Effects of Cu, Zn Doping on the Structural, Electronic, and Optical Properties of α-Ga2O3: First-Principles Calculations
Abstract
1. Introduction
2. Calculation Methods
2.1. Computational Details
2.2. Formation Energy, Transitional Level, and Optical Calculations
3. Results and Discussions
3.1. Structural Stability
3.2. Electronic Structure
3.3. Optical Property
3.4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.X.; Wu, Z.Y.; Ma, C.C.; Deng, J.N.; Zhang, H.; Xu, Y.; Ye, J.D.; Fang, Z.L.; Zhang, G.Q.; Kang, J.Y.; et al. P-type β-Ga2O3 metal-semiconductor-metal solar-blind photodetectors with extremely high responsivity and gain-bandwidth product Mater. Today Phys. 2020, 14, 100226. [Google Scholar]
- Tang, R.; Li, G.; Li, C.; Li, J.; Zhang, Y.; Huang, K.; Ye, J.; Li, C.; Kang, J.Y.; Zhang, R.; et al. Localized surface plasmon enhanced Ga2O3 solar blind photodetectors. Opt. Express 2020, 28, 5731–5740. [Google Scholar] [CrossRef] [PubMed]
- Tadjer, M.J. Toward gallium oxide power electronics. Science 2022, 378, 724–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, P.; Dang, K.; Zhang, Y.; Yan, Q.; Xiang, H.; Su, J.; Liu, Z.; Si, M.; Gao, J.; et al. Ultra-wide bandgap semiconductor Ga2O3 power diodes. Nat. Commun. 2022, 13, 3900. [Google Scholar] [CrossRef]
- Harada, T.; Tsukazaki, A. Dynamic characteristics of PdCoO2/β-Ga2O3 Schottky junctions. Appl. Phys. Lett. 2020, 116, 232104. [Google Scholar] [CrossRef]
- Harada, T.; Ito, S.; Tsukazaki, A. Electric dipole effect in PdCoO2/β-Ga2O3 Schottky diodes for high-temperature operation. Sci. Adv. 2019, 5, eaax5733. [Google Scholar] [CrossRef]
- Pang, R.; Teramura, K.; Morishita, M.; Asakura, H.; Hosokawa, S.; Tanaka, T. Enhanced CO evolution for photocatalytic conversion of CO2 by H2O over Ca modified Ga2O3. Commun. Chem. 2020, 3, 137. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Qi, D.-C.; Chen, L.; Zhang, K.H.L. Recent progress on the electronic structure, defect, and doping properties of Ga2O3. APL Mater. 2020, 8, 020906. [Google Scholar] [CrossRef]
