Thermo-Sensitive Microgel/Poly(ether sulfone) Composited Ultrafiltration Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PMO-MGs
2.3. Characterization
2.4. Preparation of PMO-MGs/PES Composited Ultrafiltration Membranes
2.5. Contact Angle Measurement of PMO-MGs/PES Composited Ultrafiltration Membranes
2.6. Membrane Porosity and Pore Size Measurement
2.7. Performance of PMO-MGs/PES Composited Ultrafiltration Membranes
2.7.1. Water Flux of the Membranes
2.7.2. BSA Rejection Property of the Membranes
2.7.3. Antifouling Performance of the Membranes
3. Results and Discussion
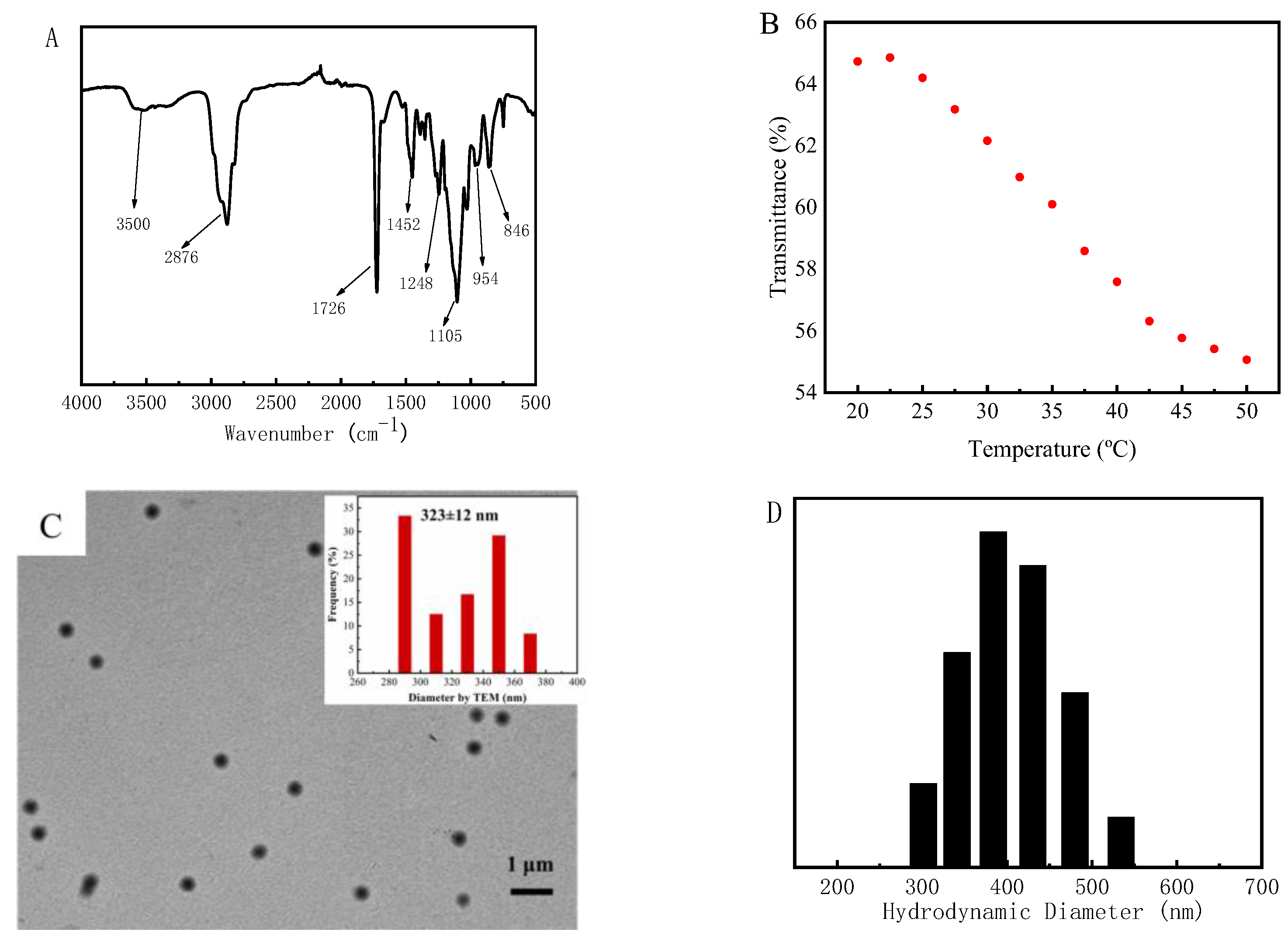
3.1. Synthesis and Characterization of PMOmicrogels
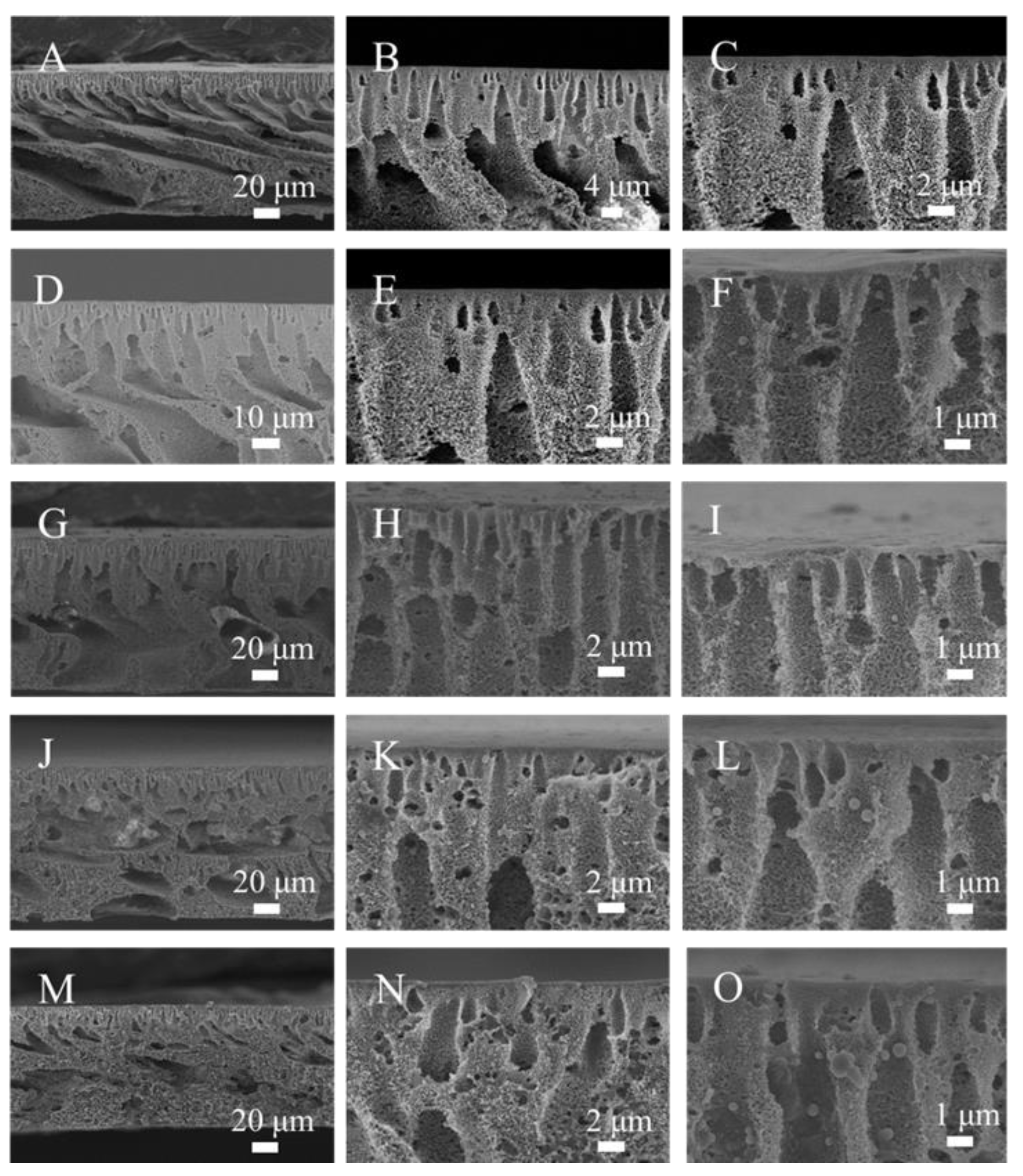
3.2. Microstructures of PMO-MGs/PES Composited Ultrafiltration Membranes
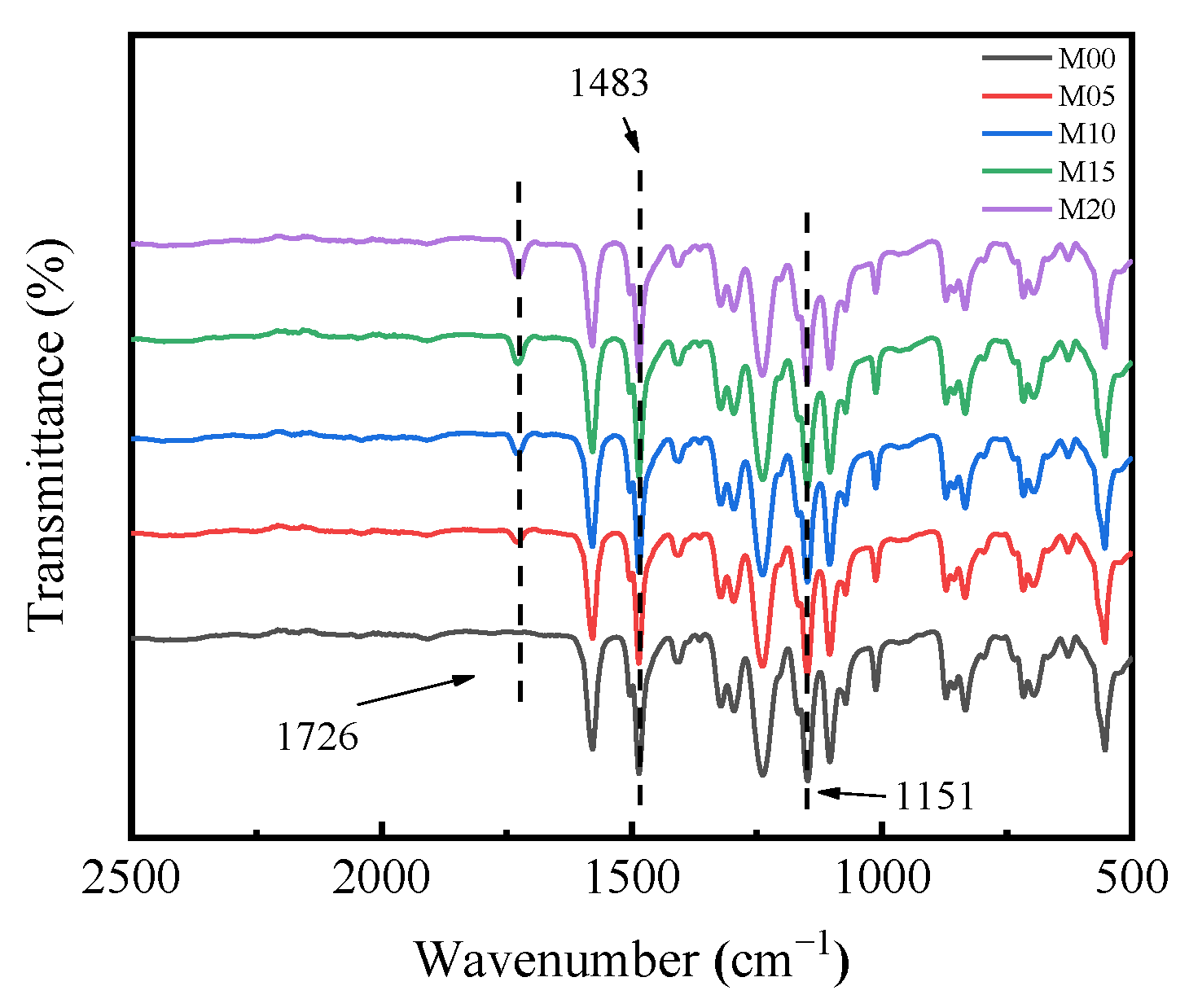
3.3. Surface Chemical Composition of PMO-MGs/PES Composited Ultrafiltration Membranes
3.4. Porosity and Pore Size of PMO-MGs/PES Composited Ultrafiltration Membranes
3.5. Surface Hydrophilicity of PMO-MGs/PES Composited Ultrafiltration Membranes
3.6. Thermo-Sensitive Water Flux of PMO-MGs/PES Composited Ultrafiltration Membranes
3.7. BSA Rejection Property of PMO-MGs/PES Composited Ultrafiltration Membranes
3.8. Antifouling Performance of PMO-MGs/PES Composited Ultrafiltration Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Q.; Jiang, J. Molecular simulations of liquid separations in polymer membranes. Curr. Opin. Chem. Eng. 2020, 28, 66–74. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Piatkovsky, M.; Acar, H.; Marciel, A.B.; Tirrell, M.; Herzberg, M. A zwitterionic block-copolymer, based on glutamic acid and lysine, reduces the biofouling of UF and RO membranes. J. Membr. Sci. 2018, 549, 507–514. [Google Scholar] [CrossRef]
