Microstructural, Mechanical, and Electrochemical Characterization of CrMoNbTiZr High-Entropy Alloy for Biomedical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Synthesis of Alloy
2.2. Alloy Preparation
2.3. Alloy Characterization
3. Results
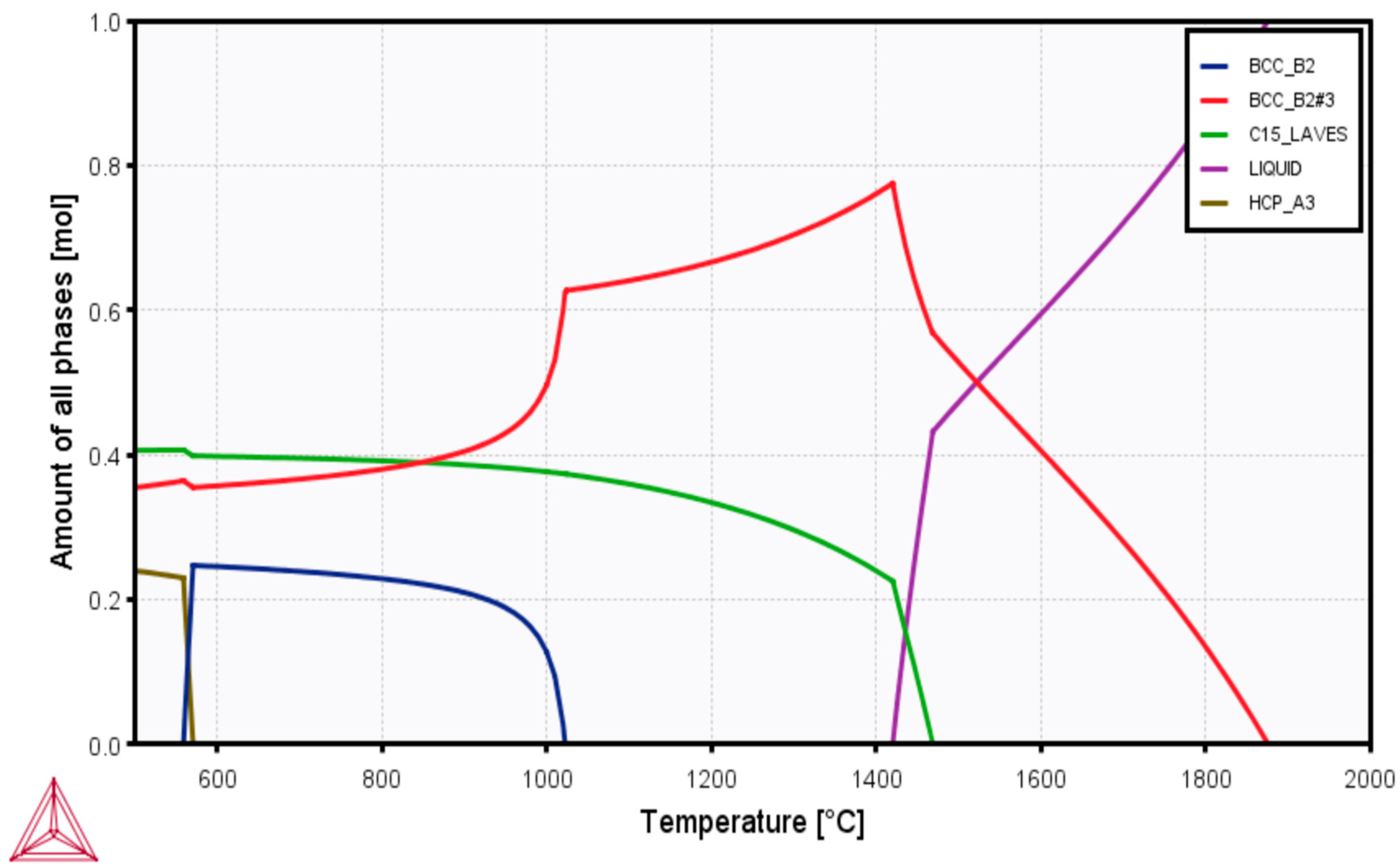
3.1. Density and Microstructural Characterizations
3.2. Microhardness and Compression Characteristics
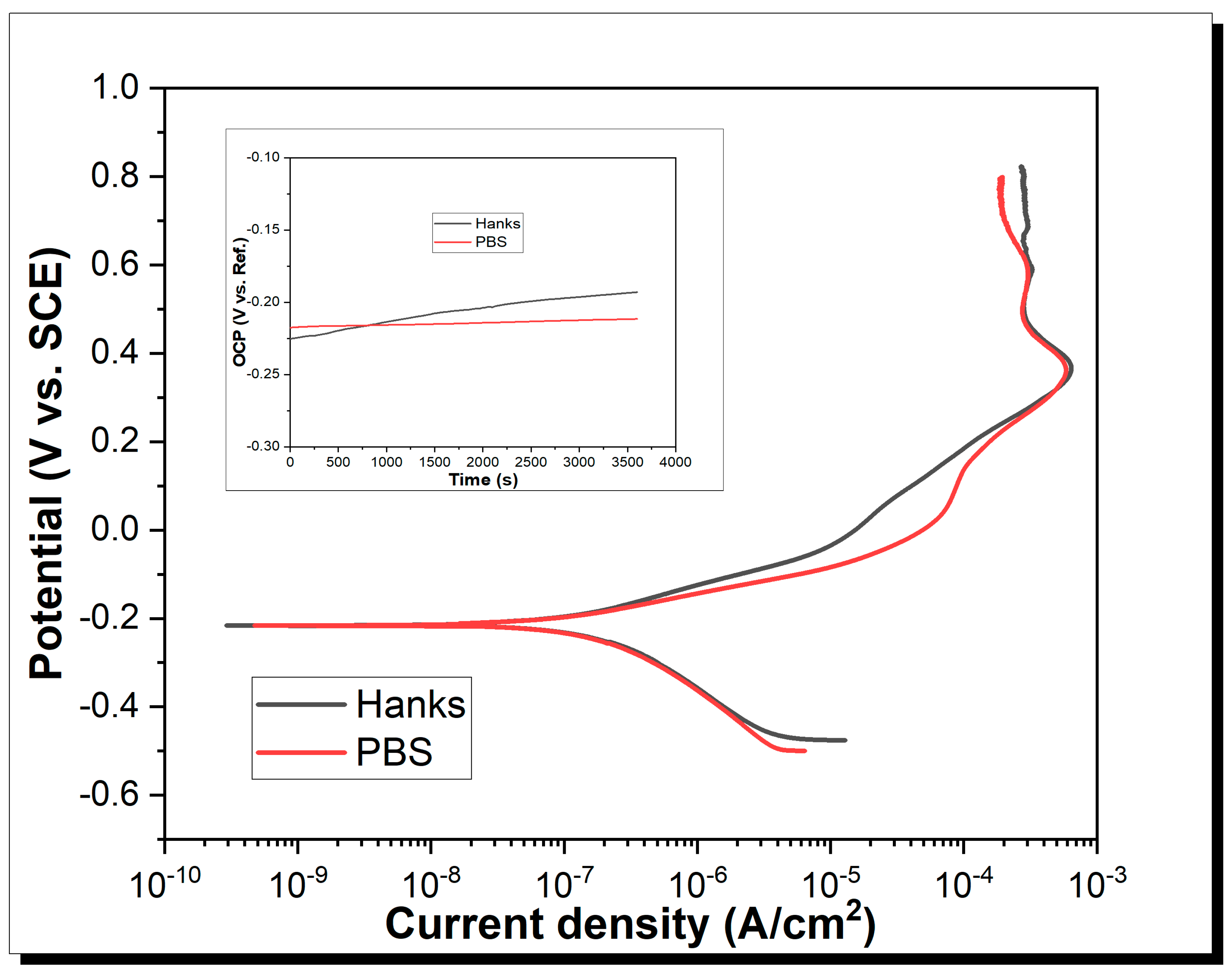
3.3. Potentiodynamic Analyses
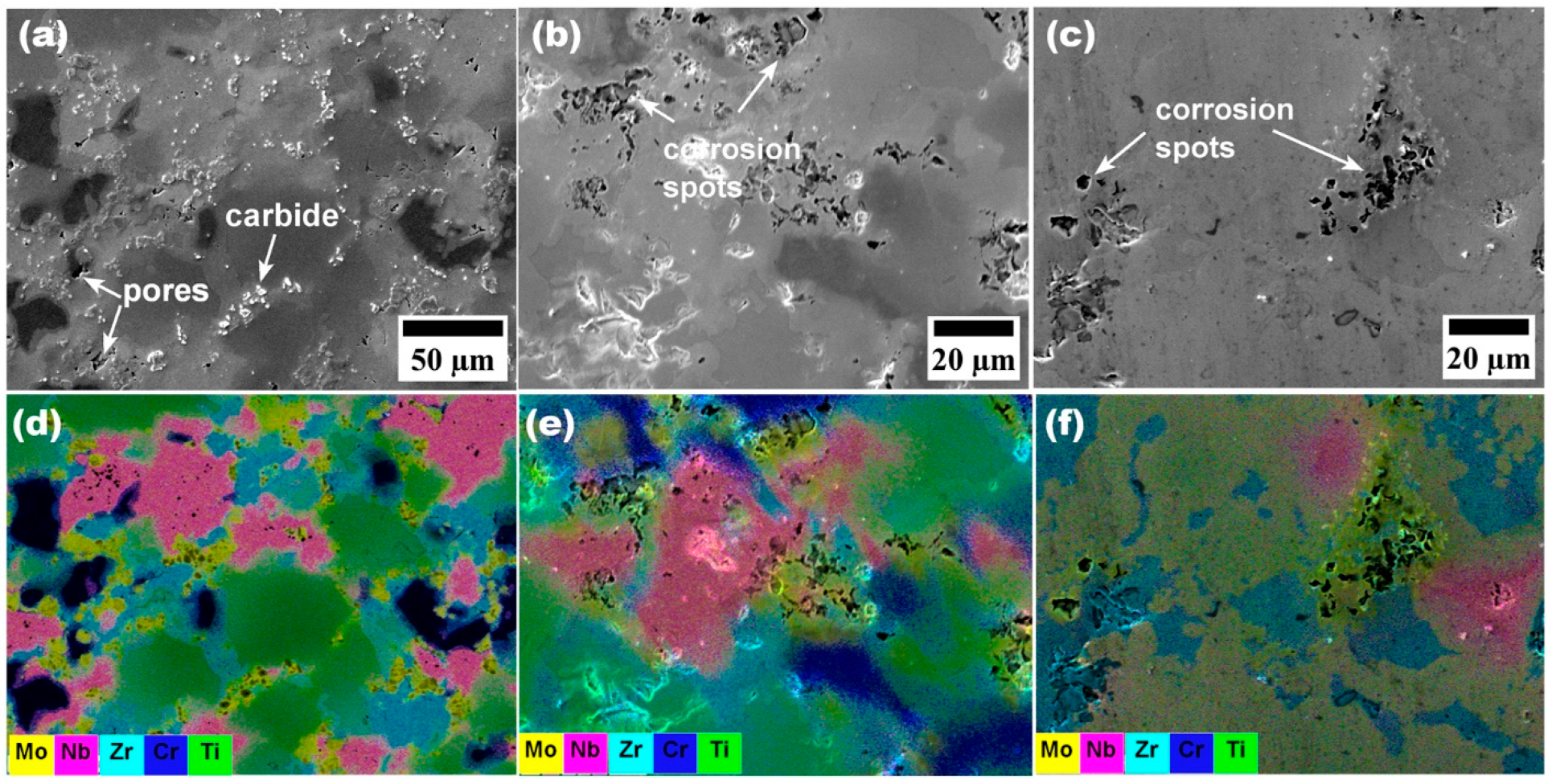
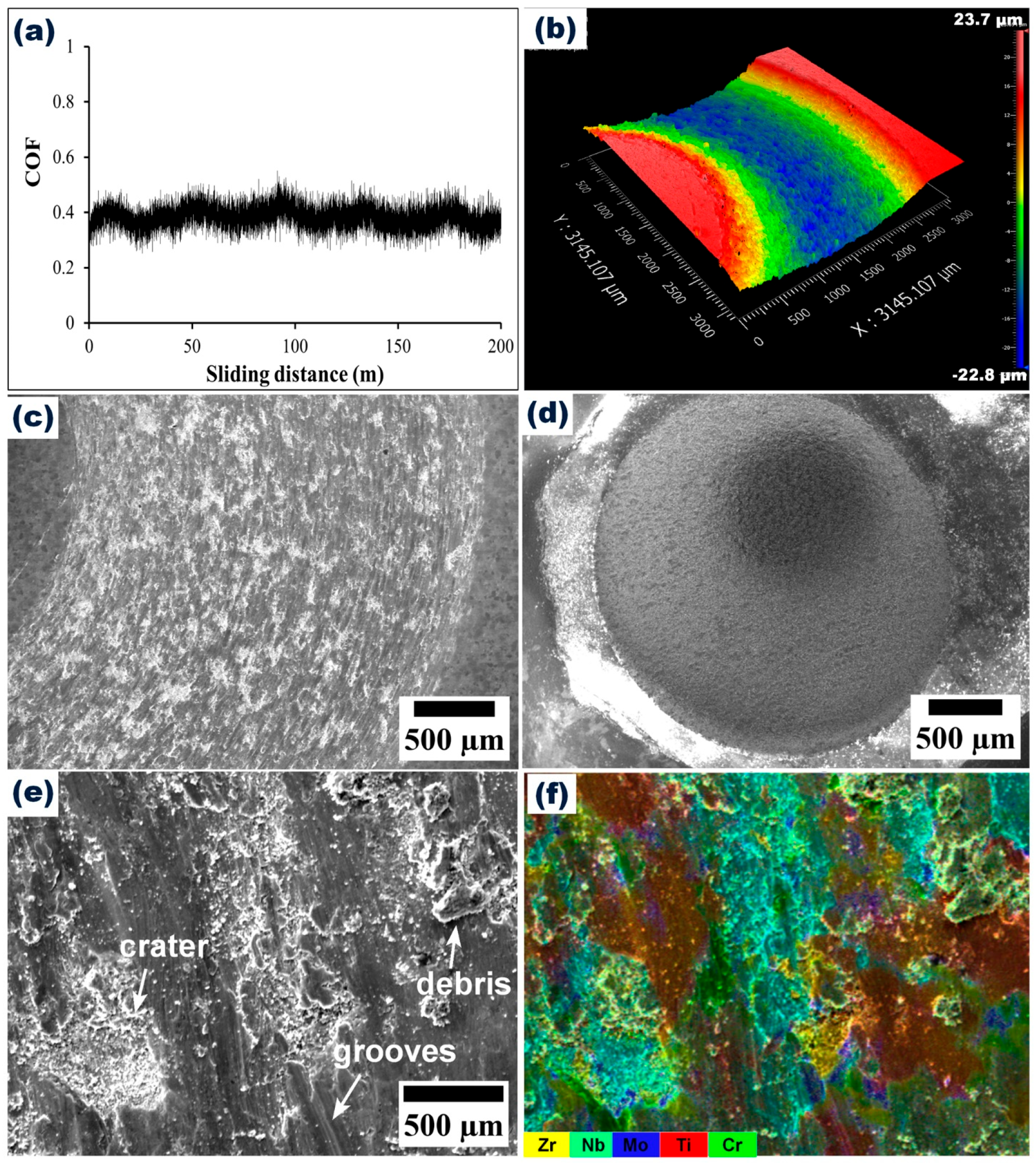
3.4. Wear Resistance of CrMoNbTiZr HEA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstein Research Global Medical Implants Market Analysis and Forecast 2016–2024. Available online: https://www.goldsteinresearch.com/report/global-medical-implants-market (accessed on 17 March 2020).
- Hynowska, A.; Blanquer, A.; Pellicer, E.; Fornell, J.; Suriñach, S.; Baró, M.D.; González, S.; Ibáñez, E.; Barrios, L.; Nogués, C.; et al. Novel Ti-Zr-Hf-Fe nanostructured alloy for biomedical applications. Materials 2013, 6, 4930–4945. [Google Scholar] [CrossRef] [Green Version]
- Istrate, B.; Munteanu, C.; Cimpoesu, R.; Cimpoesu, N.; Popescu, O.D.; Vlad, M.D. Microstructural, electrochemical and in vitro analysis of Mg-0.5Ca-xGd biodegradable alloys. Appl. Sci. 2021, 11, 981. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.; Walker, E.; Stanciu, L. Magnesium, iron and zinc alloys, the trifecta of bioresorbable orthopaedic and vascular implantation—A review. J. Biotechnol. Biomater. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloys Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Doni, Z.; Alves, A.C.; Toptan, F.; Gomes, J.R.; Ramalho, A.; Buciumeanu, M.; Palaghian, L.; Silva, F.S. Dry sliding and tribocorrosion behaviour of hot pressed CoCrMo biomedical alloy as compared with the cast CoCrMo and Ti6Al4V alloys. Mater. Des. 2013, 52, 47–57. [Google Scholar] [CrossRef]
