Chemical Pressure Effect on the Stabilization of Rock-Salt ZnO—Lin−2MeOn−1 Solid Solutions Synthesized at High Pressure
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
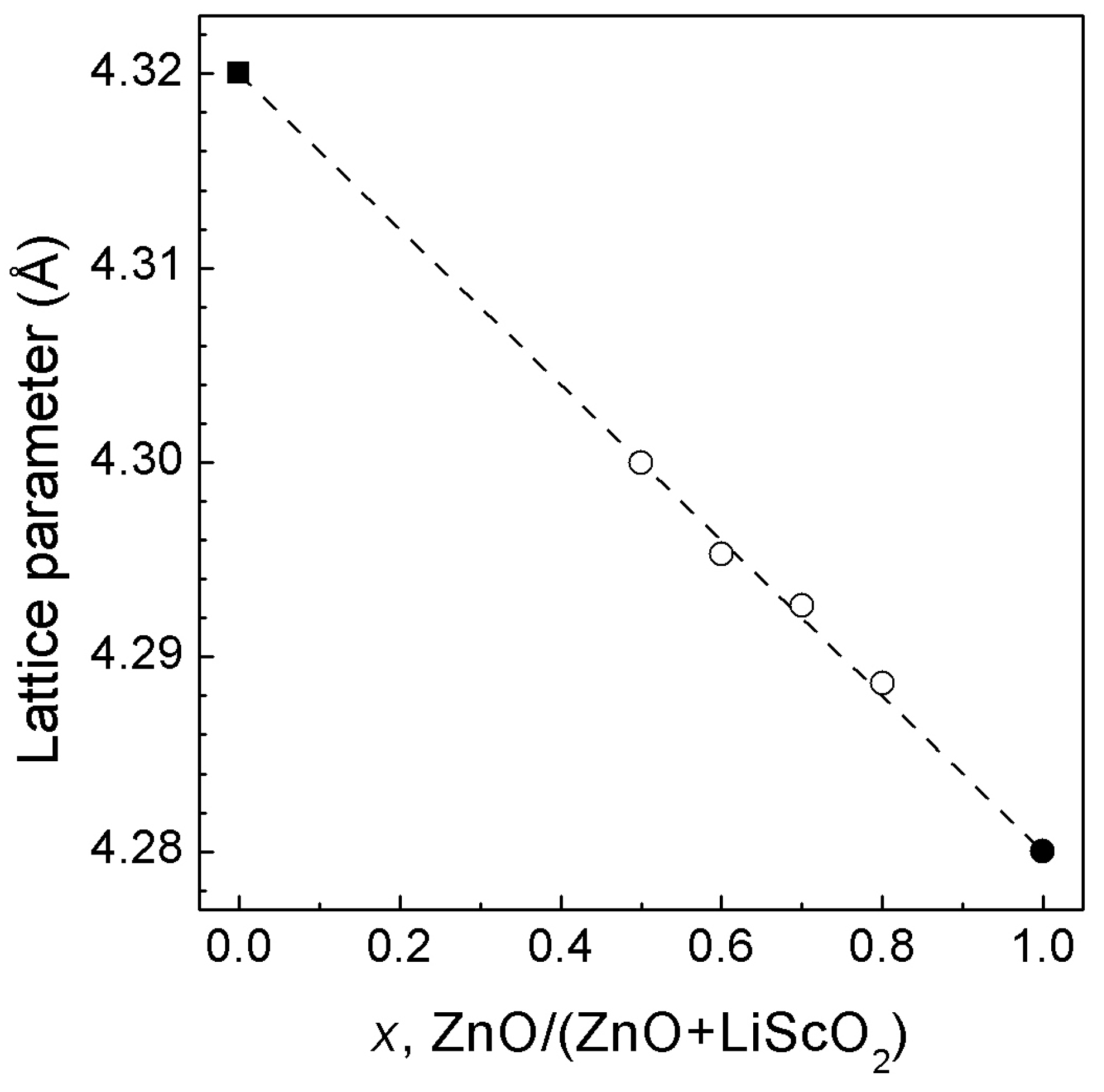
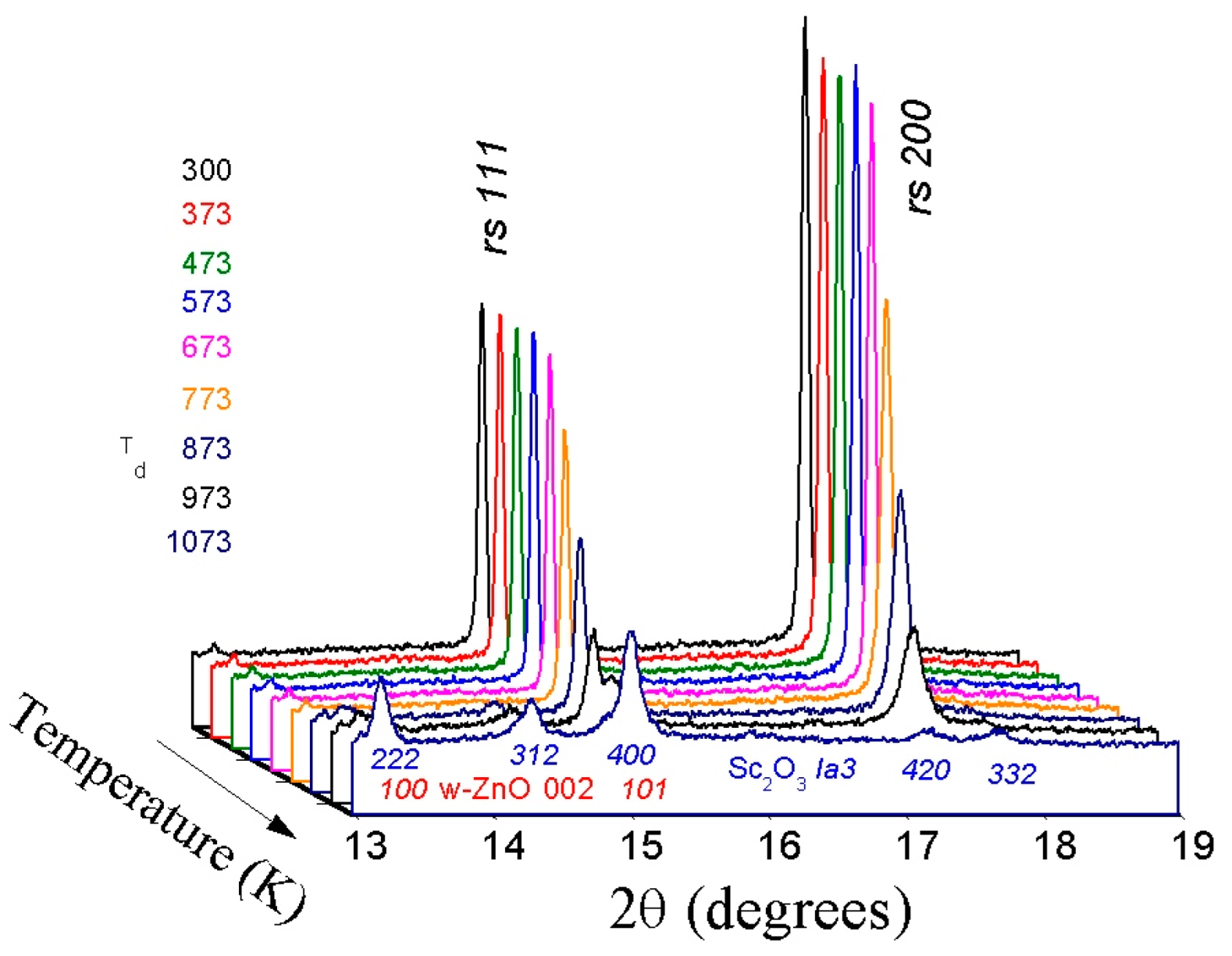
3.1. ZnO—LiScO2 System
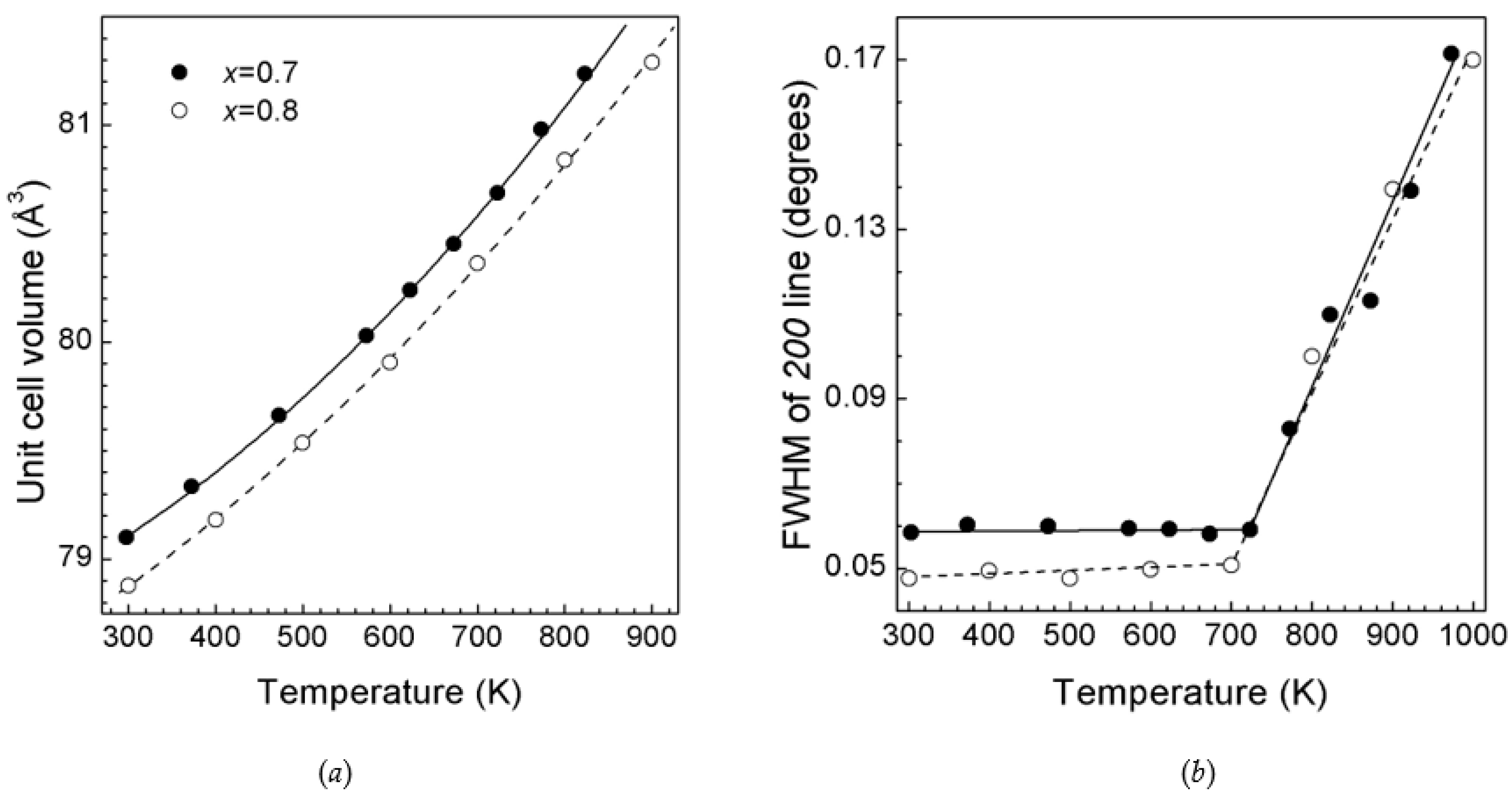
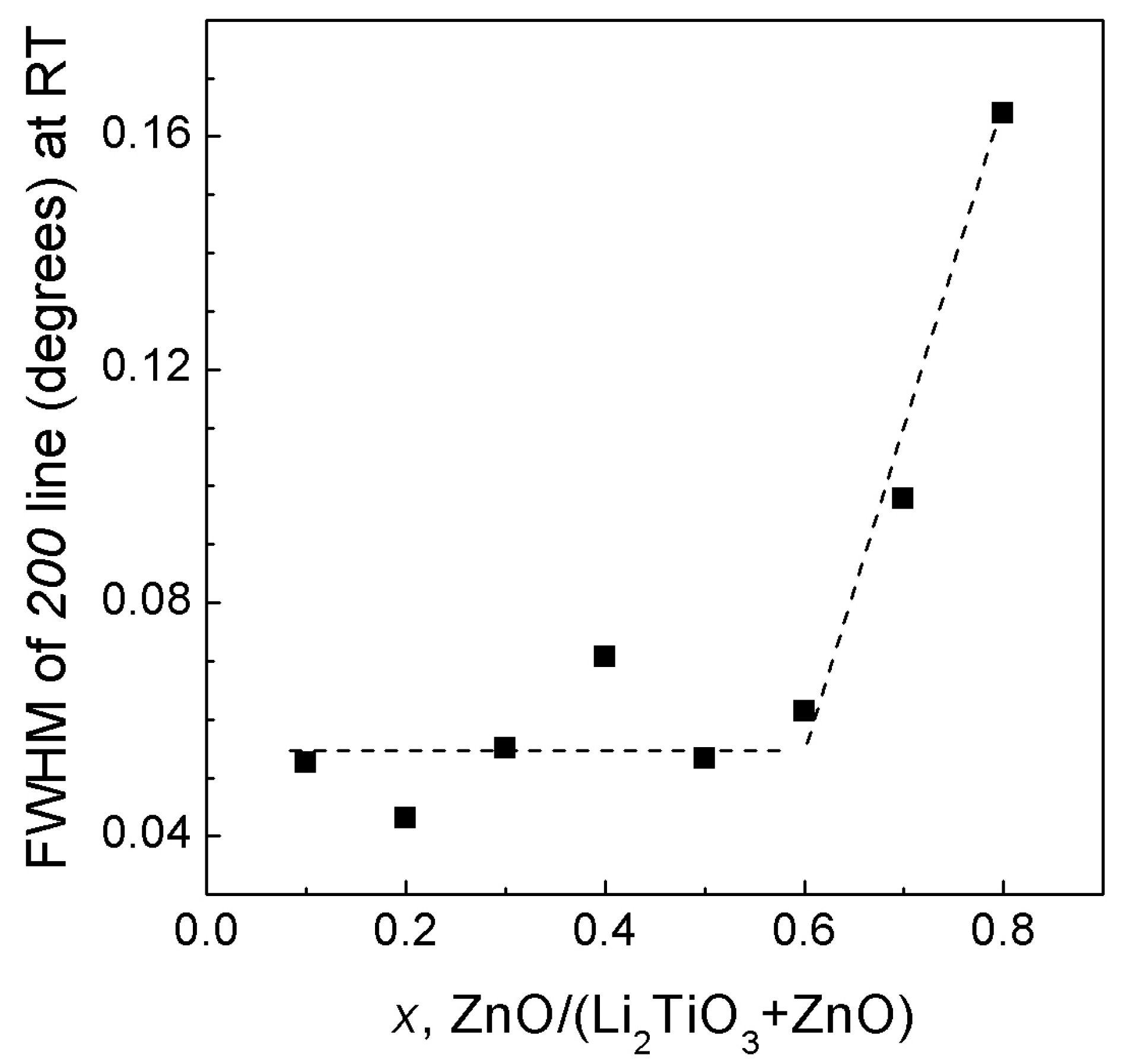
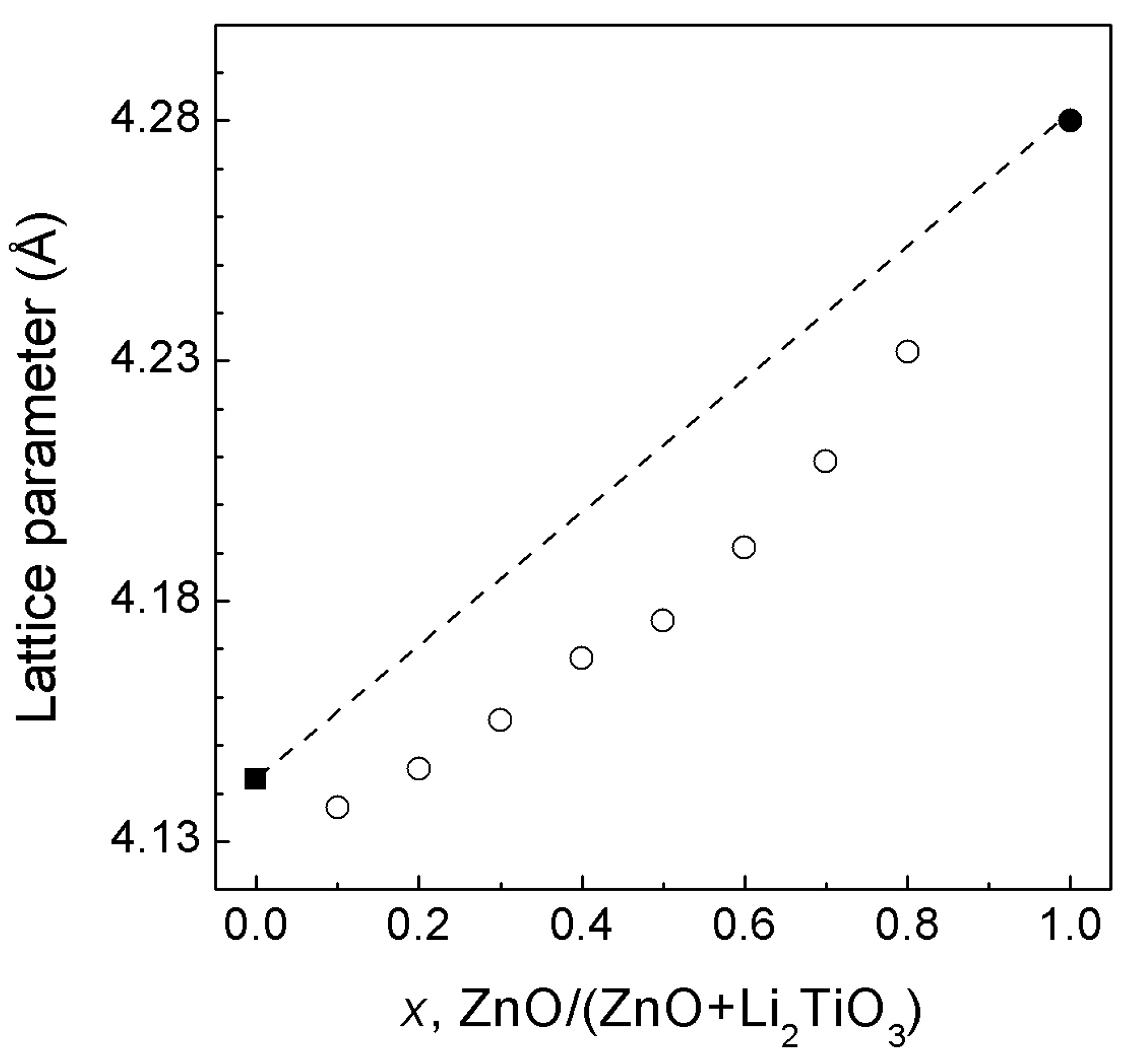
3.2. ZnO—Li2TiO3 System
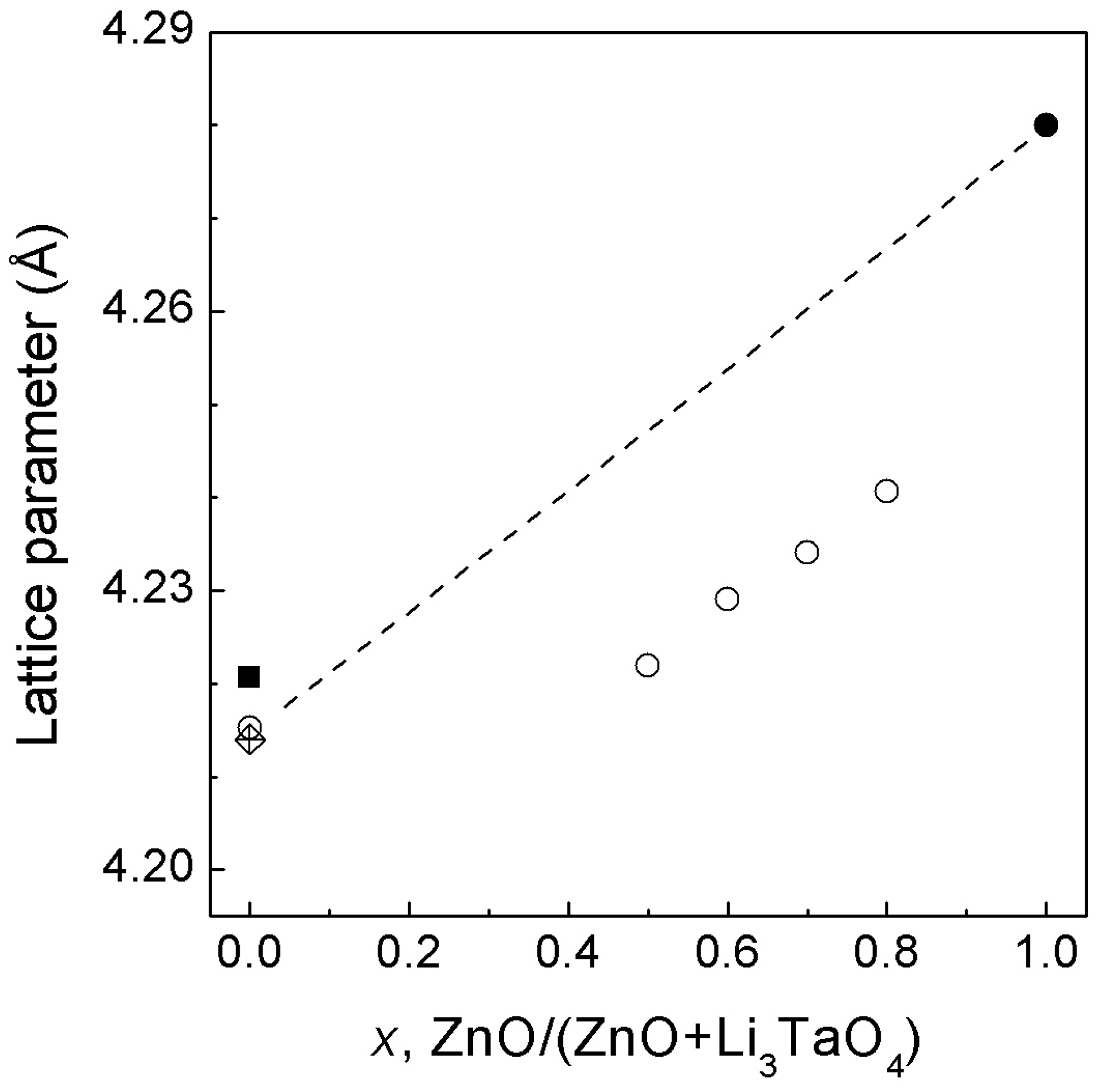
3.3. ZnO—Li3TaO4 System
3.4. General Remarks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- West, A.R. Solid State Chemistry and Its Applications; John Wiley & Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- Rao, C.N.R. Chemical Approaches to Synthesis of Inorganic Materials; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Solozhenko, V.L.; Kurakevych, O.O.; Sokolov, P.S.; Baranov, A.N. Kinetics of the wurtzite to rock-salt phase transformation in ZnO at high pressure. J. Phys. Chem. A 2011, 115, 4354–4358. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, K.; Syono, Y.; Kikegawa, T. Phase transition of ZnO under high pressure and temperature. Proc. Jpn. Acad. Ser. B 1999, 75, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Baranov, A.N.; Sokolov, P.S.; Tafeenko, V.A.; Lathe, C.; Zubavichus, Y.V.; Veligzhanin, A.A.; Chukichev, M.V.; Solozhenko, V.L. Nanocrystallinity as a route to metastable phases: Rock salt ZnO. Chem. Mater. 2013, 25, 1775–1782. [Google Scholar] [CrossRef] [Green Version]