- Ran, F.; Nie, S.; Zhao, W.; Li, J.; Su, B.; Sun, S.; Zhao, C. Biocompatibility of modified polyethersulfone membranes by blending an amphiphilic triblock co-polymer of poly(vinyl pyrrolidone)-b-poly(methyl methacrylate)-b-poly(vinyl pyrrolidone). Acta Biomater. 2011, 7, 3370–3381. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ji, J.; Li, K. Crystal nuclei templated nanostructured membranes prepared by solvent crystallization and polymer migration. Nat. Commun. 2016, 7, 12804. [Google Scholar] [CrossRef] [Green Version]
- Esfahani, M.R.; Koutahzadeh, N.; Esfahani, A.R.; Firouzjaei, M.D.; Anderson, B.; Peck, L. A novel gold nanocomposite membrane with enhanced permeation, rejection and self-cleaning ability. J. Membr. Sci. 2019, 573, 309–319. [Google Scholar] [CrossRef]
- Nie, C.; Ma, L.; Xia, Y.; He, C.; Deng, J.; Wang, L.; Cheng, C.; Sun, S.; Zhao, C. Novel heparin-mimicking polymer brush grafted carbon nanotube/PES composite membranes for safe and efficient blood purification. J. Membr. Sci. 2015, 475, 455–468. [Google Scholar] [CrossRef]
- Song, H.; Ran, F.; Fan, H.; Niu, X.; Kang, L.; Zhao, C. Hemocompatibility and ultrafiltration performance of surface-functionalized polyethersulfone membrane by blending comb-like amphiphilic block copolymer. J. Membr. Sci. 2014, 471, 319–327. [Google Scholar] [CrossRef]
- Cai, T.; Li, X.; Wan, C.; Chung, T.S. Zwitterionic polymers grafted poly(ether sulfone) hollow fiber membranes and their antifouling behaviors for osmotic power generation. J. Membr. Sci. 2016, 497, 142–152. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A smart membrane with antifouling capability and switchable oil wettability for high-efficiency oil/water emulsions separation. J. Membr. Sci. 2018, 555, 69–77. [Google Scholar] [CrossRef]
- Wu, M.; Liu, W.; Mu, P.; Wang, Q.; Li, J. Sacrifice template strategy to the fabrication of a self-cleaning nanofibrous membrane for efficient crude oil-in-water emulsion separation with high flux. ACS Appl. Mater. Interfaces 2020, 12, 53484–53493. [Google Scholar] [CrossRef]