- Pearton, S.J.; Yang, J.; Cary IV, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Ping, L.K.; Berhanuddin, D.D.; Mondal, A.K.; Menon, P.S.; Mohamed, M.A. Properties and perspectives of ultrawide bandgap Ga2O3 in optoelectronic applications. Chin. J. Phys. 2021, 73, 195–212. [Google Scholar] [CrossRef]
- Ahmadi, E.; Oshima, Y. Materials issues and devices of α- and β-Ga2O3. J. Appl. Phys. 2019, 126, 160901. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.; Yuan, H.; Lin, Z.; Zhang, J.; Hao, Y.; Chang, J. Impurity level properties in transition metal doped α-Ga2O3 for optoelectronic applications. Semicond. Sci. Technol. 2021, 36, 095026. [Google Scholar] [CrossRef]
- Guo, D.Y.; Zhao, X.L.; Zhi, Y.S.; Cui, W.; Huang, Y.Q.; An, Y.H.; Li, P.G.; Wu, Z.P.; Tang, W.H. Epitaxial growth and solar-blind photoelectric properties of corundum-structured α-Ga2O3 thin films. Mater. Lett. 2016, 164, 364–367. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Zhou, D.; Yang, S.; Ren, F.-F.; Lu, H.; Tang, K.; Gu, S.; Zhang, R.; Zheng, Y.; et al. Solar-Blind Photodetector with High Avalanche Gains and Bias-Tunable Detecting Functionality Based on Metastable Phase α-Ga2O3/ZnO Isotype Heterostructures. ACS Appl. Mater. Interfaces 2017, 9, 36997–37005. [Google Scholar] [CrossRef]
- Kim, K.-H.; Ha, M.-T.; Kwon, Y.-J.; Lee, H.; Jeong, S.-M.; Bae, S.-Y. Growth of 2-Inch α-Ga2O3 Epilayers via Rear-Flow-Controlled Mist Chemical Vapor Deposition. ECS J. Solid State Sci. Technol. 2019, 8, Q3165. [Google Scholar] [CrossRef]
- Bhuiyan, A.F.M.A.U.; Feng, Z.; Huang, H.-L.; Meng, L.; Hwang, J.; Zhao, H. Metalorganic chemical vapor deposition of α-Ga2O3 and α-(AlxGa1−x)2O3 thin films on m-plane sapphire substrates. APL Mater. 2021, 9, 101109. [Google Scholar] [CrossRef]
- Oshima, Y.; Víllora, E.G.; Shimamura, K. Halide vapor phase epitaxy of twin-free α-Ga2O3 on sapphire (0001) substrates. Appl. Phys. Express 2015, 8, 055501. [Google Scholar] [CrossRef]