- Baranov, A.N.; Sokolov, P.S.; Kurakevich, O.O.; Tafeenko, V.A.; Trots, D.; Solozhenko, V.L. Synthesis of rock-salt MeO–ZnO solid solutions (Me=Ni2+, Co2+, Fe2+, Mn2+) at high pressure and high temperature. High Press. Res. 2008, 28, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Baranov, A.N.; Sokolov, P.S.; Solozhenko, V.L. ZnO under pressure: From nanoparticles to single crystals. Crystals 2022, 12, 744. [Google Scholar] [CrossRef]
- Sokolov, P.S.; Baranov, A.N.; Solozhenko, V.L. Phase stability and thermal expansion of ZnO solid solutions with 3d transition metal oxides synthesized at high pressure. J. Phys. Chem. Solids 2023, 180, 111437. [Google Scholar] [CrossRef]
- Baranov, A.N.; Solozhenko, V.L.; Chateau, C.; Bocquillon, G.; Petitet, J.P.; Panin, G.N.; Kang, T.W.; Shpanchenko, R.V.; Antipov, E.V.; Oh, Y.J. Cubic MgxZn1–xO wide band gap solid solutions synthesized at high pressures. J. Phys. Cond. Matter. 2005, 7, 3377–3384. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Baranov, A.N.; Turkevich, V.Z. High-pressure formation of MgxZn1−xO solid solutions with rock salt structure. Solid State Comm. 2006, 138, 534–537. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, P.S.; Baranov, A.N.; Lathe, C.; Solozhenko, V.L. High-pressure synthesis of FeO–ZnO solid solutions with rock salt structure: In situ X-ray diffraction studies. High Press. Res. 2010, 30, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, P.S.; Baranov, A.N.; Lathe, C.; Turkevich, V.Z.; Solozhenko, V.L. High-pressure synthesis of MnO–ZnO solid solutions with rock salt structure: In situ X-ray diffraction studies. High Press. Res. 2011, 31, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, P.S.; Baranov, A.N.; Tafeenko, V.A.; Solozhenko, V.L. High pressure synthesis of LiMeO2–ZnO (Me=Fe3+, Ti3+) solid solutions with a rock salt structure. High Press. Res. 2011, 31, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Hanck, K.W.; Laitimen, H.A. Structural and thermal stability studies of LiZn2CrO4 and Co2CrO4. J. Inorg. Nucl. Chem. 1970, 33, 63–73. [Google Scholar] [CrossRef]
- Brixner, L.H. Preparation, structure and electrical properties of some substituted lithium-oxo-metallates. J. Inorg. Nucl. Chem. 1960, 16, 162–163. [Google Scholar] [CrossRef]
- Hernandez, V.S.; Martinez, L.M.T.; Mather, G.C.; West, A.R. Stoichiometry, structures and polymorphism of spinel-like phases, Li1.33xZn2−2xTi1+0.67xO4. J. Mater. Chem. 1996, 6, 1533–1536. [Google Scholar] [CrossRef]
- Hewston, T.A.; Chamberland, B.L. A survey of first-row ternary oxides LiMO2 (M = Sc-Cu). J. Phys. Chem. Solids 1987, 48, 97–108. [Google Scholar] [CrossRef]
- Mather, G.C.; Dussarrat, C.; Etourneau, J.; West, A.R. A review of cation-ordered rock salt superstructure oxides. J. Mater. Chem. 2000, 10, 2219–2230. [Google Scholar] [CrossRef]
- Kleykamp, H. Enthalpy, heat capacity and enthalpy of transformation of Li2TiO3. J. Inorg. Nucl. Mat. 2001, 295, 244–248. [Google Scholar] [CrossRef]
- Izquierdo, G.; West, A.R. Phase equilibria in the system Li2O-TiO2. Mater. Res. Bull. 1980, 14, 1655–1660. [Google Scholar] [CrossRef]
- Campos, A.; Quintana, P.; West, A.R. Order-disorder in rock salt-like phases and solid solutions, Li2(Ti1−xZrx)O3. J. Solid State Chem. 1990, 86, 129–130. [Google Scholar] [CrossRef]
