Experimental Investigation on a Novel Temperature-Controlled Phase Change Aggregate Concrete: Thermo-Mechanical Properties and Hydration Heat Control
Abstract
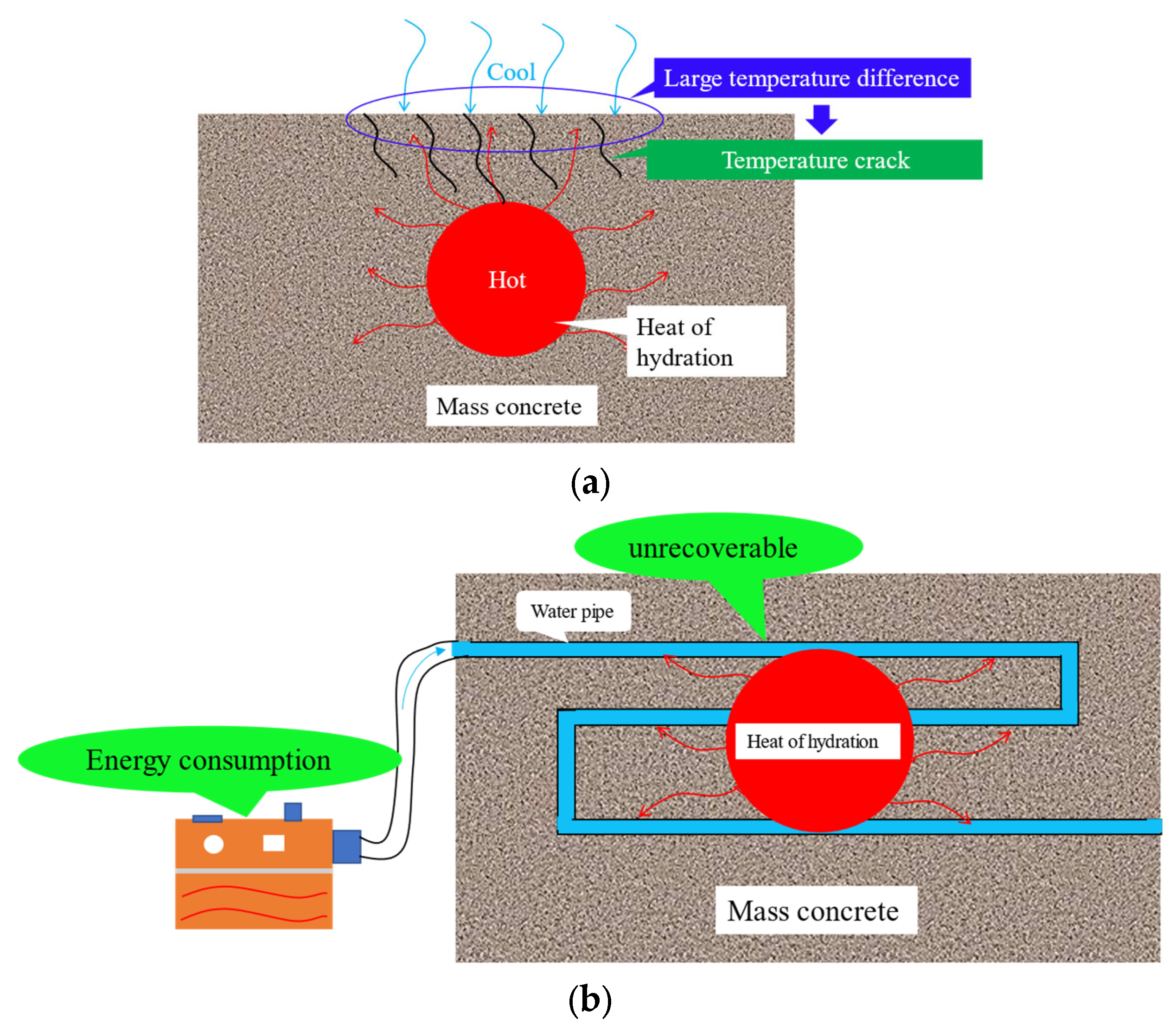
1. Introduction
2. Materials and Equipment
2.1. Aggregate
2.2. Phase Change Materials
2.3. Encapsulating Materials of PCA
2.3.1. Superfine Cement
2.3.2. Epoxy Resin
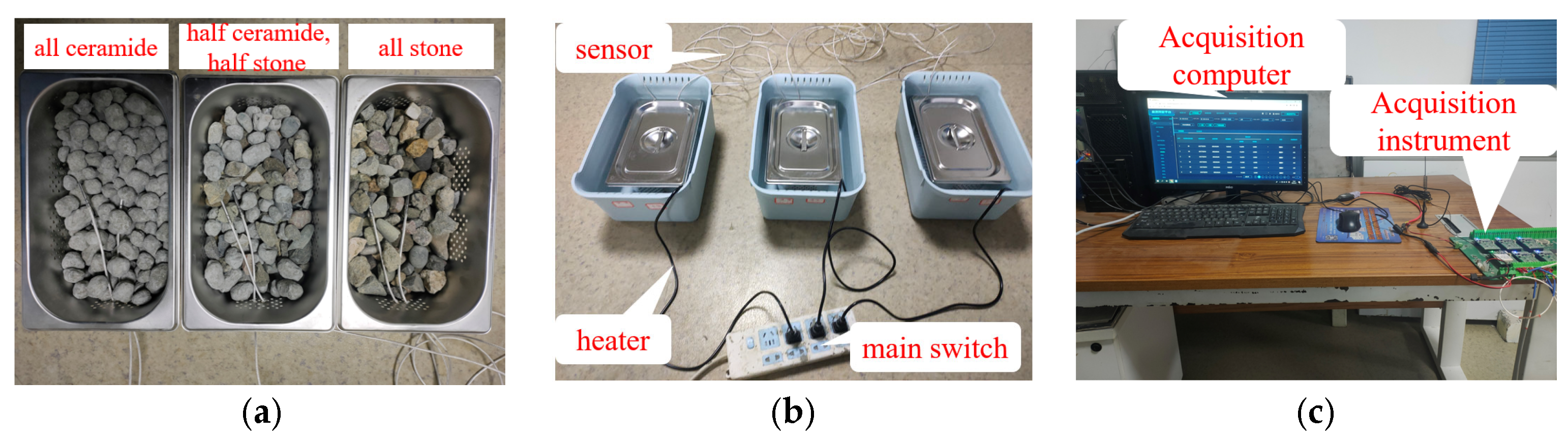
2.4. Equipment
3. Phase Change Aggregate Test Procedure
