Investigating the Corrosive Influence of Chloride Ions on Slag Recovery Machine Shells in Power Plants
Abstract
:1. Introduction
2. Experimental
2.1. Material and Sample Preparation
2.2. Characterization Methods
2.2.1. Hanging Piece Experiment
2.2.2. Q235-A Surface Corrosion Analysis
2.2.3. Electrochemical Testing
3. Results and Discussion
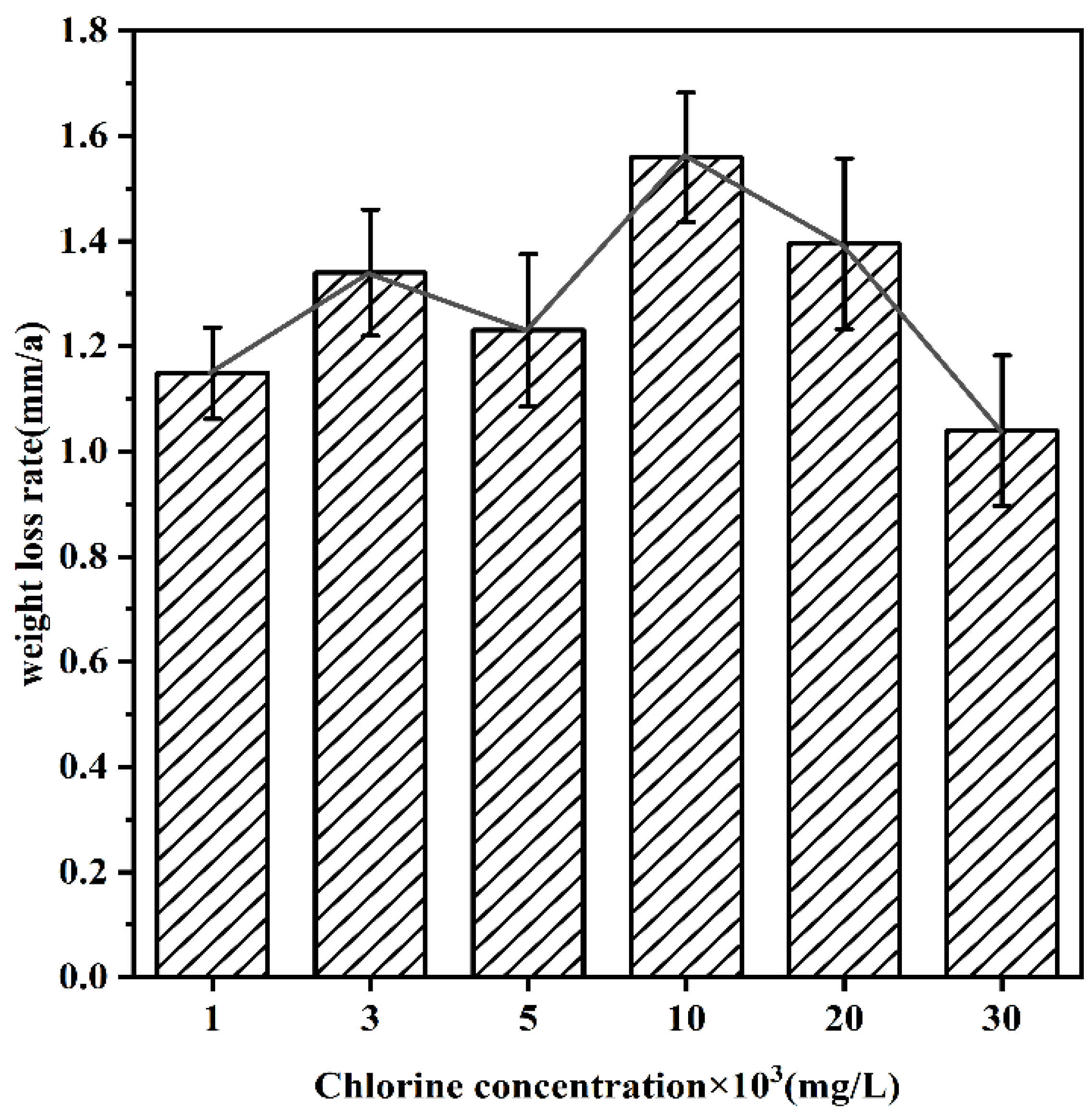
3.1. Hanging Experimental Analysis
3.2. Q235-A Surface Morphology Analysis
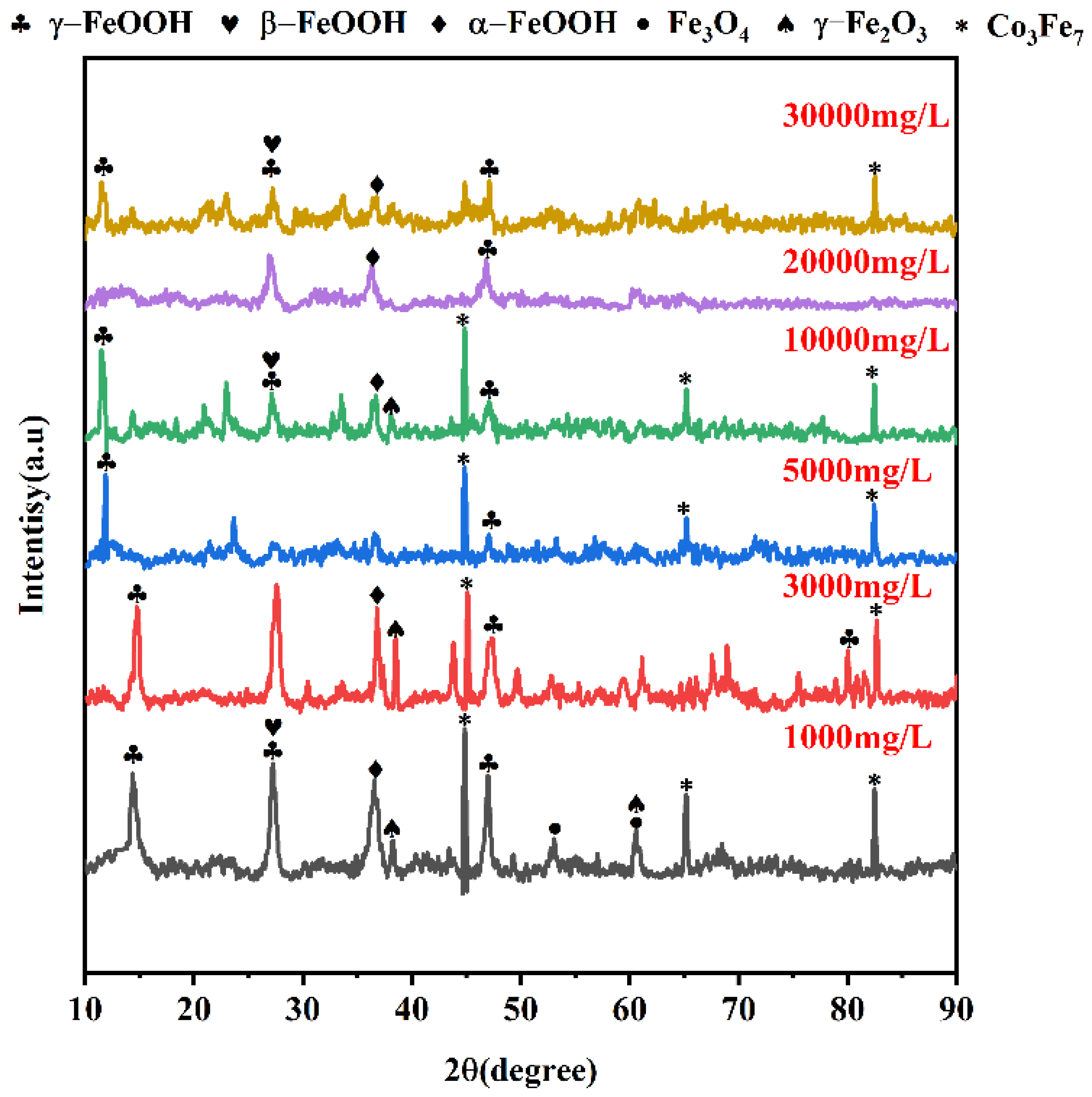
3.2.1. Composition of Corrosion-Producing Films
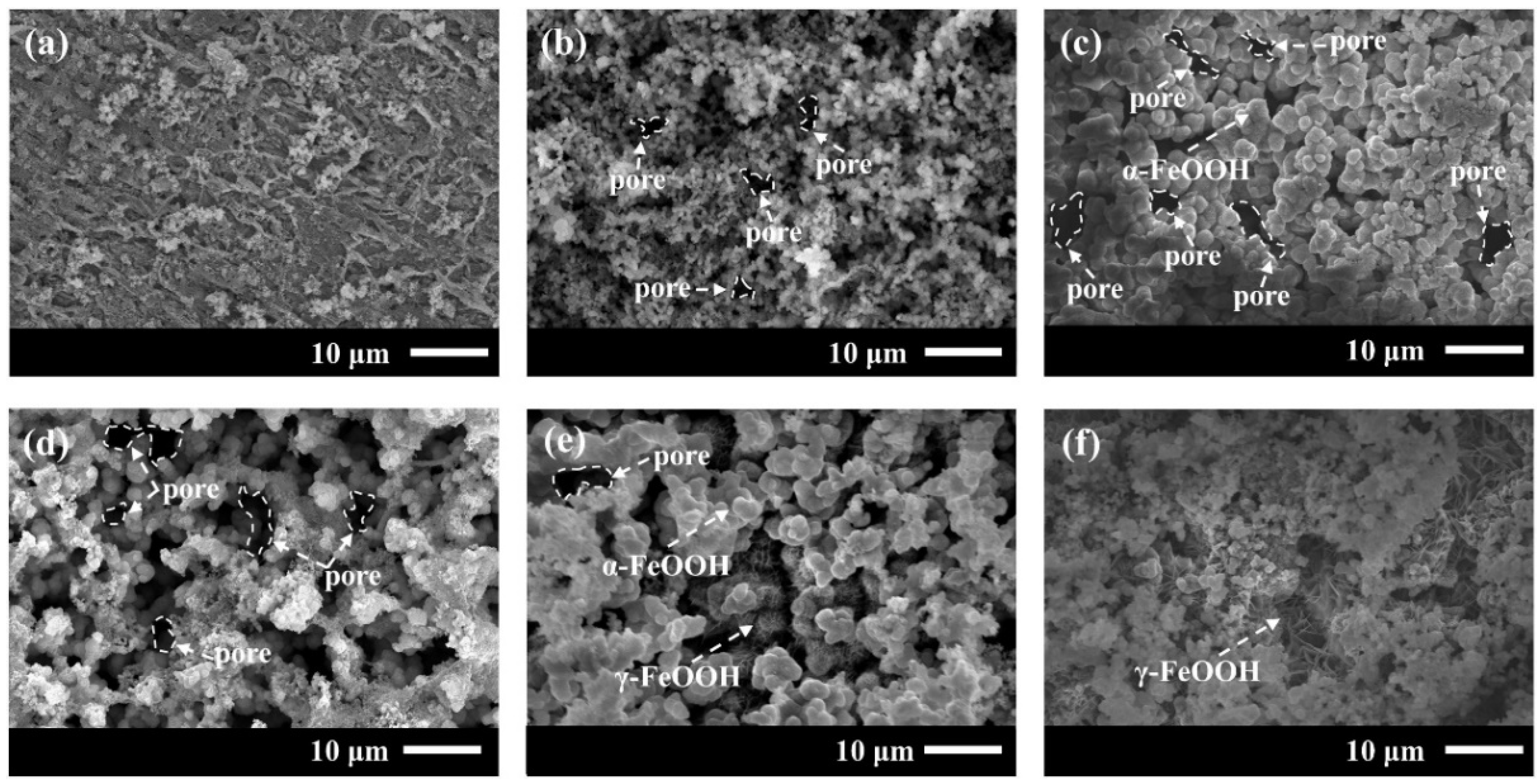
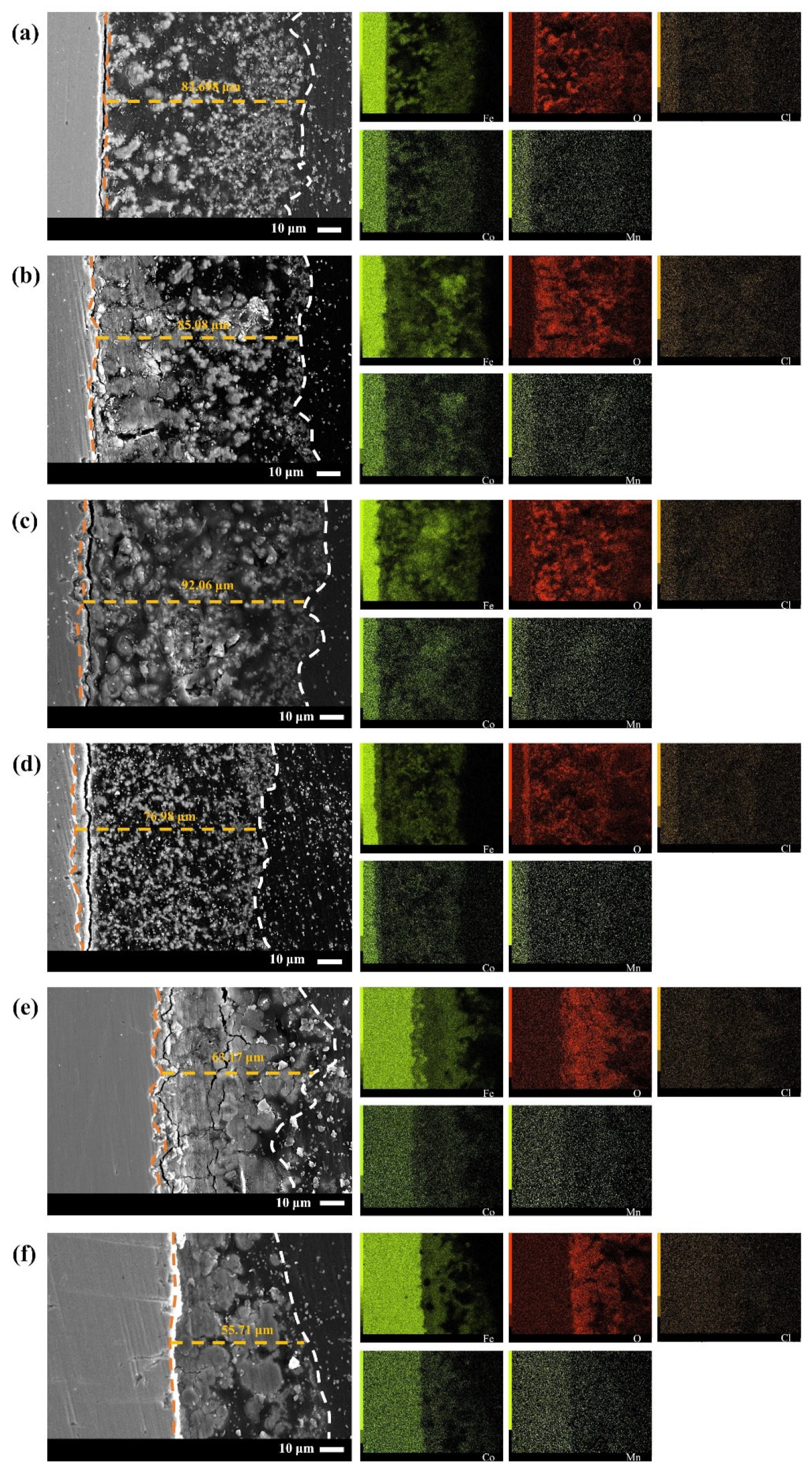
3.2.2. Corrosion Product Form
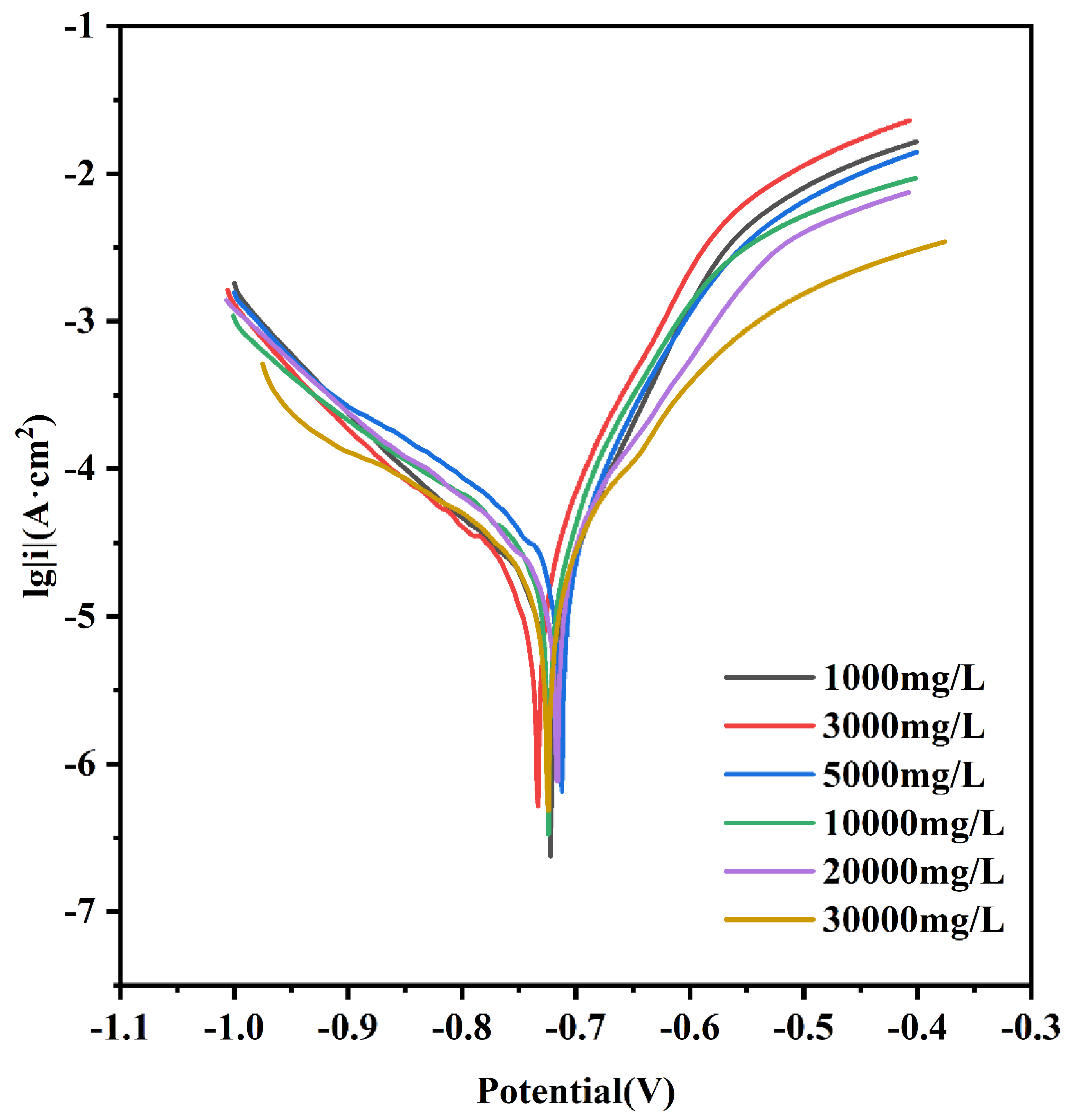
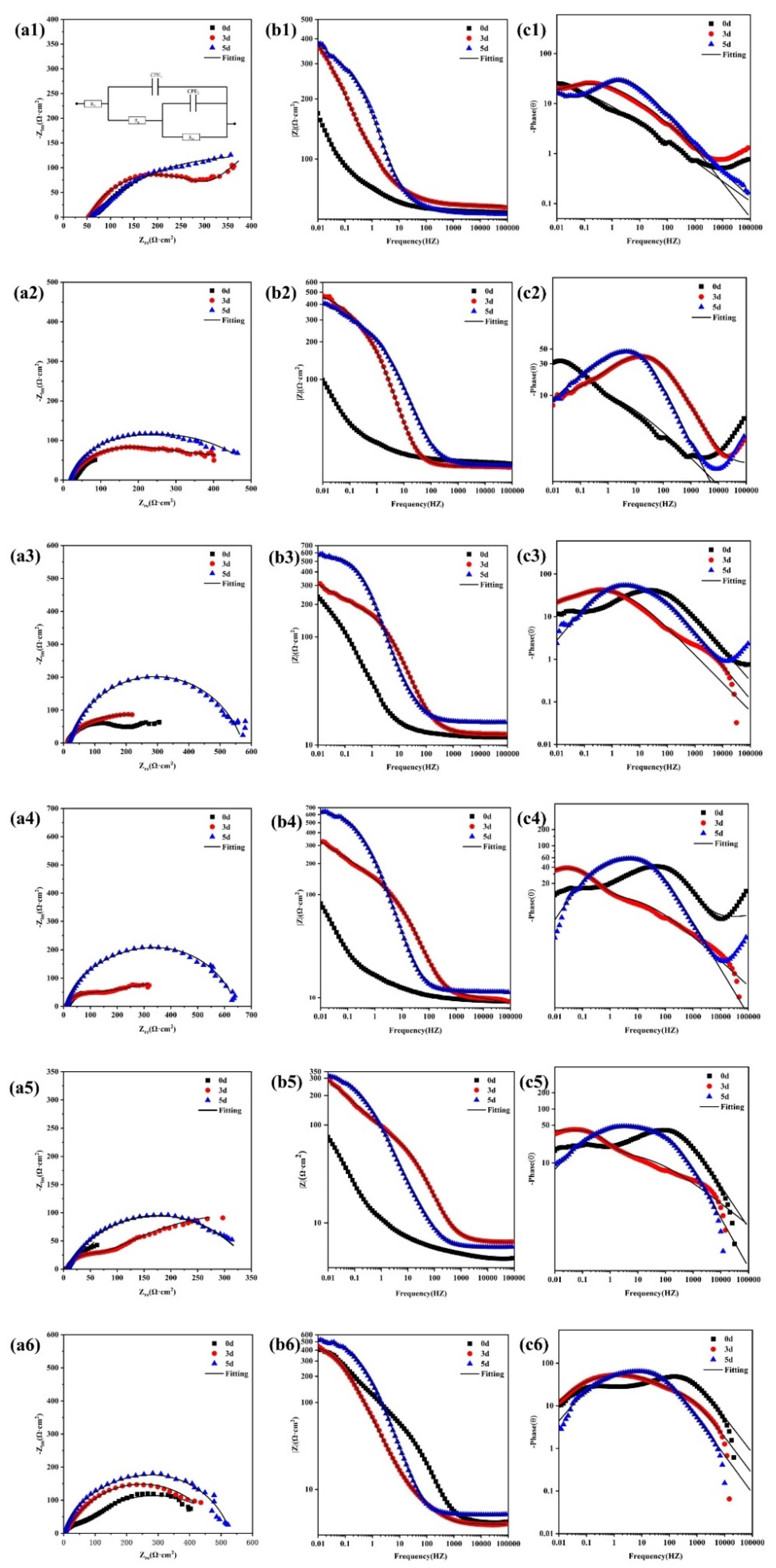
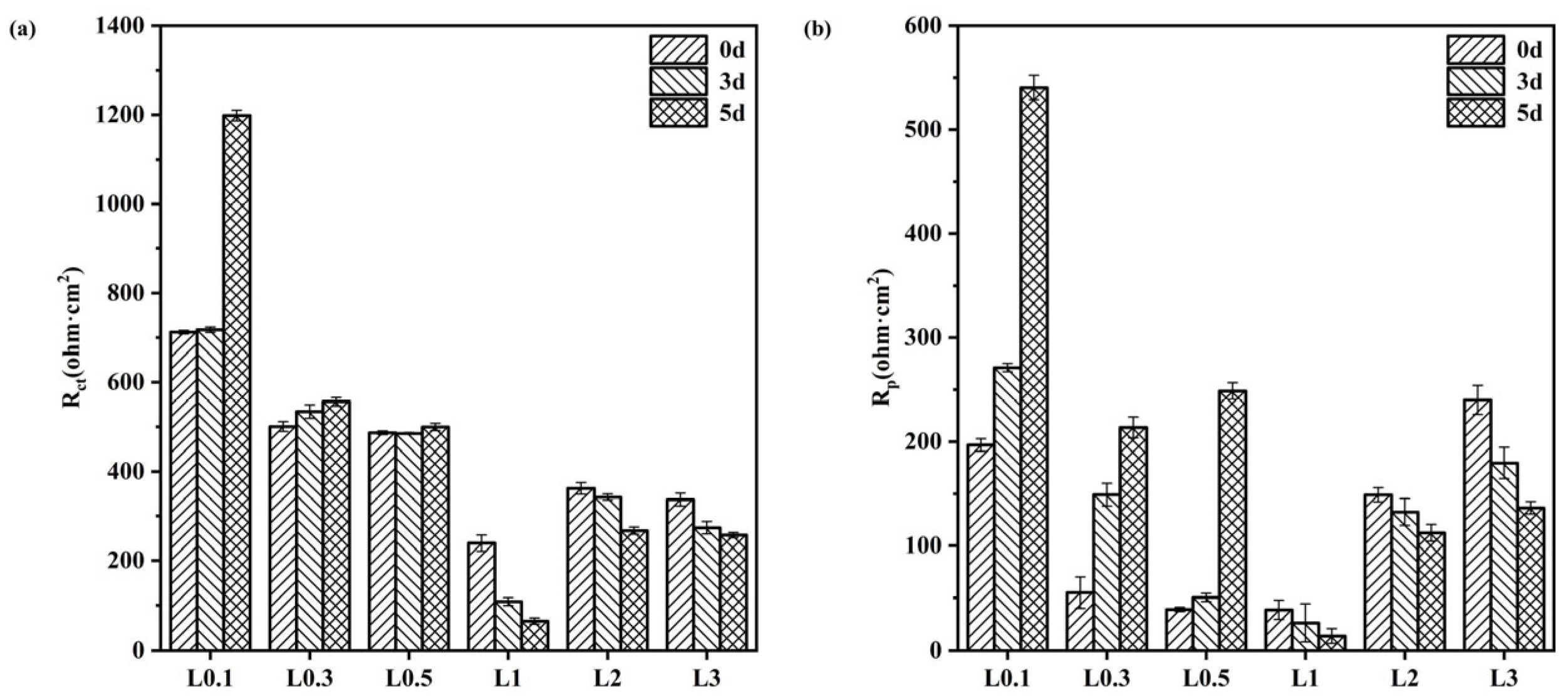
3.2.3. Electrochemical Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, W.H.; Zhao, J.; Lu, J.G.; Han, Y.; Li, D. Zero-liquid discharge technologies for desulfurization wastewater: A review. J. Environ. Manag. 2022, 321, 115953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, J.; Feng, Q. Review on occurrence, speciation, transition and fate of sulfur in typical ultra-low emission coal-fired power plants. J. Acad. Nutr. Diet. 2022, 100, 259–276. [Google Scholar] [CrossRef]
- Xin, Y.; Zhou, Z.; Ming, Q.; Sun, D.; Han, J.; Ye, X.; Dai, S.; Jiang, L.-M.; Zhao, X.; An, Y. A two-stage desalination process for zero liquid discharge of flue gas desulfurization wastewater by chloride precipitation. J. Hazard. Mater. 2020, 397, 122744. [Google Scholar] [CrossRef]
- Izadi, R.; Assarian, D.; Altaee, A.; Mahinroosta, M. Investigation of methods for fuel desulfurization wastewater treatment. Chem. Eng. Res. Des. 2023, 190, 198–219. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Shi, P.Y.; Jiang, M.F. Hydrothermal recovery and reuse of oxidated by-products in magnesium flue gas desulfurization: Experimental studies and molecular dynamics simulation. J. Environ. Chem. Eng. 2022, 10, 108099. [Google Scholar] [CrossRef]
