Production of Ceramics/Metal Oxide Nanofibers via Electrospinning: New Insights into the Photocatalytic and Bactericidal Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2 Nanofibers
2.3. Preparation of Zr0.5Sn0.5TiO3/SnO2 Ceramics Nanofibers
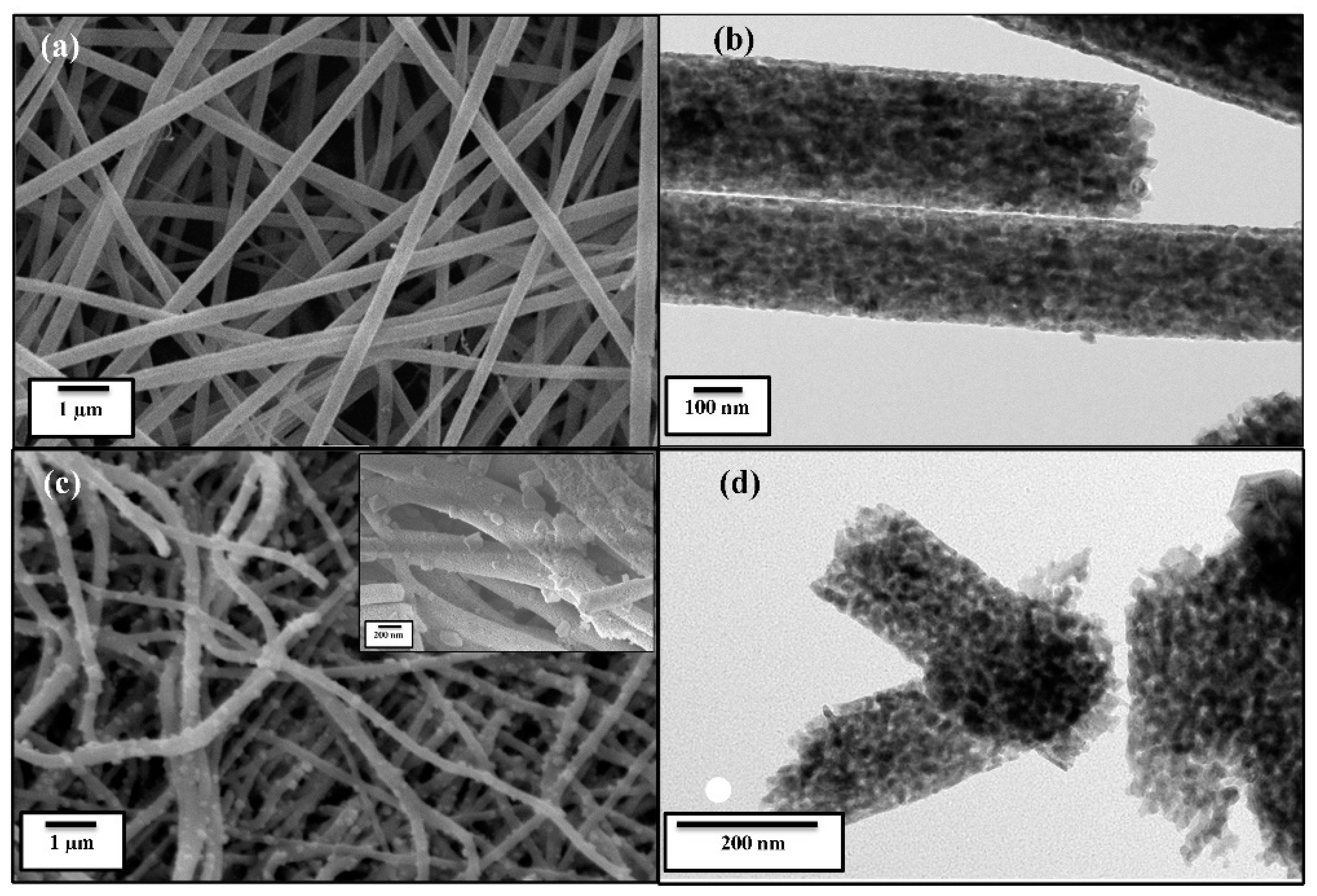
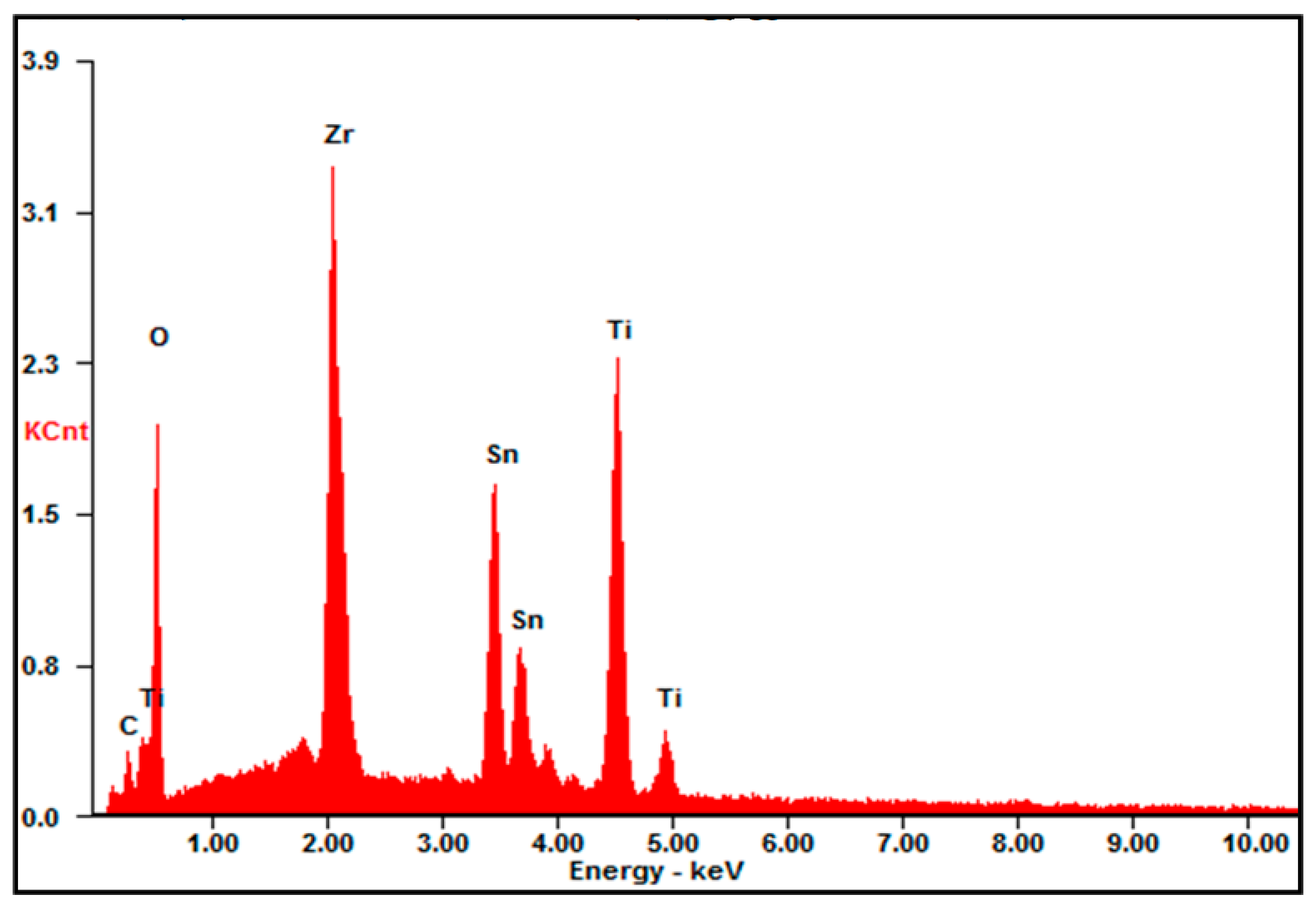
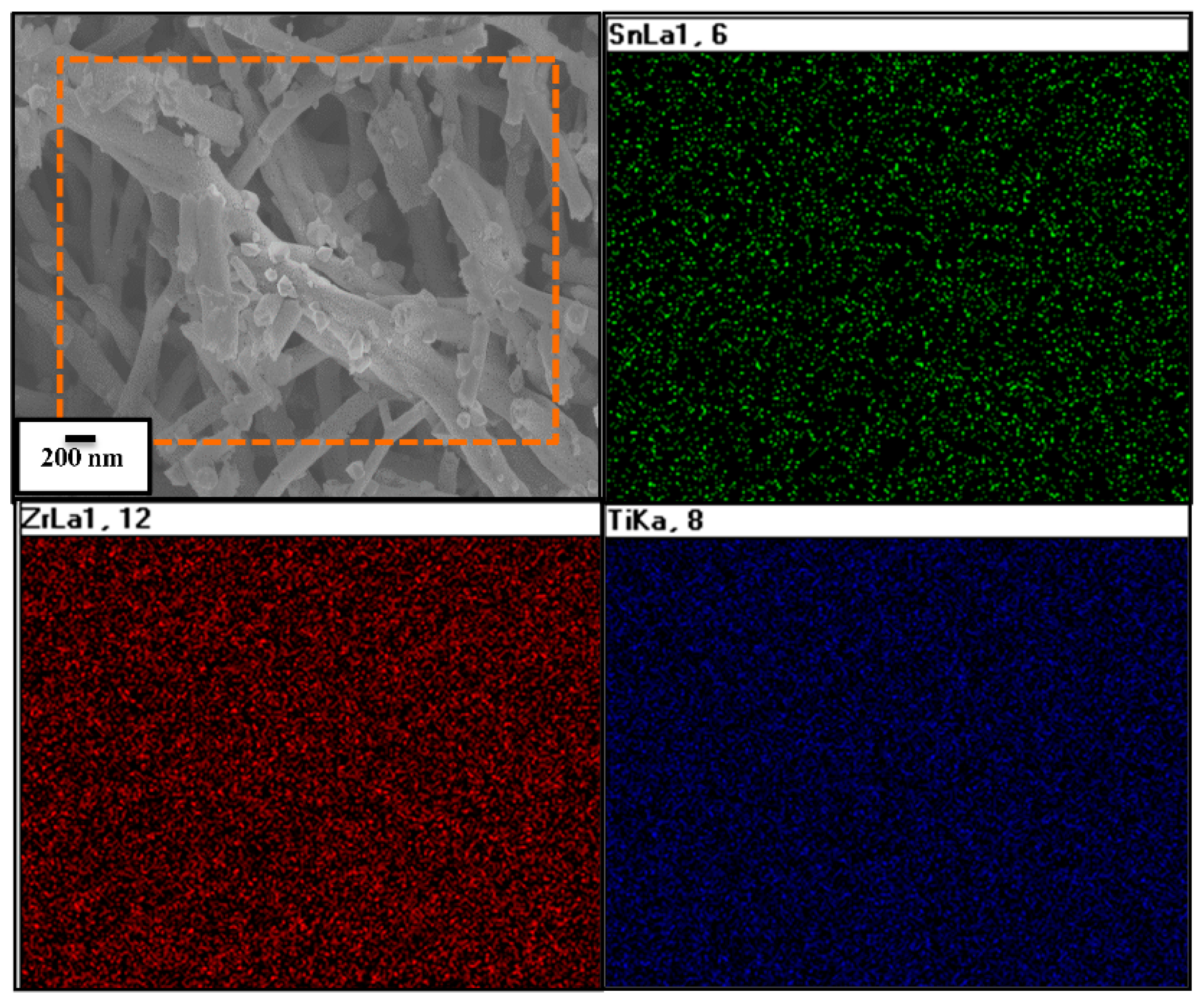
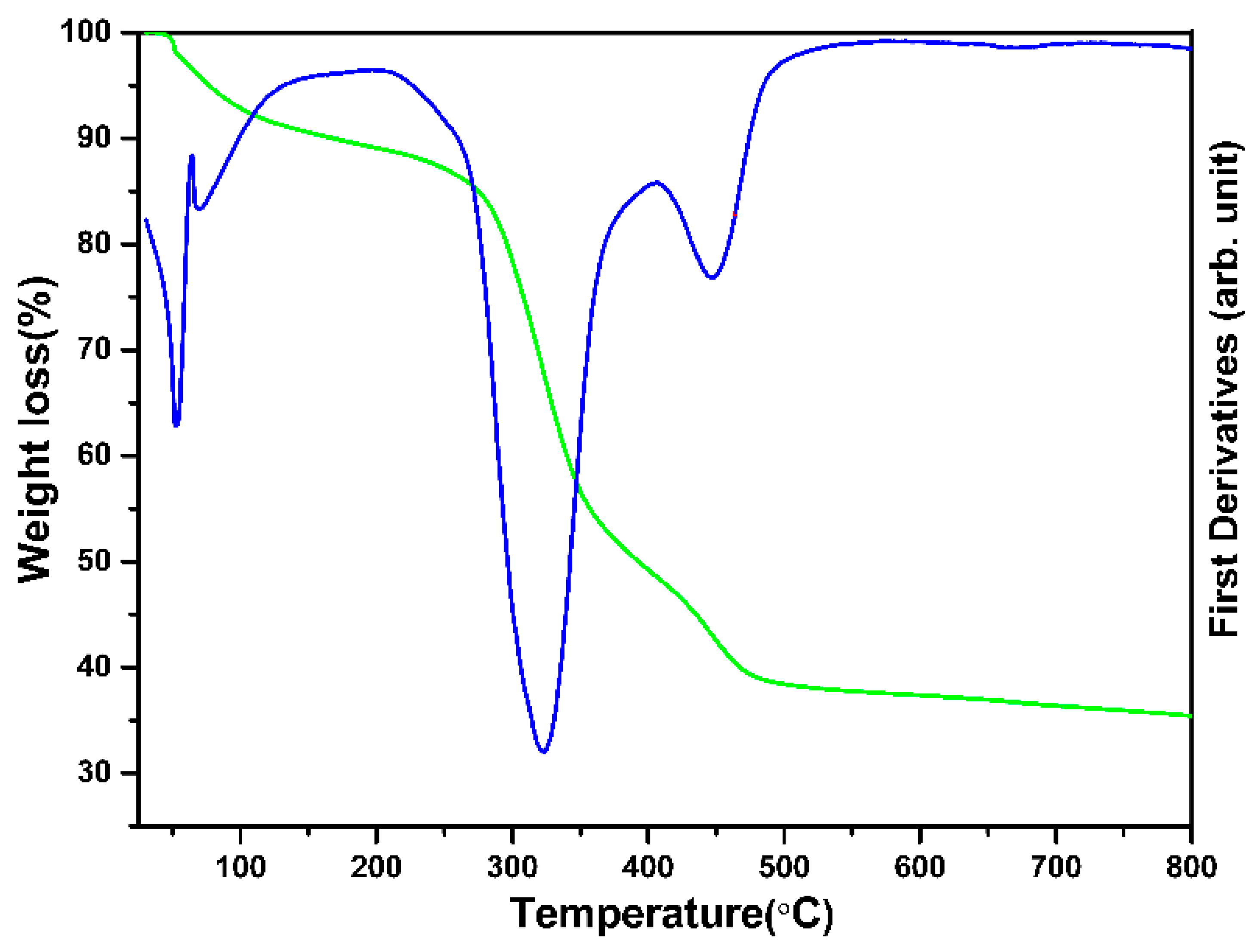
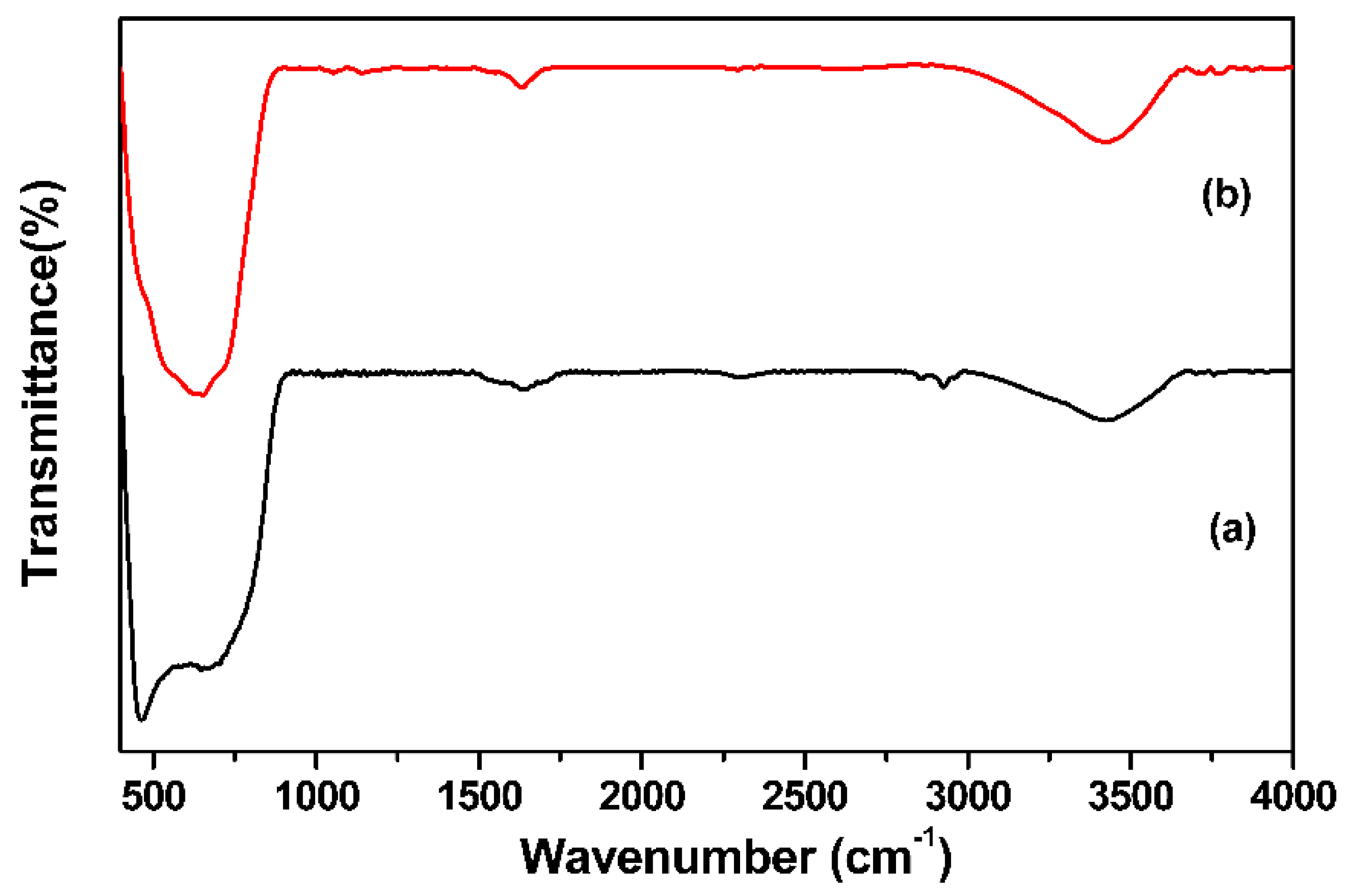
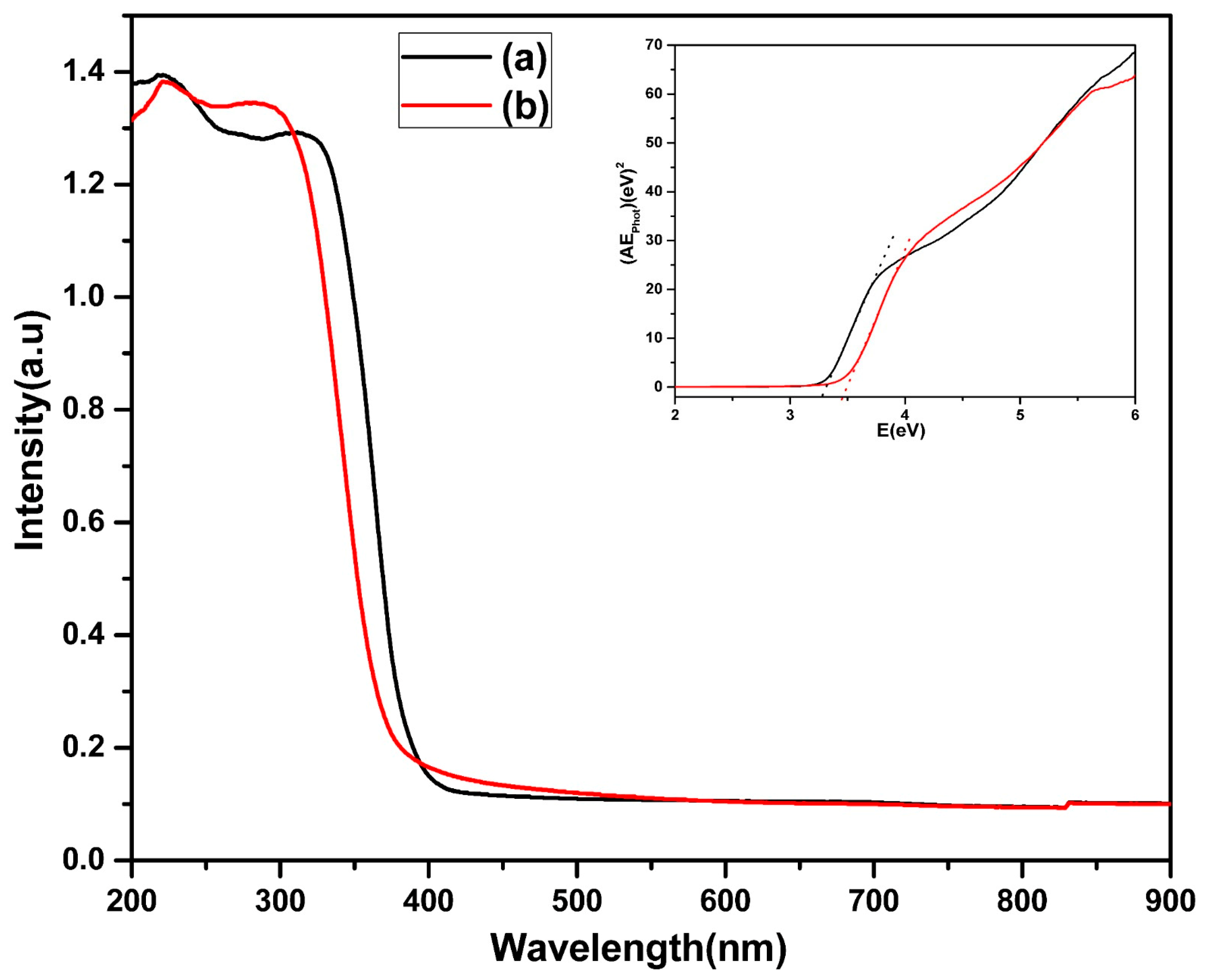
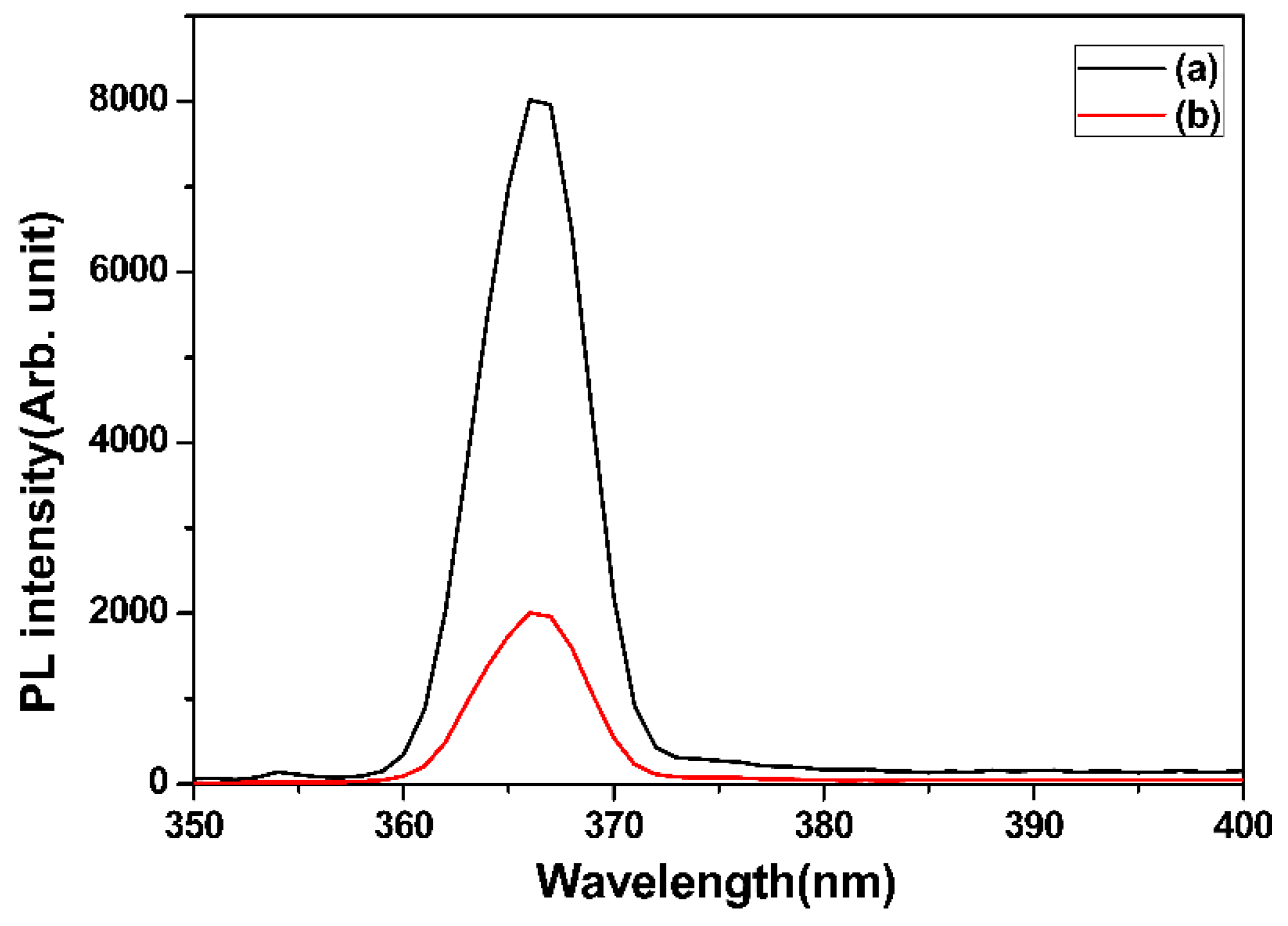
2.4. Material Characterization
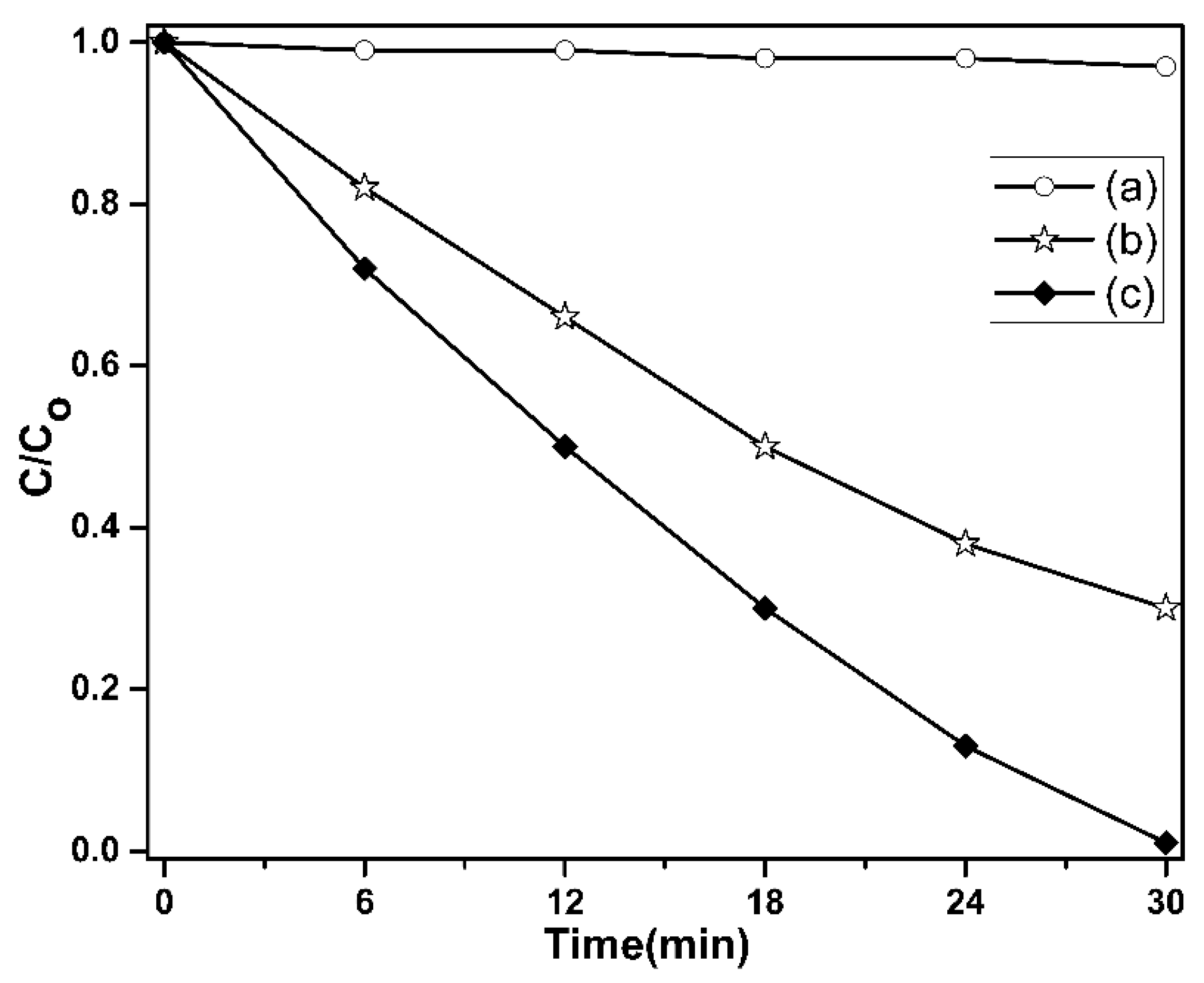
2.5. Photocatalytic Experiment
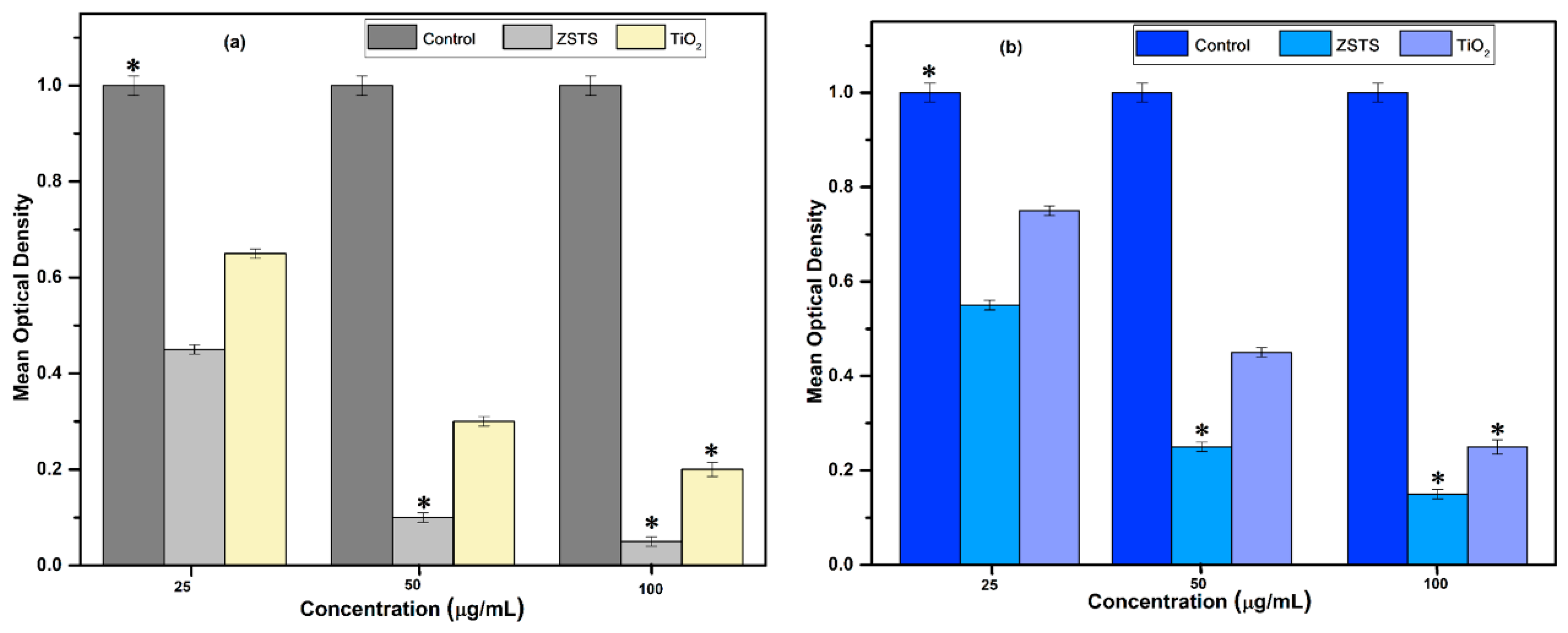
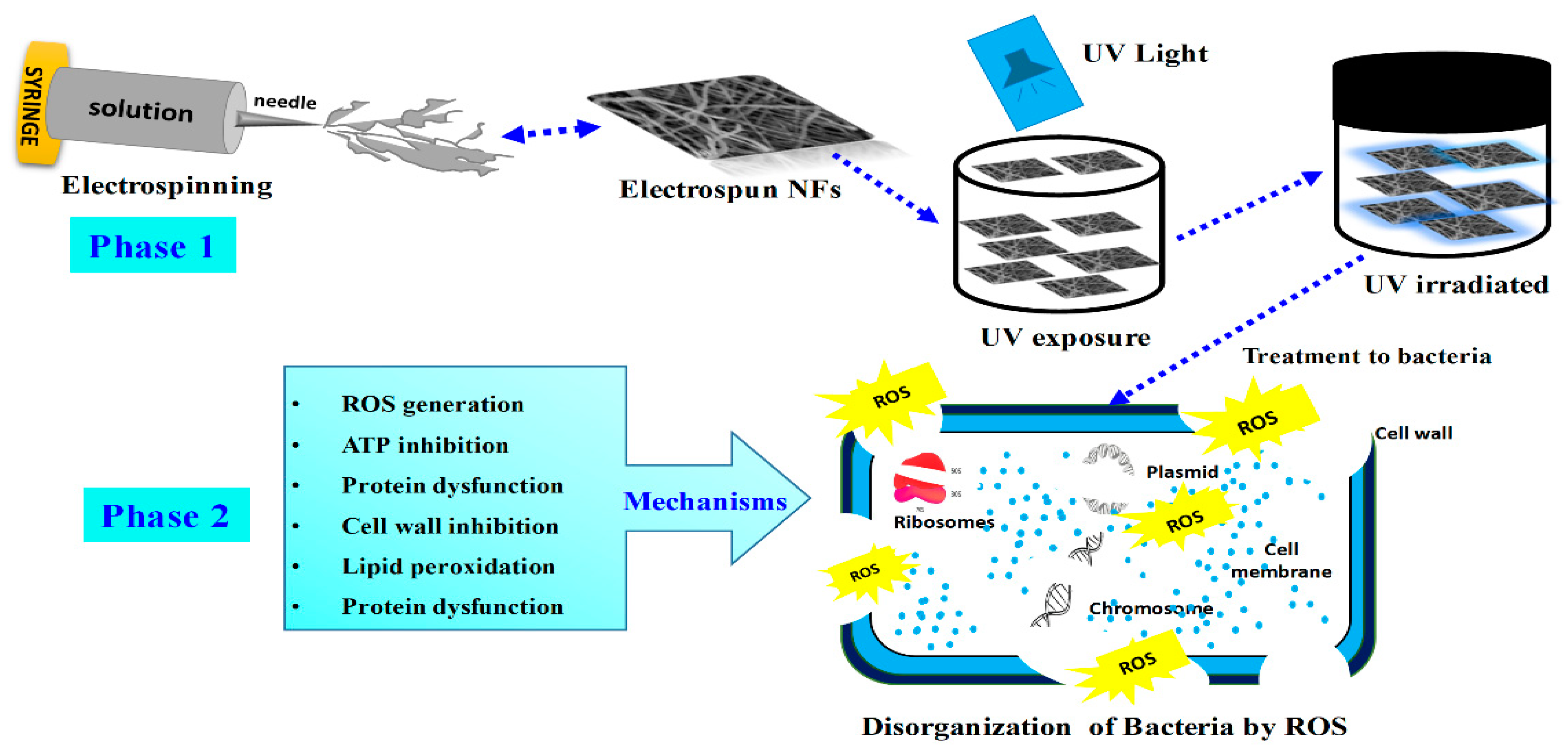
2.6. Computation of the Antibacterial Potential of TiO2 and Zr0.5Sn0.5TiO3/SnO2 (ZSTS) Nanofibers
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badgley, B.D.; Ferguson, J.; Heuvel, A.V.; Kleinheinz, G.T.; McDermott, C.M.; Sandrin, T.R.; Kinzelman, J.; Junion, E.A.; Byappanahalli, M.N.; Whitman, R.L. Multi-scale temporal and spatial variation in genotypic composition of Cladophora-borne Escherichia coli populations in Lake Michigan. Water Res. 2011, 45, 721–731. [Google Scholar] [CrossRef] [PubMed]
- DeFlorio-Barker, S.; Wing, C.; Jones, R.M.; Dorevitch, S. Estimate of incidence and cost of recreational waterborne illness on United States surface waters. Environ. Health 2018, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Korajkic, A.; McMinn, B.R.; Harwood, V.J. Relationships between microbial indicators and pathogens