- Laumann, A.; Fehr, K.T.; Wachsmann, M.; Holzapfel, M.; Iversen, B.B. Metastable formation of low temperature cubic Li2TiO3 under hydrothermal conditions—Its stability and structural properties. Solid State Ionics. 2010, 181, 1525–1529. [Google Scholar] [CrossRef]
- Laumann, A.; Fehr, K.T.; Boysen, H.; Hozel, M.; Holzapfel, M. Temperature-dependent structural transformations of hydrothermally synthesized cubic Li2TiO3 studied by in-situ neutron diffraction. Z. Kristallogr. 2011, 226, 53–61. [Google Scholar] [CrossRef]
- Du Boulay, D.; Sakaguchi, A.; Suda, K.; Ishizawa, N. Reinvestigation of β-Li3TaO4. Acta Cryst. E 2003, 59, i80–i82. [Google Scholar] [CrossRef] [Green Version]
- Zocchi, M.; Gatti, M.; Santoro, A.; Roth, R.S. Neutron and X-ray diffraction study on polymorphism in lithium orthotantalate, Li3TaO4. J. Solid State Chem. 1983, 48, 420–430. [Google Scholar] [CrossRef]
- Nyman, M.; Anderson, T.M.; Provencio, P.P. Comparison of aqueous and non-aqueous soft-chemical syntheses of lithium niobate and lithium tantalate powders. Cryst. Growth Des. 2009, 9, 1036–1040. [Google Scholar] [CrossRef]
- Blasse, G. On the structure of some compounds Li3Me5+O4, and some other mixed metal oxides containing lithium. Z. Anorg. Allg. Chem. 1964, 331, 44–50. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Kim, C.; Pham, T.L.; Lee, J.-S.; Kim, Y.-I. Synthesis, thermal analysis, and band gap of ordered and disordered complex rock salt Li3TaO4. J. Solid State Chem. 2022, 315, 123450. [Google Scholar] [CrossRef]
- Grenier, J.-C.; Martin, C.; Durif, A. Étude cristallographique des orthoniobates et orthotantalates de lithium. Bull. Soc. Franç. Minér. Crist. 1964, 87, 316–320. [Google Scholar] [CrossRef]