3.1. Preparation and Encapsulation Test of Phase Change Aggregate
3.2. Mechanical Properties of PCA
3.2.1. Cylindrical Compress Strength of PCA
3.2.2. Crushing Rate of PCA during Mixing
3.2.3. Enthalpy Value of PCA
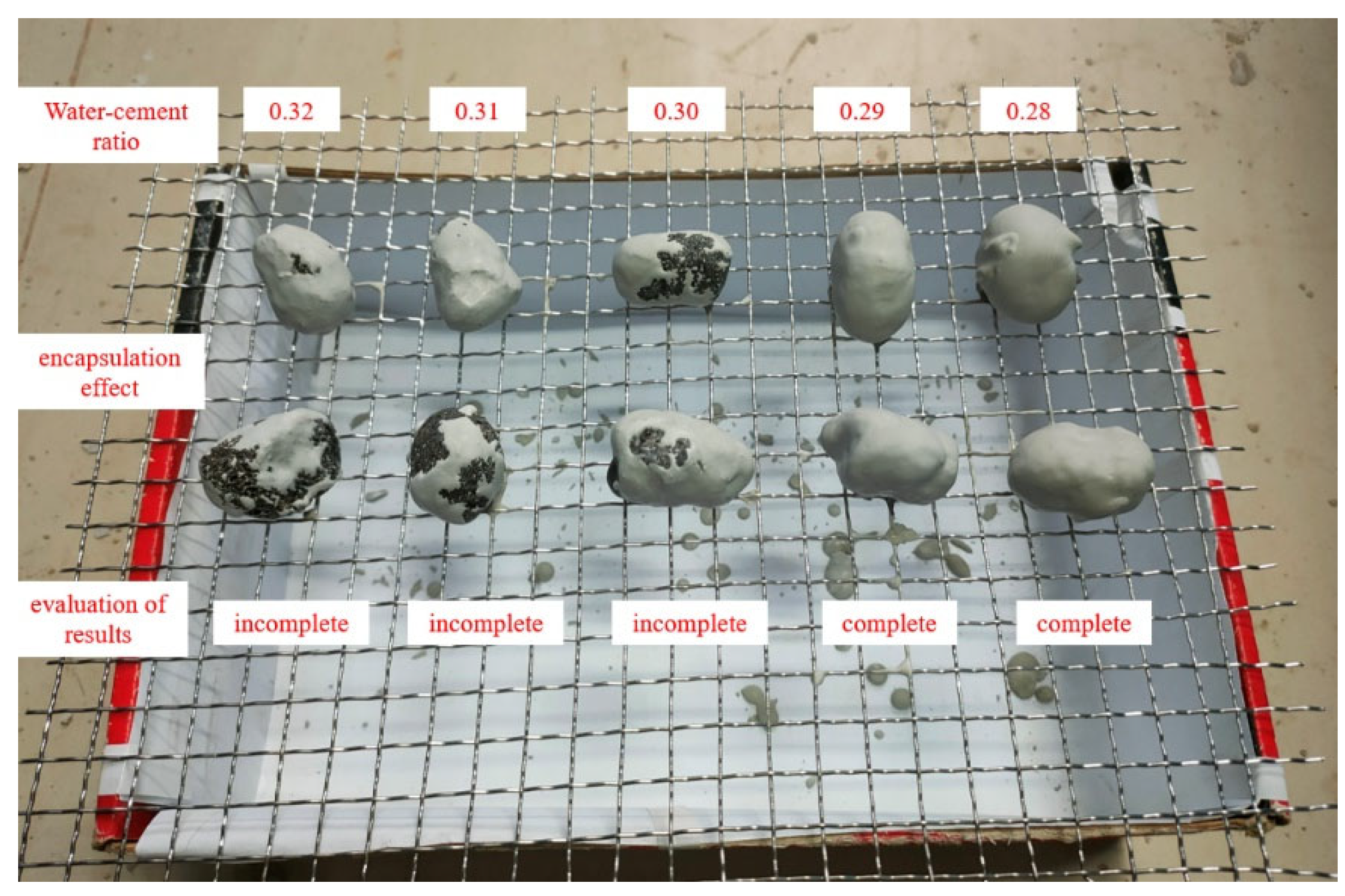
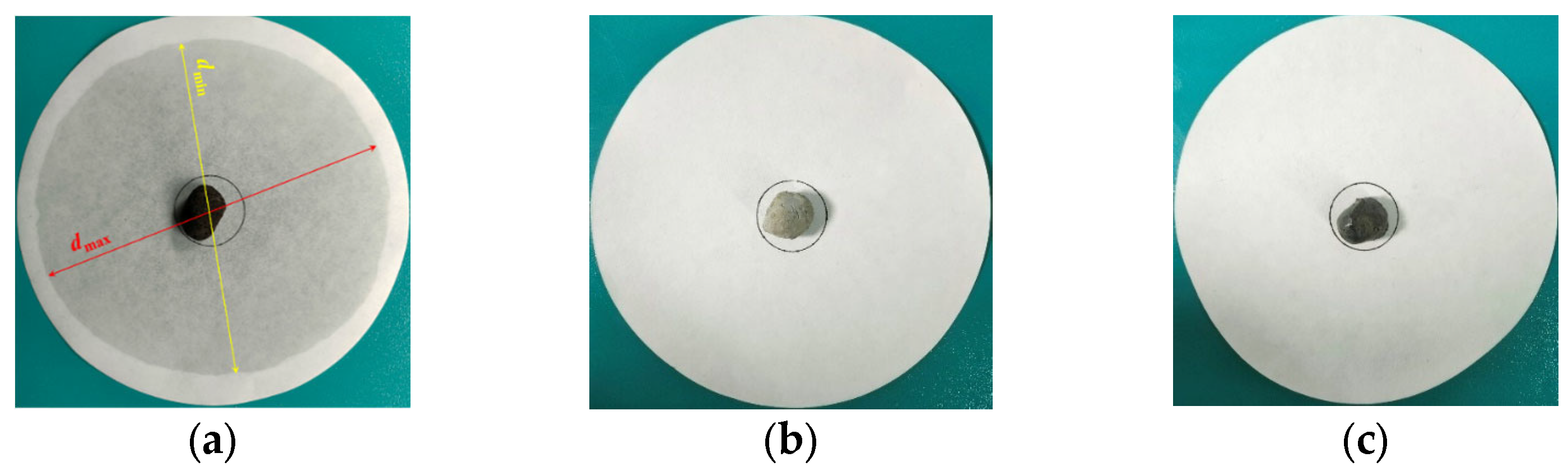
3.2.4. Thermal Stability Test of PCA
- (1)
- Draw a circle with a diameter of 30 mm in the center of the filter paper, and place the PCA in the drawing area, as shown in Figure 10a.