in recreational water settings. Int. J. Environ. Res. Public Health 2018, 15, 2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Phukan, A.; Bhattacharjee, R.P.; Dutta, D.K. Stabilization of SnO2 nanoparticles into the nanopores of modified Montmorillonite and their antibacterial activity. Adv. Powder Technol. 2017, 28, 139–145. [Google Scholar] [CrossRef]
- Otero-González, L.; García-Saucedo, C.; Field, J.A.; Sierra-Álvarez, R. Toxicity of TiO2, ZrO2, Fe0, Fe2O3, and Mn2O3 nanoparticles to the yeast, Saccharomyces cerevisiae. Chemosphere 2013, 93, 1201–1206. [Google Scholar] [CrossRef]
- Fathima, J.B.; Pugazhendhi, A.; Venis, R. Synthesis and characterization of ZrO2 nanoparticles-antimicrobial activity and their prospective role in dental care. Microb. Pathog. 2017, 110, 245–251. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef] [Green Version]
- Palit, S.; Hussain, C.M. Nanodevices applications and recent advancements in nanotechnology and the global pharmaceutical industry. In Nanomaterials in Diagnostic Tools and Devices; Elsevier: Amsterdam, The Netherlands, 2020; pp. 395–415. [Google Scholar]
- Lin, J.; Jimmy, C.Y.; Lo, D.; Lam, S. Photocatalytic Activity of Rutile Ti1− xSnxO2Solid Solutions. J. Catal. 1999, 183, 368–372. [Google Scholar] [CrossRef]
- Wang, J.; Jing, L.; Xue, L.; Qu, Y.; Fu, H. Enhanced activity of bismuth-compounded TiO2 nanoparticles for photocatalytically degrading rhodamine B solution. J. Hazard. Mater. 2008, 160, 208–212. [Google Scholar] [CrossRef]