- Mischler, S.; Muñoz, A.I. Wear of CoCrMo alloys used in metal-on-metal hip joints: A tribocorrosion appraisal. Wear 2013, 297, 1081–1094. [Google Scholar] [CrossRef]
- Gurel, S.; Nazarahari, A.; Canadinc, D.; Cabuk, H.; Bal, B. Assessment of biocompatibility of novel TiTaHf-based high entropy alloys for utility in orthopedic implants. Mater. Chem. Phys. 2021, 266, 124573. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Spataru, M.C.; Verestiuc, L.; Balan, V.; Solcan, C.; Sandu, A.V.; Geanta, V.; Voiculescu, I.; Vizureanu, P. Design, synthesis, and preliminary evaluation for ti-mo-zr-ta-si alloys for potential implant applications. Materials 2021, 14, 6806. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Gao, M.C. Progress in high-entropy alloys. JOM 2014, 66, 1964–1965. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Ma, X. High temperature deformation and dynamic recrystallization behavior of AlCrCuFeNi high entropy alloy. Mater. Sci. Eng. A 2020, 778, 139077. [Google Scholar] [CrossRef]
- Luo, S.; Gao, P.; Yu, H.; Yang, J.; Wang, Z.; Zeng, X. Selective laser melting of an equiatomic AlCrCuFeNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical behavior. J. Alloys Compd. 2019, 771, 387–397. [Google Scholar] [CrossRef]
- Qiu, X.W.; Zhang, Y.P.; He, L.; Liu, C.G. Microstructure and corrosion resistance of AlCrFeCuCo high entropy alloy. J. Alloys Compd. 2013, 549, 195–199. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Alvi, S.; Akhtar, F. High temperature tribology of CuMoTaWV high entropy alloy. Wear 2019, 426–427, 412–419. [Google Scholar] [CrossRef]
