Abstract
The influences of Mg2+ and Ca2+ on the short-term (1800 s) corrosion behavior of X100 pipeline steel were investigated in a sodium chloride (NaCl) solution saturated with CO2. Either Ca2+ or Mg2+ in the solution inhibited the short-term corrosion of X100 pipeline steel, with the corrosion current density decreasing from 262.4 μA cm−2 to 163.5 μA cm−2 or 80.8 μA cm−2. During longer-term (8−48 h) immersion, the Mg2+ inhibited the formation of the protective scale, whereas the Ca2+ accelerated the formation of the scale. Further, an experimental equation establishing the relationship between the precipitation rate of the corrosion scale and the exposure time was proposed to quantitatively study the effects of Mg2+ and Ca2+ on the precipitation rate of the corrosion scale.
1. Introduction
The global oil demand will grow from 84 million barrels per day in 2010 to 123 million barrels per day in 2025, as reported by the United States Energy Information Administration [1]. Pipelines are widely used to transport significant amounts of oil [2]. CO2 corrosion is a significant problem for pipeline steel. The results of pipeline leakage induced by corrosion are severe [3]. The pipeline operators use some predictive models of CO2 corrosion to determine operating parameters, for instance, the pressures, appropriate inhibitors, and flowing velocities [4]. One of the important factors that these prediction models take into consideration is the chemistry of CO2-containing water, which may further carry some salts such as magnesium chloride (MgCl2) and calcium chloride (CaCl2) [4]. The precise understanding of the role of Mg2+ and Ca2+ in the corrosion of pipeline steel in CO2-containing water can help improve these predictive models, which have huge commercial and environmental importance.
The precipitation of protective iron carbonate (FeCO3) on steels in CO2-containing water needs an induction time [5]. Prior to FeCO3 precipitation, general corrosion often occurs in the active zones of steels, such as ferrite [6]. As the concentration of ferrous (Fe2+) increases, FeCO3 accumulates on the specimen [7]. If Mg2+ and Ca2+ can affect the initial corrosion prior to FeCO3 formation, the addition of Mg2+ and Ca2+ may influence the subsequent scale precipitation by influencing the initial steel dissolution, which provides Fe2+ for the scale formation. Therefore, such additions may be either beneficial (accelerating the formation of the scale) or otherwise (inhibiting the production of the scale) as far as the corrosion rate is considered. However, previous studies [5,7,8,9,10,11,12] only paid attention to the influences of cations on the corrosion of steel after scale precipitation without considering the initial corrosion prior to scale formation.
Further, the role of Mg2+ and Ca2+ in the corrosion of pipeline steels after the scale formation is under dispute. Jiang et al. stated that the initiation period for localized corrosion on N80 steel in the NaCl solutions containing 1.5 wt.% CaCl2 is prolonged and the corrosion rate is inhibited by the presence of Ca2+ at 57 °C [11]. Pots reported that a more porous and less protective scale that consists of iron and calcium carbonate can precipitate in the CO2 solution with the addition of Ca2+ [12]. However, Esmaeely et al. showed that low Ca2+ concentrations (10 ppm and 100 ppm) in solutions decrease the corrosion rates of steel at 80 °C because of the production of FeCO3 [7]. When the Ca2+ concentration exceeds 1000 ppm, a non-protective calcium carbonate (CaCO3) scale forms and the corrosion of steel deteriorates [7]. With regard to magnesium chloride (MgCl2), it has been proposed that MgCl2 additions decrease the required critical supersaturation for FeCO3 precipitation in the case of carbon steel immersed in the NaCl solutions [5]. In contrast, Chen et al. argued that the additions of Mg2+ restrain the formation of scales, both in the bulk solutions as well as on the stainless steel [13]. Additionally, during the establishment of various prediction models of CO2 corrosion, the precipitation rate of the corrosion scale needs to be considered and calculated [14,15]. However, to the best of our knowledge, there is no research study concerning the quantitative effects of Ca2+ and Mg2+ on the formation rate of the corrosion scale, which is an important parameter for establishing the prediction models.
In this study, the influences of Mg2+ and Ca2+ on the short-term (prior to scale formation) corrosion behavior of X100 pipeline steel were investigated by open circuit potential (OCP), potentiodynamic polarization (PDP), and scanning electron microscopy (SEM). Further, OCP, linear polarization resistance (LPR), and electrochemical impedance spectroscopy (EIS) were employed to study the longer-term (after scale formation) corrosion behavior of X100 pipeline steel. SEM, X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) were utilized to characterize the surface information of corrosion scales. Especially, the quantitative effects of Ca2+ and Mg2+ on the formation rate of the corrosion scale were gained. The results will help improve various predictive models that are widely used to evaluate the CO2 corrosion of steel pipelines.
2. Experimental
2.1. Materials and Electrolytes
The composition of the X100 pipeline steel in this study is given in Table 1. The specimens for electrochemical measurements were connected to a copper wire. The specimens sealed by the epoxy had a 1 cm2 front face. The specimens were sequentially ground with SiC papers of 120, 600, 800, and 1200 grit.

Table 1.
The chemical composition of X100 pipeline steel.
Deionized (DI) water, NaCl (Anachemia), anhydrous MgCl2 (Amresco), anhydrous CaCl2 (Anachemia), and sodium hydroxide (NaOH, Anachemia) were used to prepare the solutions. The desired Mg2+ and Ca2+ concentrations were achieved by the addition of MgCl2 and CaCl2. Table 2 presents the chemical compositions of the solutions in this work. The addition of CaCl2 and MgCl2 introduces extra Cl− ions that may influence the corrosion rate of steel; thus, all the solutions listed in Table 2 have the same Cl− concentration, which was adjusted with the NaCl concentration. The solutions were sparged with a 1-bar CO2 for 2 h before all the measurements were taken. Then, the specimens were placed in the solutions, and CO2 sparging was continued throughout the measurements. The solution temperature was maintained at 60 ± 1 °C. The pH values of the solutions were controlled to 7 ± 0.2 by the addition of NaOH. The pH values were tested by an ALpHAⒽ series epoxy electrode manufactured by OMEGA.

Table 2.
The chemical compositions of the solutions in this study.
2.2. Electrochemical and Surface Analysis Measurements
A Princeton Applied Research Versastat 4 was utilized for the various electrochemical tests. The standard three-electrode system for electrochemical measurements used graphite as the counter electrode, a saturated calomel electrode (SCE, 0.215 V vs. SHE) as the reference electrode, and X100 as the working electrode. The short-term OCP measurements were conducted for 1800 s. The longer-term corrosion behavior of the specimens was measured by OCP with 1 point per hour. At every 8 h interval within the OCP measurements, EIS and LPR measurements were conducted to characterize the scale evolution. LPR measurements were carried out from −0.02 to 0.02 V (vs. OCP) with a scan rate of 0.167 mV s−1. EIS measurements were tested in the frequency range of 0.01 Hz−10 kHz with a sampling rate of 10 points per decade. EIS data were analyzed and fitted by ZSimpWin. Here, it is assumed that EIS and LPR measurements would not influence the real evolution of longer-term OCP since they do not significantly polarize the samples during the measurements [16]. All the electrochemical measurements were repeated at least three times in order to obtain reliable results.
Surface corrosion morphology of the specimens was characterized by a field-emission scanning electron microscope (SEM, Zeiss Σigma, Shenzhen, China). X100 pipeline steel with corrosion scales on the surface was carbon-coated to mitigate the charging effects. The SEM measurements were repeated at three different positions in the selected area to ensure reliable results. The crystal structures of the corrosion scales were identified by a Rigaku MultiFlex machine (manufactured by Hangzhou Remai Technology Co., Ltd., Hangzhou, China) using Cu Kα radiation (wavelength: 0.15406 nm). All XRD measurements were completed from 20 deg to 55 deg with a rate of 0.5 deg min−1. The XRD measurements were repeated three times to ensure consistent results. XPS was tested on an Omicron & Leybold MAX200 with a monochromated Al Kα source (1486.6 eV). The absolute binding energies were calibrated by the C 1s line of adventitious carbon at 284.8 eV binding energy. Wide spectra were collected from areas of 300 μm × 300 μm, at ~10 nm depth, using a pass energy of 100 eV. High-resolution spectra were collected at the binding energies of Fe, O, C, Ca, Mg, and Mn using a pass energy of 20 eV. Shirley and linear backgrounds were used in the curve fitting process performed in XPS Peak software (XPS Peak Fit V4.1).
3. Results and Discussion
3.1. Effects of Mg2+ and Ca2+ on the Short-Term Corrosion Behavior of X100
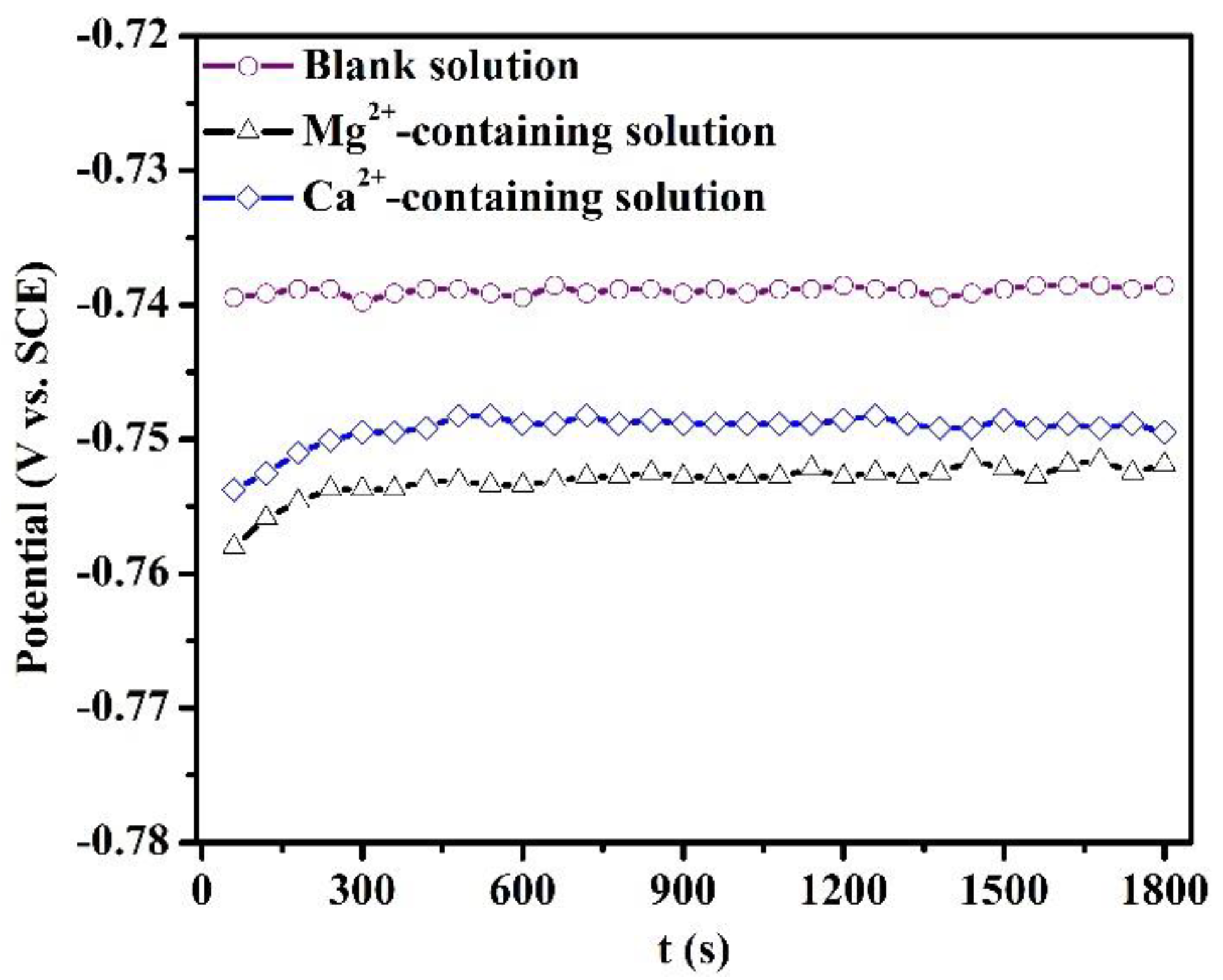
Figure 1 depicts the variation of OCP of specimens after 1800 s of immersion in the blank solutions with various additions. The fluctuation of OCP over the last 10 min of each OCP measurement in Figure 1 is less than 5 mV. The addition of Mg2+ or Ca2+ causes a shift of OCP to the cathodic direction. The maximum shift in the OCP was 15 mV for the Mg2+-containing solution. The presence of Ca2+ and Mg2+ can inhibit the cathodic reaction, thus decreasing the OCP.