- Reddy, B.M.; Sreekanth, P.M.; Reddy, E.P.; Yamada, Y.; Xu, Q.; Sakurai, H.; Kobayashi, T. Surface Characterization of La2O3− TiO2 and V2O5/La2O3− TiO2 Catalysts. J. Phys. Chem. B 2002, 106, 5695–5700. [Google Scholar] [CrossRef]
- Hong, S.-S.; Lee, M.S.; Park, S.S.; Lee, G.-D. Synthesis of nanosized TiO2/SiO2 particles in the microemulsion and their photocatalytic activity on the decomposition of p-nitrophenol. Catal. Today 2003, 87, 99–105. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Tahir, M.N.; Eberhardt, M.; Therese, H.A.; Kolb, U.; Theato, P.; Müller, W.E.; Schröder, H.C.; Tremel, W. From Single Molecules to Nanoscopically Structured Functional Materials: Au Nanocrystal Growth on TiO2 Nanowires Controlled by Surface-Bound Silicatein. Angew. Chem. Int. Ed. 2006, 45, 4803–4809. [Google Scholar] [CrossRef] [PubMed]
- Stafford, U.; Gray, K.A.; Kamat, P.V. Photocatalytic degradation of organic contaminants: Halophenols and related model compounds. Heterog. Chem. Rev. 1996, 3, 77–104. [Google Scholar] [CrossRef]
- Vassiliev, S.Y.; Yusipovich, A.; Rogynskaya, Y.E.; Chibirova, F.K.; Skundin, A.; Kulova, T. Nanostructured SnO2-TiO2 films as related to lithium intercalation. J. Solid State Electrochem. 2005, 9, 698–705. [Google Scholar] [CrossRef]
- Wu, C.; Shen, L.; Yu, H.; Huang, Q.; Zhang, Y.C. Synthesis of Sn-doped ZnO nanorods and their photocatalytic properties. Mater. Res. Bull. 2011, 46, 1107–1112. [Google Scholar] [CrossRef]