- Motallebzadeh, A.; Peighambardoust, N.S.; Sheikh, S.; Murakami, H.; Guo, S.; Canadinc, D. Microstructural, mechanical and electrochemical characterization of TiZrTaHfNb and Ti1.5ZrTa0.5Hf0.5Nb0.5 refractory high-entropy alloys for biomedical applications. Intermetallics 2019, 113, 106572. [Google Scholar] [CrossRef]
- Geanta, V.; Voiculescu, I.; Vizureanu, P.; Sandu, A.V. High Entropy Alloys for Medical Applications. In Engineering Steels and High Entropy-Alloys; Sharma, A., Duriagina, Z., Kumar, S., Eds.; InTechOpen: London, UK, 2019. [Google Scholar]
- Iijima, Y.; Nagase, T.; Matsugaki, A.; Wang, P.; Ameyama, K.; Nakano, T. Design and development of Ti–Zr–Hf–Nb–Ta–Mo high-entropy alloys for metallic biomaterials. Mater. Des. 2021, 202, 109548. [Google Scholar] [CrossRef]
- Wang, S.P.; Xu, J. TiZrNbTaMo high-entropy alloy designed for orthopedic implants: As-cast microstructure and mechanical properties. Mater. Sci. Eng. C 2017, 73, 80–89. [Google Scholar] [CrossRef]
- Shittu, J.; Pole, M.; Cockerill, I.; Sadeghilaridjani, M.; Reddy, L.V.K.; Manivasagam, G.; Singh, H.; Grewal, H.S.; Arora, H.S.; Mukherjee, S. Biocompatible high entropy alloys with excellent degradation resistance in a simulated physiological environment. ACS Appl. Bio Mater. 2020, 3, 8890–8900. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, S.; Lee, K. Thermodynamic parameters, microstructure, and electrochemical properties of equiatomic TiMoVWCr and TiMoVNbZr high-entropy alloys prepared by vacuum arc remelting. Int. J. Refract. Met. Hard Mater. 2021, 99, 105595. [Google Scholar] [CrossRef]