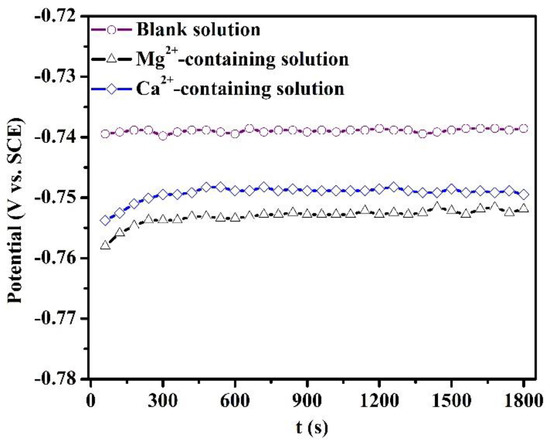
Figure 1.
Open circuit potential of the specimens after 1800 s of immersion in the blank solutions with various additions.
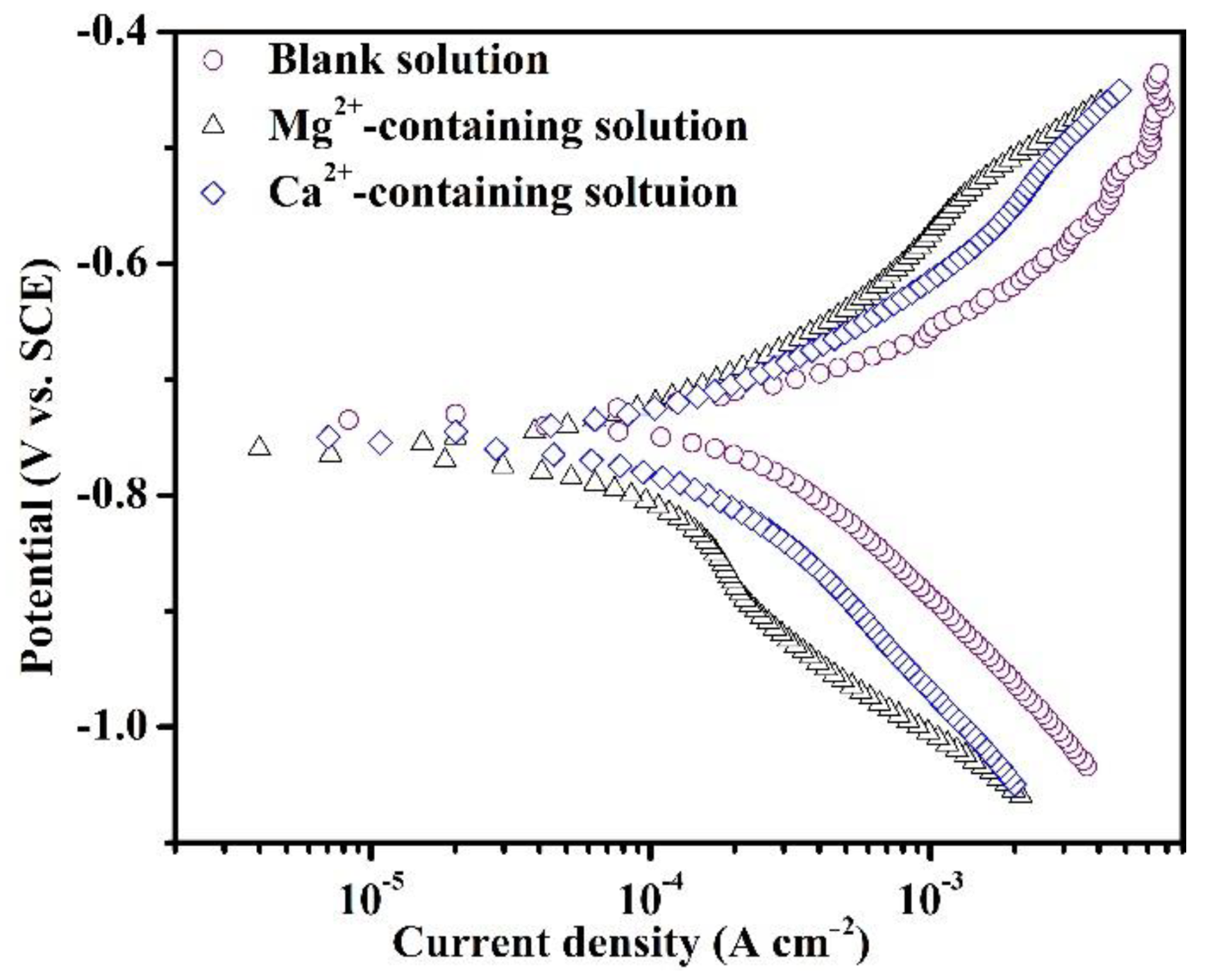
Figure 2 shows the PDP plots of the specimens in the blank solutions with various additions after 1800 s of immersion. The short immersion was selected prior to the precipitation of large amounts of corrosion products, thus enabling the study of the effects of different cations on the electrochemical reactions in the initial stage of corrosion. As reported in the literature [17], the cathodic process (1, 2, and 3) and dissolution behavior (4) should have proceeded in the blank solutions, depending on the following reactions:
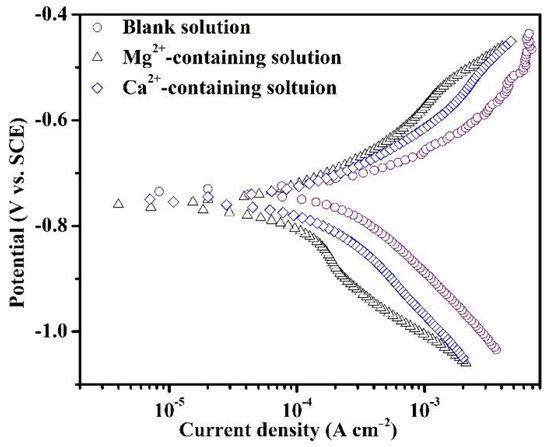
Figure 2.
Potentiodynamic polarization plots of the specimens after 1800 s of immersion in the blank solutions with various additions.
The related electrochemical parameters of PDP plots in Figure 2 are shown in Table 3. The presence of Mg2+ and Ca2+ decreases the icorr values, illustrating the inhibiting role of Mg2+ and Ca2+ in the corrosion of specimens. According to the icorr values, Mg2+ presents a better impeding effect compared to Ca2+. The negative shift of Ecorr values from −733.7 mV vs. SCE to −756.7 mV vs. SCE and −751.3 mV vs. SCE indicates an inhibition effect of cations on the cathodic reaction. The decrease in icorr after adding cations indicates a sluggish kinetics effect of cations during the initial corrosion process. The bc values for all three samples are more than 140 mV dec−1, indicating that the Volmer step is the rate-determining step of various processes involved in cathodic reactions.

Table 3.
Polarization parameters for specimens after 1800 s of immersion in the blank solutions with various additions. Ecorr is the corrosion potential, icorr stands for the corrosion current density, and bc as well as ba correspond to the Tafel slope.
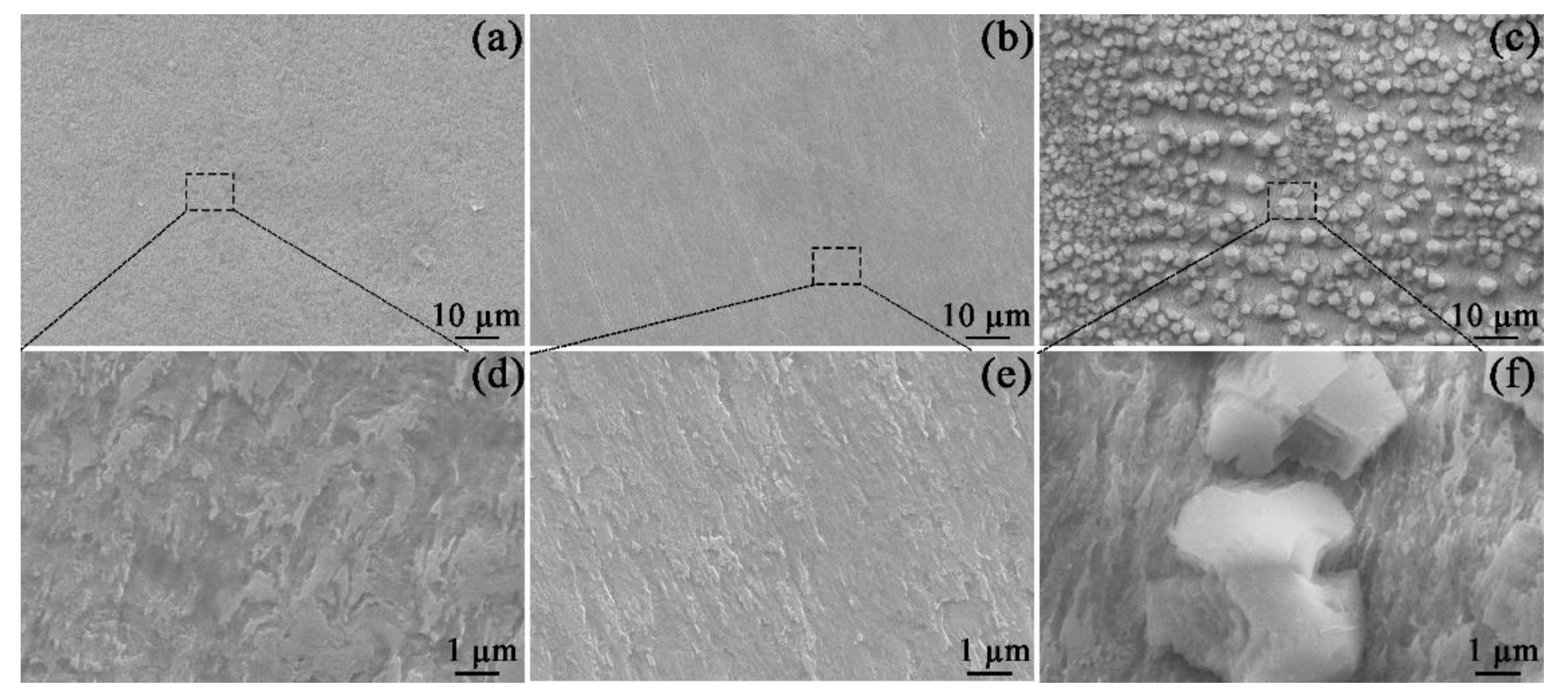
As shown in Figure 3, the SEM images of specimens were measured at different magnifications. After 1800 s of exposure, the corrosion scale did not form (Figure 3a,b). In Figure 3a,b, the specimen surface was highly corroded by virtue of the fast dissolution of the steel. When Mg2+ was added to the solution (Figure 3b,e), the steel surface after 1800 s of immersion still had scratches from the sample preparation, implying a relatively slow corrosion rate. The comparison of Figure 3d,e confirms that the presence of Mg2+ can inhibit the corrosion of the specimen. Interestingly, when Ca2+ was added to the solution (Figure 3c), large amounts of particles appeared on the steel surface. Per the Eh−pH diagram of Ca−C−H2O (see the supplementary information), at 60 °C and pH 7, the stable CaCO3 has formed in Ca2+-containing solution. However, no particle was seen in the Mg2+-containing solution (Figure 3b), owing to the higher solubility of MgCO3 compared to that of CaCO3 (see Eh−pH diagram of Mg−C−H2O in the supplementary information).