- Smirnov, A.M.; Kremleva, A.V.; Sharofidinov, S.S.; Bougrov, V.E.; Romanov, A.E. Stress–strain state in α-Ga2O3 epitaxial films on α-Al2O3 substrates. Appl. Phys. Express 2020, 13, 075502. [Google Scholar] [CrossRef]
- Kaneko, K.; Kawanowa, H.; Ito, H.; Fujita, S. Evaluation of Misfit Relaxation in α-Ga2O3 Epitaxial Growth on α-Al2O3 Substrate. Jpn J. Appl. Phys. 2012, 51, 020201. [Google Scholar] [CrossRef]
- Chen, X.H.; Chen, Y.T.; Ren, F.-F.; Gu, S.L.; Tan, H.H.; Jagadish, C.; Ye, J.D. Band alignment and band bending at α-Ga2O3/ZnO n-n isotype hetero-interface. Appl. Phys. Lett. 2019, 115, 202101. [Google Scholar] [CrossRef]
- Kobayashi, T.; Gake, T.; Kumagai, Y.; Oba, F.; Matsushita, Y.-i. Energetics and electronic structure of native point defects in α-Ga2O3. Appl. Phys. Express 2019, 12, 091001. [Google Scholar] [CrossRef]
- Pan, Y. First-principles investigation of the influence of point defect on the electronic and optical properties of α-Ga2O3. Int. J. Energy Res. 2022, 46, 13070–13078. [Google Scholar] [CrossRef]
- Simon, J.; Protasenko, V.; Lian, C.; Xing, H.; Jena, D. Polarization-Induced Hole Doping in Wide-Band-Gap Uniaxial Semiconductor Heterostructures. Science 2010, 327, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Casula, M.; Miyake, T.; Aryasetiawan, F.; Millis, A.J.; Biermann, S. Satellites and large doping and temperature dependence of electronic properties in hole-doped BaFe2As2. Nat. Phys. 2012, 8, 331–337. [Google Scholar] [CrossRef]
- Euvrard, J.; Yan, Y.; Mitzi, D.B. Electrical doping in halide perovskites. Nat. Rev. Mater. 2021, 6, 531–549. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, M.; Wang, H.-Q.; Zheng, J.-C.; Kang, J.Y. Tuning the magnetic and electronic properties of strontium titanate by carbon doping. Front. Phys. 2021, 16, 43501. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, M.; Wang, H.-Q.; Zheng, J.-C.; Kang, J.Y. Tuning the Magnetism in Boron-Doped Strontium Titanate. Materials 2020, 12, 5686. [Google Scholar] [CrossRef]