- Knapp, M.; Baehtz, C.; Ehrenberg, H.; Fuess, H. The synchrotron powder diffractometer at beamline B2 at HASYLAB/DESY: Status and capabilities. J. Synchrotron. Rad. 2004, 11, 328–334. [Google Scholar] [CrossRef]
- Knapp, M.; Joco, V.; Baehtz, C.; Brecht, H.H.; Berghäuser, A.; Ehrenberg, H.; von Seggern, H.; Fuess, H. Position-sensitive detector system OBI for high resolution X-ray powder diffraction using on-site readable image plates. Nucl. Instr. Meth. Phys. Res. A 2004, 521, 565–570. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mat. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Nolze, G.; Kraus, W. PowderCell 2.0 for Windows. Powder Diffr. 1998, 13, 256–259. [Google Scholar]
- Syassen, K. Computer Code DATLAB 1.34; Max Planck Institute: Stuttgart, Germany, 2012. [Google Scholar]
- Lin, K.; Li, Q.; Yu, R.; Chen, J.; Attfield, J.P.; Xing, X. Chemical pressure in functional materials. Chem. Soc. Rev. 2022, 51, 5351–5364. [Google Scholar] [CrossRef]
- Decremps, F.; Datchi, F.; Saitta, A.M.; Polian, A.; Pascarelli, S.; Di Cicco, A.; Itié, J.P.; Baudelet, F. Local structure of condensed zinc oxide. Phys. Rev. B 2003, 68, 104101. [Google Scholar] [CrossRef] [Green Version]
- Castellanos, M.; West, A.R. Order-disorder phenomena in oxides with rock salt structures: The system Li2TiO3-MgO. J. Mater. Sci. 1979, 14, 450. [Google Scholar] [CrossRef]
- Castellanos, M.; West, A.R. Deviations from Vegard’s law in oxide solid solutions. The systems Li2TiO3–MgO and Li2TiO3–Na2TiO3. J. Chem. Soc. Faraday Trans. 1980, 76, 2159–2169. [Google Scholar] [CrossRef]
- Adam, D.B.; Brindley, G.W. Cation solutions in crystalline MgO of the Type Mg1−x(R+, Rn+)xO. Mat. Sci. Eng. 1969, 3, 252–254. [Google Scholar] [CrossRef]