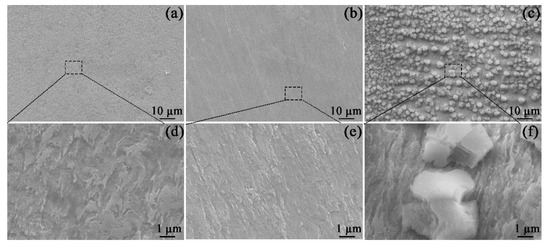
Figure 3.
SEM images of the corrosion morphology on the specimens exposed to the blank solutions with no addition (a,d), Mg2+ addition (b,e), and Ca2+ addition (c,f) for 1800 s.
As stated in the introduction, the effects of Mg2+ and Ca2+ on the CO2 corrosion of steel prior to scale formation have previously been unobtainable. Therefore, this part mainly investigated the short-term corrosion evolution of X100 steel in the blank solutions with various additions by OCP, PDP, and SEM measurements. After 1800 s of exposure, the corrosion scale was not detected on the specimens. Either Ca2+ or Mg2+ in solutions can restrain the corrosion of X100 pipeline steels when the steels are placed in these solutions for 1800 s. Collazo et al. [18] found a similar result that Mg2+ in chloride-containing electrolytes (without purging CO2) can act as a corrosion inhibitor for aluminum alloy samples by inhibiting the cathodic reactions. In addition, some metal cations were used as corrosion inhibitors for mild steel in a sulfuric acid solution [19]. Actually, the OCP shift (Figure 1) for the solutions containing Mg2+ and Ca2+ demonstrates that these cations can inhibit the corrosion rate by suppressing the cathodic reactions.
3.2. Effects of Mg2+ and Ca2+ on the Compositions of Corrosion Scale
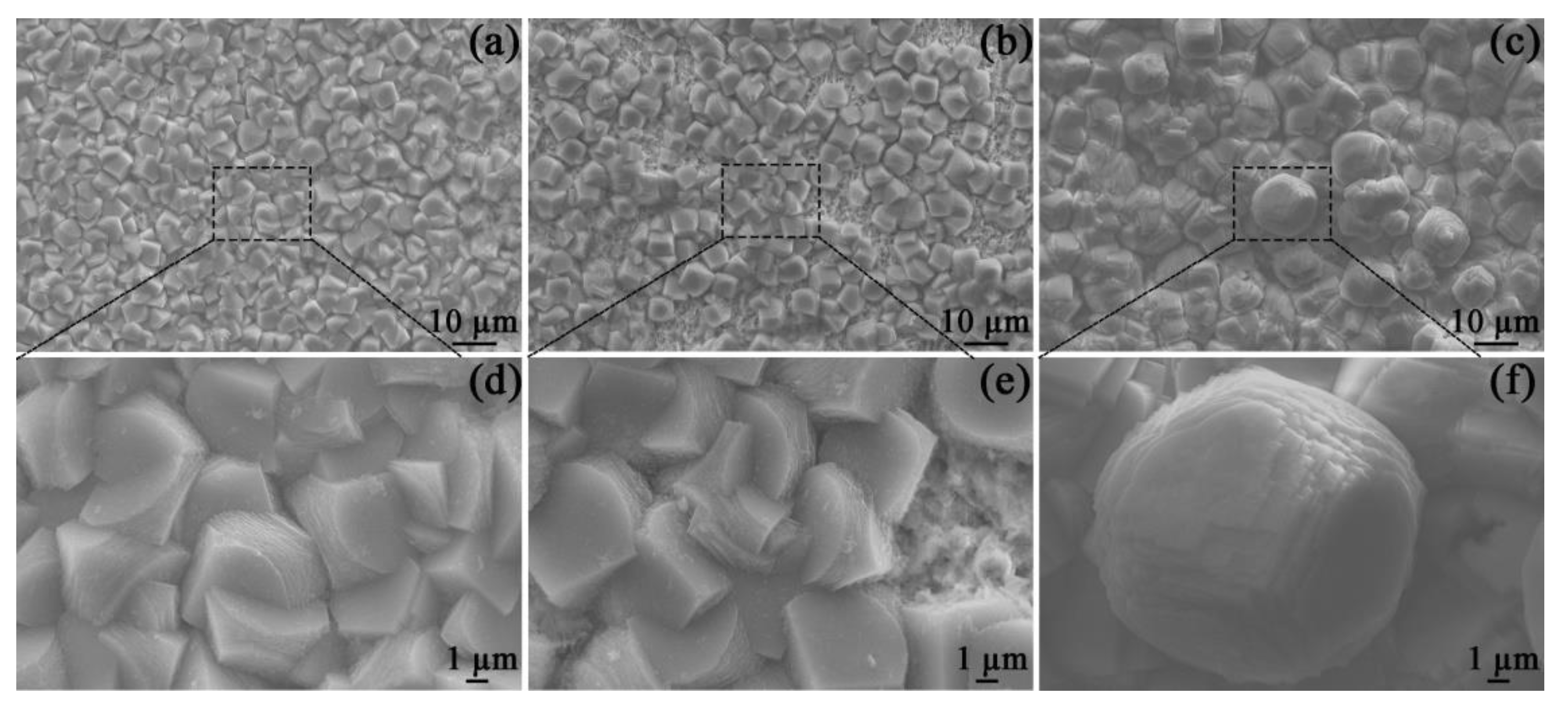
After 48 h of OCP measurements, the corrosion scales of the specimens in all solutions were observed by SEM (Figure 4). All specimen surfaces were covered with porous and prismatic crystals, which are characterized by irregular edges and stepped growth morphology. These characteristics are in line with the morphologies of FeCO3 scales that are frequently detected in similar environments [20].
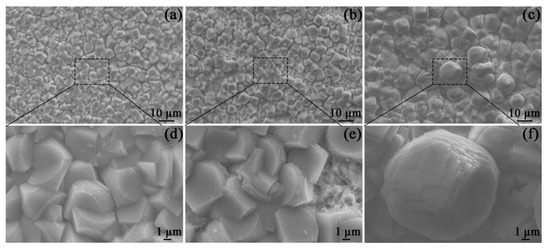
Figure 4.
SEM images of the corrosion scales on the specimens placed in the blank solutions with no addition (a,d), Mg2+ addition (b,e), and Ca2+ addition (c,f) for 48 h.
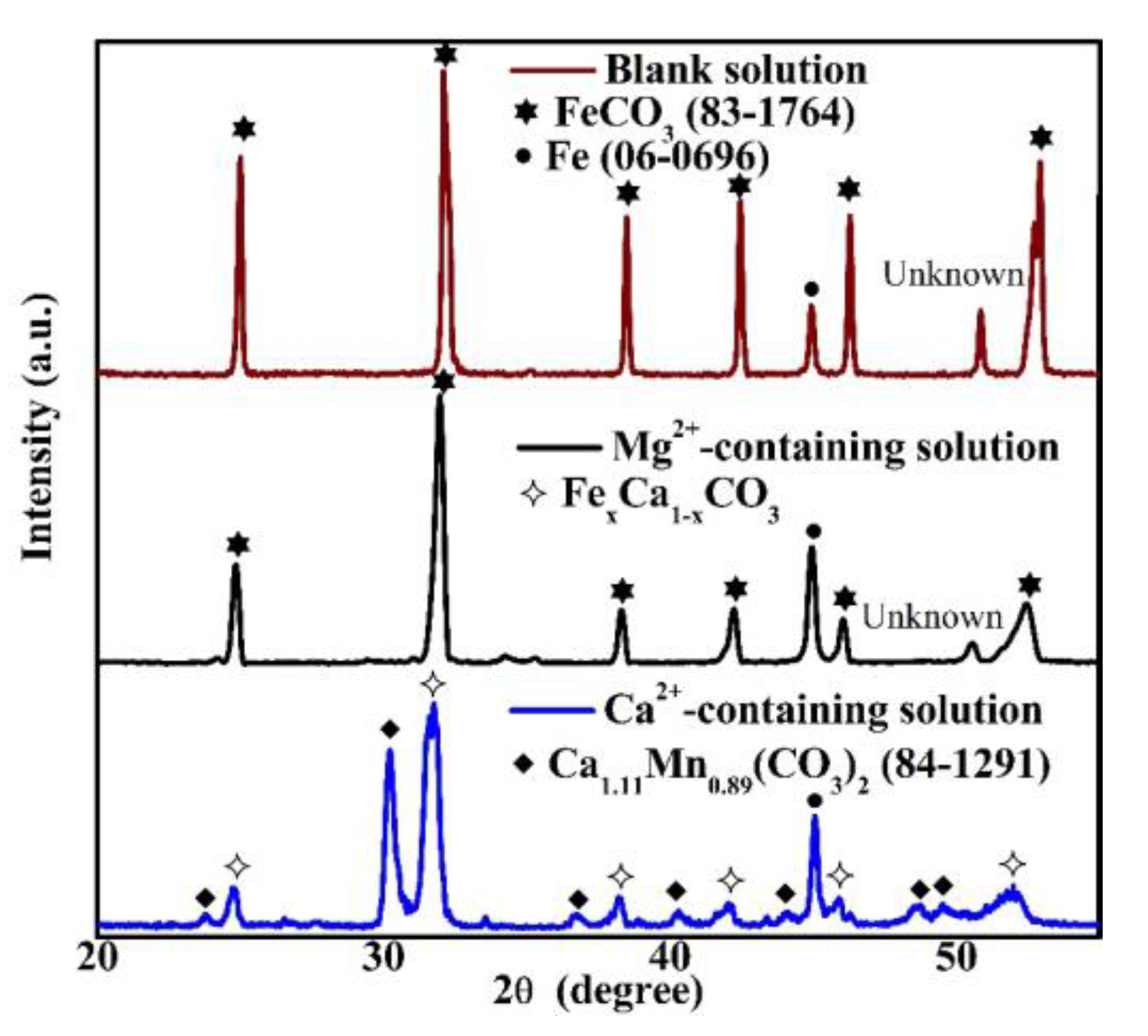
XRD measurements were conducted in order to analyze the crystal structures in Figure 4. When the specimen was put in the blank solution, the notable crystal structure in the corrosion scale was FeCO3, as shown in Figure 5. This crystal structure is determined based on the database of the International Centre for Diffraction Data [21]. For the corrosion scale in the solution containing Mg2+, only FeCO3 is recognized (Figure 5). However, if Ca2+ is present, the corrosion scale consists of FexCa1−xCO3 and Ca1.11Mn0.89(CO3)2 (84–1291) [21]. The identification of FexCa1−xCO3, which is an intermediate compound between CaCO3 and FeCO3, is obtained based on the XRD results of Esmaeely et al. [8]. The Ca1.11Mn0.89(CO3)2 identified in the corrosion scale may be related to the dissolution of MnS in X100 pipeline steel [22,23].