- Guo, Y.S.; Mi, Y.F.; Ji, Y.L.; An, Q.F.; Gao, C.J. One-step surface grafting method for preparing zwitterionic nanofiltation membrane via in situ introduction of initiator in interfacial polymerization. ACS Appl. Polym. Mater. 2019, 1, 1022–1033. [Google Scholar] [CrossRef]
- Offner, A.; Ramon, G.Z. The interaction of a particle and a polymer brush coating a permeable surface. J. Fluid Mech. 2021, 913, R3. [Google Scholar] [CrossRef]
- Suresh, D.; Goh, P.S.; Ismail, A.F.; Hilal, N. Surface design of liquid separation membrane through graft polymerization: A state of the art review. Membranes 2021, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ren, H.T.; Li, T.T.; Huang, S.Y.; Zhang, Y.; Lou, C.W.; Lin, J.H. Bioinspired design of underwater superoleophobic Poly(N-isopropylacrylamide)/polyacrylonitrile/TiO2 nanofibrous membranes for highly efficient oil/water separation and photocatalysis. Environ. Res. 2020, 186, 109494. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Hao, J.; Bao, B.; Bian, X.; Jiang, X.; Pang, J.; Zhang, H.; Jiang, Z.; Jiang, L. A Charge-Density-Tunable Three/Two-Dimensional Polymer/Graphene Oxide Heterogeneous Nanoporous Membrane for Ion Transport. ACS Nano 2017, 11, 10816–10824. [Google Scholar] [CrossRef]
- Bandehali, S.; Parvizian, F.; Hosseini, S.M.; Matsuura, T.; Drioli, E.; Shen, J.; Moghadassi, A.; Adeleye, A.S. Planning of smart gating membranes for water treatment. Chemosphere 2021, 283, 131207. [Google Scholar] [CrossRef]
- Abidin, M.N.Z.; Goh, P.S.; Ismail, A.F.; Othman, M.H.D.; Hasbullah, H.; Said, N.; Kadir, S.; Kamal, F.; Abdullah, M.S.; Ng, B.C. Antifouling polyethersulfone hemodialysis membranes incorporated with poly (citric acid) polymerized multi-walled carbon nanotubes. Mater. Sci. Eng. C 2016, 68, 540–550. [Google Scholar] [CrossRef]