- Jing, L.; Xin, B.; Yuan, F.; Xue, L.; Wang, B.; Fu, H. Effects of surface oxygen vacancies on photophysical and photochemical processes of Zn-doped TiO2 nanoparticles and their relationships. J. Phys. Chem. B 2006, 110, 17860–17865. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Wu, L.; Chen, X.; Ding, Z.; Wang, X.; Fu, X. N-doped SiO2/TiO2 mesoporous nanoparticles with enhanced photocatalytic activity under visible-light irradiation. Chemosphere 2008, 72, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Krous, E.; Patel, D.; Langston, P.; Menoni, C.; Markosyan, A.; Route, R.; Fejer, M.; Nguyen, D.; Emmert, L.; Rudolph, W. Scandium Oxide Thin Films Deposited by Dual Ion Beam Sputtering for High-Power Laser Applications. In Optical Interference Coatings; P FA10; Optica Publishing Group: Washington, DC, USA, 2010. [Google Scholar]
- Stehlin, F.; Bourgin, Y.; Spangenberg, A.; Jourlin, Y.; Parriaux, O.; Reynaud, S.; Wieder, F.; Soppera, O. Direct nanopatterning of 100 nm metal oxide periodic structures by Deep-UV immersion lithography. Opt. Lett. 2012, 37, 4651–4653. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Hong, C.K. Morphology and photoelectrochemical properties of TiO2 electrodes prepared using functionalized plant oil binders. Electrochem. Commun. 2008, 10, 1187–1190. [Google Scholar] [CrossRef]
- Ishizuka, S.; Yamada, A.; Matsubara, K.; Fons, P.; Sakurai, K.; Niki, S. Alkali incorporation control in Cu (In, Ga) Se 2 thin films using silicate thin layers and applications in enhancing flexible solar cell efficiency. Appl. Phys. Lett. 2008, 93, 124105. [Google Scholar] [CrossRef]