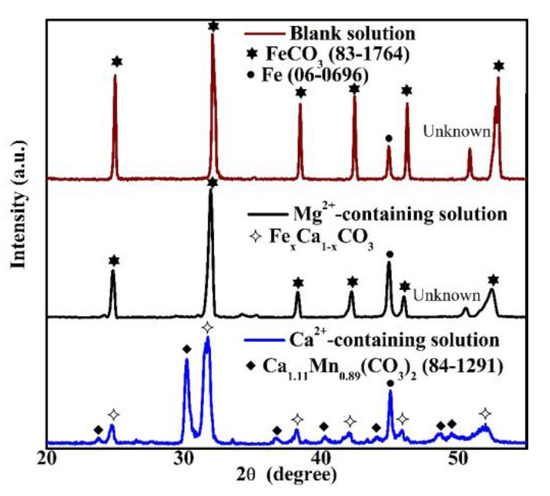
Figure 5.
The XRD pattern of the corrosion scales formed on the specimens exposed to the blank solutions with various additions for 48 h.
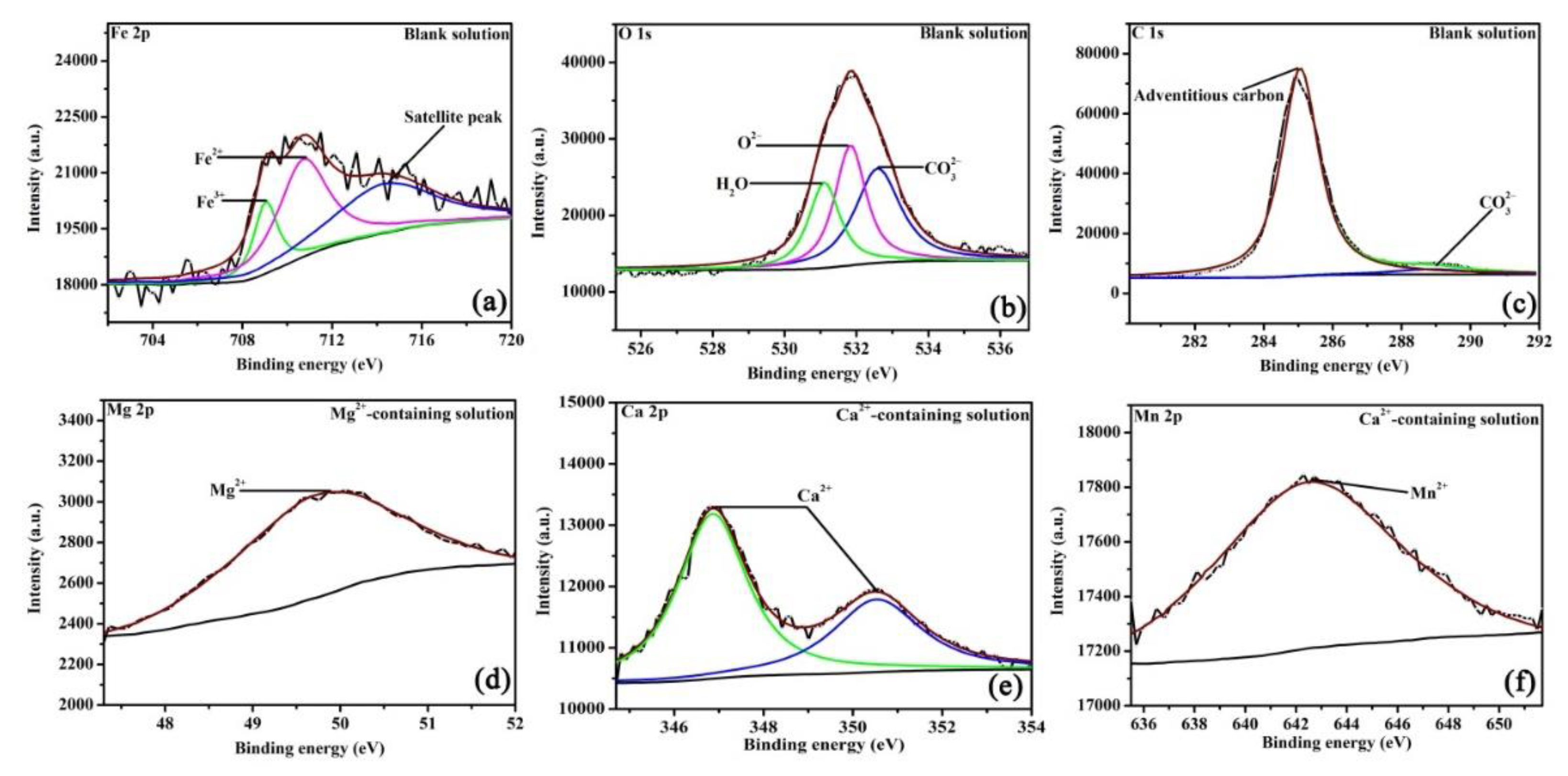
As XRD cannot detect the amorphous phase or trace amounts of crystalline structures, XPS was tested to precisely analyze the valence states of elements in the corrosion scales. Figure 6 shows the high-resolution XPS pattern of corrosion scales on specimens after 48 h of OCP measurements. Fe, O, and C elements are present on all scales and formed in different solutions. In the blank solution (Figure 6a), the Fe 2p peaks at 709.9, 710.9, and 714.5 eV, which are assigned to Fe3+, Fe2+, and the satellite peak, respectively [24,25]. In Figure 6b, the O 1s peaks at 530.9, 531.7, and 532.7 eV, which are ascribed to the presence of H2O, O2−, and CO32−, consecutively [24,25]. Fe3+ and O2− correspond to Fe2O3, while Fe2+ and CO32− are attributed to FeCO3. However, it is unclear whether Fe2O3 formed in the solution or resulted from oxidation after being removed from the solution [22]. Figure 6c shows a small peak at 289.6 eV, which is attributed to CO32−. For the Mg2+-containing solution, apart from the peaks assigned to Fe2O3 and FeCO3 (see supplementary information), the peak of Mg2+ (Figure 6d) appears at 49.9 eV, which belongs to MgCO3. MgCO3 may be produced by the local pH increase as a result of cathodic reactions (2) and (3). XRD cannot find MgCO3 in the corrosion scale, either because MgCO3 is amorphous or because the content of MgCO3 (even if it is crystalized) is low. When Ca2+ is added (Figure 6e), the fitting peaks for Ca 2p appear at 346.8 and 350.5 eV, which can reflect FexCa1−xCO3 in the corrosion products [26]. In Figure 6f, the peaks of Mn are seen in the scale in Ca2+-containing solution, which further identifies the formation of Ca1.11Mn0.89(CO3)2 [26]. The XPS plots demonstrate that either Mg2+ or Ca2+ in the NaCl solution saturated with CO2 can affect the scale structures by forming carbonate species. The concentration of various bonds identified in the current study is important for determining the corrosion process of steel; this information will be provided in a future study.
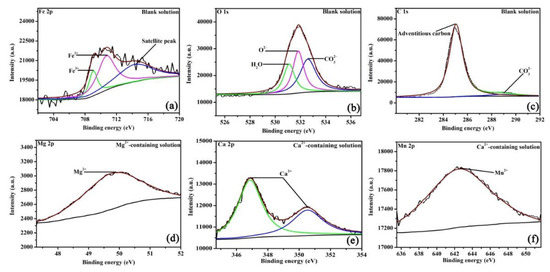
Figure 6.
The XPS pattern of the corrosion scales of the specimens exposed to the blank solutions with no addition (a–c), Mg2+ addition (d), and Ca2+ addition (e,f) for 48 h.
In the literature, it is widely accepted that Ca2+ can be solutionized within the FeCO3 crystal, and FexCa1−xCO3 formed in the solutions containing CO2 and Ca2+ [8,9,27]. As for Mg2+, Yu et al. [9] thought that carbonate species containing Mg2+ can form in the solutions containing CO2 and Mg2+, whereas Ingham et al. [5] used in situ XRD to measure the composition of the corrosion scale and reported that the Mg2+ in CO2-containing solutions cannot lead to the formation of detectable Mg2+-containing carbonate species. Therefore, both XRD and XPS were used to precisely measure the compositions of the corrosion scale to solve the current dispute about whether Mg2+-containing carbonate species form in the corrosion scale. This study reveals that either amorphous MgCO, trace amounts of crystalline MgCO3, or both exist in the corrosion scale.