- (2)
- Place the PCAs to be tested in the electric blast drying oven and heat then at 100 °C for 24 h. The unencapsulated PCA sample is placed on the top layer of the oven, the PCA encapsulated in superfine cement is in the middle layer and the PCA encapsulated in epoxy resin is placed on the bottom layer, as is shown in Figure 10b.
- (3)
- Observe the diffusion degree of the PCM on the filter paper, then measure the maximum diameter and minimum diameter of the phase-change material diffusion and calculate the mean of the maximum and minimum values.
- (4)
- Calculate the percentage of exudation by Equation (3) as follows:
3.2.5. Thermal Storage Performance Test of PCA
4. Test Procedure of PCAC
5. Results and Discussion
5.1. Mechanical Properties of PCA
5.1.1. Cylindrical Compress Strength of PCA
5.1.2. Crushing Rate of PCA during Mixing
5.2. Thermodynamic Properties of PCA
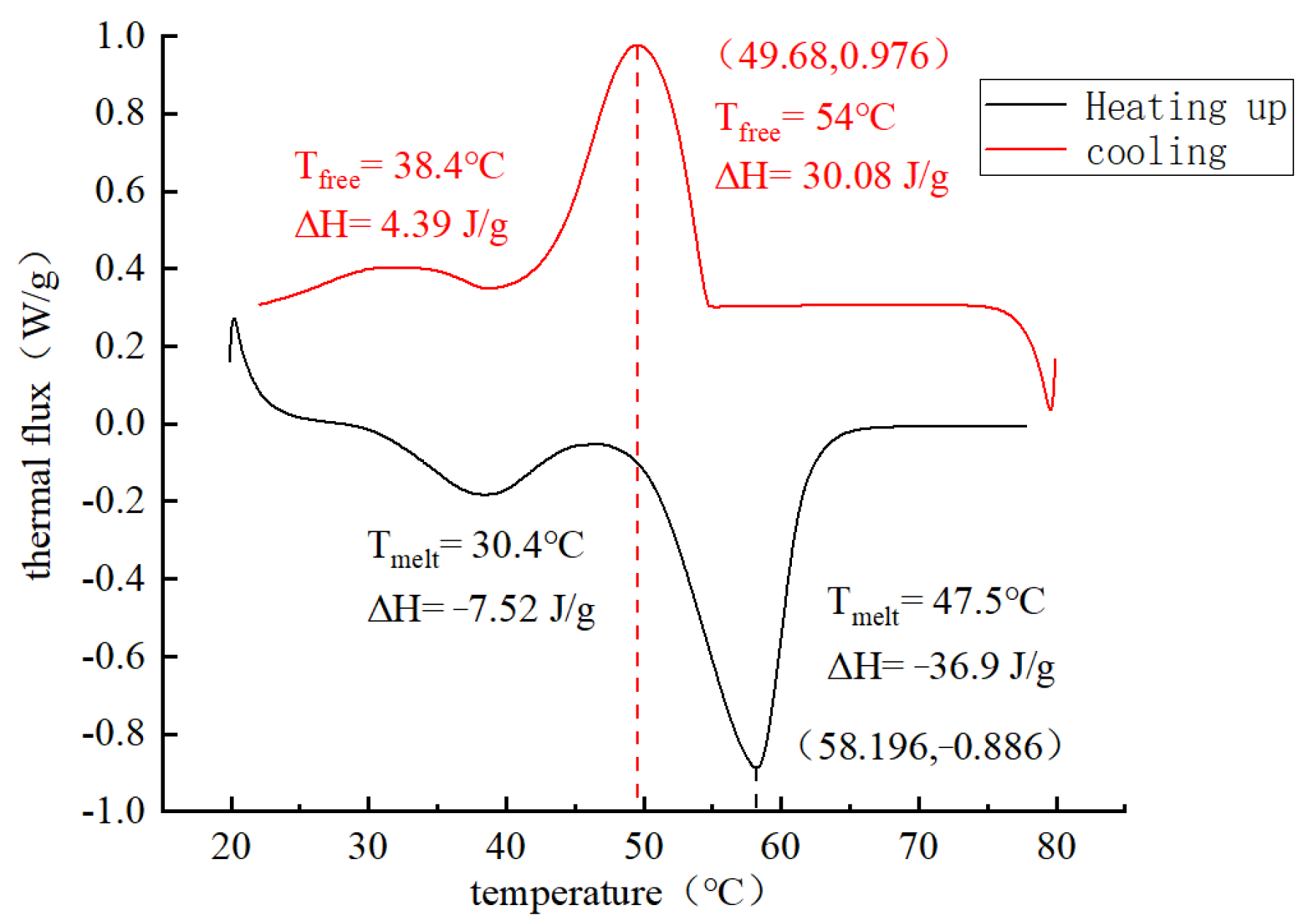
5.2.1. Enthalpy Value of PCA
5.2.2. Thermal Stability of PCA
5.2.3. Thermal Storage Performance of PCA
5.3. Mechanical Properties of PCAC
