Evaluation of Cytotoxicity of Hyaluronic Acid/Chitosan/Bacterial Cellulose-Based Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BC Membranes
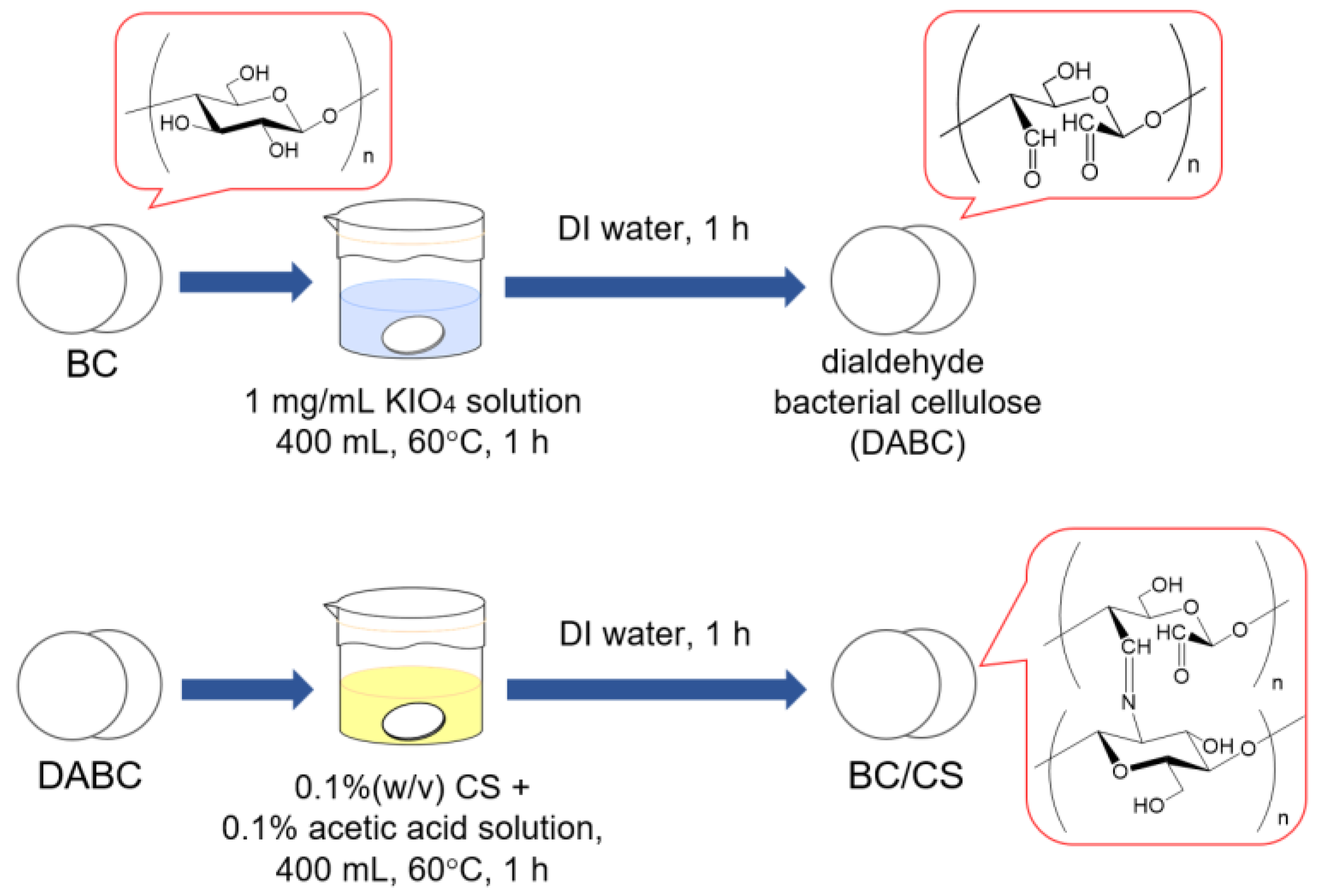
2.3. Preparation of Chitosan-Modified Bacterial Cellulose (BC/CS) Membranes by Periodate Oxidation
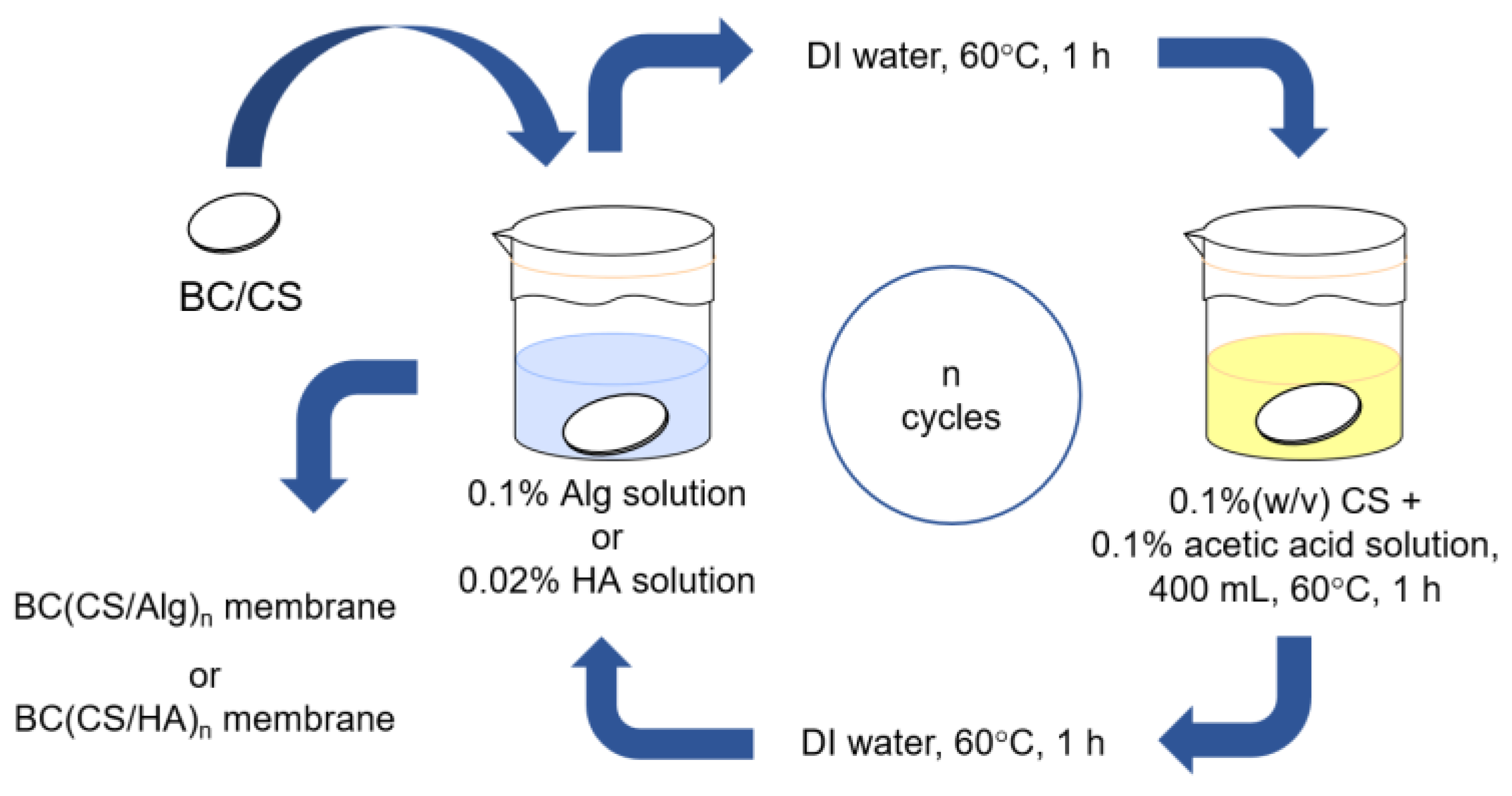
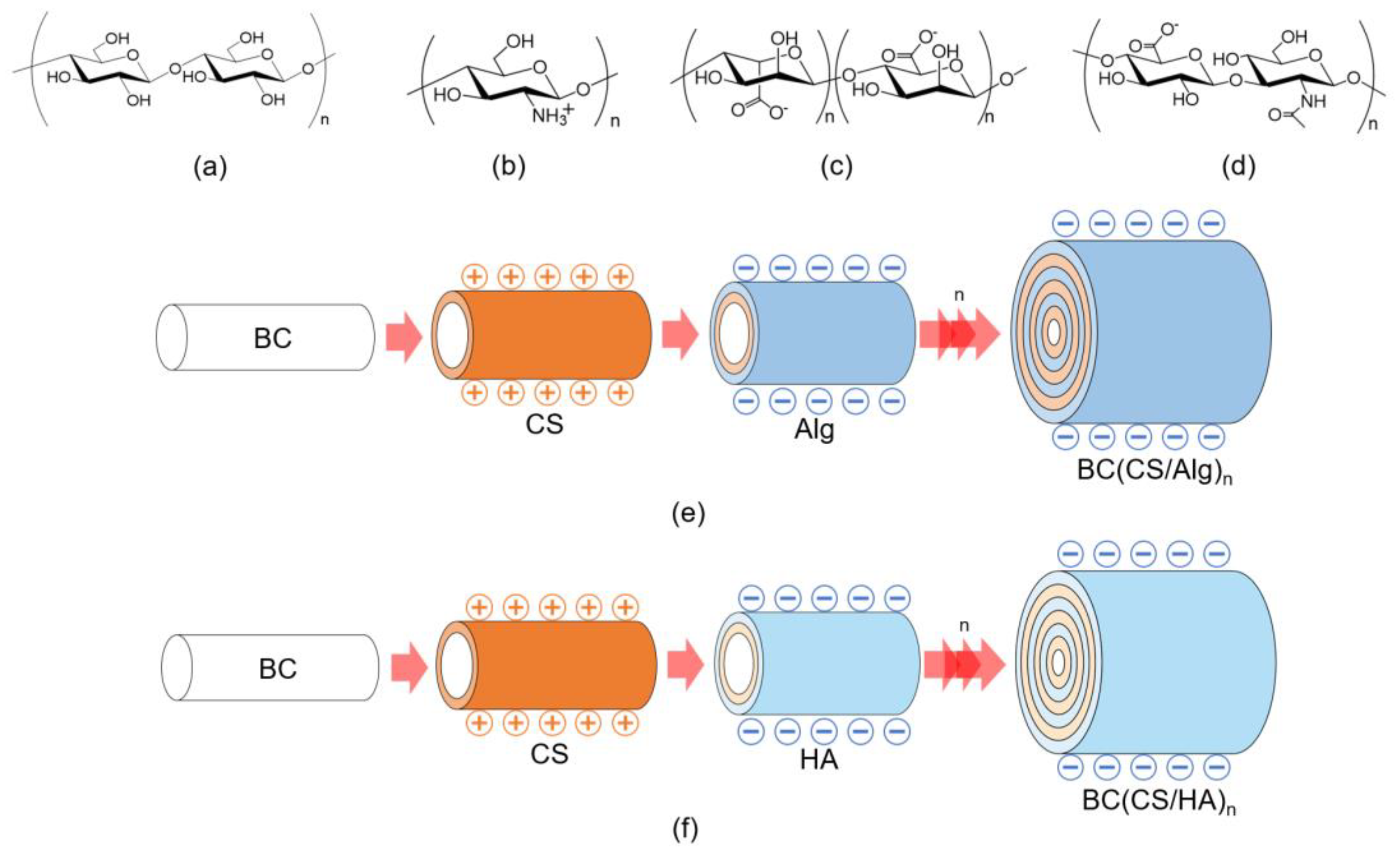
2.4. Preparation of Chitosan–Alginate and Chitosan–Hyaluronic Acid Laminated BC Membranes Using the LBL Method
2.4.1. Preparation of Chitosan-Alginate Laminated Membranes
2.4.2. Preparation of Chitosan-Hyaluronic Acid-Laminated Membranes
2.5. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.6. Thermogravimetric Analysis (TGA)
2.7. Scanning Electron Microscopy (SEM)
2.8. Thickness
2.9. Tensile Properties
2.10. Cytotoxicity Evaluation
2.10.1. Cell Propagation for Cytotoxicity Test
2.10.2. Cytotoxicity Test
3. Results and Discussion
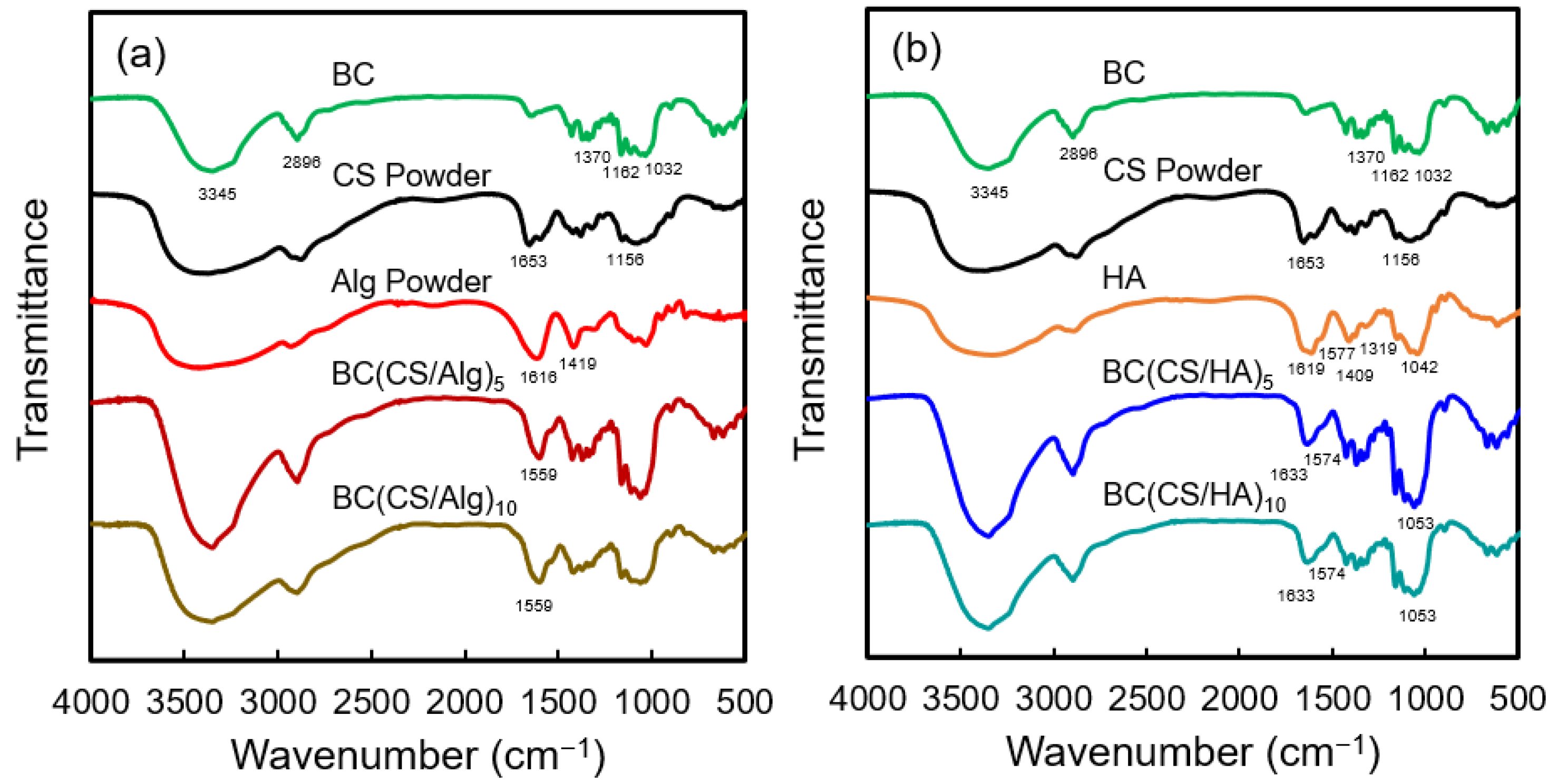
3.1. FT-IR Analysis
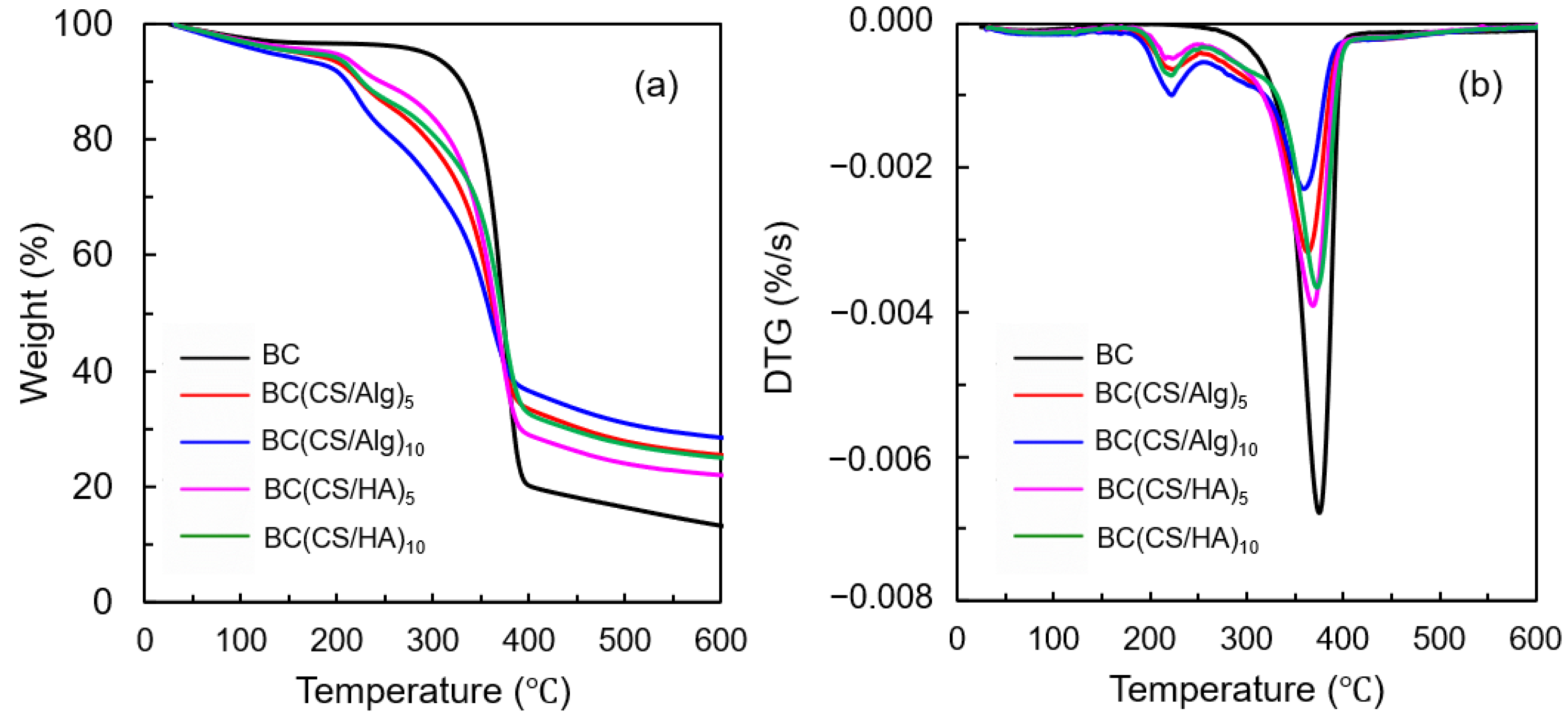
3.2. Thermal Analysis
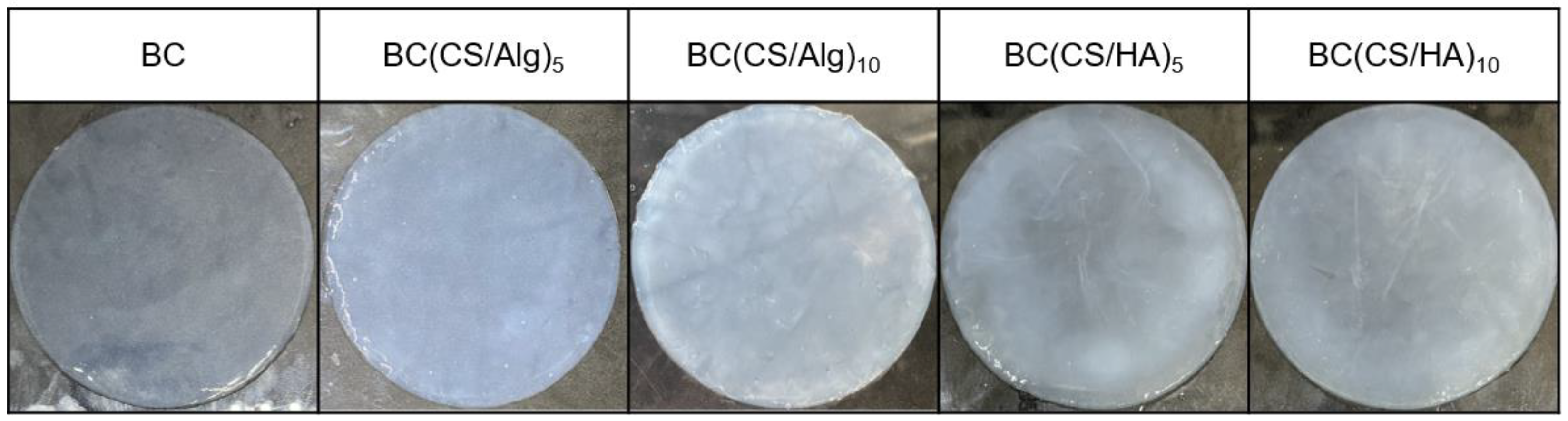
3.3. Physical Appearance and Morphology of the Membrane
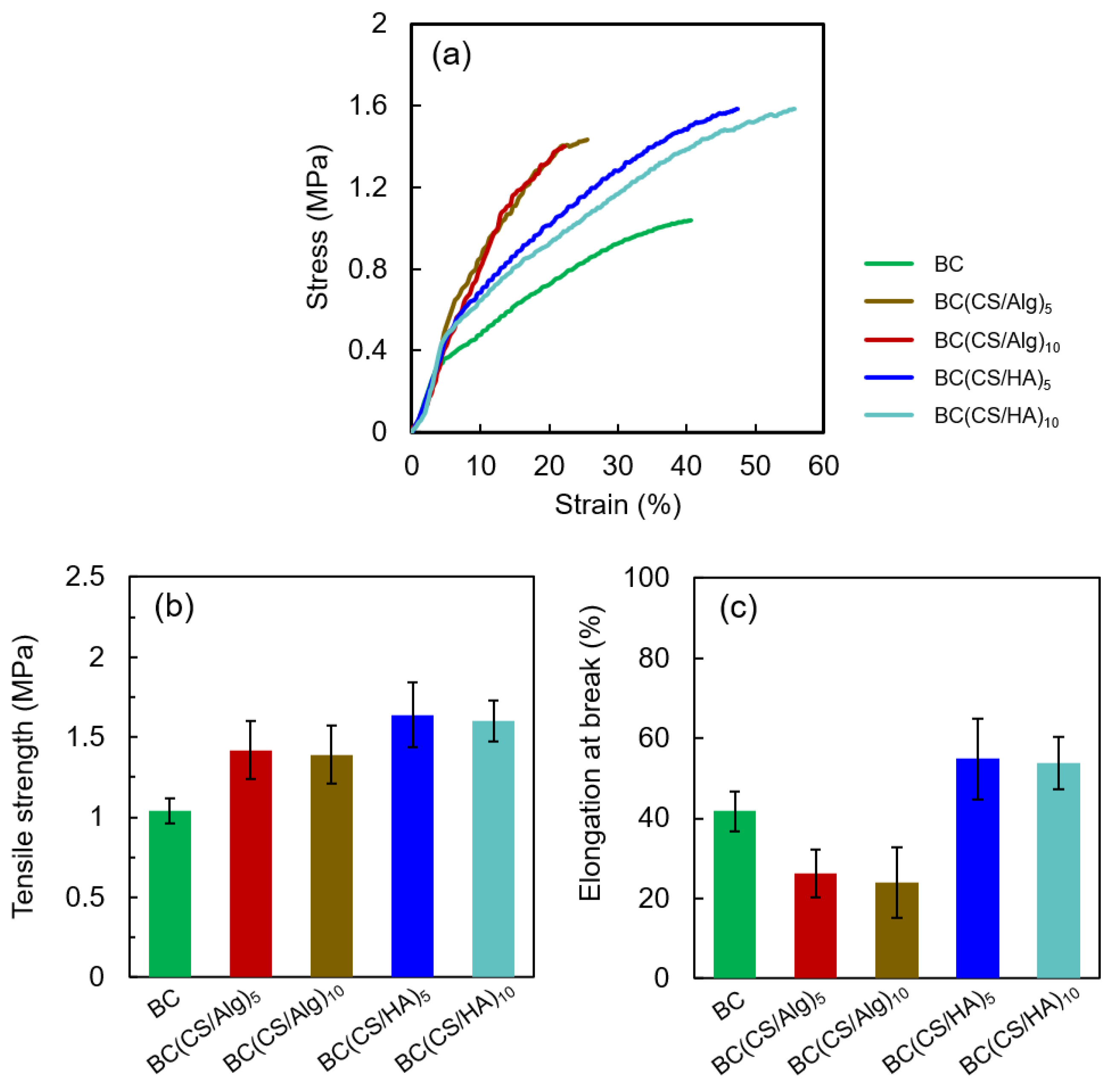
3.4. Thickness in the Swollen State, Moisture Content and Tensile Properties
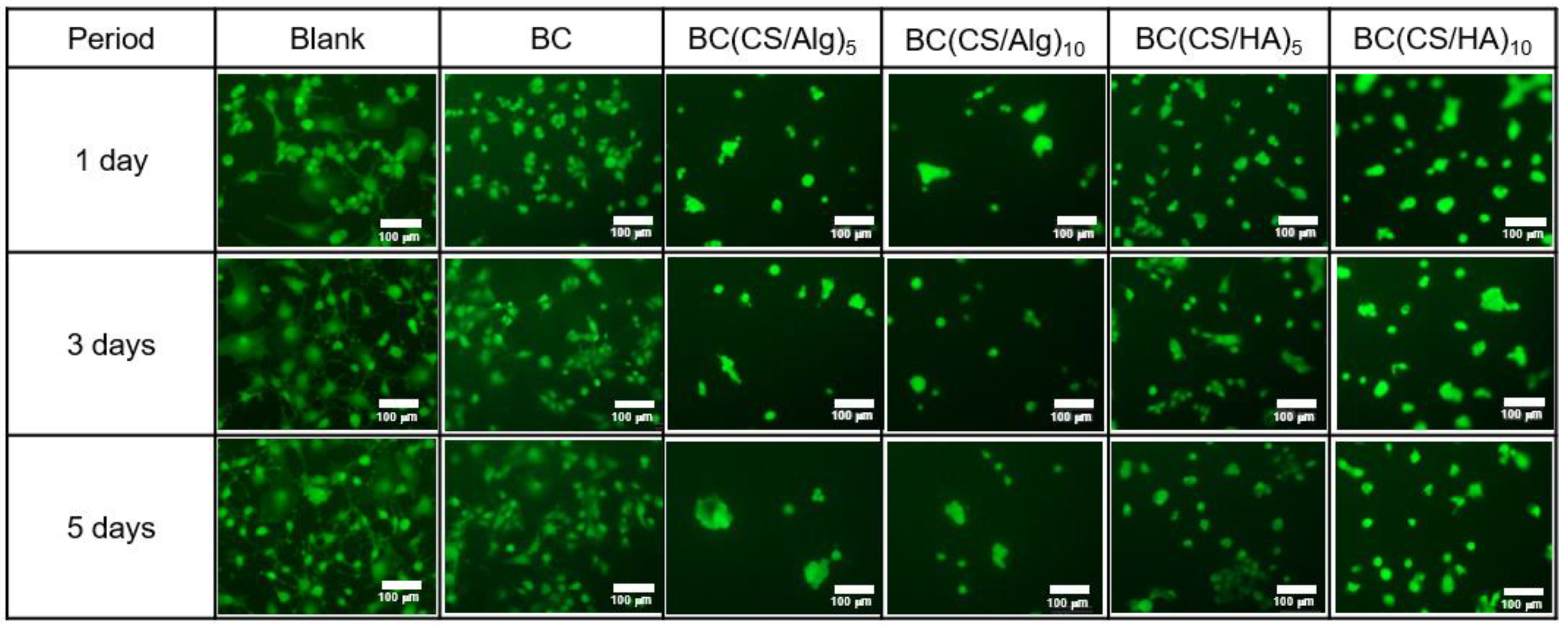
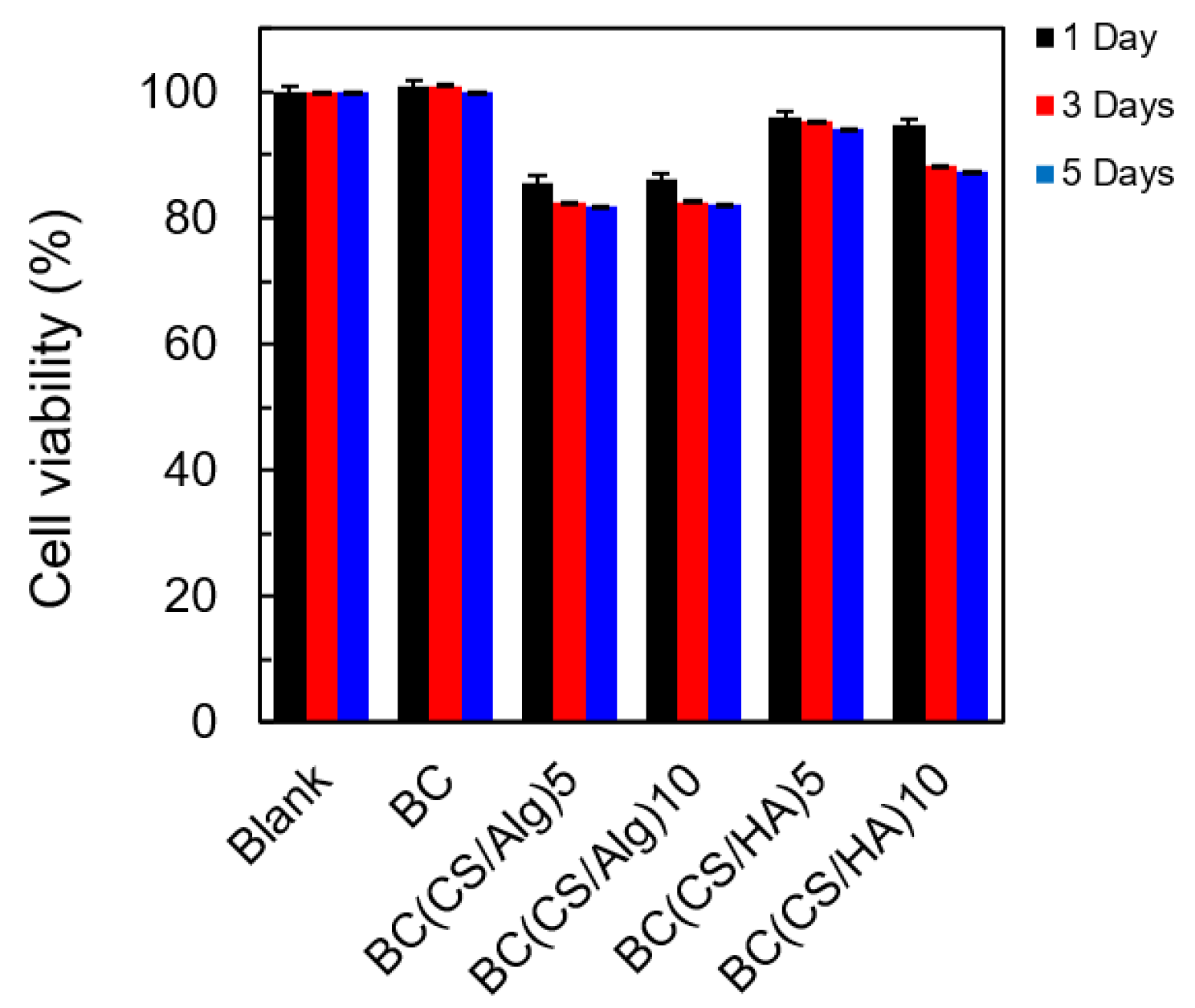