3.3. Effects of Mg2+ and Ca2+ on the Longer-Term Corrosion Behavior of X100
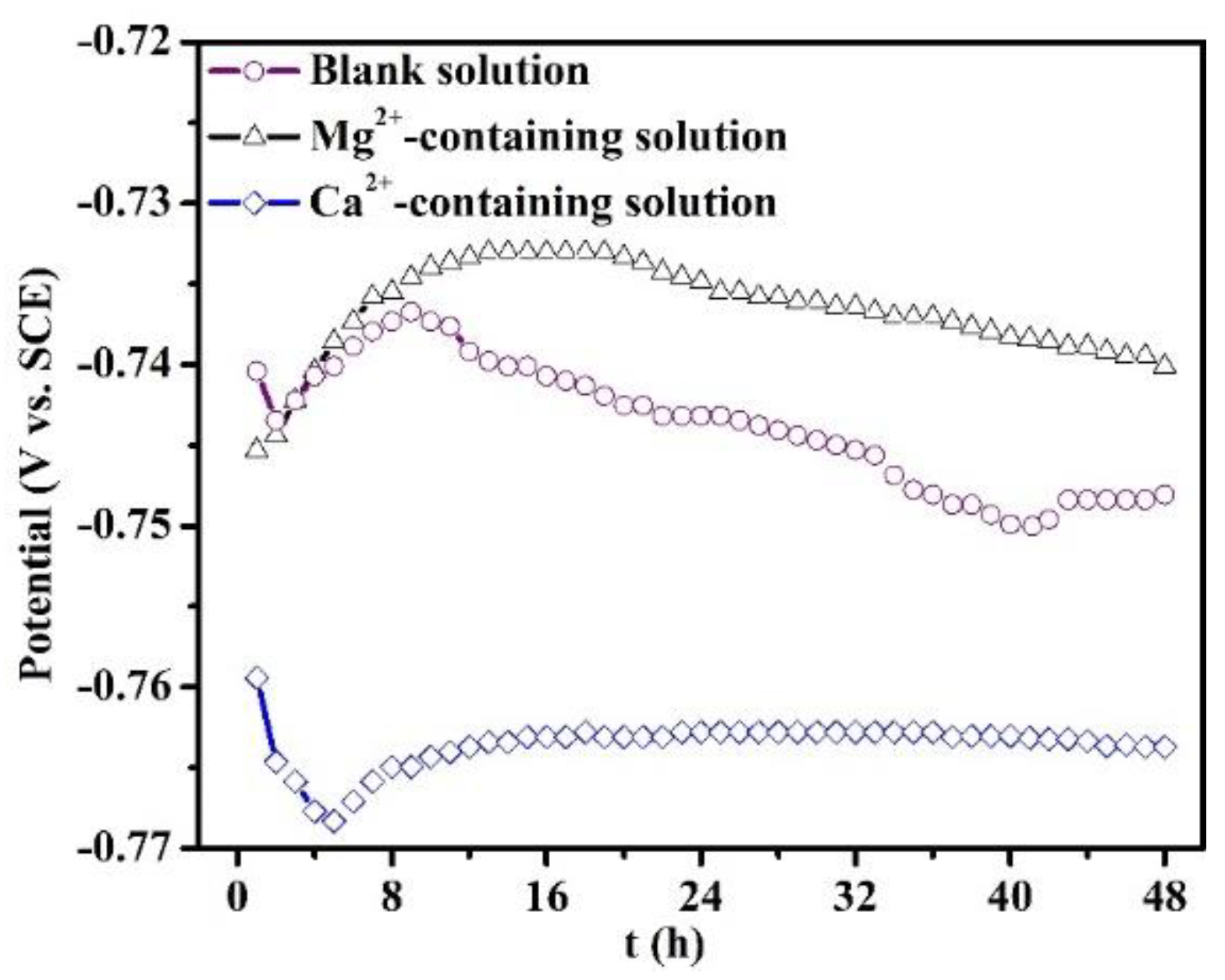
The evolution of OCP of the specimens in all the solutions for 48 h is recorded in Figure 7. The OCP curve of the specimen in the blank solution is characterized by an initial increase and reaches a maximum value at 8 h, and then drops after 8 h. When the immersion time is more than 40 h, the curve displays a stable fluctuation. The presence of Ca2+ in the solution decreases the OCP value with respect to the blank solution, and the curve presents a stable fluctuation only after 16 h of immersion. This demonstrates that the Ca2+ addition predominantly suppresses the cathodic reactions. However, the existence of Mg2+ causes a positive OCP shift, with the corrosion potential reaching a maximum value at 16 h.
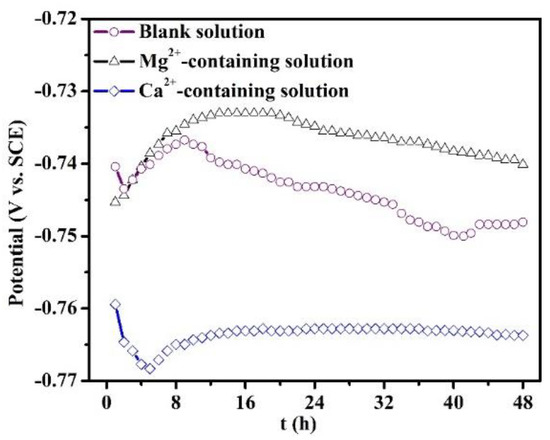
Figure 7.
Open circuit potential evolution of the specimens put in the blank solutions with various additions for 48 h.
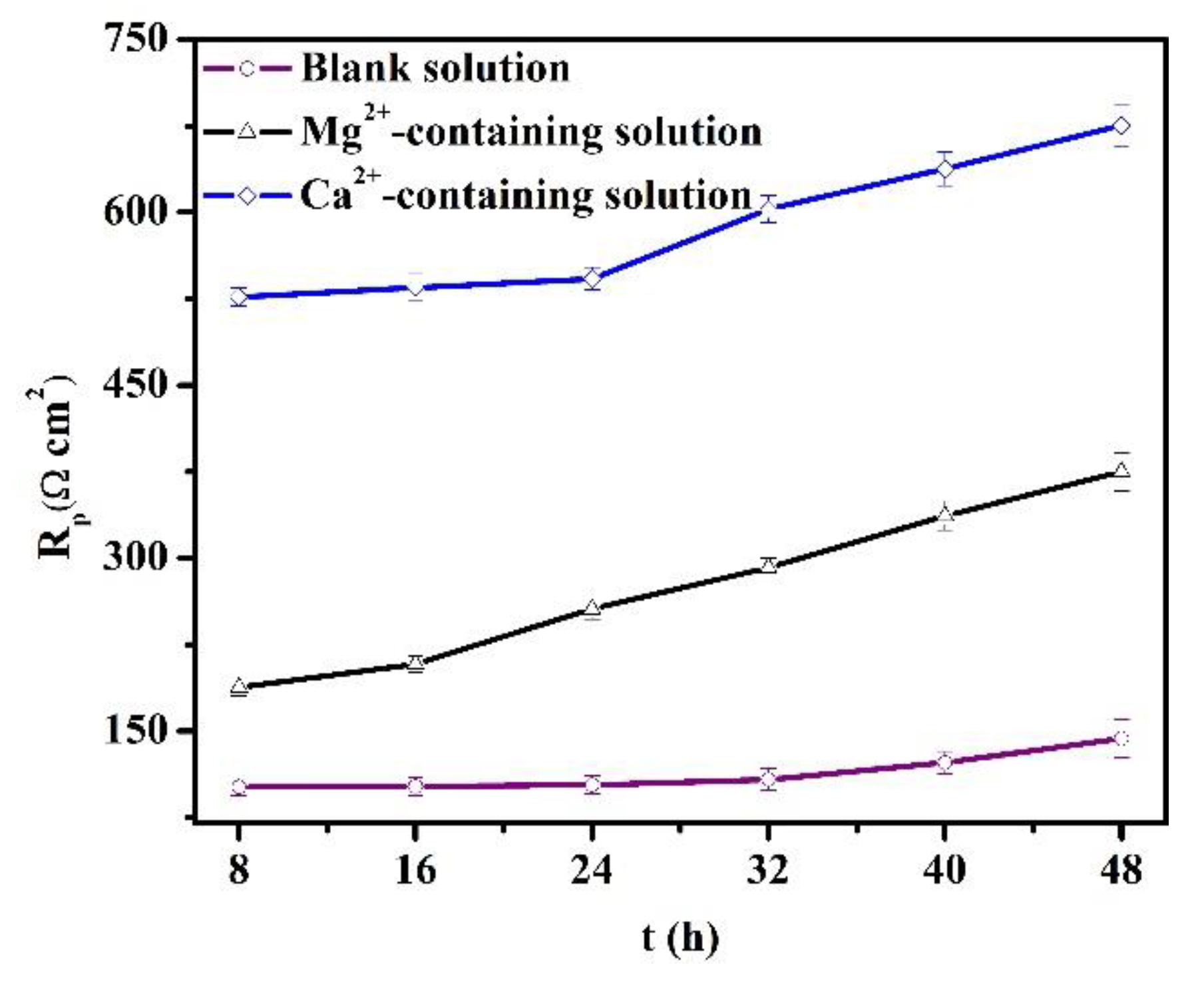
During the OCP tests, LPR was also measured to understand the evolution of longer-term corrosion. In Figure 8, the Rp values increase versus time for the specimens in all the solutions. The increase in the Rp values is usually attributed to the thickening of the protective scale [28]. Also, the Ca2+ addition significantly amplifies the Rp values, while the Mg2+ in the solution causes a negative shift of the Rp values. As indicated in Section 3.1, the Mg2+ in the solution can inhibit the short-term corrosion of specimens. Thus, the concentration of Fe2+ produced by the steel dissolution declines, and the precipitation rate of protective scale is impeded. Lower Rp values for specimens in the Mg2+-containing solution were triggered by the relatively low formation rate of the corrosion scale. In contrast, the presence of Ca2+ in the solution accelerated the formation of corrosion scale.
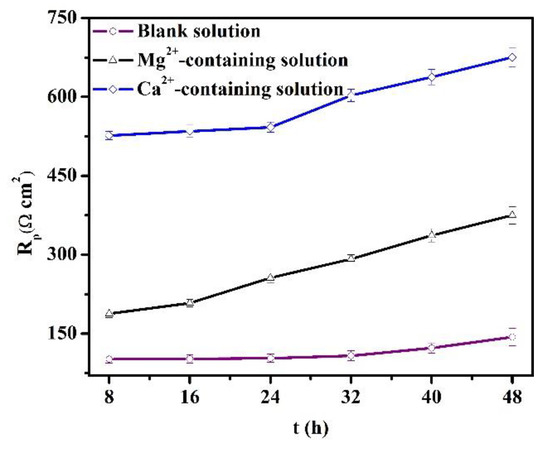
Figure 8.
Polarization resistances obtained from the linear polarization method for the specimens in the blank solutions with various additions.