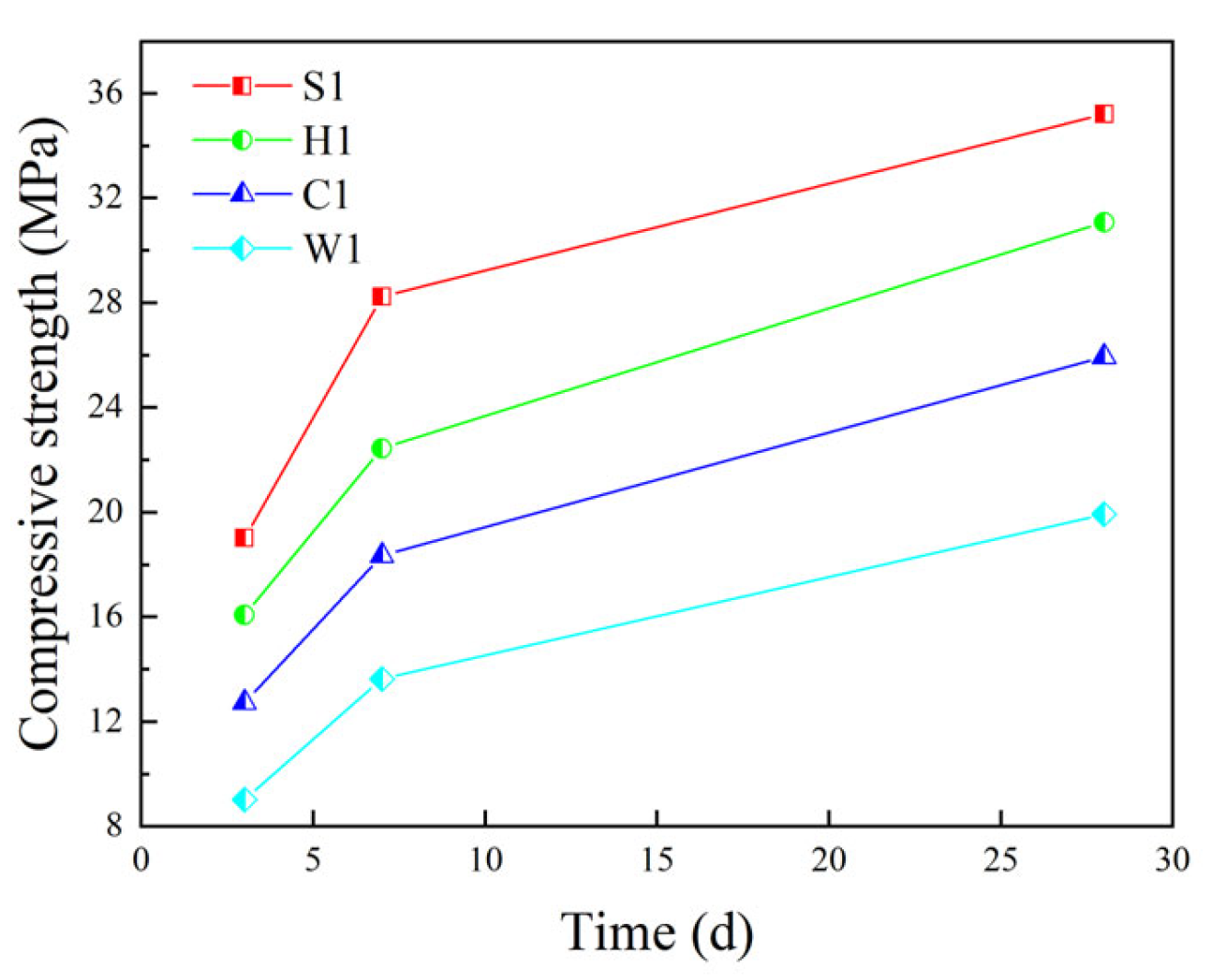
5.3.1. Compressive Performance
5.3.2. Flexural Performance
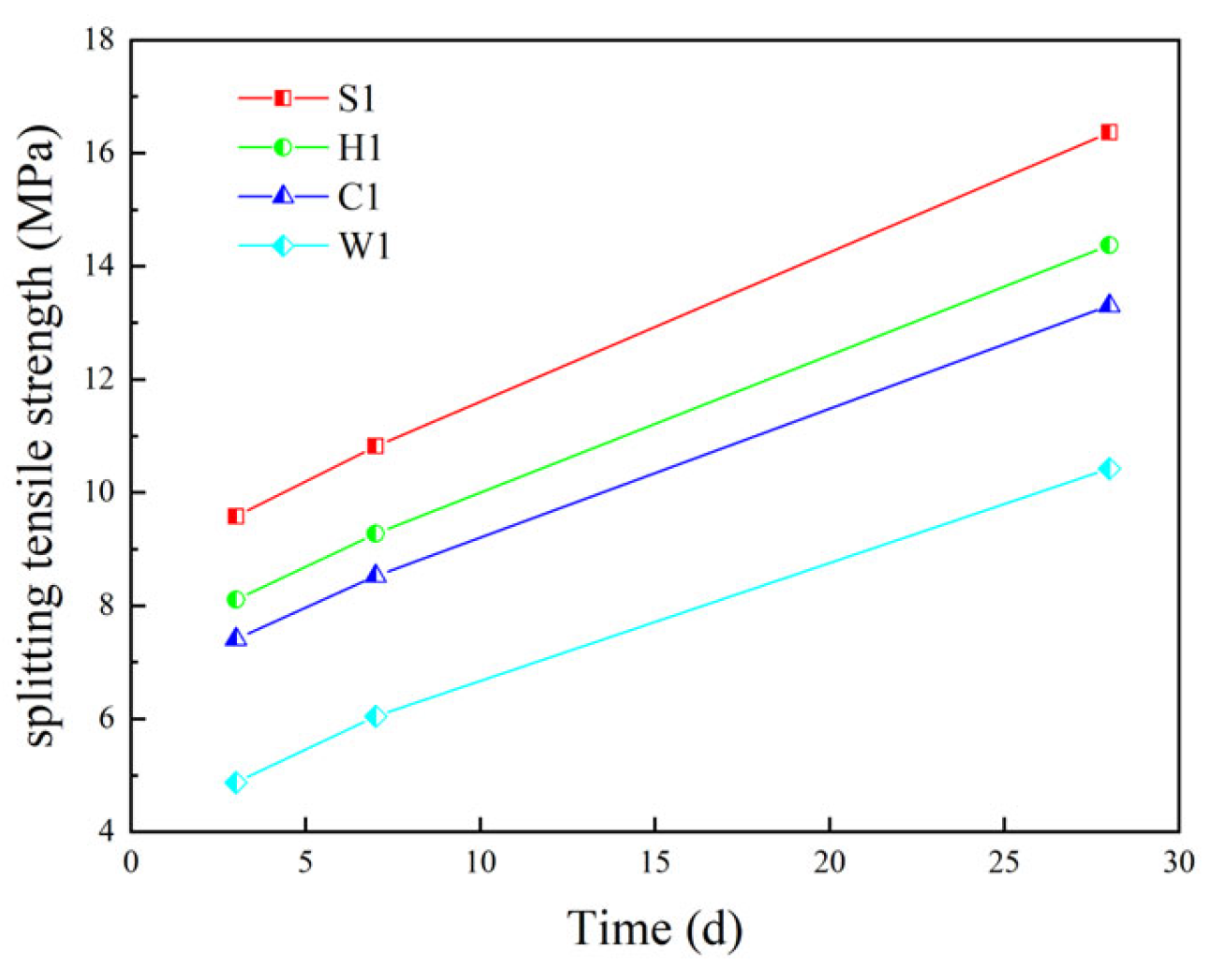
5.3.3. Splitting Tensile Performance
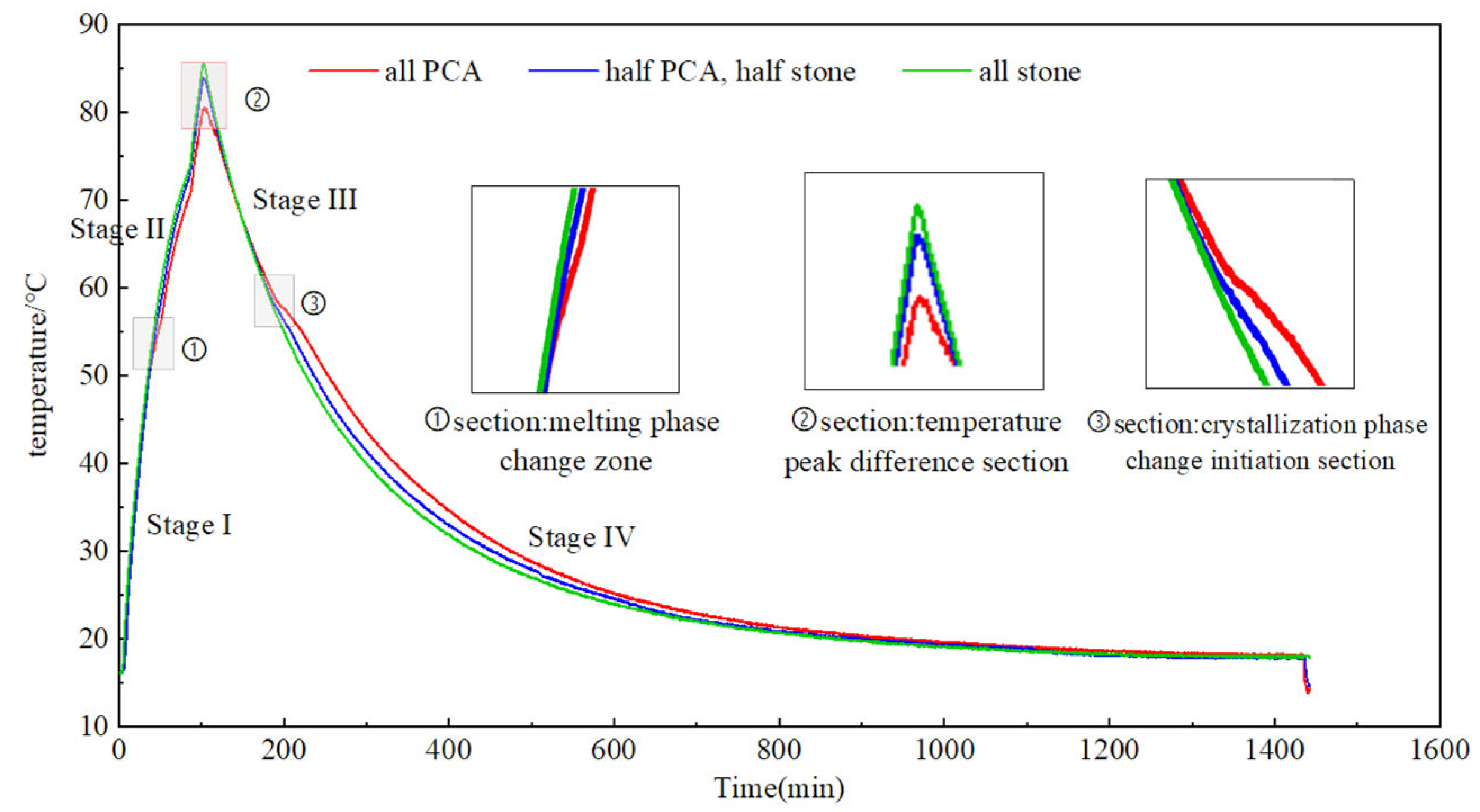
5.4. Control Effect of the Hydration Heat of PCAC
6. Conclusions
- (1)
- Both superfine cement and epoxy resin can be used as preferred materials for PCA encapsulation and shell making. When encapsulating PCA with epoxy resin, graphite powder can be added to the epoxy resin slurry to increase its thermal conductivity.