- Koralegedara, N.H.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Recent advances in flue gas desulfurization gypsum processes and applications—A review. J. Environ. Manag. 2019, 251, 109572. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W. Research and application of power plant desulfurization wastewater reuse to wet slag collector. Low Carbon World 2018, 11, 64–65. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, J.F.; Tang, M.Z.; Jia, C.X.; Zhang, C. Optimization for desulfurization wastewater pretreatment in a typical coal-fired thermal power plant. J. Qufu. Nor. Univ. (Nat. Sci.) 2019. [Google Scholar] [CrossRef]
- Du, M.; Shao, N.; Jiang, Y.S. Analysis on feasible technology about wastewater treatment and desulfurization wastewater zero discharge in thermal power plant. Shanxi Archit. 2019, 45, 189–190. [Google Scholar] [CrossRef]
- Wang, N.J.; Wei, W.J.; Zhang, B.H.; Sun, H.; Li, J.F.; Chen, Q.; Cheng, G. Feasibility analysis of zero discharge reform scheme of desulfurization wastewater in thermal power plant based on flue gas evaporation technology. Liaoning Chem. Ind. 2019, 48, 1209–1211. [Google Scholar] [CrossRef]
- Zheng, L.B.; Wei, Y.S.; Jiao, Y.Y.; Wang, G.; Yue, Z.G. Status and prospect of zero liquid discharge technologies for flue gas desulfurization wastewater in thermal power plant. Chem. Ind. Eng. 2019, 36, 24–37. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chi, Y.F.; Zou, Y.J.; Wang, B.H.; Zhang, J.Y.; Zheng, Y.M. Status and outlook of engineering applications of desulfurization wastewater treatment technology for coal-fired power plants. Ind. Water Treat. 2020. [Google Scholar] [CrossRef]
- Chen, B.; Xu, C.; Zhao, Q.; Fu, Y.Q. Experimental study of flue gas desulfurization wastewater discharge into slag water treatment system. Zhejiang Electr. Power 2010, 29, 33–36+54. [Google Scholar] [CrossRef]