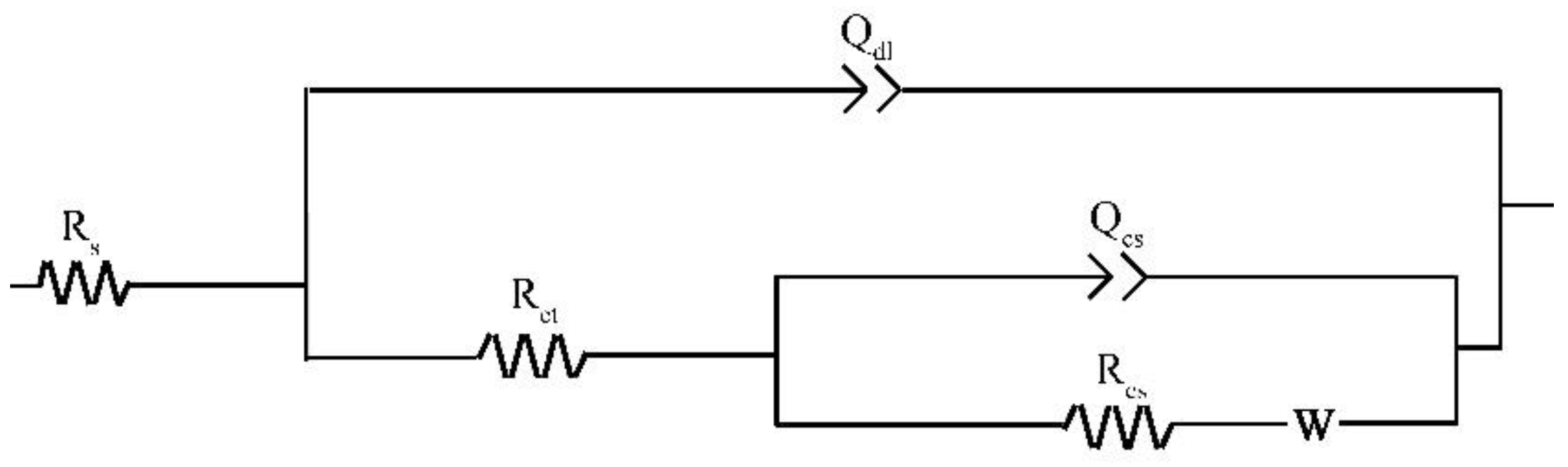
The Nyquist plots for the specimens exposed to the three solutions for 48 h are shown in Figure 9. To make a qualitative comparison of the curves presented in Figure 9, it is necessary to model the datasets with electrical equivalent circuits. Rs(Qdl(Rct(Qcs(RcsW)))) is used to interpret the steel/solution interface of this study (Figure 10) with the Warburg element selected based on a previous study [29]. The circuit elements and their notations in Figure 10 are as follows: Rs is the solution resistance, Qdl is the constant phase element (CPE) for the electrical double layer, Rct is the charge transfer resistance, Rcs is the corrosion scale resistance, Qcs is the CPE for the corrosion scale, and W is the Warburg diffusion element. The chosen equivalent circuit gives good fits between the simulated and measured results for all cases in this work. The values of Chi-Square (X2) for all the EIS data are approximate 1 × 10−4 (Supplementary information).
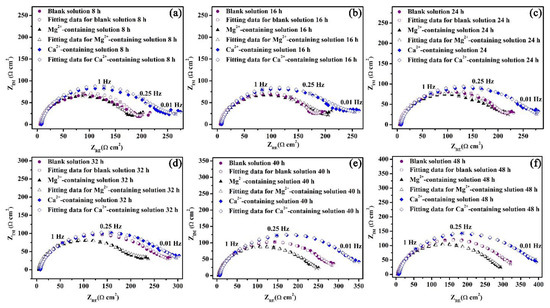
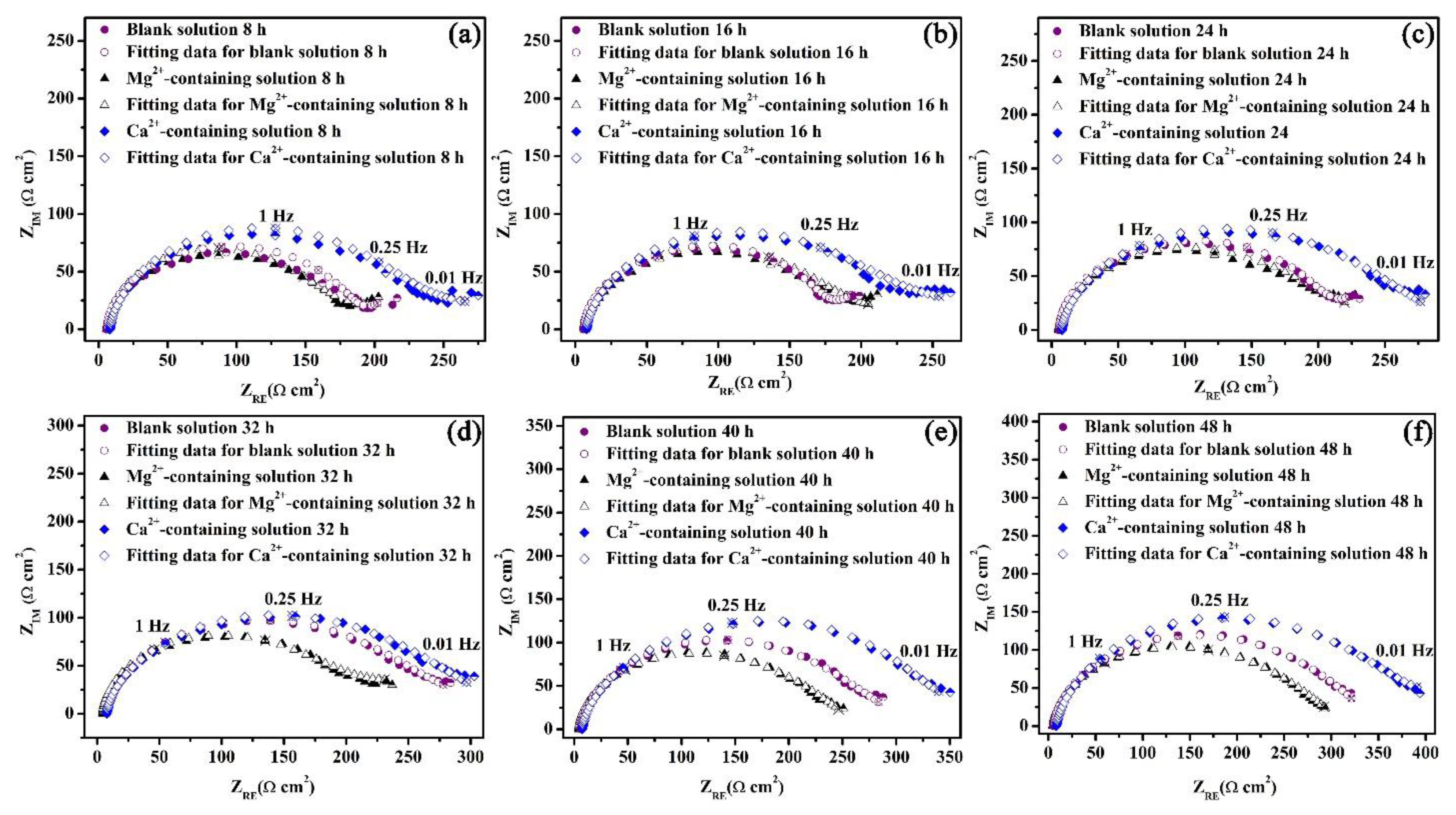
Figure 9.
Nyquist plots for the specimens in the blank solutions with various additions for 8 (a), 16 (b), 24 (c), 32 (d), 40 (e), and 48 (f) h.
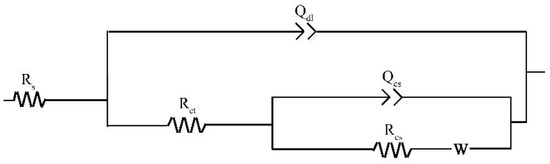
Figure 10.
Equivalent circuit model for electrochemical impedance spectroscopy.
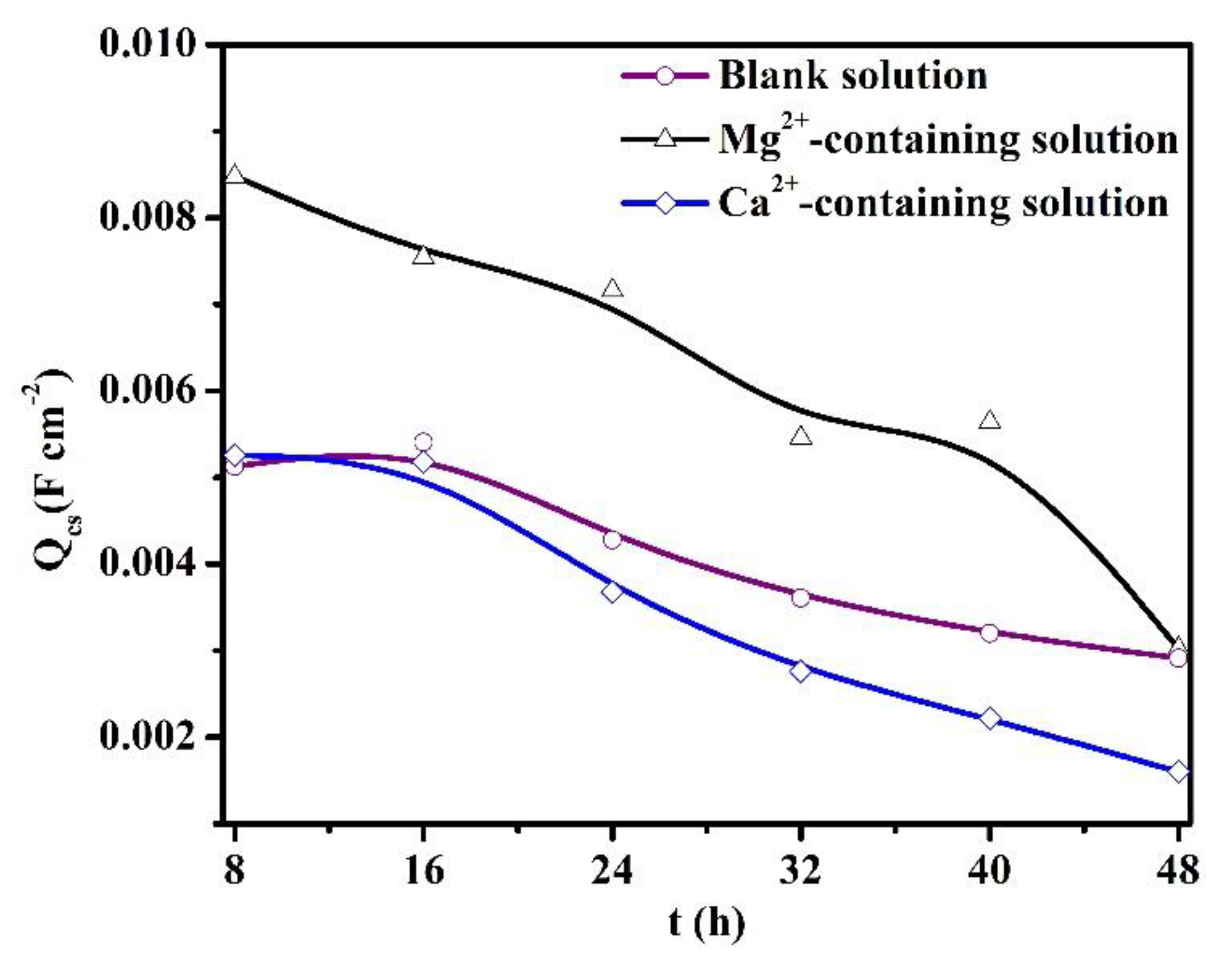
The Qcs values are presented in Figure 11. The Qcs reflects the available area for the cathodic reaction, which predominantly occurs at the cementite of carbon steel [30]. For the blank solutions, the Qcs values decline as time increases, illustrating the thickening of the FeCO3 scales and the decrement of the available cathodic area [30]. Thus, the cathodic reactions are predominantly suppressed, which is corroborated by the decline in OCP values after 8 h of immersion (Figure 7). The variations of Qcs values, Rp (Figure 8), and the thickness of the corrosion scale (see the supplementary information) validate the thickening of the protective corrosion scale over time. In addition, when the X100 pipeline steels were placed in three solutions for the same amount of time, the presence of Ca2+ decreased the Qcs values, while the addition of Mg2+ increased the Qcs values. One explanation for the variations of Qcs values would be the different formation rates of corrosion scales when Ca2+ or Mg2+ are present. The Mg2+-containing solution is characterized by the relatively low formation rate of the protective scale, while the presence of Ca2+ in a solution can accelerate the formation of the protective scale.