- Castellanos, M.; Gard, J.A.; West, A.R. Crystal data for a new family of phases, Li3Mg2XO6:X=Nb, Ta, Sb. J. Appl. Cryst. 1982, 15, 116–119. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, A.; Djenadic, R.; Usgarani, N.J.; Sanghvi, K.P.; Chakravadhanula, V.S.K.; Gandhi, A.S.; Hahn, H.; Bhattacharya, S.S. Nanocrystalline multicomponent entropy stabilised transition metal oxides. J. Eur. Ceram. Soc. 2017, 37, 747–754. [Google Scholar] [CrossRef]
- Fracchia, M.; Coduri, M.; Manzoli, M.; Ghigna, P.; Tamburini, U.A. Is configurational entropy the main stabilizing term in rock-salt Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O high entropy oxide? Nat. Commun. 2022, 13, 2977. [Google Scholar] [CrossRef] [PubMed]
- Baranov, A.N.; Kurakevych, O.O.; Tafeenko, V.A.; Sokolov, P.S.; Panin, G.N.; Solozhenko, V.L. High-pressure synthesis and luminescent properties of cubic ZnO/MgO nanocomposites. J. Appl. Phys. 2010, 107, 073519. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, P.S.; Baranov, A.N.; Dobrokhotova, Z.V.; Solozhenko, V.L. Synthesis and thermal stability of cubic ZnO in the salt nanocomposites. Russ. Chem. Bull. 2010, 59, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Urusov, S.V. Interaction of cations on octahedral and tetrahedral sites in simple spinels: A reply. Phys. Chem. Miner. 1984, 10, 194–195. [Google Scholar] [CrossRef]
- Li, B.; Sougrati, S.T.; Rouse, G.; Morozov, A.V.; Dedryvere, R.; Ladecola, A.; Senyshyn, A.; Zhang, L.; Abakumov, A.M.; Doublet, M.L.; et al. Correlating ligand-to-metal charge transfer with voltage hysteresis in a Li-rich rock-salt compound exhibiting anionic redox. Nat. Chem. 2021, 13, 1070–1108. [Google Scholar] [CrossRef] [PubMed]