- Zou, Y.J. Application and impact analysis of sludge water system to achieve zero discharge of desulfurization wastewater. J. Environ. Sci. 2022, 41, 49–52. [Google Scholar] [CrossRef]
- Motte, R.D.; Basilico, E.; Mingant, R.; Kittel, J.; Francois, R.; Combrade, P. A study by electrochemical impedance spectroscopy and surface analysis of corrosion product layers formed during CO2 corrosion of low alloy steel. Corros. Sci. 2020, 172, 108666. [Google Scholar] [CrossRef]
- Mundhenk, N.; Carrero, S.; Knauss, K.G.; Wonneberger, R.; Wu, Y. Kinetic and thermodynamic analysis of high-temperature CO2 corrosion of carbon steel in simulated geothermal NaCl fluids. Corros. Sci. 2020, 171, 108597. [Google Scholar] [CrossRef]
- He, C.C.; Xu, L.; Chen, L.; Xu, X.F.; OuYang, P.W. Mechanism prediction of flow-induced corrosion and optimization of protection measures in overhead system of atmospheric tower. Chem. Eng. J. 2019, 70, 1027–1034. [Google Scholar] [CrossRef]
- Marian, B.; Kellenberger, A.; Millet, J.P.; Muntean, C.; Vaszilcsin, N. Corrosion resistance of carbon steel in weak acid solutions in the presence of l-histidine as corrosion inhibitor. Corros. Sci. 2013, 69, 389–395. [Google Scholar] [CrossRef]
- Kadowaki, M.; Muto, I.; Sugawara, Y.; Doi, T.; Kawano, K.; Hara, N. Real-time microelectrochemical observations of very early stage pitting on ferrite-pearlite steel in chloride solutions. J. Electrochem. Soc. 2017, 164, C261–C268. [Google Scholar] [CrossRef] [Green Version]
- Kadowaki, M.; Muto, I.; Sugawara, Y.; Hara, N. Beneficial role of retained austenite in pitting corrosion resistance of Fe-C-Si-Mn steel in chloride environments. Corros. Sci. 2022, 200, 110251. [Google Scholar] [CrossRef]
- Feng, W.G.; Wei, R.Z.; Wang, S.; Yang, X.Q.; Wei, W.F.; Liu, Z.Z. Study on corrosion behavior and corrosion mechanism of carbon steel in cooling water of hydroelectric power plants. Mater. Protect. 2022, 55, 119–127. [Google Scholar] [CrossRef]