- Kang, B.; Lee, J.; Ryu, H.J.; Hong, S.H. Ultra-high strength WNbMoTaV high-entropy alloys with fine grain structure fabricated by powder metallurgical process. Mater. Sci. Eng. A 2018, 712, 616–624. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Z.; Hong, C.; Dai, P.; Chen, J. Microstructure and mechanical properties of the TiZrNbMoTa refractory high-entropy alloy produced by mechanical alloying and spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2020, 93, 105357. [Google Scholar] [CrossRef]
- Shivam, V.; Basu, J.; Pandey, V.K.; Shadangi, Y.; Mukhopadhyay, N.K. Alloying behaviour, thermal stability and phase evolution in quinary AlCoCrFeNi high entropy alloy. Adv. Powder Technol. 2018, 29, 2221–2230. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Ge, W.; Wu, B.; Wang, S.; Xu, S.; Shang, C.; Zhang, Z.; Wang, Y. Characterization and properties of CuZrAlTiNi high entropy alloy coating obtained by mechanical alloying and vacuum hot pressing sintering. Adv. Powder Technol. 2017, 28, 2556–2563. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Fang, Q.; Liu, B.; Wu, Y.; Chen, S. Preparation of superfine-grained high entropy alloy by spark plasma sintering gas atomized powder. Intermetallics 2016, 68, 16–22. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Anand Sekhar, R.; Samal, S.; Nayan, N.; Bakshi, S.R. Microstructure and mechanical properties of Ti-Al-Ni-Co-Fe based high entropy alloys prepared by powder metallurgy route. J. Alloys Compd. 2019, 787, 123–132. [Google Scholar] [CrossRef]
- Xiang, T.; Cai, Z.; Du, P.; Li, K.; Zhang, Z.; Xie, G. Dual phase equal-atomic NbTaTiZr high-entropy alloy with ultra-fine grain and excellent mechanical properties fabricated by spark plasma sintering. J. Mater. Sci. Technol. 2021, 90, 150–158. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Akmal, M.; Hussain, A.; Afzal, M.; Lee, Y.I.; Ryu, H.J. Systematic study of (MoTa) NbTiZr medium- and high-entropy alloys for biomedical implants—In vivo biocompatibility examination. J. Mater. Sci. Technol. 2021, 78, 183–191. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2012, 532, 480–486. [Google Scholar] [CrossRef]
