fachgespr. 2003, 21, 7–23. [Google Scholar]
- Sitthikhankaew, R.; Chadwick, D.; Assabumrungrat, S.; Laosiripojana, N. Effects of humidity, O2, and CO2 on H2S adsorption onto upgraded and KOH impregnated activated carbons. Fuel Process. Technol. 2014, 124, 249–257. [Google Scholar] [CrossRef]
- Council Directive of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources (91/676/EEC). Available online: https://eur-lex.europa.eu/eli/dir/1991/676/2008-12-11 (accessed on 7 June 2023).
- Allegue, L.B.; Hinge, J. Biogas Upgrading Evaluation of Methods for H2S removal. Danish Technological Institute. 2014. Available online: https://www.teknologisk.dk/_/media/60599_Biogas%20upgrading.%20Evalution%20of%20metods%20for%20H2S%20removal.pdf (accessed on 22 May 2023).
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.-H.; Jiang, L.-H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [PubMed]
- Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. Removal of Hydrogen Sulfide From Various Industrial Gases: A Review of The Most Promising Adsorbing Materials. Catalysts 2020, 10, 521. [Google Scholar] [CrossRef]
- Guo, J.; Luo, Y.; Lua, A.C.; Chi, R.-A.; Chen, Y.-L.; Bao, X.-T.; Xiang, S.-X. Adsorption of hydrogen sulphide (H2S) by activated carbons derived from oil-palm shell. Carbon 2007, 45, 330–336. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Chiung-Fen, C.; Ching-Yuan, C.; Wen-Tien, T. Effects of Burn-off and Activation Temperature on Preparation of Activated Carbon from Corn Cob Agrowaste by CO2 and Steam. J. Colloid Interface Sci. 2000, 232, 45–49. [Google Scholar] [CrossRef]
- Farma, R.; Deraman, M.; Awitdrus, A.; Talib, I.; Taer, E.; Basri, N.; Manjunatha, J.; Ishak, M.; Dollah, B.; Hashmi, S. Preparation of highly porous binderless activated carbon electrodes from fibres of oil palm empty fruit bunches for application in supercapacitors. Bioresour. Technol. 2013, 132, 254–261. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; Gonzalez, M.; Rodriguez-Reinoso, F.; Sepúlveda-Escribano, A. Effect of steam and carbon dioxide activation in the micropore size distribution of activated carbon. Carbon 1996, 34, 505–509. [Google Scholar] [CrossRef]
- Calderon, S.T.; Pampa-Quispe, N.B.; Huaranga, M.A.C. Adsorption of hydrogen sulfide by activated carbon produced by regeneration of sludge from upflow anaerobic sludge blanket. Eng. Rural. Dev. 2022, 5, 25–27. [Google Scholar] [CrossRef]
- Tuerhong, T.; Kuerban, Z. Preparation and characterization of cattle manure-based activated carbon for hydrogen sulfide removal at room temperature. J. Environ. Chem. Eng. 2022, 10, 107177. [Google Scholar] [CrossRef]
- Hussain, A. A computational study of adsorption of H2S and SO2 on the activated carbon surfaces. J. Mol. Graph. Model. 2023, 122, 108463. [Google Scholar] [CrossRef] [PubMed]
- Tangsathitkulchai, C.; Naksusuk, S.; Wongkoblap, A.; Phadungbut, P.; Borisut, P. Equilibrium and Kinetics of CO2 Adsorption by Coconut Shell Activated Carbon Impregnated with Sodium Hydroxide. Processes 2021, 9, 201. [Google Scholar] [CrossRef]
- Fomkin, A.; Pribylov, A.; Men’shchikov, I.; Shkolin, A.; Aksyutin, O.; Ishkov, A.; Romanov, K.; Khozina, E. Adsorption-Based Hydrogen Storage in Activated Carbons and Model Carbon Structures. Reactions 2021, 2, 209–226. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.A.; Ocampo-Pérez, R.; Forgionny, A.; Labrada-Delgado, G.J.; Zárate-Guzmán, A.I.; Cruz-Briano, S.A.; Flores-Ramírez, R. Insights into Equilibrium and Adsorption Rate of Phenol on Activated Carbon Pellets Derived from Cigarette Butts. Processes 2021, 9, 934. [Google Scholar] [CrossRef]
- Jurkiewicz, M.; Pełech, R. Adsorption of 1,2-Dichlorobenzene from the Aqueous Phase onto Activated Carbons and Modified Carbon Nanotubes. Int. J. Mol. Sci. 2021, 22, 13152. [Google Scholar] [CrossRef]
- Wang, R.-S.; Li, Y.; Shuai, X.-X.; Liang, R.-H.; Chen, J.; Liu, C.-M. Pectin/Activated Carbon-Based Porous Microsphere for Pb2+ Adsorption: Characterization and Adsorption Behaviour. Polymers 2021, 13, 2453. [Google Scholar] [CrossRef]
- Alberto, D.R.; Tyler, A.C.; Trabold, T.A. Phosphate adsorption using biochar derived from solid digestate. Bioresour. Technol. Rep. 2021, 16, 100864. [Google Scholar] [CrossRef]
- Plaza, M.; González, A.; Pis, J.; Rubiera, F.; Pevida, C. Production of microporous biochars by single-step oxidation: Effect of activation conditions on CO2 capture. Appl. Energy 2014, 114, 551–562. [Google Scholar] [CrossRef]
- Aworn, A.; Thiravetyan, P.; Nakbanpote, W. Preparation and characteristics of agricultural waste activated carbon by physical activation having micro- and mesopores. J. Anal. Appl. Pyrolysis 2008, 82, 279–285. [Google Scholar] [CrossRef]
- Kainourgiakis, M.; Steriotis, T.; Kikkinides, E.; Romanos, G.; Stubos, A. Adsorption and diffusion in nanoporous materials from stochastic and process-based reconstruction techniques. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 206, 321–334. [Google Scholar] [CrossRef]
- Romanos, G.; Vangeli, O.; Stefanopoulos, K.; Kouvelos, E.; Papageorgiou, S.; Favvas, E.; Kanellopoulos, N. Methods of evaluating pore morphology in hybrid organic–inorganic porous materials. Microporous Mesoporous Mater. 2008, 120, 53–61. [Google Scholar] [CrossRef]
- Pokhrel, J.; Bhoria, N.; Anastasiou, S.; Tsoufis, T.; Gournis, D.; Romanos, G.; Karanikolos, G.N. CO2 adsorption behavior of amine-functionalized ZIF-8, graphene oxide, and ZIF-8/graphene oxide composites under dry and wet conditions. Microporous Mesoporous Mater. 2018, 267, 53–67. [Google Scholar] [CrossRef]
- Aik Chong, L.; Fong Yow, L.; Jia, G. Influence of pyrolysis conditions on pore development of oil-palm-shell activated carbons. J. Anal. Appl. Pyrolysis 2006, 76, 96–102. [Google Scholar]
- Bouchelta, C.; Medjram, M.S.; Zoubida, M.; Chekkat, F.A.; Ramdane, N.; Bellat, J.-P. Effects of pyrolysis conditions on the porous structure development of date pits activated carbon. J. Anal. Appl. Pyrolysis 2012, 94, 215–222. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocel-lulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Antonakou, E.; Kalogiannis, K.; Stefanidis, S.; Karakoulia, S.; Triantafyllidis, K.; Lappas, A.; Achilias, D. Catalytic and thermal pyrolysis of polycarbonate in a fixed-bed reactor: The effect of catalysts on products yields and composition. Polym. Degrad. Stab. 2014, 110, 482–491. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).