- Lin, X.F.; Zhong, T.D.; Tong, X.H.; Chen, G.Y.; Zhang, J.R.; Zheng, Y.M. Effect of desulfurization wastewater on slag water system corrosion. J. Environ. Eng. 2021, 15, 350–359. [Google Scholar] [CrossRef]
- Deng, J.Y.; Xiao, X.M.; Li, Y.Y.; Liu, J.Y. Low-carbon nitrogen removal from power plants circulating cooling water and municipal wastewater by partial denitrification-anammox. Bioresour. Technol. 2023, 380, 129071. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.J.; Zhai, R.R.; Guo, Y.C. Water consumption of the integration of coal-fired power plant with CO2 post combustion system with different cooling methods. Int. J. Greenh. Gas. Con. 2022, 121, 103776. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, M.; Yuan, B.; Guo, Z.; Wu, Z.Y.; Li, G.Y. Multipath retrofit planning approach for coal-fired power plants in low-carbon power system transitions: Shanxi Province case in China. Energy 2023, 275, 127502. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, L.Y.; Liu, M.; Wang, J.S.; Yan, J.J. Performance analysis of coal-fired power plants integrated with carbon capture system under load-cycling operation conditions. Energy 2023, 276, 127532. [Google Scholar] [CrossRef]
- Yuan, Q.X.; Zhang, Y.X.; Wang, T.; Wang, J.W.; Romero, C.E. Mechanochemical stabilization of heavy metals in fly ash from coal-fired power plants via dry milling and wet milling. Waste Manag. 2021, 135, 428–436. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.H.; An, L.M.; Xu, G.; Zhu, X. Performance evaluation of a novel design for the waste heat recovery of a cement plant incorporating a coal-fired power plant. Energy 2022, 246, 123420. [Google Scholar] [CrossRef]
- Kondash, A.J.; Albright, E.; Vengosh, A. Quantity of flowback and produced waters from unconventional oil and gas exploration. Sci. Total. Environ. 2017, 574, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Boschee, P. Produced and flowback water recycling and reuse: Economics, limitations, and technology. Oil Gas Fac. 2014, 3, 16–21. [Google Scholar] [CrossRef]