- Cheng, J.B.; Liang, X.B.; Xu, B.S. Effect of Nb addition on the structure and mechanical behaviors of CoCrCuFeNi high-entropy alloy coatings. Surf. Coat. Technol. 2014, 240, 184–190. [Google Scholar] [CrossRef]
- Xing, Q.; Feltrin, A.C.; Akhtar, F. Processing, microstructure and high temperature dry sliding wear of a Cr-Fe-Hf-Mn-Ti-Ta-V high-entropy alloy based composite. Mater. Today Commun. 2021, 28, 102657. [Google Scholar] [CrossRef]
- Waseem, O.A.; Ryu, H.J. Powder metallurgy processing of a WxTaTiVCr high-entropy alloy and its derivative alloys for fusion material applications. Sci. Rep. 2017, 7, 1926. [Google Scholar] [CrossRef] [Green Version]
- Brama, M.; Rhodes, N.; Hunt, J.; Ricci, A.; Teghil, R.; Migliaccio, S.; Rocca, C.D.; Leccisotti, S.; Lioi, A.; Scandurra, M.; et al. Effect of titanium carbide coating on the osseointegration response in vitro and in vivo. Biomaterials 2007, 28, 595–608. [Google Scholar] [CrossRef]
- Vladescu, A.; Pruna, V.; Kulesza, S.; Braic, V.; Titorencu, I.; Bramowicz, M.; Gozdziejewska, A.; Parau, A.; Cotrut, C.M.; Pana, I.; et al. Influence of Ti, Zr or Nb carbide adhesion layers on the adhesion, corrosion resistance and cell proliferation of titania doped hydroxyapatite to the Ti6Al4V alloy substrate, utilizable for orthopaedic implants. Ceram. Int. 2019, 45, 1710–1723. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Cheng, G.; Guo, J.; Du, S.; Qiu, J.; Wang, C.; Li, C.; Yang, X.; Chen, T.; et al. Tailored Hydrogel Delivering Niobium Carbide Boosts ROS-Scavenging and Antimicrobial Activities for Diabetic Wound Healing. Small 2022, 18, 2201300. [Google