- Yerushalmi, R.; Scherz, A.; van der Boom, M.E.; Kraatz, H.-B. Stimuli responsive materials: New avenues toward smart organic devices. J. Mater. Chem. 2005, 15, 4480–4487. [Google Scholar] [CrossRef]
- Xie, R.; Li, Y.; Chu, L.Y. Preparation of thermo-responsive gating membranes with controllable response temperature. J. Membr. Sci. 2007, 289, 76–85. [Google Scholar] [CrossRef]
- Pokharel, P.; Pant, B.; Pokhrel, K.; Pant, H.R.; Lim, J.G.; Lee, D.S.; Kim, H.Y.; Choi, S. Effects of functional groups on the graphene sheet for improving the thermomechanical properties of polyurethane nanocomposites. Compos. Part B Eng. 2015, 78, 192–201. [Google Scholar] [CrossRef]
- Strandman, S.; Zhu, X.X. Thermo-responsive block copolymers with multiple phase transition temperatures in aqueous solutions. Prog. Polym. Sci. 2015, 42, 154–176. [Google Scholar] [CrossRef]
- Zhang, J.; Raza, S.; Wang, P.; Wen, H.; Zhu, Z.; Huang, W.; Mohamed, I.M.A.; Liu, C. Polymer brush-grafted ZnO-modified cotton for efficient oil/water separation with abrasion/acid/alkali resistance and temperature “switch” property. J. Colloid Interface Sci. 2020, 580, 822–833. [Google Scholar] [CrossRef]
- Dutta, K.; De, S. Smart responsive materials for water purification: An overview. J. Mater. Chem. A 2017, 5, 22095–22112. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Thermo-responsive polymers and advances in their applications in separation science. Microchem. J. 2022, 179, 107554. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, J.-H.; Yan, B.-F.; Wu, D.; Zhang, Q.-Q. Thermo-responsive and antifouling PVDF nanocomposited membranes based on PNIPAAm modified TiO2 nanoparticles. Chin. J. Polym. Sci. 2014, 32, 892–905. [Google Scholar] [CrossRef]
- Du, X.; Wang, Z.; Liu, W.; Xu, J.; Chen, Z.; Wang, C. Imidazolium-functionalized poly (arylene ether ketone) cross-linked anion exchange membranes. J. Membr. Sci. 2018, 566, 205–212. [Google Scholar] [CrossRef]