- Gueorguiev, G.K.; Pacheco, J.M. Shapes of cagelike metal carbide clusters: First-principles calculations. Phys. Rev. B 2003, 68, 241401. [Google Scholar] [CrossRef]
- dos Santos, R.B.; Rivelino, R.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Exploring 2D structures of indium oxide of different stoichiometry. CrystEngComm 2021, 23, 6661–6667. [Google Scholar] [CrossRef]
- Alves Machado Filho, M.; Hsiao, C.-L.; dos Santos, R.B.; Hultman, L.; Birch, J.; Gueorguiev, G.K. Self-Induced Core–Shell InAlN Nanorods: Formation and Stability Unraveled by Ab Initio Simulations. ACS Nanosci. Au 2023, 3, 84–93. [Google Scholar] [CrossRef]
- Yan, H.; Guo, Y.; Song, Q.; Chen, Y. First-principles study on electronic structure and optical properties of Cu-doped β-Ga2O3. Phys. B 2014, 434, 181–184. [Google Scholar] [CrossRef]
- Stehr, J.E.; Hofmann, D.M.; Schörmann, J.; Becker, M.; Chen, W.M.; Buyanova, I.A. Electron paramagnetic resonance signatures of Co2+ and Cu2+ in β-Ga2O3. Appl. Phys. Lett. 2019, 115, 242101. [Google Scholar] [CrossRef]
- Li, C.; Yan, J.-L.; Zhang, L.-Y.; Zhao, G. Electronic structures and optical properties of Zn-doped β-Ga2O3 with different doping sites. Chin. Phys. B 2012, 21, 127104. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, J.; Yang, Y.; Pan, D.; Xing, Y.; Shi, X.; Xia, X.; Liang, H. Catalytic growth and characterization of single crystalline Zn doped p-type β-Ga2O3 nanowires. J. Alloys Compd. 2016, 687, 964–968. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhang, F.B.; Saito, K.; Tanaka, T.; Nishio, M.; Guo, Q.X. Electrical properties and emission mechanisms of Zn-doped β-Ga2O3 films. J. Phys. Chem. Solids 2014, 75, 1201–1204. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, H.; Song, Q.; Chen, Y.; Guo, S. Electronic structure and magnetic interactions in Zn-doped β-Ga2O3 from first-principles calculations. Comput. Mater. Sci. 2014, 87, 198–201. [Google Scholar] [CrossRef]