- Gusmano, G.; Bianco, A.; Viticoli, M.; Kaciulis, S.; Mattogno, G.; Pandolfi, L. Study of Zr1− xSnxTiO4 thin films prepared by a polymeric precursor route. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 2002, 34, 690–693. [Google Scholar] [CrossRef]
- Hu, C.; Sun, J.; Long, C.; Wu, L.; Zhou, C.; Zhang, X. Synthesis of nano zirconium oxide and its application in dentistry. Nanotechnol. Rev. 2019, 8, 396–404. [Google Scholar] [CrossRef]
- Gupta, V.; Singh, S.; Rawat, K.; Bohidar, H.; Solanki, P.R. Cytotoxicity and antimicrobial activity of transition metal oxide nanoparticles. Adv. Sci. Lett. 2014, 20, 1650–1653. [Google Scholar] [CrossRef]
- Imran, M.; Riaz, S.; Sanaullah, I.; Khan, U.; Sabri, A.N.; Naseem, S. Microwave assisted synthesis and antimicrobial activity of Fe3O4-doped ZrO2 nanoparticles. Ceram. Int. 2019, 45, 10106–10113. [Google Scholar] [CrossRef]
- Jangra, S.L.; Stalin, K.; Dilbaghi, N.; Kumar, S.; Tawale, J.; Singh, S.P.; Pasricha, R. Antimicrobial activity of zirconia (ZrO2) nanoparticles and zirconium complexes. J. Nanosci. Nanotechnol. 2012, 12, 7105–7112. [Google Scholar] [CrossRef]
- Thirumagal, K.; Rajeshkumar, S.; ROY, A. In vitro Cytotoxic Effect of Zirconium Oxide Nanoparticle and Its Antibacterial Activity Against Oral Pathogens. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 28–36. [Google Scholar]
- Hassan, M.S.; Amna, T.; Mishra, A.; Yun, S.-I.; Kim, H.-C.; Kim, H.-Y.; Khil, M.-S. Fabrication, characterization and antibacterial effect of novel electrospun TiO2 nanorods on a panel of pathogenic bacteria. J. Biomed. Nanotechnol. 