- Wu, C.J.; Xie, R.; Wei, H.-B.; Xu, T.T.; Liu, Z.; Wang, W.; Ju, X.J.; Chu, L.Y. Fabrication of a thermo-responsive membrane with cross-linked smart gates via a ‘grafting-to’ method. RSC Adv. 2016, 6, 45428–45433. [Google Scholar] [CrossRef]
- Xie, R.; Song, X.-L.; Luo, F.; Liu, Z.; Wang, W.; Ju, X.-J.; Chu, L.-Y. Ethanol-responsive poly(vinylidene difluoride) membranes with nanogels as functional gates. Chem. Eng. Technol. 2016, 39, 841–848. [Google Scholar] [CrossRef]
- Lutz, J.F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Lutz, J.F.; Akdemir, Ö.; Hoth, A. Point by point comparison of two thermosensitive polymers exhibiting a similar LCST: Is the age of poly(NIPAM) over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Marquez, M.; Hu, Z. Monodisperse thermoresponsive microgels of poly(ethylene glycol) analogue-based biopolymers. Langmuir 2007, 23, 8663–8666. [Google Scholar] [CrossRef] [PubMed]
- Boularas, M.; Deniau-Lejeune, E.; Alard, V.; Tranchant, J.-F.; Billon, L.; Save, M. Dual stimuli-responsive oligo(ethylene glycol)-based microgels: Insight into the role of internal structure in volume phase transitions and loading of magnetic nanoparticles to design stable thermoresponsive hybrid microgels. Polym. Chem. 2016, 7, 350–363. [Google Scholar] [CrossRef]
- Dieuzy, E.; Auguste, S.; Chougrani, K.; Alard, V.; Billon, L.; Derail, C. Microgel structure-driven linear and non-linear mechanical properties of self-assembled microgel films. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126082. [Google Scholar] [CrossRef]
- Gawlitza, K.; Ivanova, O.; Radulescu, A.; Holderer, O.; von Klitzing, R.; Wellert, S. Bulk phase and surface dynamics of PEG microgel particles. Macromolecules 2015, 48, 5807–5815. [Google Scholar] [CrossRef]