- Onishi, V.C.; Carrero-Parreño, A.; Reyes-Labarta, J.A.; Ruiz-Femenia, R.; Salcedo-Díaz, R.; Fraga, E.S. LabaShale gas flowback water desalination: Single vs multiple-effect evaporation with vapor recompression cycle and thermal integration. Desalination 2017, 404, 230–248. [Google Scholar] [CrossRef]
- Zolfaghari, A.; Dehghanpour, H.; Noel, M.; Bearinger, D. Laboratory and field analysis of flowback water from gas shales. J. Unconv. Oil Gas Resour. 2016, 14, 113–127. [Google Scholar] [CrossRef]
- Rodrigues, R.T.; Rubio, J. DAF-dissolved air flotation: Potential applications in the mining and mineral processing industry. Int. J. Miner. Process. 2007, 82, 1–13. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.J.; Yao, M.Y.; Zhou, Y. Corrosion kinetics and corrosion mechanisms of BN–ZrO2–SiC composites in molten steel. Corros. Sci. 2014, 89, 93–100. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Liu, W.; Fan, Y.M.; Fan, E.D.; Dong, B.J.; Zhang, T.Y. Effect of cr content on the passivation behavior of cr alloy steel in a CO2 aqueous environment containing silty sand. Corros. Sci. 2020, 168, 108591. [Google Scholar] [CrossRef]
- Renner, F.U.; Ankah, G.N.; Bashir, A.; Ma, D.; Biedermann, P.U.; Shrestha, B.R.; Nellessen, M.; Khorashadizadeh, A.; Losada-Pérez, P.; Duarte, M.J.; et al. Star-Shaped Crystallographic Cracking of Localized Nanoporous Defects. Adv. Mater. 2015, 27, 4877–4882. [Google Scholar] [CrossRef]
- Yu, Q.; Dong, W.; Yang, X.Y.; Wang, Q.F.; Zhang, F.C. Insights into the corrosion mechanism and electrochemical properties of the rust layer evolution for weathering steel with various cl deposition in the simulated atmosphere. Mater. Res. Express. 2021, 8, 036515. [Google Scholar] [CrossRef]