- Pan, Y. Effects of Cu, Ag and Au on electronic and optical properties of α-Ga2O3 oxide according to first-principles calculations. J. Phys. Chem. Solids 2023, 174, 111152. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Gorai, P.; Peng, H.; Lany, S.; Stevanović, V. A computational framework for automation of point defect calculations. Comput. Mater. Sci. 2017, 130, 1–9. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Sun, D.; Gao, Y.; Xue, J.; Zhao, J. Defect stability and electronic structure of doped β-Ga2O3: A comprehensive ab initio study. J. Alloys Compd. 2019, 794, 374–384. [Google Scholar] [CrossRef]
- Mondal, A.K.; Mohamed, M.A.; Ping, L.K.; Mohamad Taib, M.F.; Samat, M.H.; Mohammad Haniff, M.A.S.; Bahru, R. First-principles studies for electronic structure and optical properties of p-type calcium doped α-Ga2O3. Materials 2021, 14, 604. [Google Scholar] [CrossRef]
- Dong, L.; Jia, R.; Li, C.; Xin, B.; Zhang, Y. Ab initio study of N-doped β-Ga2O3 with intrinsic defects: The structural, electronic and optical properties. J. Alloys Compd. 2017, 712, 379–385. [Google Scholar] [CrossRef]
- Choi, M.; Son, J. Doping-induced bandgap tuning of α-Ga2O3 for ultraviolet lighting. Curr. Appl. Phys. 2017, 17, 713–716. [Google Scholar] [CrossRef]
- Marezio, M.; Remeika, J.P. Bond Lengths in the α-Ga2O3 Structure and the High-Pressure Phase of Ga2−xFexO3. J. Chem. Phys. 1967, 46, 1862–1865. [Google Scholar] [CrossRef]
- Dong, L.; Yu, J.; Zhang, Y.; Jia, R. Elements (Si, Sn, and Mg) doped α-Ga2O3: First-principles investigations and predictions. Comp. Mater. Sci. 2019, 156, 273–279. [Google Scholar] [CrossRef]
- Goyal, A.; Gorai, P.; Toberer, E.S.; Stevanović, V. First-principles calculation of intrinsic defect chemistry and self-doping in PbTe. Npj Comput. Mater. 2017, 3, 42. [Google Scholar] [CrossRef]