- Gawlitza, K.; Radulescu, A.; von Klitzing, R.; Wellert, S. On the structure of biocompatible, thermoresponsive poly(ethylene glycol) microgels. Polymer 2014, 55, 6717–6724. [Google Scholar] [CrossRef]
- Li, X.; Xuan, Y.; Li, Q. Self-adaptive chip cooling with template-fabricated nanocomposite P(MEO2MA-co-OEGMA) hydrogel. Int. J. Heat Mass Transf. 2021, 166, 120790. [Google Scholar] [CrossRef]
- Aguirre, G.; Deniau, E.; Brulet, A.; Chougrani, K.; Alard, V.; Billon, L. Versatile oligo(ethylene glycol)-based biocompatible microgels for loading/release of active bio(macro)molecules. Colloids Surf. B 2019, 175, 445–453. [Google Scholar] [CrossRef]
- Ma, L.; Tang, H.; Wu, P. Volume phase transition mechanism of poly[di(ethylene glycol)ethyl ether acrylate]-Based microgels involving a thermosensitive poly(ionic liquid). Langmuir 2017, 33, 12326–12335. [Google Scholar] [CrossRef]
- Wellert, S.; Kesal, D.; Schon, S.; von Klitzing, R.; Gawlitza, K. Ethylene glycol-based microgels at solid surfaces: Swelling behavior and control of particle number density. Langmuir 2015, 31, 2202–2210. [Google Scholar] [CrossRef]
- Wilms, D.; Adler, Y.; Schroer, F.; Bunnemann, L.; Schmidt, S. Elastic modulus distribution in poly(N-isopopylacrylamide) and oligo(ethylene glycol methacrylate)-based microgels studied by AFM. Soft Matter 2021, 17, 5711–5717. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Mai, W.; Min, C.; Zhou, B.; Shan, M.; Li, Y.; Yang, C.; Wang, Z.; Qian, X. Improved hydrophilicity, permeability, antifouling and mechanical performance of PVDF composite ultrafiltration membranes tailored by oxidized low-dimensional carbon nanomaterials. J. Mater. Chem. A 2013, 1, 3101–3111. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Bi, S.; Fu, W.; Shi, C.; Chen, L. Conferring pH-sensitivity on poly(vinylidene fluoride) membrane by poly(acrylic acid-co-butyl acrylate) microgels. React. Funct. Polym. 2014, 74, 58–66. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Bi, S.; Shi, C.; Chen, L.; Li, L. Structure and pH-sensitive properties of poly (vinylidene fluoride) membrane changed by blending poly (acrylic acid) microgels. Polym. Adv. Technol. 2013, 24, 934–944. [Google Scholar] [CrossRef]