- Lun, Z.; Ouyang, B.; Kwon, D.H.; Ha, Y.; Foley, E.E.; Huang, T.-Y.; Cai, H.; Kim, H.; Balasubramanian, M.; Sun, Y.; et al. Cation-disordered rocksalt-type high-entropy cathodes for Li-ion batteries. Nat. Mater. 2021, 20, 214–221. [Google Scholar] [CrossRef]
- Yuan, L.L.; Bian, J.J. Microwave Dielectric Properties of the Lithium Containing Compounds with Rock Salt Structure. Ferroelectrics 2009, 387, 123–129. [Google Scholar] [CrossRef]
- Uyama, T.; Mukai, K.; Yamada, I. High-Pressure Synthesis of Cation-Disordered Rock-Salt Oxyfluorides with High Crystallinity. Electrochemistry 2021, 89, 94–99. [Google Scholar] [CrossRef]
- Chen, M.-M.; Xue, H.-G.; Guo, S.-P. Multinary metal chalcogenides with tetrahedral structures for second-order nonlinear optical, photocatalytic, and photovoltaic applications. Coordin. Chem. Rev. 2018, 368, 115–133. [Google Scholar] [CrossRef]
- Sakuda, A.; Takeuchi, T.; Okamura, K.; Kobayashi, H.; Sakaebe, H.; Tatsumi, K.; Ogumi, Z. Rock-salt-type lithium metal sulphides as novel positive-electrode materials. Sci. Rep. 2014, 4, 4883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchandier, T.; Mariayppan, S.; Kirsanova, M.A.; Abakunov, A.M.; Rousse, G.; Foix, D.; Sougratim, M.-T.; Doublet, M.L.; Tarascon, J.-M. Triggering Anionic Redox Activity in Li3NbS4 Through Cationic Disordering or Substitution. Adv. Energy Mater. 2022, 12, 2201417. [Google Scholar] [CrossRef]




| Precursor | Cation Radius (Å) * | Crystal Structure (Space Group) | References | |
|---|---|---|---|---|