Scholar] [CrossRef] [PubMed]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Materialia 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Hua, N.; Wang, W.; Wang, Q.; Ye, Y.; Lin, S.; Zhang, L.; Guo, Q.; Brechtl, J.; Liaw, P.K. Mechanical, corrosion, and wear properties of biomedical Ti–Zr–Nb–Ta–Mo high entropy alloys. J. Alloys Compd. 2021, 861, 157997. [Google Scholar] [CrossRef]
- Yang, W.; Pang, S.; Liu, Y.; Wang, Q.; Liaw, P.K.; Zhang, T. Design and properties of novel Ti–Zr–Hf–Nb–Ta high-entropy alloys for biomedical applications. Intermetallics 2022, 141, 107421. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, M.R.; Chen, S.K.; Yeh, J.W.; Shun, T.T.; Lin, S.J.; Chang, S.Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta 25W25 and V20Nb20Mo 20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Garbiec, D.; Siwak, P.; Mróz, A. Effect of compaction pressure and heating rate on microstructure and mechanical properties of spark plasma sintered Ti6Al4V alloy. Arch. Civ. Mech. Eng. 2016, 16, 702–707. [Google Scholar] [CrossRef]
- He, G.; Hagiwara, M. Ti alloy design strategy for biomedical applications. Mater. Sci. Eng. C 2006, 26, 14–19. [Google Scholar] [CrossRef]
- Gradzka-Dahlke, M.; Dabrowski, J.R.; Dabrowski, B. Modification of mechanical properties of sintered implant materials on the base of Co–Cr–Mo alloy. J. Mater. Process. Technol. 2008, 204, 199–205. [Google Scholar] [CrossRef]
- Wu, Y.; Liaw, P.K.; Zhang, Y. Preparation of bulk TiZrNbMoV and NbTiAlTaV high-entropy alloys by powder sintering. Metals 2021, 11, 1748. [Google Scholar] [CrossRef]
- Kailas, S.V.; Prasad, Y.V.R.K.; Biswas, S.K. Flow Instabilities and fracture in Ti-6Al-4V deformed in compression at 298 K to 673 K. Metall. Mater. Trans. A 1994, 25, 2173–2179. [Google Scholar] [CrossRef]