3.5. Cytotoxicity Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, F.; Deng, H.; Du, Y.; Shi, X.; Wang, Q. Emerging chitin and chitosan nanofibrous materials for biomedical applications. Nanoscale 2014, 6, 9477–9493. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, Y.; Wu, Y.; Shi, X.; Du, Y.; Deng, H. Chitosan/tannic acid bilayers layer-by-layer deposited cellulose nanofibrous mats for antibacterial application. Int. J. Biol. Macromol. 2019, 139, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Husteden, C.; Doberenz, F.; Goergen, N.; Pinnapireddy, S.R.; Janich, C.; Langner, A.; Syrowatka, F.; Repanas, A.; Erdmann, F.; Jedelská, J.; et al. Contact-triggered lipofection from multilayer films designed as surfaces for in situ transfection strategies in tissue engineering. ACS Appl. Mater. Interfaces 2020, 12, 8963–8977. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H. Preparation and characterization of ferulic acid-modified water soluble chitosan and poly (gamma-glutamic acid) polyelectrolyte films through layer-by-layer assembly towards protein adsorption. Int. J. Biol. Macromol. 2021, 171, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Guo, C.; Liao, W.; Liu, X.; Wang, Q. Development and characterization of chitosan/bacterial cellulose/pullulan bilayer film with sustained release curcumin. Int. J. Biol. Macromol. 2023, 226, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M.; et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and chitosan: Biopolymers for wound management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Fontana, J.D.; De Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; De Souza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F.X. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990, 24, 253–264. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M., Jr. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.H.; Kim, J.E.; Go, J.; Koh, E.K.; Song, S.H.; Son, H.J.; Kim, H.S.; Yun, Y.H.; Jung, Y.J.; Hwang, D.Y. Bacterial cellulose membrane produced by Acetobacter sp. A10 for burn wound dressing applications. Carbohydr. Polym. 2015, 122, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials. 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Silva, J.; Vanat, P.; Marques-da-Silva, D.; Rodrigues, J.R.; Lagoa, R. Metal alginates for polyphenol delivery systems: Studies on crosslinking ions and easy-to-use patches for release of protective flavonoids in skin. Bioact. Mater. 2020, 5, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.R.S.; Tamer, T.M.; El-Meligy, M.A.; Eldin, M.S.M. Poly(vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Ozaki, C.K.; Hamdan, A.D.; Barshes, N.R.; Wyers, M.; Hevelone, N.D.; Belkin, M.; Nguyen, L.L. Prospective, randomized, multi-institutional clinical trial of a silver alginate dressing to reduce lower extremity vascular surgery wound complications. J. Vasc. Surg. 2015, 61, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Dowling, M.B.; Chaturvedi, A.; MacIntire, I.C.; Javvaji, V.; Gustin, J.; Raghavan, S.R.; Scalea, T.M.; Narayan, M. Determination of efficacy of a novel alginate dressing in a lethal arterial injury model in swine. Injury 2016, 47, 2105–2109. [Google Scholar] [CrossRef] [Green Version]