2012, 8, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Amna, T.; Hassan, M.S.; Sheikh, F.A.; Seo, H.C.; Kim, H.-C.; Alotaibi, N.; Alshahrani, T.; Khil, M.-S. Natural mulberry biomass fibers doped with silver as an antimicrobial textile: A new generation fabric. Text. Res. J. 2021, 91, 00405175211013422. [Google Scholar] [CrossRef]
- Gao, Y.; Bach Truong, Y.; Zhu, Y.; Louis Kyratzis, I. Electrospun antibacterial nanofibers: Production, activity, and in vivo applications. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Ramanathan, P.T.; Abdullah, M.S.; Amalraj, L. Preparation and Characterization of Tin Dioxide Thin Film by Nebulized Spray Pyrolysis Technique. J. Bloomers Res. 2013, 5, 651–655. [Google Scholar]
- He, Z.; Que, W.; Chen, J.; He, Y.; Wang, G. Surface chemical analysis on the carbon-doped mesoporous TiO2 photocatalysts after post-thermal treatment: XPS and FTIR characterization. J. Phys. Chem. Solids 2013, 74, 924–928. [Google Scholar] [CrossRef]
- Shaposhnik, D.; Pavelko, R.; Llobet, E.; Gispert-Guirado, F.; Vilanova, X. Hydrogen sensors on the basis of SnO2-TiO2 systems. Procedia Eng. 2011, 25, 1133–1136. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Zhu, Y. Synthesis and photoactivity enhancement of ZnWO 4 photocatalysts doped with chlorine. CrystEngComm 2012, 14, 8076–8082. [Google Scholar] [CrossRef]
- Shang, J.; Yao, W.; Zhu, Y.; Wu, N. Structure and photocatalytic performances of glass/SnO2/TiO2 interface composite film. Appl. Catal. A Gen. 2004, 257, 25–32. [Google Scholar] [CrossRef]