- Hao, Y.; Moriya, A.; Maruyama, T.; Ohmukai, Y.; Matsuyama, H. Effect of metal ions on humic acid fouling of hollow fiber ultrafiltration membrane. J. Membr. Sci. 2011, 376, 247–253. [Google Scholar] [CrossRef]
- Liu, F.; Abed, M.R.M.; Li, K. Preparation and characterization of poly(vinylidene fluoride) (PVDF) based ultrafiltration membranes using nano γ-Al2O3. J. Membr. Sci. 2011, 366, 97–103. [Google Scholar] [CrossRef]
- Li, X.Y.; Xie, R.; Zhang, C.; Chen, Z.H.; Hu, J.Q.; Ju, X.J.; Wang, W.; Liu, Z.; Chu, L.Y. Effects of hydrophilicity of blended submicrogels on the microstructure and performance of thermo-responsive membranes. J. Membr. Sci. 2019, 584, 202–215. [Google Scholar] [CrossRef]
- Zhang, S.; Manasa, P.; Wang, Q.; Li, D.; Dang, X.; Ran, F. Grafting copolymer of thermo-responsive and polysaccharide chains for surface modification of high performance membrane. Sep. Purif. Technol. 2020, 240, 116585. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Liu, T.; Xu, X.; Hu, Y. Improving the perm-selectivity and anti-fouling property of UF membrane through the micro-phase separation of PSf-b-PEG block copolymers. J. Membr. Sci. 2020, 599, 117851. [Google Scholar] [CrossRef]
- Wang, G.; Xie, R.; Ju, X.J.; Chu, L.Y. Thermo-Responsive Polyethersulfone composite membranes blended with Poly(N-isopropylacrylamide) nanogels. Chem. Eng. Technol. 2012, 35, 2015–2022. [Google Scholar] [CrossRef]