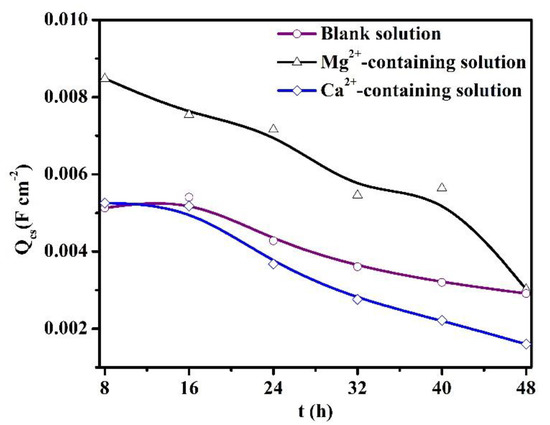
Figure 11.
The values of Qcs for the specimens exposed to the blank solutions with different additions.
Figure 8 and Figure 11 demonstrate that the thickening of the protective corrosion scale is beneficial for corrosion reduction in pipeline steels. Traditionally, it is thought that the coexistence of Mg2+ and Ca2+ in CO2-containing solutions can produce heavy carbonate precipitation, which may be (Fe, Ca, Mg)CO3 [9,31,32]. However, that does not indicate that Mg2+ alone in the solution can accelerate the formation of the scale. This study reveals that Mg2+ alone in the solution inhibits the scale precipitation, while the Ca2+ in the solution indeed accelerates the formation of the protective scale.
Although some studies on the corrosion scale have been carried out in the NaCl solutions containing CO2, these studies focused on the evolutions in the scale composition and corrosion rate by altering the pH [33], temperature [34], CO2 partial pressure [35], and chloride concentrations [36]. Further, some models were proposed to establish the relationship between the precipitation rate of the CO2 scale and the supersaturation of the solution [37,38]. For instance, van Hunnik et al. reported the following [37]:
where S is the supersaturation, KSP is the solubility product of FeCO3, and C represents the concentration. Then, the precipitation rate of FeCO3 follows the equation [37]:
where [Fe2+]prec is the precipitation rate of FeCO3, A/V corresponds to the surface area-to-volume ratio, kr stands for the kinetic constant, KSP represents the solubility product of FeCO3, R is the universal gas constant, and T corresponds to the temperature. However, during corrosion development, the concentration of Fe2+ is not constant with time. On the one hand, the formation of the corrosion scale consumes Fe2+. On the other hand, the dissolved steel is weakened due to the improved protection of the corrosion scale with time. Therefore, the precipitation rate of the corrosion scale may change with the variance of the Fe2+ concentration. It is inconvenient to constantly measure the Fe2+ concentration in pipelines, especially when the pipelines are running. Liu et al. [36] investigated the effects of the chloride content on the CO2 corrosion of carbon steel based on the point defect model (PDM), which considers the migration of oxygen vacancy and metal vacancy during scale growth. According to the PDM, when the thickness is more than 5 Å, the following equation is derived [16]:
where R is the universal gas constant, T corresponds to the temperature, ε represents the electrical field strength, F stands for the faradic constant, A and B are constant, and t is the measurement time. Nevertheless, the relationship between scale thickness and time was not checked by Liu et al. to evaluate whether PDM applied to the scale formation in their study. Collectively speaking, these studies are inconvenient for predicting the precipitation rate of the corrosion scale, and they fail to consider the effects of Mg2+ and Ca2+ on the precipitation rate of the corrosion scale.
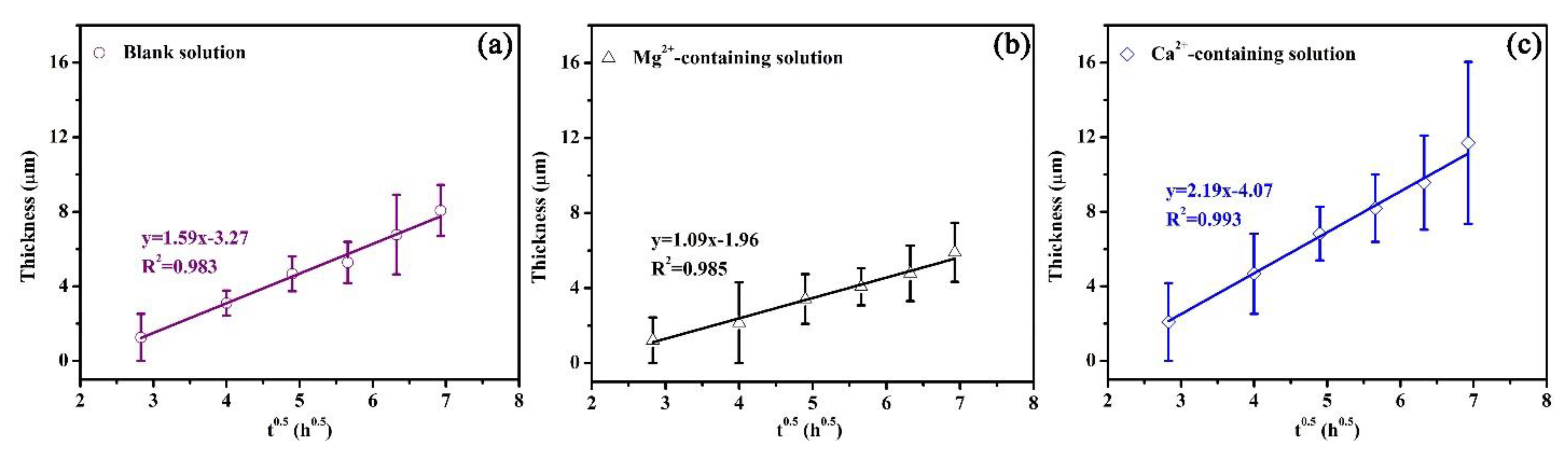
In this study, an experimental equation was proposed to establish the relationship between the thickness of the corrosion scale and exposure time, which is similar to Equation (7). Based on the experimental equation, the quantitative effects of Mg2+ and Ca2+ on the precipitation rate can be obtained. Figure 12 shows the plots of the average scale thickness (L) versus time (t) for the corrosion scales on the specimens exposed to the blank solutions with various additions. The average thicknesses of the corrosion scales were measured by SEM (Figures S4–S6 in the supplementary information). The minimum correlation coefficient (R2) resulting from L versus t0.5 was 0.983 (Figure 12a), demonstrating that L and t0.5 show a good linear relationship. The scale growth obeys the following relationship:
where L is the average scale thickness, A and B are the constant, and t is the time. From Figure 12, the values of A were calculated. The values of A for three solutions were 1.59, 1.09, and 2.19 µm/h0.5, respectively. When the specimens were exposed to the solutions for the same time, the presence of Ca2+ benefits the growth of the corrosion scale while the Mg2+ in the solution weakens the growth rate of the corrosion scale.
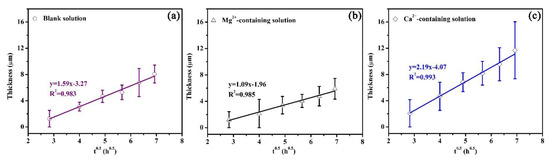
Figure 12.
Plots of average scale thickness versus time for the corrosion scales on the specimens exposed to the blank solution (a), Mg2+-containing solution (b), and Ca2+-containing solution (c).
According to the above results, it is reasonable to make the following hypothesis on the roles of Ca2+ and Mg2+ in the formation of a corrosion scale. Fe2+ produced by the dissolution of the specimen (reaction (4)) reacts with a carbonate (CO3−) that is formed due to the reduction in bicarbonate (reaction (2)), which leads to the precipitation of the FeCO3 scale on the X100 pipeline steel [28]. The corroded matrix of X100 pipeline steel behaved as a nucleation skeleton, promoting the formation of the scale [28]. Though Mg2+ addition can trigger a decrease in the formation rate of the corrosion scale, the sample with Mg2+ addition still showed a decrease in the corrosion rate due to the inhibition effect of Mg2+ in the electrolyte (Figure 2 and Figure 3). This reduced the available nucleation skeleton and the Fe2+ concentration for the growth of the corrosion scales. Correspondingly, the formation rate of the corrosion scale in Mg2+-containing solutions was prohibited. It is assumed that the inhibition effect of Mg2+ can persist even after the formation of a corrosion scale. But the specimen in the Mg2+-containing solution showed the minimum Rp values (Figure 8) and maximum Qcs values due to the low formation rate of the corrosion scale. As for the solution with Ca2+, the formation of CaCO3 particles on the surface of X100 pipeline steel predominantly benefits the precipitation of the corrosion scale by acting as the nucleation sites of the corrosion scale (Figure 3 and Figure 4). As a result, the Ca2+-containing solution presents the thickest scale (Figure 12).
4. Conclusions
The role of Mg2+ and Ca2+ in the corrosion of pipeline steels after scale formation is under dispute in the corrosion field. Thus, the current study was conducted to clarify the discrepancy between different studies and provide a deep understanding of the cation effects in the corrosion process. The following conclusions are drawn based on the results of this work:
- The corrosion of X100 pipeline steel (icorr 262.4 μA cm−2) after 1800 s of exposure was inhibited by the presence of either Ca2+ (icorr 163.5 μA cm−2) or Mg2+ (icorr 80.8 μA cm−2) in the NaCl solution saturated with CO2.
- Either Mg2+ or Ca2+ in the NaCl solution saturated with CO2 can affect the scale structures by forming carbonate species. Though Mg2+ addition can trigger a decrease in the formation rate of the corrosion scale, the sample with Mg2+ addition still shows a decrease in the corrosion rate due to the inhibition effect of Mg2+ in the electrolyte.
- The Ca2+ in the solution accelerates the formation of the protective scale in the NaCl solution saturated with CO2, thus improving the corrosion resistance of carbon steel.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma16155258/s1. References [29,39,40,41,42] were cited in supplementary materials.
Author Contributions
Conceptualization, Q.S.; Software, T.H.; Validation, Y.L.; Formal analysis, Z.Z.; Resources, Z.Z.; Data curation, Y.L.; Writing—original draft, X.Y.; Writing—review & editing, T.H.; Visualization, X.Y.; Supervision, Q.S.; Project administration, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundations of China (No. 12202498) and the Natural Science Foundations of Jiangsu Province (No. BK20210438), which are formed the fund of the State Key Laboratory of Disaster Prevention and Mitigation of Explosion and Impact (Army Engineering University of PLA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Conti, J.J.; Holtberg, P.D.; Beamon, J.; Schaal, A.M.; Ayoub, J.; Turnure, J.T. Annual Energy Outlook 2014; US Energy Information Administration: Washington, DC, USA, 2012.