- (2)
- Superfine cement shell and epoxy resin shell can increase aggregate strength, with epoxy resin having a better effect than superfine cement. The PCAs encapsulated in superfine cement and in epoxy resin both have good thermal stability.
- (3)
- For the concrete at 3d, 7d and 28d, the mechanical properties of concrete mixed with PCA are reduced to varying degrees compared to ordinary crushed stone concrete.
- (4)
- When the water bath temperature reaches 85 °C, the temperature difference between the PCA and the common stone aggregate can be up to 6 °C, and based on the law of conservation of energy, the test results will be converted to mass concrete with the same volume of the aggregate mixture, The difference of PCAC and ordinary concrete temperature can be up to 10 °C, and the temperature control effect is significant.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, H.; Bi, K.; Chen, W.; Pham, T.M.; Li, J. Towards next generation design of sustainable, durable, multi-hazard resistant, resilient, and smart civil engineering structures. Eng. Struct. 2023, 277, 115477. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Luo, Z.; Wang, K.; Shah, S.P. A critical review on phase change materials (PCM) for sustainable and energy efficient building: Design, characteristic, performance and application. Energy Build. 2022, 260, 111923. [Google Scholar] [CrossRef]
- Mei, Y.; Zhao, L.; Nong, X.; Yang, T.; Zhang, X.; Wang, R.; Wang, X. Field test study on early strain development law of mass concrete in cold weather. Case Stud. Constr. Mater. 2022, 17, e01455. [Google Scholar] [CrossRef]
- Saeed, M.K.; Rahman, M.K.; Alfawzan, M.; Basha, S.; Dahish, H.A. Recycling of date kernel powder (DKP) in mass concrete for mitigating heat generation and risk of cracking at an early age. Constr. Build. Mater. 2023, 376, 131033. [Google Scholar] [CrossRef]
- Xie, Y.; Du, W.; Xu, Y.; Peng, B.; Qian, C. Temperature field evolution of mass concrete: From hydration dynamics, finite element models to real concrete structure. J. Build. Eng. 2023, 65, 105699. [Google Scholar] [CrossRef]
- Xin, J.; Jiang, X.; Chen, Z.; Zuo, L.; Zhang, G.; Wang, Z.; Qi, C.; Zhang, L.; Liu, Y. Early age thermal cracking resistance of basalt fiber-reinforced concrete for mass concrete structures under restraint condition. Structures 2022, 45, 1189–1198. [Google Scholar] [CrossRef]
- Liu, J.; Tian, Q.; Wang, Y.; Li, H.; Xu, W. Evaluation method and mitigation strategies for shrinkage cracking of modern concrete. Engineering 2021, 7, 348–357. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Bentur, A.; Igarashi, S.-I.; Kovler, K. Prevention of autogenous shrinkage in high-strength concrete by internal curing using wet lightweight aggregates. Cem. Concr. Res. 2001, 31, 1587–1591. [Google Scholar] [CrossRef]
- Bentz, D.P.; Snyder, K.A. Protected paste volume in concrete: Extension to internal curing using saturated lightweight fine aggregate. Cem. Concr. Res. 1999, 29, 1863–1867. [Google Scholar] [CrossRef]
- Suzuki, M.; Meddah, M.S.; Sato, R. Use of porous ceramic waste aggregates for internal curing of high-performance concrete. Cem. Concr. Res. 2009, 39, 373–381. [Google Scholar] [CrossRef]
- Tziviloglou, E.; Wiktor, V.; Jonkers, H.; Schlangen, E. Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr. Build. Mater. 2016, 122, 118–125. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Farnam, Y.; Esmaeeli, H.S.; Zavattieri, P.D.; Haddock, J.; Weiss, J. Incorporating phase change materials in concrete pavement to melt snow and ice. Cem. Concr. Compos. 2017, 84, 134–145. [Google Scholar] [CrossRef]
- Farnam, Y.; Krafcik, M.; Liston, L.; Washington, T.; Erk, K.; Tao, B.; Weiss, J. Evaluating the use of phase change materials in concrete pavement to melt ice and snow. J. Mater. Civ. Eng. 2016, 28, 04015161. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, L.; Zhang, H. Study on anti-freezing functional design of phase change and temperature control composite bridge decks. Constr. Build. Mater. 2016, 122, 714–720. [Google Scholar] [CrossRef]
- Qian, C.; Gao, G. Reduction of interior temperature of mass concrete using suspension of phase change materials as cooling fluid. Constr. Build. Mater. 2012, 26, 527–531. [Google Scholar] [CrossRef]
- Qian, C.; Gao, G.; Zhu, C.; Guo, Z. Influence of phase change materials on temperature rise caused by hydration heat evolution of cement-based materials. Mag. Concr. Res. 2010, 62, 789–794. [Google Scholar] [CrossRef]
- Eddhahak, A.; Drissi, S.; Colin, J.; Caré, S.; Neji, J. Effect of phase change materials on the hydration reaction and kinetic of PCM-mortars. J. Therm. Anal. Calorim. 2014, 117, 537–545. [Google Scholar] [CrossRef]