- Ayyagari, A.; Barthelemy, C.; Gwalani, B.; Banerjee, R.; Scharf, T.W.; Mukherjee, S. Reciprocating sliding wear behavior of high entropy alloys in dry and marine environments. Mater. Chem. Phys. 2018, 210, 162–169. [Google Scholar] [CrossRef]
- Ocran, E.K.; Guenther, L.E.; Brandt, J.M.; Wyss, U.; Ojo, O.A. Corrosion and fretting corrosion studies of medical grade CoCrMo alloy in a clinically relevant simulated body fluid environment. Metall. Mater. Trans. A 2015, 46, 2696–2709. [Google Scholar] [CrossRef]
- Lodhi, M.J.K.; Deen, K.M.; Greenlee-Wacker, M.C.; Haider, W. Additively manufactured 316L stainless steel with improved corrosion resistance and biological response for biomedical applications. Addit. Manuf. 2019, 27, 8–19. [Google Scholar] [CrossRef]
- Kale, A.B.; Kim, B.K.; Kim, D.I.; Castle, E.G.; Reece, M.; Choi, S.H. An investigation of the corrosion behavior of 316L stainless steel fabricated by SLM and SPS techniques. Mater. Charact. 2020, 163, 110204. [Google Scholar] [CrossRef]
- Rumble, J. (Ed.) CRC Handbook of Chemistry and Physics, 98th ed.; CRC: Boca Raton, FL, USA, 2004; ISBN 9780849305979. [Google Scholar]
- Alvi, S.; Waseem, O.A.; Akhtar, F. High temperature performance of spark plasma sintered W0.5(TaTiVCr)0.5 alloy. Metals 2020, 10, 1512. [Google Scholar] [CrossRef]
- Mahathanabodee, S.; Palathai, T.; Raadnui, S.; Tongsri, R.; Sombatsompop, N. Dry sliding wear behavior of SS316L composites containing h-BN and MoS2 solid lubricants. Wear 2014, 316, 37–48. [Google Scholar] [CrossRef]
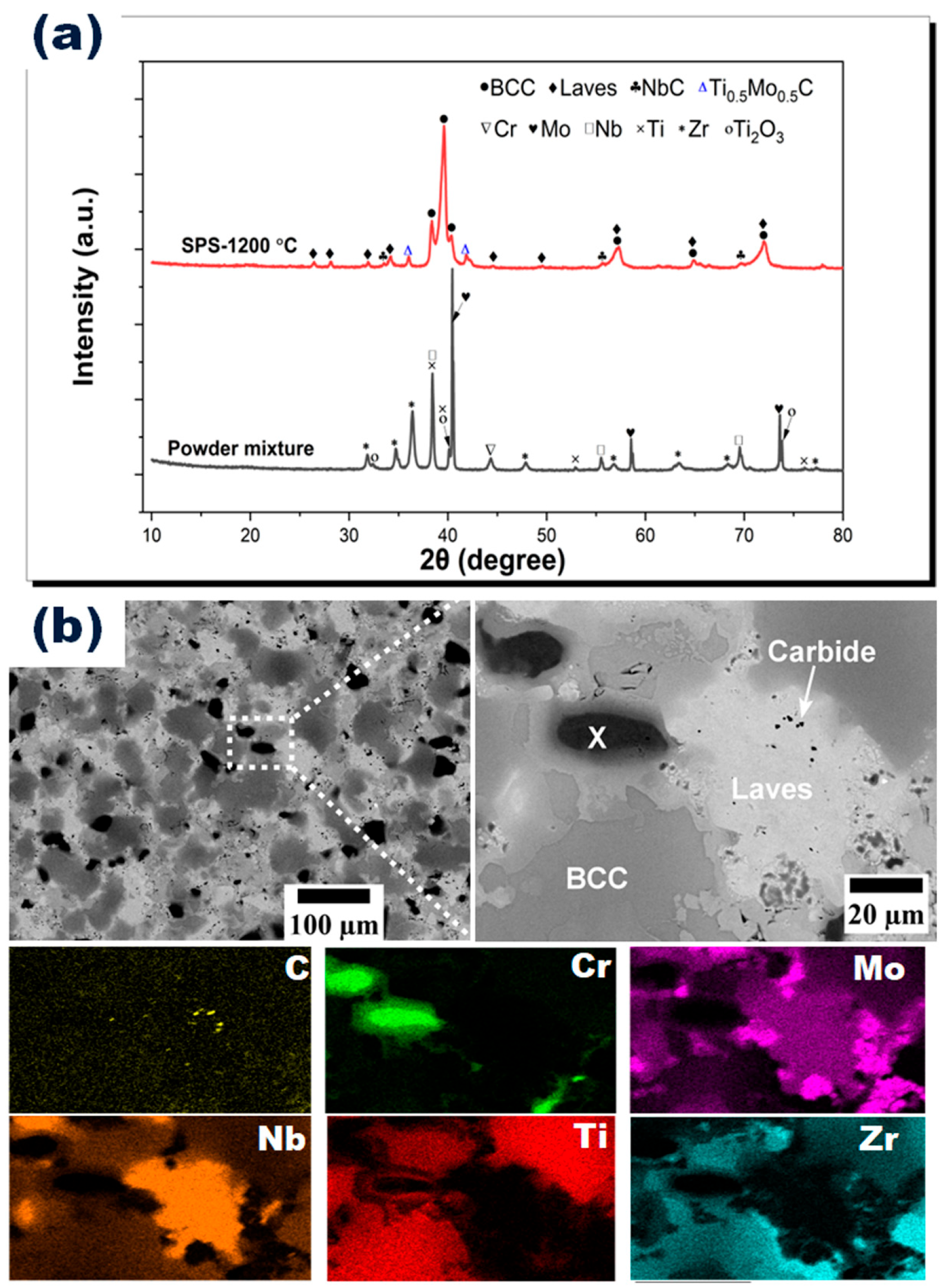

| Regions | Cr | Mo | Nb | Ti | Zr |
|---|---|---|---|---|---|
| BCC (dark gray) | 7.42 ± 1.96 | 12.08 ± 1.71 | 8.29 ± 4.77 | 51.97 ± 3.661 | 20.40 ± 5.53 |