- Romualdi, N.; Militzer, M.; Poole, W.; Collins, L.; Lazor, R. Austenite Grain Size Model for the Coarse Grain Heat Affected Zone in Line Pipe Steels. In Proceedings of the International Pipeline Conference, Virtual, 28–30 September 2020. [Google Scholar]
- Majchrowicz, K.; Brynk, T.; Wieczorek, M.; Miedzińska, D.; Pakieła, Z. Exploring the susceptibility of P110 pipeline steel to stress corrosion cracking in CO2-rich environments. Eng. Fail. Anal. 2019, 104, 471–479. [Google Scholar] [CrossRef]
- Nyborg, R. Overview of CO2 corrosion models for wells and pipelines. In Corrosion 2002; NACE International: Houston, TX, USA, 2002. [Google Scholar]
- Ingham, B.; Laycock, N.J.; Burnell, M.K.; Kappen, P.; Kimpton, J.; Williams, D. In situ synchrotron X-ray diffraction study of scale formation during CO2 corrosion of carbon steel in sodium and magnesium chloride solutions. Corros. Sci. 2012, 56, 96–104. [Google Scholar] [CrossRef]
- Wang, S.; Lamborn, L.; Chevil, K.; Gamboa, E.; Chen, W. Near-neutral pH corrosion of mill-scaled X-65 pipeline steel with paint primer. J. Mater. Sci. Technol. 2020, 49, 166–178. [Google Scholar] [CrossRef]
- Esmaeely, S.N.; Choi, Y.S.; Young, D.; Nešic, S. Effect of calcium on the formation and protectiveness of iron carbonate layer in CO2 corrosion. Corrosion 2013, 69, 912–920. [Google Scholar] [CrossRef]
- Esmaeely, S.N.; Young, D.; Brown, B.; Nešic, S. Effect of incorporation of calcium into iron carbonate protective layers in CO2 corrosion of mild steel. Corrosion 2016, 73, 238–246. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, H.; Xu, L. Corrosion behavior and in-situ pH monitoring of a 3% chromium low alloy pipeline steel welded joint in a CO2 environment. Mater. Res. Express. 2019, 6, 116573. [Google Scholar] [CrossRef]
- Zeng, D.; Dong, B.; Zhang, S. Annular corrosion risk analysis of gas injection in CO2 flooding and development of oil-based annulus protection fluid. J. Pet. Sci. Eng. 2022, 208, 109526. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, Y.; Qu, D.; Ke, W. Effect of calcium ions on pitting corrosion and inhibition performance in CO2 corrosion of N80 steel. Corros. Sci. 2006, 48, 3091–3108. [Google Scholar] [CrossRef]
- Pots, B.F. Prediction of corrosion rates of the main corrosion mechanisms in upstream applications. In Corrosion 2005; NACE International: Houston, TX, USA, 2005. [Google Scholar]
- Chen, T.; Neville, A.; Yuan, M. Influence of Mg2+ on the Kinetics and Crystal Morphology of CaCO3 Scale Formation on the Metal Surface and in Bulk Solution. In Corrosion 2004; NACE International: Houston, TX, USA, 2004. [Google Scholar]
- Olivo, J.M.D.; Brown, B.; Young, D. Electrochemical Model of CO2 Corrosion in the Presence of Quaternary Ammonium Corrosion Inhibitor Model Compounds. In Proceedings of the Corrosion Conference and Expo, Nashville, TN, USA, 24–28 March 2019. [Google Scholar]
- Xiang, Y.; Xu, M.; Choi, Y.-S. State-of-the-art overview of pipeline steel corrosion in impure dense CO2 for CCS transportation: Mechanisms and models. Corros. Eng. Sci. Technol. 2017, 52, 485–509. [Google Scholar] [CrossRef]
- Ulaganathan, J.; Ha, H. Investigation on the Effect of Lead (Pb) on the Degradation Behavior of Passive Films on Alloy 800. In Proceedings of the International Conference on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors, Boston, MA, USA, 18–22 August 2019. [Google Scholar]
- Nordsveen, M.; Nešic, S.; Nyborg, R.; Stangeland, A. A mechanistic model for carbon dioxide corrosion of mild steel in the presence of protective iron carbonate films—Part 1: Theory and verification. Corrosion 2003, 59, 443–456. [Google Scholar] [CrossRef]
- Collazo, A.; Nóvoa, X.; Pérez, C. The role of Mg2+ ions in the corrosion behaviour of AA2024-T3 aluminium alloys immersed in chloride-containing environments. Electrochim. Acta 2014, 124, 17–26. [Google Scholar] [CrossRef]
- Golabadi, M.; Foratirad, H.; Asgari, M.; Gholami, M.G.; Karimi, M. The synergistic effect of 2-mercaptobenzotiazole and zinc nitrate as corrosion inhibitor on carbon steel in saline solution. Int. J. Mater. Res. 2020, 10, 111. [Google Scholar]
- Berntsen, T.; Seiersten, M.; Hemmingsen, T. Effect of FeCO3 supersaturation and carbide exposure on the CO2 corrosion rate of carbon steel. Corrosion 2013, 69, 601–613. [Google Scholar] [CrossRef]
- Graf, D. Crystallographic tables for the rhombohedral carbonates. Am. Mineral. 1961, 46, 1283–1316. [Google Scholar]
- Wei, L.; Pang, X.; Liu, C.; Gao, K. Formation mechanism and protective property of corrosion product scale on X70 steel under supercritical CO2 environment. Corros. Sci. 2015, 100, 404–420. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Rodionova, I.G.; Arutyunyan, N.A.; Dunaev, S.F. Study of Effect of Non-Metallic Inclusions on Structural State and Properties of Low-Carbon Microalloyed Structural Steels. Metallurgist 2021, 64, 10. [Google Scholar] [CrossRef]
- Wang, L.; Shinohara, T.; Zhang, B.-P. XPS study of the surface chemistry on AZ31 and AZ91 magnesium alloys in dilute NaCl solution. Appl. Surf. Sci. 2010, 256, 5807–5812. [Google Scholar] [CrossRef]
- Ni, M.; Ratner, B.D. Differentiating calcium carbonate polymorphs by surface analysis techniques—An XPS and TOF-SIMS study. Surf. Interface Anal. 2008, 40, 1356–1361. [Google Scholar] [CrossRef]
- Wu, S.L.; Cui, Z.D.; He, F.; Bai, Z.Q.; Zhu, S.L.; Yang, X.J. Characterization of the surface film formed from carbon dioxide corrosion on N80 steel. Mater. Lett. 2004, 58, 1076–1081. [Google Scholar] [CrossRef]
- Ye, F.; Jiao, Z.; Yan, S.; Guo, L.; Feng, L.; Yu, J. Microbeam plasma arc remanufacturing: Effects of Al on microstructure, wear resistance, corrosion resistance and high temperature oxidation resistance of AlxCoCrFeMnNi high-entropy alloy cladding layer. Vacuum 2020, 174, 1. [Google Scholar] [CrossRef]
- Gao, M.; Pang, X.; Gao, K. The growth mechanism of CO2 corrosion product films. Corros. Sci. 2011, 53, 557–568. [Google Scholar] [CrossRef]
- Gadala, I.M.; Alfantazi, A. A study of X100 pipeline steel passivation in mildly alkaline bicarbonate solutions using electrochemical impedance spectroscopy under potentiodynamic conditions and Mott–Schottky. Appl. Surf. Sci. 2015, 357, 356–368. [Google Scholar] [CrossRef]
- Farelas, F.; Galicia, M.; Brown, B.; Nesic, S.; Castaneda, H. Evolution of dissolution processes at the interface of carbon steel corroding in a CO2 environment studied by EIS. Corros. Sci. 2010, 52, 509–517. [Google Scholar] [CrossRef]
- Sridhar, N.; Dunn, D.; Anderko, A.; Lencka, M.; Schutt, H. Effects of water and gas compositions on the internal corrosion of gas pipelines-modeling and experimental studies. Corrosion 2001, 57, 221–235. [Google Scholar] [CrossRef]
- De Alwis, C.; Trought, M.; Lundeen, J.; Perrine, K.A. Effect of Cations on the Oxidation and Atmospheric Corrosion of Iron Interfaces to Minerals. J. Phys. Chem. A 2021, 36, 125. [Google Scholar] [CrossRef] [PubMed]
- Tanupabrungsun, T.; Brown, B.; Nesic, S. Effect of pH on CO2 corrosion of mild steel at elevated temperatures. In Corrosion/2013 Paper; NACE International: Houston, TX, USA, 2013. [Google Scholar]
- Hussien, B.M.; Al-Sabagh, A.M.; Migahed, M.A. Corrosion Control of X-60 Type Carbon Steel in Petroleum Formation Water Under High Pressure of CO2 at High Temperature. In Proceedings of the Offshore Mediterranean Conference and Exhibition, Ravenna, Italy, 29–31 March 2017. [Google Scholar]
- Liu, Z.; Gao, X.; Du, L.; Li, J.; Zheng, C.; Wang, X. Corrosion mechanism of low-alloy steel used for flexible pipe in vapor-saturated H2S/CO2 and H2S/CO2-saturated brine conditions. Mater. Corros. 2018, 69, 9. [Google Scholar] [CrossRef]
- Liu, Q.; Mao, L.; Zhou, S. Effects of chloride content on CO2 corrosion of carbon steel in simulated oil and gas well environments. Corros. Sci. 2014, 84, 165–171. [Google Scholar] [CrossRef]
- Van Hunnik, E.; Pots, B.F.; Hendriksen, E. The Formation of Protective FeCO3 Corrosion Product Layers in CO2 Corrosion; NACE International: Houston, TX, USA, 1996. [Google Scholar]
- Wei, L.; Pang, X.; Zhou, M.; Gao, K. Effect of exposure angle on the corrosion behavior of X70 steel under supercritical CO2 and gaseous CO2 environments. Corros. Sci. 2017, 121, 57–71. [Google Scholar] [CrossRef]
- López, D.A.; Simison, S.N.; de Sánchez, S.R. The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole. Electrochim. Acta 2003, 48, 845–854. [Google Scholar] [CrossRef]
- Liu, J.; Alfantazi, A.; Asselin, E. A new method to improve the corrosion resistance of titanium for hydrometallurgical applications. Appl. Surf. Sci. 2015, 332, 480–487. [Google Scholar] [CrossRef]
- Sun, J.B.; Zhang, G.A.; Liu, W.; Lu, M.X. The formation mechanism of corrosion scale and electrochemical characteristic of low alloy steel in carbon dioxide-saturated solution. Corros. Sci. 2012, 57, 131–138. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. On the theory of CO2 corrosion reactions–Investigating their interrelation with the corrosion products and API-X100 steel microstructure. Corros. Sci. 2014, 85, 380–393. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).