- Eddhahak-Ouni, A.; Drissi, S.; Colin, J.; Neji, J.; Care, S. Experimental and multi-scale analysis of the thermal properties of Portland cement concretes embedded with microencapsulated Phase Change Materials (PCMs). Appl. Therm. Eng. 2014, 64, 32–39. [Google Scholar] [CrossRef]
- Lecompte, T.; Le Bideau, P.; Glouannec, P.; Nortershauser, D.; Le Masson, S. Mechanical and thermo-physical behaviour of concretes and mortars containing phase change material. Energy Build. 2015, 94, 52–60. [Google Scholar] [CrossRef]
- Ricklefs, A.; Thiele, A.M.; Falzone, G.; Sant, G.; Pilon, L. Thermal conductivity of cementitious composites containing microencapsulated phase change materials. Int. J. Heat Mass Transf. 2017, 104, 71–82. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Neeper, D. Thermal dynamics of wallboard with latent heat storage. Sol. Energy 2000, 68, 393–403. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Nepomuceno, M.C.; Silva, P.D. Experimental evaluation of cement mortars with phase change material incorporated via lightweight expanded clay aggregate. Constr. Build. Mater. 2014, 63, 89–96. [Google Scholar] [CrossRef]
- Sakulich, A.R.; Bentz, D.P. Incorporation of phase change materials in cementitious systems via fine lightweight aggregate. Constr. Build. Mater. 2012, 35, 483–490. [Google Scholar] [CrossRef]
- Memon, S.A.; Cui, H.; Zhang, H.; Xing, F. Utilization of macro encapsulated phase change materials for the development of thermal energy storage and structural lightweight aggregate concrete. Appl. Energy 2015, 139, 43–55. [Google Scholar] [CrossRef]
- Sakulich, A.R.; Bentz, D.P. Increasing the service life of bridge decks by incorporating phase-change materials to reduce freeze-thaw cycles. J. Mater. Civ. Eng. 2012, 24, 1034–1042. [Google Scholar] [CrossRef]
- Aguayo, M.; Das, S.; Castro, C.; Kabay, N.; Sant, G.; Neithalath, N. Porous inclusions as hosts for phase change materials in cementitious composites: Characterization, thermal performance, and analytical models. Constr. Build. Mater. 2017, 134, 574–584. [Google Scholar] [CrossRef]
- Bentz, D.P.; Turpin, R. Potential applications of phase change materials in concrete technology. Cem. Concr. Compos. 2007, 29, 527–532. [Google Scholar] [CrossRef]
- Sharifi, N.P.; Sakulich, A. Application of phase change materials to improve the thermal performance of cementitious material. Energy Build. 2015, 103, 83–95. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Zhou, J.; Wu, K. Development of thermal energy storage concrete. Cem. Concr. Res. 2004, 34, 927–934. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, J.; Sun, G.; Xu, B.; Li, Z.; Gong, C. Effects of the form-stable expanded perlite/paraffin composite on cement manufactured by extrusion technique. Energy 2015, 82, 43–53. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sanjayan, J.; Wang, X.; Alam, M.; Wilson, J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl. Energy 2015, 157, 85–94. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite composite phase change material incorporated cement-based composite for thermal energy storage. Appl. Energy 2013, 105, 229–237. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite/multi-wall carbon nanotubes composite phase change material tailor-made for thermal energy storage cement-based composites. Energy 2014, 72, 371–380. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Performance of novel thermal energy storage engineered cementitious composites incorporating a paraffin/diatomite composite phase change material. Appl. Energy 2014, 121, 114–122. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Lu, Z.; Li, Z. Paraffin/expanded vermiculite composite phase change material as aggregate for developing lightweight thermal energy storage cement-based composites. Appl. Energy 2015, 160, 358–367. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Li, X.; Sanjayan, J.G. Development of thermal energy storage composites and prevention of PCM leakage. Appl. Energy 2014, 135, 225–233. [Google Scholar] [CrossRef]
- Li, X.; Sanjayan, J.G.; Wilson, J.L. Fabrication and stability of form-stable diatomite/paraffin phase change material composites. Energy Build. 2014, 76, 284–294. [Google Scholar] [CrossRef]
- Memon, S.A.; Cui, H.; Lo, T.Y.; Li, Q. Development of structural–functional integrated concrete with macro-encapsulated PCM for thermal energy storage. Appl. Energy 2015, 150, 245–257. [Google Scholar] [CrossRef]
- GB/T 10247-2008; Methods of Viscosity Measurement. Chinese Specification Press: Beijing, China, 2008.
- GB/T 17431-2010; Lightweight Aggregates and Its Test Methods-Part 1: Lightweight Aggregates. Chinese Specification Press: Beijing, China, 2010.
- GB 50164-2011; Standard for Quality Control of Concrete. China Architecture & Building Press: Beijing, China, 2011.
- GB/T 50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. China Architecture & Building Press: Beijing, China, 2011.