- Nishikata, A.; Zhu, Q.J.; Tada, E. Long-term monitoring of atmospheric corrosion at weathering steel bridges by an electrochemical impedance method. Corros. Sci. 2014, 87, 80–88. [Google Scholar] [CrossRef]
- Dong, B.J.; Liu, W.; Chen, L.J.; Zhang, T.Y.; Fan, Y.Y.; Zhao, Y.Y.; Li, S.; Yang, W.; Banthukul, W. Optimize Ni, Cu, Mo element of low Cr-steel rebars in tropical marine atmosphere environment through two years of corrosion monitoring. Cem. Concr. Compos. 2022, 125, 104317. [Google Scholar] [CrossRef]
- Guo, X.Y.; Kang, J.F.; Zhu, J.S.; Duan, M.H. Corrosion behavior and mechanical property degradation of weathering steel in marine atmosphere. J. Mater. Civil. Eng. 2019, 31, 04019181. [Google Scholar] [CrossRef]
- Gao, X.L.; Han, Y.; Fu, G.Q.; Zhu, M.Y.; Zhang, X.Z. Evolution of the rust layers formed on carbon and weathering steels in environment containing chloride ions. Acta. Metall. 2016, 29, 1025–1036. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.M.; Liu, W.; Li, S.M.; Wongpat, B.; Zhao, Y.G.; Dong, B.J. Evolution of rust layers on carbon steel and weathering steel in high humidity and heat marine atmospheric corrosion. J. Mater. Sci. Technol. 2020, 39, 190–199. [Google Scholar] [CrossRef]
- Fu, J.D.; Wan, S.; Yang, Y.; Su, Q.; Han, W.W.; Zhu, Y.B. Accelerated corrosion behavior of weathering steel Q345qDNH for bridge in industrial atmosphere. Constr. Build. Mater. 2021, 306, 124864. [Google Scholar] [CrossRef]
- Kokalj, A.; Lozinek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Pérez, P.; Xie, C.; Stavber, S.; Crespo, D.; Renner, F.U.; et al. Simplistic correlations between molecular electronic properties and inhibition efficiencies: Do they really exist? Corros. Sci. 2021, 179, 108856. [Google Scholar] [CrossRef]
- Kamimura, T.; Kashima, K.; Sugae, K.; Miyuki, H.; Kudo, T. The role of chloride ion on the atmospheric corrosion of steel and corrosion resistance of Sn-bearing steel. Corros. Sci. 2012, 62, 34–41. [Google Scholar] [CrossRef]