- Kurczewska, J.; Pecyna, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Halloysite nanotubes as carriers of vancomycin in alginate-based wound dressing. Saudi Pharm. J. 2017, 25, 911–920. [Google Scholar] [CrossRef]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Hu, B.; Guo, Y.; Li, H.; Liu, X.; Fu, Y.; Ding, F. Recent advances in chitosan-based layer-by-layer biomaterials and their biomedical applications. Carbohydr. Polym. 2021, 271, 118427. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef]
- Ibrahim, A.; Khalil, I.A.; Mahmoud, M.Y.; Bakr, A.F.; Ghoniem, M.G.; Al-Farraj, E.S.; El-Sherbiny, I.M. Layer-by-layer development of chitosan/alginate-based platelet-mimicking nanocapsules for augmenting doxorubicin cytotoxicity against breast cancer. Int. J. Biol. Macromol. 2023, 225, 503–517. [Google Scholar] [CrossRef]
- Kotatha, D.; Morishima, K.; Uchida, S.; Ogino, M.; Ishikawa, M.; Furuike, T.; Tamura, H. Preparation and characterization of gel electrolyte with bacterial cellulose coated with alternating layers of chitosan and alginate for electric double-layer capacitors. Res. Chem. Intermed. 2018, 44, 4971–4987. [Google Scholar] [CrossRef]
- Gomes, A.P.; Mano, J.F.; Queiroz, J.A.; Gouveia, I.C. Layer-by-layer deposition of antibacterial polyelectrolytes on cotton fibres. J. Polym. Environ. 2012, 20, 1084–1094. [Google Scholar] [CrossRef]