- Ma, B.; Adhikari, S.; Chang, Y.; Ren, J.; Liu, J.; You, Z. Preparation of composite shape-stabilized phase change materials for highway pavements. Constr. Build. Mater. 2013, 42, 114–121. [Google Scholar] [CrossRef]
- Hunger, M.; Entrop, A.; Mandilaras, I.; Brouwers, H.; Founti, M. The behavior of self-compacting concrete containing micro-encapsulated phase change materials. Cem. Concr. Compos. 2009, 31, 731–743. [Google Scholar] [CrossRef]
- Fernandes, F.; Manari, S.; Aguayo, M.; Santos, K.; Oey, T.; Wei, Z.; Falzone, G.; Neithalath, N.; Sant, G. On the feasibility of using phase change materials (PCMs) to mitigate thermal cracking in cementitious materials. Cem. Concr. Compos. 2014, 51, 14–26. [Google Scholar] [CrossRef]

















| Ceramic Granules | Particle Size | Cylinder Compression Strength | Water Absorption | Bulk Density |
|---|---|---|---|---|
| Lightweight black ceramic granules | 5–30 mm | 16.99 MPa | 9% | 753 kg/m3 |
| Shape | Phase Change Temperature Peak | Density | Specific Heat | Latent Heat of Phase Transition |
|---|---|---|---|---|
| White block | 58 °C | 900 kg/m3 | 2.25 kJ·kg−1·K−1 | 225.1 J·g−1 |
| Performance Index | 3 Days Compressive Strength | 28 Days Compressive Strength | 3 Days Flexural Strength | 28 Days Flexural Strength |
|---|---|---|---|---|
| Value | ≥30 MPa | ≥60 MPa | ≥5 MPa | ≥7 MPa |
| Performance Index | Chroma | Epoxy Equivalent | Hydrolyzed Chlorine | Inorganic Chlorine | Volatile Matter | Softening Point |
|---|---|---|---|---|---|---|
| Value | ≤Pt-Co | ≤210~230 g/mol | ≤0.50% | ≤50 mg/kg | ≤0.60% | 14~23 °C |
| No. | Replacement Rate of Coarse Aggregate (%) | Cement (kg/m3) | Sand (kg/m3) | Crushed Stone (kg/m3) | Phase Change Aggregate (kg/m3) | Water (kg/m3) | Water Reducing Agent (kg/m3) |
|---|---|---|---|---|---|---|---|
| S1 | 0% | 295 | 760 | 1024 | 0 | 160 | 85 |
| W1 | 100% | 295 | 760 | 0 | 805 | 160 | 85 |
| C1 | 100% | 295 | 760 | 0 | 825 | 160 | 85 |
| H1 | 100% | 295 | 760 | 0 | 782 | 160 | 85 |
| Material | Mean Value of Cylindrical Compressive Strength (MPa) | Standard Deviation (MPa) |
|---|---|---|
| Lightweight ceramsites | 16.99 | 0.12 |
| Unencapsulated PCA | 21.95 | 0.21 |
| Superfine cement-encapsulated PCA | 22.06 | 0.21 |
| Epoxy resin-encapsulated PCA | 23.85 | 0.39 |
| Encapsulating Material | Superfine Cement Slurry | Epoxy Resin |
|---|---|---|
| Crushing rate | 42% | 0 |
| Unencapsulated Phase Change Aggregates | Phase Change Aggregates Encapsulated in Superfine Cement | Phase Change Aggregates Encapsulated in Epoxy Resin | |
|---|---|---|---|
| (mm) | 141 | 0 | 0 |
| (mm) | 157 | 0 | 0 |
| (mm) | 149 | 0 | 0 |
| Percentage of exudation | 397 | 0 | 0 |
| The Mechanical Properties of PCAC(MPa) | ||||||
|---|---|---|---|---|---|---|
| Superfine Cement-Encapsulated | Epoxy Resin-Encapsulated | |||||
| 3 d | 7 d | 28 d | 3 d | 7 d | 28 d | |
| Compressive performance | 12.74 (1.03) | 18.35 (1.54) | 25.95 (1.89) | 16.08 (0.67) | 22.45 (0.97) | 31.09 (1.24) |
| Flexural performance | 3.18 (0.23) | 4.24 (0.34) | 5.57 (0.66) | 2.76 (0.32) | 4.37 (0.27) | 5.68 (0.46) |
| Splitting tensile performance | 7.41 (0.45) | 8.53 (0.57) | 13.31 (0.36) | 8.11 (0.56) | 9.28 (0.78) | 14.38 (1.09) |
| Parameters | cw/(kJ·kg−1·K−1) | mw/(kg) | m/(kg) | c/(kJ·kg−1·K−1) | mc/(kg) | mp/(kg) |
|---|---|---|---|---|---|---|
| Value | 4.2 | 8 | 3.03 | 3.2 | 5.64 | 3.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, C.; Luo, A.; Dong, M.; Su, Q.; Zhou, C.; Zhang, Z.; Pei, Y. Experimental Investigation on a Novel Temperature-Controlled Phase Change Aggregate Concrete: Thermo-Mechanical Properties and Hydration Heat Control. Materials 2023, 16, 5269. https://doi.org/10.3390/ma16155269
Wang Y, Wang C, Luo A, Dong M, Su Q, Zhou C, Zhang Z, Pei Y. Experimental Investigation on a Novel Temperature-Controlled Phase Change Aggregate Concrete: Thermo-Mechanical Properties and Hydration Heat Control. Materials. 2023; 16(15):5269. https://doi.org/10.3390/ma16155269
Chicago/Turabian StyleWang, Yejia, Chengjin Wang, Aibo Luo, Minqi Dong, Qian Su, Chenling Zhou, Zongyu Zhang, and Yanfei Pei. 2023. "Experimental Investigation on a Novel Temperature-Controlled Phase Change Aggregate Concrete: Thermo-Mechanical Properties and Hydration Heat Control" Materials 16, no. 15: 5269. https://doi.org/10.3390/ma16155269
APA StyleWang, Y., Wang, C., Luo, A., Dong, M., Su, Q., Zhou, C., Zhang, Z., & Pei, Y. (2023). Experimental Investigation on a Novel Temperature-Controlled Phase Change Aggregate Concrete: Thermo-Mechanical Properties and Hydration Heat Control. Materials, 16(15), 5269. https://doi.org/10.3390/ma16155269