| Laves (light gray) | 2.84 ± 1.43 | - | 79.87 ± 24.26 | 2.94 ± 2.26 | - |
| Carbide | 2.35 ± 1.06 | - | 96.68 ± 5.19 | 4.50 ± 0.00 | - |
| X | 98.10 ± 0.44 | 1.80 ± 0.00 | - | 0.98 ± 0.61 | - |
| Overall composition | 20.85 ± 1.06 | 19.60 ± 0.14 | 17.65 ± 1.48 | 20.50 ± 0.99 | 21.40 ± 1.41 |
| OCP (mV vs. SCE) | (mV vs. SCE) | (µA/cm2) | Cr (mm/y) | (mA/cm2) | (µA/cm2) | (V/SCE) | |
|---|---|---|---|---|---|---|---|
| PBS | −200.17 | −216 | 0.14 | 1.24 × 10−3 | 907.25 | 600 | 0.36 |
| Hanks | −176.25 | −215 | 0.24 | 2.03 × 10−3 | 906.83 | 650 | 0.36 |
| Substrate | EDX Point | Cr | Mo | Nb | Ti | Zr | Al | O |
|---|---|---|---|---|---|---|---|---|
| Ball | 1 | 0.5 | 1.4 | 1.7 | 0.8 | 1.9 | 17.6 | 75.9 |
| Bare | 2 | 19.6 | 14.9 | 15 | 17.6 | 16.6 | - | 16.3 |
| Track | 3 | 8.6 | 10.4 | 8.9 | 22.9 | 14.3 | 3.1 | 31.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinwekomi, A.; Akhtar, F. Microstructural, Mechanical, and Electrochemical Characterization of CrMoNbTiZr High-Entropy Alloy for Biomedical Application. Materials 2023, 16, 5320. https://doi.org/10.3390/ma16155320
Akinwekomi A, Akhtar F. Microstructural, Mechanical, and Electrochemical Characterization of CrMoNbTiZr High-Entropy Alloy for Biomedical Application. Materials. 2023; 16(15):5320. https://doi.org/10.3390/ma16155320
Chicago/Turabian StyleAkinwekomi, Akeem, and Farid Akhtar. 2023. "Microstructural, Mechanical, and Electrochemical Characterization of CrMoNbTiZr High-Entropy Alloy for Biomedical Application" Materials 16, no. 15: 5320. https://doi.org/10.3390/ma16155320
APA StyleAkinwekomi, A., & Akhtar, F. (2023). Microstructural, Mechanical, and Electrochemical Characterization of CrMoNbTiZr High-Entropy Alloy for Biomedical Application. Materials, 16(15), 5320. https://doi.org/10.3390/ma16155320