- Gomes, A.P.; Mano, J.F.; Queiroz, J.A.; Gouveia, I.C. Incorporation of antimicrobial peptides on functionalized cotton gauzes for medical applications. Carbohydr. Polym. 2015, 127, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Li, W.; Lv, X.; Lei, Z.; Bian, Y.; Deng, H.; Wang, H.; Li, J.; Li, X. Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials 2015, 53, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ma, X.; Fan, L.; Gao, Y.; Deng, H.; Wang, Y. Accelerating dermal wound healing and mitigating excessive scar formation using LBL modified nanofibrous mats. Mater. Des. 2020, 185, 108265. [Google Scholar] [CrossRef]
- Mandapalli, P.K.; Labala, S.; Bojja, J.; Venuganti, V.V.K. Effect of pirfenidone delivered using layer-by-layer thin film on excisional wound healing. Eur. J. Pharm. Sci. 2016, 83, 166–174. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/hyaluronic acid/alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing—Know-how. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Luo, Z.; Dai, Y.; Gao, H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Al-Khateeb, R.; Olszewska-Czyz, I. Biological molecules in dental applications: Hyaluronic acid as a companion biomaterial for diverse dental applications. Heliyon 2020, 6, e03722. [Google Scholar] [CrossRef]
- Lafuente-Merchan, M.; Ruiz-Alonso, S.; Espona-Noguera, A.; Galvez-Martin, P.; López-Ruiz, E.; Marchal, J.A.; López-Donaire, M.L.; Zabala, A.; Ciriza, J.; Saenz-del-Burgo, L.; et al. Development, characterization and sterilisation of nanocellulose-alginate-(hyaluronic acid)- bioinks and 3D bioprinted scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 126, 112160. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, H.; Zheng, W.; Gong, N.; Chen, L.; Jiang, X.; Yang, G. Bacterial cellulose–hyaluronan nanocomposite biomaterials as wound dressings for severe skin injury repair. J. Mater. Chem. B 2015, 3, 3498–3507. [Google Scholar] [CrossRef]
- del Hoyo-Gallego, S.; Pérez-Álvarez, L.; Gómez-Galván, F.; Lizundia, E.; Kuritka, I.; Sedlarik, V.; Laza, J.M.; Vila-Vilela, J.L. Construction of antibacterial poly(ethyleneterephthalate) films via layer by layer assembly of chitosan and hyaluronic acid. Carbohydr. Polym. 2016, 143, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Oe, T.; Dechojarassri, D.; Kakinoki, S.; Kawasaki, H.; Furuike, T.; Tamura, H. Microwave-assisted incorporation of AgNP into chitosan–alginate hydrogels for antimicrobial applications. J. Funct. Biomater. 2023, 14, 199. [Google Scholar] [CrossRef]
- Wang, X.; Tang, J.; Huang, J.; Hui, M. Production and characterization of bacterial cellulose membranes with hyaluronic acid and silk sericin. Colloids Surf. B Biointerfaces 2020, 195, 111273. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Dynamic and self-healable chitosan/hyaluronic acid-based in situ-forming hydrogels. Gels 2022, 8, 477. [Google Scholar] [CrossRef]
- Yang, P.-F.; Lee, C.-K. Hyaluronic acid interaction with chitosan-conjugated magnetite particles and its purification. Biochem. Eng. J. 2007, 33, 284–289. [Google Scholar] [CrossRef]