- Shibata, K.; Kiyoura, T.; Kitagawa, J.; Sumiyoshi, T.; Tanabe, K. Acidic properties of binary metal oxides. Bull. Chem. Soc. Jpn. 1973, 46, 2985–2988. [Google Scholar] [CrossRef] [Green Version]
- Fresno, F.; Hernández-Alonso, M.D.; Tudela, D.; Coronado, J.M.; Soria, J. Photocatalytic degradation of toluene over doped and coupled (Ti,M)O2 (M = Sn or Zr) nanocrystalline oxides: Influence of the heteroatom distribution on deactivation. Appl. Catal. B Environ. 2008, 84, 598–606. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Su, M.; Wang, X.; Zou, X.; Sun, X.; Shi, J.; Zhang, H. Environmental fate and behavior of silver nanoparticles in natural estuarine systems. J. Environ. Sci. 2020, 88, 248–259. [Google Scholar] [CrossRef]
- Ann, L.C.; Mahmud, S.; Bakhori, S.K.M.; Sirelkhatim, A.; Mohamad, D.; Hasan, H.; Seeni, A.; Rahman, R.A. Antibacterial responses of zinc oxide structures against Staphylococcus aureus, Pseudomonas aeruginosa and Streptococcus pyogenes. Ceram. Int. 2014, 40, 2993–3001. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, N.; Kumar, D.; Verma, D.; Bohara, R.A.; Singh, M. Zirconium oxide (ZrO2) nanoparticles from antibacterial activity to cytotoxicity: A next-generation of multifunctional nanoparticles. Mater. Today Commun. 2021, 26, 102156. [Google Scholar] [CrossRef]
- Gowri, S.; Gandhi, R.R.; Sundrarajan, M. Structural, optical, antibacterial and antifungal properties of zirconia nanoparticles by biobased protocol. J. Mater. Sci. Technol. 2014, 30, 782–790. [Google Scholar] [CrossRef]