| LiScO2 | Sc3+ (0.74) | I41/amd (#141) | [19,20] | |
| Li2TiO3 | Ti4+ (0.60) | C2/c (#15) † | Fm-3m (#225) ‡ | [18,21,22,23,24,25] |
| Li3TaO4 | Ta5+ (0.64) | C2/c (#15) § | P2/n (#13) § | [26,27,28,29] |
| Composition, x | V0 (Å3) | α1 × 105 (K−1) | α2 × 108 (K−2) |
|---|---|---|---|
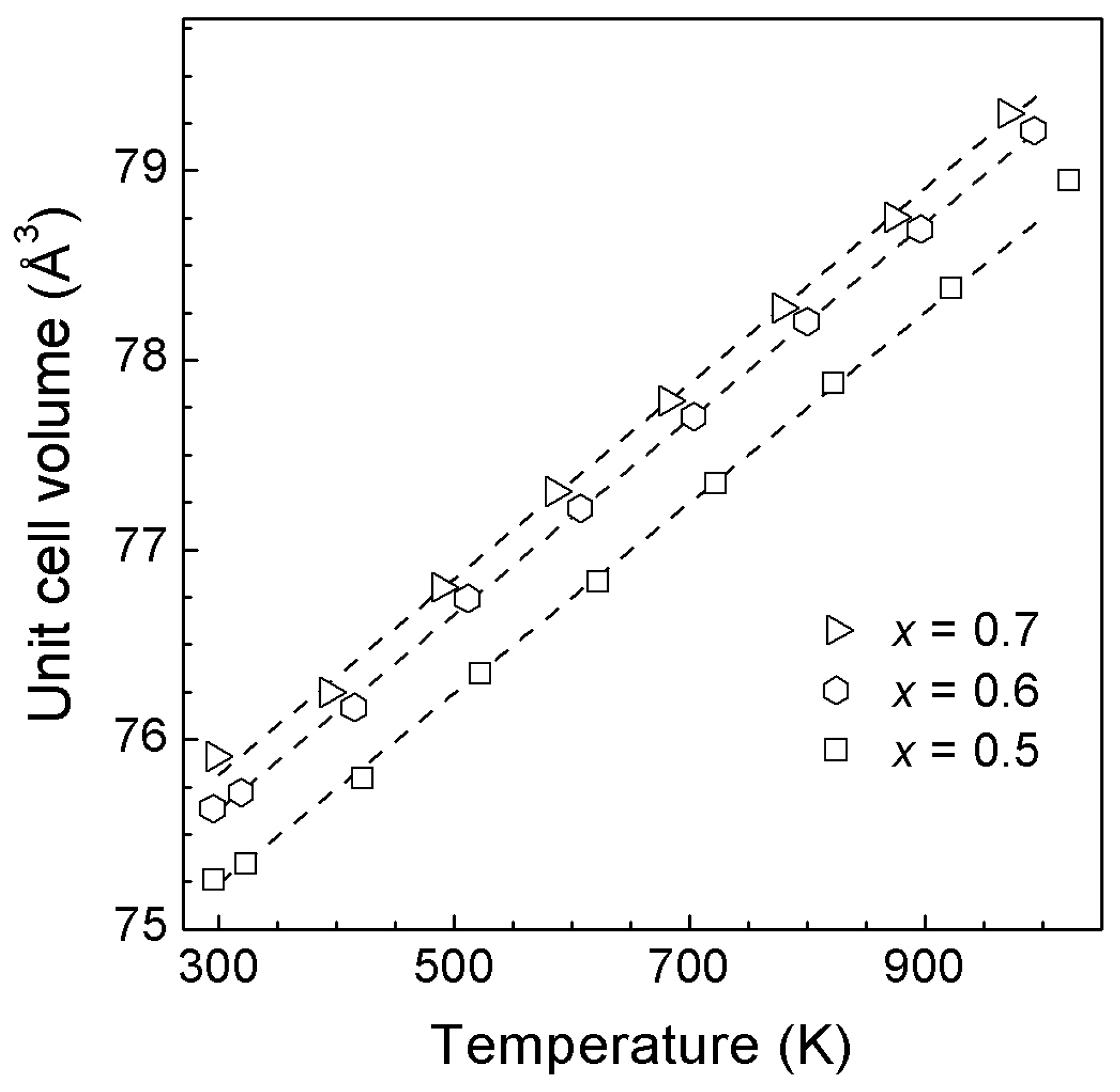
| 0.8 | 78.876(2) | 3.70(4) | 2.4(8) |
| 0.7 | 79.115(4) | 3.35(5) | 3.2(1) |
| Composition, x | V0 (Å3) | α × 105 (K−1) |
|---|---|---|
| 0.8 | 74.78(2) | 5.42(6) |
| 0.7 | 74.52(1) | 5.75(3) |
| 0.6 | 73.592(4) | 6.16(2) |
| 0.5 | 72.797(4) | 6.39(1) |
| 0.4 | 72.296(3) | 6.20(1) |
| 0.3 | 71.563(4) | 6.39(1) |
| 0.2 | 71.221(2) | 6.47(1) |
| 0.1 | 70.820(1) | 6.42(1) |
| 0.0 [25] | 71.20(2) | 6.52(4) |
| Composition, x | V0 (Å3) | α × 105 (K−1) |
|---|---|---|
| 0.7 | 75.81(1) | 6.79(3) |
| 0.6 | 75.62(1) | 6.81(2) |
| 0.5 | 75.23(1) | 6.67(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, P.S.; Baranov, A.N.; Solozhenko, V.L. Chemical Pressure Effect on the Stabilization of Rock-Salt ZnO—Lin−2MeOn−1 Solid Solutions Synthesized at High Pressure. Materials 2023, 16, 5336. https://doi.org/10.3390/ma16155336
Sokolov PS, Baranov AN, Solozhenko VL. Chemical Pressure Effect on the Stabilization of Rock-Salt ZnO—Lin−2MeOn−1 Solid Solutions Synthesized at High Pressure. Materials. 2023; 16(15):5336. https://doi.org/10.3390/ma16155336
Chicago/Turabian StyleSokolov, Petr S., Andrey N. Baranov, and Vladimir L. Solozhenko. 2023. "Chemical Pressure Effect on the Stabilization of Rock-Salt ZnO—Lin−2MeOn−1 Solid Solutions Synthesized at High Pressure" Materials 16, no. 15: 5336. https://doi.org/10.3390/ma16155336