- Gatej, I.; Popa, M.; Rinaudo, M. Role of the pH on hyaluronan behavior in aqueous solution. Biomacromolecules 2005, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Li, L. Enhanced stability and mechanical strength of sodium alginate composite films. Carbohydr. Polym. 2017, 160, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.I.; Cibrão, A.C.; Correia, C.R.; Carvalho, R.R.; Luz, G.M.; Ferrer, G.G.; Botelho, G.; Picart, C.; Alves, N.M.; Mano, J.F. Nanostructured polymeric coatings based on chitosan and dopamine-modified hyaluronic acid for biomedical applications. Small 2014, 12, 2459–2469. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Wang, S.; Wang, W.; Lin, G.; Niu, B.; Guo, R.; Yan, H.; Wang, H. Sodium alginate/chitosan-based intelligent bilayer film with antimicrobial activity for pork preservation and freshness monitoring. Food Control 2023, 148, 109615. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.-Y.; Zhang, J.; Niu, B.; Li, W. Preparation and characterization of multilayer films composed of chitosan, sodium alginate and carboxymethyl chitosan-ZnO nanoparticles. Food Chem. 2019, 283, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.-A.; Wu, H.-G. Characterization of chitosan-hyaluronic acid blended membranes and their biocompatibility with keratocytes. In Proceedings of the 3rd International Conference on Biomedical Engineering and Informatics, Yantai, China, 16–18 October 2010. [Google Scholar] [CrossRef]