- Ao, L.; Pham, A.; Xiang, X.; Li, S.; Zu, X. Defect induced charge trapping in C-doped α-Al2O3. J. Appl. Phys. 2017, 122, 025702. [Google Scholar] [CrossRef]
- Yan, C.; Su, J.; Wang, Y.; Lin, Z.; Zhang, J.; Chang, J.; Hao, Y. Reducing the acceptor levels of p-type β-Ga2O3 by (metal, N) co-doping approach. J. Alloys Compd. 2021, 854, 157247. [Google Scholar] [CrossRef]
- Gric, T. Tunable terahertz structure based on graphene hyperbolic metamaterials. Opt. Quantum Electron. 2019, 51, 202. [Google Scholar] [CrossRef]
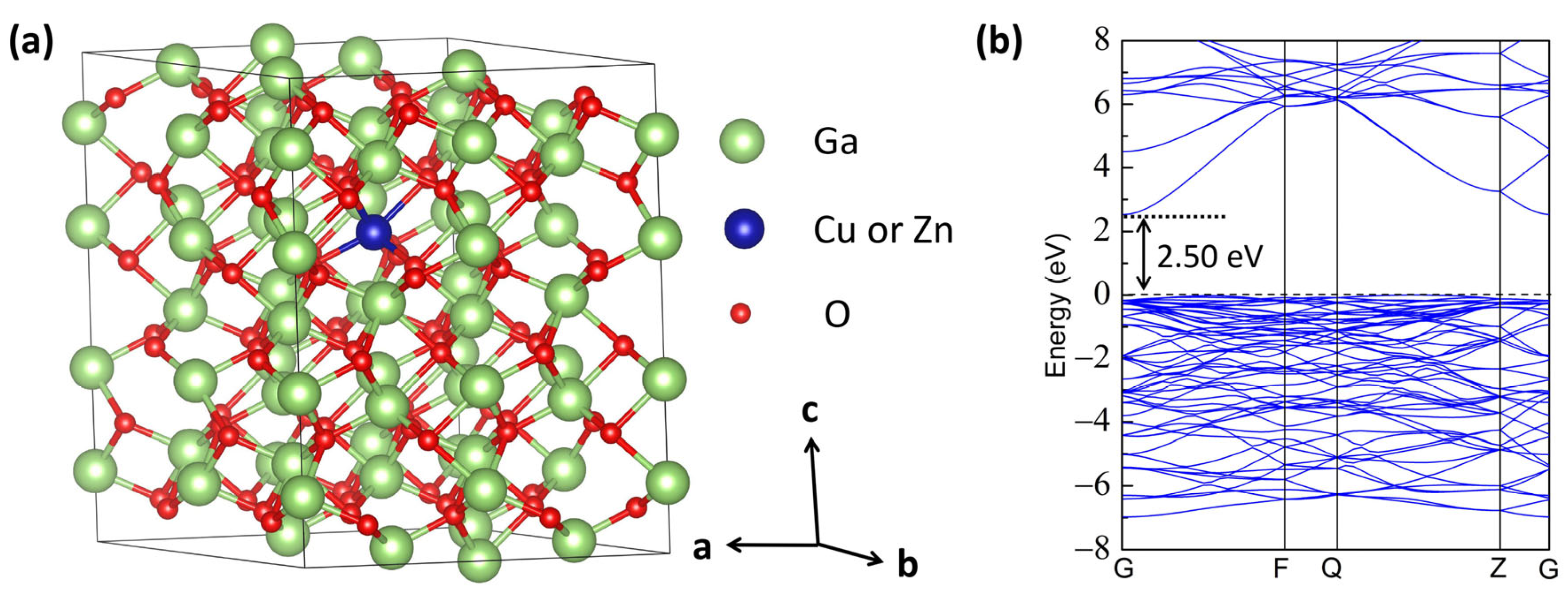


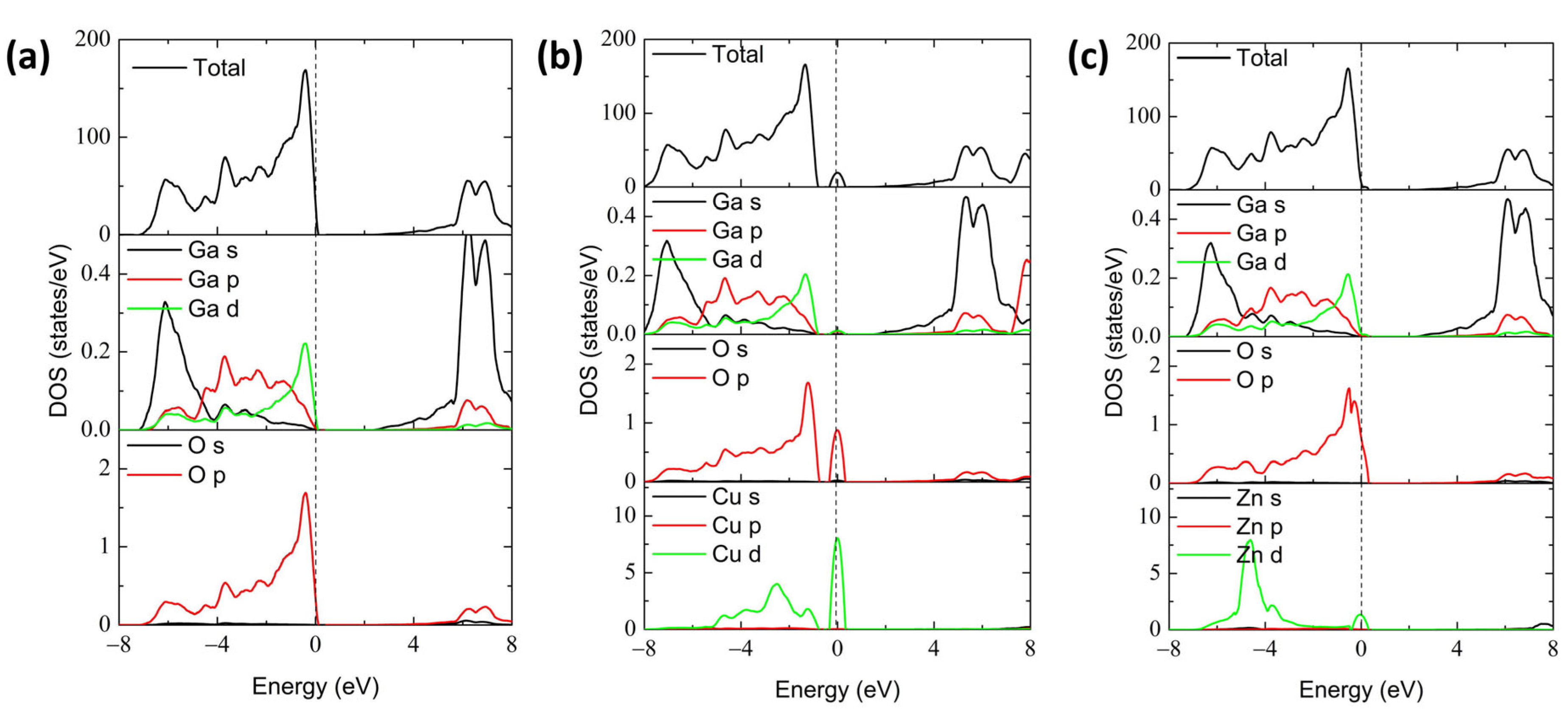
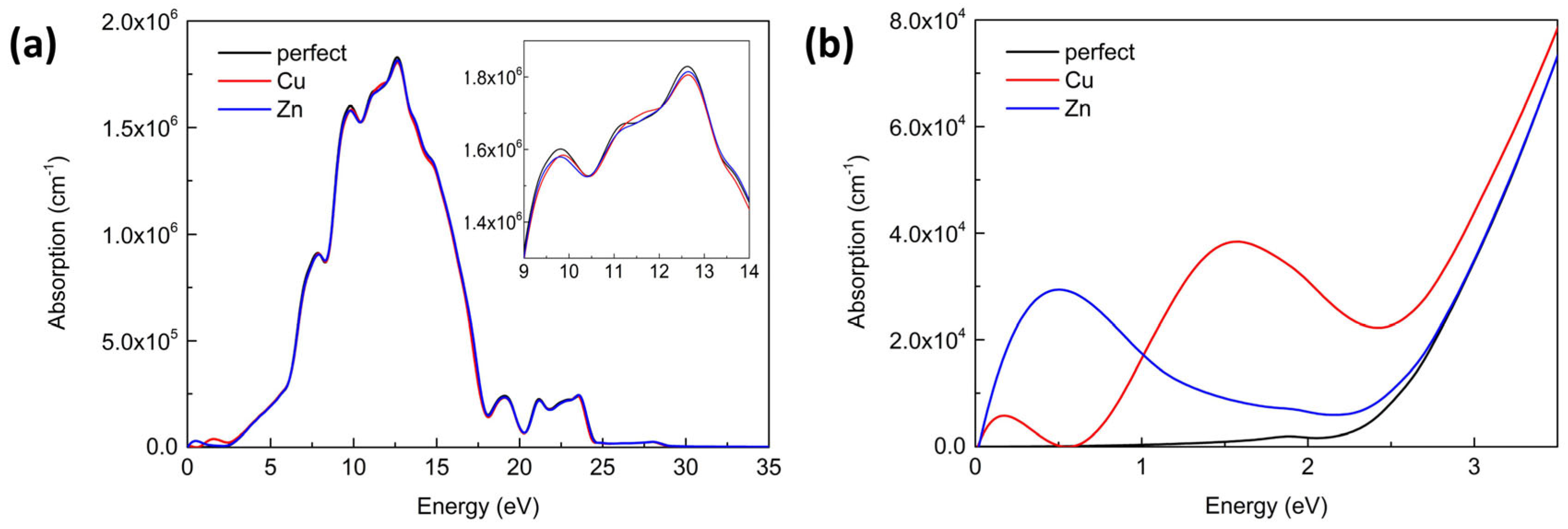
| Lattice Constants | Perfect (This Work) | Perfect (Literature) | Cu-Doped | Zn-Doped |
|---|---|---|---|---|
| a (Å) | 5.055 | 5.06 [47]/5.076 [37]/4.983 [48] | 5.056 | 5.059 |
| c (Å) | 13.586 | 13.63 [47]/13.703 [37]/13.433 [48] | 13.574 | 13.583 |
| V (Å3) | 1202.636 | 1222.8 [37] | 1202.029 | 1204.146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Wu, M.; Cheng, M.; Lin, Q. Effects of Cu, Zn Doping on the Structural, Electronic, and Optical Properties of α-Ga2O3: First-Principles Calculations. Materials 2023, 16, 5317. https://doi.org/10.3390/ma16155317
Zeng H, Wu M, Cheng M, Lin Q. Effects of Cu, Zn Doping on the Structural, Electronic, and Optical Properties of α-Ga2O3: First-Principles Calculations. Materials. 2023; 16(15):5317. https://doi.org/10.3390/ma16155317
Chicago/Turabian StyleZeng, Hui, Meng Wu, Meijuan Cheng, and Qiubao Lin. 2023. "Effects of Cu, Zn Doping on the Structural, Electronic, and Optical Properties of α-Ga2O3: First-Principles Calculations" Materials 16, no. 15: 5317. https://doi.org/10.3390/ma16155317
APA StyleZeng, H., Wu, M., Cheng, M., & Lin, Q. (2023). Effects of Cu, Zn Doping on the Structural, Electronic, and Optical Properties of α-Ga2O3: First-Principles Calculations. Materials, 16(15), 5317. https://doi.org/10.3390/ma16155317