- Peinemann, K.V.; Abetz, V.; Simon, P.F. Asymmetric superstructure formed in a block copolymer via phase separation. Nat. Mater. 2007, 6, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, A.; Madaeni, S.S. Improvement of performance and surface properties of nano-porous polyethersulfone (PES) membrane using hydrophilic monomers as additives in the casting solution. J. Membr. Sci. 2010, 360, 371–379. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, S.; Liu, C.X.; Shen, L.; Wang, S.L.; Lang, W.Z.; Wang, Y. Smart TFC membrane for simulated textile wastewater concentration at elevated temperature enabled by thermal-responsive microgels. Desalination 2021, 500, 114870. [Google Scholar] [CrossRef]

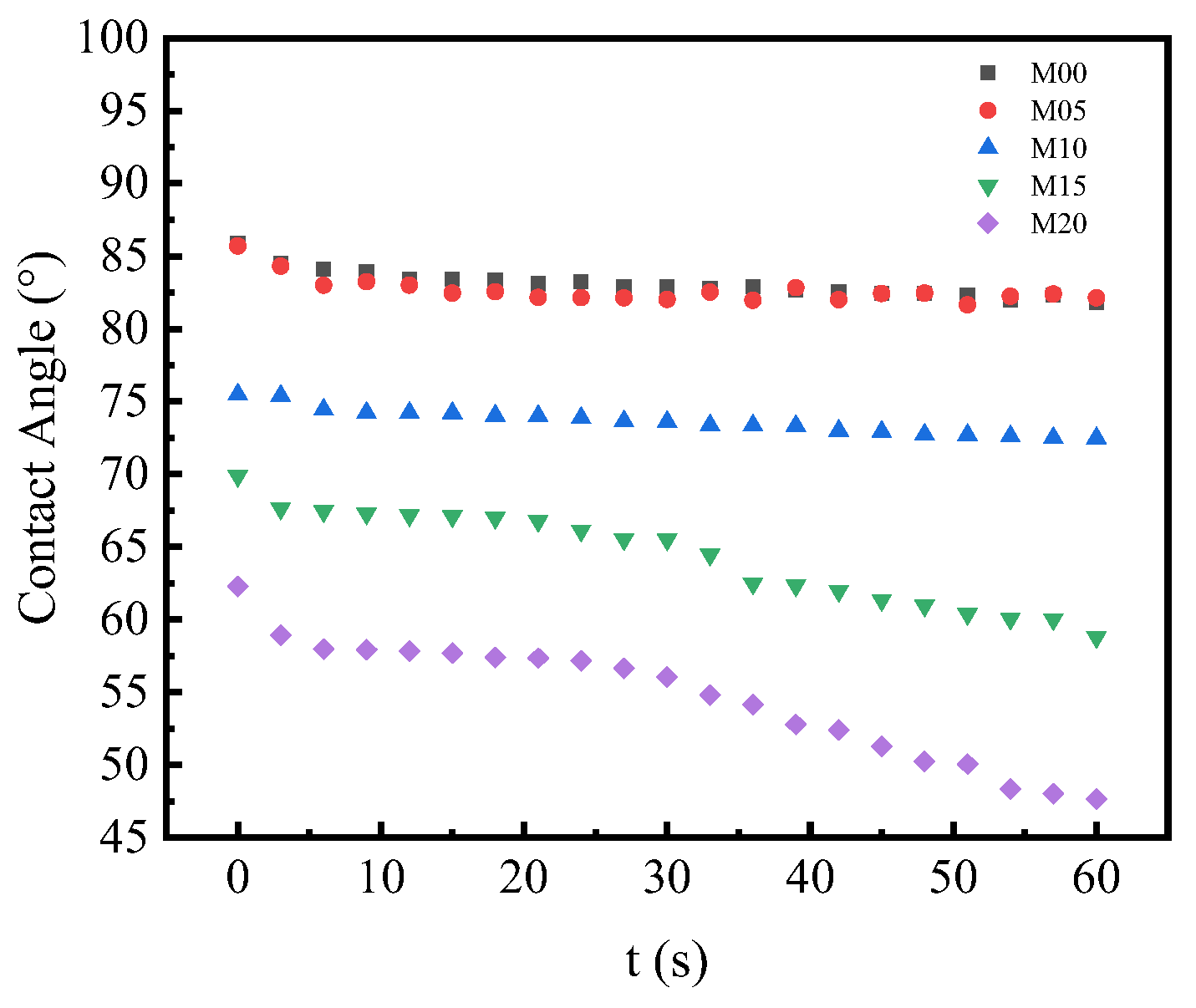
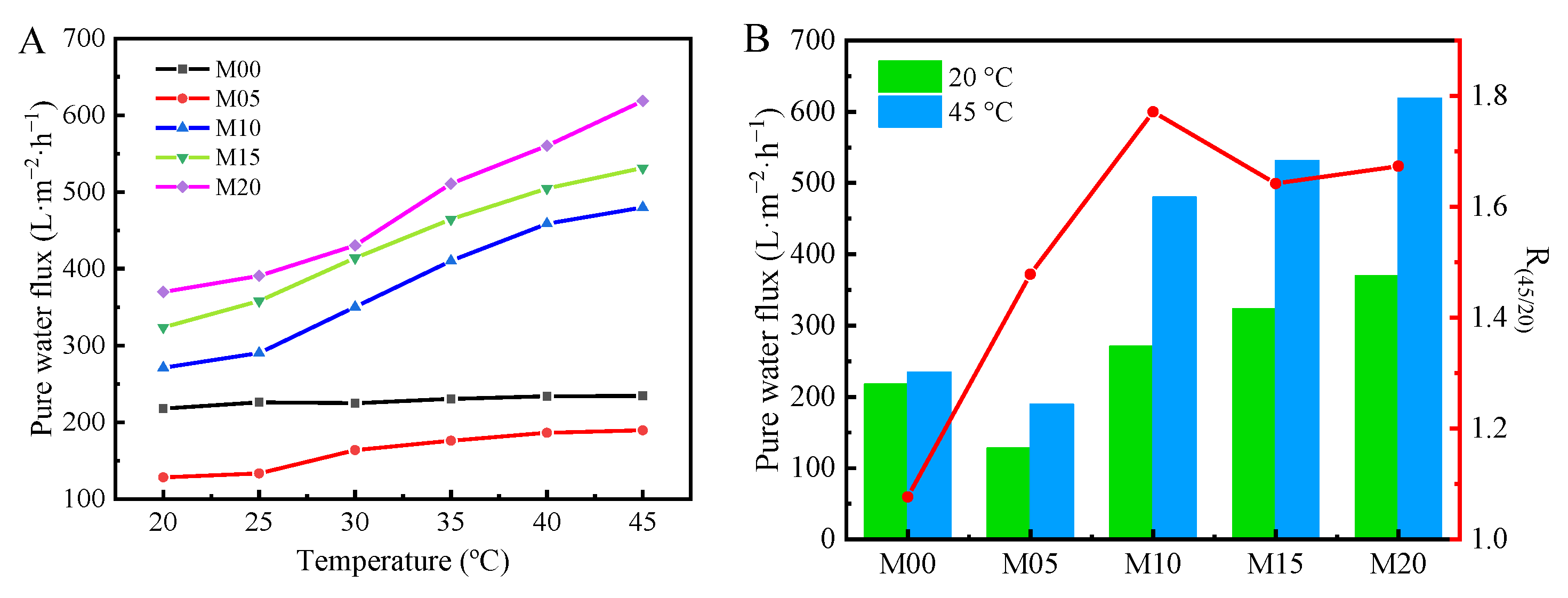

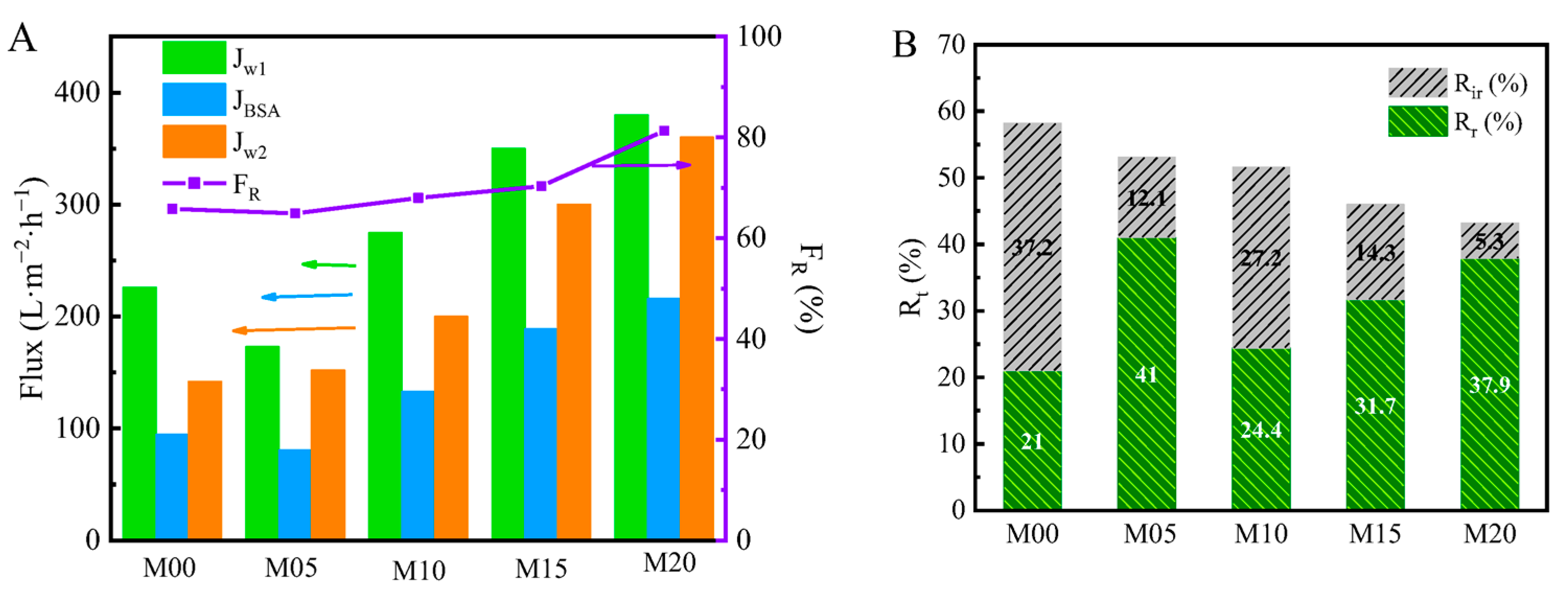
| Membrane Code | PMO Microgels (g) | PES (g) | NMP (g) | MG/PES (wt.%) |
|---|---|---|---|---|
| M00 | 0.0 | 18 | 100 | 0 |
| M05 | 0.9 | 18 | 100 | 5 |
| M10 | 1.8 | 18 | 100 | 10 |
| M15 | 2.7 | 18 | 100 | 15 |
| M20 | 3.6 | 18 | 100 | 20 |
| Membrane Code | Atomic Concentration (%) | Atomic Ratio | |||||
|---|---|---|---|---|---|---|---|
| C | O | N | S | O/C | N/C | N/O | |
| M00 | 79.02 | 15.16 | 0 | 5.82 | 0.192 | 0 | 0 |
| M20 | 71.71 | 21.99 | 2.99 | 3.30 | 0.307 | 0.042 | 0.136 |
| Membrane Code | Porosity, ε (%) | Pore Size, rm (nm) |
|---|---|---|
| M00 | 74 | 18 |
| M05 | 89 | 12 |
| M10 | 73 | 21 |
| M15 | 70 | 27 |
| M20 | 66 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Zhu, S.; Nie, J.; Du, B. Thermo-Sensitive Microgel/Poly(ether sulfone) Composited Ultrafiltration Membranes. Materials 2023, 16, 5149. https://doi.org/10.3390/ma16145149
Fan W, Zhu S, Nie J, Du B. Thermo-Sensitive Microgel/Poly(ether sulfone) Composited Ultrafiltration Membranes. Materials. 2023; 16(14):5149. https://doi.org/10.3390/ma16145149
Chicago/Turabian StyleFan, Wei, Shaoxiong Zhu, Jingjing Nie, and Binyang Du. 2023. "Thermo-Sensitive Microgel/Poly(ether sulfone) Composited Ultrafiltration Membranes" Materials 16, no. 14: 5149. https://doi.org/10.3390/ma16145149