- Tamer, T.M.; Hassan, M.A.; Valachová, K.; Omer, A.M.; El-Shafeey, M.E.A.; Mohy Eldin, M.S.; Šoltés, L. Enhancement of wound healing by chitosan/hyaluronan polyelectrolyte membrane loaded with glutathione: In Vitro and in vivo evaluations. J. Biotech. 2020, 310, 103–113. [Google Scholar] [CrossRef] [PubMed]

| Sample Name | Coating Cycles |
|---|---|
| BC | - |
| BC(CS/Alg)5 | 5 |
| BC(CS/Alg)10 | 10 |
| BC(CS/HA)5 | 5 |
| BC(CS/HA)10 | 10 |
| Sample Name | Thickness (mm) in the Swollen State | Thickness (mm) of Dried Membrane | Moisture Content (%) of Dried Membrane |
|---|---|---|---|
| BC | 0.49 ± 0.08 | 0.007 ± 0.004 | 3.20 ± 0.07 |
| BC(CS/Alg)5 | 0.49 ± 0.06 | 0.042 ± 0.013 | 4.36 ± 0.13 |
| BC(CS/Alg)10 | 0.34 ± 0.04 | 0.062 ± 0.022 | 4.73 ± 0.23 |
| BC(CS/HA)5 | 0.30 ± 0.06 | 0.015 ± 0.005 | 3.99 ± 0.06 |
| BC(CS/HA)10 | 0.32 ± 0.06 | 0.027 ± 0.015 | 4.25 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dechojarassri, D.; Okada, T.; Tamura, H.; Furuike, T. Evaluation of Cytotoxicity of Hyaluronic Acid/Chitosan/Bacterial Cellulose-Based Membrane. Materials 2023, 16, 5189. https://doi.org/10.3390/ma16145189
Dechojarassri D, Okada T, Tamura H, Furuike T. Evaluation of Cytotoxicity of Hyaluronic Acid/Chitosan/Bacterial Cellulose-Based Membrane. Materials. 2023; 16(14):5189. https://doi.org/10.3390/ma16145189
Chicago/Turabian StyleDechojarassri, Duangkamol, Tomoki Okada, Hiroshi Tamura, and Tetsuya Furuike. 2023. "Evaluation of Cytotoxicity of Hyaluronic Acid/Chitosan/Bacterial Cellulose-Based Membrane" Materials 16, no. 14: 5189. https://doi.org/10.3390/ma16145189