- Du, T.; Chen, S.; Zhang, J.; Li, T.; Li, P.; Liu, J.; Du, X.; Wang, S. Antibacterial activity of manganese dioxide nanosheets by ros-mediated pathways and destroying membrane integrity. Nanomaterials 2020, 10, 1545. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algethami, J.S.; Amna, T.; S. Alqarni, L.; Alshahrani, A.A.; Alhamami, M.A.M.; Seliem, A.F.; Al-Dhuwayin, B.H.A.; Hassan, M.S. Production of Ceramics/Metal Oxide Nanofibers via Electrospinning: New Insights into the Photocatalytic and Bactericidal Mechanisms. Materials 2023, 16, 5148. https://doi.org/10.3390/ma16145148
Algethami JS, Amna T, S. Alqarni L, Alshahrani AA, Alhamami MAM, Seliem AF, Al-Dhuwayin BHA, Hassan MS. Production of Ceramics/Metal Oxide Nanofibers via Electrospinning: New Insights into the Photocatalytic and Bactericidal Mechanisms. Materials. 2023; 16(14):5148. https://doi.org/10.3390/ma16145148
Chicago/Turabian StyleAlgethami, Jari S., Touseef Amna, Laila S. Alqarni, Aisha A. Alshahrani, Mohsen A. M. Alhamami, Amal F. Seliem, Badria H. A. Al-Dhuwayin, and M. Shamshi Hassan. 2023. "Production of Ceramics/Metal Oxide Nanofibers via Electrospinning: New Insights into the Photocatalytic and Bactericidal Mechanisms" Materials 16, no. 14: 5148. https://doi.org/10.3390/ma16145148
APA StyleAlgethami, J. S., Amna, T., S. Alqarni, L., Alshahrani, A. A., Alhamami, M. A. M., Seliem, A. F., Al-Dhuwayin, B. H. A., & Hassan, M. S. (2023). Production of Ceramics/Metal Oxide Nanofibers via Electrospinning: New Insights into the Photocatalytic and Bactericidal Mechanisms. Materials, 16(14), 5148. https://doi.org/10.3390/ma16145148