- Hai, C.; Wang, Z.; Lu, F.Y.; Zhang, S.P.; Du, C.W.; Cheng, X.Q. Analysis of corrosion evolution in carbon steel in the subtropical atmospheric environment of sichuan. J. Mater. Eng. Perform. 2021, 30, 8014–8022. [Google Scholar] [CrossRef]
- Asami, K.; Kikuchi, M. In-depth distribution of rusts on a plain carbon steel and weathering steels exposed to coastal–industrial atmosphere for 17 years. Corros. Sci. 2003, 45, 2671–2688. [Google Scholar] [CrossRef]
- Weissenrieder, J.; Leygraf, C. In situ studies of filiform corrosion of iron. J. Electrochem. Soc. 2004, 151, B165–B171. [Google Scholar] [CrossRef]
- Nie, X.H.; Li, Y.L.; Li, J.K.; Zhang, H.B. Morphology, products and corrosion mechanism analysis of Q235 carbon steel in sea-shore salty soil. J. Mater. Eng. Perform. 2010, 30, 24–28+33. [Google Scholar] [CrossRef]
- Gerwin, W.; Baumhauer, R. Effect of soil parameters on the corrosion of archaeological metal finds. Geoderma 2000, 96, 63–80. [Google Scholar] [CrossRef]
- Tamura, H. The role of rusts in corrosion and corrosion protection of iron and steel. Corros. Sci. 2008, 50, 1872–1883. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.C.; Sun, C.; Xu, J.; Dong, J.H.; Ke, W. Role of fe oxides in corrosion of pipeline steel in a red clay soil. Corros. Sci. 2014, 80, 309–317. [Google Scholar] [CrossRef]


| Projects | Numerical Value |
|---|---|
| pH | 6.53 |
| Conductivity, μS/cm | 36,200 |
| Turbidity, NTU | 24.3 |
| Tlkalinity, mmol/L | 10.51 |
| Stiffness, mmol/L | 258.00 |
| 1/2Ca2+ | 39.80 |
| 1/2Mg2+ | 218.20 |
| Lable | Icorr (A/cm2) | Ecorr (A/cm2) |
|---|---|---|
| L0.1 | 10−5 | −0.7226 |
| L0.3 | 10−5 | −0.7339 |
| L0.5 | 10−5 | −0.7138 |
| L1 | 10−5 | −0.7256 |
| L2 | 10−5 | −0.7184 |
| L3 | 10−5 | −0.7252 |
| Solution (×103 mg/L) | Time (d) | Rs (Ω·cm2) | CPE110−4 (Ω−1 cm−2S−n) | n1 | Rp (Ω·cm2) | CPE210−4 (Ω−1 cm−2S−n) | n2 | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|
| 1000 | 0 3 5 | 53.66 54.63 58.00 | 26.61 16.10 7.559 | 0.3998 0.6985 0.5055 | 196.8 271.2 540.4 | 266.1 502.6 105.32 | 0.7391 0.6977 1.000 | 712 717.4 1198 |
| 3000 | 0 3 5 | 19.21 21.51 19.95 | 3.275 5.564 3.864 | 0.7779 0.7567 0.7836 | 54.92 149.05 213.6 | 48.77 129.6 87.59 | 0.3768 0.3635 0.4189 | 500.1 533.8 556.1 |
| 5000 | 0 3 5 | 12.57 12.12 16.17 | 5.109 2.053 8.191 | 0.8690 0.8491 0.7851 | 38.86 50.22 248.6 | 36.61 38.00 31.45 | 0.6776 0.5417 0.9327 | 486.4 484.8 499.5 |
| 10,000 | 0 3 5 | 8.643 9.286 11.42 | 3.320 4.146 9.929 | 0.4271 0.4200 0.6604 | 38.43 26.16 13.74 | 22.08 19.62 22.25 | 0.8950 0.9126 0.9893 | 239.0 108.9 64.97 |
| 20,000 | 0 3 5 | 6.340 4.300 5.673 | 5.564 4.017 6.145 | 0.7567 0.9204 0.8225 | 149.0 132.3 112.3 | 129.6 26.90 25.88 | 0.3635 0.5988 0.7317 | 362.0 342.4 266.8 |
| Solution (×103 mg/L) | Time (d) | Rs (Ω·cm2) | CPE110−4 (Ω−1cm−2S−n) | n1 | Rp (Ω·cm2) | CPE210−4 (Ω−1cm−2S−n) | n2 | Rct (Ω·cm2) |
| 30,000 | 0 3 5 | 4.173 3.980 5.126 | 8.575 8.907 2.860 | 0.4747 0.8012 0.8903 | 240.1 179.6 136.3 | 43.93 25.08 19.62 | 0.5182 0.7259 0.9126 | 336.8 273.3 256.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Bai, J.; Ma, X.; Wang, S.; Hu, D.; Niu, J.; Zhang, J.; Du, A.; Sun, D.; Lu, J.; et al. Investigating the Corrosive Influence of Chloride Ions on Slag Recovery Machine Shells in Power Plants. Materials 2023, 16, 5270. https://doi.org/10.3390/ma16155270
Yu Y, Bai J, Ma X, Wang S, Hu D, Niu J, Zhang J, Du A, Sun D, Lu J, et al. Investigating the Corrosive Influence of Chloride Ions on Slag Recovery Machine Shells in Power Plants. Materials. 2023; 16(15):5270. https://doi.org/10.3390/ma16155270
Chicago/Turabian StyleYu, Yaohong, Jintao Bai, Xiaohan Ma, Shengxing Wang, Dalong Hu, Jun Niu, Jiangtao Zhang, An Du, Dongqi Sun, Jian Lu, and et al. 2023. "Investigating the Corrosive Influence of Chloride Ions on Slag Recovery Machine Shells in Power Plants" Materials 16, no. 15: 5270. https://doi.org/10.3390/ma16155270





